Abstract
Background:
Seasonal influenza imposes a significant health and economic burden in South Africa, particularly in populations vulnerable to severe consequences of influenza. This study assesses the cost-effectiveness of South Africa’s seasonal influenza vaccination strategy, which involves vaccinating vulnerable populations with trivalent inactivated influenza vaccine (TIV) during routine facility visits. Vulnerable populations included in our analysis are persons aged ≥ 65 years; pregnant women; persons living with HIV/AIDS (PLWHA), persons of any age with underlying medical conditions (UMC) and children aged 6–59 months.
Method:
We employed the World Health Organisation’s (WHO) Cost Effectiveness Tool for Seasonal Influenza Vaccination (CETSIV), a decision tree model, to evaluate the 2018 seasonal influenza vaccination campaign from a public healthcare provider and societal perspective. CETSIV was populated with existing country-specific demographic, epidemiologic and coverage data to estimate incremental cost-effectiveness ratios (ICERs) by comparing costs and benefits of the influenza vaccination programme to no vaccination.
Results:
The highest number of clinical events (influenza cases, outpatient visits, hospitalisation and deaths) were averted in PLWHA and persons with other UMCs. Using a cost-effectiveness threshold of US$ 3 400 per quality-adjusted life year (QALY), our findings suggest that the vaccination programme is cost-effective for all vulnerable populations except for children aged 6–59 months. ICERs ranged from ~US$ 1 750 /QALY in PLWHA to ~US$ 7 500/QALY in children. In probabilistic sensitivity analyses, the vaccination programme was cost-effective in pregnant women, PLWHA, persons with UMCs and persons aged ≥65 years in >80% of simulations. These findings were robust to changes in many model inputs but were most sensitive to uncertainty in estimates of influenza-associated illness burden.
Conclusion:
South Africa’s seasonal influenza vaccination strategy of opportunistically targeting vulnerable populations during routine visits is cost-effective. A budget impact analysis will be useful for supporting future expansions of the programme.
Keywords: Cost-utility analysis, Seasonal influenza vaccine, South Africa, Cost Effectiveness Tool for Seasonal, Influenza Vaccination
1. Introduction
Globally, an estimated 290 000 to 650 000 deaths are associated with respiratory diseases from seasonal influenza annually, with substantial morbidity and mortality occurring in vulnerable risk groups particularly in low and middle-income countries (LMICs) [1–3]. In South Africa, influenza accounts for over 11 000 deaths and 56 000 hospitalisations annually [4–7], imposing a high economic burden on both the health system and households [8]. The health and economic burden of seasonal influenza is further exacerbated by the high prevalence of comorbidities in South Africa including HIV and tuberculosis [9]. Influenza vaccination is an effective strategy for reducing the burden of influenza-associated illnesses, especially among individuals at risk of experiencing more severe consequences of the disease [10–14].
Following the influenza A(H1N1)pdm09 pandemic in 2009, the South African National Department of Health (NDOH) introduced the first national influenza vaccination campaign in 2010 using trivalent inactivated influenza vaccines (TIV) [15]. The campaign currently targets high-risk sub-populations including persons aged ≥ 65 years, pregnant women, persons living with HIV/AIDS (PLWHA), and persons of any age (>6 months1) with underlying medical conditions (UMC) [2,15]. However, since the introduction of the seasonal influenza vaccination programme in South Africa, coverage of high-risk population remains low. For example in 2018, only 5% (approximately 1 million doses) of the total number of doses required to cover the prioritized high-risk groups were available in the public health sector [16]. This large vaccination gap potentially limits the realisation of the full benefits of the vaccination programme in high-risk populations.
Studies from high-income settings suggest that seasonal influenza vaccination is likely to be cost-effective [17,18], particularly in high-risk groups – pregnant women [19–21], the elderly [22–24], as well as individuals with UMCs [25–30]. However, there remains a dearth of evidence on the cost-effectiveness of influenza vaccination in LMICs. Given differences in the disease profile, unit costs, and health system delivery platforms, cost-effectiveness results from high-income countries are not always transferable to LMIC settings [31]. Furthermore, differences in co-morbidities may affect vaccine efficacy and consequently, the cost-effectiveness of seasonal influenza vaccine in different contexts. Country-specific estimates are useful for informing resource allocation decisions. Two studies conducted in South Africa suggest that seasonal vaccination may be cost-effective in some risk groups [32,33]. However, these studies are limited in scope, focusing on a limited number of risk groups. There remains a dearth of evidence on the cost-effectiveness of the seasonal influenza vaccination programme across the broad range of risk groups included in South Africa’s influenza vaccination strategy. Given increasing budget constraints within the public health system, the NDOH faces difficult choices on which risk groups to continue prioritising for vaccination. This study aimed to assess the cost-effectiveness of the influenza vaccination programme in South Africa by comparing the cost and benefits of vaccinating each risk group to a no vaccination scenario. Although healthy children aged 6–59 months are no longer considered for vaccination under the current South African NDOH vaccination strategy, we analyse this subgroup, in line with WHO recommendations [34].We conducted a cost-effectiveness analysis from a public healthcare provider perspective and a societal perspective and expressed our results as incremental cost per quality-adjusted life year (QALY). This study could be useful for informing prioritisation of risk groups for vaccinations, thus ensuring optimal allocation of scarce resources.
2. Materials and Method
In this study, we used the WHO Cost-Effectiveness Tool for Seasonal Influenza Vaccination (CETSIV), a Microsoft Excel-based tool to assess the cost-effectiveness of seasonal influenza vaccine in different risk groups in South Africa. CETSIV is a new tool recently developed by researchers at the University of Groningen with support from the WHO. The tool was developed to allow the assessment of the cost-effectiveness of influenza vaccination programmes in different contexts using a decision tree model [35]. This study is the first to apply the CETSIV. The tool is flexible and allows the input of a series of context-specific data available from existing sources. Through inbuilt formulae, incremental costs and incremental effects of the vaccination programme compared to a no vaccination scenario can be estimated for a range of sub-populations. Input parameters needed include the size of the eligible population, vaccination coverage, burden of seasonal influenza-associated illness (number of influenza cases, number of influenza cases requiring outpatient visits, number of hospitalisations and number of influenza-associated deaths), health state utilities, costs of influenza-associated illness, and cost of the vaccination programme. These are described in more details below. Other inbuilt general inputs which can be overwritten by the user includes discount rates for costs and health effects, baseline health state utilities and mortality rates, as well as baseline year of analysis and currency exchange rates.
We adapted the CETSIV to align with the local context by re-specifying the vulnerable groups defined in the tool to the South African context, as well as by modifying the structure of the decision tree to reflect healthcare seeking behaviours in South Africa. The general structure of the model adopted in our analysis is displayed in Supplemental Fig. 1.
For each risk group, we modelled the 2018 seasonal vaccination campaign, which ran from approximately March to July 2018. Vaccination coverage for each risk group was estimated using country-level demographic data on the size of the population eligible for vaccination in each risk group and the number of doses administered in 2018. Given that children aged 6–59 months were not targeted in the 2018 programme, doses administered during the 2017 campaign were used to estimate vaccine coverage for this subgroup [36]. For pregnant women, we accounted for the benefits of the vaccine to unborn infants through maternal vaccination during the influenza season [32].
Using CETSIV, we estimated incremental cost per QALY (incremental cost-effectiveness ratios- ICERs) for each risk group. Costs were estimated from a public healthcare provider perspective (costs borne by the public health system) and societal perspective (costs borne by the public health systems and by patients and their caregivers). Given that influenza disease is an annual event, we estimated costs and influenza-associated health outcomes occurring within one season (i.e. one year). However, for influenza-associated death, we estimated life time age-specific QALY loss and productivity loss using discounted life expectancies obtained from WHO life tables [37]- details of the estimation of these parameter inputs are provided below. A 5% discount rate was applied to all future life years, in line with best practice guidelines for pharmacoeconomic analysis in South Africa [38].
2.1. Model inputs
The CETSIV permits inputs on burden of disease, seasonal influenza vaccine efficacy, QALY losses associated with seasonal influenza-related health states, vaccine programme costs, healthcare costs associated with influenza-associated illness, direct non-medical, and indirect costs (productivity loss). We collated model inputs from various sources (Table 1):
Table 1:
Model Input Parameters.
| Model Inputs | Pregnant women | New-borns | PLWHA | Persons with other UMC | Persons aged ≥ 65 years | Children aged 6–59 months | Source |
|---|---|---|---|---|---|---|---|
| Population size | 323 889± | - | 6 429 938 | 8 995 290 | 3 014 877 | 5 203 824 | [39,66] |
| Vaccine coverage | 48.90%± | - | 5.51% | 3.14% | 3.11% | 2.4% | [16,32,36,39] |
| Burden of disease (per 100,000 population) | |||||||
| Non-medically attended illness | 11 378 (8 306–14 450) | 18 280 (12 430–24 129) | 15 470 (11 602–19 337) | 15 226 (11 267–19 185) | 2 488 (1 018–4042) | 18,978 (13 664–24292) | [39] |
| Influenza-associated Out-patient visits | 4 676 (3 414–5939) | 8 585 (5 838–11332) | 5 290 (3 967–6612) | 5 290 (3 914–6665) | 653 (270–1 036) | 5 279 (3 801–6757) | [39] |
| Influenza-associated Hospitalisations | 425 (310–540) | 539 (366–711) | 296 (222–370) | 112 (83–142) | 329 (144–570) | 234 (168–299) | [39] |
| Influenza-associated Deaths | 16 (12–21) | 61 (41–80) | 81 (60–101) | 28 (21–36) | 138 (57–248) | 17 (12–21) | [39] |
| Vaccine-associated mild adverse events | 12 000 (6 000–18 000) | 12 000 (6 000–18 000) | 12 000 (6 000–18 000) | 12 000 (6 000–18 000) | 12 000 (6 000–18 000) | 12 000 (6 000–18 000) | [68] |
| Vaccine-associated severe adverse events | 0.15 (0.075–0.225) | 0.15 (0.075–0.225) | 0.15 (0.075–0.225) | 0.15 (0.075–0.225) | 0.15 (0.075–0.225) | 0.15 (0.075–0.225) | [68] |
| Vaccine efficacy | 33%* (9%−47%) | 17%* (4%−24%) | 44% (39%−47%) | 58% (34%−73%) | 58% (34%−73%) | 36%** (27%−44%) | [10,12,14,57,59,60] |
| Direct medical costs (2018 US$) | |||||||
| Vaccine price | 3.04 (1.26–5.01) | 0.00 (0.00–0.00) | 3.04 (1.26–5.01) | 3.04 (1.26–5.01) | 3.04 (1.26–5.01) | 6.07 (2.53–10.02) | [65] |
| Vaccine programme delivery costs | 5.42 (2.71–8.13) | 0.00 (0.00–0.00) | 5.42 (2.71–8.13) | 5.42 (2.71–8.13) | 5.42 (2.71–8.13) | 8.11 (4.06–12.17) | [65] |
| Out-patient visits | 24 (10–38) | 25 (13–36) | 25 (15–35) | 25 (15–35) | 25 (15–34) | 25 (16–33) | [66] |
| Hospitalisations | 945 (305–1855) | 649 (258–1192) | 888(406–1543) | 835 (401–1420) | 865 (402–1274) | 604 (294–1019) | [66] |
| Mild adverse events † | 1.16 (0.58–1.74) | 1.16 (0.58–1.74) | 1.16 (0.58–1.74) | 1.16 (0.58–1.74) | 1.16 (0.58–1.74) | 1.16 (0.58–1.74) | [70] |
| Severe adverse events †† | 4 981 (1 974–12688) | 4 981 (1 974–12688) | 4 981 (1 974–12688) | 4 981 (1 974–12688) | 4 981 (1 974–12688) | 4 981 (1 974–12688) | [69,70] |
| Direct non-medical costs (2018 US$) | |||||||
| Non-medically attended illness | 5 (4–6) | 5 (4–6) | 5 (4–5) | 5 (4–6) | 5 (4–6) | 5 (4–5) | [66] |
| Out-patient visits | 2 (1–2) | 2 (1–2) | 2 (1–3) | 2 (1–3) | 2 (1–2) | 3 (2–3) | [66] |
| Hospitalisations | 14 (9–20) | 14 (9–20) | 13 (8–18) | 13 (8–18) | 13 (8–18) | 14 (8–19) | [66] |
| Indirect costs (2018 US$) | |||||||
| Non-medically attended illness | 6 (3–16) | 11 (4–24) | 10 (3–25) | 9 (2–22) | 3 (1–18) | 11 (4–22) | [66] |
| Out-patient visits | 8 (4–17) | 13 (5–31) | 10 (4–26) | 10 (4–26) | 4 (1–08) | 12 (5–28) | [66] |
| Hospitalisations | 31 (11–66) | 17 (6–35) | 54 (20–113) | 54 (20–113) | 6 (2–09) | 13 (5–29) | [66] |
| Deaths | 19 624 (11 039–24 530) | 42 610 (23 968–53 263) | 32 361 (18 203–40 451) | 38 816(21 834–48 519) | 0 (0–0) | 42 001 (23 626–52502) | [66] |
| QALY loss | |||||||
| Influenza-associated mild illness | 0.009 (0.007–0.011) | 0.009 (0.007–0.011) | 0.009 (0.007–0.011) | 0.009 (0.007–0.011) | 0.009 (0.007–0.011) | 0.009 (0.007–0.011) | [61] |
| Influenza-associated severe illness | 0.031 (0.025–0.037) | 0.031 (0.025–0.037) | 0.031 (0.025–0.037) | 0.031 (0.025–0.037) | 0.031 (0.025–0.037) | 0.031 (0.025–0.037) | [61] |
| Influenza-associated mortality | 8.79 | 17.45 | 9.16 | 11.45 | 3.72 | 17.35 | [33,37,63] |
| Vaccine-associated mild adverse event‡ | 0.009 (0.007–0.011) | 0.009 (0.007–0.011) | 0.009 (0.007–0.011) | 0.009 (0.007–0.011) | 0.009 (0.007–0.011) | 0.009 (0.007–0.011) | Assumption |
| Vaccine-associated severe adverse event | 0.090 (0.050–0.067) | 0.107 (0.060–0.080) | 0.090 (0.051–0.068) | 0.090 (0.051–0.068) | 0.066 (0.037–0.050) | 0.107 (0.060–0.080) | [33,63,69,87] |
| Ave. life expectancy | 30.3 | 63.3 | 27.4 ‡‡ | 34.2 | 7.7 | 62.6 | [62,64] |
Vaccine efficacy for pregnant women and new-borns weighted by probability of still being pregnant or born, respectively during the influenza season and at risk
Two-dose vaccine efficacy for children. We assume that all children received two doses of trivalent inactivated influenza vaccine
Cost of vaccine mild adverse event assumed to be the same as costs associated with an out-patient visit for a mild influenza case. In the model we assumed that 10% of individuals with mild adverse event will seek outpatient care
Cost of severe adverse event assumed to be same as cost of hospitalisation for a severe acute respiratory infection
In absence of QALY estimate in the literature, assumed to be similar to mild influenza illness event, due to similarity of presentation (pain and fever)
Assumed to be 80% of the rest of the population [51]
PLWHA: Persons living with HIV/AIDS
UMC: Underlying Medical Conditions
Population of women pregnant during the 2018 influenza season. Total population of pregnant women in 2018 ≈925,000
2.1.1. Burden of influenza disease
Key burden of disease inputs required for the CETSIV, include the annual number (per 100 000 population) of non-medically attended influenza cases, medically attended mild cases (requiring only outpatient care), medically attended severe illness (requiring hospitalisation), and deaths. In our base case analysis, we used previously published estimates of disease burden inputs [39,40] that met the broad case definition for any influenza-associated illness [8]. This includes all-respiratory, all-circulatory and all-medical, non-respiratory, and non-circulatory influenza-associated illness. However, in a sensitivity analysis, we employed a narrower case definition for acute respiratory illness which met the WHO definitions for severe acute respiratory infection (SARI) and influenza-like illness (ILI), both of which are subsets of all-respiratory illness [8,41]. These estimates were extracted from published studies reporting laboratory-confirmed influenza cases in South Africa and ecological analyses (non-laboratory confirmed cases) of hospitalization and outpatient diagnosis data [39,40].
We used the broad case definition for the base case analysis because the WHO SARI and ILI case definitions substantially underestimates the disease burden associated with influenza virus infection. In a study conducted in South Africa with systematic laboratory confirmation of influenza among patients hospitalised with respiratory illness, the WHO SARI case definition underestimated the disease burden by 19% in children aged < 5 years and 34% among individuals aged ≥ 5 years [42]. In addition, another study conducted in South Africa reported a substantial number of influenza-associated hospitalisation and deaths among individuals with non-respiratory clinical presentation [40] and several other studies have reported atypical (i.e., non-respiratory) clinical presentation of influenza infection such as acute myocardial infarction or exacerbation of diabetes mellitus and chronic liver and kidney diseases [43–53].
2.1.2. Vaccine efficacy
Efficacy of TIV in high-risk groups were obtained from published studies (Table 1). Due to annual variations of circulating influenza, vaccine efficacy is likely to vary annually depending on the extent of mismatch between the vaccine and circulating influenza strains [54–56]. Therefore, we extracted vaccine efficacy inputs from meta-analyses that included several studies from multiple years [10,12,14,54,57]. This includes vaccine efficacy against laboratory-confirmed, symptomatic influenza-like-illness, hospitalisation and death, inputted into CETSIV as the relative risk reduction of influenza-associated illnesses and deaths. Estimates of vaccine efficacy were available for children aged 6–59 months [14,58,59], persons aged ≥ 65 years [12], pregnant women [54], and healthy adults [54]. However, due to paucity of meta-analyses on PLWHA and persons with other UMCs, vaccine efficacy in these risk groups were inferred using previously published data. For PLWHA, vaccine efficacy was estimated by applying the relative vaccine efficacy between healthy pregnant and pregnant women living with HIV/AIDS2 [57] to vaccine efficacy in healthy adults [54,57]. For persons with UMCs, we assumed comparable vaccine efficacy to persons aged ≥ 65 years [14,60].
2.1.3. QALY loss
In the absence of South Africa-specific studies on QALY loss due to influenza illness, we obtained data on QALY loss from a study conducted in Spain which estimated QALY loss associated with severe (hospitalised) and mild (non-hospitalised) cases of influenza A(H1N1)pdm09 [61]. To estimate QALY loss associated with influenza-associated deaths, we weighted age-specific life expectancies obtained from CETSIV inbuilt WHO life table [62] to baseline age-specific health related quality of life (HRQOL) for the general population [33,63]. We assumed that life expectancy in PLWHA was 80% the life expectancy of the general population [64].
2.1.4. Vaccination programme cost
During each vaccination campaign, primary healthcare facility nurses administer influenza vaccines opportunistically to eligible individuals visiting primary health care facilities for other routine or acute health services. All risk groups receive one dose of TIV with the exception of children aged 6–59 months who, we assume, will receive two doses, administered at an approximately one-month interval [59]. Planning for the seasonal influenza campaign commences every October preceding the influenza season and involves a wide range of stakeholder from all levels of the public health system (national, provincial, district and health facilities) as well as pharmaceutical companies contracted by the NDOH to supply the influenza vaccine to regional pharmacies. Each actor is involved in a range of activities involving microplanning, procurement, distribution, training, communication, social mobilisation, supervision, and monitoring and vaccine service delivery/administration. A detailed description of these activities, including quantity of resources used and unit costs are provided in Fraser et al. [65]. We obtained estimates of economic cost per person vaccinated from Fraser et al. [65], inputted into CETSIV as vaccine price and other programme costs per person vaccinated. Given similar delivery platforms (health facility-based), cost per person vaccinated was constant across all risk groups.
2.1.5. Cost of influenza-associated illness
In South Africa, healthcare services are provided free of charge at the point of care to the majority of individuals utilising the public health system. From a public healthcare providers’ perspective, we included all costs borne by the public healthcare system. These include direct medical cost associated with outpatient visits and hospitalisation. We obtained healthcare providers costs from a study estimating the economic burden of seasonal influenza in South Africa by risk groups. Tempia et al. [66] estimated direct medical cost per illness episode by multiplying quantities of resources used (e.g. length of hospitalisation, admissions to intensive care units and the duration, chest X-rays, oxygen therapy, medications, and laboratory tests) to unit costs of each item. Tempia et al. [66] obtained resource quantity estimates associated with outpatient consultation (for mild illness) and hospitalisation (for severe illness) from influenza-positive patients presenting with ILI or hospitalised with SARI in routine surveillance sites across South Africa [8,42,67]. Variations in direct medical costs between risk groups was largely driven by differences in the severity of the disease in each risk group which in turn affected the quantity of resources used and consequently, direct medical cost [66].
From a societal perspective, in addition to direct medical cost incurred by the public healthcare provider, we included direct non-medical and indirect costs incurred by patients and their caregivers when seeking health care for influenza-associated illness. These include transportation costs, other out-of-pocket payment costs for non-medically attended cases and productivity losses due to absenteeism and death. Estimates of transportation costs per illness episode, out-of-pocket payment cost per illness episode and productivity losses due to absenteeism were obtained from Tempia et al. [66]. To estimate indirect costs due to influenza-associated deaths, CETSIV inbuilt life table based on baseline mortality rate for South Africa was used to estimate the number of productive days lost at time of death. This was multiplied by median daily wage rates and adjusted for unemployment rate in South Africa [8,66].
2.1.6. Vaccine adverse event costs
Influenza vaccination is associated with both mild and severe adverse events. Mild adverse events include local injection site pain and systemic reactions such as fever and muscle pain. These occur at a rate of approximately 5–64 per 100 persons vaccinated [68]. Reported severe events include anaphylaxis and Guillain-Barre Syndrome which occur at a rate of approximately 0.7–2 per million vaccinations [68]. We assumed that 10% of patients with a mild adverse event would visit an outpatient facility and be attended by a nurse while all patients with a severe adverse event would require hospitalisation
To estimate the cost of severe adverse event, inpatient care daily costs (general ward facility fee and specialist fee) was multiplied by length of hospital stay (53 days [69]) and the incidence of Guillain-Barre Syndrome following influenza vaccination. Unit cost for mild adverse events was based on outpatient facility fee and nurse professional fee. Facility and healthcare professional fees were obtained from the South Africa Uniform Patient Fee Schedule (UPFS) [70].
All costs inputs used in the CETSIV were expressed in 2018 ZAR and converted to US$ using average 2018 exchange rate (US$ 1 = ZAR 13.25) [71].
2.2. Cost-effectiveness threshold
To assess the cost-effectiveness of the influenza vaccination programme, ICERs were compared to a cost-effectiveness threshold. The one-to three times GDP (gross domestic product) per capita thresholds has been the most widely used threshold for determining cost-effectiveness of interventions, particularly in LMICs [72,73]. However following widespread criticism of its use as a decision rule for informing resource allocation decisions, the WHO in 2016, revised its recommendations on the use of the one to three time GDP per capita [73–76]. A growing body of evidence has now emerged on empirically estimated thresholds [77–82]. In this study, we use a cost-effectiveness threshold recently estimated for South Africa that reflects the health opportunity cost of health spending [82]. This threshold, although estimated as a cost per DALY averted threshold (US$ 3400 in 2018 prices) is a close approximation of a cost per QALY threshold [81]. Therefore, in this study, cost-effectiveness of the influenza vaccination programme in each risk group was determined by comparing incremental cost per QALYs to a threshold of US$ 3400 per QALY gained.
2.3. Sensitivity analyses
We conducted a series of deterministic and probabilistic sensitivity analyses to assess the robustness of our results to uncertainty in our model inputs. For the deterministic sensitivity analysis (DSA), we conducted a series of one-way sensitivity analyses, sequentially varying each model input over a given range (Table 1). Model inputs tested include vaccination programme costs; costs associated with outpatient visits and hospitalisations; burden of influenza-associated outpatient visits, hospitalisations and deaths; QALY loss associated with influenza disease; and vaccine efficacy. All parameters (except vaccination programme cost) were varied over a 95% confidence interval reported in previous studies (Table 1). In the absence of confidence intervals, we varied vaccine programme costs by +/−50% the mean value.
Given potential mismatch between TIV and circulating influenza virus [30,54], vaccine efficacy is likely to vary annually, consequently affecting the cost-effectiveness of the influenza vaccination programmes. Therefore, we conducted a sensitivity analysis to assess the robustness of our findings to potential mismatch of TIV3. In addition, a sensitivity analysis was conducted to assess the robustness of our findings to the narrower case definition for acute respiratory illness meeting the WHO definitions for SARI and ILI.
Finally, a probabilistic sensitivity analysis (PSA) was conducted to assess the robustness of our findings to uncertainty in all parameters simultaneously. The PSA is a Bayesian approach that involves specifying probability distributions for each model parameter and running a series of Monte Carlo simulations that drew parametric inputs randomly from these distributions [83,84]. We run 1 000 Monte Carlo simulations specifying a beta distribution for burden of disease inputs, vaccine efficacy, population baseline utilities and QALY loss from non-fatal events, and a gamma distribution for all cost inputs.
3. Results
Vaccination coverage was relatively low for all risk groups except for pregnant women (Table 1). The population size for pregnant woman was weighted to reflect the fact that only 35% of all pregnant women in a given year are pregnant during the influenza campaign and therefore receive the vaccine [32].
Table 2 displays clinical events averted by the influenza vaccination programme at 2018 coverage levels, disaggregated by risk group. The highest number of influenza-associated clinical events were averted in PLWHA and persons with underlying medical conditions compared to other risk groups.
Table 2:
Vaccines Administered and Clinical Events Averted by Influenza Vaccination in South Africa, 2017–2018.
| Target Group | Vaccines administered | Influenza cases averted (% reduction) | Outpatient visits averted (% reduction) | Hospitalizations averted (% reduction) | Deaths averted (% reduction) |
|---|---|---|---|---|---|
| Pregnant women | 158 521 | 15 692 (11.1) | 4 701 (10.9) | 364 (11.7) | 24 (9.79) |
| PLWHA | 354 290 | 32 496 (2.40) | 8 180 (2.40) | 458 (2.40) | 125 (2.40) |
| Persons with UMC | 282 452 | 33 763 (1.82) | 8 666 (1.82) | 183 (1.82) | 46 (1.82) |
| Persons aged ≥ 65 years | 93 763 | 1 888 (1.80) | 355 (1.80) | 179 (1.80) | 75 (1.80) |
| Children 6–59 months* | 124 892 | 10 966 (0.86) | 2 373 (0.86) | 105 (0.86) | 8 (0.86) |
2017 estimates of vaccine administered in children aged 6–59 months. 2018 estimates for all other target groups
PLWHA: Persons living with HIV/AIDS
UMC: Underlying Medical Conditions
Table 3 shows the incremental costs incurred by the public healthcare provider, patients and their caregivers. Substantial cost savings were observed across all risk groups. From a public healthcare provider’s perspective, substantial reductions in influenza treatment costs were observed although these were largely offset by vaccination programme costs (Table 3). From the perspective of patients and their caregivers, we observed reductions in out-of-pocket expenditure and productivity losses across all influenza-associated clinical events except in the elderly population. We assumed no productivity losses due to influenza-associated deaths in persons aged ≥ 65 years given the retirement age of 60 years in South Africa. However, we estimated productivity losses due to non-medically attended and medically attended (outpatient and hospitalisation) illness in this age group due to productivity losses experienced by their caregivers. As a result, the impact of the vaccination programme on productivity loss is lowest in persons aged ≥ 65 years compared to the other risk groups (Table 3).
Table 3:
Incremental* Costs (2018 US$), by Risk Groups in South Africa.
| Cost Categories | Pregnant women | PLWHA | Persons with UMC | Persons aged ≥ 65 years | Children 6‒59 months |
|---|---|---|---|---|---|
| Vaccine Programme Costs | |||||
| Vaccine cost | 481 372 | 1 075 853 | 857 708 | 284 724 | 758 505 |
| Vaccine delivery | 859 121 | 1 920 114 | 1 530 782 | 508 158 | 1 012 923 |
| Vaccine adverse events | 46 598 | 52 073 | 41 514 | 13 781 | 18 356 |
| Influenza Treatment Costs Averted | |||||
| Outpatient visits | 115 028 | 204 333 | 214 792 | 8 805 | 58 449 |
| Hospitalisations | 302 074 | 406 373 | 153 227 | 154 786 | 63 511 |
| Deaths | 8 233 | 46 678 | 16 417 | 28 228 | 2 171 |
| Out-of-Pocket Expenditure Averted | |||||
| Non-medically attended illness | 53 264 | 118 494 | 123 557 | 6 702 | 148 041 |
| Outpatient visit | 9 314 | 16 208 | 17 171 | 704 | 7 054 |
| Hospitalisation | 5 047 | 5 895 | 2 363 | 2 304 | 1 459 |
| Productivity Loss Averted | |||||
| Non-medically attended illness | 90 481 | 236 964 | 219 635 | 4 289 | 92 969 |
| Outpatient visit | 47 203 | 81 030 | 85 847 | 1 407 | 27 850 |
| Hospitalisation | 9 409 | 24 685 | 9 896 | 993 | 1 411 |
| Death | 846 637 | 4 020 483 | 1 784 246 | 0 | 321 387 |
Estimated as the difference between costs of TIV vaccination programme and no vaccination programme
PLWHA: Persons living with HIV/AIDS
UMC: Underlying Medical Conditions
TIV: Trivalent inactivated influenza Vaccine
Table 4 shows ICERs disaggregated by risk group from both the healthcare provider and societal perspective. These are also presented diagrammatically on a cost-effectiveness plane in Supplemental Figure 2. ICERs estimated from a public healthcare provider’s perspective ranged from ~ US$ 1 700/QALY for PLWHA to ~ US$ 7 500/QALY for children aged 6–59 months. Using a cost-effectiveness threshold of US$ 3 400/QALY [82], the influenza vaccination programme was cost-effective for all risk groups except in children. When out-of-pocket costs and productivity losses were considered in a societal perspective, the influenza vaccination programme was observed to be cost saving in pregnant women as well as in PLWHA and persons with other UMCs. Overall, from a societal perspective, the vaccination programme was cost-effective for all risk groups, except in children aged 6–59 months where the ICER remained above the cost-effectiveness threshold (Table 4; Supplemental Figure 2).
Table 4:
Incremental Cost-Effectiveness Ratio (TIV vaccination programme vs No vaccination programme) by Risk Groups in South Africa.
| Pregnant women | PLWHA | Persons with UMC | Persons aged ≥ 65 years | Children 6–59 months | |
|---|---|---|---|---|---|
| Public healthcare provider perspective | |||||
| Incremental Cost (2018 US$) | 961 756 | 2 390 656 | 2 045 569 | 614 844 | 1 665 653 |
| Incremental QALY | 478 | 1 367 | 781 | 294 | 222 |
| ICER | 2 010 | 1 749 | 2 618 | 2 090 | 7 490 |
| Societal perspective | |||||
| Incremental Cost (2018 US$) | −99 666 | −2 113 316 | −197 255 | 598 388 | 1 171 228 |
| Incremental QALY | 478 | 1 367 | 781 | 294 | 222 |
| ICER | Dominant | Dominant | Dominant | 2 034 | 5 267 |
ICER: Incremental Cost-Effectiveness Ratio
PLWHA: Persons living with HIV/AIDS
UMC: Underlying Medical Conditions
TIV: Trivalent inactivated influenza Vaccine
QALY: Quality-Adjusted Life Year
PSA: Probabilistic sensitivity analysis
Negative values imply cost savings
3.1. Deterministic sensitivity analysis (DSA)
The results of the one-way sensitivity analyses are presented in tornado diagrams for each risk group (Supplemental Figures 3A–E). The figures show changes in the ICERs associated with increasing and decreasing model inputs over the ranges specified in Table 1. For persons aged ≥ 65 years, persons living with UMC and children aged 6–59 months, uncertainty in estimates of vaccine delivery costs, vaccine price, vaccine efficacy against influenza mortality, and the incidence of influenza- associated mortality had the highest impact on the ICER. For pregnant women, variations in vaccine efficacy against influenza-associated mortality, vaccine delivery costs, vaccine efficacy against hospitalisation and vaccine price had the highest impact on the ICER while vaccine delivery costs, vaccine price and incidence of influenza-associated mortality had the largest impact of the ICER for PLWHA.
To assess the impact of potential variations in vaccine efficacy due to mismatch of TIV with circulating influenza virus strains, vaccine efficacy was varied multiplicatively around the base case input for each risk group. Supplemental Figure 4 shows how the ICERs varied as a function of vaccine efficacy. We observed that cost-effectiveness of the vaccination programme increased with vaccine efficacy. The results suggest that in years with a high mismatch between the vaccine and circulating influenza virus strains (for example at vaccine efficacy multiplier = 0.5), the vaccine will not be cost-effective for any risk group. For children aged 6–59 months, even with large increases in vaccine efficacy relative to the base case input, the vaccination programme is not likely to be cost-effective.
Finally, the scenario analysis assessing the robustness of our findings to variations in the case definition of influenza-associated illness suggest that the ICERs are sensitive to the burden of disease estimates (Table 5). When a narrower case definition of SARI and ILI were applied, the ICERs for all risk group increased dramatically and the influenza vaccination programme was no longer cost-effective from both the public healthcare provider and societal perspectives (Table 5).
Table 5:
Scenario analysis showing the impact of disease burden on the ICER from a Public Healthcare Provider and Societal Perspective in South Africa.
| Public Healthcare Provider Perspective ICER (2018 US$) | Societal Perspective ICER (2018 US$) | |||
|---|---|---|---|---|
| Base case analysis | Scenario analysis* | Base case analysis | Scenario analysis* | |
| Pregnant women | 2 010 | 11 015 | Dominant | 8 362 |
| PLWHA | 1 749 | 11 361 | Dominant | 8 012 |
| Persons with UMC | 2 618 | 11 541 | Dominant | 8 621 |
| Persons aged ≥ 65 years | 2 090 | 15 888 | 2 034 | 15 801 |
| Children 6–59 months | 7 490 | 23 620 | 5 267 | 21 161 |
PLWHA: Persons living with HIV/AIDS
UMC: Underlying Medical Conditions
Base case analysis: Incremental Cost-Effectiveness Ratio (ICER) estimated using the broad case definition of influenza-associated illness which includes all respiratory, all circulatory, non-respiratory/non-circulatory influenza-associated illness
Scenario analysis: ICER estimated using the narrower case definition of influenza-associated illness which includes only ILI and SARI, a subset of all respiratory influenza-associated illness
Supplementary Figure 5 presents the results of the probabilistic sensitivity analyses for the narrower case definition scenario
3.2. Probabilistic sensitivity analysis (PSA)
The results from the PSA are presented for each risk group in cost-effectiveness scatter plots and cost-effectiveness acceptability curves (Fig. 1A–E). The cost-effectiveness acceptability curves shows the probability of the vaccination programme being cost-effective over a wide range of potential cost-effectiveness thresholds. From a public healthcare provider’s perspective, over 90% of the simulations fall below the cost-effectiveness threshold of US$ 3 400/QALY for pregnant women and PLWHA (Fig. 1A and B). For persons aged ≥ 65 years and persons with UMCs, the vaccination programme was cost-effective in >80% of the simulations (Fig. 1C and D). However, for children aged 6–59 months, the vaccination programme had a very low probability of being cost-effective from both study perspectives (Fig. 1E).
Fig. 1.
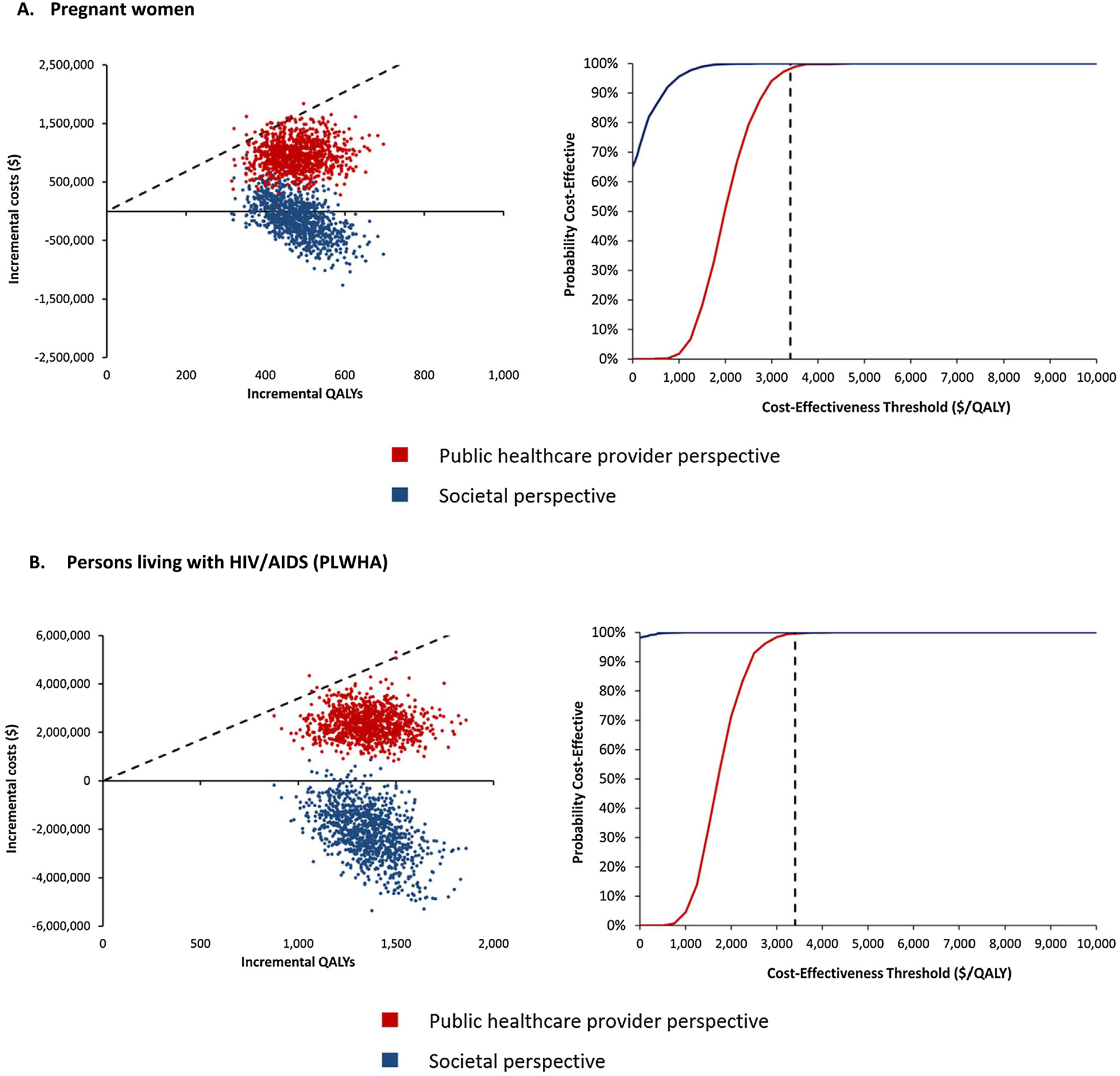
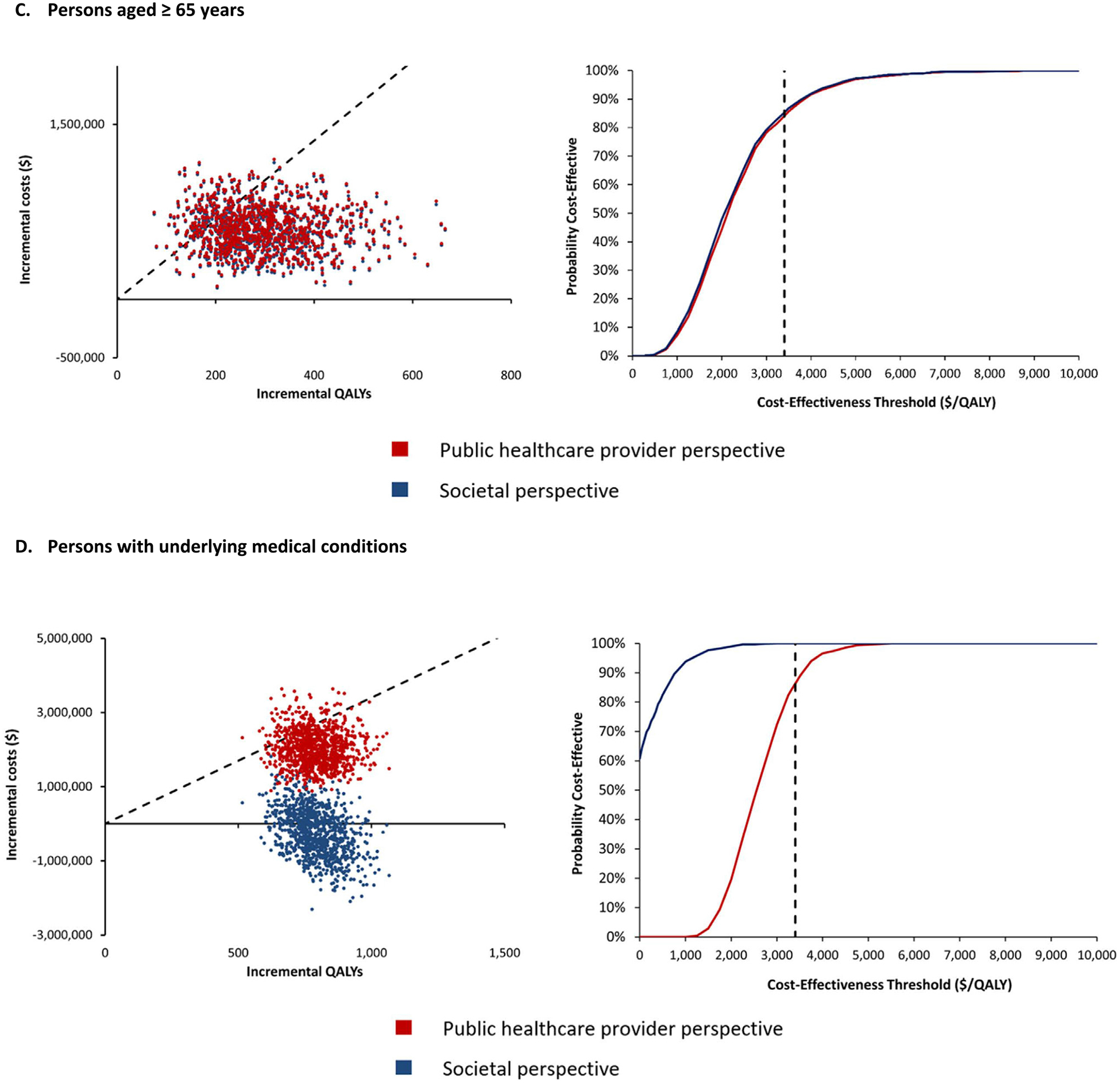
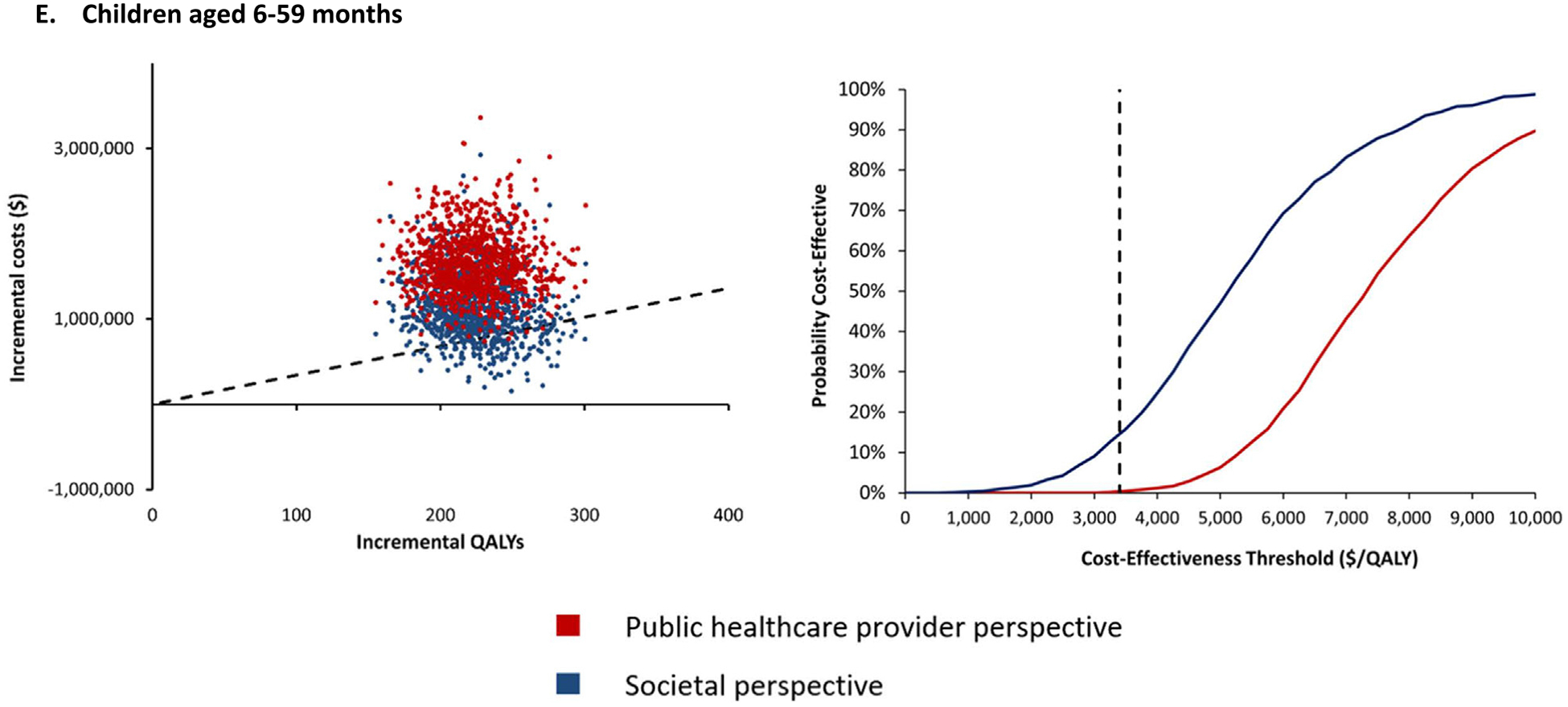
Cost-Effectiveness Scatter Plots (left graphs) & Cost-Effectiveness Acceptability Curves (right graphs) of TIV vs No Vaccine.
4. Discussion
Seasonal influenza imposes a significant health [40] and economic burden [8] in South Africa, particularly in populations vulnerable to severe consequences of the virus [6,7]. We assessed the cost-effectiveness of South Africa’s seasonal influenza vaccination strategy to inform the prioritisation of risk groups for vaccination. We modelled the 2018 vaccination campaign using a cost-effectiveness tool, the CETSIV, populated with country-specific demographic, epidemiologic and coverage data to estimate incremental costs and incremental effects associated with the vaccination programme. The highest clinical benefits of the vaccination programme were observed in PLWHA and persons with other UMCs.
Our findings suggest that it is cost-effective to vaccinate all risk groups except children aged 6–59 months. Limited efficacy of the vaccine in children and the higher number of vaccine doses (2 doses) required to achieve viral protection in children aged 6–59 months may explain the higher ICER observed in this age group.
Resource allocation decisions in South Africa’s public health system are currently limited to the perspective of the healthcare provider, excluding costs borne by individuals and their caregivers [38]. However, in our study, we adopted a broader (societal) perspective that incorporates such costs, in part, to allow for comparisons with other recent studies conducted in South Africa that had adopted a societal perspective [32,33]. As expected, from a societal perspective, the ICER reduced substantially in all risk groups except in persons aged ≥ 65 years due to the lower productivity losses experienced in this age group. From a societal perspective, the vaccination programme became the dominant strategy compared to no vaccination programme for pregnant women, PLWHA and persons with other UMCs due to averting influenza-associated clinical events and associated productivity losses and out-of-pocket expenditure incurred by patients and their caregivers. Although cost savings were observed in children aged 6–59 months, particularly from averting productivity losses due to deaths, cost savings were not sufficient to completely offset the cost of the vaccination programme in children.
Our base case results from a societal perspective appear more favourable compared to two other studies conducted in South Africa [32,33]. Differences in our model structures and input parameters may explain differences between prior studies and ours. Notably, in our study, we used a broader case definition of influenza-associated illness, which includes all respiratory, circulatory, non-respiratory, and non-circulatory cases [39,40]. As a result, our estimate of the proportion of infected individuals (medically or non-medically attended), symptomatic attack rate as well as case hospitalisation and fatality ratios were considerably higher than estimates reported in previous studies. However, when we applied the narrower case definition of ILI and SARI, our results became comparable to previous studies. For example, Biggerstaff et al. [32] using a static model, estimated an ICER for pregnant women of ~ US$ 5 900/QALY which similar to our findings with the narrower case definition is not cost-effective for pregnant women.
More broadly, our base case ICERs also compares favourably with estimates of the cost-effectiveness of other vaccination programmes currently provided in South Africa. For example, an ICER of US$1078 and US$1460 per QALY gained (from a societal and health systems perspective, respectively) was estimated for a human papilloma virus vaccination programme targeting girls aged 12 years old in South Africa [85].
Overall, our findings should be interpreted taking into consideration some limitations of our study. The CETSIV is a static tool and therefore models only the direct benefits of the vaccination programme to vaccinated individuals. However, given the dynamic nature of the influenza disease, the vaccine may have an indirect ‘herd-immunity’ effect through a reduction in the risk of infection in unvaccinated individuals. Therefore the CETSIV may have underestimated the impact of the vaccine and as a result, underestimated the cost-effectiveness of the seasonal influenza vaccination programme [86]. This may explain the difference between our finding and de Boer et al. [33], who adopted a dynamic approach in modelling the effect of the influenza vaccination programme. Therefore, our ICERs should be interpreted as conservative estimates. Nevertheless, our findings show that for all study risk groups considered except for children aged 6–59 months, the vaccine represents good value for money. Although we found that the direct benefits of vaccinating young children do not offset the associated cost in a static approach, given high transmission rates seen in young children, a vaccination programme that targets only school age children may have wider benefits to the general population. This would potentially include vulnerable groups at risk of more severe consequences of influenza. Furthermore, TIV efficacy has been shown to be higher in school-age children compared to children aged 6–59 months, which may increase the benefits of vaccinating this sub-population [13]. A reassessment of the cost-effectiveness of the influenza vaccination programme may be warranted to identify subgroups, including school-age children, who are likely to have the highest direct and indirect benefits, as well as to identify maximum coverage levels required to achieve herd protection. A dynamic transmission model will be required to answer these questions and should be considered for future studies.
5. Conclusion
The WHO Cost Effectiveness Tool for Seasonal Influenza Vaccination (CETSIV) proved to be useful for assessing the cost-effectiveness of seasonal influenza vaccination strategies in South Africa. CETSIV can potentially be adapted to reflect other country-specific decisions. The tool helped to demonstrate that South Africa’s seasonal influenza vaccination strategy of opportunistically targeting vulnerable populations during visits to health facilities for routine care is cost-effective in most target groups. Scaling up the programme have to be weighed against potential costs associated with a comprehensive vaccination programme and the budget implications of achieving higher coverage levels. Whilst the protection of health should remain the primary argument for decision makers to prioritize risk groups, in settings of scarce health resources, the results of this study may complement national policy considerations, with arguments from an economic perspective.
Supplementary Material
Acknowledgement
The authors acknowledge the input of members of a steering committee set up to provide strategic guidance throughout the project. These include Trudy Leong (Essential Drugs Programme, National Department of Health); Gary Reubenson (Rahima Moosa Mother & Child Hospital), Caroline Kesebilwe & Chika Asomugha (Gauteng Department of Health); Mmakopa Mohlala & Pinkie Lesolang (City of Tshwane); Mphaka Makgomo, Mpho Moshime & Angy Rabothata (Tshwane District Department of Health); Haroon Saloojee (University of Witwatersrand Paediatrics & Child Health – Chris Hani Baragwanath Hospital); Lee Fairlie (Wits Reproductive Health and HIV Institute) ; Shabir Madhi & Marta Nunes (Respiratory and Meningeal Pathogens Research Unit); Kerrigan McCarthy (National Institute of Communicable Disease); and Mandla Zwane (Mpumalanga Department of Health). In addition, the authors would like to acknowledge the contributions made by facility managers and nurses at the facilities visited.
Funding Source
The development and piloting of the Cost Effectiveness Tool for Seasonal Influenza Vaccination (CETSIV), was funded by a grant from the WHO Department of Immunization, Vaccines and Biologicals. This work was supported by the U. S. Centers for Disease Control and Prevention, under the terms of Cooperative Agreement Number U50CK000431. Edoka I, Kohli-Lynch C, Fraser H and Hofman K were also supported by the South African Medical Research Council (23108).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics clearance
Ethical approval for this study was obtained from the Human Research Ethics Committee of the University of the Witwatersrand, certificate number M180246.
Publisher's Disclaimer: Disclaimer
The authors have no interests to declare. Hutubessy R and Lambach P work for the World Health Organization. McMorrow M and Tempia S work for the US CDC. The findings and conclusions in this study are those of the author(s) and do not necessarily represent the official position of the funding agencies or of the World Health Organization.
Prior to 2017, the policy also targeted healthy children aged 6–59 months.
This was done using vaccine seroconversion rates reported for pregnant women with and without HIV infection. We estimated that vaccine efficacy in HIV pregnant women was 74% the vaccine efficacy in non-HIV pregnant women. Vaccine efficacy in HIV individuals was then estimated by applying this proportion to estimates of vaccine efficacy in healthy adults obtained from existing meta-analyses.
Each year, TIV includes three stains of influenza virus – two stains of influenza A virus (H1N1 and H3N2) and one influenza B virus (Victoria or Yamagata lineages). Based on WHO recommendations, one lineage of the B virus is chosen for inclusion into TIV. However, in some years, a mismatch may occur when the circulating influenza B lineage during a season differs from the influenza B lineage contained in TIV or when both influenza B lineages are in circulation. de Boer et al 2018 estimated a probability of mismatch of ~50% over an 11-year time period [33].
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.11.028.
References
- [1].Iuliano AD et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. The Lancet 2018;391(10127):1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Blumberg L, Cohen C, Dawood H. Influenza NICD Recommendations for the diagnosis, prevention, management and public health response. National Institute for Communicable Diseases; 2017. [Google Scholar]
- [3].Fischer WA II et al. Global burden of influenza: contributions from resource limited and low-income settings. Global heart 2014;9(3):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Massyn N, et al. , District Health Barometer 2015/16 2016, Health Systems Trust: Durban.
- [5].Statistics South Africa, Mortality and causes of death in South Africa, 2015: Findings from death notification. 2017: Pretoria. [Google Scholar]
- [6].Tempia S et al. Mortality Associated With Seasonal and Pandemic Influenza and Respiratory Syncytial Virus Among Children <5 Years of Age in a High HIV Prevalence Setting—South Africa, 1998–2009. Clin Infect Dis 2014;58 (9):1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tempia S et al. Mortality Associated With Seasonal and Pandemic Influenza Among Pregnant and Nonpregnant Women of Childbearing Age in a High-HIV-Prevalence Setting—South Africa, 1999–2009. Clin Infect Dis 2015;61 (7):1063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tempia S et al. Health and economic burden of influenza-associated illness in South Africa, 2013–2015. Influenza Other Respir Viruses 2019;13(5):484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cohen C et al. Epidemiology of Severe Acute Respiratory Illness (SARI) among Adults and Children Aged ≥5 Years in a High HIV-Prevalence Setting, 2009–2012. PLoS ONE 2015;10(2):e0117716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Madhi SA et al. Influenza Vaccination of Pregnant Women and Protection of Their Infants. N Engl J Med 2014;371(10):918–31. [DOI] [PubMed] [Google Scholar]
- [11].Schwarze-Zander C et al. How successful is influenza vaccination in HIV infected patients? Results from an influenza A(H1N1)pdm09 vaccine study. HIV & AIDS Review 2016;15(3):111–5. [Google Scholar]
- [12].Demicheli V et al. Vaccines for preventing influenza in the elderly. Cochrane Database of Systematic Reviews 2018;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jefferson T, et al. , Vaccines for preventing influenza in healthy children. The Cochrane database of systematic reviews, 2018. 2: p. CD004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McMorrow ML et al. Prioritization of risk groups for influenza vaccination in resource limited settings – A case study from South Africa. Vaccine 2019;37 (1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].National Department of Health, National Influenza Policy and Strategic Plan 2017 to 2021, National Department of Health, Editor. 2017. [Google Scholar]
- [16].National Department of Health, Influenza Vaccinations: Post Vaccination Evaluation 2018, National Department of Health: Communicable Diseases. [Google Scholar]
- [17].Jit M, Newall AT, Beutels P. Key issues for estimating the impact and cost-effectiveness of seasonal influenza vaccination strategies. Human Vaccines & Immunotherapeutics 2013;9(4):834–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Peasah SK et al. Influenza cost and cost-effectiveness studies globally – A review. Vaccine 2013;31(46):5339–48. [DOI] [PubMed] [Google Scholar]
- [19].Jit M et al. The cost-effectiveness of vaccinating pregnant women against seasonal influenza in England and Wales. Vaccine 2010;29(1):115–22. [DOI] [PubMed] [Google Scholar]
- [20].Beigi RH et al. Economic Value of Seasonal and Pandemic Influenza Vaccination during Pregnancy. Clin Infect Dis 2009;49(12):1784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Myers ER, Misurski DA, Swamy GK. Influence of timing of seasonal influenza vaccination on effectiveness and cost-effectiveness in pregnancy. Am J Obstet Gynecol 2011;204(6, Supplement):S128–40. [DOI] [PubMed] [Google Scholar]
- [22].Newall AT, Dehollain JP. The cost-effectiveness of influenza vaccination in elderly Australians: An exploratory analysis of the vaccine efficacy required. Vaccine 2014;32(12):1323–5. [DOI] [PubMed] [Google Scholar]
- [23].Maciosek MV et al. Influenza Vaccination: Health Impact and Cost Effectiveness Among Adults Aged 50 to 64 and 65 and Older. Am J Prev Med 2006;31(1):72–9. [DOI] [PubMed] [Google Scholar]
- [24].Nichol KL et al. The Efficacy and Cost Effectiveness of Vaccination against Influenza among Elderly Persons Living in the Community. N Engl J Med 1994;331(12):778–84. [DOI] [PubMed] [Google Scholar]
- [25].Lee BY et al. Cost-effectiveness of adjuvanted versus nonadjuvanted influenza vaccine in adult hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation 2011;57(5):724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Abbott KC, Yuan CM, Lee JL. Nothing to Sneeze At: Efficacy and Cost-Effectiveness of the Influenza Vaccine in Patients Receiving Long-term Dialysis. Am J Kidney Dis 2011;57(5):651–3. [DOI] [PubMed] [Google Scholar]
- [27].Nosyk B et al. The Cost-Effectiveness and Value of Information of Three Influenza Vaccination Dosing Strategies for Individuals with Human Immunodeficiency Virus. PLoS ONE 2011;6(12):e27059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Teufel II RJ, Basco WT Jr, Simpson KN. Cost effectiveness of an inpatient influenza immunization assessment and delivery program for children with asthma. Journal of Hospital Medicine 2008;3(2):134–41. [DOI] [PubMed] [Google Scholar]
- [29].Trogdon JG, Nurmagambetov TA, Thompson HF. The Economic Implications of Influenza Vaccination for Adults with Asthma. Am J Prev Med 2010;39 (5):403–10. [DOI] [PubMed] [Google Scholar]
- [30].Osterholm MT et al. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012;12(1):36–44. [DOI] [PubMed] [Google Scholar]
- [31].Alshreef A et al. Cost-Effectiveness of Docetaxel and Paclitaxel for Adjuvant Treatment of Early Breast Cancer: Adaptation of a Model-Based Economic Evaluation From the United Kingdom to South Africa. Value in Health Regional Issues 2019;19:65–74. [DOI] [PubMed] [Google Scholar]
- [32].Biggerstaff M et al. A cost-effectiveness analysis of antenatal influenza vaccination among HIV-infected and HIV-uninfected pregnant women in South Africa. Vaccine 2019;37(46):6874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].de Boer PT et al. The cost-effectiveness of trivalent and quadrivalent influenza vaccination in communities in South Africa. Vietnam and Australia. Vaccine 2018;36(7):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Weekly Epidemiological Record= Relevé épidémiologique hebdomadaire 2012;87(47):461–76. [PubMed] [Google Scholar]
- [35].World Health Organization. Influenza economics: Economic guidance. 2019. [cited 2019; Available from: https://www.who.int/immunization/research/development/influenza_economics/en/.
- [36].National Department of Health. Influenza Vaccinations: Post Vaccination Evaluation. Communicable Diseases: National Department of Health; 2017. [Google Scholar]
- [37].World Health Organization. Global Health Observatory data repository, Life tables by country: South Africa. 2017. [cited 2019 18 September 2019]; Available from: https://apps.who.int/gho/data/?theme=main&vid=61540.
- [38].Department of Health, Guidelines for Pharmacoeconomic Submissions 2012, Health, Editor. 2013, Government Gazette. [Google Scholar]
- [39].Tempia S et al. Influenza Disease Burden among Potential Target Risk Groups for Immunization in South Africa, 2013–2015. Vaccine 2020;38(27):4288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tempia S et al. Quantifying How Different Clinical Presentations, Levels of Severity, and Healthcare Attendance Shape the Burden of Influenza-associated Illness: A Modeling Study From South Africa. Clin Infect Dis 2018;69 (6):1036–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].World Health Organization. Global Epidemiological Surveillance Standards for Influenza. Geneva: World Health Organization; 2014. [Google Scholar]
- [42].Tempia S et al. The effects of the attributable fraction and the duration of symptoms on burden estimates of influenza-associated respiratory illnesses in a high HIV prevalence setting, South Africa, 2013–2015. Influenza Other Respir Viruses 2018;12(3):360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sellers SA et al. The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses 2017;11(5):372–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nguyen JL et al. Seasonal Influenza Infections and Cardiovascular Disease Mortality. JAMA Cardiology 2016;1(3):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Barnes M et al. Acute myocardial infarction and influenza: a meta-analysis of case–control studies. Heart 2015;101(21):1738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ebdrup L, Druey K, Mogensen TH. Severe capillary leak syndrome with cardiac arrest triggered by influenza virus infection. BMJ Case Reports 2018;2018 p. bcr-2018–226108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Onozuka D, Hagihara A. Extreme influenza epidemics and out-of-hospital cardiac arrest. Int J Cardiol 2018;263:158–62. [DOI] [PubMed] [Google Scholar]
- [48].Nichol KL et al. Influenza Vaccination and Reduction in Hospitalizations for Cardiac Disease and Stroke among the Elderly. N Engl J Med 2003;348 (14):1322–32. [DOI] [PubMed] [Google Scholar]
- [49].Vamos EP et al. Effectiveness of the influenza vaccine in preventing admission to hospital and death in people with type 2 diabetes. Can Med Assoc J 2016;188(14):E342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tignanelli CJ et al. Outcomes of Acute Kidney Injury in Patients With Severe ARDS Due to Influenza A(H1N1) pdm09 Virus. Am J Crit Care 2018;27 (1):67–73. [DOI] [PubMed] [Google Scholar]
- [51].Bitzan M, Zieg J. Influenza-associated thrombotic microangiopathies. Pediatric Nephrology 2018;33(11):2009–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nonaka K et al. Acute Liver Failure Associated with Influenza A Virus Infection: an Autopsy Case Report. Japanese Journal of Infectious Diseases 2019;72 (5):347–9. [DOI] [PubMed] [Google Scholar]
- [53].Zhang S et al. Influenza A virus infection induces liver injury in mice. Microb Pathog 2019;137:103736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Demicheli V et al. Vaccines for preventing influenza in healthy adults. Cochrane Database of Systematic Reviews 2018;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fielding JE et al. Estimation of type- and subtype-specific influenza vaccine effectiveness in Victoria, Australia using a test negative case control method, 2007–2008. BMC Infect Dis 2011;11(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Reed C et al. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012;30(11):1993–8. [DOI] [PubMed] [Google Scholar]
- [57].Nunes MC et al. Neutralization and hemagglutination-inhibition antibodies following influenza vaccination of HIV-infected and HIV-uninfected pregnant women. PLoS ONE 2019;13(12):e0210124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jefferson T et al. Assessment of the efficacy and effectiveness of influenza vaccines in healthy children: systematic review. The Lancet 2005;365 (9461):773–80. [DOI] [PubMed] [Google Scholar]
- [59].McMorrow M, Meta-analysis output for vaccine efficacy in children 6–23 months. 2019: Unpublished update to Jefferson 2018 meta-analysis.. [Google Scholar]
- [60].Michiels B et al. A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine 2011;29(49):9159–70. [DOI] [PubMed] [Google Scholar]
- [61].Hollmann M et al. Impact of Influenza on Health-Related Quality of Life among Confirmed (H1N1)2009 Patients. PLoS ONE 2013;8(3):e60477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].World Health Organization. Global Health Observatory data repository: Life tables by country. 2017. [cited 2018 18 Jan 2018]; Available from: http://apps.who.int/gho/data/view.main.61540?lang=en.
- [63].Jelsma J et al. How do Zimbabweans value health states?. Population Health Metrics 2003;1(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Johnson LF et al. Life Expectancies of South African Adults Starting Antiretroviral Treatment: Collaborative Analysis of Cohort Studies. PLoS Med 2013;10(4):e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fraser H, et al. , Cost of Seasonal Influenza Vaccination Programme in South Africa. unpublished manuscript: Unpublished manuscript.
- [66].Tempia S, et al. , Influenza Economic Burden among Potential Target Risk Groups for Immunization in South Africa, 2013–2015. 2020: Under review by Vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cohen C et al. Severe influenza-associated respiratory infection in high HIV prevalence setting, South Africa, 2009–2011. Emerg Infect Dis 2013;19 (11):1766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].World Health Organization, Information sheet: Observed rate of vaccine reactions, influenza vaccine. 2012.
- [69].Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome. The Lancet 1997;349 (9047):225–30. [PubMed] [Google Scholar]
- [70].National Department of Health. Uniform Patient Fee Schedule. 2018. [cited 2019 15 August 2019]; Available from: http://www.health.gov.za/index.php/shortcodes/2015-03-29-10-42-47/2015-04-30-09-10-23/uniform-patient-fee-schedule/category/448-upfs-2018#.
- [71].South African Reserve Bank. Selected historical rates: Rand per US Dollar. 2018. [cited 2020 12 September 2020]; Available from: https://www.resbank.co.za/Research/Rates/Pages/SelectedHistoricalExchangeAndInterestRates.aspx.
- [72].Hutubessy R et al. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Effectiveness and Resource Allocation 2003;1(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Leech AA et al. Use and Misuse of Cost-Effectiveness Analysis Thresholds in Low- and Middle-Income Countries: Trends in Cost-per-DALY Studies. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research 2018;21(7):759–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Marseille E et al. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ 2015;93(2):118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Newall AT, Jit M, Hutubessy R. Are Current Cost-Effectiveness Thresholds for Low- and Middle-Income Countries Useful? Examples from the World of Vaccines. PharmacoEconomics 2014;32(6):525–31. [DOI] [PubMed] [Google Scholar]
- [76].Bertram MY et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ 2016;94(12):925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Claxton K et al. Methods for the Estimation of the NICE Cost Effectiveness Threshold. Health Technol Assess 2015;19(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Edney LC et al. Estimating the Reference Incremental Cost-Effectiveness Ratio for the Australian Health System. PharmacoEconomics 2018;36(2):239–52. [DOI] [PubMed] [Google Scholar]
- [79].Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ 2018;27(4):746–61. [DOI] [PubMed] [Google Scholar]
- [80].Ochalek J, Lomas J, Claxton K. Estimating health opportunity costs in low-income and middle-income countries: a novel approach and evidence from cross-country data. BMJ Global Health 2018;3(6):e000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Woods B et al. Country-Level Cost-Effectiveness Thresholds: Initial Estimates and the Need for Further Research. Value in Health 2016;19(8):929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Edoka IP, Stacey NK. Estimating a cost-effectiveness threshold for health care decision-making in South Africa. Health Policy and Planning 2020;35 (5):546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Doubilet P et al. Probabilistic Sensitivity Analysis Using Monte Carlo Simulation: A Practical Approach. Med Decis Making 1985;5(2):157–77. [DOI] [PubMed] [Google Scholar]
- [84].Oakley JE, O’Hagan A. Probabilistic sensitivity analysis of complex models: a Bayesian approach. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2004;66(3):751–69. [Google Scholar]
- [85].Sinanovic E et al. The potential cost-effectiveness of adding a human papillomavirus vaccine to the cervical cancer screening programme in South Africa. Vaccine 2009;27(44):6196–202. [DOI] [PubMed] [Google Scholar]
- [86].Yang K-C et al. Cost-effectiveness analysis of universal influenza vaccination: Application of the susceptible–infectious–complication–recovery model. International Journal of Infectious Diseases 2018;73:102–8. [DOI] [PubMed] [Google Scholar]
- [87].Skedgel C et al. An Incremental Economic Evaluation of Targeted and Universal Influenza Vaccination in Pregnant Women. Can J Public Health 2011;102 (6):445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


