Abstract
Late Onset Alzheimer’s Disease is the most common cause of dementia, characterized by extracellular deposition of plaques primarily of amyloid-β (Aβ) peptide and tangles primarily of hyperphosphorylated tau protein. We present data to suggest a noninvasive strategy to decrease potentially toxic Aβ levels, using repeated electromagnetic field stimulation (REMFS) in primary human brain (PHB) cultures. We examined effects of REMFS on Aβ levels (Aβ40 and Aβ42, that are 40 or 42 amino acid residues in length, respectively) in PHB cultures at different frequencies, powers, and specific absorption rates (SAR). PHB cultures at day in vitro 7 (DIV7) treated with 64 MHz, and 1 hour daily for 14 days (DIV 21) had significantly reduced levels of secreted Aβ40 (p = 001) and Aβ42 (p = 0.029) peptides, compared to untreated cultures. PHB cultures (DIV7) treated at 64 MHz, for 1 or 2 hour during 14 days also produced significantly lower Aβ levels. PHB cultures (DIV28) treated with 64 MHz 1 hour/day during 4 or 8 days produced a similar significant reduction in Aβ40 levels. 0.4 W/kg was the minimum SAR required to produce a biological effect. Exposure did not result in cellular toxicity nor significant changes in secreted Aβ precursor protein-α (sAPPα) levels, suggesting the decrease in Aβ did not likely result from redirection toward the α-secretase pathway. EMF frequency and power used in our work is utilized in human magnetic resonance imaging (MRI, thus suggesting REMFS can be further developed in clinical settings to modulate Aβ deposition.
Subject terms: Neurological disorders, Psychiatric disorders, Neuroscience, Alzheimer's disease
Introduction
In the United States alone, there are over 5.8 million individuals with AD, and numbers are expected to rise in parallel with life expectancy1. The number of people living with Alzheimer’s disease (AD) and other dementias worldwide was estimated at 46 million in 2015, with estimated prevalence reaching 131 million in 20502. The total estimated worldwide cost of dementia was $604 billion in 2010. Barring development of medical breakthroughs to prevent, slow down, or stop the disease, potential impacts on health, society, and the global economy will be enormous.
AD is a complex and heterogeneous disorder that includes both familial autosomal dominant early-onset (EOAD), and sporadic late-onset AD (LOAD); the latter being far more common2. Although age is the most closely associated factor, the specific etiology of LOAD, distinct from overall aging is presently unknown, several factors, including genetic, epigenetic, lifestyle and environment, are thought to be associated with AD3,4. AD is characterized by neuritic plaques of amyloid-β (Aβ) peptide, neurofibrillary tangles of hyperphosphorylated microtubule-associated protein τ, gliosis, neuroinflammation, and synaptic loss5–7. Aβ is cleaved sequentially from the Aβ precursor protein (APP) by β-secretase (BACE1) and γ-secretase complex. This “amyloidogenic” processing pathway is neurodegenerative. In contrast, the “anabolic” pathway wherein APP is processed first by one of the α-secretases, followed by γ-secretase activity, is neuroprotective and neurotrophic8–12.
Currently available treatments for AD have demonstrated limited efficacy. The drugs approved by the U.S. Food and Drug Administration (FDA) for the treatment of some symptoms of AD at best only improve them temporarily13, and their effectiveness varies across patients. Although none of the available treatments significantly alter progression of the disease, lessons from recent failed clinical drug trials have provided important clues and prompted researchers to reexamine some of these strategies, such as antibody treatment against Aβ or inhibition of BACE114,15. In addition to pharmacological approaches, researchers are examining alternative modalities to slow or halt the disease process. These include “holistic” approaches and lifestyle modifications that seek to improve diet, exercise, and social enrichment3.
Other non-pharmacological interventions for management of neuropsychiatric disorders, including AD, major depressive disorder and autism spectrum disorder16,17 include transcranial magnetic stimulation (TMS). TMS is approved for the treatment of treatment resistant depression. Given its tolerability, there is a growing interest to explore other potential applications and their mechanisms. Several well written reviews summarize TMS and other stimulation modalities18.
Recently a 2-month phase 1 clinical trial of electromagnetic exposure (915 MHz) to AD patients for 1 hour (h) twice a day found no deleterious behavioral effects, discomfort, or physiologic changes19. Such noninvasive, non-pharmacological approaches are inherently appealing if they were to improve cognition in AD. Active research in the use of noninvasive brain stimulation as a potential therapy for AD has included a number of pilot studies and small clinical trials that have highlighted the potential for neuroenhancement and improvement in cognitive function in healthy individuals via noninvasive brain stimulation (NBS)20. However, consensus is lacking on their mechanism of action, efficacy and reproducibility21–24.
Our goal is to explore the use of NBS, such as repeated electromagnetic field stimulation (REFMS), as a potential non-invasive strategy to lower Aβ peptide load observed in AD. The present work aims at studying the neurobiological effects of REFMS on neuronal cell viability, and its effect on levels of potentially toxic Aβ peptide in primary human brain cultures. Recent AD animal experiments suggest that REMFS could potentially be a disease modifying and safe strategy25–29. In mouse models REMFS at a frequency of 918 MHz27–29 and 1950 MHz25,26 protected against and reversed cognitive impairment by decreasing Aβ amyloid deposition. Also, other investigators have found that REMFS exposure attenuates tau phosphorylation in the hippocampus of AD mice30, thus suggesting beneficial in vivo effects of REMFS in age-related AD-like mouse pathology.
The influence of REMFS on biological systems entails thermal and non-thermal effects. Its thermal effect depends primarily on the specific absorption rates (SAR). Whereas its non-thermal biological effect occurs at the molecular level, and involves multitarget interactions between signaling pathways31–34, including those between EMF-DNA35, EMF-RNA36, in addition to changes in Ca2+ regulation37,38, channel activity39, enzyme activity40, nucleic acid synthesis41–43, and microRNA expression44–46. Other changes include free radical gene expression47–49, oxidative stress reduction50–54, heat shock response55, heat shock factor 1 activation56, and mTOR activation57. Likewise, other molecular effects are noteworthy, such as histone acetylation58, cell protection59, growth behavior60,61, ubiquitin–proteasome system activation62–64, autophagy-lysosome systems36, inflammation65–67, mitochondrial enhancement27, neuronal activity28. Particularly, in the context of our studies on the APP pathway, BACE1 mRNA reduction44, regulation of gene expression68, and epigenetic alterations69,70, are important.
Many different types of cells that respond to EMF exposures71,72, the present study adds primary human neurons and glia to the growing list, and attempts to establish the lowest SAR capable of producing potentially specific, non-thermal effects. As discussed in our mathematical model and computer simulation articles, we calculated the applied SAR to our cell cultures34,73, a 64 MHz frequency allowed us to minimize power needed to obtain the minimum SAR with biological effect, often called “MSBE”, permitting the use of an average SAR that was well below the permitted values of 2 W/kg (Watts per kilogram) set forth by the International Electrotechnical Commission (IEC). Thus, our conditions provide an established framework for safe human exposure (Table 1).
Table 1.
Electromagnetic frequencies and human tissue penetration77.
| Frequency | Depth of Penetration (cm) into various tissues | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin (Dry) | Fat | Muscle | Skeletal System | Nervous System | |||||||||
| (Bone) | CNS | Peripheral | |||||||||||
| Cancellous | Cortical | Marrow | Dura | Cerebellum | Gray Matter | White Matter | Spinal Cord | CSF | Nerve | ||||
| 64 MHz | 13.5 | 45.6 | 9.1 | 21.6 | 40.0 | 72.4 | 9.0 | 9.8 | 12.1 | 17.0 | 15.1 | 4.8 | 15.1 |
| 100 MHz | 10.5 | 40.0 | 7.7 | 18.1 | 34.3 | 62.1 | 7.3 | 7.6 | 9.7 | 13.6 | 12.4 | 3.9 | 12.4 |
| 918 MHz | 4.0 | 24.2 | 4.2 | 7.1 | 13.0 | 30.7 | 3.7 | 3.0 | 4.1 | 5.6 | 5.3 | 1.9 | 5.3 |
When applied experimentally within these parameters, REMFS resulted in significant reductions in levels of both Aβ40 and Aβ42 peptides, with possible little perturbation of cell culture vitality and health. Interestingly, no alterations in total processed APP levels, as measured by secreted APP-α (sAPPα) or total secreted APP (sAPP), were observed. A mechanism for this biophysical interaction currently remains unknown, but may involve increased Aβ degradation due to activation of several proteolytic pathways34,36,62–64,74,75. Alternatively, a complex readjustment of secretase activity may also play a role. EMF frequency and power used in our work is typical of that already utilized in human magnetic resonance imaging (MRI, thus suggesting REMFS can be further developed in appropriate animal models and clinical settings to modulate Aβ deposition. Our work is, thus, both mechanistic and translational, and would advance the field of neuroscience as well as AD.
Results
REMFS treatment was not toxic in primary human brain cells
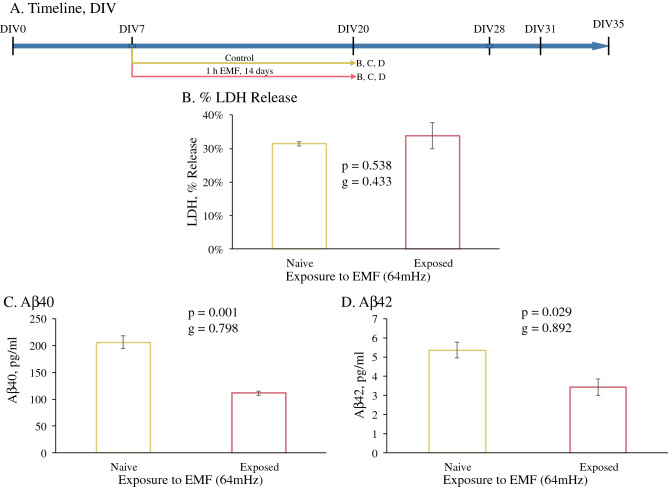
PHB cultures were utilized to investigate the effects on levels of potentially toxic secreted Aβ peptide. An example of PHB culture morphology and cell type distribution has been published76. PHB cultures were subjected to REMFS at 64 MHz with a SAR of 0.6 W/kg every day for 1 h in the TEM (Transversal Electromagnetic) cell chamber, which was performed initially in an incubator and after determining no significant difference, at room temperature (Fig. 1A). Cell membrane damage and integrity were measured by assaying lactate dehydrogenase (LDH) release into the conditioned medium (CM) in comparison to LDH present in cell lysates collected at the end of the experiment. We observed no significant difference in relative %LDH released between non-treated and REMFS-treated samples at DIV21 (14 days EMF treatment) (Fig. 1B).
Figure 1.
REMFS effects on cellular toxicity and Aβ40 and 42 levels. (A) Schedule of cell growth and treatments for data presented in figure. (B) REMFS treatment at 64 MHz with a SAR of 0.6 W/kg daily for 1 h for 14 days did not show significant cellular toxicity and/or membrane damage by LDH assay. (C) REMFS at 64 MHz with a SAR 0.6 W/kg daily for 1 h for 14 days reduced Aβ40 levels in PHB tissue culture conditioned media (p = 0.001). (D) REMFS reduced Aβ42 levels in PHB cell culture conditioned media (p = 0.029).
REMFS lowered Aβ40 and Aβ42 levels in PHB cultures
We measured levels of Aβ40 peptide in CM samples via ELISA after 14 day of exposure in treated and non- treated cultures, beginning at DIV 7 (Fig. 1A). The REMFS dose tested in this study, 64 MHz with a SAR of 0.6 W/kg, 1 h daily over a 14-day period, yielded a 46% decrease in Aβ40 levels in the three independent experiments examined (Fig. 1C, p = 0.001, g = 0.798), compared to the non-treated cultures. The same treatment produced a 36% reduction in Aβ42 levels (Fig. 1D, p = 0.029, g = 0.892).
Daily REMFS for 14 days at different lengths of exposure was non-toxic to cells and reduced Aβ40 and Aβ42 levels
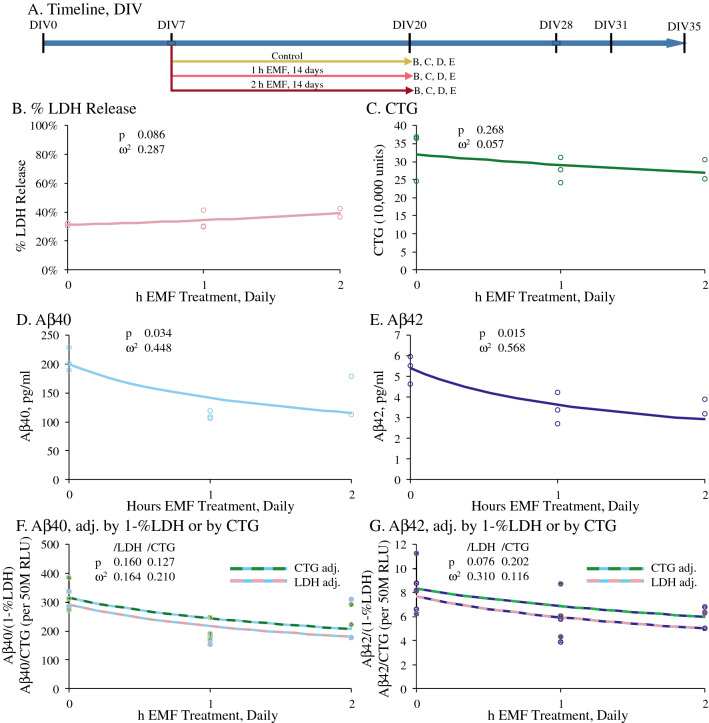
As per the timeline shown in Fig. 2A, we also examined if REMFS effects on cell viability (measured by the CellTiter Glo (CTG) assay) and toxicity (LDH) and on secretion of Aβ40 and Aβ42 peptides depended upon length of individual exposure sessions. We treated DIV 7 cultures at 64 MHz with a SAR of 0.4 W/kg, for 1 or 2 h, for 14 days, with exposures at 64 MHz and 100 MHz (100 MHz data not shown). CTG data shows no significant change in cell viability dependent on exposure time, while LDH was not significantly elevated (Fig. 2B,C). When Aβ40 and Aβ42 were assayed (Fig. 2D–E), we found that both levels were significantly reduced by REMFS treatment in a time dose-dependent fashion This relationship was not significant when adjusting by either 1-% LDH or CTG as an approximation of overall culture health (Fig. 2F,G). Visual examination of the plots suggested a possible diminishing returns trend, wherein dosage in excess of 1 h or higher than 64 MHz resulted in less optimal results. However, insufficient data points were generated to explicitly test non-linear models.
Figure 2.
REMFS effects on Aβ40 and Aβ versus different exposure times in human brain cultures. PHB cultures were treated for 1 or 2 h at 64 MHz with a SAR of 0.4 W/kg and secreted Aβ40 and Aβ42 were measured in CM by ELISA as described in the text. Data is presented as individual measurements and corresponding regression lines. (A) Schedule of cell growth and treatments for data presented in figure. (B) LDH assay showed that REMFS treatment did not cause cell toxicity. (C) CTG data showed no significant change in cell viability dependent on exposure time. (D) Aβ40 versus daily exposure time. Aβ40 significantly (p = 0.034) decreased as EMF exposure time increased. (E) Aβ42 versus daily exposure time. Aβ42 significantly (p = 0.015) decreased as EMF exposure time increased. (F) Aβ40 adjusted by %LDH or CTG. When adjusted by 1-%LDH or CTG, reduction of Aβ40 was not significant (p = 0.160 or 0.127, respectively) (G) Aβ42 adjusted by %LDH or CTG. When adjusted by 1-%LDH or CTG, reduction of Aβ42 was not significant (p = 0.076 or 0.202, respectively).
REMFS treatments after 7 days of differentiation did not alter sAPPα levels
PHB cultures at DIV7 were treated with 1-h daily REMFS at 64 MHz with a SAR of 0.4 W/kg for 14 days (Fig. 3A). On the 14th day of treatment, all conditioned media above the cells was replaced by fresh medium, and total soluble APPα levels were analyzed using ELISA. At exposure day 14, no significant changes in sAPPα levels were observed for the REMFS-treated culture (Fig. 3B).
Figure 3.
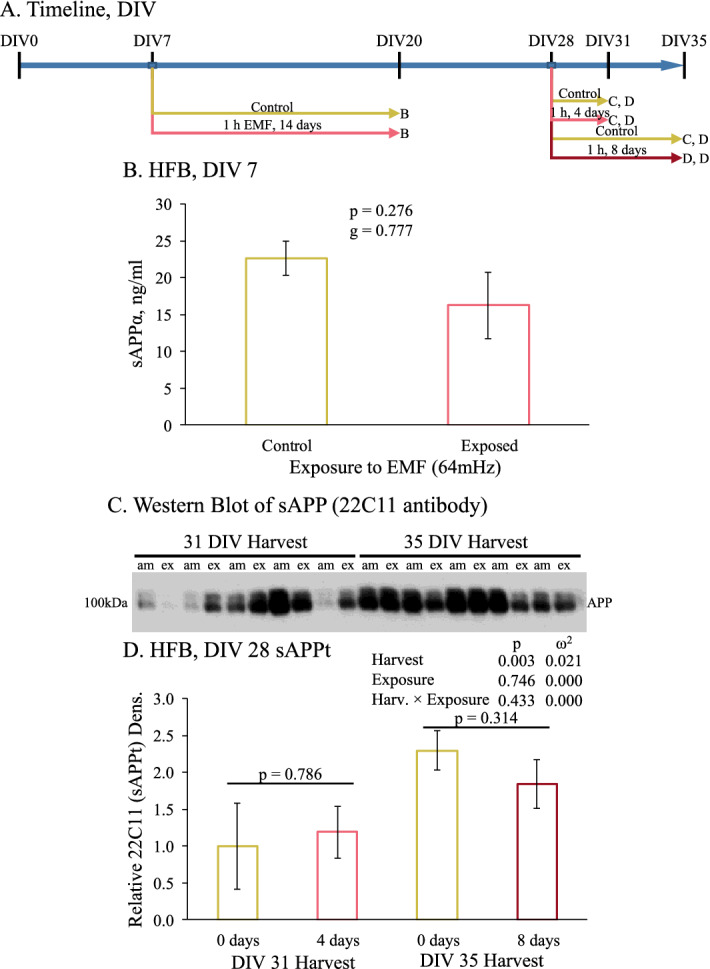
Effect of REMFS on levels of sAPPα and sAPP in human brain cultures at DIV7 and DIV28. Cultures were grown for 7 or 28 days and then exposed as described in the text for 14 (DIV7) or 4 or 8 days (DIV28), as described in the text. CM was assayed for levels of sAPP or sAPPα. sAPPα was measured by ELISA. Total sAPP was measured by semi-quantitative western blotting. (A) Schedule of cell growth and treatments for data presented in figure. (B) sAPPα was measured by ELISA of CM from PHB-DIV7 exposed to REMF for 14 day as described in the text. REMFS does not cause a significant change in levels of sAPPα. (C) Total sAPP was measured by semiquantitative western blotting of CM from PHB-DIV28 exposed to REMFS at 64 MHz with SAR of 0.9 W/kg for 4 or 8 days, as described in the text. (D) Analysis of blot densitometry revealed that, while the interval between 28 + 4 and 28 + 8 days significantly increased sAPP, there was no effect of REMFS treatment.
REMFS treatments after 28 days of differentiation alter did not alter total APP levels
PHB cultures at DIV 28 cultures were treated with 64 MHz with a SAR of 0.9 W/kg for 4 or 8 days but found no REMF treatment effect on total sAPP levels. As cultures aged overall sAPP increased, regardless of REMFS treatment (Fig. 3C,D).
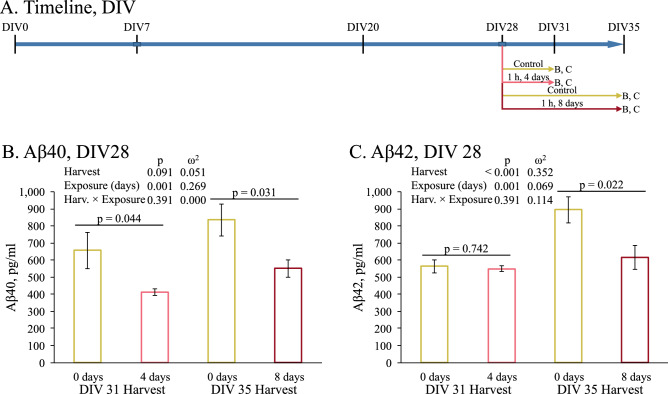
PHB cultures were allowed to grow for 28 days then exposed daily to REMFS at 64 MHz with a SAR of 0.9 W/kg for 1 hour (Fig. 4A). Notably, EMF exposure achieved a significant decrease in the Aβ40 levels (Fig. 4B). This difference was primarily due to length of treatment, as shown by 2-way glm that compared cell harvest at DIV 31 or 35 vs length of REMF exposure (0, 4, or 8 days). While longer additional growth resulted in greater overall Aβ40 (“Day” p = 0.048) and REMF exposure reduced Aβ40 levels on each day (p = 0.002), the extent of reduction was approximately the same regardless of exposure length (p = 0.799 for interaction). Aβ42 levels after 4 and 8 days of REMFS treatments had a different pattern, where culture age (Days) and REMF exposure significantly (p = 0.020) interacted. At day four we did not find any difference in Aβ42 levels between the REMFS exposed and control (Naïve) cultures (Fig. 4C). After 8 days of treatment the REMFS cultures showed a significant reduction of the Aβ42 levels compared to the ambient control culture (p = 0.022).
Figure 4.
Effect of REMFS on levels of Aβ40 and Aβ42 in human brain cultures at DIV28. Cultures were exposed to REMFS as described in the text (A) Schedule of cell growth and treatments for data presented in figure. (B) Both culture age (28 + 4 vs 28 + 8 days) and EMF treatment produced significant differences in Aβ40 levels. However, the effect of REMFS treatment was the same regardless of culture age. (C) Both culture age (28 + 4 vs 28 + 8 days) and REMFS treatment produced significant differences in Aβ42 levels. However, the effect of REMFS treatment significantly differed by culture age. Reduction only appeared in the oldest cultured cells.
Discussion
The current study is the first, to our knowledge, to show a potentially safe and effective strategy to decrease potentially toxic Aβ levels in primary human brain cultures through application of REMFS. Ultimately, our results revealed that REMFS at 64 MHz with a SAR of 0.4 W/K for 1 hour could reduce levels of secreted Aβ peptides. This minimal energy has valuable clinical implications for the treatment of Alzheimer’s patients, since higher energy levels would induce thermal injuries as well as other potential adverse effects77 (Table 1). Importantly, we also found that these treatments did not cause cellular toxicity in PHB cultures, as was noted through analysis of LDH levels.
Separate REMFS schedules were carefully studied to determine the degree of reduction of Aβ40 and Aβ42 levels in PHB cultures, and the treatment chamber and control cultures were maintained in an incubator at all times so as to prevent changes in temperature or environmental electromagnetic frequencies that could potentially alter outcomes. Results in CM samples revealed a 46% reduction of Aβ40 levels when cultures were subjected to REMFS at 64 MHz with a SAR of 0.6 W/kg daily for 1 hour for 14 days and a corresponding 36% reduction in Aβ42. Additional modifiable variables, such as exposure time and frequency were also considered, and the impact of these different EMF settings was studied relative to the reduction in Aβ40 and Aβ42 peptides levels.
While there are differences between mouse models and human tissues, it bears noting that REMFS studies with SAR of 0.25–1.05 W/kg (similar to our study with SAR values of 0.4–0.9 W/kg) reported decreased Aβ levels in older AD mouse models27–29. Therefore, we treated PHB cultures differentiated for 28 days to determine if REMFS also reduced Aβ levels in cells near the end of primary culture survival on the dish78,79. Results revealed REMFS at 64 MHz with SAR of 0.9 W/kg daily for 1 h after 4 and 8 days produced a significant reduction of Aβ40 levels in the media cultures. Interestingly, a SAR of 0.4 W/kg produced similar results, although a significant reduction of the Aβ42 levels was only noted at day 8. Nevertheless, an overall shorter treatment duration also reduced Aβ levels (4 or 8 vs. 14 or 21 days). This is an advance from our prior results following 21 days of exposure, leading us to believe that through additional fine tuning of REMFS settings in future, the desired biological effects of REMFS may ultimately be achieved after only a few treatments. Conveniently, effects on Aβ deposition could be measured early through analysis of several AD biomarkers, such as amyloid positronic emission tomography (PET), cerebrospinal fluid (CSF)-Aβ (42) and CSF tau levels in patients80. Our SAR calculations made specific assumptions (see Methods) regarding density and conductivity. These may not perfectly reflect the differences between brain in living patients vs. monolayer cell culture. Nevertheless, our work gives proof of concept that can be further refined by translational experiments.
Interestingly, we also found that REMFS did not cause a significant change in levels of the sAPPα or total sAPP in PHB cultures. Because sAPPβ (not assayed here) is a unique product of amyloidogenic processing, and sAPPα is a unique product of anabolic APP processing, our findings may suggest several interesting and testable hypotheses for the REMFS-mediated lowering of Aβ levels. One such hypothesis suggests the lowering of Aβ levels may be due to activation of Aβ degradation pathways34,36,62–64,81–83 rather than a reduction of APP expression. Other potential pathways could involve inflammation and microglial activation. However, the literature is not yet clear. Exposure of primary neurogenic cell cultures resulted in reduced microglial phagocytic ability and reduced axon lengths and branchpoints84. On the other hand, 8-month whole-body exposure of aged mice to REMF had no effects on oxidative stress, apoptosis, or microglia markers versus un-exposed animals85. However, the levels of sAPPα and sAPPβ were not compared side-by-side to determine relative changes. Given that sAPPα and total sAPP levels in our conditioned medium did not show a significant reduction, we expected the changes in this product would have pointed to the redirection of APP processing pathway selection86.
One such Aβ degradation pathway involves heat shock factor 1 (HSF1); however, it is speculative at this time. Some studies suggest that REMFS decreases Aβ production25,44. Given that decreased clearance of Aβ87 and loss of proteostasis due to age-related attenuation of the HSF1 pathway are early molecular events in LOAD88–91, one could expect that upregulation of the HSF1 pathway in senescent cells34 would increase levels of HSPs and chaperones that transport Aβ40 and Aβ42 to the proteasome for degradation, thereby reducing Aβ levels and potentially preventing or ameliorating AD92–95.
Overexpression of HSF1 significantly reduced Aβ levels in AD mouse models96. Additional evidence suggests REMFS may reactivate the HSF1 pathway and recover its proteostasis activity in senescent cells34, and organisms such as old AD mouse models28. REMFS may induce these effects by causing structural changes of heat-induced long non-coding RNA 1 (HSR1)83, which ultimately binds and activates HSF1 thereby increasing the expression of chaperones such as HSP70 that promote Aβ degradation97.
Interestingly, when human peripheral blood mononuclear cells derived from AD patients were exposed to pulsed EMF, upregulation of microRNA (miR)-107 and reduced levels of BACE1 mRNA were observed44. Also, whole-body exposure of rats to EMF upregulated miR-107 in brain tissues46. MiR-107has been shown to downregulate BACE1 translation98,99.
As mentioned, other electromagnetic stimulation methods may also be useful versus AD, but they would operate through different pathways than REMF. TMS, for example, induces an electric current that depolarizes neurons and trigger action potentials using a field strength of about 1 tesla (T), it allows stimulating the brain areas located up to 2 cm from its surface100. REMFS may not depolarize neurons. It radiates low energy coupled electromagnetic fields with non-thermal effects at a frequency of 64 MHz that activate intracellular biomolecules; it allows to stimulate the brain areas located up to 13.49 cm from its surface73. Secondly, TMS treatment significantly decreased levels of APP in AD mice treated with TMS: 67.1 ± 10.0% relative to non-treated mice, p < 0.05101. There were no significant changes in the APP levels in our REMFS experiments, also suggesting a different mechanism of decreasing Aβ aggregates; however, models are different.
Another type of non-pharmacological intervention is Deep Brain Stimulation (DBS), which also uses electrical currents for stimulation. DBS is a well-established neurosurgical technique used to treat neurological disorders such as Parkinson’s disease102,103. A recent study on the effects of the electrical stimulation on neural precursor cells found that there was a twofold increase in the neural stem cell pool and increase in neurogenesis under direct current stimulation of 250 mV/mm, these findings suggest a regenerative strategy to neural repair105. Finally, REMFS approach might complement within a broad context of other strategies, such as diazoxide, melatonin, resveratrol, and nanocurcumin, tested in different models105–107.
In short, precise mechanisms by which REMFS lowers Aβ levels in the PHB culture are unclear by any measure. Also, we recognize the limitation of our present work on several fronts. First, the number of samples is low (n = 3–4). This is partly due to the small area of the TCM chamber, which has limited room to accommodate several multi-well plates at the same time. Since we aimed at performing every experiment under identical conditions, we avoided doing experiments in batches with time intervals and then pooling samples. We were, thus, constrained regarding testing different conditions such as power, frequency and SAR settings. Therefore, future work with a redesigned large TCM chamber is needed. Second, we used primary human brain cultures throughout our experiments. This is an important innovation as primary culture derived from human fetal brain tissue is much closer to human AD than humanized transgenic animal models. We must note that PHB has become a regulatory challenge. Third, we could not measure several other proteins, such as HSTF1 (which requires nuclear extracts), as discussed in the text, due to the low amount of proteins derived from each well of the dish containing primary neurons. Nevertheless, the preliminary results from this experiment would encourage other investigators of the field to move this idea further. If REMFS produces the effects we observed on Aβ primarily through increased protein turnover, our findings could have implications for the treatment of other protein-associated neurodegenerative disorders associated with aberrant protein accumulations108. On the other hand, there is also a possibility of an AD-specific mechanism44,109. Finally, given the multitarget nature of REMFS, a synergistic modulation of both pathways is possible, as evidenced in previous studies.
Methods
Culture of primary human brain (PHB) cells
The protocol was approved by the Indiana University School of Medicine Institutional Review Board (IRB) and complied with state and federal regulations. Primary cultures of mixed human fetal brain cells were prepared from the brain parenchyma of aborted fetuses (80–110 days gestational age), as described previously76. The tissues were obtained from the Laboratory of Developmental Biology, University of Washington, Seattle, WA, after shipping overnight in chilled Hibernate-E medium (Invitrogen) supplemented with B27 (Invitrogen), GlutaMAX (Invitrogen), and antibiotic/antimycotic solution (Cellgro). All samples were collected under the supervision of the IRB of the University of Washington, which collected and keeps on file all appropriate informed consent. The meninges and blood vessels were stripped off; the brain tissue was washed in minimum essential medium and enzymatically dissociated by incubation in 0.05% Trypsin- 0.53 mM EDTA solution at 37 °C in a shaking water bath set to 150RPM. Tissue was subsequently mechanically dissociated by trituration through a siliconized (Sigma-Cote; Sigma-Aldrich, St Louis, MO), fire-polished Pasteur pipette.
Cells were then centrifuged at 800×g for 10 min, resuspended and seeded at an initial density of 2.2 × 105 cells/cm2 in Neurobasal (plus GlutaMAX, B27, antibiotic cocktail, normocin, bFGF) and allowed to attach overnight in poly-d-lysine (PDL) coated 24-well tissue culture plates. The following day, media and non-cellular debris were aspirated from the plate and media replaced with Neurobasal medium (Invitrogen), supplemented with 1× B27, 0.5 mM GlutaMAX, 5 ng/ml basic FGF (Invitrogen), and antibiotic/antimycotic mixture. Half-media changes were performed every 3rd day of culture. PHB cultures have been shown previously to comprise approximately 60 to 70% neurons with 30 to 40% mixed glial cells and have been established as a physiological model for growth of neurons and supporting cells79. In these cultures there is initially rapid neuronal growth, followed by a plateau/small decline before marked decline coupled with gliosis at 70 to 84 days in vitro76. Sample sizes were chosen to provide adequate power based on our prior work with PHB cultures110. Post-treatment as indicated, conditioned media was collected by pipette and stored, cells were washed with 1× PBS and lysed using 100 µL of Mammalian protein extraction reagent (M-Per, Life technologies) containing one tablet of protease inhibitor cocktail (Roche). The cell lysate was centrifuged for 10 mins at 30,000g and the supernatant was collected and used for further assays.
Electromagnetic field exposures and treatment conditions
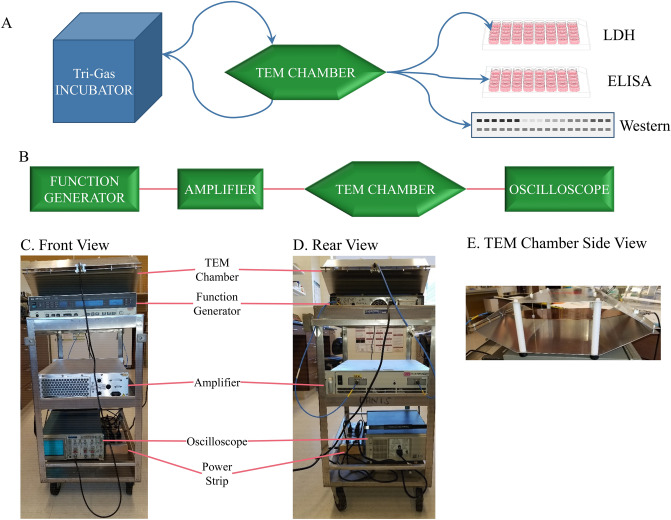
Electromagnetic field exposures were carried out using a vertically-mounted IFI TEM Cell (Transversal Electromagnetic Cell, model CC110-SPEC, DC to 1000 MHz, Test Equipment Corporation, Mountain View, CA, IFI Ronkonkoma NY). This chamber is an expanded coaxial transmission line operating in the TEM mode, consisting of a main rectangular waveguide that contains a flat-metal-strip center conductor located in the middle between the top and bottom walls. The wall and center conductor are tapered at both ends to provide 50-Ω impedance along the entire length of the chamber. One port was connected to the RF source (HP 8656B/57A/57B synthesized signal generator) via coaxial cable and the other end to a matched load impedance of 50-ohms (provided by an oscilloscope), which is the characteristic impedance to mimic free space or plane wave irradiation. The complete array was mounted on a compact and portable cart (Fig. 5). The wave impedance throughout the chamber is the 377-ohms intrinsic impedance of free space34,111. We used the constants of 1030 kg/m3 for density of culture medium and 1.15 S/m for conductivity (derived from brain tissues) for our calculation of SAR values34,73.
Figure 5.
REMFS experiment workflow and apparatus. (A) Workflow began with culturing cells in tri-gas incubator, alternating with treatments in TEM chamber. After final treatments, cells were processed and extracts used for analysis by LDH (cell death), ELISA (Aβ) and western blotting (APP). (B) Schematic diagram illustrating the
source of the electromagnetic fields (function generator). The signal is then sent through an amplifier, then through the TEM chamber. Signal is monitored through the TEM chamber with an oscilloscope. (C) Front view photograph of a compact and convenient equipment system. The TEM chamber, being very light, rests upon the function generator. The next lower shelf holds the amplifier. The oscilloscope rests on the bottom shelf. Appropriate cables link each component. Power is supplied by a permanently affixed power strip on the bottom cart shelf. (D) Rear view of the compact cart setup. (E) Side view of the TEM chamber, showing shelf running across middle.
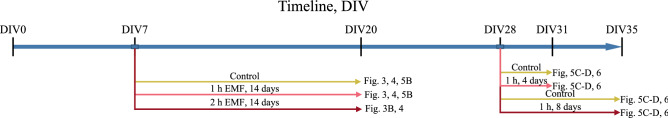
PHB cultures were subjected to REMFS (Fig. 6) at 64 MHz and 100 MHz, with different times (1 or 2 h) and exposure schedules (daily for 4, 8, or 14 days). We used power levels of 0.125, 0.5, and 1 Watts for our experiments with SAR of 0.4, 0.6, and 0.9, respectively. Levels of Aβ were measured in conditioned medium (CM) samples after 4, 8, 14, and 21 days of exposure in treated and control cultures. Temperature and SAR exposures were derived from computer simulations in our recent paper73. The 14- and 21-day exposures were performed on PHB cultures beginning DIV 7, while 4- and 8-day exposures were on HFB cultures beginning DIV28.
Figure 6.
Schedule of treatments of tissue cultures by REMFS. Our study used different aged cultures (measured by days in vitro, DIV), different exposure times per exposure, and different days of repeated exposure. Figure keys each combination to specific results figures (Fig. 3–4). DIV0 is day tissues were triturated and initially seeded.
ELISA of Aβ40 and Aβ42 peptides
Levels of Aβ40 and Aβ42 were measured using specific ELISA kits obtained from IBL (catalogue #s 27713 and 27711, respectively), and the assay was performed as per the manufacturer’s protocol. Briefly, an equal volume (50 μl) of conditioned medium was added onto the well, which was pre-coated with monoclonal anti-human Aβ (35–40) antibody (clone 1A10) for Aβ40 or polyclonal rabbit IgG to Aβ (38–42) for Aβ42 and incubated overnight. HRP-conjugated monoclonal anti-human Aβ (11–28, clone 12B2) or mouse polyclonal anti-Aβ (11–28) were used as detection antibodies for Aβ40 or Aβ42, respectively78,112,113. The assays can detect as low as 5 pg/ml of Aβ40 or 4 pg/ml of Aβ42 in a typical culture sample with cross-type reactivities (Aβ 40 vs. 42) of < 0.2%. Absolute Aβ values (pg/ml of CM) were measured and corrected for well-to-well variations in cell number by either normalizing to total protein as measured by BCA. We read colorimetric signals of all ELISAs at 450 nm on a microplate reader (BioRad, Model 550).
Determination of cellular toxicity and viability
LDH enzyme is a cytosolic component of the glycolytic pathway, and leakage from the cytoplasm into the cell culture medium is an indication of membrane permeability, which results from cellular toxicity. The CTG assay measures ATP presence by luminescent reaction. ATP is taken as an indicator of cell viability. For LDH, after 14 days of REMFS treatments, CM samples (50 µl) were collected from treated and control cultures. To determine cellular toxicity and/or membrane damage, LDH was measured in the CM as well as cell lysate samples using the Tox-7 kit (Sigma-Aldrich, St. Louis, MO). Leakage of cytosolic LDH enzyme from the membrane would indicate toxicity and membrane damage113. For CTG, after 14 days of REMFS treatments, cells from the same wells used for LDH and other measurements of conditioned media were harvested and lysed in M-PER buffer, clarified, and lysates used for CTG (Promega G7570) assay and measured with Glomax luminometer.
Statistical analyses
Data are presented as means ± SEM. We performed hypothesis testing with generalized linear models (glm) followed by Dunnett’s test or (Šidak-protected) Student’s t test, as appropriate and considered p ≤ 0.05 to indicate statistical significance. In addition, we calculated Hedge’s g or ω2 as appropriate for standardized effect sizes114. Further testing by second-order polynomial models was performed if the second-order Akaike information criterion (AICc) for a model with an orthogonal polynomial.
Supplementary information
Acknowledgements
We sincerely thank Jason Bailey for performing some experiments reported here, when he was working in the lab. Likewise, we thank Balmiki Ray and Justin Long for their help. We also acknowledge inputs from Joseph Bandeira and Maher Rizkalla (Indiana University).
Abbreviations
- AD
Alzheimer’s disease
- APP
Aβ precursor protein
- Aβ
Amyloid-β
- BACE1
Beta-site APP cleaving enzyme 1 or β-Secretase
- CM
Conditioned medium
- CSF
Cerebrospinal fluid
- EOAD
Early-onset
- FDA
Food and Drug Administration
- PHB
Primary human brain
- HSF1
Heat shock factor 1
- IEC
International Electrotechnical Commission
- IRB
Institutional Review Board
- LDH
Lactate dehydrogenase
- LOAD
Late Onset Alzheimer’s Disease
- MRI
Magnetic resonance imaging
- MSBE
Minimum SAR with biological effect
- NBS
Noninvasive brain stimulation
- PET
Positronic emission tomography
- REMFS
Repeated electromagnetic field stimulation
- sAPPα
Secreted Aβ precursor protein-α
- SAR
Specific absorption rate
- TEM
Transversal electromagnetic
- TMS
Transcranial magnetic stimulation
- τ
Microtubule-associated protein tau
Author contributions
F.P.P. performed study design and manuscript editing. N.C. performed study design, carried out cell culture experiments and manuscript editing. J.J.M. built and set up experimental devices. B.M. performed data analysis and manuscript writing. D.K.L. planned and performed study design and manuscript writing, editing and submission.
Funding
DKL was supported by National Institute on Aging (US NIH) and Indiana Alzheimer Disease Center (IADC).
Data availability
Data is available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-77808-2.
References
- 1.Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement.16, 391–460. 10.1002/alz.12068 (2020).
- 2.Prince M, et al. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75 e62. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Maloney B, Lahiri DK. Epigenetics of dementia: Understanding the disease as a transformation rather than a state. Lancet Neurol. 2016;15:760–774. doi: 10.1016/S1474-4422(16)00065-X. [DOI] [PubMed] [Google Scholar]
- 4.Lahiri DK, Maloney B, Zawia NH. The LEARn model: An epigenetic explanation for idiopathic neurobiological diseases. Mol. Psychiatry. 2009;14:992–1003. doi: 10.1038/mp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom GS. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 6.Terry RD, et al. Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 7.Nilson AN, et al. Tau oligomers associate with inflammation in the brain and retina of tauopathy mice and in neurodegenerative diseases. J. Alzheimer's Dis. 2017;55:1083–1099. doi: 10.3233/JAD-160912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saftig P, Lichtenthaler SF. The alpha secretase ADAM10: A metalloprotease with multiple functions in the brain. Prog. Neurobiol. 2015;35:1–20. doi: 10.1016/j.pneurobio.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Siopi E, et al. Etazolate, an alpha-secretase activator, reduces neuroinflammation and offers persistent neuroprotection following traumatic brain injury in mice. Neuropharmacology. 2013;67:183–192. doi: 10.1016/j.neuropharm.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Obregon D, et al. Soluble amyloid precursor protein-alpha modulates beta-secretase activity and amyloid-beta generation. Nat. Commun. 2012;3:777. doi: 10.1038/ncomms1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey JA, Ray B, Greig NH, Lahiri DK. Rivastigmine lowers Abeta and increases sAPPalpha levels, which parallel elevated synaptic markers and metabolic activity in degenerating primary rat neurons. PLoS ONE. 2011;6:e21954. doi: 10.1371/journal.pone.0021954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng J, et al. Soluble amyloid precursor protein alpha inhibits tau phosphorylation through modulation of GSK 3β signaling pathway. J. Neurochem. 2015;135:630–637. doi: 10.1111/jnc.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mielke MM, et al. Effects of food and drug administration-approved medications for Alzheimer's disease on clinical progression. Alzheimers Dement. 2012;8:180–187. doi: 10.1016/j.jalz.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahiri DK, Maloney B, Long JM, Greig NH. Lessons from a BACE1 inhibitor trial: Off-site but not off base. Alzheimers Dement. 2014;10:S411–419. doi: 10.1016/j.jalz.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahiri DK, Ray B. Intravenous immunoglobulin treatment preserves and protects primary rat hippocampal neurons and primary human brain cultures against oxidative insults. Curr. Alzheimer Res. 2014;11:645–654. doi: 10.2174/1567205011666140812113851. [DOI] [PubMed] [Google Scholar]
- 16.Perera T, et al. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9:336–346. doi: 10.1016/j.brs.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberman LM, et al. Transcranial magnetic stimulation in autism spectrum disorder: Challenges, promise, and roadmap for future research. Autism Res. 2016;9:184–203. doi: 10.1002/aur.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freitas C, Mondragon-Llorca H, Pascual-Leone A. Noninvasive brain stimulation in Alzheimer's disease: Systematic review and perspectives for the future. Exp. Gerontol. 2011;46:611–627. doi: 10.1016/j.exger.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arendash G, et al. A clinical trial of transcranial electromagnetic treatment in Alzheimer’s disease: Cognitive enhancement and associated changes in cerebrospinal fluid, blood, and brain imaging. J. Alzheimer's Dis. 2019;71:57–82. doi: 10.3233/JAD-190367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brem AK, Fried PJ, Horvath JC, Robertson EM, Pascual-Leone A. Is neuroenhancement by noninvasive brain stimulation a net zero-sum proposition? NeuroImage. 2014;85(Pt 3):1058–1068. doi: 10.1016/j.neuroimage.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderkova L, Rektorova I. Cognitive effects of repetitive transcranial magnetic stimulation in patients with neurodegenerative diseases - clinician's perspective. J. Neurol. Sci. 2014;339:15–25. doi: 10.1016/j.jns.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 22.Santarnecchi E, et al. Frequency-dependent enhancement of fluid intelligence induced by transcranial oscillatory potentials. Curr. Biol. 2013;23:1449–1453. doi: 10.1016/j.cub.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Martis B, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin. Neurophysiol. 2003;114:1125–1132. doi: 10.1016/S1388-2457(03)00046-4. [DOI] [PubMed] [Google Scholar]
- 24.Hausmann A, et al. No deterioration of cognitive performance in an aggressive unilateral and bilateral antidepressant rTMS add-on trial. J. Clin. Psychiatry. 2004;65:772–782. doi: 10.4088/JCP.v65n0608. [DOI] [PubMed] [Google Scholar]
- 25.Jeong YJ, et al. 1950 MHz electromagnetic fields ameliorate abeta pathology in Alzheimer's disease mice. Curr. Alzheimer Res. 2015;12:481–492. doi: 10.2174/156720501205150526114448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Son Y, et al. 1950 MHz radiofrequency electromagnetic fields do not aggravate memory deficits in 5xFAD mice. Bioelectromagnetics. 2016;37:391–399. doi: 10.1002/bem.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arendash GW, et al. Electromagnetic treatment to old Alzheimer's mice reverses beta-amyloid deposition, modifies cerebral blood flow, and provides selected cognitive benefit. PLoS ONE. 2012;7:e35751. doi: 10.1371/journal.pone.0035751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arendash GW. Transcranial electromagnetic treatment against Alzheimer's disease: Why it has the potential to trump Alzheimer's disease drug development. J. Alzheimer's Dis. 2012;32:243–266. doi: 10.3233/JAD-2012-120943. [DOI] [PubMed] [Google Scholar]
- 29.Arendash GW, et al. Electromagnetic field treatment protects against and reverses cognitive impairment in Alzheimer's disease mice. J. Alzheimers Dis. 2010;19:191–210. doi: 10.3233/JAD-2010-1228. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y, et al. Long-term exposure to ELF-MF ameliorates cognitive deficits and attenuates tau hyperphosphorylation in 3xTg AD mice. Neurotoxicology. 2016;53:290–300. doi: 10.1016/j.neuro.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Leszczynski D, Nylund R, Joenvaara S, Reivinen J. Applicability of discovery science approach to determine biological effects of mobile phone radiation. Proteomics. 2004;4:426–431. doi: 10.1002/pmic.200300646. [DOI] [PubMed] [Google Scholar]
- 32.Pall ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell Mol. Med. 2013;17:958–965. doi: 10.1111/jcmm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao S, Henderson AS. Regulation of c-fos is affected by electromagnetic fields. J. Cell. Biochem. 1996;63:358–365. doi: 10.1002/(SICI)1097-4644(19961201)63:3<358::AID-JCB11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 34.Perez FP, Zhou X, Morisaki J, Jurivich D. Electromagnetic field therapy delays cellular senescence and death by enhancement of the heat shock response. Exp. Gerontol. 2008;43:307–316. doi: 10.1016/j.exger.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Lin H, Blank M, Rossol-Haseroth K, Goodman R. Regulating genes with electromagnetic response elements. J. Cell. Biochem. 2001;81:143–148. doi: 10.1002/1097-4644(20010401)81:1<143::AID-JCB1030>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Marchesi N, et al. Autophagy is modulated in human neuroblastoma cells through direct exposition to low frequency electromagnetic fields. J. Cell. Physiol. 2014;229:1776–1786. doi: 10.1002/jcp.24631. [DOI] [PubMed] [Google Scholar]
- 37.Conti P, et al. A role for Ca2+ in the effect of very low frequency electromagnetic field on the blastogenesis of human lymphocytes. FEBS Lett. 1985;181:28–32. doi: 10.1016/0014-5793(85)81107-8. [DOI] [PubMed] [Google Scholar]
- 38.Rozek RJ, Sherman ML, Liboff AR, McLeod BR, Smith SD. Nifedipine is an antagonist to cyclotron resonance enhancement of 45Ca incorporation in human lymphocytes. Cell Calcium. 1987;8:413–427. doi: 10.1016/0143-4160(87)90025-X. [DOI] [PubMed] [Google Scholar]
- 39.Fesenko EE, Geletyuk VI, Kazachenko VN, Chemeris NK. Preliminary microwave irradiation of water solutions changes their channel-modifying activity. FEBS Lett. 1995;366:49–52. doi: 10.1016/0014-5793(95)98629-W. [DOI] [PubMed] [Google Scholar]
- 40.Byus CV, Pieper SE, Adey WR. The effects of low-energy 60-Hz environmental electromagnetic fields upon the growth-related enzyme ornithine decarboxylase. Carcinogenesis. 1987;8:1385–1389. doi: 10.1093/carcin/8.10.1385. [DOI] [PubMed] [Google Scholar]
- 41.Liboff AR, Williams T, Jr, Strong DM, Wistar R., Jr Time-varying magnetic fields: Effect on DNA synthesis. Science. 1984;223:818–820. doi: 10.1126/science.6695183. [DOI] [PubMed] [Google Scholar]
- 42.Grundler W, Abmayr W. Differential inactivation analysis of diploid yeast exposed to radiation of various LET. I. Computerized single-cell observation and preliminary application to X-ray-treated Saccharomyces cerevisiae. Radiat. Res. 1983;94:464–479. doi: 10.2307/3575905. [DOI] [PubMed] [Google Scholar]
- 43.Blank M, Findl E. Mechanistic Approaches to Interactions of Electric and Electromagnetic Fields with Living Systems. New York: Springer; 2013. pp. 134–137. [Google Scholar]
- 44.Capelli E, et al. Low-frequency pulsed electromagnetic field is able to modulate miRNAs in an experimental cell model of Alzheimer’s disease. J. Healthc. Eng. 2017 doi: 10.1155/2017/2530270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, et al. Extremely low-frequency electromagnetic fields affect the miRNA-mediated regulation of signaling pathways in the GC-2 cell line. PLoS ONE. 2015;10:e0139949. doi: 10.1371/journal.pone.0139949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dasdag S, et al. Effects of 2.4 GHz radiofrequency radiation emitted from Wi-Fi equipment on microRNA expression in brain tissue. Int. J. Radiat. Biol. 2015;91:555–561. doi: 10.3109/09553002.2015.1028599. [DOI] [PubMed] [Google Scholar]
- 47.Luukkonen J, Liimatainen A, Juutilainen J, Naarala J. Induction of genomic instability, oxidative processes, and mitochondrial activity by 50Hz magnetic fields in human SH-SY5Y neuroblastoma cells. Mutat. Res. 2014;760:33–41. doi: 10.1016/j.mrfmmm.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Jouni FJ, Abdolmaleki P, Ghanati F. Oxidative stress in broad bean (Vicia faba L.) induced by static magnetic field under natural radioactivity. Mutat. Res. 2012;741:116–121. doi: 10.1016/j.mrgentox.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Campisi A, et al. Reactive oxygen species levels and DNA fragmentation on astrocytes in primary culture after acute exposure to low intensity microwave electromagnetic field. Neurosci. Lett. 2010;473:52–55. doi: 10.1016/j.neulet.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Hajnorouzi A, Vaezzadeh M, Ghanati F, Jamnezhad H, Nahidian B. Growth promotion and a decrease of oxidative stress in maize seedlings by a combination of geomagnetic and weak electromagnetic fields. J. Plant Physiol. 2011;168:1123–1128. doi: 10.1016/j.jplph.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Maaroufi K, et al. Spatial learning, monoamines and oxidative stress in rats exposed to 900 MHz electromagnetic field in combination with iron overload. Behav. Brain Res. 2014;258:80–89. doi: 10.1016/j.bbr.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 52.Osera C, et al. Pre-exposure of neuroblastoma cell line to pulsed electromagnetic field prevents H2O2-induced ROS production by increasing MnSOD activity. Bioelectromagnetics. 2015;36:219–232. doi: 10.1002/bem.21900. [DOI] [PubMed] [Google Scholar]
- 53.Osera C, et al. Cytoprotective response induced by electromagnetic stimulation on SH-SY5Y human neuroblastoma cell line. Tissue Eng. Part A. 2011;17:2573–2582. doi: 10.1089/ten.TEA.2011.0071. [DOI] [PubMed] [Google Scholar]
- 54.Ehnert S, et al. Extremely low frequency pulsed electromagnetic fields cause antioxidative defense mechanisms in human osteoblasts via induction of O2 and H2O2. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-14983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodman R, Blank M. Magnetic field stress induces expression of hsp70. Cell Stress Chaperon. 1998;3:79–88. doi: 10.1379/1466-1268(1998)003<0079:MFSIEO>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin H, Opler M, Head M, Blank M, Goodman R. Electromagnetic field exposure induces rapid, transitory heat shock factor activation in human cells. J. Cell. Biochem. 1997;66:482–488. doi: 10.1002/(SICI)1097-4644(19970915)66:4<482::AID-JCB7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 57.Patruno, A. et al. mTOR activation by PI3K/Akt and ERK signaling in short ELF-EMF exposed human keratinocytes. PLoS ONE10. 10.1371/journal.pone.0139644 (2015). [DOI] [PMC free article] [PubMed]
- 58.Leone L, et al. Epigenetic modulation of adult hippocampal neurogenesis by extremely low-frequency electromagnetic fields. Mol. Neurobiol. 2014;49:1472–1486. doi: 10.1007/s12035-014-8650-8. [DOI] [PubMed] [Google Scholar]
- 59.Carmody S, et al. Cytoprotection by electromagnetic field-induced hsp70: A model for clinical application. J. Cell. Biochem. 2000;79:453–459. doi: 10.1002/1097-4644(20001201)79:3<453::AID-JCB100>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 60.Goodman R, Henderson AS. Mechanistic Approaches to Interactions of Electric and Electromagnetic Fields with Living Systems. New York: Plenum Press; 1987. p. 217. [Google Scholar]
- 61.Blank M, Goodman R. DNA is a fractal antenna in electromagnetic fields. Int. J. Radiat. Biol. 2011;87:409–415. doi: 10.3109/09553002.2011.538130. [DOI] [PubMed] [Google Scholar]
- 62.Eleuteri AM, et al. 50 Hz extremely low frequency electromagnetic fields enhance protein carbonyl groups content in cancer cells: Effects on proteasomal systems. J. Biomed. Biotechnol. 2009;2009:834239. doi: 10.1155/2009/834239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caraglia M, et al. Electromagnetic fields at mobile phone frequency induce apoptosis and inactivation of the multi-chaperone complex in human epidermoid cancer cells. J. Cell. Physiol. 2005;204:539–548. doi: 10.1002/jcp.20327. [DOI] [PubMed] [Google Scholar]
- 64.Hirai T, et al. Stimulation of ubiquitin-proteasome pathway through the expression of amidohydrolase for N-terminal asparagine (Ntan1) in cultured rat hippocampal neurons exposed to static magnetism. J. Neurochem. 2006;96:1519–1530. doi: 10.1111/j.1471-4159.2006.03655.x. [DOI] [PubMed] [Google Scholar]
- 65.Pena-Philippides JC, et al. Effect of pulsed electromagnetic field (PEMF) on infarct size and inflammation after cerebral ischemia in mice. Transl. Stroke Res. 2014;5:491–500. doi: 10.1007/s12975-014-0334-1. [DOI] [PubMed] [Google Scholar]
- 66.Kubat NJ, Moffett J, Fray LM. Effect of pulsed electromagnetic field treatment on programmed resolution of inflammation pathway markers in human cells in culture. J. Inflamm. Res. 2015;8:59–69. doi: 10.2147/JIR.S78631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rohde CH, et al. Pulsed electromagnetic fields reduce postoperative interleukin-1beta, pain, and inflammation: A double-blind, placebo-controlled study in TRAM flap breast reconstruction patients. Plast. Reconstr. Surg. 2015;135:808e–817e. doi: 10.1097/PRS.0000000000001152. [DOI] [PubMed] [Google Scholar]
- 68.Nittby H, et al. Exposure to radiation from global system for mobile communications at 1,800 MHz significantly changes gene expression in rat hippocampus and cortex. Environmentalist. 2008;28:458–465. doi: 10.1007/s10669-008-9170-8. [DOI] [Google Scholar]
- 69.Aypar U, Morgan WF, Baulch JE. Radiation-induced epigenetic alterations after low and high LET irradiations. Mutat. Res. 2011;707:24–33. doi: 10.1016/j.mrfmmm.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 70.Gherardini L, Ciuti G, Tognarelli S, Cinti C. Searching for the perfect wave: The effect of radiofrequency electromagnetic fields on cells. Int. J. Mol. Sci. 2014;15:5366–5387. doi: 10.3390/ijms15045366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Group, B. W. BioInitiative 2012: A Rationale for Biologically-based Exposure Standards for Low-Intensity Electromagnetic Radiation. (2012). http://www.bioinitiative.org/report/wp-content/uploads/pdfs/sec07_2012_Evidence_for_Stress_Response_Cellular.pdf.
- 72.Calabro E, et al. Modulation of heat shock protein response in SH-SY5Y by mobile phone microwaves. World J. Biol. Chem. 2012;3:34–40. doi: 10.4331/wjbc.v3.i2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perez F, et al. Electromagnetic and thermal simulations of human neurons for SAR applications. J. Biomed. Sci. Eng. 2016;9:437–444. doi: 10.4236/jbise.2016.99039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perez FP, et al. Longevity pathways: HSF1 and FoxO pathways, a new therapeutic target to prevent age-related diseases. Curr. Aging Sci. 2012;5:87–95. doi: 10.2174/1874609811205020087. [DOI] [PubMed] [Google Scholar]
- 75.Fragopoulou AF, et al. Brain proteome response following whole body exposure of mice to mobile phone or wireless DECT base radiation. Electromagn. Biol. Med. 2012;31:250–274. doi: 10.3109/15368378.2011.631068. [DOI] [PubMed] [Google Scholar]
- 76.Long JM, Ray B, Lahiri DK. MicroRNA-153 physiologically inhibits expression of amyloid-beta precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J. Biol. Chem. 2012;287:31298–31310. doi: 10.1074/jbc.M112.366336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gabriel S, Lau R, Gabriel C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996;41:2251. doi: 10.1088/0031-9155/41/11/002. [DOI] [PubMed] [Google Scholar]
- 78.Ray, B. et al. Rivastigmine modifies the α-secretase pathway and potentially early Alzheimer's disease. Transl. Psychiatry.10. 10.1038/s41398-020-0709-x (2020). [DOI] [PMC free article] [PubMed]
- 79.Ray B, Chopra N, Long JM, Lahiri DK. Human primary mixed brain cultures: Preparation, differentiation, characterization and application to neuroscience research. Mol. Brain. 2014;7:63. doi: 10.1186/s13041-014-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McKhann GM, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y, et al. Effect of 50 Hz extremely low-frequency electromagnetic fields on the DNA methylation and DNA methyltransferases in mouse spermatocyte-derived cell line GC-2. Biomed. Res. Int. 2015;2015:10. doi: 10.1155/2015/237183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin H, Blank M, Goodman R. A magnetic field-responsive domain in the human HSP70 promoter. J. Cell. Biochem. 1999;75:170–176. doi: 10.1002/(SICI)1097-4644(19991001)75:1<170::AID-JCB17>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 83.Shamovsky I, Nudler E. Isolation and characterization of the heat shock RNA 1. Methods Mol. Biol. 2009;540:265–279. doi: 10.1007/978-1-59745-558-9_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Su L, Yimaer A, Xu Z, Chen G. Effects of 1800 MHz RF-EMF exposure on DNA damage and cellular functions in primary cultured neurogenic cells. Int. J. Radiat. Biol. 2018;94:295–305. doi: 10.1080/09553002.2018.1432913. [DOI] [PubMed] [Google Scholar]
- 85.Jeong YJ, et al. Impact of long-term RF-EMF on oxidative stress and neuroinflammation in aging brains of C57BL/6 mice. Int. J. Mol. Sci. 2018;19:50. doi: 10.3390/ijms19072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y-W, Thompson R, Zhang H, Xu H. APP processing in Alzheimer's disease. Mol. Brain. 2011;4:3. doi: 10.1186/1756-6606-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mawuenyega kg, et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14914–14919. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Udelsman R, Blake MJ, Stagg CA, Holbrook NJ. Endocrine control of stress-induced heat shock protein 70 expression in vivo. Surgery. 1994;115(611):616. [PubMed] [Google Scholar]
- 90.Liu AY, Lin Z, Choi HS, Sorhage F, Li B. Attenuated induction of heat shock gene expression in aging diploid fibroblasts. J. Biol. Chem. 1989;264(12037):12045. [PubMed] [Google Scholar]
- 91.Campanini C, Petronini PG, Alfieri R, Borghetti AF. Decreased expression of heat shock protein 70 mRNA and protein in WI-38 human fibroblasts aging in vitro. Ann. N. Y. Acad. Sci. 1992;663(442):443. doi: 10.1111/j.1749-6632.1992.tb38695.x. [DOI] [PubMed] [Google Scholar]
- 92.David DC, et al. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8:e1000450. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015;84:435–464. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reis-Rodrigues P, et al. Proteomic analysis of age-dependent changes in protein solubility identifies genes that modulate lifespan. Aging Cell. 2012;11:120–127. doi: 10.1111/j.1474-9726.2011.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perez FP, et al. Late-Onset Alzheimer's Disease, heating up and foxed by several proteins: Pathomolecular effects of the aging process. J. Alzheimer's Dis. JAD. 2014;40(1):17. doi: 10.3233/JAD-131544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pierce A, et al. Over-expression of heat shock factor 1 phenocopies the effect of chronic inhibition of TOR by rapamycin and is sufficient to ameliorate Alzheimer's-like deficits in mice modeling the disease. J. Neurochem. 2013;124:880–893. doi: 10.1111/jnc.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shamovsky I, Nudler E. New insights into the mechanism of heat shock response activation. Cell. Mol. Life Sci. 2008;65:855–861. doi: 10.1007/s00018-008-7458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parsi S, Smith PY, Goupil C, Dorval V, Hebert SS. Preclinical evaluation of miR-15/107 family members as multifactorial drug targets for Alzheimer's disease. Mol. Ther. Nucleic Acids. 2015;4:e256. doi: 10.1038/mtna.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang WX, et al. The expression of microRNA miR-107 decreases early in Alzheimer's disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008;28:1213–1223. doi: 10.1523/jneurosci.5065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Premi E, et al. Modulation of long-term potentiation-like cortical plasticity in the healthy brain with low frequency-pulsed electromagnetic fields. BMC Neurosci. 2018;19(1):34. doi: 10.1186/s12868-018-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang Z, et al. Low-frequency repetitive transcranial magnetic stimulation ameliorates cognitive function and synaptic plasticity in APP23/PS45 mouse model of Alzheimer’s disease. Front. Aging Neurosci. 2017;9:292. doi: 10.3389/fnagi.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsuboi T, Wong JK, Okun MS, Ramirez-Zamora A. Quality of life outcomes after deep brain stimulation in dystonia: A systematic review. Parkinsonism Relat. Disord. 2020;70:82–93. doi: 10.1016/j.parkreldis.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heumann R, et al. Dyskinesia in Parkinson's disease: Mechanisms and current non-pharmacological interventions. J. Neurochem. 2014;130:472–489. doi: 10.1111/jnc.12751. [DOI] [PubMed] [Google Scholar]
- 104.Sefton, E. et al. Electric field application in vivo regulates neural precursor cell behaviour in the adult mammalian forebrain. eNeuro. 10.1523/ENEURO.0273-20.2020 (2020). [DOI] [PMC free article] [PubMed]
- 105.Liu D, et al. The KATP channel activator diazoxide ameliorates amyloid-β and Tau pathologies and improves memory in the 3xTgAD mouse model of Alzheimer's disease. J. Alzheimer's Dis. 2010;22:443–457. doi: 10.3233/JAD-2010-101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bisht S, et al. A polymeric nanoparticle formulation of curcumin (NanoCurc) ameliorates CCl 4-induced hepatic injury and fibrosis through reduction of pro-inflammatory cytokines and stellate cell activation. Lab. Invest. 2011;91:1383–1395. doi: 10.1038/labinvest.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song W, Lahiri DK. Melatonin alters the metabolism of the β-amyloid precursor protein in the neuroendocrine cell line PC12. J. Mol. Neurosci. 1997;9(75):92. doi: 10.1007/BF02736852. [DOI] [PubMed] [Google Scholar]
- 108.Terro F, et al. GSM-900MHz at low dose temperature-dependently downregulates α-synuclein in cultured cerebral cells independently of chaperone-mediated-autophagy. Toxicology. 2012;292:136–144. doi: 10.1016/j.tox.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 109.Liu, X. et al. Improvement of spatial memory disorder and hippocampal damage by exposure to electromagnetic fields in an Alzheimer’s disease rat model. PloS One10 (2015). [DOI] [PMC free article] [PubMed]
- 110.Long JM, Maloney B, Rogers JT, Lahiri DK. Novel upregulation of amyloid-beta precursor protein (APP) by microRNA-346 via targeting of APP mRNA 5'-untranslated region: Implications in Alzheimer's disease. Mol. Psychiatry. 2019;24:345–363. doi: 10.1038/s41380-018-0266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perez FP, et al. Engineered repeated electromagnetic field shock therapy for cellular senescence and age-related diseases. Rejuvenation Res. 2008;11:1049–1057. doi: 10.1089/rej.2008.0793. [DOI] [PubMed] [Google Scholar]
- 112.Lahiri DK, Chen D, Ge YW, Bondy SC, Sharman EH. Dietary supplementation with melatonin reduces levels of amyloid beta-peptides in the murine cerebral cortex. J. Pineal Res. 2004;36(224):231. doi: 10.1111/j.1600-079X.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- 113.Lahiri DK, Farlow MR, Sambamurti K. The secretion of amyloid beta-peptides is inhibited in the tacrine-treated human neuroblastoma cells. Brain Res. Mol. Brain Res. 1998;62:131–140. doi: 10.1016/S0169-328X(98)00236-8. [DOI] [PubMed] [Google Scholar]
- 114.Ialongo C. Understanding the effect size and its measures. Biochem. Med. (Zagreb) 2016;26:150–163. doi: 10.11613/bm.2016.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available from the corresponding author on request.