Abstract
Tumor cells often switch from mitochondrial oxidative metabolism to glycolytic metabolism even under aerobic conditions. Tumor cell glycolysis is accompanied by several nonenzymatic activities among which induction of drug resistance has important therapeutic implications. In this article, we review the main aspects of glycolysis-induced drug resistance. We discuss the classes of antitumor drugs that are affected and the components of the glycolytic pathway (transporters, enzymes, metabolites) that are involved in the induction of drug resistance. Glycolysis-associated drug resistance occurs in response to stimuli, either cell-autonomous (e.g., oncoproteins) or deriving from the tumor microenvironment (e.g., hypoxia or pseudohypoxia, mechanical cues, etc.). Several mechanisms mediate the induction of drug resistance in response to glycolytic metabolism: inhibition of apoptosis, induction of epithelial-mesenchymal transition, induction of autophagy, inhibition of drug influx and increase of drug efflux. We suggest that drug resistance in response to glycolysis comes into play in presence of qualitative (e.g., expression of embryonic enzyme isoforms, post-translational enzyme modifications) or quantitative (e.g., overexpression of enzymes or overproduction of metabolites) alterations of glycolytic metabolism. We also discern similarities between changes occurring in tumor cells in response to stimuli inducing glycolysis-associated drug resistance and those occurring in cells of the innate immune system in response to danger signals and that have been referred to as danger-associated metabolic modifications. Eventually, we briefly address that also mitochondrial oxidative metabolism may induce drug resistance and discuss the therapeutic implications deriving from the fact that the main energy-generating metabolic pathways may be both at the origin of antitumor drug resistance.
Keywords: Glycolysis, Tumors, Drug resistance, EMT, Danger, Apoptosis
Abbreviations: ABC, ATP-binding cassette; ALDO, fructose biphosphate aldolase; ATG, autophagy related; ATP, adenosine triphosphate; DDR, DNA damage repair; EGFR, epidermal growth factor receptor; EMT, epithelial-mesenchymal transition; ENO, enolase; ENO1, ENO isoform 1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GLUT, glucose transporter; HIF, hypoxia-inducible factor; HK, hexokinase; HK2, HK isoform 2; LDH, lactate dehydrogenase; LDHA, LDH isoform A; mAb, monoclonal antibody; mTOR, mechanistic target of rapamycin; NADPH, nicotinamide adenine dinucleotide phosphate reduced; OXPHOS, oxidative phosphorylation; PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase; PDK1, PDK isoform 1; PFKFB, phosphofructo-2-kinase/fructose-2,6-biphosphatase; PFKFB3, PFKFB isoform 3; PFKP, PFK platelet type; PGK, phosphoglycerate kinase; PGK1, PGK isoform 1; PI3K, phosphoinositide 3-kinase; PK, pyruvate kinase; PKM2, PK isoform M2; PPP, pentose phosphate pathway; ROS, reactive oxygen species; TKI, tyrosine kinase inhibitor; TME, tumor microenvironment
Introduction
Reprogramming of metabolism is now considered one of the hallmarks of cancer [1]. While most normal cells rely mainly on mitochondrial oxidative metabolism for energy generation under the form of adenosine triphosphate (ATP), tumor cells often switch to the much less efficient glycolytic metabolism (Fig. 1), even under oxygen-sufficient conditions, a phenomenon referred to as aerobic glycolysis or the Warburg effect [2,3]. In fact, the end product of glycolysis under anaerobic conditions is lactate. Under aerobic conditions, the penultimate metabolite, pyruvate, is normally redirected towards the citric acid cycle and oxidative metabolism upon transformation into acetyl-CoA. Alternatively, pyruvate is metabolized to lactate also under aerobic conditions, thereby conforming to the Warburg effect.
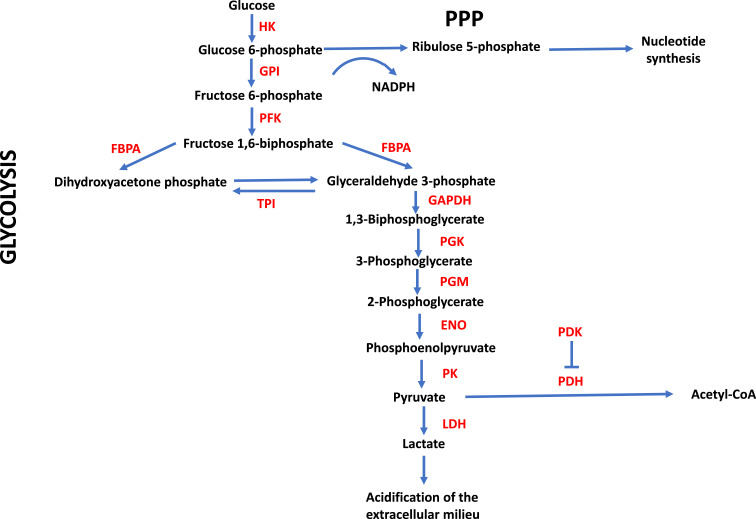
Fig. 1.
Glycolytic metabolism. Enzymes and metabolites are depicted as well as some of the pathways that branch off from glycolysis and are discussed in the text. ENO, enolase; FBPA, fructose-biphosphate aldolase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GPI, glucose 6-phosphate isomerase; HK, hexokinase; LDH, lactate dehydrogenase; NADPH, nicotinamide adenine dinucleotide phosphate reduced; PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase; PFK, phosphofructo-2-kinase; PGK, phosphoglycerate kinase; PGM, phosphoglycerate mutase; PK, pyruvate kinase; PPP, pentose phosphate pathway; TPI, triosephosphate isomerase.
The switch from oxidative metabolism toward glycolysis, however, does not occur necessarily in the entire tumor cell population but, rather, it may be about a fraction of tumor cells [4]. In fact, lactate-producing tumor cells relying on aerobic glycolysis for energy generation may coexist with nonlactate producing tumor cells relying on oxidative metabolism [5]. Moreover, the latter cells can internalize and utilize lactate and fuel it into the citric acid cycle and oxidative phosphorylation (OXPHOS) [6]. Vice versa, tumor cells can also switch back from aerobic glycolysis to mitochondrial oxidative metabolism at low glucose concentrations, as has been demonstrated with some glycolytic glioblastoma cell lines [7]. Eventually, it has also been proposed that individual tumor cells can exist in a hybrid metabolic state in which both aerobic glycolysis and OXPHOS are used [8].
As to why tumor cells use glycolysis for energy generation even under oxygen-sufficient conditions, 3 main reasons have been identified. First, to facilitate the generation and incorporation of biomass precursors through the fueling of glycolytic intermediates into the pentose phosphate pathway (PPP). These precursors are required for rapidly proliferating tumor cells [9]. Second, the rate of glycolysis is much faster than that of OXPHOS [10,11]. This may be particularly advantageous in situations where a sudden increase of energy demand occurs, like in tumor cells undergoing an epithelial-mesenchymal transition (EMT) [12,13]. Third, using glycolytic metabolism tumor cells are passively and actively protected against oxidative stress. Passively, because this allows to avoid OXPHOS, a major cellular source of reactive oxygen species (ROS) [9]. Actively, because fueling of intermediates into the PPP leads to the generation of nicotinamide adenine dinucleotide phosphate reduced (NADPH) which gives rise to the reduced form of glutathione, a compound that protects cells from ROS-induced damage [14].
To the extent that our understanding of the role of glycolysis in tumor cell biology and metabolism increased, it was realized that glycolysis was associated with drug resistance in tumor cells, and that there was even a causal relationship between glycolysis and drug resistance. In this review, we summarize current knowledge in this field. We will first address the classes of antitumor drugs that are involved in glycolysis-induced drug resistance, then we will discuss the components (transporters, enzymes, or metabolites) of glycolytic metabolism that induce drug resistance, the mechanisms that have been found to underlie such resistance and, eventually, we will try to put forward some possible reasons as to how and why an energy-generating metabolic pathway may induce drug resistance.
Classes of antitumor drugs that are associated with glycolysis-induced resistance
Strikingly, many different classes of antitumor drugs have been found being associated with glycolysis-induced resistance: a large number of different chemotherapeutics [15], [16], [17], [18], [19], [20], [21], [22], [23], large molecule therapeutics like monoclonal antibodies (mAb) targeting a variety of different antigens, including immune checkpoint inhibitors [1,[24], [25], [26], [27], [28], [29], [30], [31], hormone antagonists [1,32], targeted, small molecule therapeutics like tyrosine kinase inhibitors (TKI) [1,[33], [34], [35], [36], glucocorticoids [1,37], and ionizing radiation [1,38]. While the mechanisms underlying glycolysis-induced drug resistance will be discussed in a later section, it can be anticipated that the broad range of therapeutic agents associated with glycolysis-induced resistance, spanning from small molecule drugs to large-sized molecules like mAbs, likely involves different mechanisms that may vary according to the different classes of molecules.
Glycolysis-induced drug resistance has been investigated by a variety of different approaches. Thus, glycolysis and glycolytic enzymes or metabolites have been studied in isogenic, drug-sensitive and drug resistant cell lines [15,17,22,23,33,36]. Alternatively, cell lines with intrinsic or acquired drug resistance have been used [34,35,37,38], as well as cell lines before or after overexpression or down-regulation of a glycolytic enzyme [15,16,20]. Also in vivo experiments in mice have been performed for this purpose as, for example, with immune checkpoint inhibitors in mice lacking or overexpressing a glycolytic enzyme [27], or with an inhibitor of a glycolytic enzyme or metabolite in order to resensitize mice to a given drug [29,31]. In several cases these observations were accompanied by the demonstration of overexpression of the investigated enzyme or metabolite in patient-derived, drug-resistant tumor tissues [16,17].
Elements of glycolytic metabolism involved in the induction of drug resistance
Glycolysis is a complex chain of enzymatic reactions that encompasses transporters that internalize glucose into cells as well as several enzymes and metabolites, and many of these players have been shown being involved in the induction of drug resistance. As regards glucose transporters, glucose transporter (GLUT) 1, GLUT3, GLUT4, and GLUT5 have been reported to induce antitumor drug resistance [39], [40], [41], [42], [43]. As to glycolytic enzymes, hexokinase (HK) [23,[44], [45], [46], 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase (PFKFB) [34,[47], [48], [49], [50], fructose biphosphate aldolase (ALDO) [51,52], glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [1,37], phosphoglycerate kinase (PGK) [53,54], enolase (ENO) [1,20,[55], [56], [57], pyruvate kinase (PK) [4,15,[58], [59], [60], [61], [62], and lactate dehydrogenase (LDH) [1,22,27,29,31,[63], [64], [65], [66], [67], have all been shown being involved in the induction of antitumor drug resistance.
An important question that arises at this point is whether drug resistance is induced either directly by one of these elements, whether transporters, enzymes or metabolites, or indirectly, through an overall enhancement of the glycolytic metabolism in tumor cells induced, for example, by the upregulation of one of the enzymes listed above. In fact, both situations can occur. First, the upregulation of an individual enzyme has been shown to induce an overall elevation of the glycolytic metabolism and it is this elevation that is at the origin of the drug resistance through mechanisms that will be discussed later [16,33,45,49]. Second, it is an individual element of the metabolic pathway that is directly responsible for the induction of drug resistance, whether or not this may be accompanied by an overall elevation of glycolytic metabolism [44,46,48,51,54]. The latter situation occurs because glycolytic enzymes are also endowed with nonenzymatic activities and these nonenzymatic activities are actually those responsible for the induction of drug resistance [44,51,54,55]. The direct involvement of such nonenzymatic activities has been documented in different ways. Thus, the post-translational modification of a glycolytic enzyme was shown to be directly responsible for the induction of different nonenzymatic activities, including chemoresistance [44] and inhibition of such a post-translational modification abrogated the induction of drug resistance [46], induction of drug resistance depended on the noncytoplasmic (nuclear) localization of a glycolytic enzyme [48], mutant forms of a glycolytic enzyme that had lost their enzymatic activity still induced drug resistance [51], and a glycolytic enzyme interacted with proteins unrelated to glycolytic metabolism in order to induce drug resistance [54,55].
Another important point regarding glycolysis-induced drug resistance in tumor cells is that several of the glycolytic enzymes expressed in tumor cells and involved in the induction of drug resistance are particular isoforms (e.g., HK isoform 2 [HK2], PFKFB isoform 3 [PFKFB], ALDO isoform A [ALDOA], PGK isoform 1 [PGK1], ENO isoform 1 [ENO1], LDH isoform A [LDHA]) [44,47,51,53,56,63] and some of these isoforms are expressed in normal cells only during embryonic development [9]. In some cases, functional differences between isoforms expressed preferentially by normal adult cells and those expressed by tumor cells have been described. A very interesting case is the PK isoform M2 (PKM2). PKM2 is normally expressed only in embryonic cells and becomes re-expressed and overexpressed in tumor cells [68]. PKM2 exists in a tetrameric form that has high enzymatic activity and a low-activity dimeric form [68], which is the prevalent form in tumor cells. The switch between the tetrameric and dimeric form is promoted by phosphorylation at Tyr105 [69]. Due to its low enzymatic activity, dimeric PKM2 promotes the shunt of upstream accumulating glucose 6-phosphate into the PPP, thereby favoring generation of biomass precursors and antioxidant molecules over ATP. In addition, PKM2 has other activities unrelated to its role in glycolytic metabolism, such as serving as a cytosolic receptor for thyroid hormone and activities that are the consequence of its entrance into the nucleus, where it acts as a protein kinase and is involved in the epigenetic regulation of gene transcription [70].
In addition to enzymes that are directly involved in glycolytic metabolism, some other enzymes that have indirect effects on glycolysis have also been associated with drug resistance. Thus, the pyruvate dehydrogenase (PDH) complex decarboxylates pyruvate to acetyl-CoA, which enters the citric acid cycle in mitochondria and eventually fuels OXPHOS. Pyruvate dehydrogenase kinase (PDK) phosphorylates one of the subunits of PDH and inactivates it. Consequently, inhibition of PDH or overexpression of PDK are expected to promote glycolysis and, in accordance with what has been observed with enzymes directly involved in glycolysis, to induce drug resistance. In fact, PDH inhibition [71,72] as well as PDK overexpression [73], [74], [75], [76], [77] have been found to induce antitumor drug resistance.
As regards glycolytic metabolites, pyruvate [1,32,78,79] and lactate [1,27,[80], [81], [82], [83], [84], and ATP itself [85] have been associated with drug resistance. These are other examples of elements of the glycolytic metabolism that are directly involved in drug resistance beyond their role in the metabolic pathway. Moreover, extracellularly released lactate leads to acidification of the tumor microenvironment (TME) because of the contemporary release of stoichiometric amounts of H+ ions [86]. Acidosis of the TME is also responsible for the induction of resistance to antitumor drugs [87,88].
While most of the evidence brought so far bears on tumor cell-autonomous forms of glycolysis-associated drug resistance, some work suggests also that aerobic glycolysis may occur in tumor accessory cells and the end-product, lactate, may then induce drug resistance in tumor cells. In this form of symbiotic relationship aerobic glycolysis is induced in cancer-associated fibroblasts (CAF). Lactate then feeds mitochondrial oxidative metabolism in tumor cells, thereby conferring drug resistance [1,25,32,89]. This effect is commonly referred to as the “reverse Warburg effect” [90]. Importantly, in this setting, it is actually the mitochondrial activity which utilizes lactate as fuel for ATP generation and induction of drug resistance. In fact, as we will see in the final part of this article, also mitochondrial oxidative metabolism can be involved in the induction of drug resistance and we will discuss possible commonalities between glycolysis- and oxidative metabolism-associated drug resistance.
Stimuli promoting glycolysis-induced drug resistance
Glycolysis-induced drug resistance in tumor cells has been reported to occur either in response to stimuli from the TME or to tumor cell-autonomous stimuli (Fig. 2). As regards stimuli deriving from the TME, one class is represented by antitumor drugs such as chemotherapeutics [18,49,73,91], TKIs [47,82,92], or ionizing radiation [59]. In other words, the same drugs that induce tumor cell glycolysis are also victims of glycolysis-induced drug resistance. Similarly, also mechanical cues [93] and hypoxia or pseudohypoxia [56,94] can promote glycolysis-induced drug resistance. As regards cell-autonomous stimuli, oncoproteins, whether mutated or overexpressed [39,47,[95], [96], [97], [98], [99], [100], [101], [102], tumor-associated immune checkpoint molecules [45] and long noncoding (lnc) RNAs/micro (mi) RNAs [16] are examples of stimuli that induce glycolysis-associated drug resistance.
Fig. 2.
Stimuli promoting glycolysis-induced drug resistance. Cell-autonomous stimuli or stimuli from the TME induce tumor cells to undergo a switch from mitochondrial oxidative metabolism towards glycolytic metabolism. When this switch is accompanied by «qualitative» (e.g., expression of embryonic isoforms, post-translational modifications) or «quantitative» (overexpression of enzymes or overproduction of metabolites) changes, drug resistance and, possibly, other nonenzymatic activities are induced in the affected cell(s). LncmRNA, long noncoding RNA; miRNA, microRNA; TME, tumor microenvironment.
Many of these stimuli lead, directly or indirectly, to the activation of transcription factors that induce the expression of genes encoding glycolytic enzymes. In this regard, the phosphoinositide 3-kinase (PI3K)-AKT-mechanistic target of rapamycin (mTOR) signaling pathway [98,103,104] as well as the transcription factors hypoxia-inducible factor (HIF)-1α and c-Myc [67,94,96,105] play a prominent, but not exclusive [106] role.
Glycolysis-induced drug resistance and EMT: A close relationship
In the previous sections, we have addressed the association of glycolysis and its individual components with the induction of resistance towards different drug classes. At this point, one is led to ask whether a predominantly glycolytic metabolism is associated with other changes in tumor cells. The answer is yes and, in general, the changes that occur are characteristic of increased tumor aggressiveness. Thus, glycolytic metabolism in general [107,108] as well as individual glycolytic enzymes or metabolites are associated with increased tumor cell proliferation and tumor growth [9,19,50,54,55,87,97,109], invasion and metastasis [19,39,54,55,72,74,97,109,110] and generation of cancer stem-like cells [4,44,59,74,111,112]. Of note, these biological effects as well as the induction of drug resistance are hallmarks of tumor cells undergoing an EMT [9,[113], [114], [115]. Moreover, tumor cell EMT is induced, by and large, by stimuli similar to those previously discussed that induce upregulation of glycolysis and glycolysis-associated drug resistance [12]. On these bases, it is not surprising to note that glycolysis is now recognized as a hallmark of tumor cell EMT [116,117].
Whether glycolysis and glycolysis-associated drug resistance can occur in the absence of other traits characteristic of tumor cell EMT is a difficult question to answer. In fact, to the best of our knowledge, no dedicated studies have been published on this issue. Moreover, tumor cell EMT is a heterogeneous process that can encompass different stages, from a fully epithelial to a fully mesenchymal phenotype [9,[113], [114], [115],[118], [119], [120]. Therefore, even the observation of glycolysis-associated drug resistance of tumor cells in the absence of other EMT markers may reflect a “partial” EMT.
Mechanisms underlying glycolysis-induced drug resistance
Given that glucose transporters, glycolytic enzymes and metabolites can induce resistance toward a large number of antitumor drugs, one wants to know which are the mechanism(s) that underlie such resistance. On the basis of the studies that have been published on this issue, the following can be identified (Fig. 3).
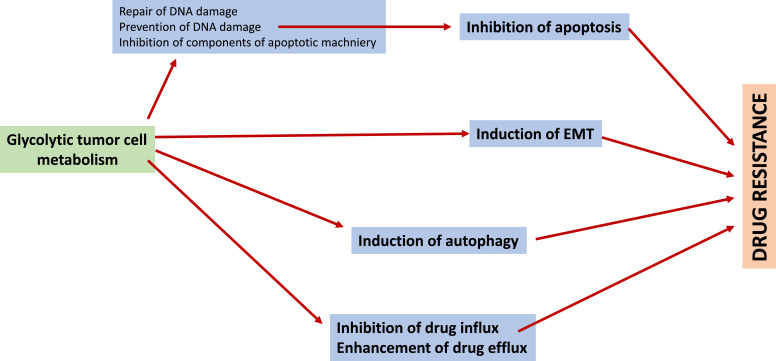
Fig. 3.
Mechanisms underlying glycolysis-induced drug resistance. Four basic mechanisms underlying glycolysis-induced drug resistance are depicted: inhibition of apoptosis, induction of EMT, induction of autophagy, inhibition of drug influx and enhancement of drug efflux. Apoptosis can be inhibited through different mechanisms: repair of DNA damage, prevention of DNA damage, inhibition of components of the apoptotic machinery. EMT, epithelial-mesenchymal transition.
First, and not surprisingly, antiapoptotic effects. In fact, apoptosis is the main mechanism for tumor cell death in response to antitumor drugs and, therefore, a molecule that induces drug resistance is expected to have antiapoptotic effects. Antiapoptotic effects have been demonstrated for glycolytic metabolism in general [49,98,112] and for individual components of it: enzymes like HK2 [16], PFKFB3 [49], PFK platelet type P (PFKP) [121], ENO1 [56,95], PKM2 [18,59,94,109,122], LDHA [123], PDK isoform 1 (PDK1) [73,124], metabolites like pyruvate [78], lactate and lactate-induced acidosis [87]. Antiapoptotic effects are achieved in several ways. The first is DNA damage repair (DDR) [125]. DDR allows cells to avoid accumulation of DNA damages to a point that may lead to activation of mechanisms promoting the demise of the cell(s) [126]. Induction of DDR has been demonstrated for PGK1 [54], PFKFB3 [48], GLUT1 [41], and lactate [83]. The molecular mechanisms whereby DDR is promoted, however, may not be the same for all of these molecules. Thus, PGK1 has been shown to promote DDR through upregulation of DDR-related proteins and methylation-related enzymes [54]. PFKFB3, on the other hand, promotes localization of DNA damage and homologous recombination proteins to nuclear foci induced by ionizing radiation [48]. GLUT1 induces DDR indirectly, through chromatin remodeling [41]. Similarly, lactate induces a transcriptionally permissive chromatin conformational state leading to overexpression of proteins involved in the repair of DNA double-strand breaks [83]. Elevated glycolysis following ionizing radiation promotes DDR by activating both non-homologous end joining and homologous recombination pathways of repair leading to reduced cytogenetic damage [38]. Elevation of ALDOA upregulates glycolysis and the PPP and this leads to increased nucleotide metabolism which protects breast cancer cells from chemotherapeutic-induced DNA damage [52]. Avoidance of the DNA damaging effects of free radicals is another way glycolysis inhibits apoptosis and promotes drug resistance [56,73]. In fact, the prevalence of glycolysis over OXPHOS entails, per se, an antiapoptotic effect. This is because, as already mentioned, OXPHOS is accompanied by substantial generation of ROS which may lead to DNA damage and, eventually, when exceeding a certain threshold, induce cell death [127]. Moreover, when glycolytic intermediates enter the PPP, NAPDH is generated which, in turn, promotes the generation of the reduced form of glutathione and thioredoxin, both of which can scavenge ROS [108]. A related, but different mechanism has been demonstrated for pyruvate. Its antiapoptotic effect is due to its ability to directly scavenge free radicals [78,128]. A ROS-scavenging activity has also been reported for PGK1 and shown to promote drug resistance [129]. Glycolysis may inhibit apoptosis also by directly impacting on individual components of the apoptotic machinery. Thus, glycolysis was found to cause overexpression of the antiapoptotic molecule myeloid cell leukemia-1 (Mcl-1) [130], while PKM2 causes overexpression of B-cell lymphoma 2 (Bcl-2) [59] and of B-cell lymphoma extra large (Bcl-xL) [131]. Eventually, HK2 can inhibit mitochondrial apoptosis by direct insertion in the outer mitochondrial membrane and inhibition of cytochrome c release upon interaction with the voltage-dependent anion channel [132].
A second mechanism underlying glycolysis-induced drug resistance is through the induction of EMT. We have already addressed the close relationship between glycolysis and glycolysis-induced drug resistance on one hand, and EMT on the other hand [116,117]. Important, in the present context, is the finding showing that glycolysis can induce EMT and, consequently, a drug-resistant phenotype [133].
Autophagy induction is still another mechanism whereby glycolysis can promote drug resistance. Autophagy is a catabolic process of self-digestion that provides nutrients to the cell in order to maintain vital cellular functions during fasting and other forms of stress, including that induced by antitumor drugs [134]. Autophagy confers tumor cell resistance toward different classes of antitumor drugs [135], [136], [137], [138], [139]. Glycolytic metabolism can induce autophagy and, consequently, autophagy-associated drug resistance, in different ways. HK2, for example, was shown to interact with mTOR and inhibit its activity in breast cancer MCF-7 cells [140]. mTOR is a negative regulator of autophagy [134] and lowering its activity by HK2 augments autophagy, thereby conferring resistance to the estrogen receptor antagonist tamoxifen. LDHA promotes autophagy by interacting with and activating Beclin-1, a key protein involved in autophagosome formation, thereby leading to resistance towards tamoxifen in breast cancer cells [63]. GAPDH elevates glycolysis and enhances mitophagy (mitochondrial autophagy) in a manner dependent on autophagy related (ATG) 5 and these 2 effects cooperate to protect cells from caspase-independent cell death [141]. PGK1 induces autophagy and chemoresistance by promoting the formation of the autophagy initiator ATG5-ATG12 conjugate [142].
Eventually, glycolysis-induced drug resistance can also be due to inhibition of drug influx or increase of drug efflux. As regards inhibition of drug influx, the inversed pH gradient that is induced on each side of the plasma membrane of tumor cells reduces the penetration of weakly basic antitumor drugs (e.g., doxorubicin or mitoxantrone) into tumor cells [143]. Acidification of the extracellular milieu may also lower the cytotoxicity of these drugs [144,145]. Drug efflux is controlled by the activity of plasma membrane transporter proteins. In particular, the ATP-binding cassette (ABC) transporter family of transmembrane proteins promotes the efflux of many structurally unrelated chemotherapeutic agents. Out of the 49 members of this protein family, the most intensively investigated are multidrug resistance protein 1 (MDR1, also known as P-glycoprotein and ABCB1), MDR-associated protein 1 (MRP1, also known as ABCC1) and breast cancer resistance protein (BCRP; also known as ABCG2) [146]. Efflux activity of ABC transporters is highly dependent on cellular ATP levels [147]. Consequently, the ATP generated in tumor cells due to glycolytic metabolism activates ABC transporters and induces drug resistance and, vice versa, glycolysis inhibition inactivates them and restores drug sensitivity [148,149]. Another way whereby glycolysis leads to enhanced ABC transporter activity is through pyruvate-induced increase of the expression of MDR1 which was paralleled by an increased efflux of doxorubicin [79] or oxaliplatin [122]. Eventually, acidification of the extracellular tumor milieu may increase the activity of efflux pumps with the consequent ejection of drugs out of the tumor cells [150].
We have previously discussed that glycolysis-associated drug resistance may be induced, either directly by one of the elements of the glycolytic metabolism or, indirectly, through an overall enhancement of glycolysis. In this section, we have discussed several cases which fit to one or the other of the 2 possibilities. Thus, for example, protection from DNA damage may be the result of a direct, nonenzymatic activity of several glycolytic enzymes [38,41,48,54], but also the consequence of an overall enhancement of glycolysis and PPP leading to increased synthesis of nucleotides that prevent chemotherapy-induced damage [52], of reduced generation of ROS because of the avoidance of OXPHOS or increased scavenging of ROS through increased generation of NADPH [108] or through a direct ROS-scavenging activity of glycolytic metabolites [78,128], or of elevated lactate levels leading to overexpression of proteins involved in DDR [83]. Vice versa, some glycolytic elements can directly induce the expression of some antiapoptotic proteins or inhibit apoptosis [131,132], while drug resistance due to inhibition of drug influx or increased drug efflux is, in most cases, a consequence of an overall elevation of glycolysis [143], [144], [145],[148], [149], [150].
When do these mechanisms come into play?
In the previous section, we have addressed the molecular mechanisms that are conducive to glycolysis-induced drug resistance. Even more importantly, however, is to know whether such drug resistance does inevitably occur whenever glycolytic metabolism is active. We suggest that this is not the case and that glycolysis-associated drug resistance occurs in tumor cells when one of the 2 following, nonmutually exclusive conditions are satisfied.
First, drug resistance may be due to “qualitative” differences between glycolytic enzymes that are normally expressed and those that are expressed upon a metabolic switch in tumor cells. Thus, in some experimental systems, some (e.g., HK2, PKM2, PFKFB3), but not other isoforms of glycolytic enzymes were able to induce drug resistance [46,49,60,151]. In other instances, drug resistance was induced upon post-translational modifications of glycolytic enzymes [46,49]. In one case, the post-translational modification (phosphorylation) resulted in higher glycolytic rates in the absence of any overexpression of enzymes [35]. Here, we may include also glycolysis-induced drug resistance resulting from an interaction between a glycolytic enzyme and another protein involved in oncogenesis. Thus, Yang et al [152] showed that a glycolytic enzyme (dimeric PKM2) directly interacted with a mutant epidermal growth factor receptor (EGFR) protein and heat shock protein 90 (HSP90), and by so doing stabilized mutant EGFR by maintaining its binding with HSP90 and co-chaperones. Mutant EGFR stabilization promoted resistance toward TKIs. On the other hand, PKM2 silencing led to reduced mutant EGFR expression and inhibition of tumor growth. Along similar lines, it was shown that Src inactivated PDH through direct phosphorylation of Tyr289 of the PDH E1α subunit thereby promoting aerobic glycolysis and therapy resistance on one hand, and inhibition of mitochondrial respiration and oxidative stress on the other hand [72].
Second, although there are exceptions (see [35]), in many instances, glycolysis-induced drug resistance occurs upon overexpression of one or more glucose transporters or glycolytic enzymes [16,24,51,97,153] leading to an overall enhancement of glycolytic metabolism in affected tumor cells. This suggests that the degree of enhancement of glycolytic metabolism in a given population of tumor cells may dictate whether glycolysis is accompanied by drug resistance or not. Thus, only above a certain threshold concentration, glycolytic enzymes, and/or metabolites would be able to activate the molecular mechanisms leading to drug resistance that have been described in the previous section.
Overall and on the basis of what we have discussed so far, we suggest the following model (Fig. 4), which may accommodate most, if not all available data on glycolysis-induced resistance. At the 2 extremes, we find situations where quantitative (i.e., overexpression above a certain threshold concentration) or qualitative factors (e.g., expression of an embryonic enzyme isoform or a posttranslational modification of a glycolytic enzyme), respectively, may induce drug resistance in their own right. In most cases, however, the 2 variables may contribute each, to a variable degree, to drug resistance [15,51,53,56,97]. In fact, the expression of a particular isoform of a glycolytic enzyme may be linked to the tumorigenic process per se and its overexpression may be the result of intracellular or extracellular stimuli [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106]. Such overexpression would then represent a sufficient condition for setting in motion the molecular mechanisms that ultimately lead to drug resistance.
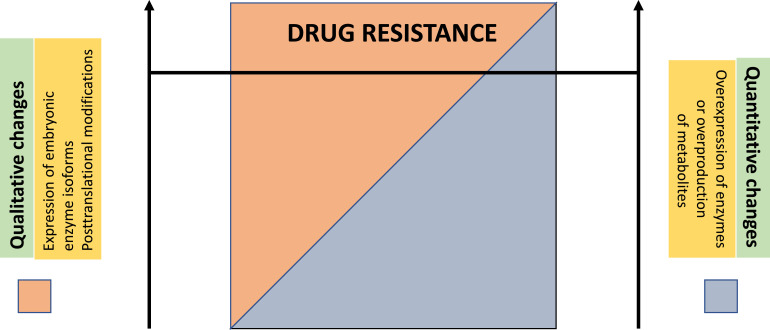
Fig. 4.
Model to explain the occurrence of glycolysis-induced drug resistance. Qualitative changes (e.g., expression of an embryonic enzyme isoform or a post-translational modification of a glycolytic enzyme) or quantitative changes (i.e., overexpression of an enzyme or overproduction of a metabolite above a certain threshold concentration) in glycolytic metabolism may induce drug resistance in their own right (extreme left or extreme right of the image) but, in most cases, the 2 variables may contribute each, to a variable degree, to drug resistance.
Are stimuli that induce glycolysis-associated drug resistance danger signals?
In previous sections, we have discussed that glycolysis and glycolysis-associated drug resistance are induced by intracellular (mutated or overexpressed proteins) or extracellular stimuli (antitumor drugs, hypoxia, mechanical cues, etc.). We have also discussed the mechanisms that are activated by glycolytic enzymes or metabolites in order to induce drug resistance. Antiapoptotic effects, induction of autophagy, induction of EMT, and inhibition of drug influx or enhancement of drug efflux were identified as the main mechanisms.
The signals that induce glycolytic metabolism in tumor cells, whether intracellular or extracellular, can be considered danger signals that induce cellular modifications allowing to avoid harmful consequences like those induced by antitumor drugs. The concept of danger signals inducing cellular responses was introduced in order to explain stimulus-induced changes observed in cells of the innate immune system [154]. Innate immune responses against pathogens are initiated upon recognition of specific components of microorganisms by pattern-recognition receptors (PRR). Recognition and binding then trigger responses aimed at eliminating the invading pathogens [155]. Moreover, an increasing number of endogenous molecules, termed damage-associated molecular patterns are now recognized being able to interact with PRRs and other, non-PRRs [156], thereby causing a sterile inflammatory response aimed at repairing and regenerating damaged tissues [156]. While damage-associated molecular patterns are involved in the recognition of molecules which, typically, are released from damaged cells, the more inclusive term danger-associated molecular pattern has been coined to include also signals which, while not necessarily being released from damaged cells, alert cells in view of harmful consequences of these signals [156]. Importantly, it has also been proposed that metabolic changes in macrophages might function as mediators of danger signals leading to the activation of inflammasomes, a class of PRR, in response to invading bacteria. These modifications have been called danger-associated metabolic modifications [157]. We propose that a similar situation may exist in tumor cells, that is, intracellular or extracellular stimuli/danger signals induce danger-associated metabolic modifications in tumor cells such as an altered glycolytic metabolism displaying qualitative (e.g., expression of embryonic enzyme isoforms or posttranslational modifications) [35,46,49,60,151,152] or quantitative changes (overexpression of enzymes or overproduction of metabolites) [16,24,51,97,153] that activate molecular mechanisms (discussed under section "Mechanisms underlying glycolysis-induced drug resistance") which eventually lead to drug resistance. While such a model rests on an apparent symmetry between tumor cells and innate immune cells, known target molecules of glycolytic enzymes or metabolites that are involved in the induction drug resistance in tumor cells cannot be classified as PRR or non-PRR, as currently defined [156]. However, it is interesting to note that some products of glycolytic metabolism, like lactate and acidosis [158,159], and ATP itself [160] have been shown to interact with a classical PRR like the NLRP3 inflammasome [160]. These observations suggest the possibility that these interactions might be involved in changes related to drug resistance.
Finally, as already mentioned in this article, drug resistance is but one of the numerous nonenzymatic activities induced by glycolysis (for a review, see [161]). The relationship of these activities with the induction of drug resistance is unclear. Nevertheless, it is reasonable to assume that the molecular mechanisms that have been discussed inducing glycolysis-associated drug resistance may be involved in at least part of the other nonenzymatic activities of glycolytic metabolism. For this reason, the model that we have just proposed leading to glycolysis-associated drug resistance may apply also to other, nonmetabolic effects associated with glycolysis.
Conclusions and perspectives—From glycolysis- to OXPHOS-associated drug resistance: Are there commonalities?
In this article, we have reviewed the basic issues of glycolysis-induced drug resistance. We have also proposed a model whereby glycolysis-associated drug resistance is induced in response to an altered glycolytic metabolism.
An important aspect that remains to be addressed in future work is the relationship between glycolysis- and mitochondrial oxidative metabolism-associated drug resistance. In fact, over the years, the view that aerobic glycolysis is the main energy source for tumor cells has been considerably mitigated. We have already mentioned in the initial section of this article that glycolytic and oxidative metabolism can prevail over the other between tumors and within tumors and they can even coexist in a symbiotic relationship between tumor cells and accessory cells or between different tumor cells [4], [5], [6], [7], [8]. Similarly, also drug resistance has been found being associated not only with glycolytic metabolism, but also with oxidative metabolism (reviewed in [108]).
Thus, for example, cisplatin-resistant lung cancer and ovarian cells have been shown to rely on increased OXPHOS for energy generation, and such increase was accompanied by decreased glycolytic activity, and higher dependence on glutamine and fatty acid oxidation for feeding the citric acid cycle in these cells [162,163]. The causal relationship between enhanced OXPHOS and cisplatin resistance was inferred from the observation that inhibition of glutaminase and fatty acid synthase allowed to recover sensitivity to cisplatin by ovarian cancer cells in which glutamine and β-oxidation fuel the citric acid cycle and OXPHOS [164,165]. A similar situation has been described for melanoma cells expressing mutant BRAF and which became resistant toward BRAF inhibitors. These cells showed increased OXPHOS and, not surprisingly, higher levels of ROS [166,167]. Inhibiting OXPHOS directly or indirectly by inhibiting glutaminase enhanced the antitumor activity of the BRAF inhibitor, again suggesting a causal role of OXPHOS in the induction of drug resistance [166,168]. Mechanistically, it has been proposed that glutamine-fueled OXPHOS represents an essential source of energy for the proliferation of drug-resistant tumor cells and, accordingly, blockade of OXPHOS with an inhibitor of complex I of the electron transport chain overcame the drug resistance [169]. Very recently, it has been shown that OXPHOS blockade through complex I inhibition leads to inhibition of the autophagic process that was induced by drug treatment and led to tumor cell survival and drug resistance [170].
For the future, it will be of obvious interest to know whether the conditions leading to OXPHOS-associated drug resistance are similar or dissimilar to those that underlie glycolysis-associated drug resistance. A quantitatively or qualitatively dysregulated mitochondrial oxidative metabolism as precondition for the induction of drug resistance would appear even more important than for glycolysis because of its almost ubiquitous functioning throughout organs and tissues under normal circumstances. Hence, if oxidative metabolism would be always accompanied by drug resistance, then most living cells should be, ab initio, drug resistant.
This knowledge is of considerable interest also as regards therapeutic implications. In fact, the coexistence of glycolysis- and oxidative metabolism-induced drug resistance suggests that it may not be sufficient to block one of the 2 in order to circumvent metabolically induced drug resistance. Arguably, one should act on both of these 2 main branches of the metabolism with the obvious possibility that such a regimen may be accompanied by an unacceptable side effect profile. For this reason, a more detailed knowledge of the stimuli, conditions, and mechanisms that underlie these 2 forms of drug resistance is required in order to tailor therapeutic interventions that are efficacious while not being burdened by unacceptable toxicity.
Author contributions
FM was the originator of this work. FM and CR contributed to the elaboration and writing of the manuscript.
Competing interests
All authors declare no conflict of interest.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–673. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 3.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 4.Shang D, Wu J, Guo L, Xu Y, Liu L, Lu J. Metformin increases sensitivity of osteosarcoma stem cells to cisplatin by inhibiting expression of PKM2. Int J Oncol. 2017;50:1848–1856. doi: 10.3892/ijo.2017.3950. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima EC, Van Houten B. Metabolic symbiosis in cancer: refocusing the Warburg lens. Mol Carcinog. 2013;52:329–337. doi: 10.1002/mc.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouzier AK, Voisin P, Goodwin R, Canioni P, Merle M. Glucose and lactate metabolism in C6 glioma cells: evidence for the preferential utilization of lactate for cell oxidative metabolism. Dev Neurosci. 1998;20:331–338. doi: 10.1159/000017328. [DOI] [PubMed] [Google Scholar]
- 8.Jia D, Lu M, Jung KH, Park JH, Yu L, Onuchic JN, Kaipparettu BA, Levine H. Elucidating cancer metabolic plasticity by coupling gene regulation with metabolic pathways. Proc Natl Acad Sci U S A. 2019;116:3909–3918. doi: 10.1073/pnas.1816391116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vidal RS, Quarti J, Rodrigues MF, Rumjanek FD, Rumjanek VM. Metabolic reprogramming during multidrug resistance in leukemias. Front Oncol. 2018;8:90. doi: 10.3389/fonc.2018.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcucci F, Stassi G, De Maria R. Epithelial–mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov. 2016;15:311–325. doi: 10.1038/nrd.2015.13. [DOI] [PubMed] [Google Scholar]
- 13.Marcucci F, Rumio C, Corti A. Tumor cell-associated immune checkpoint molecules—drivers of malignancy and stemness. Biochim Biophys Acta. 2017;1868:571–583. doi: 10.1016/j.bbcan.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Sattler UGA, Mueller-Klieser W. The anti-oxidant capacity of tumour glycolysis. Int J Radiat Biol. 2009;85:963–971. doi: 10.3109/09553000903258889. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Zhang H, Yang H, Bai M, Ning T, Deng T, Liu R, Fan Q, Zhu K, Li J. Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer. Mol Oncol. 2020;14:539–555. doi: 10.1002/1878-0261.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Chen G, Gao Y, Liang H. HOTAIR/miR-125 axis-mediated Hexokinase 2 expression promotes chemoresistance in human glioblastoma. J Cell Mol Med. 2020;24:5707–5717. doi: 10.1111/jcmm.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T, Ning K, Sun X, Zhang C, Jin LF, Hua D. Glycolysis is essential for chemoresistance induced by transient receptor potential channel C5 in colorectal cancer. BMC Cancer. 2018;18:207. doi: 10.1186/s12885-018-4123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian Y, Bi L, Yang Y, Wang D. Effect of pyruvate kinase M2-regulating aerobic glycolysis on chemotherapy resistance of estrogen receptor-positive breast cancer. Anticancer Drugs. 2018;29:616–627. doi: 10.1097/CAD.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 19.Wagner W, Kania KD, Blauz A, Ciszewski WM. The lactate receptor (HCAR1/GPR81) contributes to doxorubicin chemoresistance via ABCB1 transporter up-regulation in human cervical cancer HeLa cells. J Physiol Pharmacol. 2017;68:555–564. [PubMed] [Google Scholar]
- 20.Qian X, Xu W, Xu J, Shi Q, Li J, Weng Y, Jiang Z, Feng L, Wang X, Zhou J. Enolase 1 stimulates glycolysis to promote chemoresistance in gastric cancer. Oncotarget. 2017;8:47691–47708. doi: 10.18632/oncotarget.17868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zechner D, Albert AC, Bürtin F, Vollmar B. Metformin inhibits gemcitabine-induced apoptosis in pancreatic cancer cell lines. J Cancer. 2017;8:1744–1749. doi: 10.7150/jca.17972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hua G, Liu Y, Li X, Xu P, Luo Y. Targeting glucose metabolism in chondrosarcoma cells enhances the sensitivity to doxorubicin through the inhibition of lactate dehydrogenase-A. Oncol Rep. 2014;31:2727–2734. doi: 10.3892/or.2014.3156. [DOI] [PubMed] [Google Scholar]
- 23.Jiang JX, Gao S, Pan YZ, Yu C, Sun CY. Overexpression of microRNA-125b sensitizes human hepatocellular carcinoma cells to 5-fluorouracil through inhibition of glycolysis by targeting hexokinase II. Mol Med Rep. 2014;10:995–1002. doi: 10.3892/mmr.2014.2271. [DOI] [PubMed] [Google Scholar]
- 24.Van Wilpe S, Koornstra R, Den Brok M, De Groot JW, Blank C, De Vries J, Gerritsen W, Mehra N. Lactate dehydrogenase: a marker of diminished antitumor immunity. Oncoimmunology. 2020;9 doi: 10.1080/2162402X.2020.1731942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen E, Miéville P, Warren CM, Saghafinia S, Li L, Peng MW, Hanahan D. Metabolic symbiosis enables adaptive resistance to anti-angiogenic therapy that is dependent on mTOR signaling. Cell Rep. 2016;15:1144–1460. doi: 10.1016/j.celrep.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N, Kang Y, Wang L, Huff S, Tang R, Hui H, Agrawal K, Gonzalez GM, Wang Y, Patel SP. ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc Natl Acad Sci U S A. 2020;117:20159–20170. doi: 10.1073/pnas.1918986117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daneshmandi S, Wegiel B, Seth P. Blockade of lactate dehydrogenase-A (LDH-A) improves efficacy of anti-programmed cell death-1 (PD-1) therapy in melanoma. Cancers (Basel) 2019;11:450. doi: 10.3390/cancers11040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eriksson JA, Wanka C, Burger MC, Urban H, Hartel I, von Renesse J, Harter PN, Mittelbronn M, Steinbach JP, Rieger J. Suppression of oxidative phosphorylation confers resistance against bevacizumab in experimental glioma. J Neurochem. 2018;144:421–430. doi: 10.1111/jnc.14264. [DOI] [PubMed] [Google Scholar]
- 29.Fu J, Jiang H, Wu C, Jiang Y, Xiao L, Tian Y. Overcoming cetuximab resistance in Ewing's sarcoma by inhibiting lactate dehydrogenase-A. Mol Med Rep. 2016;14:995–1001. doi: 10.3892/mmr.2016.5290. [DOI] [PubMed] [Google Scholar]
- 30.Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, Damaghi M, Wojtkowiak JW, Mulé JJ, Ibrahim-Hashim A. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 2016;76:1381–1390. doi: 10.1158/0008-5472.CAN-15-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y, Liu H, Liu Z, Ding Y, Ledoux SP, Wilson GL, Voellmy R, Lin Y, Lin W, Nahta R. Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res. 2011;71:4585–4597. doi: 10.1158/0008-5472.CAN-11-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu T, Yang G, Hou Y, Tang X, Wu C, Wu XA, Guo L, Zhu Q, Luo H, Du YE. Cytoplasmic GPER translocation in cancer-associated fibroblasts mediates cAMP/PKA/CREB/glycolytic axis to confer tumor cells with multidrug resistance. Oncogene. 2017;36:2131–2145. doi: 10.1038/onc.2016.370. [DOI] [PubMed] [Google Scholar]
- 33.Lorito N, Bacci M, Smiriglia A, Mannelli M, Parri M, Comito G, Ippolito L, Giannoni E, Bonechi M, Benelli M. Glucose metabolic reprogramming of ER breast cancer in acquired resistance to the CDK4/6 inhibitor palbociclib. Cells. 2020;9:668. doi: 10.3390/cells9030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Dai W, Mo W, Li J, Feng J, Wu L, Liu T, Yu Q, Xu S, Wang W. By inhibiting PFKFB3, aspirin overcomes sorafenib resistance in hepatocellular carcinoma. Int J Cancer. 2017;141:2571–2584. doi: 10.1002/ijc.31022. [DOI] [PubMed] [Google Scholar]
- 35.Ruprecht B, Zaal EA, Zecha J, Wu W, Berkers CR, Kuster B, Lemeer S. Lapatinib resistance in breast cancer cells is accompanied by phosphorylation-mediated reprogramming of glycolysis. Cancer Res. 2017;77:1842–1853. doi: 10.1158/0008-5472.CAN-16-2976. [DOI] [PubMed] [Google Scholar]
- 36.Klawitter J, Kominsky DJ, Brown JL, Klawitter J, Christians U, Leibfritz D, Melo JV, Eckhardt SG, Serkova NJ. Metabolic characteristics of imatinib resistance in chronic myeloid leukaemia cells. Br J Pharmacol. 2009;158:588–600. doi: 10.1111/j.1476-5381.2009.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hulleman E, Kazemier KM, Holleman A, VanderWeele DJ, Rudin CM, Broekhuis MJ, Evans WE, Pieters R, Den Boer ML. Inhibition of glycolysis modulates prednisolone resistance in acute lymphoblastic leukemia cells. Blood. 2009;113:2014–2021. doi: 10.1182/blood-2008-05-157842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatt AN, Chauhan A, Khanna S, Rai Y, Singh S, Soni R, Kalra N, Dwarakanath BS. Transient elevation of glycolysis confers radio-resistance by facilitating DNA repair in cells. BMC Cancer. 2015;15:335. doi: 10.1186/s12885-015-1368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park GB, Jeong J-Y, Kim D. GLUT5 regulation by AKT1/3-miR-125b-5p downregulation induces migratory activity and drug resistance in TLR-modified colorectal cancer cells. Carcinogenesis. 2020;41:1329–1340. doi: 10.1093/carcin/bgaa074. [DOI] [PubMed] [Google Scholar]
- 40.Ryu MJ, Han J, Kim SJ, Lee MJ, Ju X, Lee YL, Son JH, Cui J, Jang Y, Chung W. PTEN/AKT signaling mediates chemoresistance in refractory acute myeloid leukemia through enhanced glycolysis. Oncol Rep. 2019;42:2149–2158. doi: 10.3892/or.2019.7308. [DOI] [PubMed] [Google Scholar]
- 41.Kim J, Xu S, Xiong L, Yu L, Fu X, Xu Y. SALL4 promotes glycolysis and chromatin remodeling via modulating HP1α-Glut1 pathway. Oncogene. 2017;36:6472–6479. doi: 10.1038/onc.2017.265. [DOI] [PubMed] [Google Scholar]
- 42.Wei C, Bajpai R, Sharma H, Heitmeier M, Jain AD, Matulis SM, Nooka AK, Mishra RK, Hruz PW, Schiltz GE. Development of GLUT4-selective antagonists for multiple myeloma therapy. Eur J Med Chem. 2017;139:573–586. doi: 10.1016/j.ejmech.2017.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Calvé B, Rynkowski M, Le Mercier M, Bruyère C, Lonez C, Gras T, Haibe-Kains B, Bontempi G, Decaestecker C, Ruysschaert JM. Long-term in vitro treatment of human glioblastoma cells with temozolomide increases resistance in vivo through up-regulation of GLUT transporter and aldo-keto reductase enzyme AKR1C expression. Neoplasia. 2010;12:727–739. doi: 10.1593/neo.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee HJ, Li CF, Ruan D, He J, Montal ED, Lorenz S, Girnun GD, Chan CH. Non-proteolytic ubiquitination of hexokinase 2 by HectH9 controls tumor metabolism and cancer stem cell expansion. Nat Commun. 2019;10:2625. doi: 10.1038/s41467-019-10374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi T, Ma Y, Cao L, Zhan S, Xu Y, Fu F, Liu C, Zhang G, Wang Z, Wang R. B7-H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis. 2019;10:308. doi: 10.1038/s41419-019-1549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang T, Ren C, Qiao P, Han X, Wang L, Lv S, Sun Y, Liu Z, Du Y, Yu Z. PIM2-mediated phosphorylation of hexokinase 2 is critical for tumor growth and paclitaxel resistance in breast cancer. Oncogene. 2018;37:5997–6009. doi: 10.1038/s41388-018-0386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lypova N, Telang S, Chesney J, Imbert-Fernandez Y. Increased 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 activity in response to EGFR signaling contributes to non-small cell lung cancer cell survival. J Biol Chem. 2019;294:10530–10543. doi: 10.1074/jbc.RA119.007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gustafsson NMS, Färnegårdh K, Bonagas N, Ninou AH, Groth P, Wiita E, Jönsson M, Hallberg K, Lehto J, Pennisi R. Targeting PFKFB3 radiosensitizes cancer cells and suppresses homologous recombination. Nat Commun. 2018;9:3872. doi: 10.1038/s41467-018-06287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li FL, Liu JP, Bao RX, Yan G, Feng X, Xu YP, Sun YP, Yan W, Ling ZQ, Xiong Y. Acetylation accumulates PFKFB3 in cytoplasm to promote glycolysis and protects cells from cisplatin-induced apoptosis. Nat Commun. 2018;9:508. doi: 10.1038/s41467-018-02950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi L, Pan H, Liu Z, Xie J, Han W. Roles of PFKFB3 in cancer. Signal Transduct Target Ther. 2017;2:17044. doi: 10.1038/sigtrans.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang YC, Yang YF, Chiou J, Tsai HF, Fang CY, Yang CJ, Chen CL, Hsiao M. Nonenzymatic function of Aldolase A downregulates miR-145 to promote the Oct4/DUSP4/TRAF4 axis and the acquisition of lung cancer stemness. Cell Death Dis. 2020;11:195. doi: 10.1038/s41419-020-2387-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Q, Qin T, Bi Z, Hong H, Ding L, Chen J, Wu W, Lin X, Fu W, Zheng F. Rac1 activates non-oxidative pentose phosphate pathway to induce chemoresistance of breast cancer. Nat Commun. 2020;11:1456. doi: 10.1038/s41467-020-15308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Y, Luo Y, Zhang D, Wang X, Zhang P, Li H, Ejaz S, Liang S. PGK1-mediated cancer progression and drug resistance. Am J Cancer Res. 2019;9:2280–2302. [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou JW, Tang JJ, Sun W, Wang H. PGK1 facilities cisplatin chemoresistance by triggering HSP90/ERK pathway mediated DNA repair and methylation in endometrial endometrioid adenocarcinoma. Mol Med. 2019;25:11. doi: 10.1186/s10020-019-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun L, Lu T, Tian K, Zhou D, Yuan J, Wang X, Zhu Z, Wan D, Yao Y, Zhu X. Alpha-enolase promotes gastric cancer cell proliferation and metastasis via regulating AKT signaling pathway. Eur J Pharmacol. 2019;845:8–15. doi: 10.1016/j.ejphar.2018.12.035. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Bi R, Yin H, Liu H, Li L. ENO1 silencing impairs hypoxia-induced gemcitabine chemoresistance associated with redox modulation in pancreatic cancer cells. Am J Transl Res. 2019;11:4470–4480. [PMC free article] [PubMed] [Google Scholar]
- 57.Liu CC, Wang H, Wang WD, Wang L, Liu WJ, Wang JH, Geng QR, Lu Y. ENO2 promotes cell proliferation, glycolysis, and glucocorticoid-resistance in acute lymphoblastic leukemia. Cell Physiol Biochem. 2018;46:1525–1535. doi: 10.1159/000489196. [DOI] [PubMed] [Google Scholar]
- 58.Martin SP, Fako V, Dang H, Dominguez DA, Khatib S, Ma L, Wang H, Zheng W, Wang XW. PKM2 inhibition may reverse therapeutic resistance to transarterial chemoembolization in hepatocellular carcinoma. J Exp Clin Cancer Res. 2020;39:99. doi: 10.1186/s13046-020-01605-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin Y, Zhai H, Ouyang Y, Lu Z, Chu C, He Q, Cao X. Knockdown of PKM2 enhances radiosensitivity of cervical cancer cells. Cancer Cell Int. 2019;19:129. doi: 10.1186/s12935-019-0845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng C, Xie Z, Li Y, Wang J, Qin C, Zhang Y. PTBP1 knockdown overcomes the resistance to vincristine and oxaliplatin in drug-resistant colon cancer cells through regulation of glycolysis. Biomed Pharmacother. 2018;108:194–200. doi: 10.1016/j.biopha.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Zhang F, Wu X-R. Inhibition of pyruvate kinase M2 markedly reduces chemoresistance of advanced bladder cancer to cisplatin. Sci Rep. 2017;7:45983. doi: 10.1038/srep45983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He J, Xie G, Tong J, Peng Y, Huang H, Li J, Wang N, Liang H. Overexpression of microRNA-122 re-sensitizes 5-FU-resistant colon cancer cells to 5-FU through the inhibition of PKM2 in vitro and in vivo. Cell Biochem Biophys. 2014;70:1343–1350. doi: 10.1007/s12013-014-0062-x. [DOI] [PubMed] [Google Scholar]
- 63.Das CK, Parekh A, Parida PK, Bhutia SK, Mandal M. Lactate dehydrogenase A regulates autophagy and tamoxifen resistance in breast cancer. Biochim Biophys Acta Mol Cell Res. 2019;1866:1004–1018. doi: 10.1016/j.bbamcr.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Koukourakis M, Tsolou A, Pouliliou S, Lamprou I, Papadopoulou M, Ilemosoglou M, Kostoglou G, Ananiadou D, Sivridis E, Giatromanolaki A. Blocking LDHA glycolytic pathway sensitizes glioblastoma cells to radiation and temozolomide. Biochem Biophys Res Commun. 2017;491:932–938. doi: 10.1016/j.bbrc.2017.07.138. [DOI] [PubMed] [Google Scholar]
- 65.Maiso P, Huynh D, Moschetta M, Sacco A, Aljawai Y, Mishima Y, Asara JM, Roccaro AM, Kimmelman AC, Ghobrial IM. Metabolic signature identifies novel targets for drug resistance in multiple myeloma. Cancer Res. 2015;75:2071–2082. doi: 10.1158/0008-5472.CAN-14-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X, Zhao H, Zhou X, Song L. Inhibition of lactate dehydrogenase A by microRNA-34a resensitizes colon cancer cells to 5-fluorouracil. Mol Med Rep. 2015;11:577–582. doi: 10.3892/mmr.2014.2726. [DOI] [PubMed] [Google Scholar]
- 67.Lu H, Li X, Luo Z, Liu J, Fan Z. Cetuximab reverses the Warburg effect by inhibiting HIF-1-regulated LDH-A. Mol Cancer Ther. 2013;12:2187–2199. doi: 10.1158/1535-7163.MCT-12-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–234. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 69.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 70.Zahra K, Dey T, Ashish, Mishra SP, Pandey U. Pyruvate kinase M2 and cancer: the role of PKM2 in promoting tumorigenesis. Front Oncol. 2020;10:159. doi: 10.3389/fonc.2020.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chakraborty PK, Mustafi SB, Xiong X, Dwivedi SKD, Nesin V, Saha S, Zhang M, Dhanasekaran D, Jayaraman M, Mannel R. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat Commun. 2017;8:14634. doi: 10.1038/ncomms14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin Y, Cai Q, Shenoy AK, Lim S, Zhang Y, Charles S, Tarrash M, Fu X, Kamarajugadda S, Trevino JG. Src drives the Warburg effect and therapy resistance by inactivating pyruvate dehydrogenase through tyrosine-289 phosphorylation. Oncotarget. 2016;7:25113–25124. doi: 10.18632/oncotarget.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Zhang D, Li Y, Fang F. MiR-138 suppresses the PDK1 expression to decrease the oxaliplatin resistance of colorectal cancer. Onco Targets Ther. 2020;13:3607–3618. doi: 10.2147/OTT.S242929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sradhanjali S, Reddy MM. Inhibition of pyruvate dehydrogenase kinase as a therapeutic strategy against cancer. Curr Top Med Chem. 2018;18:444–453. doi: 10.2174/1568026618666180523105756. [DOI] [PubMed] [Google Scholar]
- 75.Xuan Y, Hur H, Ham IH, Yun J, Lee JY, Shim W, Kim YB, Lee G, Han SU, Cho YK. Dichloroacetate attenuates hypoxia-induced resistance to 5-fluorouracil in gastric cancer through the regulation of glucose metabolism. Exp Cell Res. 2014;321:219–230. doi: 10.1016/j.yexcr.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 76.Shen YC, Ou DL, Hsu C, Lin KL, Chang CY, Lin CY, Liu SH, Cheng AL. Activating oxidative phosphorylation by a pyruvate dehydrogenase kinase inhibitor overcomes sorafenib resistance of hepatocellular carcinoma. Br J Cancer. 2013;108:72–81. doi: 10.1038/bjc.2012.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hur H, Xuan Y, Kim YB, Lee G, Shim W, Yun J, Ham IH, Han SU. Expression of pyruvate dehydrogenase kinase-1 in gastric cancer as a potential therapeutic target. Int J Oncol. 2013;42:44–54. doi: 10.3892/ijo.2012.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang CY, Kuo WT, Huang CY, Lee TC, Chen CT, Peng WH, Lu KS, Yang CY, Yu LC. Distinct cytoprotective roles of pyruvate and ATP by glucose metabolism on epithelial necroptosis and crypt proliferation in ischaemic gut. J Physiol. 2017;595:505–521. doi: 10.1113/JP272208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wartenberg M, Richter M, Datchev A, Günther S, Milosevic N, Bekhite MM, Figulla HR, Aran JM, Pétriz J, Sauer H. Glycolytic pyruvate regulates P-Glycoprotein expression in multicellular tumor spheroids via modulation of the intracellular redox state. J Cell Biochem. 2010;109:434–446. doi: 10.1002/jcb.22422. [DOI] [PubMed] [Google Scholar]
- 80.Morrot A, da Fonseca LM, Salustiano EJ, Gentile LB, Conde L, Filardy AA, Franklim TN, da Costa KM, Freire-de-Lima CG, Freire-de-Lima L. Metabolic symbiosis and immunomodulation: how tumor cell-derived lactate may disturb innate and adaptive immune responses. Front Oncol. 2018;8:81. doi: 10.3389/fonc.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ge X, Cao Z, Gu Y, Wang F, Li J, Han M, Xia W, Yu Z, Lyu P. PFKFB3 potentially contributes to paclitaxel resistance in breast cancer cells through TLR4 activation by stimulating lactate production. Cell Mol Biol (Noisy-le-grand) 2016;30(62):119–125. [PubMed] [Google Scholar]
- 82.Apicella M, Giannoni E, Fiore S, Ferrari KJ, Fernández-Pérez D, Isella C, Granchi C, Minutolo F, Sottile A, Comoglio PM. Increased lactate secretion by cancer cells sustains non-cell-autonomous adaptive resistance to MET and EGFR targeted therapies. Cell Metab. 2018;28:848–865. doi: 10.1016/j.cmet.2018.08.006. e6. [DOI] [PubMed] [Google Scholar]
- 83.Wagner W, Ciszewski WM, Kania KD. L- And D-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell Commun Signal. 2015;13:36. doi: 10.1186/s12964-015-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tavares-Valente D, Baltazar F, Moreira R, Queirós O. Cancer cell bioenergetics and pH regulation influence breast cancer cell resistance to paclitaxel and doxorubicin. J Bioenerg Biomembr. 2013;45:467–475. doi: 10.1007/s10863-013-9519-7. [DOI] [PubMed] [Google Scholar]
- 85.Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, Gao G, Zhang A, Xia X, Brasher H. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72:304–314. doi: 10.1158/0008-5472.CAN-11-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corbet C, Feron O. Tumour acidosis: from the passenger to the driver's seat. Nat Rev Cancer. 2017;17:577–593. doi: 10.1038/nrc.2017.77. [DOI] [PubMed] [Google Scholar]
- 87.Faes S, Duval AP, Planche A, Uldry E, Santoro T, Pythoud C, Stehle JC, Horlbeck J, Letovanec I, Riggi N. Acidic tumor microenvironment abrogates the efficacy of mTORC1 inhibitors. Mol Cancer. 2016;15:78. doi: 10.1186/s12943-016-0562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 89.Martinez-Outschoorn UE, Goldberg A, Lin Z, Ko YH, Flomenberg N, Wang C, Pavlides S, Pestell RG, Howell A, Sotgia F. Anti-estrogen resistance in breast cancer is induced by the tumor microenvironment and can be overcome by inhibiting mitochondrial function in epithelial cancer cells. Cancer Biol Ther. 2011;12:924–938. doi: 10.4161/cbt.12.10.17780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 91.Xi Y, Yuan P, Li T, Zhang M, Liu MF, Li B. hENT1 reverses chemoresistance by regulating glycolysis in pancreatic cancer. Cancer Lett. 2020;479:112–122. doi: 10.1016/j.canlet.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 92.Cardoso HJ, Vaz CV, Carvalho TMA, Figueira MI, Socorro S. Tyrosine kinase inhibitor imatinib modulates the viability and apoptosis of castrate-resistant prostate cancer cells dependently on the glycolytic environment. Life Sci. 2019;218:274–283. doi: 10.1016/j.lfs.2018.12.055. [DOI] [PubMed] [Google Scholar]
- 93.Park JS, Burckhardt CJ, Lazcano R, Solis LM, Isogai T, Li L, Chen CS, Gao B, Minna JD, Bachoo R. Mechanical regulation of glycolysis via cytoskeleton architecture. Nature. 2020;578:621–626. doi: 10.1038/s41586-020-1998-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feng J, Dai W, Mao Y, Wu L, Li J, Chen K, Yu Q, Kong R, Li S, Zhang J. Simvastatin re-sensitizes hepatocellular carcinoma cells to sorafenib by inhibiting HIF-1α/PPAR-γ/PKM2-mediated glycolysis. J Exp Clin Cancer Res. 2020;39:24. doi: 10.1186/s13046-020-1528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen R, Li D, Zheng M, Chen B, Wei T, Wang Y, Li M, Huang W, Tong Q, Wang Q. FGFRL1 affects chemoresistance of small-cell lung cancer by modulating the PI3K/Akt pathway via ENO1. J Cell Mol Med. 2020;24(3):2123–2134. doi: 10.1111/jcmm.14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma L, Liu W, Xu A, Ji Q, Ma Y, Tai Y, Wang Y, Shen C, Liu Y, Wang T. Activator of thyroid and retinoid receptor increases sorafenib resistance in hepatocellular carcinoma by facilitating the Warburg effect. Cancer Sci. 2020;111(6):2028–2040. doi: 10.1111/cas.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shao M, Zhang J, Zhang J, Shi H, Zhang Y, Ji R, Mao F, Qian H, Xu W, Zhang X. SALL4 promotes gastric cancer progression via hexokinase II mediated glycolysis. Cancer Cell Int. 2020;20:188. doi: 10.1186/s12935-020-01275-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zong S, Dai W, Fang W, Guo X, Wang K. SIK2 promotes cisplatin resistance induced by aerobic glycolysis in breast cancer cells through PI3K/AKT/mTOR signaling pathway. Biosci Rep. 2020 doi: 10.1042/BSR20201302. [DOI] [PubMed] [Google Scholar]
- 99.Ma D, Huang Y, Song S. Inhibiting the HPV16 oncogene-mediated glycolysis sensitizes human cervical carcinoma cells to 5-fluorouracil. Onco Targets Ther. 2019;12:6711–6720. doi: 10.2147/OTT.S205334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng M, Xiong G, Cao Z, Yang G, Zheng S, Qiu J, You L, Zheng L, Zhang T, Zhao Y. LAT2 regulates glutamine-dependent mTOR activation to promote glycolysis and chemoresistance in pancreatic cancer. J Exp Clin Cancer Res. 2018;37:274. doi: 10.1186/s13046-018-0947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu M, Chen S, Yang W, Cheng X, Ye Y, Mao J, Wu X, Huang L, Ji J. FGFR4 links glucose metabolism and chemotherapy resistance in breast cancer. Cell Physiol Biochem. 2018;47:151–160. doi: 10.1159/000489759. [DOI] [PubMed] [Google Scholar]
- 102.Zhang J, Gao Q, Zhou Y, Dier U, Hempel N, Hochwald SN. Focal adhesion kinase-promoted tumor glucose metabolism is associated with a shift of mitochondrial respiration to glycolysis. Oncogene. 2016;35:1926–1942. doi: 10.1038/onc.2015.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu X, Gao X, Mao X, Shi Z, Zhu B, Xie L, Di S, Jin L. Knockdown of FOXO6 inhibits glycolysis and reduces cell resistance to paclitaxel in HCC cells via PI3K/Akt signaling pathway. Onco Targets Ther. 2020;13:1545–1556. doi: 10.2147/OTT.S233031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang X, Chen J, Ai Z, Zhang Z, Lin L, Wei H. Targeting glycometabolic reprogramming to restore the sensitivity of leukemia drug-resistant K562/ADM cells to adriamycin. Life Sci. 2018;215:1–10. doi: 10.1016/j.lfs.2018.10.050. [DOI] [PubMed] [Google Scholar]
- 105.Singh L, Aldosary S, Saeedan AS, Ansari MN, Kaithwas G. Prolyl hydroxylase 2: a promising target to inhibit hypoxia-induced cellular metabolism in cancer cells. Drug Discov Today. 2018;23:1873–1882. doi: 10.1016/j.drudis.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y, Wu S, Huang C, Li Y, Zhao H, Kasim V. Yin Yang 1 promotes the Warburg effect and tumorigenesis via glucose transporter GLUT3. Cancer Sci. 2018;109:2423–2434. doi: 10.1111/cas.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Icard P, Shulman S, Farhat D, Steyaert JM, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat. 2018;38:1–11. doi: 10.1016/j.drup.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 108.Zaal EA, Berkers CR. The influence of metabolism on drug response in cancer. Front Oncol. 2018;8:500. doi: 10.3389/fonc.2018.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yan XL, Zhang XB, Ao R, Guan L. Effects of shRNA-mediated silencing of PKM2 gene on aerobic glycolysis, cell migration, cell invasion, and apoptosis in colorectal cancer cells. J Cell Biochem. 2017;118:4792–4803. doi: 10.1002/jcb.26148. [DOI] [PubMed] [Google Scholar]
- 110.Wang G, Wang JJ, Yin PH, Xu K, Wang YZ, Shi F, Gao J, Fu XL. New strategies for targeting glucose metabolism-mediated acidosis for colorectal cancer therapy. J Cell Physiol. 2018;234:348–368. doi: 10.1002/jcp.26917. [DOI] [PubMed] [Google Scholar]
- 111.Hou GX, Liu PP, Zhang S, Yang M, Liao J, Yang J, Hu Y, Jiang WQ, Wen S, Huang P. Elimination of stem-like cancer cell side-population by auranofin through modulation of ROS and glycolysis. Cell Death Dis. 2018;9:89. doi: 10.1038/s41419-017-0159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dai J, Ji Y, Wang W, Kim D, Fai LY, Wang L, Luo J, Zhang Z. Loss of fructose-1,6-bisphosphatase induces glycolysis and promotes apoptosis resistance of cancer stem-like cells: an important role in hexavalent chromium-induced carcinogenesis. Toxicol Appl Pharmacol. 2017;331:164–173. doi: 10.1016/j.taap.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marcucci F, Bellone M, Caserta CA, Corti A. Pushing tumor cells towards a malignant phenotype. Stimuli from the microenvironment, intercellular communications and alternative roads. Int J Cancer. 2014;135:1265–1276. doi: 10.1002/ijc.28572. [DOI] [PubMed] [Google Scholar]
- 114.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 115.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 116.Marcucci F, Romeo E, Caserta CA, Rumio C, Lefoulon F. Context-dependent pharmacological effects of metformin on the immune system. Trends Pharmacol Sci. 2020;41:162–171. doi: 10.1016/j.tips.2020.01.003. doi.org/ [DOI] [PubMed] [Google Scholar]
- 117.Qin W, Li C, Zheng W, Guo Q, Zhang Y, Kang M, Zhang B, Yang B, Li B, Yang H. Inhibition of autophagy promotes metastasis and glycolysis by inducing ROS in gastric cancer cells. Oncotarget. 2015;6:39839–39854. doi: 10.18632/oncotarget.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kröger C, Afeyan A, Mraz J, Eaton EN, Reinhardt F, Khodor YL, Thiru P, Bierie B, Ye X, Burge CB. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc Natl Acad Sci USA. 2019;116:7353–7362. doi: 10.1073/pnas.1812876116. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D, Moers V, Lemaire S. Identification of the tumour transition states occurring during EMT. Nature. 2018;556:463–468. doi: 10.1038/s41586-018-0040-3. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 120.Tan TZ, Miow QH, Miki Y, Noda T, Mori S, Huang RY, Thiery JP. Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol Med. 2014;6:1279–1293. doi: 10.15252/emmm.201404208. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen L, Hu N, Wang C, Zhao H. HOTAIRM1 knockdown enhances cytarabine-induced cytotoxicity by suppression of glycolysis through the Wnt/β-catenin/PFKP pathway in acute myeloid leukemia cells. Arch Biochem Biophys. 2020;680 doi: 10.1016/j.abb.2019.108244. [DOI] [PubMed] [Google Scholar]
- 122.Lu WQ, Hu YY, Lin XP, Fan W. Knockdown of PKM2 and GLS1 expression can significantly reverse oxaliplatin-resistance in colorectal cancer cells. Oncotarget. 2017;8:44171–44185. doi: 10.18632/oncotarget.17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li L, Liu H, Du L, Xi P, Wang Q, Li Y, Liu D. miR-449a suppresses LDHA-mediated glycolysis to enhance the sensitivity of non-small cell lung cancer cells to ionizing radiation. Oncol Res. 2018;26:547–556. doi: 10.3727/096504017X-15016337254605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liang Y, Zhu D, Zhu L, Hou Y, Hou L, Huang X, Li L, Wang Y, Li L, Zou H. Dichloroacetate overcomes oxaliplatin chemoresistance in colorectal cancer through the miR-543/PTEN/Akt/mTOR pathway. J Cancer. 2019;10:6037–6047. doi: 10.7150/jca.34650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Efimova EV, Takahashi S, Shamsi NA, Wu D, Labay E, Ulanovskaya OA, Weichselbaum RR, Kozmin SA, Kron SJ. Linking cancer metabolism to DNA repair and accelerated senescence. Mol Cancer Res. 2016;14:173–184. doi: 10.1158/1541-7786.MCR-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Checa J, Aran JM. Reactive oxygen species: drivers of physiological and pathological processes. J Inflamm Res. 2020;13:1057–1073. doi: 10.2147/JIR.S275595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16:20–33. doi: 10.1038/nrc.2015.2. [DOI] [PubMed] [Google Scholar]
- 128.Frenzel J, Richter J, Eschrich K. Pyruvate protects glucose-deprived Müller cells from nitric oxide-induced oxidative stress by radical scavenging. Glia. 2005;52:276–288. doi: 10.1002/glia.20244. [DOI] [PubMed] [Google Scholar]
- 129.Sun R, Meng X, Pu Y, Sun F, Man Z, Zhang J, Yin L, Pu Y. Overexpression of HIF-1α could partially protect K562 cells from 1,4-benzoquinone induced toxicity by inhibiting ROS, apoptosis and enhancing glycolysis. Toxicol In Vitro. 2019;55:18–23. doi: 10.1016/j.tiv.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 130.Meynet O, Bénéteau M, Jacquin MA, Pradelli LA, Cornille A, Carles M, Ricci JE. Glycolysis inhibition targets Mcl-1 to restore sensitivity of lymphoma cells to ABT-737-induced apoptosis. Leukemia. 2012;26:1145–1147. doi: 10.1038/leu.2011.327. [DOI] [PubMed] [Google Scholar]
- 131.Kwon OH, Kang TW, Kim JH, Kim M, Noh SM, Song KS, Yoo HS, Kim WH, Xie Z, Pocalyko D. Pyruvate kinase M2 promotes the growth of gastric cancer cells via regulation of Bcl-xL expression at transcriptional level. Biochem Biophys Res Commun. 2012;423:38–44. doi: 10.1016/j.bbrc.2012.05.063. [DOI] [PubMed] [Google Scholar]
- 132.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 133.Lee SY, Ju MK, Jeon HM, Lee YJ, Kim CH, Park HG, Han SI, Kang HS. Oncogenic metabolism acts as a prerequisite step for induction of cancer metastasis and cancer stem cell phenotype. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/1027453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marcucci F, Ghezzi P, Rumio C. The role of autophagy in the cross-talk between epithelial-mesenchymal transitioned tumor cells and cancer stem-like cells. Mol Cancer. 2017;16:3. doi: 10.1186/s12943-016-0573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liao YX, Yu HY, Lv JY, Cai YR, Liu F, He ZM, He SS. Targeting autophagy is a promising therapeutic strategy to overcome chemoresistance and reduce metastasis in osteosarcoma. Int J Oncol. 2019;55:1213–1222. doi: 10.3892/ijo.2019.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, Han W, Lou F, Yang J, Zhang Q. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 2008;68:1485–1494. doi: 10.1158/0008-5472. [DOI] [PubMed] [Google Scholar]
- 138.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Autophagy facilitates the development of breast cancer resistance to the anti-HER2 monoclonal antibody trasbuzumab. PloS One. 2009;4:e6251. doi: 10.1371/journal.pone.0006251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fitzwalter BE, Towers CG, Sullivan KD, Andrysik Z, Hoh M, Ludwig M, O'Prey J, Ryan KM, Espinosa JM, Morgan MJ. Autophagy inhibition mediates apoptosis sensitization in cancer therapy by relieving FOXO3a turnover. Dev Cell. 2018;44:555–565. doi: 10.1016/j.devcel.2018.02.014. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu X, Miao W, Huang M, Li L, Dai X, Wang Y. Elevated hexokinase II expression confers acquired resistance to 4-hydroxytamoxifen in breast cancer cells. Mol Cell Proteomics. 2019;18:2273–2284. doi: 10.1074/mcp.RA119.001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Colell A, Ricci JE, Tait S, Milasta S, Maurer U, Bouchier-Hayes L, Fitzgerald P, Guio-Carrion A, Waterhouse NJ, Li CW. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 142.Cai Q, Wang S, Jin L, Weng M, Zhou D, Wang J, Tang Z, Quan Z. Long non-coding RNA GBCDRlnc1 induces chemoresistance of gallbladder cancer cells by activating autophagy. Mol Cancer. 2019;18:82. doi: 10.1186/s12943-019-1016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 144.Vukovic V, Tannock IF. Influence of low pH on cytotoxicity of paclitaxel, mitoxantrone and topotecan. Br J Cancer. 1997;75:1167–1172. doi: 10.1038/bjc.1997.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Greijer AE, de Jong MC, Scheffer GL, Shvarts A, van Diest PJ, van der Wall E. Hypoxia-induced acidification causes mitoxantrone resistance not mediated by drug transporters in human breast cancer cells. Cell Oncol. 2005;27:43–49. doi: 10.1155/2005/236045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 147.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 148.Nakano A, Tsuji D, Miki H, Cui Q, El Sayed SM, Ikegame A, Oda A, Amou H, Nakamura S, Harada T. Glycolysis inhibition inactivates ABC transporters to restore drug sensitivity in malignant cells. PLoS One. 2011;6:e27222. doi: 10.1371/journal.pone.0027222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fanciulli M, Bruno T, Giovannelli A, Gentile FP, Di Padova M, Rubiu O, Floridi A. Energy metabolism of human LoVo colon carcinoma cells: correlation to drug resistance and influence of lonidamine. Clin Cancer Res. 2000;6:1590–1597. [PubMed] [Google Scholar]
- 150.Breedveld P, Pluim D, Cipriani G, Dahlhaus F, van Eijndhoven MA, de Wolf CJ, Kuil A, Beijnen JH, Scheffer GL, Jansen G. The effect of low pH on breast cancer resistance protein (ABCG2)-mediated transport of methotrexate, 7-hydroxymethotrexate, methotrexate diglutamate, folic acid, mitoxantrone, topotecan, and resveratrol in in vitro drug transport models. Mol Pharmacol. 2007;71:240–249. doi: 10.1124/mol.106.028167. [DOI] [PubMed] [Google Scholar]
- 151.Li WC, Huang CH, Hsieh YT, Chen TY, Cheng LH, Chen CY, Liu CJ, Chen HM, Huang CL, Lo JF. Regulatory role of hexokinase 2 in modulating head and neck tumorigenesis. Front Oncol. 2020;10:176. doi: 10.3389/fonc.2020.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang YC, Cheng TY, Huang SM, Su CY, Yang PW, Lee JM, Chen CK, Hsiao M, Hua KT, Kuo ML. Cytosolic PKM2 stabilizes mutant EGFR protein expression through regulating HSP90-EGFR association. Oncogene. 2016;35:3387–3398. doi: 10.1038/onc.2015.397. [DOI] [PubMed] [Google Scholar]
- 153.Song K, Li M, Xu X, Xuan LI, Huang G, Liu Q. Resistance to chemotherapy is associated with altered glucose metabolism in acute myeloid leukemia. Oncol Lett. 2016;12:334–342. doi: 10.3892/ol.2016.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 155.Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol. 2016;16:35–50. doi: 10.1038/nri.2015.8. [DOI] [PubMed] [Google Scholar]
- 156.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 157.Dramé M, Buchrieser C, Escoll P. Danger-associated metabolic modifications during bacterial infection of macrophages. Int Immunol. 2020;32:475–483. doi: 10.1093/intimm/dxaa035. [DOI] [PubMed] [Google Scholar]
- 158.Rajamäki K, Nordström T, Nurmi K, Åkerman KE, Kovanen PT, Öörni K, Eklund KK. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J Biol Chem. 2013;288:13410–13419. doi: 10.1074/jbc.M112.426254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng L, Sun X, Yang M, Billiar TR, Wang H. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun. 2016;7:13280. doi: 10.1038/ncomms13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 161.Yu X, Li S. Non-metabolic functions of glycolytic enzymes in tumorigenesis. Oncogene. 2017;36:2629–2636. doi: 10.1038/onc.2016.410. [DOI] [PubMed] [Google Scholar]
- 162.Wangpaichitr M, Wu C, Li YY, Nguyen DJM, Kandemir H, Shah S, Chen S, Feun LG, Prince JS, Kuo MT. Exploiting ROS and metabolic differences to kill cisplatin resistant lung cancer. Oncotarget. 2017;8:49275–49292. doi: 10.18632/oncotarget.17568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sullivan EJ, Kurtoglu M, Brenneman R, Liu H, Lampidis TJ. Targeting cisplatin-resistant human tumor cells with metabolic inhibitors. Cancer Chemother Pharmacol. 2014;73:417–427. doi: 10.1007/s00280-013-2366-8. [DOI] [PubMed] [Google Scholar]
- 164.Papaevangelou E, Almeida GS, Box C, deSouza NM, Chung YL. The effect of FASN inhibition on the growth and metabolism of a cisplatin-resistant ovarian carcinoma model. Int J Cancer. 2018;143:992–1002. doi: 10.1002/ijc.31392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Masamha CP, LaFontaine P. Molecular targeting of glutaminase sensitizes ovarian cancer cells to chemotherapy. J Cell Biochem. 2018;119:6136–6145. doi: 10.1002/jcb.26814. [DOI] [PubMed] [Google Scholar]
- 166.Baenke F, Chaneton B, Smith M, Van Den Broek N, Hogan K, Tang H, Viros A, Martin M, Galbraith L, Girotti MR. Resistance to BRAF inhibitors induces glutamine dependency in melanoma cells. Mol Oncol. 2016;10:73–84. doi: 10.1016/j.molonc.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cesi G, Walbrecq G, Zimmer A, Kreis S, Haan C. ROS production induced by BRAF inhibitor treatment rewires metabolic processes affecting cell growth of melanoma cells. Mol Cancer. 2017;16:102. doi: 10.1186/s12943-017-0667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Corazao-Rozas P, Guerreschi P, Jendoubi M, André F, Jonneaux A, Scalbert C, Garçon G, Malet-Martino M, Balayssac S, Rocchi S. Mitochondrial oxidative stress is the Achille's heel of melanoma cells resistant to Braf-mutant inhibitor. Oncotarget. 2013;4:1986–1998. doi: 10.18632/oncotarget.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang L, Yao Y, Zhang S, Liu Y, Guo H, Ahmed M, Bell T, Zhang H, Han G, Lorence E. Metabolic reprogramming toward oxidative phosphorylation identifies a therapeutic target for mantle cell lymphoma. Sci Transl Med. 2019;11:eaau1167. doi: 10.1126/scitranslmed.aau1167. [DOI] [PubMed] [Google Scholar]
- 170.Lee JS, Lee H, Jang H, Woo SM, Park JB, Lee SH, Kang JH, Kim HY, Song J, Kim SY. Targeting oxidative phosphorylation reverses drug resistance in cancer cells by blocking autophagy recycling. Cells. 2020;99:2013. doi: 10.3390/cells9092013. [DOI] [PMC free article] [PubMed] [Google Scholar]