Abstract
Whether the lipid profile in diabetic patients is associated with diabetic neuropathy (DN) development remains ambiguous, as does the predictive value of serum lipid levels in the risk of DN. Here, we performed the first meta-analysis designed to investigate the relationship between DN and the serum levels of triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL), and low-density lipoprotein cholesterol (LDL). Candidate studies were comprehensively identified by searching PubMed, Embase, Cochrane Library and Web of Science databases up to May 2020. Observational methodological meta-analysis was conducted to assess the relationships of TG, TC, HDL, and LDL levels with DN. Changes in blood lipids were used to estimate the effect size. The results were pooled using a random-effects or fixed-effects model. Potential sources of heterogeneity were explored by subgroup analysis. Various outcomes were included, and statistical analyses were performed using STATA (Version 12.0). Mean differences (MDs) and odds ratios (ORs) with 95% confidence intervals (CIs) were estimated. The Newcastle–Ottawa Scale (NOS) was applied to assess the methodological quality. I2 statistics were calculated to evaluate statistical heterogeneity. Funnel plots were utilized to test for publication bias. A sensitivity analysis was performed by omitting each study one by one. Thirty-nine clinical trials containing 32,668 patients were included in the meta-analysis. The results demonstrated that DN patients showed higher TG and lower HDL levels (MD = 0.34, 95% CI: 0.20–0.48 for TG; MD = -0.05, 95% CI: -0.08–-0.02, I2 = 81.3% for HDL) than controls. Subgroup analysis showed that patients with type 1 diabetes mellitus (T1DM) neuropathy had elevated TG levels in their serum (MD = 0.25, 95% CI: 0.16–0.35,I2 = 64.4% for T1DM). However, only patients with T1DM neuropathy had reduced serum HDL levels, and there was no significant difference in serum HDL levels between patients with T2DM neuropathy and controls (MD = -0.07, 95% CI: -0.10–-0.03, I2 = 12.4% for T1DM; MD = -0.02, 95% CI: -0.07–0.03, I2 = 80.2% for T2DM). TC and LDL levels were not significantly different between DN patients and controls (MD = -0.03, 95% CI: -0.14–0.09, I2 = 82.9% for TC; MD = -0.00, 95% CI: -0.08–0.08, I2 = 78.9% for LDL). In addition, compared with mild or painless DN patients, those with moderate or severe pain DN pain had significantly reduced serum TC and LDL levels (MD = -0.31, 95% CI: -0.49–-0.13, I2 = 0% for TC; MD = -0.19, 95% CI: -0.32–-0.08, I2 = 0% for LDL). TG levels and HDL levels did not vary considerably between patients with mild or painless DN and those with moderate or severe DN pain patients (MD = 0.12, 95% CI: -0.28–0.51, I2 = 83.2% for TG; MD = -0.07, 95% CI:-0.14–0.01, I2 = 58.8% for HDL). Furthermore, people with higher TG and LDL levels had higher risk of DN (OR = 1.36, 95% CI: 1.20–1.54, I2 = 86.1% for TG and OR = 1.10, 95% CI: 1.02–1.19, I2 = 17.8% for LDL). Conversely, high serum HDL levels reduced the risk of DN (OR = 0.85, 95% CI: 0.75–0.96, I2 = 72.6%), while TC levels made no significant difference with the risk of DN (OR = 1.02, 95% CI: 1.00–1.04, I2 = 84.7%). This meta-analysis indicated that serum lipid profile changes are among the biological characteristics of DN. Lipid levels should be explored as routine laboratory markers for predicting the risk of DN, as they will help clinicians choose appropriate therapies, and thus optimize the use of available resources.
Subject terms: Endocrinology, Risk factors
Introduction
Diabetic neuropathy (DN) is a highly common but often neglected complication of both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), affecting an estimated 50% of individuals with diabetes1. Its prevalence is more than 2% in the general population2,3 and approximately 15% among people over 40 years old4. Importantly, patients with peripheral and autonomic neuropathy have a more than twofold increase in their risk of death5. DN is a progressive and debilitating disease that can seriously reduce a patient's quality of life and is a key cause of non-trauma related amputations of the lower limbs6,7, which means that DN is recognized as the leading contributor to disability in people with diabetes8. In addition, one-third of subjects with DN report burning, tingling, shooting or lancing sensations as a symptom and need help alleviating this symptom9,10. Painful diabetic neuropathy (PDN) can seriously negatively affect patients’ psychological and physical health, leading to anxiety, depression and sleep disorders11,12. The treatment of PDN can be a major challenge for both the clinician and the patient, as such pain is unresponsive or only partially responsive to existing management approaches13,14. Owing to the pressing need for a solution to DN, many clinical studies have been carried out to prevent or cure this complication. To date, there is no treatment to prevent the onset of DN other than intensive glycaemic control, which substantially reduces the incidence of DN only in T1DM patients and is minimally effective in preventing DN among patients with T2DM15–17. The cause of DN is more complex than dysregulated glucose levels alone. Even patients with good glycaemic control (HbA1c < 5.4%) can still develop DN, suggesting that components other than glycaemic control may be involved in the onset and progression of DN18. Recently, some studies have implicated cardiovascular risk factors, such as obesity19 and triglycerides (TG)20, in the pathogenesis of DN. Therefore, it is essential to understand whether lipid levels modulate DN progression.
Changes in serum lipid profiles and lipid metabolism are at the root of at least some disease mechanisms21. Numerous studies have confirmed that some biomarkers may be associated with DN, among which serum lipid levels might play a significant role . Despite the mass of evidence accumulated in the last few years and the considerable contribution of serum lipid profiles to DN, there are still some considerable contradictions regarding the relationship between serum lipid levels and DN in observational and epidemiological studies23–26. Some studies have shown a positive association between a high level of total cholesterol (TC) and DN in diabetic patients27. In contrast, other studies have found a lack of significant changes in serum lipid profiles or even an inverse association between TG levels and DN18,28,29. Ascertaining whether a relation exists between serum lipid profiles and DN might lead to new disease-modifying therapies.
To comprehensively investigate the relationship between DN and the serum levels of TG, TC, high-density lipoprotein cholesterol (HDL), and low-density lipoprotein cholesterol (LDL), we conducted a meta-analysis quantitatively assessing the role of serum lipid levels in DN. The results provide new knowledge regarding the treatment of DN and may help in the development of clinical biomarker guidelines for DN.
Methods
Literature search strategy
This meta-analysis was conducted using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines30. This systematic review was prospectively registered in PROSPERO (CRD42020191400); the registration is available at http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42020191400.
Relevant articles were identified through an electronic search of PubMed, Embase, Cochrane Library and Web of Science using the following search terms: (“Diabetic Neuropathy” OR “Neuropathies, Diabetic” OR “Neuropathy, Diabetic” OR “Diabetic Autonomic Neuropathy” OR “Autonomic Neuropathies, Diabetic” OR “Autonomic Neuropathies, Diabetic” OR “Diabetic Autonomic Neuropathies” OR “Neuropathies, Diabetic Autonomic” OR “Neuropathy, Diabetic Autonomic” OR “Diabetic Neuralgia” OR “Diabetic Neuralgias” OR “Neuralgias, Diabetic” OR “Diabetic Neuropathy, Painful” OR “Diabetic Neuropathies, Painful” OR “Neuropathies, Painful Diabetic” OR “Neuropathy, Painful Diabetic” OR “Painful Diabetic Neuropathies” OR “Painful Diabetic Neuropathy” OR “Neuralgia, Diabetic” OR “Symmetric Diabetic Proximal Motor Neuropathy” OR “Asymmetric Diabetic Proximal Motor Neuropathy” OR “Diabetic Asymmetric Polyneuropathy” OR “Asymmetric Polyneuropathies, Diabetic” OR “Asymmetric Polyneuropathy, Diabetic” OR “Diabetic Asymmetric Polyneuropathies” OR “Polyneuropathies, Diabetic Asymmetric” OR “Polyneuropathy, Diabetic Asymmetric” OR “Diabetic Mononeuropathy” OR “Diabetic Mononeuropathies” OR “Mononeuropathies, Diabetic” OR “Mononeuropathy, Diabetic” OR “Diabetic Mononeuropathy Simplex” OR “Diabetic Mononeuropathy Simplices” OR “Mononeuropathy Simplex, Diabetic” OR “Mononeuropathy Simplices, Diabetic” OR “Simplex, Diabetic Mononeuropathy” OR “Simplices, Diabetic Mononeuropathy” OR “Diabetic Amyotrophy” OR “Amyotrophies, Diabetic” OR “Amyotrophy, Diabetic” OR “Diabetic Amyotrophies” OR “Diabetic Polyneuropathy” OR “Diabetic Polyneuropathies” OR “Polyneuropathies, Diabetic” OR “Polyneuropathy, Diabetic”) and (“serum lipid profiles” OR “lipid profiles” OR “lipid levels” OR “triglycerides” OR “total cholesterol” OR “high-density lipoprotein cholesterol” OR “low-density lipoprotein cholesterol”). Relevant articles published up to May 2020 were included in this study. We also manually screened the reference lists of retrieved articles to identify any potentially relevant studies. The summarized search strategy and the full electronic search strategies for multiple international databases are presented in Supplementary Files 2 and 3.
Selection criteria
Studies were included in this meta-analysis only if any all of the following criteria were met: (1) The study was published as an original article; (2) There were at least 2 groups (a DN group and a healthy control group); (3) The study evaluated the serum levels of TG, TC, HDL, or/and LDL of these 2 groups. Studies were excluded from this meta-analysis if any of the following criteria were met: (1) The study was a review, commentary, case report, case series or letter to the editor; (2) The study was performed in animals or in vitro; (3) The article was not in English (this restriction was imposed because English is the international language of science); (4) There was a significant difference in baseline age, gender, or body mass index (BMI) between the 2 groups. Studies were selected by two reviewers (ZC and YY) for inclusion in our analysis using the aforementioned criteria, and disagreements were resolved by consensus or with the help of a third reviewer (JZ).
Data extraction and quality assessment
Clinical information was robustly extracted from all eligible studies: number, first author, year, country, N (case/control), age, M%, outcome reported, diabetes and Newcastle–Ottawa scale (NOS) score. Two investigators (ZC and YY) independently extracted study characteristics from the selected studies based on the predetermined inclusion and exclusion criteria. Any disagreements were settled with the help of a third reviewer (JZ) when necessary. For each study, the risk of bias was assessed using the NOS quality assessment instrument, which is used for assessing the quality of nonrandomized studies in a meta-analysis31. The measures on this scale comprise three items: the selection of participants, the comparability of cases and controls, and the ascertainment of outcomes. The scale has a minimum score of 0 and a maximum score of 9. Studies scoring at least 7 (corresponding to 78% of the maximum score) were regarded as having a low risk of bias (‘good’ quality); those that scored 4–6 were deemed to have a modest risk of bias (‘fair’ quality); and those that scored < 3 were considered to have a substantial risk of bias (‘poor’ quality)32.
Statistical analysis
Statistical analysis was performed using the STATA software package (version 12.0, STATA Corp, College Station, TX). The results are expressed as mean differences (MDs) and odds ratios (ORs) with 95% confidence intervals (CIs). Lipid levels were extracted as continuous variables for statistical analysis and reported as the mean and standard deviation (SD). We also used the following approximations: if a study provided lipid levels with the mean and standard error, we converted the standard error into an SD by the following equation: standard error × square root of the sample size. If a study provided medians and interquartile ranges, we converted them to means and SDs as described by Hozo et al.33. For discrete data, if the OR and 95% CI were not available, a 2 × 2 table was used to obtain the value of OR and 95% CI. A random-effects model was used to calculate the pooled results if the inconsistency index (I2) statistic was > 50%, and a fixed-effects model was applied if I2 ≤ 50%. Data with p ≥ 0.10 and I2 ≤ 50% were defined as having low heterogeneity. We assessed potential publication bias using funnel plots. Sensitivity analysis was performed by the leave-one-out method to assess whether the results were sufficiently robust and verify that they were not excessively influenced by any single study34.
Results
Study characteristics
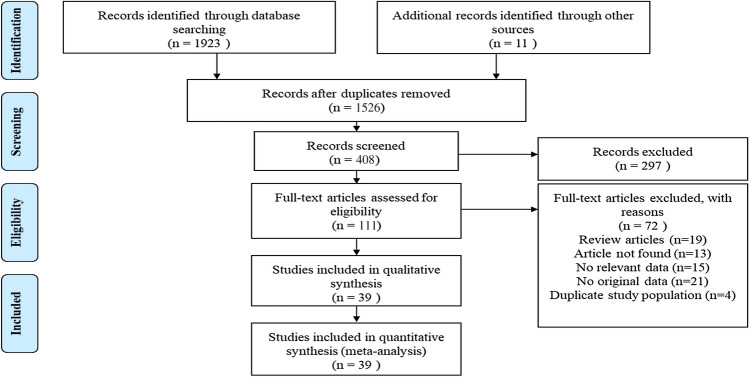
We identified 1923 studies through electronic searches. We also identified and read 11 potentially relevant articles that we found by browsing the reference lists of related articles and reviews. Of the candidate studies, we excluded 1526 after reading the abstracts and titles because they were duplicate studies, review articles, animal studies, commentaries, proceedings, case observations, or irrelevant to the present analysis. By further analysing the full text of the 111 remaining papers, the remaining 39 eligible studies were included in our meta-analysis18,22–29,35–65. A flow chart showing our selection process is presented in Fig. 1. Of these articles, 35 studies with 32,198 patients presented data for TG, 29 studies with 22,141 patients reported data for TC, 34 studies with 28,681 patients reported data for HDL and 30 studies with 22,615 patients presented data for LDL. There were 16 studies of patients with T2DM, 10 studies of patients with T1DM, 4 studies of patients with either T1DM or T2DM and 9 studies that did not specify the type of diabetes. Detailed characteristics of these eligible studies are described in Table 1.
Figure 1.
Flow of study selection.
Table 1.
Studies included in the meta-analysis.
| Number | References | Country | N (case/control) | Age | M% | Study design | Outcome reported | Diabetes |
|---|---|---|---|---|---|---|---|---|
| 1 | Jende35 | Germany | 100(64/36) | 64.6 ± 0.9 | 68% | cross-sectional cohort | TC, LDL-C, HDL-C, TG | T2DM |
| 2 | Hosny36 | Egypt | 60(30/30) | 51.238 ± 7.784 | NA | a case control study | TC, LDL, HDL, TG | T2DM |
| 3 | Song29 | China | 455 | 62.8 ± 8.61 | 46% | a case control study | TC,TG,HDL,LDL | DM |
| 4 | Vural37 | Turkey | 165(90/75) | 64.0 ± 10.3/66.6 ± 14.7 | 58.90% | retrospective study | TC,TG,LDL-C,HDL-C | DM |
| 5 | Andersen38 | Denmark | 144(27/117) | 62.59 ± 17.31/52.36 ± 17.52 | 81.50% | cross-sectional study | TC, LDL-C, HDL-C, TG | T2DM |
| 6 | Mizokami-Stout39 | USA | 5936(630/5306) | 39 ± 18 | 45% | cross-sectional study | TG, HDL-C, LDL-C,TC | T1DM |
| 7 | Litzelman40 | African-American 76% | 352 | 60.4 ± 9.6 | 19% | randomized controlled trial | HDL/cholesterol, HDL,TG,TC | T2DM |
| 8 | Aryan41 | Iran | 939(444/495) | 43.28 ± 14.42 | 48% | A nested case–control stud | TG, HDL-C, LDL-C,TC | T2DM |
| 9 | Akinci42 | Turkey | 74(31/43) | 28.26 ± 10.33/18.26 ± 7.42 | 12.9%/44.2% | cross-sectional study | TC, TG,LDL-C, HDL-C, | DM |
| 10 | Akbar25 | India | 202(62/140) | 55.7 ± 10.0/51.3 ± 10.8 | 33%/61% | cross-sectional cohort | TC,HDL,TG,TyG index | T2DM |
| 11 | Aktaş26 | Turkey | 50(27/23) | 56.85 ± 9.87/55.39 ± 8.84 | 7%/10% | cross-sectional cohort | LDL,HDL,TC | DM |
| 12 | Hwang23 | Korea | 530(239/291) | 20–80 | 58.5%/51.2%/64.4%/44.3% | retrospective study | TC, TG,LDL-C, HDL-C, | T2DM |
| 13 | Jane43 | China | 628 | < 65 22.9%/28.1%; ≥ 65 77.1%/71.9% | 37%/46.1% | cross-sectional study | TC, TG | T2DM |
| 14 | Najafi44 | Iran | 192 | 58.8 ± 8.2/57.9 ± 8.8 | 48.9%44.1% | cross-sectional study | TG,TC,LDL-C,HDL-C | T2DM |
| 15 | Zoppini45 | Italy | 557(461/96) | 58 ± 9.6/56.7 ± 6.6/57.8 ± 9.8 | 68.75%/66.16% | A cohort | LDL-C, HDL-C, TGs | T2DM |
| 16 | Ishibashi46 | Iapan | 107(78/28) | 55.7 ± 1.5/54.8 ± 2.2/58.7 ± 2.2/48.1 ± 2.2 | 54.55%/65.22% | cross-sectional cohort | LDL-C,HDL-C,TG | T2DM |
| 17 | Cho47 | Korea | 48(15/33) | 2006(55.93 ± 9.23/54.26 ± 10.55);2012(66.60 ± 5.47/63.15 ± 8.81) | NA | retrospective study | TC, TG,LDL-C, HDL-C, | T2DM |
| 18 | Rosales-Hernandez48 | Canada | 82(60/12) | 61.1 ± 10.0/59.8 ± 10.3/53.6 ± 14.4 | 69%/64%/50% | a case control study | TC, TG,LDL-C, HDL-C, | DM |
| 19 | Jende49 | Germany | 120(84/36) | 60.83 ± 1.61/62.55 ± 1.29 | 60% | cross-sectional cohort | TC, TG,LDL-C, HDL-C, | T1DM and T2DM |
| 20 | Smith22 | USA | 218 | 58.5 ± 9.1/60 ± 7.9/57.5 ± 9.8 | 50%/53%/55% | retrospective study | TC,LDL,HDL | T2DM |
| 21 | Katulanda50 | Sri Lanka | 528 | 55.0 ± 12.4 | 0.373 | a case control study | TC, TG,LDL-C, HDL-C, | T1DM and T2DM |
| 22 | Hsu24 | China | 326(85/241) | 65.7 ± 9.3/62.8 ± 9.5 | 38.8%/31.1% | A cohort | TC, TG | T2DM |
| 23 | Hsiao51 | China | 271 | 55.33 ± 11.04/56.49 ± 6.94 | 57.93%/60% | cross-sectional study | TC | T2DM |
| 24 | Ylitalo52 | USA | 100(9/91) | 63.2 ± 0.7/55.8 ± 0.4 | 65.5%/46.2% | A cohort | TG,LDL-C, HDL-C, | DM |
| 25 | Spallone53 | Italy | 191(135/56) | 59.9 ± 9.7/57.2 ± 10.5/58.1 ± 9.6 | 44.87%/71.93%/57.14% | a case control study | TC, TG,LDL-C, HDL-C, | T1DM and T2DM |
| 26 | Terekeci65 | USA | 42(25/17) | 58.80 ± 8.60/55.18 ± 6.41 | 50% | A cohort | TC,TG | T2DM |
| 27 | Faisal27 | Bahrain | 1225(526/689) | 54/52 | 42.99% | cross-sectional cohort | TC,TG,HDL-C | T1DM and T2DM |
| 28 | Coppini55 | UK | 300(100/200) | 66.2 ± 9.4 /49.2 ± 16.3 | Gender (M:F) 1.3 : 1 /2 : 1 | a retrospective case–control study | TC, TG,LDL-C, HDL-C, | DM |
| 29 | Kempler28 | 31 centres in 16 European countries | 3270 | 32.7 ± 10.2 | 51.62% | A cohort | TC, TG,LDL-C, HDL-C, | T1DM |
| 30 | Christen64 | USA | 407 | 31.4 ± 1.4 | 75.30% | a prospective cohort study | LDL,HDL,TG | T1DM |
| 31 | Maser57 | Pittsburgh | 363(228/135) | 34 ± 6/28 ± 6 | 52%/50% | A cohort | LDL,HDL,TG | T1DM |
| 32 | Maser58 | Pittsburgh | 168(105/63) | 30 ± 3/29 ± 3 | 49%/57% | A cohort | LDL,HDL,TG | T1DM |
| 33 | Orchard59 | Pittsburgh | 325(57/268) | < 17 | 47%/56% | A cohort | TC,TG,LDL-C, HDL-C, | T1DM |
| 34 | Tesfaye18 | 31 centres in 16 European countries | 3250 | NA | 51.32% | A cohort | LDL,HDL,VLDL | T1DM |
| 35 | Simmons60 | UK | 33(20/13) | 49 ± 5/50 ± 11/50 ± 6 | 63.64%/66.67%/46.15% | cross-sectional cohort | TC, TG | T1DM |
| 36 | Tesfaye9 | 31 centers in the European Diabetes | 1172(276/896) | 29.8 ± 8.1/33.6 ± 10.0 | 51.1%/48.6% | prospective study | TC, TG,LDL-C, HDL-C, | T1DM |
| 37 | Witte56 | UK | 956(163/793) | 34.5 ± 10.3/30.7 ± 8.4 | 35.58%/6.68% | A cohort | TC, TG,LDL, HDL | T1DM |
| 38 | Callaghan62 | USA | 2382 | 73.5 ± 2.9 | 48.30% | A cohort | TG,HDL | DM |
| 39 | Callaghan63 | China | 4002 | 51.6 ± 11.8 | 51% | A cross-sectional | TG,HDL | DM |
Quality assessment
All thirty-nine studies had NOS quality scores greater than or equal to 5, indicating that all these studies had ‘good’ or ‘fair’ methodological quality. Details on the risk of bias among those 39 studies are summarized in Table 2.
Table 2.
Risk of bias analysis in each study.
| Number | References | Selection | Comparability | Outcome ascertainment | Bias risk (Total scores) | Final quality conclusion |
|---|---|---|---|---|---|---|
| 1 | Jende35 | 4 | 1 | 1 | 6 | Fair |
| 2 | Hosny36 | 4 | 2 | 1 | 7 | Good |
| 3 | Song29 | 3 | 2 | 1 | 6 | Fair |
| 4 | Vural37 | 2 | 2 | 1 | 5 | Fair |
| 5 | Andersen38 | 3 | 2 | 3 | 8 | Good |
| 6 | Mizokami-Stout39 | 3 | 2 | 3 | 8 | Good |
| 7 | Litzelman40 | 4 | 2 | 2 | 8 | Good |
| 8 | Aryan41 | 3 | 2 | 2 | 7 | Good |
| 9 | Akinci42 | 2 | 2 | 1 | 5 | Fair |
| 10 | Akbar25 | 3 | 1 | 2 | 6 | Fair |
| 11 | Aktaş26 | 2 | 1 | 2 | 5 | Fair |
| 12 | Hwang23 | 4 | 2 | 1 | 7 | Good |
| 13 | Jane43 | 4 | 1 | 3 | 8 | Good |
| 14 | Najafi44 | 3 | 1 | 2 | 6 | Fair |
| 15 | Zoppini45 | 4 | 2 | 3 | 9 | Good |
| 16 | Ishibashi46 | 2 | 1 | 2 | 5 | Fair |
| 17 | Cho47 | 2 | 1 | 2 | 5 | Fair |
| 18 | Rosales-Hernandez48 | 3 | 1 | 1 | 5 | Fair |
| 19 | Jende49 | 3 | 2 | 1 | 6 | Fair |
| 20 | Smith22 | 3 | 2 | 2 | 7 | Good |
| 21 | Katulanda50 | 4 | 2 | 2 | 8 | Good |
| 22 | Hsu24 | 4 | 2 | 1 | 7 | Good |
| 23 | Hsiao51 | 4 | 3 | 1 | 8 | Good |
| 24 | Ylitalo52 | 3 | 2 | 1 | 6 | Fair |
| 25 | Spallone53 | 3 | 1 | 2 | 6 | Fair |
| 26 | Terekeci65 | 2 | 2 | 1 | 5 | Fair |
| 27 | Faisal27 | 4 | 2 | 3 | 9 | Good |
| 28 | Coppini55 | 3 | 2 | 3 | 8 | Good |
| 29 | Kempler28 | 4 | 2 | 3 | 9 | Good |
| 30 | Christen64 | 4 | 1 | 1 | 6 | Fair |
| 31 | Maser57 | 3 | 2 | 2 | 7 | Good |
| 32 | Maser58 | 3 | 1 | 1 | 5 | Fair |
| 33 | Orchard59 | 3 | 1 | 2 | 6 | Fair |
| 34 | Tesfaye18 | 4 | 2 | 3 | 9 | Good |
| 35 | Simmons60 | 3 | 1 | 1 | 5 | Fair |
| 36 | Tesfaye9 | 4 | 2 | 3 | 9 | Good |
| 37 | Witte56 | 3 | 2 | 3 | 8 | Good |
| 38 | Callaghan62 | 4 | 2 | 3 | 9 | Good |
| 39 | Callaghan63 | 4 | 2 | 3 | 9 | Good |
Serum TG levels between DN and non-DN patients/healthy controls
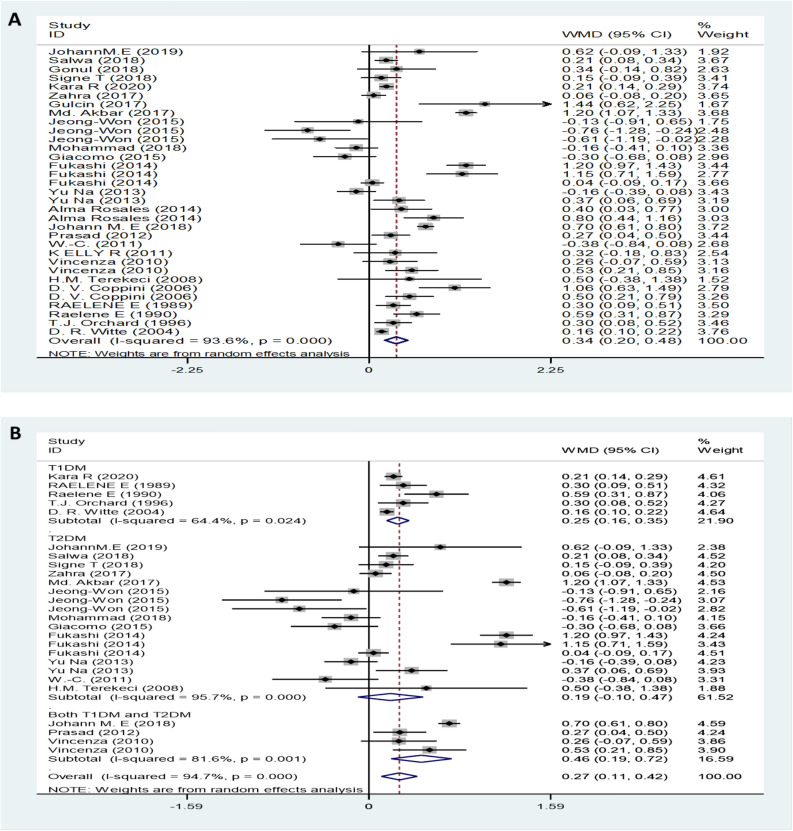
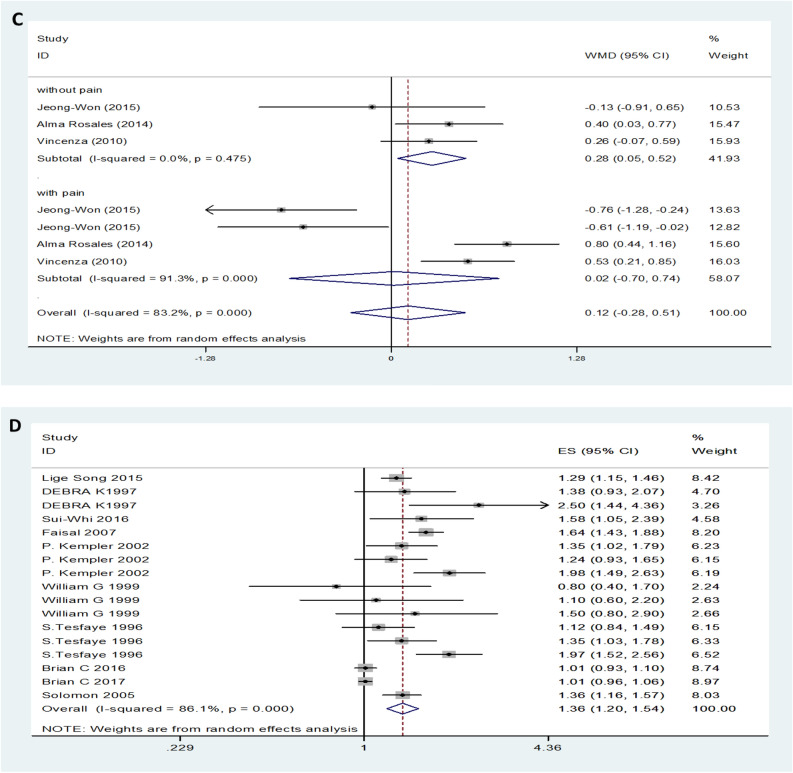
The pooled TG results with of 35 studies on TG showed a significantly increased serum TG levels in DN patients compared to non-DN patients with a random-effects model (MD (95% CI): 0.34 (0.20–0.48), I2 = 93.6%, p < 0.001) (Fig. 2A). Moreover, the serum TG levels of neuropathy patients with T1DM were higher than those of control patients (Fig. 2B). Patients with moderate or severe pain had no significant difference in TG levels of TG than compared with patients with mild or painless controls patients (MD (95% CI): 0.12 (-0.28– 0.51), I2 = 83.2%, p < 0.001) (Fig. 2C). Furthermore, compared with the lower serum TG level category, the highest serum TG level showed an increased the risk of DN (OR (95% CI): 1.36 (1.20–1.54), I2 = 86.1%, p < 0.001) (Fig. 2D).
Figure 2.
TG levels (A) in people with DN versus those without DN. TG levels in the subgroup analysis stratified by the type of diabetes (B) and symptom severity (C). OR (D) for DN in patients according to serum TG levels.
Serum TC between DN and non-DN patients
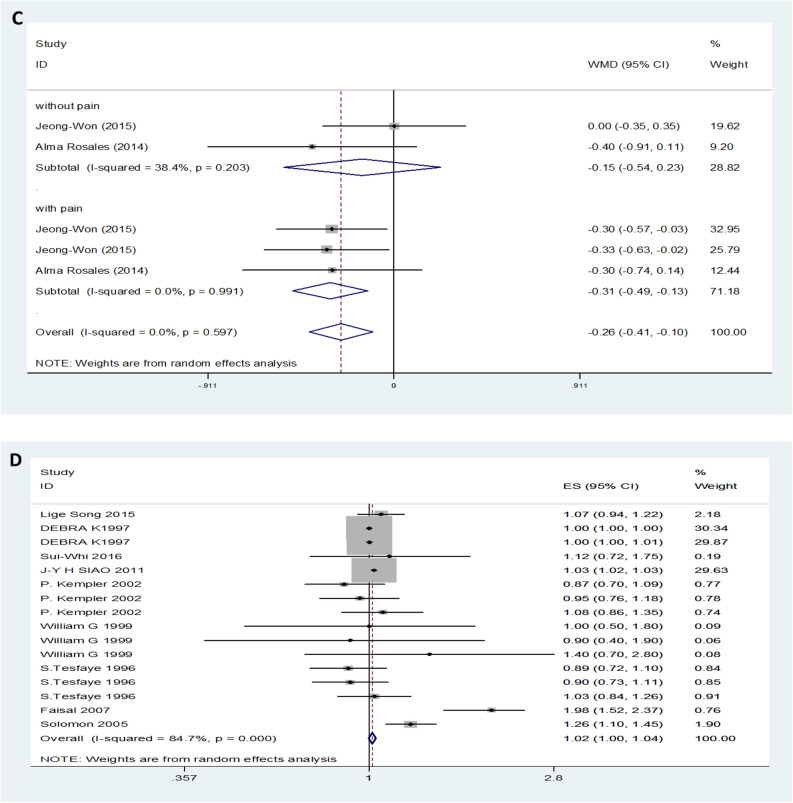
Twenty-nine studies, provided data on TC. Forest plot indicated that the expression level of TC in DN patients was not significantly different from that in control patients with a random-effects model (MD (95% CI): -0.03 (-0.14–0.09), I2 = 82.9%; p < 0.001) (Fig. 3A). The expression levels of TC in both T1DM neuropathy and T2DM neuropathy were not significantly different from those in the control group (Fig. 3B). Additionally, for symptomatic DN, moderate or severe DN patients had lower levels of serum TC compared to mild or painless DN patients (MD (95% CI): -0.31 (-0.49– -0.31), I2 = 0%, p = 0.991) (Fig. 3C). Patients with high levels of TC level had no influence on the risk of DN (OR (95% CI): 1.02 (1.00–1.04), and there was obvious evidence of significant heterogeneity among studies (I2 = 84.7%, p < 0.001) (Fig. 3D).
Figure 3.
TC levels (A) in people with DN versus those without DN. TC levels in the subgroup analysis stratified by the type of diabetes (B) and symptom severity (C). OR (D) for DN in patients according to serum TC levels.
Serum HDL levels between of DN and non-DN patients/healthy controls
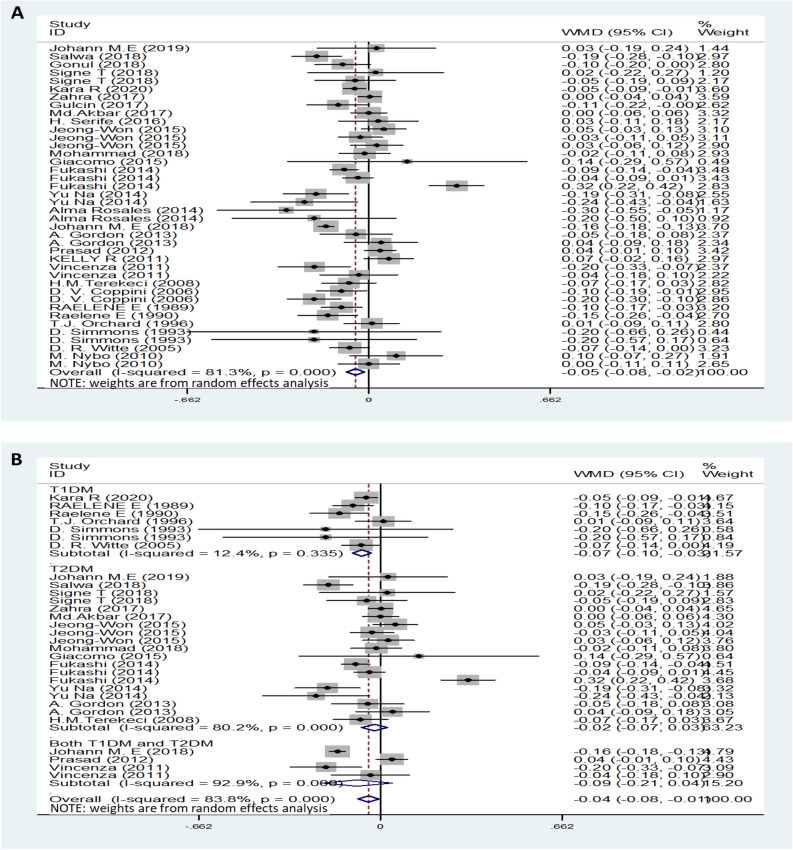
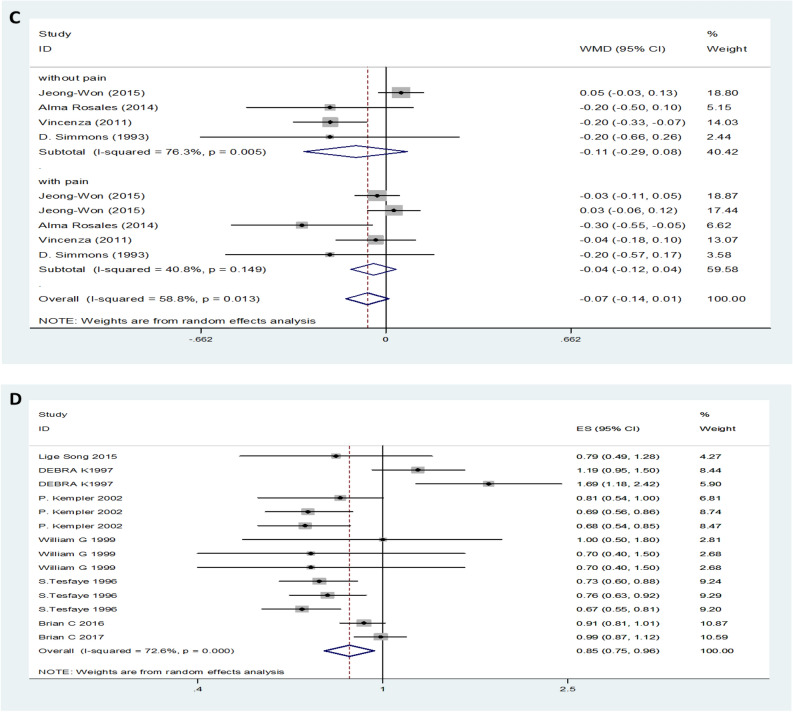
Data on HDL levels for DN were obtained from 34 studies. The results showed that serum HDL levels in patients with DN were lower than those in the control group with a random-effects model (MD (95% CI): -0.05 (-0.08‐ -0.02, I2 = 81.3%; p < 0.001) (Fig. 4A). Interestingly, subgroup analysis found that only patients with T1DM neuropathy had lower HDL levels than patients in the control group, while serum HDL levels in patients with T2DM were not different from those in the control group (Fig. 4B). The change in the MD for serum HDL levels was not significantly different between DPN and painless neuropathy (MD (95% CI): -0.07 (-0.04–0.01), I2 = 58.8%, p = 0.013 (Fig. 4C). In addition, high levels of HDL were observed to decrease the risk of DN (OR (95% CI):0.85 (0.75–0.96), I2 = 72.6%, p < 0.001 (Fig. 4D).
Figure 4.
HDL levels (A) in people with DN versus those without DN. HDL levels in the subgroup analysis stratified by the type of diabetes (B) and symptom severity (C). OR (D) for DN in patients according to serum HDL levels.
Serum LDL levels between DN and non-DN patients/healthy controls
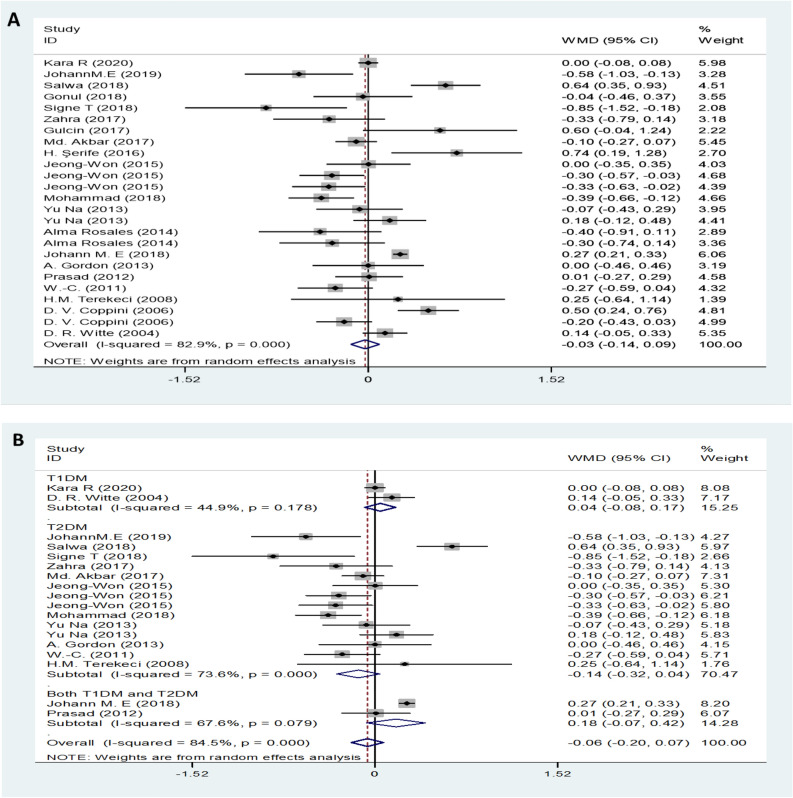
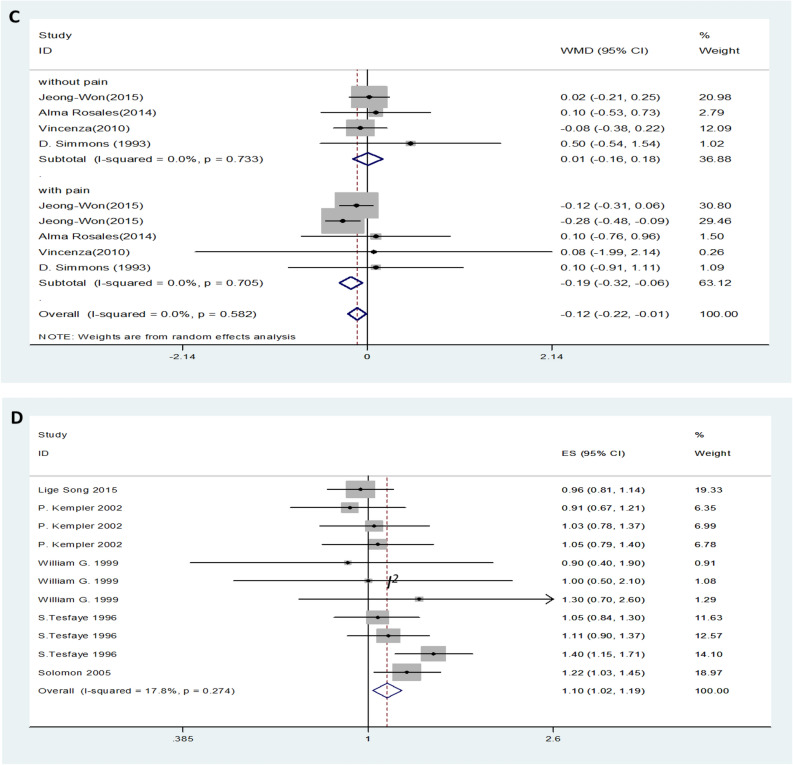
Twenty-nine trials showed no effect of LDL levels on DN. DN patients showed no difference in LDL levels compared to the control group with the random-effects model (MD (95% CI): -0.00 (-0.08–0.08, I2 = 78.9%; p < 0.001) (Fig. 5A). There was no significant difference in LDL levels in either type 1 or type 2 diabetic neuropathy patients compared with the control group (Fig. 5B). Moderate or severe pain DN patients had lower levels of LDL compared to than mild or painless DN patients (MD (95% CI): − 0.19 (− 0.32‐ − 0.06), I2 = 0%, p = 0.705) (Fig. 5C). Serum LDL levels increased the risk of DN (OR (95% CI): 1.10 (1.02‐1.19, I2 = 17.8%, p = 0.274) (Fig. 5D).
Figure 5.
LDL levels (A) in people with DN versus those without DN. LDL levels in the subgroup analysis stratified by the type of diabetes (B) and symptom severity (C). OR (D) for DN in patients according to serum LDL levels.
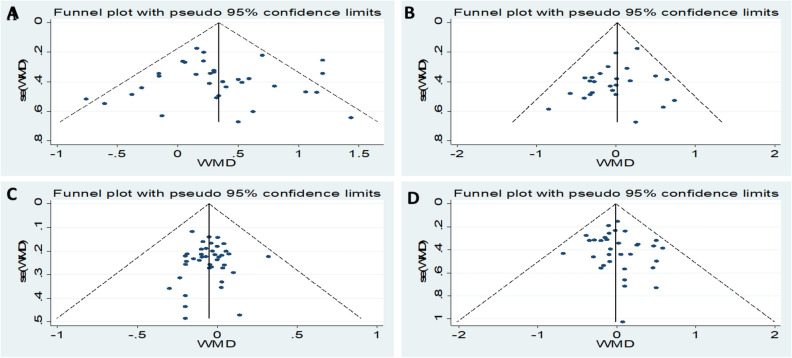
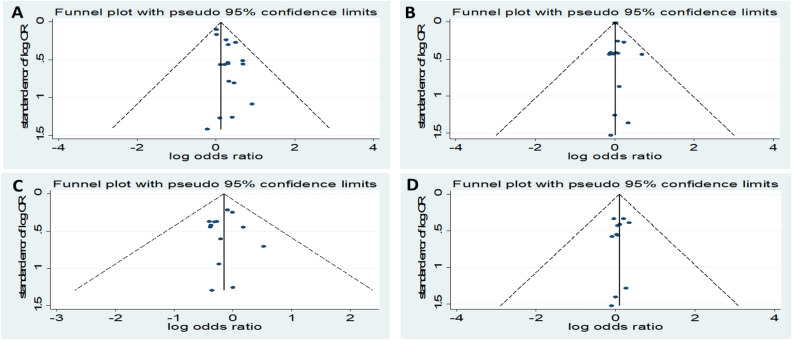
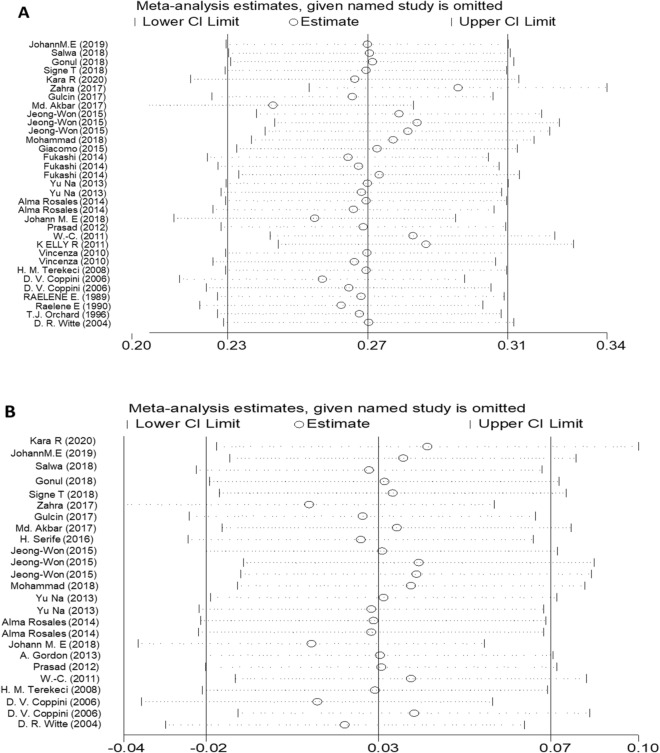
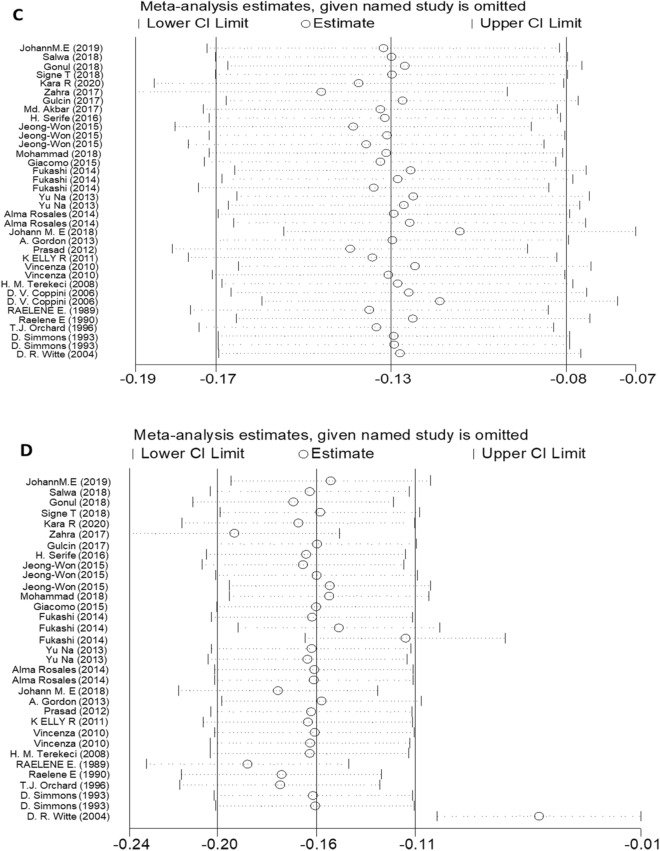
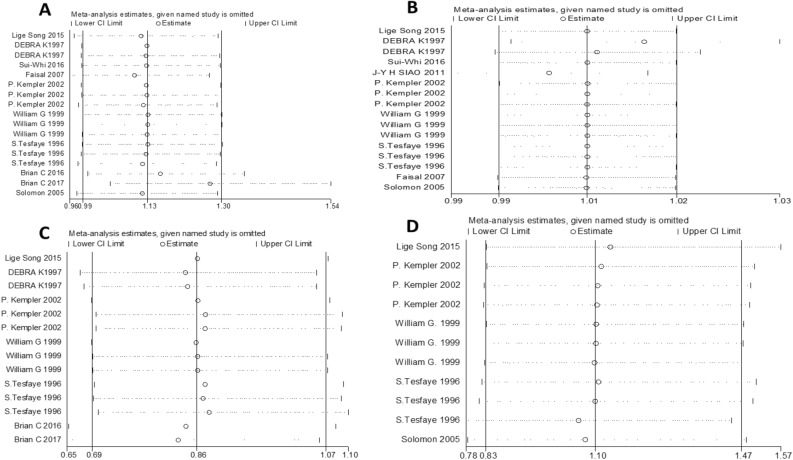
Sensitivity analyses and publication bias
Visual inspection of the funnel plot did not reveal remarkable asymmetry (Figs. 6, 7). Sensitivity analysis was performed to analyse the pooled results of the remaining studies by sequential removal of individual studies. There was no significant change in the overall outcomes after removing any single study, suggesting the stability and reliability of our results and that the data were not influenced by any given study (Figs. 8, 9). Thus, the above results suggest that publication bias was not apparent in this meta-analysis.
Figure 6.
Publication bias funnel plots of the MD for (A) TG, (B) TC, (C) HDL, and (D) LDL and DN.
Figure 7.
Publication bias funnel plots of the OR for (A) TG, (B) TC, (C) HDL, and (D) LDL and DN.
Figure 8.
Sensitivity analysis of the pooled MD for (A) TG, (B) TC, (C) HDL, and (D) LDL.
Figure 9.
Sensitivity analysis of the pooled OR for (A) TG, (B) TC, (C) HDL, and (D) LDL.
Discussion
DN is a common cause of morbidity and death among patients with diabetes66; this form of neuropathy is characterized by pain, paraesthesia, sensory loss, an increased frequency of falls, and reduced quality of life (QOL)9,67. It is obvious that DN poses a heavy health challenge to individuals. The duration and level of hyperglycaemia are important determinants of diabetic complications, including DN68. The risk of DN can be reduced with intensive blood glucose control in T1DM patients. Intensive blood glucose control has little influence in patients with T2DM15–17. One study showed that DN remained substantial, despite intensive control of the glucose level68. Moreover, except for optimal glycaemic control, there have been no definite positive prevention studies of other risk factor modifications for DN. Thus, there may be risk factors aside from hyperglycaemia involved in the development of DN. Identifying them, particularly if they are modifiable, might lead to new risk-reduction strategies. Accumulated evidence has shown a correlation between DN and serum lipid profiles but has shown inconsistent results18,28,29,40. Here, we performed a systematic review and meta-analysis and found that serum lipid profile changes are correlated with DN. To our knowledge, this is the first meta-analysis to provide evidence of a close relationship between TG, TC, HDL, LDL levels and DN risk.
Exploration of heterogeneity
Because of the apparent heterogeneity of our study, subgroup analysis was performed. We investigated whether the different diabetic types (T1DM or T2DM) and different symptoms (mild and painless or moderate and severe pain) affected the results. These factors may partly explain the origin of heterogeneity.
On the basis of the results, we performed a subgroup analysis with the different diabetic types. In the pooled analysis of the studies, regardless of T1DM or T2DM, the trend of TG change was consistent, and the TG levels of patients with DN were increased compared with those of the control group (Fig. 2B). However, serum HDL levels decreased only in T1DM neuropathy patients, and there was no difference between the control group patients and T2DM neuropathy patients (Fig. 4B). Furthermore, this approach did not obviously change the heterogeneity between the individual efficacy estimates. In addition, another subgroup was performed regarding the different symptoms. Heterogeneity was significantly reduced when analyses were stratified by different symptoms (Fig. 2C, 3C and 5C). We also separately analysed location and study design, as these were assumed to be potential sources of bias (data not shown). These methods slightly change the heterogeneity between the individual efficacy estimates, although they do not eliminate it.
Due to the limited number of included studies, subgroup analysis could not be conducted to explore the heterogeneity of some outcomes. Other uncharacteristic or unexplained underlying factors may contribute to the heterogeneity.
Relationship between lipid profile and DN
Changes in lipid levels are obvious biological characteristics of DN. After pooled data from 39 studies, our results showed that DN patients had higher levels of serum TG than control patients (Fig. 2A), while HDL levels were lower in DN patients than in control patients (Fig. 4A). The above results indicated that serum detection by TG and HDL may be serological markers for diabetic patients with or without DN. Patients with moderate or severe pain had lower levels of TC and LDL compared to mild or painless DN patients (Figs. 3C and Fig. 5C). It is worth mentioning that TC and LDL level changes in serum may be markers of symptomatic diabetes neuropathy. In addition, the results of our meta-analysis showed that higher levels of TG and LDL significantly increased the risk of DN (Fig. 2D and Fig. 5D). These findings suggest that changes in lipid profiles, especially TG and LDL serum levels, may be risk factors for DN. High-quality randomized controlled trials (RCTs) in the future are needed to better understand the causality between the serum lipid profile and DN.
Underlying mechanisms of lipid effects on DN
There is a high incidence of dyslipidaemia in both T1DM and T2DM patients, and dyslipidaemia is linked to DN (Figs. 2, 3, 4, 5). The mechanisms by which plasma lipids influence DN have not been fully elucidated, but certain factors may be involved. First, patients with dyslipidaemia are characterized by insulin resistance and a chronic inflammation status69 that can also contribute to insulin resistance70. Furthermore, insulin resistance has shown a positive association with peripheral neuropathy71.
Second, oxidative stress is an important risk factor for DN. Neurons express scavenger receptors for oxidized LDLs, such as oxidized LDL receptor 172. Elevated LDL has increased susceptibility to oxidation and oxidized LDLs (oxLDLs), and these modified LDLs can bind to extracellular receptors, triggering signalling cascades that activate oxidative stress. oxLDL-induced oxidative stress has been shown to mediate nerve injury in a mouse model of dyslipidaemia-induced neuropathy73. In addition, oxLDL is involved in neuron injury through nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation, which leads to increased superoxide production73. Additionally, free fatty acids (FFAs) bind to excess intramitochondrial pyruvate, leading to the production of reactive oxygen species. FFAs have been shown to directly cause damage to Schwann cells in vitro, and they can also cause proinflammatory factors to be released from adipocytes and macrophages71.
Third, demyelination due to lipid profile disorders is another potential mechanism for lipid-induced nerve injury. Segmental demyelination is an important feature of DN patients, and myelin breakdown with focal demyelination has been shown to occur in high-fat fed mice74. Thus, it is plausible to suggest that dyslipidaemia negatively impacts myelination status in nerves and contributes to the development of DN. Moreover, some studies have indicated that TC can be oxidized to oxysterols, which have been shown to lead to neuronal apoptosis72,75.
Of these, insulin resistance, inflammation, oxidative stress, and demyelination are possible mechanisms linking lipid profile disorder to DN.
Surprisingly, TC and LDL serum levels were reduced in patients with severe pain compared with asymptomatic conditions. However, at present, there is no reasonable explanation to explain this phenomenon.
Why intensive blood glucose control has little influence in patients with T2DM
Hyperglycaemia is a key factor underlying DN, but other changes also contribute, including dyslipidaemia and changes in insulin signalling17.
Patients with type 2 diabetes have an elevated incidence of dyslipidaemia, which is associated with the occurrence of DN. A study demonstrated that obesity, LDL, HDL, and hypertriglyceridemia were independently associated with neuropathy17. A series of bioinformatics analyses identified 532 differentially expressed genes between patient samples with progressive versus nonprogressive diabetic polyneuropathy and found that these were functionally enriched in pathways involving lipid metabolism and inflammatory responses76.
T2DM patients are insulin resistant. Disruption of insulin signalling due to insulin resistance makes neurons more vulnerable to metabolic insults and may contribute to the development of DN77.
Hyperglycaemia and dyslipidaemia, together with altered insulin signalling, lead to several pathological alterations in neurons, glia and vascular cells, which can lead to nerve dysfunction and ultimately DN.
Theoretical and practical implications
Our meta-analysis provides good guidance for clinical and scientific research. The treatment of DN has largely been directed at the control of symptoms and glucose control rather than to treat the underlying mechanisms17. To date, clinical interventions to treat DN have mainly focused on glycaemic control, while even proper control of blood sugar is a poor efficacy for T2DM neuropathy patients17. Along with substantial research into the relationship between serum lipid profiles and DN, the results of our present meta-analytic investigation indicate a clear direction: high levels of TG and LDL increased the risk of DN. In addition, DN patients had higher serum TG and lower HDL levels. Therefore, routine examination of serum TG and HDL levels in diabetic patients to predict the risk of DN is essential. Elevated levels of TG and low levels of HDL may be biomarkers for DN, except for the influence of lifestyle and other factors on blood lipid levels. The clinical use of lipid-lowering drugs may be an effective way to prevent and treat DN. PDN is associated with considerable morbidity, mortality and diminished quality of life9. The results of our meta-analysis showed that the presence of decreased TC and LDL in patients with DN may indicate a transition from asymptomatic status to severe pain status in DN patients. To the best of our knowledge, this is the first meta-analysis to explore the relationship between DN and serum lipid profiles; the results may be helpful for future clinical diagnosis and treatment. Blood lipid profiles are an appropriate source of biomarkers for DN screening; additionally, these profiles are widely applicable and easily measured.
The implication of our meta-analysis for scientific research is as follows: first, since the included primary studies had different cut-off values of serum lipids, we strongly suggest that the cut-off value be made uniform in subsequent studies. Second, blood lipid levels can be affected by many conditions, such as the environment, lifestyle and diet, and more detailed characteristics of patients should be recorded. Third, TG, TC, HDL and LDL levels provided a state of lipid levels. However, the other parameters may be considered; for instance, TC/HDL and LDL/HDL ratios are also considered indexes for the prediction of DN. Finally, identifying the mechanism of DN is the basis of treating DN.
Limitations of our study
Our current meta-analysis provides stable evidence of the relationship between the serum lipid profile and DN. Several limitations should be recognized. First, we found significant heterogeneity in the relationship between serum lipid profile and DN risk, which might result from a very large number of included studies and differences in study quality and basic participant characteristics. Second, the mechanisms underlying the decline in serum TC and LDL levels in moderate or severe pain are unclear. Third, there is a shortage of RCTs investigating the impact of lipid levels on DN risk. Thus, in order to obtain a more precise assessment of the impact of lipids on DN risk, well-designed RCTs are necessary. Fourth, DN represents a heterogeneous syndrome, and various definitions and methods are used to formulate the diagnosis. Screening tests for subjective symptoms or objective instrumental assessments, such as vibration perception threshold measurement, are used in the diagnosis of DN74. This increases the heterogeneity between studies. Finally, the included studies were all non-RCTs; thus, causality cannot be shown.
Conclusion
This meta-analysis supports that higher TG and lower HDL levels may be markers for predicting the development of DN in diabetic patients. Higher TG and LDL levels increase the risk of DN. Reduced TC and LDL serum levels in patients with DN may be a marker of an asymptomatic condition to severe pain condition in patients with DN. The mechanisms underlying the effect of serum TG and LDL levels on the increased risk of DN in recent tobacco quitters need to be further explored, since an improved understanding of this effect could contribute to the development of targeted pharmaceuticals that could be used to aid lipid profile regulation and prediction or treatment of DN.
Supplementary Information
Author's contributions
J.Z. coordinated the study. Z.C. developed the idea for the study and, along with Y.Y. and J.Z., contributed to the study design, literature search, figures, statistical analysis, outcome data synthesis, and drafting and editing of the final paper. All authors critically revised the report. All members have approved and agreed to submit the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82070807, 91749118, 81770775, 81730022), the Planned Science and Technology Project of Hunan Province (2017RS3015) and National key research and development program (2019YFA0801903, 2018YFC2000100).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zixin Cai and Yan Yang
Supplementary Information
is available for this paper at 10.1038/s41598-020-79276-0.
References
- 1.Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA. 2015;314(20):2172–2181. doi: 10.1001/jama.2015.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savettieri G, et al. Prevalence of diabetic neuropathy with somatic symptoms: a door-to-door survey in two Sicilian municipalities. Sicilian Neuro-Epidemiologic Study (SNES) Group. Neurology. 1993;43(6):1115–1120. doi: 10.1212/WNL.43.6.1115. [DOI] [PubMed] [Google Scholar]
- 3.Bharucha NE, Bharucha AE, Bharucha EP. Prevalence of peripheral neuropathy in the Parsi community of Bombay. Neurology. 1991;41(8):1315–1317. doi: 10.1212/WNL.41.8.1315. [DOI] [PubMed] [Google Scholar]
- 4.Gregg EW, et al. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004;27(7):1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 5.Soedamah-Muthu SS, et al. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS) Diabetes Care. 2008;31(7):1360–1366. doi: 10.2337/dc08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nather A, et al. Epidemiology of diabetic foot problems and predictive factors for limb loss. J. Diabetes Complic. 2008;22(2):77–82. doi: 10.1016/j.jdiacomp.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Kioskli K, et al. Psychosocial factors in painful diabetic neuropathy: a systematic review of treatment trials and survey studies. Pain Med. 2019;20(9):1756–1773. doi: 10.1093/pm/pnz071. [DOI] [PubMed] [Google Scholar]
- 8.Costa AF, et al. Burden of type 2 diabetes mellitus in Brazil. Cad SaudePublica. 2017;33(2):e00197915. doi: 10.1590/0102-311X00197915. [DOI] [PubMed] [Google Scholar]
- 9.Tesfaye S. Recent advances in the management of diabetic distal symmetrical polyneuropathy. J. Diabetes Investig. 2011;2(1):33–42. doi: 10.1111/j.2040-1124.2010.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies M, et al. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29(7):1518–1522. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 11.Gore M, et al. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J. Pain Symptom Manag. 2005;30(4):374–385. doi: 10.1016/j.jpainsymman.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Vinik A, et al. Relationship between pain relief and improvements in patient function/quality of life in patients with painful diabetic peripheral neuropathy or postherpetic neuralgia treated with pregabalin. Clin. Ther. 2013;35(5):612–623. doi: 10.1016/j.clinthera.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Jose VM, et al. Randomized double-blind study comparing the efficacy and safety of lamotrigine and amitriptyline in painful diabetic neuropathy. Diabet Med. 2007;24(4):377–383. doi: 10.1111/j.1464-5491.2007.02093.x. [DOI] [PubMed] [Google Scholar]
- 14.Morello CM, et al. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch. Intern. Med. 1999;159(16):1931–1937. doi: 10.1001/archinte.159.16.1931. [DOI] [PubMed] [Google Scholar]
- 15.Ohkubo Y, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res. Clin. Pract. 1995;28(2):103–117. doi: 10.1016/0168-8227(95)01064-K. [DOI] [PubMed] [Google Scholar]
- 16.Linn T, et al. Intensive therapy in adult insulin-dependent diabetes mellitus is associated with improved insulin sensitivity and reserve: a randomized, controlled, prospective study over 5 years in newly diagnosed patients. Metabolism. 1996;45(12):1508–1513. doi: 10.1016/S0026-0495(96)90180-8. [DOI] [PubMed] [Google Scholar]
- 17.Callaghan BC, et al. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tesfaye S, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia. 1996;39(11):1377–1384. doi: 10.1007/s001250050586. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler D, et al. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31(3):464–469. doi: 10.2337/dc07-1796. [DOI] [PubMed] [Google Scholar]
- 20.Wiggin TD, et al. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58(7):1634–1640. doi: 10.2337/db08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khovidhunkit W, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 2004;45(7):1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Smith AG, Singleton JR. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J. Diabetes Complic. 2013;27(5):436–442. doi: 10.1016/j.jdiacomp.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang JW, Pyun SB, Kwon HK. Relationship of vascular factors on electrophysiologic severity of diabetic neuropathy. Ann. Rehabil. Med. 2016;40(1):56–65. doi: 10.5535/arm.2016.40.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu WC, et al. Somatic neuropathy is an independent predictor of all- and diabetes-related mortality in type 2 diabetic patients: a population-based 5-year follow-up study (KCIS No. 29) Eur. J. Neurol. 2012;19(9):1192–1198. doi: 10.1111/j.1468-1331.2011.03659.x. [DOI] [PubMed] [Google Scholar]
- 25.Akbar M, et al. Potential association of triglyceride glucose index with cardiac autonomic neuropathy in type 2 diabetes mellitus patients. J. Korean Med. Sci. 2017;32(7):1131–1138. doi: 10.3346/jkms.2017.32.7.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aktaş HŞ, et al. The relation of protein C and protein S levels with cardiovascular risk in patients with diabetic neuropathy. Diabetes Metab. Syndr. 2016;10(4):234–237. doi: 10.1016/j.dsx.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Al-Mahroos F, Al-Roomi K. Diabetic neuropathy, foot ulceration, peripheral vascular disease and potential risk factors among patients with diabetes in Bahrain: a nationwide primary care diabetes clinic-based study. Ann. Saudi Med. 2007;27(1):25–31. doi: 10.5144/0256-4947.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kempler P, et al. Autonomic neuropathy is associated with increased cardiovascular risk factors: the EURODIAB IDDM Complications Study. Diabet. Med. 2002;19(11):900–909. doi: 10.1046/j.1464-5491.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- 29.Song L, Zhou L, Tang Z. An association analysis of lipid profile and diabetic cardiovascular autonomic neuropathy in a Chinese sample. Lipids Health Dis. 2016;15:122. doi: 10.1186/s12944-016-0287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swartz MK. The PRISMA statement: a guideline for systematic reviews and meta-analyses. J. Pediatr. Health Care. 2011;25(1):1–2. doi: 10.1016/j.pedhc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 32.Sabbagh HJ, et al. Passive smoking in the etiology of non-syndromicorofacial clefts: a systematic review and meta-analysis. PLoS ONE. 2015;10(3):e0116963. doi: 10.1371/journal.pone.0116963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int. J. Epidemiol. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jende J, et al. Association of serum cholesterol levels with peripheral nerve damage in patients with type 2 diabetes. JAMA Netw. Open. 2019;2(5):e194798. doi: 10.1001/jamanetworkopen.2019.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosny SS, et al. Relation between plasma Apelin level and peripheral neuropathy in Type 2 diabetic patients. Diabetes Metab. Syndr. 2019;13(1):626–629. doi: 10.1016/j.dsx.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 37.Vural G, Gümüsyayla Ş. Monocyte-to-high density lipoprotein ratio is associated with a decreased compound muscle action potential amplitude in patients with diabetic axonal polyneuropathy. Medicine (Baltimore) 2018;97(42):e12857. doi: 10.1097/MD.0000000000012857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen ST, et al. Corneal confocal microscopy as a tool for detecting diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes: ADDITION-Denmark. J. Diabetes Complic. 2018;32(12):1153–1159. doi: 10.1016/j.jdiacomp.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Mizokami-Stout KR, et al. The contemporary prevalence of diabetic neuropathy in type 1 diabetes: findings from the T1D exchange. Diabetes Care. 2020;43(4):806–812. doi: 10.2337/dc19-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litzelman DK, Marriott DJ, Vinicor F. Independent physiological predictors of foot lesions in patients with NIDDM. Diabetes Care. 1997;20(8):1273–1278. doi: 10.2337/diacare.20.8.1273. [DOI] [PubMed] [Google Scholar]
- 41.Aryan Z, et al. Conflicting interactions of apolipoprotein A and high density lipoprotein cholesterol with microvascular complications of type 2 diabetes. Diabetes Res. Clin. Pract. 2017;133:131–141. doi: 10.1016/j.diabres.2017.07.037. [DOI] [PubMed] [Google Scholar]
- 42.Akinci G, et al. Clinical spectra of neuromuscular manifestations in patients with lipodystrophy: a multicenter study. Neuromuscul. Disord. 2017;27(10):923–930. doi: 10.1016/j.nmd.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 43.Jane SW, et al. Prevalence, discomfort and self-relief behaviours of painful diabetic neuropathy in Taiwan: a cross-sectional study. BMJ Open. 2016;6(10):e011897. doi: 10.1136/bmjopen-2016-011897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Najafi MT, et al. Ambulatory blood pressure monitoring and diabetes complications: targeting morning blood pressure surge and nocturnal dipping. Medicine (Baltimore) 2018;97(38):e12185. doi: 10.1097/MD.0000000000012185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zoppini G, et al. Prevalence of cardiovascular autonomic neuropathy in a cohort of patients with newly diagnosed type 2 diabetes: the Verona newly diagnosed type 2 diabetes study (VNDS) Diabetes Care. 2015;38(8):1487–1493. doi: 10.2337/dc15-0081. [DOI] [PubMed] [Google Scholar]
- 46.Ishibashi F, et al. Correlation between sudomotor function, sweat gland duct size and corneal nerve fiber pathology in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2014;5(5):588–596. doi: 10.1111/jdi.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho YN, et al. The role of insulin resistance in diabetic neuropathy in Koreans with type 2 diabetes mellitus: a 6-year follow-up study. Yonsei Med. J. 2014;55(3):700–708. doi: 10.3349/ymj.2014.55.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosales-Hernandez A, et al. Absence of clinical relationship between oxidized low density lipoproteins and diabetic peripheral neuropathy: a case control study. Lipids Health Dis. 2014;13:32. doi: 10.1186/1476-511X-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jende J, et al. Diabetic neuropathy differs between type 1 and type 2 diabetes: insights from magnetic resonance neurography. Ann. Neurol. 2018;83(3):588–598. doi: 10.1002/ana.25182. [DOI] [PubMed] [Google Scholar]
- 50.Katulanda P, et al. The prevalence, patterns and predictors of diabetic peripheral neuropathy in a developing country. Diabetol. Metab. Syndr. 2012;4(1):21. doi: 10.1186/1758-5996-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsiao JY, et al. The relationship between diabetic autonomic neuropathy and diabetic risk factors in a Taiwanese population. J. Int. Med. Res. 2011;39(4):1155–1162. doi: 10.1177/147323001103900403. [DOI] [PubMed] [Google Scholar]
- 52.Ylitalo KR, Sowers M, Heeringa S. Peripheral vascular disease and peripheral neuropathy in individuals with cardiometabolic clustering and obesity: National Health and Nutrition Examination Survey 2001–2004. Diabetes Care. 2011;34(7):1642–1647. doi: 10.2337/dc10-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spallone V, et al. Clinical correlates of painful diabetic neuropathy and relationship of neuropathic pain with sensorimotor and autonomic nerve function. Eur. J. Pain. 2011;15(2):153–160. doi: 10.1016/j.ejpain.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 54.Nybo M, et al. Plasma osteoprotegerin concentrations in peripheral sensory neuropathy in Type 1 and Type 2 diabetic patients. Diabet. Med. 2010;27(3):289–294. doi: 10.1111/j.1464-5491.2010.02940.x. [DOI] [PubMed] [Google Scholar]
- 55.Coppini DV, et al. Established diabetic neuropathy seems irreversible despite improvements in metabolic and vascular risk markers–a retrospective case-control study in a hospital patient cohort. Diabet. Med. 2006;23(9):1016–1020. doi: 10.1111/j.1464-5491.2006.01934.x. [DOI] [PubMed] [Google Scholar]
- 56.Witte DR, et al. Risk factors for cardiac autonomic neuropathy in type 1 diabetes mellitus. Diabetologia. 2005;48(1):164–171. doi: 10.1007/s00125-004-1617-y. [DOI] [PubMed] [Google Scholar]
- 57.Maser RE, et al. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes. 1989;38(11):1456–1461. doi: 10.2337/diab.38.11.1456. [DOI] [PubMed] [Google Scholar]
- 58.Maser RE, et al. Diabetic autonomic neuropathy and cardiovascular risk. Pittsburgh Epidemiology of Diabetes Complications Study III. Arch. Intern. Med. 1990;150(6):1218–1222. doi: 10.1001/archinte.1990.00390180056009. [DOI] [PubMed] [Google Scholar]
- 59.Orchard TJ, et al. Why does diabetic autonomic neuropathy predict IDDM mortality? An analysis from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Res. Clin. Pract. 1996;34(Suppl):S165–S171. doi: 10.1016/S0168-8227(96)90025-X. [DOI] [PubMed] [Google Scholar]
- 60.Simmons D, Ng LL, Bomford J. Relationship between myoinositol influx and lipids in diabetic neuropathy. ActaDiabetol. 1993;30(4):233–237. doi: 10.1007/BF00569934. [DOI] [PubMed] [Google Scholar]
- 61.Sone, H., S. Mizuno and N. Yamada, Vascular risk factors and diabetic neuropathy. N Engl J Med, 2005. 352(18): p. 1925–7; author reply 1925–7. [PubMed]
- 62.Callaghan BC, et al. Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care. 2016;39(5):801–807. doi: 10.2337/dc16-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Callaghan BC, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann. Clin. Transl. Neurol. 2018;5(4):397–405. doi: 10.1002/acn3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Christen WG, et al. Risk factors for progression of distal symmetric polyneuropathy in type 1 diabetes mellitus. Sorbinil Retinopathy Trial Research Group. Am. J. Epidemiol. 1999;150(11):1142–1151. doi: 10.1093/oxfordjournals.aje.a009941. [DOI] [PubMed] [Google Scholar]
- 65.Terekeci HM, et al. Plasma osteoprotegerin concentrations in type 2diabetic patients and its association with neuropathy. Exp. Clin. Endocrinol. Diabetes. 2009;117(3):119–123. doi: 10.1055/s-0028-1085425. [DOI] [PubMed] [Google Scholar]
- 66.Vinik AI, et al. Diabetic neuropathies. Diabetologia. 2000;43(8):957–973. doi: 10.1007/s001250051477. [DOI] [PubMed] [Google Scholar]
- 67.Pop-Busui R, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.The effect of intensive diabetes therapy on the development and progression of neuropathy. The Diabetes Control and Complications Trial Research Group. Ann Intern Med, 1995. 122(8): 561–568. [DOI] [PubMed]
- 69.Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015;14:121. doi: 10.1186/s12944-015-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim, J.J. and D.D. Sears, TLR4 and Insulin Resistance. Gastroenterol Res Pract, 2010. 2010. [DOI] [PMC free article] [PubMed]
- 71.Han L, et al. Peripheral neuropathy is associated with insulin resistance independent of metabolic syndrome. Diabetol. Metab. Syndr. 2015;7:14. doi: 10.1186/s13098-015-0010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vincent AM, et al. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat. Rev. Neurol. 2011;7(10):573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- 73.Vincent AM, et al. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58(10):2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie F, et al. High energy diets-induced metabolic and prediabetic painful polyneuropathy in rats. PLoS ONE. 2013;8(2):e57427. doi: 10.1371/journal.pone.0057427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jang ER, Lee CS. 7-ketocholesterol induces apoptosis in differentiated PC12 cells via reactive oxygen species-dependent activation of NF-κB and Akt pathways. Neurochem. Int. 2011;58(1):52–59. doi: 10.1016/j.neuint.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 76.Hur J, et al. The identification of gene expression profiles associated with progression of human diabetic neuropathy. Brain. 2011;134(Pt 11):3222–3235. doi: 10.1093/brain/awr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim B, et al. Hyperinsulinemia induces insulin resistance in dorsal root ganglion neurons. Endocrinology. 2011;152(10):3638–3647. doi: 10.1210/en.2011-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.