Abstract
The effect of chrysophanic acid (CA) (2, 4, and 8 mg kg−1) on the immunity and immune-related gene profile of Catla catla against Aeromonas hydrophila is reported. In both control and treated groups fed with 2 mg kg−1 (2 CA), the phagocytosis, hemolytic, myeloperoxidase content, and superoxide anion production decreased significantly between 6th and 8th weeks, whereas when fed with 4 mg kg−1 CA (4 CA) the H2O2 production and nitric oxide synthase increased significantly between 4th and 8th week. When fed with 2 CA and 4 CA diets, the total protein, bactericidal, and antibody titer increased significantly from the 4th week onwards. When fed with 2 CA, the IL-1β and IL-10 mRNA expression of head kidney leucocytes were significant between weeks 6 and 8. The expressions of toll-like receptors significantly increased when fed with a 4 CA diet from 4th week onwards. The 4 CA group significantly increased in TNF-α, TNF receptor-associated factor 6 (NOD), which influences protein expression, after the 4th week. The mRNA transcription of MHCI, lysozyme-chicken and goose type expressions significantly increased in 4 CA group within the 4th week. In summary, the dietary administration of 4 mg kg−1 of CA (4 CA) provides better immunity and enhances the up-regulation of immune-related genes in Catla against A. hydrophila.
Subject terms: Immunology, Adaptive immunity, Gene regulation in immune cells, Innate immunity
Introduction
Today’s growing world population has led to increasing demand for aquaculture as a luxury and cheap protein source1. Correspondingly, the current aquaculture practice has shifted from extensive to semi- or intensive systems. In the year 2016, the global aquatic food production has exceeded 171 million tons2. Fish production in the first two quarters of 2017 and 2018 increased to 5.80 million tons3. Among the Indian major carps (IMCs) Catla catla is the most commonly farmed freshwater fish due to its size, good flavor, high protein content, omega-3 fatty acids, yet with fewer triglycerides, which promote brain function4; besides species like C. catla are also a cheap source of aqua-protein (about 2 US dollars/kg in countries like India). However, an intensive aquaculture system triggers a highly stressful environment that adversely affects the immune system, making the cultivated fish more vulnerable to infectious agents5. Besides, any culture system with maximum rearing density triggers frequent outbreaks of several infectious diseases, increasing the host susceptibility, virulence of the pathogen, and health-related problems6–8. Like other IMCs, Catla suffers from several infections, including aeromoniasis, edwardsiellosis, and epizootic ulcerative syndrome (EUS)9. Among these, Aeromonas hydrophila is a leading bacterial pathogen known to cause symptoms like haemorrhagic septicaemia, infectious dropsy, ulcerative lesion, and fin rot resulting in mass mortality10,11 affecting the quality and quantity of the size of harvest significantly. To manage these diseases, fish farmers conventionally use broad-spectrum antibiotics and chemotherapeutics, which often lead to frequent outbreaks and spread of resistant strains and environmental threats, creating further problems12,13. Vaccines are an effective prophylactic measure in aqua-practice to inhibit or control of infectious diseases; however, its success rate varies since, as they are pathogen specific14. Furthermore, the vaccines developed for intracellular fish pathogens are yet to become successful15,16.
In this regard, natural immunostimulants have become an attractive alternative. In fish disease management, they are widely accepted to sustain aquaculture, as they are biocompatible, biodegradable, often readily available, eco-friendly, and safe for human health17,18. Several immune-stimulants are known to afford disease protection by boosting non-specific and specific immune systems19–22. The use of herbals and their bioactive constituents can circumvent the use of traditional chemotherapies. In plants, polyphenols like anthraquinones, lignans, flavonoids, and aromatic acids are widely distributed; they exhibit antioxidant properties23; in human health management, the herbal medicine also play a progressive role in controlling cardiac disease24, cancer25, and viral infection26. Chrysophanic acid or chrysophanol (1, 8-dihydroxy-3-methyl-anthraquinone) that come under the anthraquinone family is widely distributed in Chinese herbs (Rheum officinale and Polygpnum cuspidatum), and exhibit anti-bacterial and anti-fungal properties27,28. Chrysophanic acid (CA) induces reactive oxygen species (ROS) production, dysfunction of mitochondria, and damage of ATP and DNA, causing necrotic cell death in human liver J5 cancer cells29–31. CA is also known to stimulate cytosolic Ca21 production, and cause a decrease in Dilated Cardiomyopathy (DCM) levels32. However, no detailed research has been conducted on CA’s effect with reference to immunity and cytokine gene modulations in aquatic species. This work aims to find out the effect of diets containing CA on the innate and adaptive immunity and expression of immune-related gene expression in C. catla against A. hydrophila.
Results
Immunological response
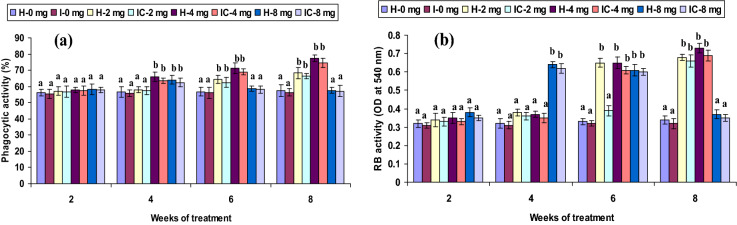
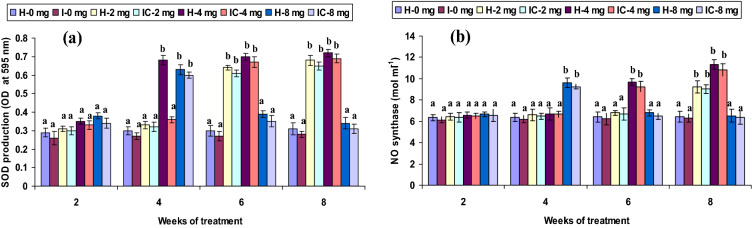
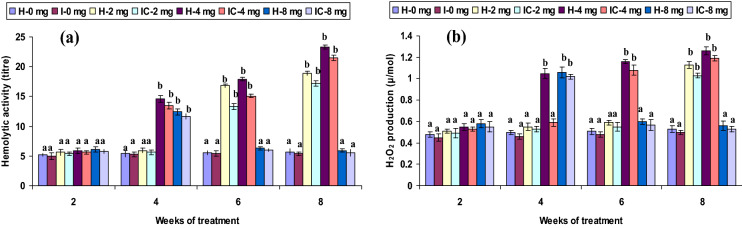
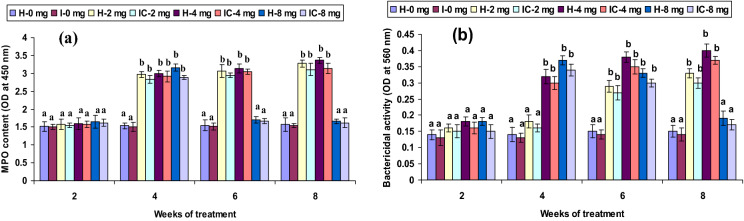
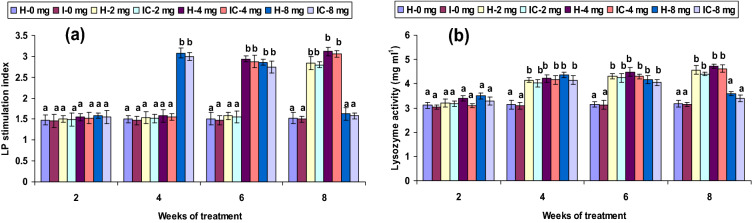
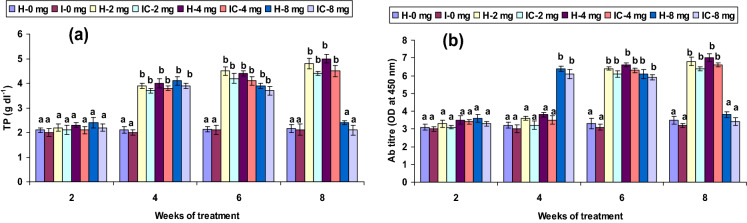
Both healthy and infected groups fed with the 2 CA enriched diet had increased phagocytic and hemolytic activity and SOD generation (P < 0.05) between the 6th and 8th weeks, but not in the 2nd or 4th week. Fish fed with 4 CA diet in both groups exhibited these activities at the 4th, 6th, and 8th weeks. However, the 8 CA administration significantly enhanced these parameters in the 2nd week only; no differences were observed in either the 1st week or after the 6th week (Figs. 1a, 2a, 3a). In both 2 CA groups, enhanced LP index, MPO content, bactericidal action, and antibody levels were observed between the 6th and 8th weeks. A similar trend existed in both groups fed with 4 CA from week 2 to 8. The maximum values of the immune parameters were obtained when both groups were fed the 8 CA diets between weeks 2 and 4; however, no increases were observed in the initial study period (week 1) or after prolonged periods (after week 8) (Figs. 4a,b, 5a, 6b).
Figure 1.
(a) Phagocytic activity and (b) respiratory burst (RB) activity of catla (n = 6) head kidney leucocytes against A. hydrophila. H healthy, I infected. Data are expressed as mean ± SD and the statistically significant difference (P < 0.05) between mean values as indicated in different letters in each column.
Figure 2.
(a) Superoxide anion (SOD) radical production and (b) nitric oxide (NO) synthase of catla (n = 6) head kidney leucocytes against A. hydrophila. H healthy, I infected. Data are expressed as mean ± SD and the statistically significant difference (P < 0.05) between mean values as indicated in different letters in each column.
Figure 3.
(a) Hemolytic activity and (b) hydrogen peroxide (H2O2) production of catla (n = 6) head kidney leucocytes against A. hydrophila. H healthy, I infected. Data are expressed as mean ± SD and the statistically significant difference (P < 0.05) between mean values as indicated in different letters in each column.
Figure 4.
(a) Myeloperoxidase (MPO) content and (b) bactericidal activity of catla (n = 6) head kidney leucocytes against A. hydrophila. H healthy, I infected. Data are expressed as mean ± SD and the statistically significant difference (P < 0.05) between mean values as indicated in different letters in each column.
Figure 5.
(a) Lymphocyte proliferate (LP) stimulate index and (b) lysozyme activity of catla (n = 6) head kidney leucocytes against A. hydrophila. H healthy, I infected. Data are expressed as mean ± SD and the statistically significant difference (P < 0.05) between mean values as indicated in different letters in each column.
Figure 6.
(a) Total protein (TP) level and (b) antibody (Ab) titre of catla (n = 6) head kidney leucocytes against A. hydrophila. H healthy, I infected. Data are expressed as mean ± SD and the statistically significant difference (P < 0.05) between mean values as indicated in different letters in each column.
Low respiratory burst (RB) activity was observed in both 2 CA and 4 CA groups in the 2nd and 4th weeks; however, the highest (P < 0.05) values were noted after week 6. In both 8 CA groups, the highest RB activity (P < 0.05) manifested between weeks 4 and 6, but these values were insignificant in the 1st week and after week 8 (Fig. 1b). The nitric oxide (NO) synthase was low in both 2 CA groups (weeks 2–6); increased significantly after the 8th week. Similarly, both 4 CA groups had slightly higher levels (P > 0.05) of NO synthase in the 2nd and 4th weeks, and significantly (P < 0.05) higher levels between weeks 6 and 8. NO synthase levels varied moderately (P > 0.05), except in week 2 (Fig. 2b).
Hydrogen peroxide (H2O2) production did not differ (P > 0.05) significantly in the 2 CA fed groups from weeks 2 to 6 but attained significant levels (P < 0.05) in the 8th week. The highest H2O2 production was observed in both 4 CA groups between the 4th and 8th weeks, and the lowest production activity (P > 0.05) occurred in the 8 CA groups (Fig. 3b). Lysozyme levels and TP levels decreased slightly (P > 0.05) in the 2nd week in the 2 CA and 4 CA groups. These values rose significantly (P < 0.05) in both 8 CA groups from 4 to 8 weeks, though increases did not vary in either the 1st or after the 8th weeks (Figs. 5b, 6a).
Immune gene expression
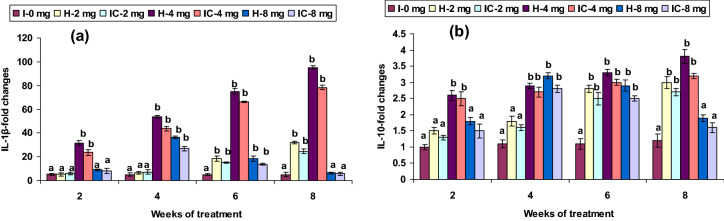
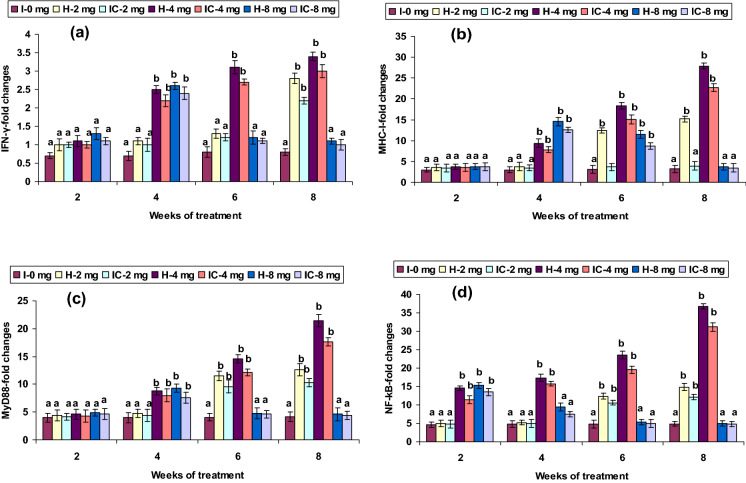
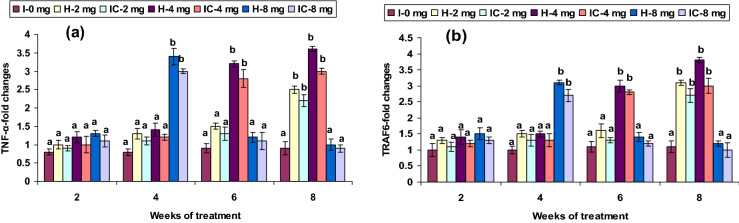
The mRNA transcripts of IL-1β and 10, TLR-4, as well as MHC-I in head kidney leucocytes, were significantly low between weeks 2 and 4 when fed with 2 CA in both groups, whereas its expression was up-regulated between the 6th and 8th weeks. Feeding with 4 CA could induced IL-1β, IL-10, TLR-4, and MHC-I mRNA expression in both groups significantly from weeks 4 to 8, compared with that of the control. However, the IL-1β, IL-10, TLR-4, and MHC-I mRNA expression were significant in both groups in the 4th and 6th weeks in the 8 CA groups; yet no up-regulation was observed in the first or after the 8th week (Figs. 7a,b, 8b, 10c). The expression levels of TRAF6 and TNF-α were not significantly up-regulated in the 2 CA groups from week 2 to 6, while significant up-regulation was observed in the 8th week. Both groups fed with 4 CA showed down-regulation in the 2nd and 4th weeks, whereas up-regulation was observed in the 6th and 8th weeks. Nevertheless, the TRAF6 and TNF-α expression levels were slightly higher in the groups fed with 8 CA from week 4 to 8 (Fig. 9a,b).
Figure 7.
Expression pattern of (a) IL-1β and (b) IL-10 gene relative to β-actin in the head kidney of catla (n = 3) against A. hydrophila. H healthy, I infected. Values are expressed as mean ± SD and the statistical difference between means (P < 0.05) indicated in different letters in each column.
Figure 8.
Expression pattern of (a) IFN-γ, (b) MHC-I, (c) MyD88, and (d) NF-kB relative to β-actin in the head kidney of catla (n = 3) against A. hydrophila. H healthy, I infected. Values are expressed as mean ± SD and the statistical difference between means (P < 0.05) indicated in different letters in each column.
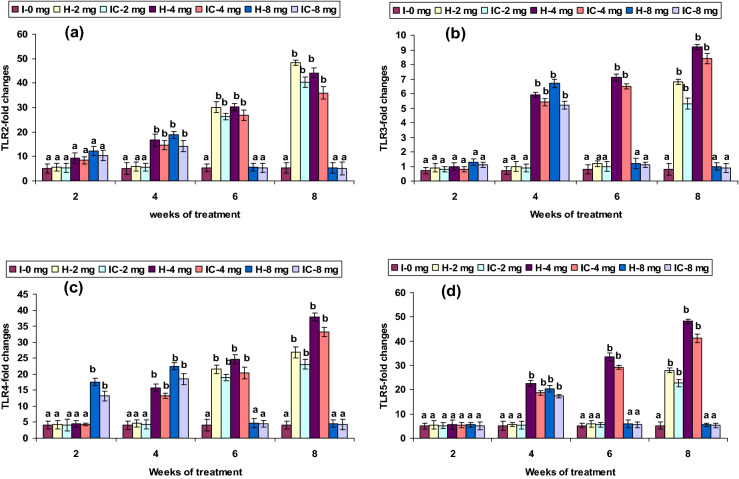
Figure 10.
Expression pattern of (a) TLR-2, (b) TLR-3, (c) TLR-4, and (d) TLR-5 relative to β-actin in the head kidney of catla (n = 3) against A. hydrophila. H healthy, I infected. Values are expressed as mean ± SD and the statistical difference between means (P < 0.05) indicated in different letters in each column.
Figure 9.
Expression pattern of (a) TNF-α and (b) TRAF6 relative to β-actin in the head kidney of catla (n = 3) against A. hydrophila. H healthy, I infected. Values are expressed as mean ± SD and the statistical difference between means (P < 0.05) indicated in different letters in each column.
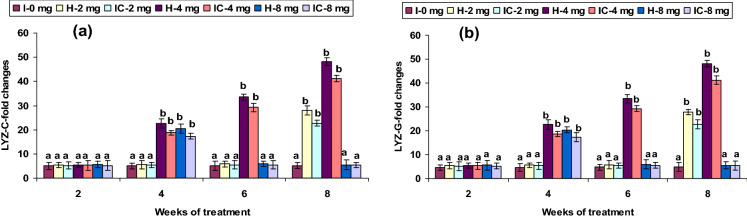
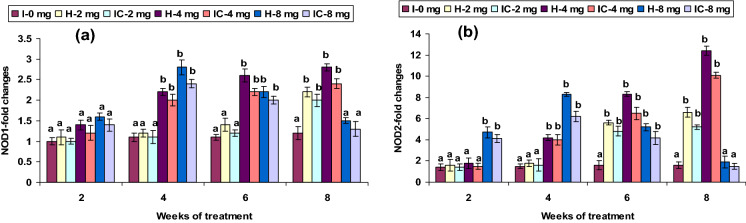
The expression of INF-γ, TLR3, TLR5, Lyz-C, and Lyz-G genes showed no up-regulation in both 2 CA groups from weeks 2 to 6; however, mRNA gene expressions were up-regulated in the 8th week. The 4 CA groups had a significant induction of INF-γ, MyD88, TLR3, TLR5, Lyz-C, and Lyz-G genes expression between weeks 4 and 8, but not in the 1st week (Figs. 8a,c, 10b,d, 11a,b). Interestingly, a slight up-regulation of NF-kB and TLR2 expression was observed in weeks 2 and 4 in the 2 CA group. However, there was a significant up-regulation between weeks 6 and 8. Similar results were found with the 4 CA and 8 CA groups in the 2nd and 4th weeks; however, all the values decreased after the 6th week (Figs. 8d, 10a). The expression of NOD1 and NOD2 were not significantly improved in the 2 CA group (weeks 2–6), yet there was a sudden up-regulation in the 8th week. Significant NOD1 and NOD2 mRNA expressions were also observed in the 4 CA group after the 4th week, as well as in the 8 CA groups (weeks 4 and 6), but not in the 1st week or after the 8th week (Fig. 12a,b).
Figure 11.
Expression pattern of (a) LYZ-C and (b) LYZ-G relative to β-actin in the head kidney of catla (n = 3) against A. hydrophila. H healthy, I infected. Values are expressed as mean ± SD and the statistical difference between means (P < 0.05) indicated in different letters in each column.
Figure 12.
Expression pattern of (a) NOD1 and (b) NOD2 relative to β-actin in the head kidney of catla (n = 3) against A. hydrophila. H healthy, I infected. Values are expressed as mean ± SD and the statistical difference between means (P < 0.05) indicated in different letters in each column.
RPS
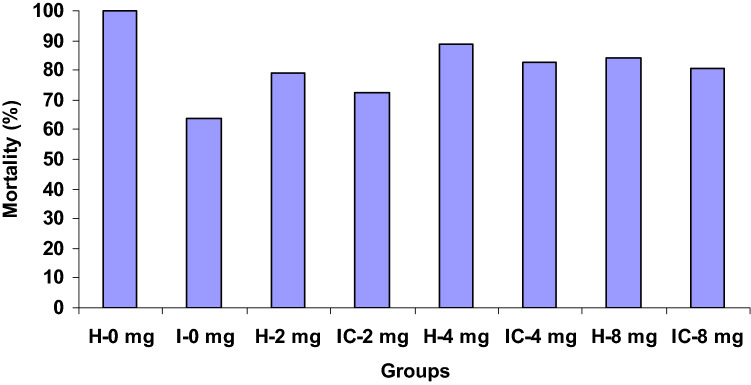
Survival was 100% in group I (H-0 mg). In both post-challenged and un-challenged groups (groups III and IV), treated with 4 CA and 8 CA resulted in higher survival rates between 80.5 and 89%. On the other hand, post-challenged or un-challenged (groups II and VI) treated with 2 CA had low survival between 72.5 and 79%. However, post-challenged group V treated with the control diet had the least survival of 64% (Fig. 13).
Figure 13.
Survival rate in C. catla post-challenged and un-challenged A. hydrophila groups treated with different concentration of (0 mg, 2 mg, 4 mg, 8 mg) of chrysophanic acid (CA) observed at the end of experiment. H healthy, I infected.
Discussion
The ability of phagocytic cells to kill the invading pathogens is an essential innate defense mechanism33. In the present study, phagocytic activity significantly improved in both groups (P < 0.05) with doses of 2 mg kg−1 CA (2 CA) between weeks 6 and 8; and in the 4 CA group between weeks 4 and 8. The phagocytic leucocytes harvested from HK of the 2 CA and 4 CA groups revealed high RB activity, attributed as a metabolic function due to CA in the 6th and 8th weeks, resulting in higher amounts of SOD synthesis. The HK leukocytes RB activity is another important innate immune function widely considered a bio-indicator of immune-competence, exclusively triggered by immunostimulants33,34. Extracellular O2− production was highly significant in the 2 CA groups (6 and 8 weeks) and the 4 CA groups (4–8 weeks). Similar results were found in rohu, in which a higher level of O2− production was reported (Days 4, 6, and 14) post-vaccination with A. hydrophila35. The O2− production in macrophages exhibits immunity after the initiation of phagocytes in fish that generate reactive oxygen products; such as H2O2 and OH, during a period of robust O2 intake, termed as RB36. This ability to kill microorganisms by phagocytes has an essential role in anti-microbicidal mechanisms in fish37. The trout HK leucocytes collected after zymosan exposure showed maximum oxygen consumption or RB activity that produced 12 nM or 13 nM superoxide anion for 107 cells38. In the present study, both groups fed 2 and 4 mg kg−1 CA diets (2 CA and 4 CA) showed no significant change in RB activity in weeks 4–6. These results were in agreement with a recent study in which a significant observance of RB activity was reported in Atlantic salmon and rainbow trout against Lepeophtheirus salmonis39. However, significantly high RB activity was observed in Pangasianodon hypophthalmus against pathogen40. Similarly, Munoz, et al.41 reported that a higher O2− level afforded protection to Dicentrarchus labrax against Sphaerospora dicentrarchi. Natural immunostimulants do not significantly affect the innate defense response in fish42,43; numerous studies have reported a higher RB activity in fish through various immunostimulants44,45. H2O2 production was significantly high in the 8th week of the 4 CA feeding, whereas the RB activity in the 8 CA group was manifested in the 2nd week. Similar increases in H2O2 production were observed in Psetta maxima and Sparus aurata via the administration of glucan46. High ROS production due to glucan may be associated with augmented bactericidal and phagocytosis, as reported in Atlantic salmon and rainbow trout47,48. The ability to generate H2O2 was reported in neutrophils due to O2 breakdown during RB in channel catfish49. However, in the present study, the H2O2 production was low in both groups fed 2 mg kg−1 CA (2 CA) from week 2 to 6. Similarly, the native or heat-denatured lectin of Abrus precatorius induced low phagocytosis levels, bactericidal, and H2O2 production in mice macrophages50.
A significantly higher proliferative response was observed in both 2 CA groups after week 6, while in the 4 CA group, the same effect was observed after week 4. Consequently, with the administration of the A. hydrophila vaccine, the proliferative response in fish increased51. A lower response was observed in the earlier stage, week 2, in the 2 CA group, suggesting the induction of low memory. However, the potential influence of leukocyte function on enhancing specific immunity related to immunostimulant is yet to be explored. Notably, NO production was directly related to the granulocytes associated with the innate immune system52. In this study, NO synthase production was low in both 2 CA groups between weeks 2 and 6, which became significant in the 8th week. However, the 4 CA groups produced a significantly higher level of NO synthase in weeks 6 and 8. Das et al.35 reported similar effects of NO production in leukocytes in rohu against A. hydrophila. Consequently, the present results suggest that CA potentially influences NO synthesis of ROS and RNS in stimulated leukocytes as an immediate response to pathogens.
Neutrophil granules release MPO enzymes during oxidative RB that produce toxic hypochlorous acids that react with pathogenic microorganisms53. MPO content was highly significant (P < 0.05) in both 2 CA groups at 6 and 8 weeks, similar to that of the 4 CA groups from weeks 2 to 8. A significantly elevated level of MPO content was reported in P. hypophthalmus against monogenean infection40. Conversely, a recent study in rohu found that MPO activity was not significantly different between healthy and infected fish54. The present results indicate that a relatively lower concentration (2 mg kg−1 CA) stimulates MPO after 6 weeks, whereas a medium concentration (4 mg kg−1 CA) stimulated MPO earlier, at week 4. Hemolytic activity was statistically influenced (P < 0.05) in the 2 CA groups in weeks 6 and 8, whereas the 4 CA groups induced MPO between weeks 4 and 8. Among various immune mechanisms, only the complement pathway has the potential to avert microbial infection. Therefore, the hemolytic exertion in fish serum is recognized as an intersperse complement pathway, which plays a vital role against infectious pathogens55. In this study, a significant hemolysin titer was obtained with the 4 mg kg−1 CA, suggesting the influence of complement pathways against pathogens56. However, the groups fed with a low dose (2 mg kg−1 CA) produced a high hemolysin titre in the later stages, whereas a high dose (8 mg kg−1 CA) produced similar results in the earlier stages. Additionally, a more recent study reported less hemolysin titre in rohu against dactylogyrid monogenean54. However, these changes may be dependent upon fish species, the pathogenicity of the microbes, or the blocking of certain microbe epitopes, capable of influencing the alternate complement cascade.
Apart from the RB mechanism, lysozyme is a lysis enzyme produced by granulocytes during non-specific oxygen-independent pathways that play an essential role in innate immunity57–59. In vertebrates, lysozyme is an imperative humoral component in the systemic and mucosal immune systems; it further acts as a defensive factor against pathogenic microorganisms57; lysozyme secreted by human granulocytes attach to the hyphae of Candida albicans60. These studies strongly suggest the existence of a lysozyme-conflict defence mechanism in fish against pathogens. TP is also an important compound involved in the immune system. Both lysozyme activity and TP concentration reached significant levels (P < 0.05) in both groups fed with 2 CA and 4 CA diets between the 4th and 8th weeks. Elevated serum lysozyme activity was also reported in the immunostimulant administration in rainbow trout and rohu61,62.
Bactericidal and antibody levels were statistically improved (P < 0.05) in both 2 CA groups after week 6 and in the 4 CA groups from weeks 4 to 8. The relationship between antibody production and protection against pathogens has been demonstrated in Catla against furunculosis51. However, the significance of protection or resistance to pathogen within antibodies further suggests cellular immunity following immunostimulants. Antibody synthesis depends primarily on effector-cell proliferation and its specific antibody secretion or memory cell differentiation, which representing the complex progression needed for cytokine secretion and cell cooperation63.
In the present study, immune defense system changes when infected with A. hydrophila led to both up- and down-regulation of selected immune genes. The immune-related gene expression patterns in fish have been investigated only recently; and, consequently, the understanding of immunity to pathogen is limited. IL-1β is an important inflammatory mediator in microbial infections64. In the 2 mg kg−1 CA diets, the IL-1β and IL-10 expression levels in HK were significantly low between weeks 2 and 4, and relatively high in weeks 6 and 8. The gene’s significant up-regulation was also present in the 4 CA groups in the 6th and 8th weeks. IL-1β was up-regulated in the skin of trout against Gyrodactylus derjavini65. However, in the present study, IL-1β showed a significant down-regulation in Catla infected with A. hydtophila; suggesting the release of pathogen toxins in different phases of pathogen attachment, which play a significant role in arbitrating inflammation, thereby leading to down-regulation62; an increase in IL-1β and IL-10 expression. The exact actions of CA involvement in fish against A. hydtophila may be contained within the immune system. IL-10 is involved in the synthesis of the multifunctional cytokine inhibitory factor, which promotes immune-suppressive function66, and plays a major role in undermining the proinflammatory cytokines responses; such as IL-1β, TNFα, and IFN-γ; as well as preventing tissue damage67,68. Increased IL-10 mRNA expression levels have been reported in fish due to different immunostimulants related to the transcription arising in innate immune cells, such as macrophages69,70.
TLRs facilitate inflammatory immune responses when binding to molecules with PAMPs, and trigger an inflammation-related genes expression through signal transduction71,72, also referred to as endogenous TLR ligands, which acts as an ominous early alarm to innate and adaptive immunities73. The TLR2, TLR3, TLR4, and TLR5 expressions were significantly up-regulated in the 4 CA groups after 4 weeks and in the very early and late stages in the 2 CA and 8 CA groups. Modulation of negating innate immune gene receptors, like TLRs and NLRs, plays a fundamental role in forming an Ig repository74. A recent study reported that similar functions were demonstrated in the embryo stages in different organs or tissues of L. rohita TLR2 and TLR5 genes75. The TLR4 has been recently demonstrated with the ability to recognize viral or bacterial motifs76.
TRAF6 and TNF-α were up-regulation in the HK in both 4 CA groups after the 6th week and after the 8th week in the 2 CA groups. A similar expression pattern of TNF-α was recorded in zebrafish embryos and adults against Edwardsiella tarda infection77 and in carp HK, due to LPS78. However, TRAF6 and TNF-α expression presented low in the HK in both 4 CA groups between the 2nd and 4th weeks and between the 4th and 6th weeks in the 8 CA groups. Similarly, a low TNF-α expression was reported in rohu HK against E. tarda79. The TRAF6 response to different PAMPs treatments in Epinephelus tauvina revealed its contribution in influencing immune responses80. MHC receptors are associated with antigen-presenting cells (APCs), which assist T-cells in initiating the immune system81. In HK, leucocyte MHC-I expression was up-regulated in the 2 CA groups in the 6th and 8th weeks and the 4 CA groups on or after the 4th week. The high level of MHC expression in Catla, due to A. hydrophila, suggests that MHC contains cells (macrophages) within the HK that mediate the inflammation. However, no such change in MHC-I expression was observed in the 2 CA groups between weeks 2 and 4. Gharbi et al.82 reported the MHC receptors susceptibility in Atlantic salmon to L. salmonis.
The up-regulation of NF-kB occurred in both 2 mg kg−1 CA groups in weeks 6 and 8, whereas MyD88 expression significantly was up-regulated in the same period, in the 4 CA groups in weeks 4–8, and the 8 CA in weeks 4–6. The IL-10 mechanism was described in Catla by blocking the NF-kB-signals in the HK. Association of PAMP-TLR induced oligomerization, triggered intracellular signaling cascade via recruitment of the myeloid differentiation factor 88 (MyD88)-dependent or -independent pathways83. Combined stimulation of intracellular MAP kinase pathway and Iκβ deprivation irritates the triggering of transcription factors, like AP-1 and NF-κB; which triggers proinflammatory cytokine secretion in the B and T cells leading to B cell proliferation84. In zebrafish, the TLR4-induced MyD88 dependent signals triggered the regulation of anti-inflammation85. The results herein suggest that both MyD88-dependent and independent TLR-induced signaling pathways together to regulate the activation of catla lymphocytes and lymphoid organs. NF-κB is capable of binding to Ig kappa light-chain of pathogens encountered within the B cells86. Therefore, the tight NF-κB phosphorylation and adhesion regulation are necessary to inhibit dysfunction of immune function87. Thus, the sudden increase in NF-κB levels observed in this study indicates their vital role in the pathophysiology linked to TLR signaling.
When fed with 4 mg kg−1 CA, both NOD1 and NOD2 demonstrated significant expression on or after week 4. In addition to TLRs, NLRs is also responded to microbial components88 and endogenous ligands, as a result of tissue or cellular injuries89. The TLR2 and TLR5, NOD1, and NOD2 genes were up-regulated carp’s embryonic stages90,91. The TLR and NOD constitutive expression suggest the possibility of innate immune receptors in the early stage of CA treatment in Catla.
The chicken (Lyz-C) and goose (Lyz-G) gene expressions were not up-regulated in the 2 CA groups in the 8th week and in the 4 CA groups between weeks 4 and 8. Similar Lyz-C and Lyz-G up-regulation was recorded in Japanese flounder kidney, intestine, heart, whole blood, spleen, and ovary against E. tarda92,93. Hence, the Lyz gene up-regulation may be responsible for the rise in serum lysozyme concentration in Catla fed with CA diets against A. hydrophila infection.
There was 100% survival in H-0 mg, but only 64% of survival rate was observed in the infected group fed with the control diet. Both post-challenged and un-challenged groups treated with median dose (4 mg kg−1) and high doses (8 mg kg−1) of CA had high survival (between 80.5 and 89%), but the low survival rate was observed in both groups treated with low dose (2 mg kg−1) of CA resulting in low survival (between 72.5 and 79%). Similar reports in different fishes when fed with diets enriched with various active constituents indicate the same trends94–96.
In conclusion, to the best of our knowledge, this is the first report on the positive influence of CA in C. catla on innate and adaptive immune responses. The optimum level of CA is 4 mg kg−1, which manifested during the 2nd week of treatment. The pathway related to the inflammation and immunomodulation of genes also triggered a similar response. The 8 mg kg−1 of CA resulted in such response much later, after the 6th week. Further comprehensive investigations are necessary to explain immune gene expressions’ ability to elucidate the mechanism of action in other fish species, with different chrysophanol doses, against different pathogens.
Materials and methods
Formulation of experimental diet
The basal/control diet contained maize grain, fish meal, finger millet, and pearl millet as protein sources; rice-bran and wheat-flour as carbohydrate sources; and groundnut oil cake and vegetable oil as lipid sources (Table 1). Each ingredient was finely pounded and mixed with the required water volume to make a soft bread. The prepared feed was kept in an aluminum vessel and steamed in a pressure cooker for 15 min (at 15 psi). The formulated blend was cooled at room temperature (RT); then incorporated with the pre-mix of vitamins and minerals. Four experimental diets were prepared, by mixing chrysophanic acid (CA) in four different concentrations: (1) 0 mg kg−1, (2) 2 mg kg−1 (2CA), (3) 4 mg kg−1 (4CA), and (4) 8 mg kg−1 (8CA). Pellet feeds were prepared using a manual pelletizer (2 mm). The prepared diets were immediately oven-dried at 60 °C for 12 h. and tightly packed, stored in appropriate containers, and labeled. The experimental pellet feed constitutions were analyzed using regular procedures.
Table 1.
Ingredients on dry matter basis and proximate composition experimental feed used in this study.
| Ingredients | Chrysophanol | |||
|---|---|---|---|---|
| 0 mg | 5 mg | 10 mg | 15 mg | |
| Maize grain | 10.000 | 10.000 | 10.000 | 10.000 |
| Fish meal | 10.000 | 10.000 | 10.000 | 10.000 |
| Finger millet | 10.000 | 10.000 | 10.000 | 10.000 |
| Pearl millet | 10.000 | 10.000 | 10.000 | 10.000 |
| Rice bran | 25.000 | 24.992 | 24.996 | 25.992 |
| Wheat flour | 10.000 | 10.000 | 10.000 | 10.000 |
| Groundnut oil cake | 20.000 | 20.000 | 20.000 | 20.000 |
| Vegetable oil | 2.000 | 2.000 | 2.000 | 2.000 |
| Vitamin + mineral mixa | 2.000 | 2.000 | 2.000 | 2.000 |
| Common salt | 1.000 | 1.000 | 1.000 | 1.000 |
| Chrysophanic acid | 0.000 | 0.002 | 0.004 | 0.008 |
| Proximate composition (dry matter basis, g kg−1) | ||||
| Crude protein | 38.96 | 38.22 | 38.45 | 37.92 |
| Crude lipid | 11.36 | 11.27 | 11.08 | 10.89 |
| Crude fiber | 2.57 | 2.38 | 2. 23 | 2.02 |
| Ash | 9.33 | 9.26 | 9.18 | 9.04 |
| Moisture | 6.63 | 6.46 | 6.31 | 6.15 |
aVitamin and minerals pre mix: Vitamin A: 700,000 IU, Vitamin D3: 140,000 IU, Vitamin E: 500 mg, Vitamin B12: 1000 mcg, Folic acid: 100 mg, Nicotinamide: 1000 mg, Copper: 1200 mg, Cobalt: 150 mg, Iron: 1500 mg, Zinc: 3000 mg, Iodine: 325 mg, Selenium: 10 mg, Magnesium: 6000 mg, Manganese: 1500 mg, Potassium: 100 mg, Calcium: 27 mg, Phosphorus: 13 mg, Sulphur: 0.72 mg, Fluorine: 300 mg.
Aeromonas hydrophila
Aeromonas hydrophila (MTCC 1739) was acquired at Himedia (India), and sub-cultured at 37 °C for 24 h. in a nutrient broth. The bacterial suspension obtained was centrifuged at 3000 × g for 10 min, and the supernatant was then discarded. The remaining pellet bacteria was re-dissolved in phosphate buffered saline (PBS, pH 7.4); until an optical density (OD) of the suspension [0.5 at 456 nm at 1 × 106 colony forming unit (CFU)] was achieved using a microplate reader and stored in a deep freezer for further use.
Fish
Healthy, Catla catla (36.7 ± 2.1 g, 20.3 ± 2.9 cm) were procured in a local farm and kept in 500 L fiber-reinforced plastic (FRP) containers, sufficiently aerated, and filtered with de-chlorinated freshwater. The fish were immediately immersion in a KMnO4 solution for 2 min to evade any dermal infection and then kept for 2 weeks under standard laboratory conditions within natural photoperiod. During the acclimation period, fish were provided the control diet (Table 1). Fecal and unfed materials were siphoned off daily to avoid the accumulation of ammonia content in the tanks. The following measurements were recorded: pH 7.2 ± 0.05, dissolved oxygen 8.8 ± 0.02 mg l−1, temperature 24 ± 1 °C, and CaCO3 190 ± 0.2 ppm.
Experimental setup
Fish were arbitrarily strewn into eight groups of 25 fish (8 × 25 = 200 fish), in three replicates (3 × 200 = 600 fish): Group I, healthy (non-challenged) fish fed with the basal control diet (0 mg kg−1) CA [0CA or H-0 mg]; Group II, the healthy fish fed the dietary inclusion of 2 mg kg−1 [2CA or H-2 mg], Group III: fed the 4 mg kg−1 [4CA or H-4 mg] CA diet, Group IV: fed the 8 mg kg−1 [8CA or H-8 mg] CA diet; Group V, infected (or challenged) fish fed the basal control diet (0 mg kg−1) CA [0CA or I-0 mg]; and the challenged infected fish fed dietary inclusion of 2 mg kg−1 [2CA or IC-2 mg], Group VI; 4 mg kg−1 [4CA or IC-4 mg], Group VII; and 8 mg kg−1 [8CA or IC-8 mg] CA, Group VIII. Groups I through IV represented non-infected or non-challenge fish, injected with 0.5 mL PBS; whereas groups V through VIII contained fish challenged with 0.5 mL PBS containing A. hydrophila at 1 × 106 CFU. The specified diets were provided twice daily at 10:00 and 17:00 throughout the experiment.
Blood and tissues sampling procedure
Six fish from each group were arbitrarily chosen at the end of weeks 2, 4, 6, and 8 post-challenged with A. hydrophila. Post-anesthetized (MS-222, Sigma, USA), blood samples were drawn separately from the cardinal vein via a 1 mL plastic syringe. Each blood sample was equally divided into two separate sterile tubes with and without heparin, respectively. The heparinized blood was immediately analysed for hemato-biochemical analysis and immunological study, whereas the non-heparinized blood samples were permitted to clot at RT, then preserved at 4 °C for 4 h. All samples were centrifuged (2300 × g) for 5 min at 4 °C. The resulting serum was collected and stored in individual sterile tubes (− 80 °C) for further analyses. Lastly, the anterior kidney was dissected out aseptically from each fish, and RNAlater was added (Ambion, USA) and then preserved at − 80 °C awaiting RNS extraction.
Immunological assays
Non-specific immune parameter
Phagocytosis was analysed via Aeromonas hydrophila, whereas the respiratory bursts were determined by the reduction of nitroblue tetrazolium (NBT) assay (Sigma, MO, USA) to measure neutrophils reactive oxygen radical production97. Bactericidal activity was determined using a 96-well microtiter plate with 3-(4, 5 dimethyl-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT), according to the method of Kampen et al.98. Serum lysozyme levels were measured via turbidimetric assay Ellis57. Nitric oxide synthase (NOS) was analysed as prescribed by Lee et al.99. The production of hydrogen peroxide (H2O2) was measured by phenol red oxidation method using horseradish peroxidase33. The HK leukocytes proliferative reactions were measured via MTT assay100,101. The synthesis of superoxide anion (SOD) was estimated following51. Respiratory burst (RB) in terms of oxygen radical generation was measured by phagocytes using NBT reduction assay97. Total serum myeloperoxidase (MPO) and total protein contents were measured by the technique employed by Mohanty and Sahoo79 and Gornall et al.102. Serum hemolytic activity was estimated by microtitre plate54.
Specific immune parameters
The serum antibody levels of fish infected with A. hydrophila was determined using an indirect enzyme linked immunosorbant assay (ELISA)103 with some modifications104.
Immune-related gene expression study
Total RNA extraction
Roughly 50–100 mg of HK tissues was dissected in each fish for isolation of total RNA using TRI reagent (Sigma), per manufacturer’s instruction. The total RNA concentration was determined using spectrophotometer (Nanodrop ND-1000, Thermo Scientific, USA). The sample’s purity was achieved by quantifying the ratio of OD 260 nm/OD 280 nm (1.8–2.0). The purified RNA was then used for cDNA synthesis.
Expression study
Two µg of total RNA, which utilized the first strand of cDNA synthesis, were obtained via an Enhanced Avian HS RT-PCR kit (Sigma) through the use of a thermocycler (Mycycler Thermal cycler, BioRad, USA). RNA was kept for 5 min at 80 °C, containing 1 µL of 2.5 µM random hexamer, then kept another 5 min at 4 °C, allowing the primers to anneal of the RNA. A mixture of 10 × MMLV-RT buffer (2 µL), 0.4 U/µL of RNase inhibitor (1 µL), 10 mM dNTPs (1 µL), DEPC water (5 µL), and RT enzyme (1.0 µL) was softly agitated, and kept for 1 h at 42 °C, followed by 10 min at 70 °C. The resulting cDNA was synthesized and kept at 4 °C for further analyses. The constitutive expression of β-actin housekeeping gene was used in both positive control and experimental sample for normalization. The size of cloned PCR products and the primer sequences used for β-actin, as well as the immune-related genes, were investigated. The PCR mixtures contained 2 µL of 10 × PCR buffer, 13.1 µL of dH2O, 1.5 µL of MgCl2, 0.5 µL of 10 mM dNTPs, 0.5 µL of 0.05 U/µL DNA polymerase (JumpStart Accu-Taq la), 0.2 µL of 10 pmol of both primers (forward and reverse), and 2 µL cDNA. The extension parameters of the PCR as followed: 95 °C for 3 min; followed by denaturation for 30 cycles for 45 s (94 °C), and 45 s for appropriate annealing temperature (Table 2), followed by additional 45 s extension at 72 °C, and a 10-min for final extension (72 °C). The synthesized PCR products were confirmed by 1.0% agarose gel electrophoresis. The relative expression profile of β-actin and immune-relevant genes were evaluated by densitometry (Gel DOC, BIO RAD Laboratories, India).
Table 2.
List of gene primers used for tissue specific expression pattern of selected immune related genes in C. catla.
| Primers no | Sequence (5′ → 3′) | Annealing temperature (°C) | PCR amplicon size (bp) | Accession |
|---|---|---|---|---|
| β-actin | F: ACCCACACTGTGCCCATCTACG | 60 | 146 | JQ991014 |
| R: ATTTCCCTCTCGGCTGTGGTGG | ||||
| IL-1β | F: ACCCCACAAAACATCGGCCAACC | 60 | 156 | AM932525 |
| R: TCTTCTCCATTTCCACCCTCTC | ||||
| IL-10 | F: CGCAGTGCAGAAGAGTCGAC | 64 | 310 | GU256643 |
| R: CCCGCTTGAGATCCTGAAATAT | ||||
| IFN-γ | F: AAGGGTTCCTGCTCTTGTCA | 54 | 210 | KF590042 |
| R: GCCATTTTTCACCTCGACTG | ||||
| MHC-I | F:AGGAGATGCCGAATGGAG | 56 | 256 | MG859931 |
| R: GATGATTCCCAGCACCAG | ||||
| MyD88 | F: CTTCCAGTTTGTGCATGAGA | 51 | 146 | JN247432 |
| R: CCATCCTCTTGCACCTTTTT | ||||
| NF-kB | F: TTTACAGGAGCGGCGGATAC | 59 | 453 | KY089040 |
| R: GTGCGAAACACGATAGCCAC | ||||
| TNF-α | F: CCAGGCTTTCACTTCAGG | 56 | 181 | FN543477 |
| R: GCCATAGGAATCGGAGTAG | ||||
| TRAF6 | F: CAGTTGACAATGAGGTGCTG | 53 | 328 | MF766465 |
| R: CACACTGTATTGGCGAAAGG | ||||
| TLR2 | F: GACGGTCATGGATGGTTCTTCTTTA | 58 | 131 | HQ293022 |
| R: CAAGATTGCGTATGTAGGCCGTATG | ||||
| TLR3 | F: GCTCCACAGGGTTGAAGACA | 53 | 310 | MF766464 |
| R: GCACGGCCAAGCTTTAGAAT | ||||
| TLR4 | F: ATGATGGAGCGCAATGCCAA | 55 | 140 | GU248418 |
| R: ATGTTACTCAAAGGGTCTCTGCTCC | ||||
| TLR5 | F: CAGGGTAAACATTTCACGCTTCT | 58 | 162 | GU230763 |
| R: ACGCTTTGCCATGGGAACTTT | ||||
| LYZ-C | F: GCTGTGATGTTGTTCGTATCTTC | 66 | 343 | JQ230329 |
| R: GACAGCTTACGCCCATTACAG | ||||
| LYZ-G | F: CATGGGACAGTGAGGAACATC | 66 | 204 | JQ230328 |
| R: CATGTGCTCATATGTACGGACG | ||||
| NOD1 | F: GTTGGTGGGAAATACCTTGCC | 56 | 217 | KC542884 |
| R: TGCTTTCGCCAGACTTCTTCC | ||||
| NOD2 | F: GGCGGGACAGGACGTTTCTCC | 60 | 261 | KC542885 |
| R: GCGGCAACTGAAGGGGAATA |
IL-1β/10 interleukins1β/10, IFN-γ interferon-gamma, TNF-α tumor necrosis factor-alpha, TRAF6 TNF receptor-associated factor 6, TLR2/3/4/5 toll-like receptors 2/3/4/5, LYZ-C/G lysozyme-C/G, NOD1/2 nucleotide binding oligomerization domain containing 1/2, NF-kB nuclear factor kappa-light-chain-enhancer of activated B cells, MyD88 myeloid differentiation primary response 88, MHC-I major histocompatibility complex-class I.
Relative percentage survival (RPS)
Analysis of RPS was studied in all the experimental groups as mentioned in “Experimental setup”. Each experimental group was maintained triplicate, and 20 fish were used in each group. The bacterial culture and concentration of bacterial density were the same as mentioned in “Aeromonas hydrophila”. The bacterial challenge and administration of pathogen were the same as mentioned in “Experimental setup”. The survival rate was determined at the end of the experiment. Relative percentage survival (RPS) was calculated following standard formula:
Statistical analysis
Data of each blood and tissue sample were computed as mean ± SEM in triplicate. The percentages of β-actin and the immune gene amplifying products, were later determined and further examined through one-way analysis of variance (ANOVA). Differences were subsequently calculated between the treated groups using Duncan’s Multiple Range (DMR) test, in which significance was considered at P < 0.05.
Ethical approval
The present study follows institutional guidelines mandatory for human and animal treatment and complies with relevant legislation ethical approval from the institute for conducting the research. The animal ethical committee (approval no. 791/03/b/CPCSEA) was approved by the Tamil University, Faculty of Sciences, Department of Siddha Medicine, C-4 Quarters, Thanjavur, 613 005 Tamil Nadu.
Acknowledgements
This research work was partially supported by Chiang Mai University.
Author contributions
R.H.: Conception, design, and writing the article. G.D.: Analysis and interpretation. C.B.: Contribution to sample preparation. H.D.: Critical revision of the article. S.J.: Literature search. E.R.: Final approval of the article. C.F.: Statistical expertise.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Little DC, Newton R, Beveridge M. Aquaculture: A rapidly growing and significant source of sustainable food? Status, transitions and potential. Proc. Nutr. Soc. 2016;75:274–286. doi: 10.1017/S0029665116000665. [DOI] [PubMed] [Google Scholar]
- 2.Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. Rome. ISBN 978-92-5-130562-1. https://creativecommons.org/licenses/by-nc-sa/3.0/igo (2018).
- 3.Shabana M, Karthika M, Ramasubramanian V. Effect of dietary Citrus sinensis peel extract on growth performance, digestive enzyme activity, muscle biochemical composition, and metabolic enzyme status of the freshwater fish, Catla catla. J. Basic Appl. Zool. 2019;80:51. doi: 10.1186/s41936-019-0119-x. [DOI] [Google Scholar]
- 4.Vanitha M, Dhanapal K, Reddy GVS. Quality changes in fish burger from Catla (Catla Catla) during refrigerated storage. J. Food Sci. Technol. 2015;52:1766–1771. doi: 10.1007/s13197-013-1161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieke T, et al. Sustainable aquaculture requires environmental-friendly treatment strategies for fish diseases. Rev. Aquacult. 2019 doi: 10.1111/raq.12365. [DOI] [Google Scholar]
- 6.Assefa A, Abunna F, et al. Maintenance of fish health in aquaculture: review of epidemiological approaches for prevention and control of infectious disease of fish. Vet. Med. Int. 2018 doi: 10.1155/2018/5432497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henriksson PJG, et al. Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: A review from a systems perspective. Sustain. Sci. 2018;13:1105–1120. doi: 10.1007/s11625-017-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tavares-Dias M, Martins ML. An overall estimation of losses caused by diseases in the Brazilian fish farms. J. Parasitic Diseases. 2017;41:913–918. doi: 10.1007/s12639-017-0938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saikia D, Kamilya D. Immune responses and protection in catla (Catla catla) vaccinated against epizootic ulcerative syndrome. Fish Shellfish Immunol. 2012;32:353–359. doi: 10.1016/j.fsi.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Harikrishnan R, Balasundaram C. Modern trends in Aeromonas hydrophila disease management with fish. Rev. Fish. Sci. 2005;13:281–320. doi: 10.1080/10641260500320845. [DOI] [Google Scholar]
- 11.Karunasagar I, Ali A, Otta S, Karunasagar I. Immunization with bacterial antigens: Infections with motile aeromonads. Dev. Biol. Stand. 1997;90:135. [PubMed] [Google Scholar]
- 12.Miranda CD, Godoy FA, Lee MR. Current status of the use of antibiotics and the antimicrobial resistance in the chilean salmon farms. Front. Microbiol. 2018;9:1284. doi: 10.3389/fmicb.2018.01284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watts JEM, Schreier HJ, Lanska L, Hale MS. The rising tide of antimicrobial resistance in aquaculture: Sources, sinks and solutions. Mar. Drugs. 2017;15:158. doi: 10.3390/md15060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nayak SK. Current status of Aeromonas hydrophila vaccine development in fish: An Indian perspective. Fish Shellfish Immunol. 2020 doi: 10.1016/j.fsi.2020.01.064. [DOI] [PubMed] [Google Scholar]
- 15.Ma J, Bruce TJ, Jones EM, Cain KD. A review of fish vaccine development strategies: Conventional methods and modern biotechnological approaches. Microorganisms. 2019;7:569. doi: 10.3390/microorganisms7110569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sommerset I, Krossøy B, Biering E, Frost P. Vaccines for fish in aquaculture. Exp. Rev. Vaccines. 2005;4:89–101. doi: 10.1586/14760584.4.1.89. [DOI] [PubMed] [Google Scholar]
- 17.Labh SN, Shakya SR. Application of immunostimulants as an alternative to vaccines for health management in aquaculture. Int. J. Fisheries Aquat. Stud. 2013;2:153–156. [Google Scholar]
- 18.Wang W, Sun J, Liu C, Xue Z. Application of immunostimulants in aquaculture: Current knowledge and future perspectives. Aquac. Res. 2017;48:1–23. doi: 10.1111/are.13161. [DOI] [Google Scholar]
- 19.Hoseinifar SH, et al. Mucosal immune parameters, immune and antioxidant defence related genes expression and growth performance of zebrafish (Danio rerio) fed on Gracilaria gracilis powder. Fish Shellfish Immunol. 2018;83:232–237. doi: 10.1016/j.fsi.2018.09.046. [DOI] [PubMed] [Google Scholar]
- 20.Mişe Yonar S. Growth performance, haematological changes, immune response, antioxidant activity and disease resistance in rainbow trout (Oncorhynchus mykiss) fed diet supplemented with ellagic acid. Fish Shellfish Immunol. 2019;95:391–398. doi: 10.1016/j.fsi.2019.10.056. [DOI] [PubMed] [Google Scholar]
- 21.Mohan K, et al. Potential uses of fungal polysaccharides as immunostimulants in fish and shrimp aquaculture: A review. Aquaculture. 2019;500:250–263. doi: 10.1016/j.aquaculture.2018.10.023. [DOI] [Google Scholar]
- 22.Wu C, et al. Effects of dietary Radix Rehmanniae Preparata polysaccharides on the growth performance, immune response and disease resistance of Luciobarbus capito. Fish Shellfish Immunol. 2019;89:641–646. doi: 10.1016/j.fsi.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Hsu C-Y, Chan Y-P, Chang J. Antioxidant activity of extract from Polygonum cuspidatum. Biol. Res. 2007;40:13–21. doi: 10.4067/S0716-97602007000100002. [DOI] [PubMed] [Google Scholar]
- 24.Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr. Cancer. 1993;20:21–29. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- 25.Kundu JK, Surh Y-J. Cancer chemopreventive and therapeutic potential of resveratrol: Mechanistic perspectives. Cancer Lett. 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 26.Hsiang CY, Ho TY. Emodin is a novel alkaline nuclease inhibitor that suppresses herpes simplex virus type 1 yields in cell cultures. Br. J. Pharmacol. 2008;155:227–235. doi: 10.1038/bjp.2008.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prateeksha, et al. Chrysophanol: A natural anthraquinone with multifaceted biotherapeutic potential. Biomolecules. 2019;9:68. doi: 10.3390/biom9020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coopoosamy R, Magwa M. Antibacterial activity of chrysophanol isolated from Aloe excelsa (Berger) Afr. J. Biotechnol. 2006;5:1508–1510. [Google Scholar]
- 29.Lu J, et al. Chrysophanol protects against doxorubicin-induced cardiotoxicity by suppressing cellular PARylation. Acta Pharm. Sin. B. 2019;9:782–793. doi: 10.1016/j.apsb.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu CC, et al. Chrysophanol induces necrosis through the production of ROS and alteration of ATP levels in J5 human liver cancer cells. Mol. Nutr. Food Res. 2010;54:967–976. doi: 10.1002/mnfr.200900265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie L, et al. Chrysophanol: A review of its pharmacology, toxicity and pharmacokinetics. J. Pharm. Pharmacol. 2019;71:1475–1487. doi: 10.1111/jphp.13143. [DOI] [PubMed] [Google Scholar]
- 32.Baigi MG, et al. Apoptosis/necrosis switch in two different cancer cell lines: Influence of benzoquinone-and hydrogen peroxide-induced oxidative stress intensity, and glutathione. Toxicol. In Vitro. 2008;22:1547–1554. doi: 10.1016/j.tiv.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Secombes CJ, et al. Isolation of salmonid macrophages and analysis of their killing activity. Tech. Fish Immunol. 1990;1:137–154. [Google Scholar]
- 34.Burgos-Aceves MA, Lionetti L, Faggio C. Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci. Total Environ. 2019;670:1170–1183. doi: 10.1016/j.scitotenv.2019.03.275. [DOI] [PubMed] [Google Scholar]
- 35.Das P, Joardar S, Abraham T, Kamilya D, Batabyal S. Dynamic changes in immune-effector characteristics of Indian major carp, rohu (Labeo rohita) sensitized with Aeromonas hydrophila. Ind. J. Comp. Microbiol. Immunol. Infect. Diseases. 2009;30:45–49. [Google Scholar]
- 36.Secombes C. The nonspecific immune system: Cellular defenses. Fish Immune Syst. Organ. Pathogen Environ. 1996;15:63–103. doi: 10.1016/S1546-5098(08)60272-1. [DOI] [Google Scholar]
- 37.Graham S, Secombes C. The production of a macrophage-activating factor from rainbow trout Salmo gairdneri leucocytes. Immunology. 1988;65:293. [PMC free article] [PubMed] [Google Scholar]
- 38.Nagelkerke L, Pannevis M, Houlihan D, Secombes C. Oxygen uptake of rainbow trout Oncorhynchus mykiss phagocytes following stimulation of the respiratory burst. J. Exp. Biol. 1990;154:339–353. [Google Scholar]
- 39.Fast MD, et al. Susceptibility of rainbow trout Oncorhynchus mykiss, Atlantic salmon Salmo salar and coho salmon Oncorhynchus kisutch to experimental infection with sea lice Lepeophtheirus salmonis. Diseases Aquat. Organ. 2002;52:57–68. doi: 10.3354/dao052057. [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, et al. Modulation of innate immune responses and induction of oxidative stress biomarkers in Pangasianodon hypophthalmus following an experimental infection with dactylogyrid monogeneans. Fish Shellfish Immunol. 2017;63:334–343. doi: 10.1016/j.fsi.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 41.Munoz P, Sitja-Bobadilla A, Alvarez-Pellitero P. Cellular and humoral immune response of European sea bass (Dicentrarchus labrax L.) (Teleostei: Serranidae) immunized with Sphaerospora dicentrarchi (Myxosporea: Bivalvulida) Parasitology. 2000;120:465–477. doi: 10.1017/S0031182099005855. [DOI] [PubMed] [Google Scholar]
- 42.Díaz-Rosales P, et al. Effects of two closely related probiotics on respiratory burst activity of Senegalese sole (Solea senegalensis, Kaup) phagocytes, and protection against Photobacterium damselae subsp. piscicida. Aquaculture. 2009;293:16–21. doi: 10.1016/j.aquaculture.2009.03.050. [DOI] [Google Scholar]
- 43.Sharifuzzaman SM, Austin B. Influence of probiotic feeding duration on disease resistance and immune parameters in rainbow trout. Fish Shellfish Immunol. 2009;27:440–445. doi: 10.1016/j.fsi.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Salinas I, Cuesta A, Esteban MÁ, Meseguer J. Dietary administration of Lactobacillus delbrüeckii and Bacillus subtilis, single or combined, on gilthead seabream cellular innate immune responses. Fish Shellfish Immunol. 2005;19:67–77. doi: 10.1016/j.fsi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y-Z, Yang H-L, Ma R-L, Lin W-Y. Probiotic applications of two dominant gut Bacillus strains with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immunol. 2010;29:803–809. doi: 10.1016/j.fsi.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Castro R, Couso N, Obach A, Lamas J. Effect of different β-glucans on the respiratory burst of turbot (Psetta maxima) and gilthead seabream (Sparus aurata) phagocytes. Fish Shellfish Immunol. 1999;9:529–541. doi: 10.1006/fsim.1999.0210. [DOI] [Google Scholar]
- 47.Jørgensen J, Lunde H, Robertsen B. Peritoneal and head kidney cell response to intraperitoneally injected yeast glucan in Atlantic salmon, Salmo salar L. J. Fish Dis. 1993;16:313–325. doi: 10.1111/j.1365-2761.1993.tb00865.x. [DOI] [Google Scholar]
- 48.Jørgensen JB, Sharp GJ, Secombes CJ, Robertsen B. Effect of a yeast-cell-wall glucan on the bactericidal activity of rainbow trout macrophages. Fish Shellfish Immunol. 1993;3:267–277. doi: 10.1006/fsim.1993.1026. [DOI] [Google Scholar]
- 49.Dexiang C, Ainsworth AJ. Assessment of metabolic activation of channel catfish peripheral blood neutrophils. Dev. Comp. Immunol. 1991;15:201–208. doi: 10.1016/0145-305X(91)90011-M. [DOI] [PubMed] [Google Scholar]
- 50.Tripathi S, Maiti TK. Stimulation of murine macrophages by native and heat-denatured lectin from Abrus precatorius. Int. Immunopharmacol. 2003;3:375–381. doi: 10.1016/S1567-5769(02)00291-6. [DOI] [PubMed] [Google Scholar]
- 51.Kamilya D, Maiti T, Joardar S, Mal B. Adjuvant effect of mushroom glucan and bovine lactoferrin upon Aeromonas hydrophila vaccination in catla, Catla catla (Hamilton) J. Fish Dis. 2006;29:331–337. doi: 10.1111/j.1365-2761.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 52.Yin Z, Lam T, Sin Y. Cytokine-mediated antimicrobial immune response of catfish, Clarias gariepinus, as a defence against Aeromonas hydrophila. Fish Shellfish Immunol. 1997;7:93–104. doi: 10.1006/fsim.1996.0066. [DOI] [Google Scholar]
- 53.Dalmo R, Ingebrigtsen K, Bøgwald J. Non-specific defence mechanisms in fish, with particular reference to the reticuloendothelial system (RES) J. Fish Dis. 1997;20:241–273. doi: 10.1046/j.1365-2761.1997.00302.x. [DOI] [Google Scholar]
- 54.Dash P, Kar B, Mishra A, Sahoo P. Effect of Dactylogyrus catlaius (Jain 1961) infection in Labeo rohita (Hamilton 1822): innate immune responses and expression profile of some immune related genes. Indian J. Exp. Biol. 2014;52:267–280. [PubMed] [Google Scholar]
- 55.Lange S, Guđmundsdottir BK, Magnadottir B. Humoral immune parameters of cultured Atlantic halibut (Hippoglossus hippoglossus L.) Fish Shellfish Immunol. 2001;11:523–535. doi: 10.1006/fsim.2000.0333. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez-Pellitero P. Fish immunity and parasite infections: From innate immunity to immunoprophylactic prospects. Vet. Immunol. Immunopathol. 2008;126:171–198. doi: 10.1016/j.vetimm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 57.Ellis AE. Lysozyme assays. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, van Muiswinkel WB, editors. Techniques in fish immunology. New Haven, NJ: SOS Publications; 1990. [Google Scholar]
- 58.Gobi N, Vaseeharan B, Rekha R, Vijayakumar S, Faggio C. Bioaccumulation, cytotoxicity and oxidative stress of the acute exposure selenium in Oreochromis mossambicus. Ecotoxicol. Environ. Saf. 2018;162:147–159. doi: 10.1016/j.ecoenv.2018.06.070. [DOI] [PubMed] [Google Scholar]
- 59.Aliko V, Qirjo M, Sula E, Morina V, Faggio C. Antioxidant defense system, immune response and erythron profile modulation in gold fish, Carassius auratus, after acute manganese treatment. Fish Shellfish Immunol. 2018;76:101–109. doi: 10.1016/j.fsi.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 60.Diamond RD, Krzesicki R, Epstein B, Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am. J. Pathol. 1978;91:313. [PMC free article] [PubMed] [Google Scholar]
- 61.Panigrahi A, et al. Immune responses in rainbow trout Oncorhynchus mykiss induced by a potential probiotic bacteria Lactobacillus rhamnosus JCM 1136. Vet. Immunol. Immunopathol. 2004;102:379–388. doi: 10.1016/j.vetimm.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Saurabh S, Sahoo P. Non-specific immune responses of the Indian major carp Labeo rohita (Hamilton) to infestation by the freshwater fish louse Argulus siamensis (Wilson) Indian J. Fish. 2010;57:45–53. [Google Scholar]
- 63.Marsden MJ, Secombes CJ. The influence of vaccine preparations on the induction of antigen specific responsiveness in rainbow trout Oncorhynchus mykiss. Fish Shellfish Immunol. 1997;7:455–469. doi: 10.1006/fsim.1997.0098. [DOI] [Google Scholar]
- 64.Secombes C, Zou J, Laing K, Daniels G, Cunningham C. Cytokine genes in fish. Aquaculture. 1999;172:93–102. doi: 10.1016/S0044-8486(98)00441-4. [DOI] [Google Scholar]
- 65.Lindenstrøm T, Buchmann K, Secombes C. Gyrodactylus derjavini infection elicits IL-1β expression in rainbow trout skin. Fish Shellfish Immunol. 2003;15:107–115. doi: 10.1016/S1050-4648(02)00142-0. [DOI] [PubMed] [Google Scholar]
- 66.Savan R, Igawa D, Sakai M. Cloning, characterization and expression analysis of interleukin-10 from the common carp, Cyprinus carpio L. Eur. J. Biochem. 2003;270:4647–4654. doi: 10.1046/j.1432-1033.2003.03854.x. [DOI] [PubMed] [Google Scholar]
- 67.Moore KW, Malefyt RW, Coffman RL, O'Garra A. Interleukin-10 and the Interleukin-10 Receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 68.Burmeister AR, Marriott I. The interleukin-10 family of cytokines and their role in the CNS. Front. Cell. Neurosci. 2018 doi: 10.3389/fncel.2018.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pengal RA, et al. Lipopolysaccharide-induced production of interleukin-10 is promoted by the serine/threonine kinase Akt. Mol. Immunol. 2006;43:1557–1564. doi: 10.1016/j.molimm.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 70.Burgos-Aceves MA, Cohen A, Smith Y, Faggio C. MicroRNAs and their role on fish oxidative stress during xenobiotic environmental exposures. Ecotoxicol. Environ. Saf. 2018;148:995–1000. doi: 10.1016/j.ecoenv.2017.12.001. [DOI] [Google Scholar]
- 71.Dalmo RA, Bøgwald J. ß-Glucans as conductors of immune symphonies. Fish Shellfish Immunol. 2008;25:384–396. doi: 10.1016/j.fsi.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Lauriano E, et al. Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish Shellfish Immunol. 2016;59:250–255. doi: 10.1016/j.fsi.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Matzinger P. The danger model: A renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 74.Basu M, et al. B cell activating factor is induced by toll-like receptor and NOD-like receptor-ligands and plays critical role in IgM synthesis in Labeo rohita. Mol. Immunol. 2016;78:9–26. doi: 10.1016/j.molimm.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 75.Samanta M, et al. Molecular characterization of toll-like receptor 2 (TLR2), analysis of its inductive expression and associated down-stream signaling molecules following ligands exposure and bacterial infection in the Indian major carp, rohu (Labeo rohita) Fish Shellfish Immunol. 2012;32:411–425. doi: 10.1016/j.fsi.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 76.Kurt-Jones EA, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 77.Pressley ME, Phelan PE, III, Witten PE, Mellon MT, Kim CH. Pathogenesis and inflammatory response to Edwardsiella tarda infection in the zebrafish. Dev. Comp. Immunol. 2005;29:501–513. doi: 10.1016/j.dci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 78.Savan R, Sakai M. Presence of multiple isoforms of TNF alpha in carp (Cyprinus carpio L.): Genomic and expression analysis. Fish Shellfish Immunol. 2004;17:87–94. doi: 10.1016/j.fsi.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 79.Mohanty B, Sahoo P. Immune responses and expression profiles of some immune-related genes in Indian major carp, Labeo rohita to Edwardsiella tarda infection. Fish Shellfish Immunol. 2010;28:613–621. doi: 10.1016/j.fsi.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 80.Wei J, et al. Isolation and characterization of tumor necrosis factor receptor-associated factor 6 (TRAF6) from grouper, Epinephelus tauvina. Fish Shellfish Immunol. 2014;39:61–68. doi: 10.1016/j.fsi.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 81.Klein J, Sato A. The HLA system. N. Engl. J. Med. 2000;343:702–709. doi: 10.1056/NEJM200009073431006. [DOI] [PubMed] [Google Scholar]
- 82.Gharbi K, et al. Genetic dissection of MHC-associated susceptibility to Lepeophtheirus salmonis in Atlantic salmon. BMC Genet. 2009;10:20. doi: 10.1186/1471-2156-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McGettrick AF, O’Neill LA. The expanding family of MyD88-like adaptors in Toll-like receptor signal transduction. Mol. Immunol. 2004;41:577–582. doi: 10.1016/j.molimm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 84.Takeda K, Akira S. TLR signaling pathways. Sem. Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 85.Srivastava N, et al. Aeromonas hydrophila utilizes TLR4 topology for synchronous activation of MyD88 and TRIF to orchestrate anti-inflammatory responses in zebrafish. Cell Death Discov. 2017;3:1–9. doi: 10.1038/cddiscovery.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 87.Sun S-C, Ley SC. New insights into NF-κB regulation and function. Trends Immunol. 2008;29:469–478. doi: 10.1016/j.it.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012;13:325. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krishnaswamy JK, Chu T, Eisenbarth SC. Beyond pattern recognition: NOD-like receptors in dendritic cells. Trends Immunol. 2013;34:224–233. doi: 10.1016/j.it.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Swain B, Basu M, Samanta M. Molecular cloning and characterization of nucleotide binding and oligomerization domain-1 (NOD1) receptor in the Indian Major Carp, rohu (Labeo rohita), and analysis of its inductive expression and down-stream signalling molecules following ligands exposure and Gram-negative bacterial infections. Fish Shellfish Immunol. 2012;32:899–908. doi: 10.1016/j.fsi.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 91.Swain B, Basu M, Samanta M. Nucleotide binding and oligomerization domain (NOD) 1 and NOD2 receptors in mrigal (Cirrhinus mrigala): Inductive expression and down-stream signaling in ligand stimulation and bacterial infections. J. Biosci. 2013;38:533–548. doi: 10.1007/s12038-013-9330-y. [DOI] [PubMed] [Google Scholar]
- 92.Hikima J, Hirono I, Aoki T. Characterization and expression of c-type lysozyme cDNA from Japanese flounder (Paralichthys olivaceus) Mol. Mar. Biol. Biotech. 1997;6:339–344. [PubMed] [Google Scholar]
- 93.Hikima J-I, Minagawa S, Hirono I, Aoki T. Molecular cloning, expression and evolution of the Japanese flounder goose-type lysozyme gene, and the lytic activity of its recombinant protein. Biochimica et Biophysica Acta (BBA)-Gene Struct. Expression. 2001;1520:35–44. doi: 10.1016/S0167-4781(01)00248-2. [DOI] [PubMed] [Google Scholar]
- 94.Harikrishnan R, Jawahar S, Srikanthan C, Paray BA, Al-Sadoon MK, Balasundaram C. Kaolin incorporated diet on growth and immune response in Ctenopharyngodon idellus against Aeromonas hydrophila. Fish Shellfish Immunol. 2018;77:364–373. doi: 10.1016/j.fsi.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 95.Devi G, Harikrishnan R, Paray BA, Al-Sadoon MK, Hoseinifar SH, Balasundaram C. Effects of aloe-emodin on innate immunity, antioxidant and immune cytokines mechanisms in the head kidney leucocytes of Labeo rohita against Aphanomyces invadans. Fish Shellfish Immunol. 2019;87:669–678. doi: 10.1016/j.fsi.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 96.Leya T, Raman RP, Srivastava PP, Kumar K, Ahmad I, Gora AH, Poojary N, Kumar S, Dar SA. Effects of Curcumin supplemented diet on growth and nonspecific immune parameters of Cirrhinus mrigala against Edwardsiella tarda Infection. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:1230–1243. [Google Scholar]
- 97.Anderson, D. & Siwicki, A. Basic haematology and serology for fish health programs. (1995).
- 98.Kampen AH, Tollersrud T, Lund A. Staphylococcus aureus capsular polysaccharide types 5 and 8 reduce killing by bovine neutrophils in vitro. Infect. Immun. 2005;73:1578–1583. doi: 10.1128/IAI.73.3.1578-1583.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee D-U, et al. Effects of 13-alkyl-substituted berberine alkaloids on the expression of COX-II, TNF-α, iNOS, and IL-12 production in LPS-stimulated macrophages. Life Sci. 2003;73:1401–1412. doi: 10.1016/S0024-3205(03)00435-1. [DOI] [PubMed] [Google Scholar]
- 100.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1988 doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 101.Siwicki AK, Miyazaki T, Komatsu I, Matsusato T. In vitro influence of heat extract from firefly squid Watasenia scintillans on the phagocyte and lymphocyte activities in rainbow trout Oncorhynchus mykiss. Fish Pathol. 1996;31:1–7. doi: 10.3147/jsfp.31.1. [DOI] [Google Scholar]
- 102.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177:751–766. doi: 10.1016/S0021-9258(18)57021-6. [DOI] [PubMed] [Google Scholar]
- 103.Delamare A, Echeverrigaray S, Duarte K, Gomes L, Costa S. Production of a monoclonal antibody against Aeromonas hydrophila and its application to bacterial identification. J. Appl. Microbiol. 2002;92:936–940. doi: 10.1046/j.1365-2672.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- 104.Binuramesh C, Prabakaran M, Steinhagen D, Michael RD. Effect of chronic confinement stress on the immune responses in different sex ratio groups of Oreochromis mossambicus (Peters) Aquaculture. 2005;250:47–59. doi: 10.1016/j.aquaculture.2005.03.043. [DOI] [Google Scholar]