Abstract
Background:
Completely resected stage IIIA(N2) non-small cell lung cancer (NSCLC) comprises a heterogeneous population according to discrepancies in survival prognosis. Accumulating evidence suggests that tumor-infiltrating lymphocytes (TILs) are clinically significant, despite a lack of consensus regarding the immunoscore (IS) in NSCLC. Here, we determined the prognostic value of the immune microenvironment as an IS in a uniform cohort of patients with completely resected stage IIIA(N2) NSCLC.
Methods:
Consecutive patients with pathologically confirmed stage IIIA(N2) NSCLC and who underwent complete resection (2005–2012) were retrospectively reviewed. Tissue microarrays (TMAs) were constructed from surgical paraffin-embedded primary lung tumor specimen. For each case, two representative regions from the tumor center (CT) and two from the invasive margin (IM) containing the highest density of lymphocytes were selected. Densities of CD3+, CD45RO+, and CD8+ lymphocytes were assessed using immunohistochemistry (IHC) by specialized pathologists according to predefined scoring scales. Patients were classified according to IS definition based on TIL type, density, and distribution, and relationships between IS and prognosis were evaluated.
Results:
Patients (N = 288) with complete IHC-based TMA spots were included. Univariate analyses showed that CD3+ T cell density was associated with neither overall survival (OS) nor distant metastasis-free survival (DMFS), whereas CD45RO+ T cell density in the IM was a significant prognostic factor for DMFS (p = 0.02) and was predictive of OS (p = 0.05). Combined CD45RO+ and CD8+ cell infiltration in tumor regions (CT and IM) significantly improved IS prognostic impact. Multivariate analyses revealed IS as an independent prognostic predictor for both DMFS (p = 0.001) and OS (p = 0.002).
Conclusion:
The proposed IS might provide valuable prognostic information, including prediction of DMFS and OS in stage IIIA(N2) NSCLC patients. Larger patient cohorts are needed to validate this IS classification, which might assist with accurate risk stratification and treatment decisions.
Keywords: immunoscore, non-small cell lung cancer (NSCLC), prognostic biomarker, stage IIIA(N2), tumor immune microenvironment
Introduction
The prognosis of patients with completely resected stage IIIA(N2) non-small cell lung cancer (NSCLC) remains poor, with 5-year survival rates ranging from 10% to 30% and a high degree of heterogeneity in postoperative recurrence risk,1,2 despite the same classifications used for TNM staging.3 Previous studies and our data have consistently found that systemic recurrence following surgery is a major concern in stage IIIA(N2) NSCLC.4–6 Recently, major breakthroughs in targeted therapy and immunotherapy have introduced substantially more effective treatment options for patients at high risk of systemic recurrences and enabling opportunities to improve patient prognosis.7,8 Therefore, novel strategies for identifying patient risk stratification in order to optimize personalized adjuvant treatment decision making are urgently needed.
Mounting evidence indicates that the biologic behavior of NSCLC correlates with the heterogeneous tumor immune microenvironment according to analyses of in situ immune components.9,10 This suggests that the presence and prevalence of tumor-infiltrating lymphocytes (TILs) supplementary to tumor status would represent clinically informative prognostic biomarkers for metastasis and clinical outcome.11–13 In colorectal cancer (CRC), a clinically simple and useful classification system involving an immunoscore (IS) that integrated TIL type, density, and distribution was proposed by Galon et al.14 Importantly, the IS, defined by the combined evaluation of the density of TILs (CD3+ T cells: a pan-lymphocyte marker; CD45RO+ T cells: memory T cells; and CD8+ T cells: cytotoxic T cells) in both the center of the tumor (CT) and the invasive margin (IM), is reportedly a more valuable indicator for predicting the survival prognosis of CRC patients than TNM staging.15,16 Accordingly, given the distinct clinical outcomes of stage IIIA(N2) NSCLC following complete resection, the profound and precise evaluation of the in situ host immune response to a tumor (key type, density, and distribution of immune components) in stage IIIA(N2) NSCLC could improve prognostic prediction, thereby guiding personalized adjuvant treatment selection.17
In NSCLC, numerous studies have focused on determining the prognostic impact of TILs;18,19 however, strategies for the development of a reliable IS system in NSCLC remain under investigation and have gradually become a major clinical issue.20 Our data suggest that a high density of TILs correlates with a decreased incidence of tumor spread and improved survival.13 A recent study by Donnem et al. showed that stromal CD8+ TILs have an independent positive prognostic impact in patients with resected NSCLC and might play a critical role in the development of an IS.21 In addition, Paulsen et al. identified CD45RO as a promising candidate marker following evaluation in a large NSCLC study.22 However, no information concerning precise localization of the analyzed tumor regions (CT and IM) was provided in these studies.19,21,22 Therefore, there remains no consensus regarding the IS based on combined evaluation of TIL type, density, and distribution for future clinical implementation in surgically resected stage IIIA(N2) NSCLC.
In this study, we investigated the prognostic significance of immune components and the proposed IS by incorporating combined evaluation of in situ T lymphocytes (CD3+), memory T cells (CD45RO+), and cytotoxic T cells (CD8+) in CT and IM in a uniform cohort of patients with pathologically confirmed stage IIIA(N2) NSCLC following complete resection.
Materials and methods
Patients and clinical samples
Records of consecutive patients with pathological stage T1-3N2M0 NSCLC [stage IIIA(N2) according to the TNM classification in the Union for International Cancer Control 7th edition3] and who underwent complete resection with clear surgical margins at Fudan University Shanghai Cancer Center from January 2005 to June 2012 were identified and reviewed. Patients were eligible for inclusion if they had a complete surgical resection, defined as a surgical procedure of lobectomy or pneumonectomy and systematic lymphadenectomy, or sampling with a minimum of three mediastinal lymph node (LN) stations completely dissected or sampled (one of which should be the subcarinal station).23 We excluded patients for the following reasons: they (1) underwent wedge resection; (2) received neoadjuvant therapy (chemotherapy, radiotherapy, or targeted therapy); (3) had severe postoperative complications and died within 4 months after surgery; or (4) presented with other prior or concurrent malignancies within the previous 5 years. This retrospective study was approved by the institutional review board of our center (IRB#090977-1).
Clinicopathological data were extracted from the hospital database (including clinical history and routine pathologic reports). The administrations of postoperative chemotherapy (POCT) determined at the discretion of their treating medical oncologists based on patient’s comorbidity were recorded. In addition, permanent full-face hematoxylin and eosin (H&E)-stained slides were retrieved from the pathology archives, and several clinically meaningful pathologic characteristics other than pathology reports were reevaluated by two qualified specialized pathologists (L.Y. and S.L.) for each case. Extracapsular extension (ECE) of mediastinal LN was defined as the extension of the tumor through the LN capsule microscopically.24 TILs were analyzed based on the TIL scoring method in the H&E-stained slides, as previously described.13 Using surgical specimens, gene mutations associated with lung adenocarcinoma were detected in epidermal growth factor receptor (EGFR) exons 18–21 by the amplification-refractory mutation system (ARMS). Data regarding the treatment of subsequent EGFR-tyrosine kinase inhibitors (TKIs) for patients with relapse or progressive disease were recorded.6
The follow-up interval was regularly every 3 months during the first 2 years after surgery and subsequently every 6–12 months. At each follow-up visit, physical examination, blood tests, chest computed tomography scans, ultrasonography of the abdomen, and brain magnetic resonance imaging were generally conducted. Other necessary examinations were optionally performed based on the clinical symptoms. The first patterns of failure were assessed and classified as either locoregional and/or distant. Recurrences beyond the bronchial stump, hilum, mediastinum, and/or supraclavicular regions were considered distant metastases.25
Microarray construction
We constructed tissue microarrays (TMAs) in collaboration with the Department of Pathology according to established methods.26 For each patient, all archived H&E-stained slides and the corresponding formalin-fixed paraffin-embedded (FFPE) tissue specimens were collected. All samples were from the surgical specimen at baseline. Two specialized pathologists (L.Y. and S.L.) reviewed each H&E slide of the surgical specimens in two representative regions from the CT and two regions from the IM presenting the highest infiltration of TILs. We used the UATM-272A tissue microarray system (Unitma, Seoul, Korea) to extract two representative areas of the CT and two areas of the IM from the FFPE tissue blocks (1.5-mm diameter tissue cores) for arrangement and deposit into a recipient paraffin TMA block, respectively.
Immunohistochemistry
Immunohistochemistry (IHC) of 4-μm paraffin-embedded slides from the TMA blocks was performed using the standardized EnVision system (Agilent Technologies, Santa Clara, CA, USA), as previously reported.27 The TMA tissue slides were first deparaffinized with xylene and rehydrated with an ethanol gradient. Antigen retrieval was conducted by placing the TMA slides in 10 mM citrate buffer (pH 6.0) and boiling for 15–17 min. After incubation in 0.3% hydrogen peroxide, followed by incubation with IHC background blocker (ADI-950-230-0100; Enzo Life Sciences, Farmingdale, NY, USA), the slides were respectively incubated with the following primary antibodies overnight at 4°C: mouse anti-human monoclonal CD45RO (UCH-L1; 1:100; SC-1183; Santa Cruz Biotechnology, Dallas, TX, USA), rabbit anti-human monoclonal CD8 (SP16; 1:100; Maixin Biotechnology, Fuzhou, China), and rabbit anti-human monoclonal CD3 (SP7; 1:100; Abcam, Cambridge, MA, USA). The ImmPRESS HRP IgG polymer detection kit (MP-7452 and MP-7451; Vector Laboratories, Burlingame, VT, USA) and the DAB substrate kit (SK-4105; Vector Laboratories) were applied to obtain the target antigens. Hematoxylin was used to counterstain the TMA slides.
IHC scoring
Samples were evaluated independently and without knowledge of clinicopathological data and patient outcomes by two experienced pathologists (L.Y. and S.L.). For each histospot, the densities of CD3+, CD45RO+, and CD8+ lymphocyte infiltration were assessed separately using a four-category scale based on semi-quantitative estimation of the percentages of stained lymphocytes relative to the total number of nucleated cells in the whole region of the epithelial and stromal compartments. According to a previous report,28 scoring cut-off points at 5%, 25%, or 50% were used for each T cell marker in each tumor region: score 0 (0–5%), 1 (>5–25%), 2 (>25–50%), and score 3 (>50%). The mean density of the duplicate cores was used to assign one score per tumor region. For statistical analysis, categories of scores from 0 to 1 (⩽25%) were designated as the low-infiltration group, and those with scores from 2 to 3 (>25%) were designated as high-infiltration group for the CD3+, CD45RO+, and CD8+ lymphocyte densities in each respective tumor region (CT and IM). Patients were then classified according to the definition of the IS based on the combined assessment of TIL type, density, and distribution, as previously described by Galon et al. in CRC.14
Statistical analysis
Overall survival (OS) was calculated from the date of surgery to the date of death or the last follow-up. We defined distant metastasis-free survival (DMFS) as the time from the date of surgery to the documented distant metastases or the last follow-up. OS and DMFS were estimated with the Kaplan–Meier method. The log-rank test was used to analyze the association between T lymphocytes (CD3CT/IM, CD45ROCT/IM, and CD8CT/IM alone or in combination) and survival endpoints. After identifying the prognostic immune markers and determining the IS combination, we generated a multivariate Cox proportional hazards model (backward conditional step-wise) to control confounding variables in the analysis. All statistical analyses were performed using SPSS (v.20.0; IBM Corp., Armonk, NY, USA), and a p < 0.05 was considered statistically significant unless otherwise stated.
Results
Patient characteristics
Of the 357 patients who met the eligibility criteria, 69 were excluded due to loss to follow-up (n = 18), incomplete H&E slides (n = 37), and inaccessible FFPE tissue specimens (n = 14). Therefore, 288 patients with complete IHC-based TMA histospots were assessed and included in this study [median age: 59 (range: 22–86) years] (Table 1). Of the 288 patients, 247 (86%) received POCT, 213 (74%) received ⩾4 cycles of POCT, 89 (31%) had evidence of mediastinal LN-ECE, 120 (42%) showed positive TIL infiltration according to the H&E slides, and 182 had information concerning mutation status (EGFR exons 18–21) according to ARMS, and 77 (42%) positive for EGFR mutation.
Table 1.
Patient characteristics.
| Characteristics | Data N = 288 |
|---|---|
| Age, years | Median: 59; range: 22–86 |
| Gender | |
| Male | 187 (65%) |
| Female | 101 (35%) |
| ECOG PS | |
| 0 | 17 (6%) |
| 1 | 247 (86%) |
| 2 | 24 (8%) |
| Clinical N stage | |
| cN0,1 | 158 (55%) |
| cN2 | 130 (45%) |
| Smoking history* | |
| Never/light | 133 (46%) |
| Current/heavy | 155 (54%) |
| Surgical procedure | |
| Lobectomy/sleeve lobectomy | 254 (88%) |
| Pneumonectomy | 34 (12%) |
| Histology | |
| Adenocarcinoma | 171 (59.4%) |
| Squamous cell | 87 (30.2%) |
| Adenosquamous | 21 (7.3%) |
| Large cell | 7 (2.4%) |
| Pleomorphic | 2 (0.7%) |
| Tumor differentiation | |
| Well | 2 (0.7%) |
| Moderate | 136 (47.2%) |
| Poor | 150 (52.1%) |
| LN-ECE | |
| Positive | 89 (31%) |
| Negative | 199 (69%) |
| TILs | |
| Positive | 120 (42%) |
| Negative | 168 (58%) |
| Pathologic T stage | |
| T1 | 65 (22%) |
| T2 | 189 (66%) |
| T3 | 34 (12%) |
| Number of positive nodes | |
| ⩽4 | 160 (56%) |
| >4 | 128 (44%) |
| EGFR mutation status | |
| Positive | 77 (42%) |
| Negative | 105 (58%) |
| Cycles of POCT | |
| <4 | 75 (26%) |
| ⩾4 | 213 (74%) |
Smoking history was categorized as never/light ex-smokers (<100 cigarettes smoked in their lifetime or ⩽10 pack-years, having stopped for ⩾15 years) or current/heavy ex-smokers.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; LN-ECE, lymph node extracapsular extension; POCT, postoperative chemotherapy; TIL, tumor infiltrating lymphocyte.
Of the 247 patients who received POCT, the median cycle of POCT was 4 (range, 1–6) and platinum-based doublet chemotherapy was administered. The most common reasons for not completing the planned four cycles of POCT (n = 75) were the patient’s refusal (n = 38, 50.7%), excessive toxicity (n = 17, 22.7%), poor general condition due to pneumonectomy (n = 10, 13.3%), medical non-compliance reason of old age (n = 8, 10.6%), and progressive disease during treatment (n = 2, 2.7%). In our follow-up data, 55 patients were treated with subsequent EGFR-TKIs for relapse or progressive disease, of which 37 received gefitinib, 16 received erlotinib, and two received icotinib.
Prognostic impact of immune cell densities in NSCLC
Among the entire cohort, the median follow-up time was 36.9 months (range: 4.4–132 months) for all patients and 54.9 months (range: 23.9–132 months) for living patients. For all patients, the median survival time was 37.8 months, and the 5-year OS and DMFS rates were 34% and 26%, respectively. As shown in Table 2, CD45RO+ TIL density in the IM was a significant prognostic factor for DMFS (p = 0.02) and showed a trend predictive of OS (p = 0.05). In addition, we found a high density of CD8+ TILs in the CT was associated with improved survival, although the difference was not significant (DMFS p = 0.23; and OS p = 0.24). However, no significant association was observed between densities of CD3+ TILs in either the CT or the IM with survival prognosis (OS or DMFS) in this group.
Table 2.
The prognostic impact of the CD3+, CD45RO+, and CD8+ T cells on DMFS and OS in all patients (N = 288).
| n (%) | 5-year DMFS | p | 5-year OS | p | |
|---|---|---|---|---|---|
| CD3CT | 0.43 | 0.92 | |||
| Low | 140 | 24.4 | 32.3 | ||
| High | 148 | 27.9 | 35.5 | ||
| CD3IM | 0.80 | 0.43 | |||
| Low | 142 | 27.2 | 37.0 | ||
| High | 146 | 25.4 | 31.6 | ||
| CD45ROCT | 0.06 | 0.43 | |||
| Low | 157 | 21.7 | 32.0 | ||
| High | 131 | 31.5 | 36.8 | ||
| CD45ROIM | 0.02 | 0.05 | |||
| Low | 135 | 19.7 | 30.8 | ||
| High | 153 | 32.0 | 36.8 | ||
| CD8CT | 0.23 | 0.24 | |||
| Low | 194 | 24.0 | 31.5 | ||
| High | 94 | 30.2 | 39.5 | ||
| CD8IM | 0.58 | 0.57 | |||
| Low | 170 | 23.8 | 32.9 | ||
| High | 118 | 29.8 | 35.7 |
CT, center of the tumor; DMFS, distant metastasis-free survival; IM, invasive margin; OS, overall survival.
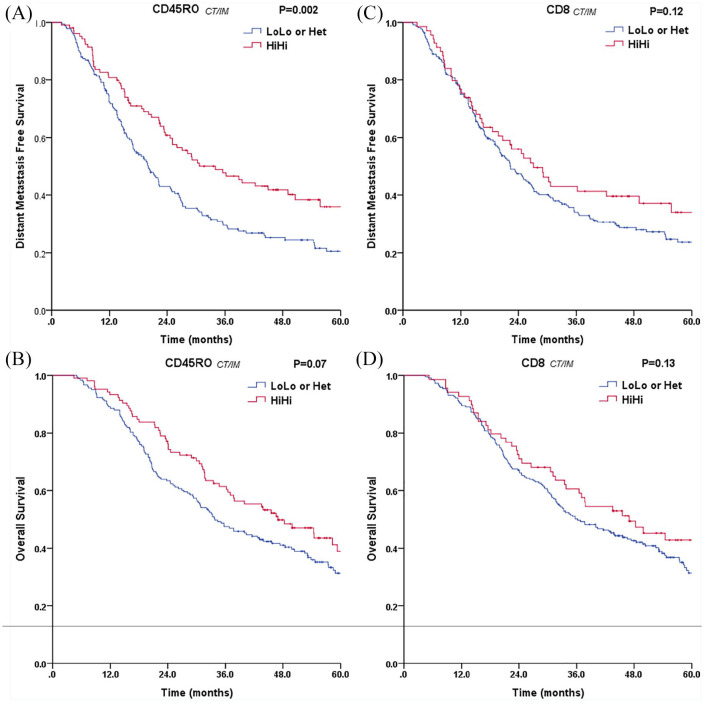
The combined analysis based on TIL density and distribution in the CT and IM better discriminated patients grouped for CD45RO+ and CD8+ TILs, respectively (Figure 1 and Supplemental material Figure 1 online). Patients with high densities of CD45RO+ T cells in both tumor regions (CD45ROCT/IMHiHi) showed a longer DMFS (p = 0.002) and an improved OS (p = 0.07) (Figure 1A and B). The beneficial prognostic impact was obviously decreased in patients with a low density of CD45RO+ cells in any one of the tumor regions (heterogeneous pattern LoHi or HiLo; CD45ROCT/IMHet) or low densities in both of tumor regions (CD45ROCT/IMLoLo) (Supplemental Figure 1A and B). In addition, high densities of CD8+ T cells in both tumor regions (CD8CT/IMHiHi) were associated with longer DMFS (p = 0.12) and better OS (p = 0.13), but the differences did not reach the level of statistical significance (Figure 1C and D). Moreover, no significant differences were found with DMFS and OS for densities of CD3+ T cells in the combined analysis of the CT and IM (Supplemental Figure 1E and F). These findings indicated that the density and distribution of CD45RO+ memory TILs significantly influenced the prognosis of patients with stage IIIA(N2) disease, especially in terms of DMFS.
Figure 1.
Plot of distant metastasis-free survival and overall survival for all patients stratified into scores for (A, B) CD45ROCT/IM and (C, D) CD8CT/IM in combined tumor regions (CT and IM) (N = 288). For each marker, high and low infiltration in the CT and IM (HiHi, red curve; and LoLo, blue curve; respectively) or heterogeneous infiltration with CTLo and IMHi or CTHi and IMLo (Het, blue curve) are presented for all patients (N = 288).
CT, center of the tumor; IM, invasive margin.
Combined analysis of IS to predict clinical outcomes
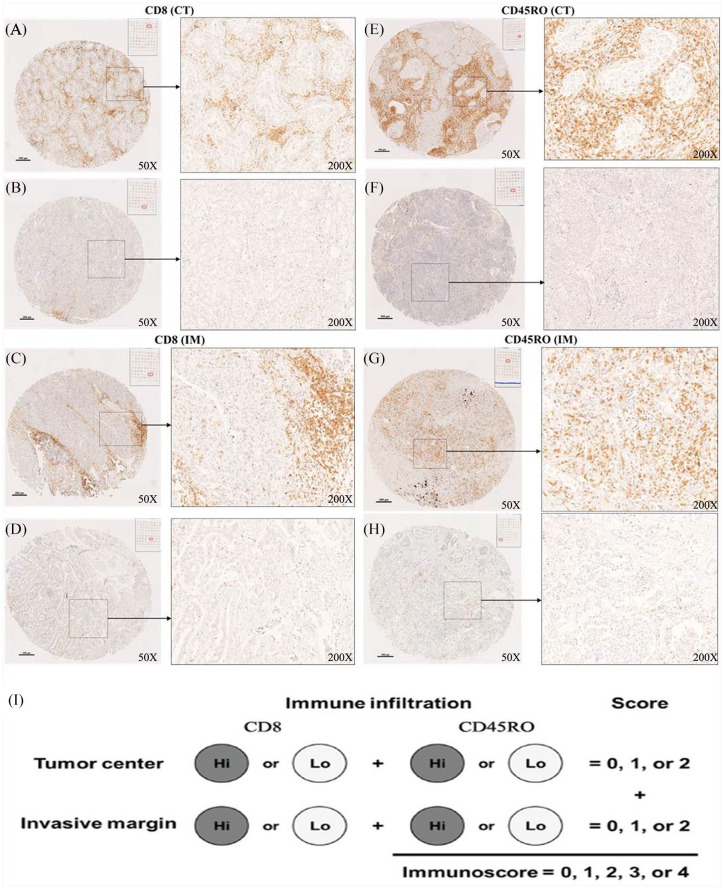
Based on combined analysis of the densities of CD45RO+ and CD8+ T cells in both tumor regions, the scoring system of the proposed IS was implemented and evaluated. Scoring was performed using the two-category IS,29 where a density between 0% and 25% was scored as low (Lo; score 0) and that from 25% to 100% was scored as high (Hi; score 1) for CD45RO+ and CD8+ T cells in each tumor region (CT and IM), respectively. Representative IHC slides showing high and low infiltration of CD45RO+ and CD8+ TILs in the CT and the IM are displayed in Figure 2, respectively. Patients were stratified according to IS scoring groups ranging from IS0 to IS4.
Figure 2.
Tumor-infiltrating lymphocytes were stained with CD8+ and CD45RO+ antibodies [(A, E) high and (B, F) low infiltration in the CT; and (C, G) high and (D, H) low infiltrations in the IM]. Magnifications: 50× and 200×. (I) Representation of immunoscore definition.
CT, center of the tumor; IM, invasive margin.
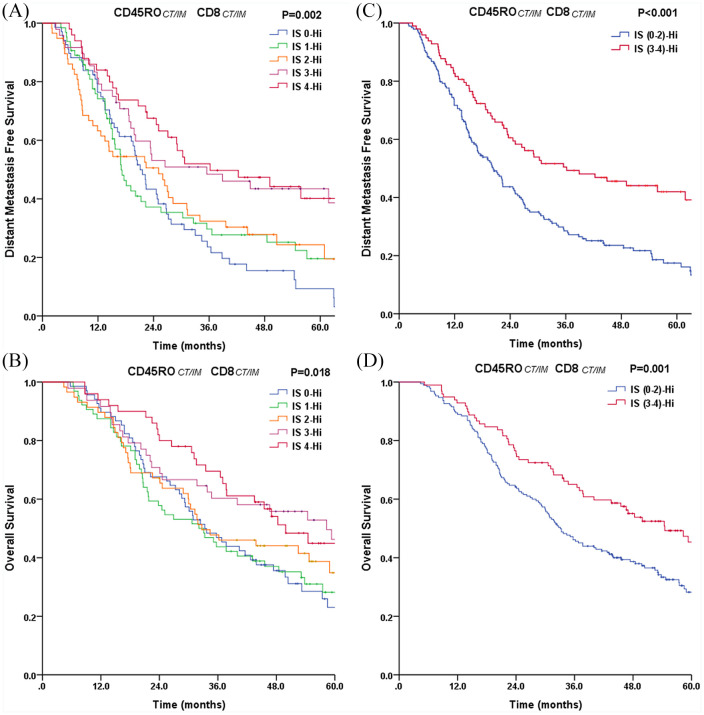
According to this criterion, there were 68 (24%) patients with IS0, 64 (22%) with IS1, 58 (20%) with IS2, 48 (17%) with IS3, and 50 (17%) with IS4. Significant differences in DMFS and OS stratified with patient IS groups were determined by univariate analysis (p = 0.002 and p = 0.018) (Figure 3A and B). Patients with IS4 (high densities of CD45RO+ and CD8+ cells in both tumor regions) had prolonged OS (5-year OS rate: 44.9%) and DMFS (5-year DMFS rate: 40.2%). Similarly, patients with IS3 experienced a similarly better postoperative outcome (5-year DMFS: 43.5%; and 5-year OS: 46.3%). By contrast, the cumulative 5-year DMFS and OS rates were only 9.3% and 23.1%, respectively, for patients with IS0 (low densities of CD45RO+ and CD8+ cells in both tumor regions). Patients with an IS of 1 or 2 had 5-year DMFS and OS rates of 21.6% and 31.1%, respectively.
Figure 3.
Plot of (A, C) distant metastasis-free survival and (B, D) overall survival for all patients stratified into the IS (N = 288).
CT, center of the tumor; IM, invasive margin; IS, immunoscore.
In addition, a better discrimination between high IS (IS3–4) and low IS (IS0–2) was demonstrated for DMFS and OS in stage IIIA(N2) patients (Figure 3C and D). The 5-year DMFS and OS rates were only 17% and 28% for the group with low IS (IS0–2; n = 190) as compared with 42% and 45% for the group with high IS (IS3–4; n = 98), respectively (DMFS p < 0.001; and OS p = 0.001). All significant clinicopathologic features from the univariate analyses were adopted as covariates when multivariate Cox regression analyses were performed (Table 3). Multivariate analyses identified the IS as an independent prognostic variable for both DMFS (p = 0.001) and OS (p = 0.002).
Table 3.
Multivariate analyses of the DMFS and OS in all patients (N = 288).
| Characteristics | n | DMFS |
OS |
||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Gender | 0.99 | 0.48 | |||
| Female | 101 | 1 | 1 | ||
| Male | 187 | 1.00 (0.62–1.62) | 1.16 (0.77–1.76) | ||
| Age, years | 0.64 | 0.18 | |||
| ⩽60 | 169 | 1 | 1 | ||
| >60 | 119 | 1.10 (0.74–1.62) | 1.33 (0.87–2.04) | ||
| ECOG PS | 0.56 | 0.003* | |||
| 0–1 | 264 | 1 | 1 | ||
| 2 | 24 | 1.32 (0.53–3.28) | 3.26 (1.50–7.09) | ||
| Smoking history | 0.08 | 0.62 | |||
| Never/light smoker | 133 | 1 | 1 | ||
| Current/heavy smoker | 155 | 1.38 (0.97–1.96) | 0.88 (0.51–1.49) | ||
| Clinical N stage | 0.004 * | 0.02* | |||
| cN0,1 | 158 | 1 | 1 | ||
| cN2 | 130 | 1.70 (1.18–2.45) | 1.58 (1.06–2.35) | ||
| Surgical procedure | 0.33 | 0.73 | |||
| Lobectomy | 254 | 1 | 1 | ||
| Pneumonectomy | 34 | 1.44 (0.69–3.04) | 1.16 (0.50–2.66) | ||
| Histology | 0.67 | 0.55 | |||
| Non-SCC | 201 | 1 | 1 | ||
| SCC | 87 | 0.78 (0.24–2.53) | 1.38 (0.48–3.96) | ||
| Tumor differentiation | 0.95 | 0.05 | |||
| Well/Moderate | 138 | 1 | 1 | ||
| Poor | 150 | 0.99 (0.70–1.41) | 1.45 (0.99–2.12) | ||
| LN-ECE | 0.018 * | 0.06 | |||
| ECE− | 199 | 1 | 1 | ||
| ECE+ | 89 | 1.61 (1.09–2.38) | 1.49 (0.98–2.26) | ||
| No. of positive nodes | 0.01 * | <0.001 * | |||
| ⩽4 | 160 | 1 | 1 | ||
| >4 | 128 | 1.68 (1.14–2.50) | 2.34 (1.55–3.52) | ||
| Pathologic T stage | 0.14 | 0.09 | |||
| T1 | 65 | 1 | 1 | ||
| T2 | 189 | 1.23 (0.83–1.81) | 1.66 (1.06–2.62) | ||
| T3 | 34 | 0.64 (0.30–1.36) | 1.36 (0.63–2.94) | ||
| Immunoscore | 0.001 * | 0.002 * | |||
| IS0–2 | 190 | 1 | 1 | ||
| IS3–4 | 98 | 0.52 (0.35–0.78) | 0.50 (0.33–0.77) | ||
| EGFR mutation | 0.82 | 0.02 * | |||
| Negative | 105 | 1 | 1 | ||
| Positive | 77 | 0.96 (0.68–1.35) | 0.64 (0.44–0.94) | ||
| Cycles of POCT | 0.017 * | 0.01 * | |||
| <4 | 75 | 1 | 1 | ||
| ⩾4 | 213 | 0.62 (0.42–0.92) | 0.57 (0.36–0.88) | ||
A number marked in bold indicates that its corresponding variable is statistically significant for the DMFS or OS.
CI, confidence interval; DMFS, distant metastasis-free survival; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; HR, hazard ratio; LN-ECE, lymph node extracapsular extension; OS, overall survival; POCT, postoperative chemotherapy; SCC, squamous cell carcinoma.
Discussion
In this study, we performed analyses of the immune contexture (TIL type, density, and distribution) and the IS in completely resected stage IIIA(N2) NSCLC patients, demonstrating a positive correlation between the IS (CD45RO+ and CD8+ T cell infiltration in the CT and IM) and postoperative clinical outcomes in a consistent population with stage IIIA(N2) NSCLC in terms of DMFS and OS. Although the dynamics occur over time because the tumor infiltration by T lymphocytes is shaped by a combination of the invasive growth of tumor and a coordinated immune reaction of the host,30 the IS described here reflects the pre-existing state of the tumor immune microenvironment has been generated in a patient at a single, static time point of tumor resection associated with patient survival prognosis. Briefly, evaluation of CD45RO+ memory and CD8+ cytotoxic T cells in the combined tumor regions (CT and IM) at the static situation of resection time provided a means for predicting postoperative distant metastatic risk and survival prognosis of stage IIIA(N2) disease. Therefore, the proposed IS (defined as CD45RO+ and CD8+ T cells within the CT and IM) effectively outlined the orientation of the tumor immune microenvironment in pathological stage IIIA(N2) patients in an accessible and easy-to-apply manner, the understanding of which could be useful for risk stratification in clinical settings and in future prospective clinical trials assessing outcomes and therapeutic efficacies in surgically resected NSCLC.
Previous studies reported the prognostic roles of TILs in NSCLC; however, because immune infiltration in tumors is heterogeneous, their prognostic impact conflicted in terms of immune cell type, density, and distribution.18,31 Other studies found that the presence of CD3+, CD8+, and CD45RO+ T cells was associated with beneficial survival outcomes for NSCLC.18–22,31 Data from the present study generally agreed with these findings. We demonstrated that the density of CD45RO+ TILs in the IM was a significant prognostic factor for clinical outcome (DMFS and OS). In addition, a high density of CD8+ TILs in the CT was associated with prolonged survival but did not reach statistical significance. In contrast to a previous study,32 we found that the density of CD3+ TILs was associated with neither OS nor DMFS in this cohort. This inconsistency might be due to the tumor cells and/or the heterogeneous tumor immune contexture including other immunosuppressive subsets of T cell populations and/or cytokines that potentially blunted the effector function of CD3+ TILs in stage IIIA(N2) NSCLC.33 There remains a need for further functional studies to explain these discrepancies. In addition to TIL subtype, our results confirmed that TIL distribution is another critical prognostic factor. Notably, accurate delineation of the analyzed tumor regions (two representative regions from the CT and two from the IM) was completed by specialized pathologists and included in our analysis in order to better reflect the TIL distribution and enhance their prognostic impact.
We showed that a higher infiltration of CD45RO+ memory TILs (CD45ROCT/IM) and the IS exhibited robust predictive values for DMFS. This is supported by several previous reports that suggested a strong association between memory T cells and metastasis in CRC,11,12 which could be explained by the durable antitumor properties of memory T cells playing an essential role in controlling tumor micrometastases following complete resection. For patients with pathological stage IIIA(N2) disease, micrometastases, occult tumor cells might be commonly detected, even after curative resection. Therefore, analysis of in situ memory T lymphocytes could provide information regarding the ability of the host immune response to eliminate occult tumor cells, which could help attenuate the metastatic potential of lung tumor cells.
For routine IHC evaluation, there remain unsolved issues concerning measuring levels of immune infiltrate.20 Based on a study of CRC,14 a simple scoring method (the IS) was established and could predict patient prognosis in a clinical setting. A previous study suggested a protective role for CD3+ pan T cells, CD8+ cytotoxic T cells, and CD45RO+ memory T cells, as well as TILs evaluated by H&E staining, with these having since become candidate markers for the development of an IS for NSCLC.20,29 With the aim to establish an NSCLC TNM-IS (TNM-I) classification for NSCLC, several in situ immunology studies were performed to explore a T lymphocyte-based IS in order to add prognostic impact to the clinical setting (Supplemental Table 1). However, there still exists discrimination against the establishment of an IS for NSCLC in terms of TIL type, the localization for scoring, and the scoring system.20,29 Overall, TIL localization (CT versus IM) was unavailable in previous studies,19,21,22 an understanding of which could be essential to optimizing the prognostic value of an IS for NSCLC.20,29 A recent study by Ishii et al. that accounted for TIL localization included a relatively small number of patients for the analysis.32 In the present study, we found that evaluation of CD45RO+ T cells and CD8+ T cells in combined tumor regions (CT and IM) allowed successful stratification of patients into two prognostic categories with dramatically different DMFS and OS prognoses. The IS demonstrated a significant prognostic impact with regard to 5-year DMFS rates [42% (IS3–4) and 17% (IS0–2); p < 0.001], as well as 5-year OS rates [45% (IS3–4) and 28% (IS0–2); p = 0.001]. Furthermore, multivariate analysis revealed that the IS remained an independent prognostic factor in terms of both DMFS and OS.
The establishment of an IS has a dual advantage. First, it demonstrated efficacy as a robust prognostic factor for DMFS and OS in completely resected stage IIIA(N2) NSCLC and might facilitate identification of patients at high risk of distant metastasis and prognostic prediction using IHC staining in routine diagnostic pathology. Second, it carries biological meaning for pathological stage IIIA(N2) NSCLC, suggesting that the state of in situ adaptive immune response to tumors plays a role in preventing systemic recurrences after surgery. CD45RO+ T cells and the IS could reflect the local immune microenvironment, which might be vital parameters that could be integrated with other tumor features to potentially enable determination of the biological behaviors of tumors.
This study has several limitations. First, this study was retrospective in nature and included a relatively small study cohort of 288 cases, although multivariable analysis was performed to control for potential confounding variables. Therefore, this study can be regarded as an initial exploration of the IS for stage IIIA(N2) NSCLC that offers a foundation for further external validation on a larger scale in order to establish TNM-I staging for this group. Second, the proposed IS for NSCLC was developed through semi-quantitative evaluation of CD8+ and CD45RO+ density by expert pathologists, under which conditions the individual expertise of a given pathologist might cause bias. However, it should be noted that the conventional IHC scoring method for common T cells is considered a standardized and reproducible procedure in pathologic practice, whereas digital quantification scoring by image-analysis software has not maintained consistency in the results, even when applied by expert pathologists.29 In addition, given the spatial heterogeneity of TILs, the TMAs used in this study tended to not reflect the complete picture for each patient. To better reflect TIL localization and attenuate the sampling effect of TMAs, a precise description of the analyzed tumor regions (CT and IM) was undertaken by selecting two representative regions from the CT and two from the IM, each containing the highest TIL density. Finally, the proposed IS, largely supported by findings in a consistent population of patients with stage IIIA(N2) NSCLC, included the T lymphocyte-based scoring without the immunogenomic analyses performed in this study, suggesting that more information might be necessary in order to construct an IS for NSCLC. Unfortunately, RNA sequencing (RNA-seq) analysis has stricter requirements on the type and quality of tissue samples, including storage for ⩽2 years from the time of surgery, which indicates that RNA-seq analysis would not have been possible using the follow-up data in this study. Therefore, a more comprehensive immunological characterization needs to be integrated into the proposed IS for NSCLC and explored in an on-going multicenter prospective clinical study led by our center [ClinicalTrials.gov identifiers: NCT02977169 and NCT02974426].
In conclusion, the proposed IS (involving combined assessment of CD45RO+ and CD8+ T cells in the CT and IM) for NSCLC might provide valuable prognostic information, including prediction of DMFS and OS in patients with completely resected stage IIIA(N2) NSCLC. Our results identified CD45RO+ memory cells and CD8+ cytotoxic cells as viable candidate markers that should be included in subsequent studies focused on establishing an IS for NSCLC. However, studies with larger patient cohorts are needed to validate these findings and evaluate the role of the IS in guiding clinical decisions for adjuvant therapy, especially in the era of immunotherapy.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_1758835920984975 for Clinical impact of the tumor immune microenvironment in completely resected stage IIIA(N2) non-small cell lung cancer based on an immunoscore approach by Wen Feng, Yuan Li, Lei Shen, Qin Zhang, Xu-Wei Cai, Zheng-Fei Zhu, Meng-Hong Sun, Hai-Quan Chen and Xiao-Long Fu in Therapeutic Advances in Medical Oncology
Acknowledgments
Thanks to Dr. Shyamal Goswami, from the Institut Pasteur of Shanghai Chinese Academy of Sciences, for his support to the IHC experiment in this study.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics and informed consent statement: This study was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center (No. 090977-1). The requirement for informed consent was waived because this was a retrospective study.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Key Research and Development Program of China (grant no. 2016YFC0905502), the Project of Shanghai Science and Technology Commission (grant no. 18YF1421500), and Project of Multi-center Clinical Research, Shanghai Jiao Tong University School of Medicine (grant no. DLY201619).
ORCID iD: Xiao-Long Fu  https://orcid.org/0000-0001-8127-3884
https://orcid.org/0000-0001-8127-3884
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Wen Feng, Department of Radiation Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China; Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.
Yuan Li, Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, China.
Lei Shen, Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, China.
Qin Zhang, Department of Radiation Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China; Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.
Xu-Wei Cai, Department of Radiation Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China; Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.
Zheng-Fei Zhu, Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.
Meng-Hong Sun, Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, China.
Hai-Quan Chen, Department of Thoracic Surgery, Fudan University Shanghai Cancer Center, Shanghai, China.
Xiao-Long Fu, Department of Radiation Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University, 241 West Huaihai Road, Shanghai, 200030, China; Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.
References
- 1. Andre F, Grunenwald D, Pignon JP, et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for subclassification and implications. J Clin Oncol 2000; 18: 2981–2989. [DOI] [PubMed] [Google Scholar]
- 2. Deng W, Xu T, Xu Y, et al. Survival patterns for patients with resected N2 non-small cell lung cancer and postoperative radiotherapy: a prognostic scoring model and heat map approach. J Thorac Oncol 2018; 13: 1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009; 136: 260–271. [DOI] [PubMed] [Google Scholar]
- 4. Wagner H., Jr. Postoperative adjuvant therapy for patients with resected non-small cell lung cancer: still controversial after all these years. Chest 2000; 117: 110S–S118. [DOI] [PubMed] [Google Scholar]
- 5. Feng W, Fu XL, Cai XW, et al. Patterns of local-regional failure in completely resected stage IIIA(N2) non-small cell lung cancer cases: implications for postoperative radiation therapy clinical target volume design. Int J Radiat Oncol Biol Phys 2014; 88: 1100–1107. [DOI] [PubMed] [Google Scholar]
- 6. Feng W, Zhang Q, Fu XL, et al. The emerging outcome of postoperative radiotherapy for stage IIIA(N2) non-small cell lung cancer patients: based on the three-dimensional conformal radiotherapy technique and institutional standard clinical target volume. BMC Cancer 2015; 15: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG 1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018; 19: 139–148. [DOI] [PubMed] [Google Scholar]
- 8. Kimura H, Matsui Y, Ishikawa A, et al. Randomized controlled phase III trial of adjuvant chemoimmunotherapy with activated cytotoxic T cells and dendritic cells from regional lymph nodes of patients with lung cancer. Patterns and risks of postoperative recurrence in completely resected EGFR-mutant non-small cell lung cancer. Cancer Immunol Immunother 2018; 67: 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogino S, Galon J, Fuchs CS, et al. Cancer immunology analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol 2011; 8: 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12: 298–306. [DOI] [PubMed] [Google Scholar]
- 11. Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353: 2654–2666. [DOI] [PubMed] [Google Scholar]
- 12. Mlecnik B, Bindea G, Kirilovsky A, et al. The tumor microenvironment and immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med 2016; 8: 327ra26. [DOI] [PubMed] [Google Scholar]
- 13. Feng W, Li Y, Shen L, et al. Prognostic value of tumor-infiltrating lymphocytes for patients with completely resected stage IIIA(N2) non-small cell lung cancer. Oncotarget 2016; 7: 7227–7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘immunoscore’ in the classification of malignant tumours. J Pathol 2014; 232: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313: 1960–1964. [DOI] [PubMed] [Google Scholar]
- 16. Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009; 27: 5944–5951. [DOI] [PubMed] [Google Scholar]
- 17. Angell H, Galon J. From the immune contexture to the immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol 2013; 25: 261–267. [DOI] [PubMed] [Google Scholar]
- 18. Remark R, Becker C, Gomez JE, et al. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am J Respir Crit Care Med 2015; 191: 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schalper KA, Brown J, Carvajal-Hausdorf D, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst 2015; 107: dju435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donnem T, Kilvaer TK, Andersen S, et al. Strategies for clinical implementation of TNM-Immunoscore in resected non-small-cell lung cancer. Ann Oncol 2016; 27: 225–232. [DOI] [PubMed] [Google Scholar]
- 21. Donnem T, Hald SM, Paulsen EE, et al. Stromal CD8+ T-cell density—a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res 2015; 21: 2635–2643. [DOI] [PubMed] [Google Scholar]
- 22. Paulsen EE, Kilvaer T, Khanehkenari MR, et al. CD45RO+ memory T lymphocytes–a candidate marker for TNM-Immunoscore in squamous non-small cell lung cancer. Neoplasia 2015; 17: 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-small-cell lung cancer, v.3.2020, https://www.nccn.org/ (2020, accessed 11 February 2020).
- 24. Lee YC, Wu CT, Kuo SW, et al. Significance of extranodal extension of regional lymph nodes in surgically resected non-small cell lung cancer. Chest 2007; 131: 993–999. [DOI] [PubMed] [Google Scholar]
- 25. Kelsey CR, Light KL, Marks LB. Patterns of failure after resection of non-small-cell lung cancer: implications for postoperative radiation therapy volumes. Int J Radiat Oncol Biol Phys 2006; 65: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 26. Xiao X, Wang L, Wei P, et al. Role of MUC20 overexpression as a predictor of recurrence and poor outcome in colorectal cancer. J Transl Med 2013; 11: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cai XW, Yu WW, Yu W, et al. Tissue-based quantitative proteomics to screen and identify the potential biomarkers for early recurrence/metastasis of esophageal squamous cell carcinoma. Cancer Med 2018; 7: 2504–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 2008; 14: 5220–5227. [DOI] [PubMed] [Google Scholar]
- 29. Ros-Martínez S, Navas-Carrillo D, Alonso-Romero JL, et al. Immunoscore: a novel prognostic tool. Association with clinical outcome, response to treatment and survival in several malignancies. Crit Rev Clin Lab Sci 2020: 57: 432–443. [DOI] [PubMed] [Google Scholar]
- 30. Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013; 39: 782–795. [DOI] [PubMed] [Google Scholar]
- 31. Bremnes RM, Busund LT, Kilvær TL, et al. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J Thorac Oncol 2016; 11: 789–800. [DOI] [PubMed] [Google Scholar]
- 32. Ishii H, Azuma K, Kawahara A, et al. Programmed cell death-ligand 1 expression and immunoscore in stage II and III non-small cell lung cancer patients receiving adjuvant chemotherapy. Oncotarget 2017; 8: 61618–61625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011; 331: 1565–1570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_1758835920984975 for Clinical impact of the tumor immune microenvironment in completely resected stage IIIA(N2) non-small cell lung cancer based on an immunoscore approach by Wen Feng, Yuan Li, Lei Shen, Qin Zhang, Xu-Wei Cai, Zheng-Fei Zhu, Meng-Hong Sun, Hai-Quan Chen and Xiao-Long Fu in Therapeutic Advances in Medical Oncology