Abstract
Currently, it is unclear whether treating Helicobacter pylori (H. pylori) infection is safe among adolescents. This study aimed to evaluate the safety of H. pylori eradication therapy by examining gut microbiota changes in adolescents 3 months after the therapy. H. pylori-infected adolescents were enrolled in this study. Their stool samples were collected at the following three time points: before treatment, 1–2 days after completion of treatment, and time of eradication successful judgment. We assessed the relative abundance, alpha-diversity, and beta-diversity of the gut microbiota and adverse events. The number of isolated Actinobacteria decreased immediately after eradication therapy in the 16 students included in the study, and it returned to pretreatment condition at the eradication judgment point. There was no change in the relative abundance at genus level. The alpha-diversity was lost immediately after eradication therapy; however, it recovered at the time of eradication judgment, and it was restored to pretreatment condition. Meanwhile, none of the participants experienced serious adverse events. H. pylori eradication therapy is safe for adolescents with respect to gut microbiota changes associated with H. pylori eradication therapy. Therefore, further long-term evaluations of gut microbiota changes following eradication therapy are warranted.
Subject terms: Microbiology, Gastroenterology
Introduction
Helicobacter pylori (H. pylori) infection is generally established at the age of ≤ 5 years in Japanese adolescents1, and it causes atrophic gastritis in childhood2–5. H. pylori eradication therapy is effective in preventing gastric cancer if initiated immediately after infection. Because H. pylori eradication reduces the damage caused to the gastric mucosa6, it can reduce the risk of developing gastric cancer at adulthood7. Considering that the current infection route of H. pylori is person-to-person transmission, particularly within the same family1,8, young individuals must take measures against H. pylori infection from the viewpoint of preventing this infection in the next-generation. Considering these circumstances, as a new method of preventing gastric cancer, the screening and treatment of H. pylori infection among young individuals have been initiated as a primary prophylactic measure in Japan9–12.
In Japan, triple therapy with proton-pump inhibitor (PPI), amoxicillin, and clarithromycin is an effective first-line treatment for H. pylori infection in children13. In Japan, potassium-competitive acid blocker (P-CAB) may be used instead of PPI in adults7,14,15, and P-CAB may be used because of high frequency of CAM-resistance H. pylori, even in adolescents9,12,16. To date, H. pylori eradication therapy has no serious side effects in children9,12,16,17; however, there is a need to conduct a long-term evaluation. Therefore, the safety of H. pylori eradication therapy using PPI or P-CAB in adolescents remains controversial.
Sustained infection of H. pylori decreases and/or increases gastric acid secretion, which might affect the gastric microbiota in adults and children. Several reports have suggested that H. pylori infection significantly affects the intestinal microbiota18–21. Antibiotic agents administered for H. pylori are known to quantitatively and qualitatively alter the human gut microbiota22,23. It has been reported that, even in children, dysbiosis is directly linked to various systemic conditions such as allergic diseases, autism spectrum disorders, and inflammatory bowel diseases24–28. Considering that the antibiotic agents used to eradicate H. pylori can affect the intestinal microbiota, it is necessary to study the safety of eradication therapy for H. pylori. A previous study reported the effect of probiotics during vonoprazan-containing triple therapy on the intestinal microbiota in individuals with H. pylori infection29; however, the study only examined the intestinal microbiota before and immediately after eradication therapy. Thus, this study aimed to evaluate the safety of H. pylori eradication therapy by examining the gut microbiota changes 3 months after therapy in adolescents.
Results
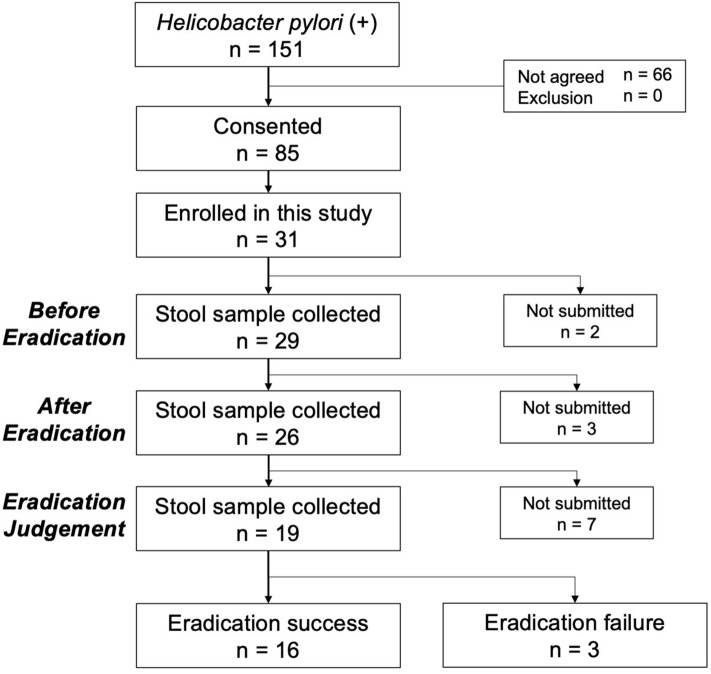
We enrolled students with two positive test results for H. pylori between June 2017 and March 2019. The flow of patient enrolment is presented in Fig. 1. Among the 151 patients with an H. pylori infection, 31 provided consent and participated in this study. There were 29 students who provided stool samples before eradication, 26 immediately after eradication, and 19 at the time of eradication judgment. Of these 19 patients, 3 experienced primary treatment failure; therefore, 16 (n = 9, male, n = 7, female; median age: 15.6 [15.1–15.9] years) were considered eligible for this study. This study was conducted as a sub analysis of H. pylori infection screening of junior high school third-year students in Saga Prefecture. Considering this, we could not thoroughly examine the background of the students. These students submitted their stool samples at each of the three time points. The H. pylori eradication assessments were conducted on median day 92.5 (range 89–107 days) after the eradication therapy, and the stool samples were collected on the median day 99 (range 91.5–108.5 days).
Figure 1.
Flow chart of patient enrollment.
Relative abundance
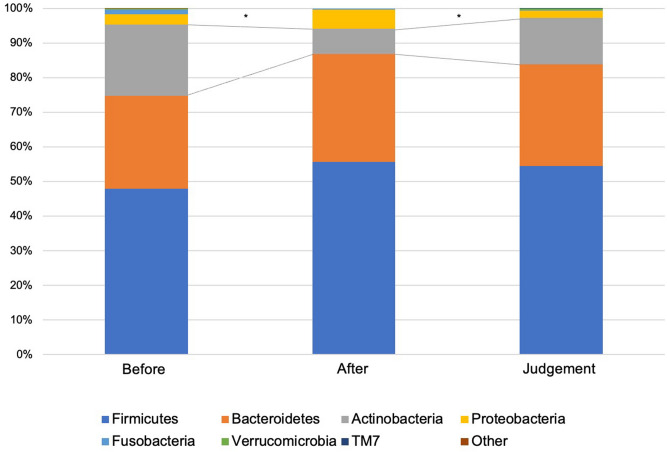
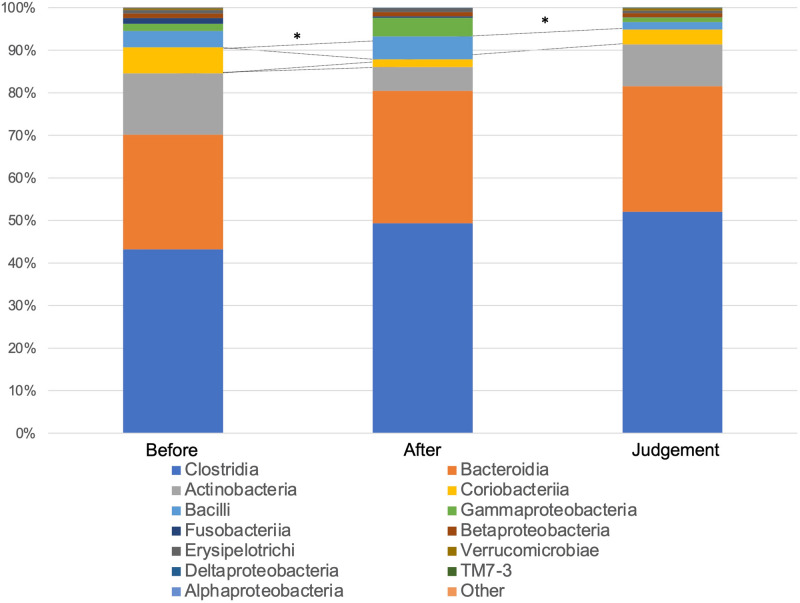
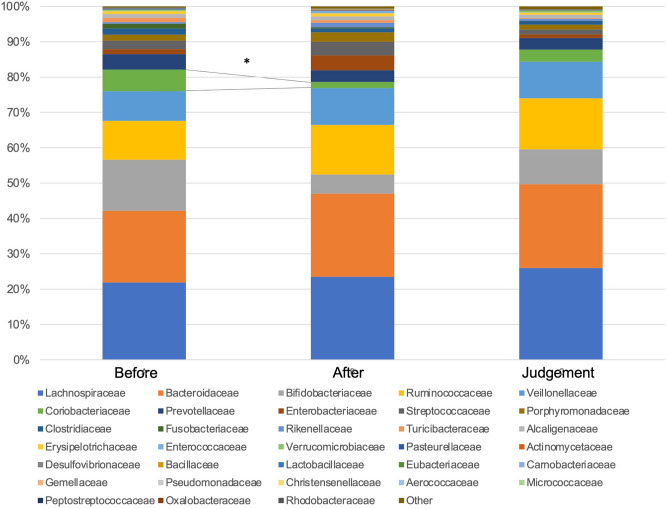
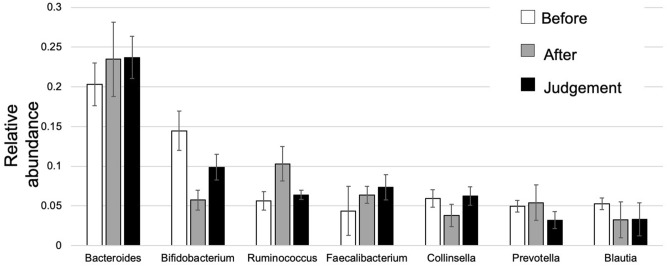
Figure 2 shows the compositions and relative abundance of the gut microbiota at the phyla level. The proportion of Actinobacteria significantly decreased after eradication therapy compared with before eradication therapy (P = 0.006924). On the contrary, the proportion of Actinobacteria significantly increased at the time of eradication assessment compared with after eradication therapy (P = 0.047065), and no significant difference was observed between before eradication therapy and at the time of eradication assessment (P = 0.214554). The compositions and relative abundance of the gut microbiota at the class level are shown in Fig. 3. Compared with before eradication therapy, the proportion of Coriobacteriia significantly decreased after eradication therapy (P = 0.003564) and eradication assessment (P = 0.025209). The compositions and relative abundance of the gut microbiota at the family level are shown in Fig. 4. Compared with before eradication therapy, the proportion of Coriobacteriaceae significantly decreased after eradication therapy (P = 0.008656). Figure 5 shows the top seven genus compositions of the relative abundance of gut microbiota. In each of the seven genuses, no significant changes were observed in the comparison results before eradication therapy, after eradication therapy, and during eradication judgment.
Figure 2.
Relative abundance of the intestinal microbiota at the phyla at the following three time points: before eradication therapy, after eradication therapy, and at the time of eradication therapy judgment.
Figure 3.
Relative abundance of the intestinal microbiota at the class level at the following three time points: before eradication therapy, after eradication therapy, and at the time of eradication therapy judgment.
Figure 4.
Relative abundance of the intestinal microbiota at the family level at the following three time points: before eradication therapy, after eradication therapy, and at the time of eradication therapy judgment.
Figure 5.
Relative abundance of the intestinal microbiota larger than 3% at the following three time points: before eradication therapy, after eradication therapy, and at the time of eradication therapy judgment.
Alpha-diversity
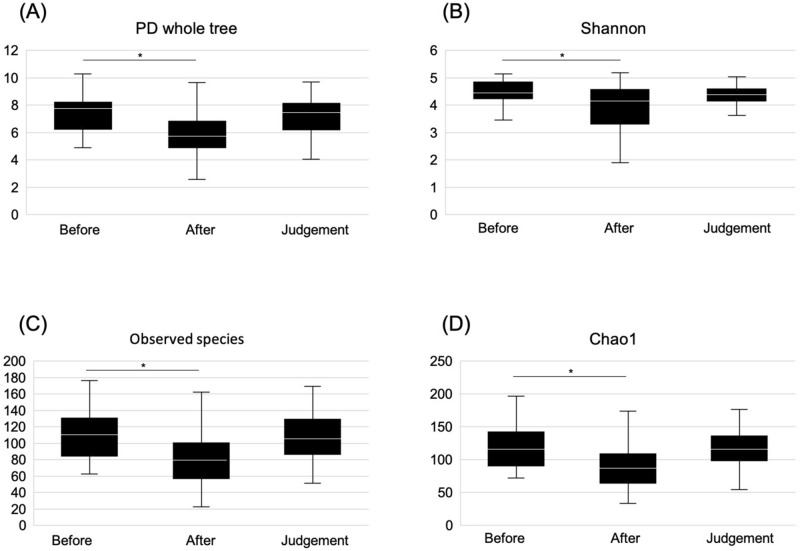
We calculated the α-diversity according to the PD whole tree, Shannon index, observed OTUs, and Chao1 at the three time points. Phylogenetic richness (PD whole tree; P = 0.003), diversity of microbiota (Shannon index; P = 0.023), and microbial species richness (observed OTUs; P = 0.003, Chao1; P = 0.001) after eradication therapy were significantly lower than those before eradication therapy. However, there were no significant differences between the PD whole tree (P = 1.000), Shannon index (P = 1.000), observed OTUs (P = 1.000), and Chao1 (P = 1.000) at the time of eradication judgment and those before eradication therapy. Moreover, the PD whole tree (P = 0.063), Shannon index (P = 0.492), observed OTUs (P = 0.106), and Chao1 (P = 0.061) at eradication judgment point did not significantly differ from those after eradication therapy (shown in Fig. 6).
Figure 6.
Boxplot depicting α-diversity before eradication therapy, after eradication therapy, and at the time of eradication judgment. (A) PD whole tree, (B) Shannon index, (C) Observed OTUs, and (D) PD whole tree. Comparison before and after eradication therapy (PD whole tree, P = 0.003; Shannon index, P = 0.023; observed OTUs, P = 0.003; and Chao1, P = 0.001). Comparison between before eradication therapy and at the time of eradication therapy judgment (PD whole tree, P = 1.000; Shannon index, P = 1.000; observed OTUs, P = 1.000; and Chao1, P = 1.000). Comparison between after eradication treatment and at the time of eradication therapy judgment (PD whole tree, P = 0.063; Shannon index, P = 0.492; observed OTUs, P = 0.106; and Chao1, P = 0.061).
Beta-diversity
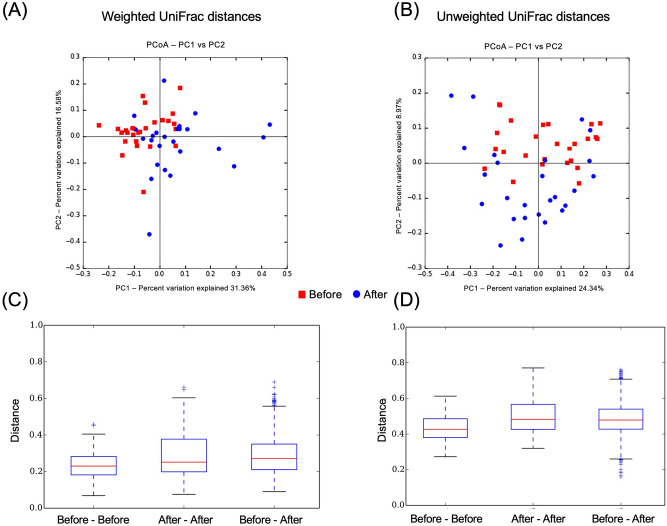
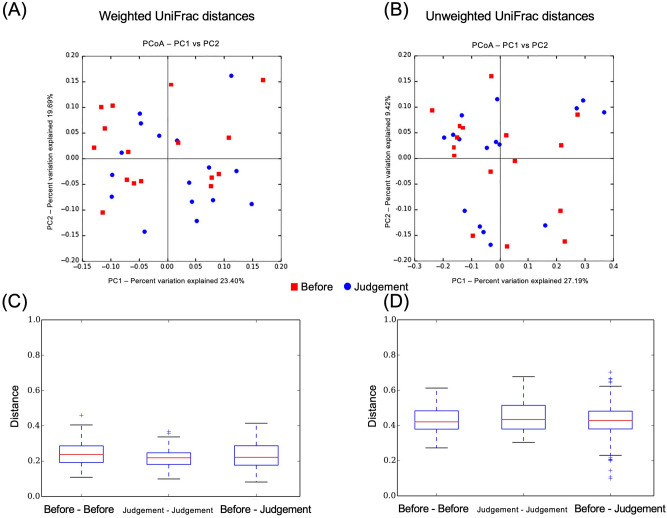
The β-diversity, calculated based on the weighted and unweighted UniFrac distances, revealed that the gut microbiota structures were similar before and after eradication therapy (weighted UniFrac distances: Before–Before vs. Before–After, P = 0.03; After–After vs. Before–After, P = 1.00. unweighted UniFrac distances: Before–Before vs. Before–After, P = 0.03; After–After vs. Before–After, P = 0.98) (shown in Fig. 7). Similarly, the β-diversity revealed that the gut microbiota structures were similar between before eradication therapy and judgment of eradication therapy (weighted UniFrac distances: Before–Before vs. Before–Judgment, P = 1.00; Judgment–Judgment vs. Before–Judgment, P = 1.00. unweighted UniFrac distances; Before–Before vs. Before–Judgment, P = 1.00; Judgment–Judgment vs. Before–Judgment, P = 0.87) (shown in Fig. 8).
Figure 7.
Principal coordinates analysis revealed clustered communities of gut microbiota after eradication in the before and after eradication therapy groups. (A) Weighted UniFrac distances, (B) Unweighted UniFrac distances. Bar diagram showing the Mean UniFrac distances for Before–Before, After–After, and Before–After participants. (C) Weighted UniFrac distances (Before–Before vs. Before–After, P = 0.03; After–After vs. Before–After, P = 1.00), (D) Unweighted UniFrac distances (Before–Before vs. Before–After, P = 0.03; After–After vs. Before–After, P = 0.98).
Figure 8.
Principal coordinates analysis revealed clustered communities of gut microbiota after eradication in the before and after eradication therapy groups. (A) Weighted UniFrac distances, (B) Unweighted UniFrac distances. Bar diagram showing the Mean UniFrac distances for Before–Before, Judgment–Judgment, and Before–Judgment participants. (C) Weighted UniFrac distances (Before–Before vs. Before–Judgment, P = 1.00; Judgment–Judgment vs. Before–Judgment, P = 1.00), (D) Unweighted UniFrac distances (Before–Before vs. Before–Judgment, P = 1.00; Judgment–Judgment vs. Before–Judgment, P = 0.87).
Adverse events
Among the 16 patients 1 (6.35%) presented with diarrhea and 2 (12.5%) presented with abdominal pain. None of the adverse events were serious. Therefore, therapy was not required, and treatment for the eradication of H. pylori was not interrupted.
Discussion
The present study revealed changes in the gut microbiota before and after H. pylori eradication therapy using 16S rRNA gene/DNA/amplicon sequencing with next-generation sequencing and amplicon analysis in Japanese adolescents. Two important clinical suggestions were obtained. First, change in the gut microbiota during eradication therapy of H. pylori with vonoprazan fumarate containing triple therapy in adolescents led to dysbiosis immediately after eradication therapy; however, it returned to pretreatment conditions 3 months after the eradication therapy (shown in Figs. 2 and 6). Second, the eradication therapy of H. pylori with vonoprazan fumarate containing triple therapy was found safe for children from the perspective of adverse events.
Yap TW et al. reported that the eradication of H. pylori caused perturbation of the gut microbiota, and it may indirectly affect the health of the patients30. By contrast, Gotoda T et al. reported that although H. pylori eradication therapy caused short-term dysbiosis, microbial diversity was restored in healthy Japanese adolescents31. To the best of our knowledge, this was the first study to evaluate the changes in the gut microbiota before and after H. pylori eradication therapy in adolescents. Moreover, α-diversity analysis revealed that species microbial richness and evenness were recovered to pretreatment levels at 2 months after eradication therapy. The proportion of Actinobacteria also significantly decreased immediately after eradication therapy, and it recovered similarly 2 months after eradication. Our results were considerably similar to those of a previous study (shown in Figs. 2 and 6)31. Cornejo-Pareja et al. observed a significant decrease in the number of Actinobacteria after the administration of omeprazole, clarithromycin, and amoxicillin in patients with H. pylori infection32. Our results also showed a decrease in Actinobacteria, which was due to a decrease in Coriobacteriia and Coriobacteriaceae at the class and family levels, respectively. We assumed that this occurred due to the effect of drug susceptibility to the antibiotics used in the eradication therapy, but we are unsure of the exact etiology. In the present study, the safety of eradication therapy among adolescents may be recommended with respect to gut microbiota because the dysbiosis of gut microbiota recovered after several months to pretreatment levels. Liou JM et al. reported that although the eradication therapy for H. pylori infection causes minimal disruption in the microbiota, their results support the long-term safety of H. pylori eradication therapy33.
Several studies showed that H. pylori infection in children caused the changes in the gut microbiota18,34–36. The diversity of intestinal microbiota is particularly reduced in adults with H. pylori infection37. Benavides-Ward et al. reported that children with H. pylori infection presented with an increase in the number of bacteria, such as Proteobacteria, Clostridium, Firmicutes, and Prevotella, in the gut microbiota34. However, information related to the relationship between H. pylori infection and intestinal bacteria included in the gut microbiota is still limited. Owing to the presence of differences in gut microbiota between H. pylori-infected and non-infected children, it cannot be denied that H. pylori infection affects children. From this perspective as well, H. pylori eradication in children appears to be necessary. To confirm the long-term safety of H. pylori eradication, future studies must be conducted to directly compare gut microbiota between the children who have remained healthy for a long time after H. pylori eradication therapy and healthy children without H. pylori infection.
The adverse events observed in our study included diarrhea and abdominal pain. However, these were not serious adverse events. The safety of eradication therapy of H. pylori with triple therapy containing vonoprazan fumarate for adolescents remains unclear; however, reports have shown no major problems in short-term evaluations9,12,16. In the present study, the number of cases was limited, and only adverse events during oral administration of antibacterial drugs and P-CAB were evaluated. Therefore, future studies with a larger sample size are warranted.
In Japan, the screening and treatment of H. pylori infection among adolescents are performed as a primary preventive measure against gastric cancer. By contrast, the clinical practice guidelines do not recommend screening and treatment for asymptomatic H. pylori-infected children to prevent gastric cancer38,39. However, these guidelines are for children and adolescents living in Europe and North America, and they may not be applicable to those living in other continents, particularly in developing countries with a high H. pylori infection rate and limited health care resources40. The safety of H. pylori screening and treatment for adolescents is debated in Japan13. According to the guidelines for the management of H. pylori infection in childhood, H. pylori eradication therapy for children younger than 15 years is not recommended owing to safety concerns. Our study showed that dysbiosis developed immediately after H. pylori eradication therapy (shown in Figs. 2 and 6). However, it returned to pretreatment condition after 3 months. This may be one factor for the safety of H. pylori eradication treatment. To prevent gastric cancer, H. pylori infection must be eradicated at a young age6. However, from the viewpoint of gastric cancer prevention, it is controversial whether the ideal period of H. pylori eradication is childhood or adulthood. It is certain that gastric mucosal atrophy associated with H. pylori infection is a high risk of well-differentiated gastric cancer41. Furthermore, gastric mucosal atrophy is known to occur since childhood42–45, and a small proportion of gastric cancers that occur in childhood46. Therefore, it seems unsafe to conclude that eradication in adulthood is absolutely sufficient to prevent gastric cancer. Although the risk of H. pylori reinfection after eradication therapy must also be considered, the reinfection rate is considered to be extremely low47–49. Moreover, the safety of H. pylori eradication therapy for adolescents must be further validated7,50.
This study had several limitations. First, the sample size was relatively small. Second, this study did not include placebo treatments for comparison. Third, the altered intestinal microbiota could be evaluated; the functionality of the bacteria itself and how it affected humans were also not evaluated. Intestinal microbiota analysis could not be performed by further analysis methods, such as UPGMA, LEfSe, and KEGG pathway. Fourth, because diet was important for microbiota evaluation, it was not possible to grasp the meal contents of each participant. However, because they were Japanese adolescents of almost the same age living in a single prefecture, it was presumed that there would be no major dietary differences.
H. pylori eradication therapy may be safe for adolescents with respect to gut microbiota. Therefore, further long-term evaluations of the changes in gut microbiota after eradication therapy must be performed. If the safety of H. pylori eradication therapy in adolescents is validated, it will promote screening and treatment among adolescents to prevent gastric cancer.
Materials and methods
Study design and participants
The present study performed a sub analysis of H. pylori infection screening as part of the junior high school health screening system among third-year students in Saga Prefecture9. Using local governmental grants, a screening and treatment program for eradicating H. pylori infection among third-grade junior high students was started in Saga Prefecture in 2016. The students underwent urinary anti-H. pylori antibody tests (RAPIRAN; Otsuka Pharmaceutical Co., Ltd. Tokyo, Japan), followed by H. pylori stool antigen tests (TESTMATE RAPID RYLORI ANTIGEN; Wakamoto Pharmaceutical Co., Ltd. Tokyo, Japan). To eradicate H. pylori infection, those who tested positive on both tests received triple therapy comprising 20 mg vonoprazan fumarate (Takeda Pharmaceutical Co., Ltd. Tokyo, Japan), 750 mg amoxicillin, and 200 mg clarithromycin twice a day for 7 days. No probiotics were added to the eradication treatment nor were they used during the subsequent follow-up period. Then, 8–12 weeks after the eradication therapy, 13C-urea breath test (13C-UBT) was conducted at one of the cooperating medical institutions to assess for treatment efficacy. Breath samples were obtained 4 h after a meal and 20 min after the ingestion of 100 mg 13C-urea (UBIT tablet, 100 mg). An infrared spectrometer (POC ONE; Otsuka Electronics Co., Ltd., Hirakata, Japan) was used for the test (negative ≤ 2.5%).
Students were excluded from receiving eradication therapy if they were allergic to any study drugs, had impaired liver or kidney function, were receiving colchicine, had infectious mononucleosis (or suspected infection with Epstein–Barr virus), or weighed < 30 kg. Students who had taken antibiotics or antacids (PPI, P-CAB, and histamine H2-receptor antagonist) within a year were excluded because of their effects on gut microbiota. The stool samples were collected prior to treatment (Before), 1–2 days after completion of treatment (After), and after 8–12 weeks of eradication therapy when it was determined that H. pylori had been eradicated (Judgment). At the time of judgment of eradication therapy, students with unsuccessful treatment outcomes were excluded to rule out the effect of the presence of H. pylori on the gut microbiota34. The outcome of H. pylori eradication therapy was confirmed using the 13C-UBT. The stools were immediately stored at − 20 °C until DNA extraction. Adverse events associated with the eradication therapy were collected through a self-report questionnaire during the eradication treatment and by interview with the attending physician during the follow-up period from the eradication treatment at 13C-UBT for eradication judgment.
The institutional review board of Saga University Hospital (approval numbers: 2017-03-01, approval date: June 5, 2017) approved the present study. As a sub analysis of the previous studies13, we examined the gut microbiota changes 3 months after therapy related to H. pylori eradication with vonoprazan fumarate containing triple therapy among adolescents. All methods were carried out in accordance with the relevant guidelines and regulations or the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants and their parents or guardians.
DNA extraction
The specimens were stored at − 20 °C until DNA extraction. Bacterial DNA was extracted using the NucleoSpin Microbial DNA kit (MACHERY-NAGEL, Düren, Germany) according to the manufacturer’s instructions. The total DNA was eluted in 50 μL of elution buffer and was stored at − 20 °C. The V3–V4 hypervariable regions of 16S ribosomal DNA (rDNA) were amplified using the 16S (V3–V4) Metagenomic Library Construction Kit for NGS (Takara Bio Inc., Kusatsu, Japan) with primer pairs (341F 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′, 806R 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT-3′). The amplicon was purified using AMPure XP magnetic beads (Beckman Coulter, Brea, CA, the USA). The index PCR was performed using the Nextera XT Index Kit (Illumina, San Diego, CA, the USA). After purification with AMPure XP beads, sequencing was conducted on a MiSeq platform with the MiSeq Reagent Kit v3 and Phix Control Kit v3 (Illumina) from Takara Bio Inc.
16 s rDNA sequence analysis
The 16S rRNA gene/DNA/amplicon sequencing results with next-generation sequencing were analyzed as follows: low-quality sequences were removed, chimeras were checked, operational taxonomic units (OTUs) were constructed, and taxonomy was assigned using CD-HIT-OTU, Quantitative Insights Into Microbial Ecology pipeline (http://qiime.org/). OTUs were established by clustering with a 97% identity threshold and were completed using an RDP classifier with the Green genes database. The observed OTUs, Chao1 (microbial species richness), and Shannon indices (microbial evenness) and PD whole tree (phylogenetic richness) were calculated to determine the alpha (α)-diversity of the microbiota in the samples. The beta (β)-diversity of our samples was calculated using the default beta-diversity metrics of the weighted and unweighted UniFrac distance. To compare the differences in the overall bacterial gut microbiota structure, a principal coordinates analysis was performed to reduce the dimensionality of the resulting distance.
Statistical analysis
The raw data were expressed as percentage, mean, and standard deviation whenever applicable. For skewed data, the Mann–Whitney U test and Wilcoxon signed-rank test were used to compare variables between and within groups, respectively. The comparison of α-diversity and group distance for β-diversity was performed using the Monte Carlo two-sample test, followed by the false discovery rate and Bonferroni correction. A P value < 0.05 was considered statistically significant. Statistical analyses of the microbiota were performed using Python. Other statistical analyses were performed using R (R Core Team 2018]. R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria; URL: https://www.R-project.org/).
Study endpoint
The study endpoint was the change in the intestinal microbiota during H. pylori eradication with vonoprazan-containing triple therapy. In particular, the relative abundance, α-diversity, and β-diversity of the gut microbiota were compared before eradication, immediately after eradication, and during assessment of treatment efficacy. In addition, the adverse effects of H. pylori eradication with vonoprazan-containing triple therapy in children were evaluated.
Acknowledgements
We would also like to thank the outpatient nurses and medical support staff at the joint research institutes. We are also grateful to Ms. Kozue Kakiuchi, Ms. Tomomi Ito, and Ms. Hiromi Beppu for providing support for the project. The Biofermin Pharmaceutical Co. Ltd. provided support for this study. However, this company was not involved in the analysis or interpretation of the test results. We would like to thank Enago (https://www.enago.jp) for editing and reviewing this manuscript for English language.
Abbreviations
- H. pylori
Helicobacter pylori
- PPI
Proton-pump inhibitor
- P-CAB
Potassium-competitive acid blocker
- rDNA
Ribosomal DNA
- OTUs
Operational taxonomic units
Author contributions
T.K. and M.O. conceptualized, analyzed data, and prepared the manuscript; T.K., K.Y., I.I., K.H., H.K., D.Y., and Y.F. collected data; and M.O. critically reviewed the manuscript. All authors approved the final version of the article, including the authorship list.
Funding
This study was supported by the Biofermin Pharmaceutical Co., Ltd. (Kobe, Japan).
Competing interests
TK received research funding from Biofermin Pharmaceutical Co., Ltd. The other authors declare no competing interests. This study was supported by the Biofermin Pharmaceutical Co., Ltd.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Konno M, et al. Five-year follow-up study of mother-to-child transmission of Helicobacter pylori infection detected by a random amplified polymorphic DNA fingerprinting method. J. Clin. Microbiol. 2005;43:2246–2250. doi: 10.1128/jcm.43.5.2246-2250.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozawa K, et al. Gastric epithelial cell turnover and mucosal protection in Japanese children with Helicobacter pylori infection. J. Gastroenterol. 2005;40:236–246. doi: 10.1007/s00535-004-1530-7. [DOI] [PubMed] [Google Scholar]
- 3.Brigic E, Hadzic D, Mladina N. Childhood and Coress model of carcinogenesis. Med. Arch. 2012;66:375–377. doi: 10.5455/medarh.2012.66.375-377. [DOI] [PubMed] [Google Scholar]
- 4.Boukthir S, et al. Chronic gastritis in children. Tunis Med. 2007;85:756–760. [PubMed] [Google Scholar]
- 5.Yu Y, Su L, Wang X, Wang X, Xu C. Association between Helicobacter pylori infection and pathological changes in the gastric mucosa in Chinese children. Intern. Med. 2014;53:83–88. doi: 10.2169/internalmedicine.53.0918. [DOI] [PubMed] [Google Scholar]
- 6.Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int. J. Cancer. 2013;132:1272–1276. doi: 10.1002/ijc.27965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato M, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 Revised Edition. Helicobacter. 2019;24:e12597. doi: 10.1111/hel.12597. [DOI] [PubMed] [Google Scholar]
- 8.Yokota S, et al. Intrafamilial, preferentially mother-to-child and intraspousal, Helicobacter pylori infection in Japan determined by mutilocus sequence typing and random amplified polymorphic DNA fingerprinting. Helicobacter. 2015;20:334–342. doi: 10.1111/hel.12217. [DOI] [PubMed] [Google Scholar]
- 9.Kakiuchi T, et al. A Helicobacter pylori screening and treatment program to eliminate gastric cancer among junior high school students in Saga Prefecture: a preliminary report. J. Gastroenterol. 2019;54:699–707. doi: 10.1007/s00535-019-01559-9. [DOI] [PubMed] [Google Scholar]
- 10.Kusano C, Gotoda T, Ishikawa H, Moriyama M. The administrative project of Helicobacter pylori infection screening among junior high school students in an area of Japan with a high incidence of gastric cancer. Gastric Cancer. 2017;20:16–19. doi: 10.1007/s10120-017-0688-7. [DOI] [PubMed] [Google Scholar]
- 11.Akamatsu T, et al. Introduction of an examination and treatment for Helicobacter pylori infection in high school health screening. J. Gastroenterol. 2011;46:1353–1360. doi: 10.1007/s00535-011-0450-6. [DOI] [PubMed] [Google Scholar]
- 12.Kaji E, et al. Helicobacter pylori test-and-treat strategy for second-year junior high school students aimed at the prevention of gastric cancer in Takatsuki City. Helicobacter. 2020 doi: 10.1111/hel.12696. [DOI] [PubMed] [Google Scholar]
- 13.Kato S, et al. The updated JSPGHAN guidelines for the management of Helicobacter pylori infection in childhood. Pediatr Int. 2020 doi: 10.1111/ped.14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakami K, et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65:1439–1446. doi: 10.1136/gutjnl-2015-311304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki S, Gotoda T, Kusano C, Iwatsuka K, Moriyama M. The efficacy and tolerability of a triple therapy containing a potassium-competitive acid blocker compared with a 7-day PPI-based low-dose clarithromycin triple therapy. Am. J. Gastroenterol. 2016;111:949–956. doi: 10.1038/ajg.2016.182. [DOI] [PubMed] [Google Scholar]
- 16.Kusano C, Gotoda T, Suzuki S, Ikehara H, Moriyama M. Safety of first-line triple therapy with a potassium-competitive acid blocker for Helicobacter pylori eradication in children. J. Gastroenterol. 2018;53:718–724. doi: 10.1007/s00535-017-1406-2. [DOI] [PubMed] [Google Scholar]
- 17.Okuda M, et al. Nationwide survey of Helicobacter pylori treatment for children and adolescents in Japan. Pediatr. Int. 2017;59:57–61. doi: 10.1111/ped.13038. [DOI] [PubMed] [Google Scholar]
- 18.Brawner KM, et al. Helicobacter pylori infection is associated with an altered gastric microbiota in children. Mucosal Immunol. 2017;10:1169–1177. doi: 10.1038/mi.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llorca L, et al. Characterization of the gastric microbiota in a pediatric population according to Helicobacter pylori status. Pediatr. Infect. Dis. J. 2017;36:173–178. doi: 10.1097/inf.0000000000001383. [DOI] [PubMed] [Google Scholar]
- 20.Oh B, et al. The effect of probiotics on gut microbiota during the Helicobacter pylori eradication: randomized controlled trial. Helicobacter. 2016;21:165–174. doi: 10.1111/hel.12270. [DOI] [PubMed] [Google Scholar]
- 21.Buhling A, Radun D, Muller WA, Malfertheiner P. Influence of anti-Helicobacter triple-therapy with metronidazole, omeprazole and clarithromycin on intestinal microflora. Aliment. Pharmacol. Ther. 2001;15:1445–1452. doi: 10.1046/j.1365-2036.2001.01033.x. [DOI] [PubMed] [Google Scholar]
- 22.Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut. 2016;65:1906–1915. doi: 10.1136/gutjnl-2016-312297. [DOI] [PubMed] [Google Scholar]
- 23.Rizzatti G, Ianiro G, Gasbarrini A. Antibiotic and modulation of microbiota: a new paradigm? J. Clin. Gastroenterol. 2018;52(Suppl 1):S74–S77. doi: 10.1097/mcg.0000000000001069. [DOI] [PubMed] [Google Scholar]
- 24.Pulikkan J, Mazumder A, Grace T. Role of the gut microbiome in autism spectrum disorders. Adv. Exp. Med. Biol. 2019;1118:253–269. doi: 10.1007/978-3-030-05542-4_13. [DOI] [PubMed] [Google Scholar]
- 25.Blázquez AB, Berin MC. Microbiome and food allergy. Transl. Res. 2017;179:199–203. doi: 10.1016/j.trsl.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vangay P, Ward T, Gerber JS, Knights D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe. 2015;17:553–564. doi: 10.1016/j.chom.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fattorusso A, Di Genova L, Dell'Isola GB, Mencaroni E, Esposito S. Autism spectrum disorders and the gut microbiota. Nutrients. 2019;11:521. doi: 10.3390/nu11030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017;14:573–584. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakiuchi T, et al. Effect of probiotics during vonoprazan-containing triple therapy on gut microbiota in Helicobacter pylori infection: a randomized controlled trial. Helicobacter. 2020 doi: 10.1111/hel.12690. [DOI] [PubMed] [Google Scholar]
- 30.Yap TW, et al. Helicobacter pylori eradication causes perturbation of the human gut microbiome in young adults. PLoS ONE. 2016;11:e0151893. doi: 10.1371/journal.pone.0151893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotoda T, et al. Gut microbiome can be restored without adverse events after Helicobacter pylori eradication therapy in teenagers. Helicobacter. 2018;23:e12541. doi: 10.1111/hel.12541. [DOI] [PubMed] [Google Scholar]
- 32.Cornejo-Pareja I, et al. H. pylori eradication treatment alters gut microbiota and GLP-1 secretion in humans. J. Clin. Med. 2019 doi: 10.3390/jcm8040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liou JM, et al. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. Lancet Infect. Dis. 2019;19:1109–1120. doi: 10.1016/s1473-3099(19)30272-5. [DOI] [PubMed] [Google Scholar]
- 34.Benavides-Ward A, et al. Helicobacter pylori and its relationship with variations of gut microbiota in asymptomatic children between 6 and 12 years. BMC Res. Notes. 2018;11:468. doi: 10.1186/s13104-018-3565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz C, et al. The active bacterial assemblages of the upper GI tract in individuals with and without Helicobacter infection. Gut. 2018;67:216–225. doi: 10.1136/gutjnl-2016-312904. [DOI] [PubMed] [Google Scholar]
- 36.Ye Q, Shao X, Shen R, Chen D, Shen J. Changes in the human gut microbiota composition caused by Helicobacter pylori eradication therapy: a systematic review and meta-analysis. Helicobacter. 2020;25:e12713. doi: 10.1111/hel.12713. [DOI] [PubMed] [Google Scholar]
- 37.Dash NR, Khoder G, Nada AM, Al Bataineh MT. Exploring the impact of Helicobacter pylori on gut microbiome composition. PLoS ONE. 2019;14:e0218274. doi: 10.1371/journal.pone.0218274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourke B, et al. Canadian Helicobacter Study Group Consensus Conference: update on the approach to Helicobacter pylori infection in children and adolescents—an evidence-based evaluation. Can. J. Gastroenterol. 2005;19:399–408. doi: 10.1155/2005/732369. [DOI] [PubMed] [Google Scholar]
- 39.Jones NL, et al. Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (update 2016) J. Pediatr. Gastroenterol. Nutr. 2017;64:991–1003. doi: 10.1097/mpg.0000000000001594. [DOI] [PubMed] [Google Scholar]
- 40.Koletzko S, et al. Evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children. J. Pediatr. Gastroenterol. Nutr. 2011;53:230–243. doi: 10.1097/MPG.0b013e3182227e90. [DOI] [PubMed] [Google Scholar]
- 41.Take S, et al. Baseline gastric mucosal atrophy is a risk factor associated with the development of gastric cancer after Helicobacter pylori eradication therapy in patients with peptic ulcer diseases. J. Gastroenterol. 2007;42(Suppl 17):21–27. doi: 10.1007/s00535-006-1924-9. [DOI] [PubMed] [Google Scholar]
- 42.Kato S, et al. Association between gastric atrophy and Helicobacter pylori infection in Japanese children: a retrospective multicenter study. Dig. Dis. Sci. 2006;51:99–104. doi: 10.1007/s10620-006-3091-5. [DOI] [PubMed] [Google Scholar]
- 43.Dimitrov G, Gottrand F. Does gastric atrophy exist in children? World J. Gastroenterol. 2006;12:6274–6279. doi: 10.3748/wjg.v12.i39.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ricuarte O, et al. Atrophic gastritis in young children and adolescents. J. Clin. Pathol. 2005;58:1189–1193. doi: 10.1136/jcp.2005.026310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kakiuchi T, Nakayama A, Shimoda R, Matsuo M. Atrophic gastritis and chronic diarrhea due to Helicobacter pylori infection in early infancy: a case report. Medicine. 2019;98:e17986. doi: 10.1097/md.0000000000017986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okuda M, et al. Gastric cancer in children and adolescents in Japan. Pediatr. Int. 2019;61:80–86. doi: 10.1111/ped.13720. [DOI] [PubMed] [Google Scholar]
- 47.Okimoto T, et al. Is the recurrence of Helicobacter pylori infection after eradication therapy resultant from recrudescence or reinfection in Japan. Helicobacter. 2003;8:186–191. doi: 10.1046/j.1523-5378.2003.00143.x. [DOI] [PubMed] [Google Scholar]
- 48.Take S, et al. Reinfection rate of Helicobacter pylori after eradication treatment: a long-term prospective study in Japan. J. Gastroenterol. 2012;47:641–646. doi: 10.1007/s00535-012-0536-9. [DOI] [PubMed] [Google Scholar]
- 49.Adachi M, et al. Reinfection rate following effective therapy against Helicobacter pylori infection in Japan. J. Gastroenterol. Hepatol. 2002;17:27–31. doi: 10.1046/j.1440-1746.2002.02666.x. [DOI] [PubMed] [Google Scholar]
- 50.Asaka M, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1–20. doi: 10.1111/j.1523-5378.2009.00738.x. [DOI] [PubMed] [Google Scholar]