Abstract
Objectives
Advanced thymic epithelial tumors (TETs) lack adequate treatment options in part due to absence of well characterized tumor-specific antigens. Mesothelin, a cell surface antigen, has been used successfully as a target for tumor-directed therapy. We sought to determine tumor expression and serum levels of mesothelin in patients with TETs.
Patients and Methods
Tissue samples were obtained from 71 patients with histologically confirmed, unresectable advanced TETs and evaluated for mesothelin expression by immunohistochemistry. The evaluation was blinded for clinical data and outcome. Mesothelin expression and its association with clinico-pathological parameters and survival were assessed.
Results
Thymic carcinoma, thymoma, and thymic neuroendocrine tumors (NETs) accounted for 34 (48%), 29 (41%), and 8 (11%) cases respectively. Mesothelin expression was seen in a significantly larger proportion of thymic carcinoma (27/34, 79%) than thymoma (3/29, 10%) (P<0.0001) and was absent in thymic NETs. Among thymic carcinomas 13/34 (38%) showed expression in nearly all tumor cells. Immunoreactivity was membranous, strong, and homogenous. Patients with thymic carcinoma and high mesothelin expression (in >50% of tumor cells) had significantly improved overall survival (median not reached, n=19) compared to patients with no or low mesothelin expression (1.60 years; 95% CI: 1.24 to 4.94 years; n=15; HR=4.46, 95% CI: 1.55 to 12.80; p=0.0026).
Conclusion
Mesothelin expression is frequently observed in advanced thymic carcinomas, infrequently in thymomas and is absent in thymic NETs. Due to strong, membranous expression mesothelin is a potential therapeutic target in thymic carcinoma.
Keywords: Thymic carcinoma, Mesothelin, Immunohistochemistry, Therapeutic target, Immunotherapy
1. Introduction
Although the overall prevalence of thymic epithelial tumors (TETs) is very low, they are the most common cause of anterior mediastinal tumors in adults. The World Health Organization (WHO) system, which subdivides thymic epithelial tumors into thymoma subtypes A, AB, B1, B2, and B3 and thymic carcinomas is widely used to classify TETs based on their histologic appearance.1 The histologic appearance correlates with the biology of the tumor and its prognosis.2 Other important prognostic factors include the stage of the disease and the completeness of surgical resection.
No standard systemic treatments exist for relapsed or refractory TETs. In phase II trials of experimental agents, response rates have been low and progression-free survival limited.3 The lack of effective therapeutic options is particularly obvious in thymic carcinoma. Less than a quarter of patients with inoperable thymic carcinoma are alive 5 years after diagnosis.4 Unlike many other epithelial tumors, tumor DNA sequencing has not identified targetable genes in TETs.5 There is an unmet need to develop new and effective therapies for TETs, in particular for thymic carcinoma.
Mesothelin is a cell surface antigen that is present on normal mesothelial cells lining the pleura, peritoneum and pericardium.6 Mesothelin is highly expressed in several cancers, including epithelioid mesotheliomas, pancreatic and biliary adenocarcinomas, gastric and ovarian cancers.7–10 Several drugs targeting mesothelin are in various phases of clinical evaluation. They aim to exploit the differential expression of mesothelin in cancers compared with normal tissue.11–13 Only two previous studies have assessed mesothelin expression in TETs wherein it was used as an immunohistochemical (IHC) marker for pathological differential diagnosis.14, 15 These studies were retrospective in nature, had a limited number of samples, did not provide clinical information and did not study the patterns of expression in detail. Given the paucity of data available, we sought to determine the expression patterns and prognostic value of mesothelin in TETs and the association of mesothelin expression in tumor cells with serum mesothelin, clinico-pathologic variables and survival.
2. Patients and methods
2.1 Patients
Tissue samples from patients with histologically confirmed, unresectable advanced (Masaoka stage III or stage IV)16 TETs enrolled in clinical trials at the National Cancer Institute (NCI) between December 2007 and December 2012 (ClinicalTrials.gov Identifier: NCT 00965250, NCT00589290, NCT01100944, NCT01621568)3, 17–19 were included in this analysis. Patients who did not have tumor samples available for mesothelin IHC were excluded. Clinical and radiographic evidence was consistent with a diagnosis of a TET in all cases and there was no evidence to support a diagnosis of an alternative primary tumor source in any patient included in this study. All patients were followed for survival. All patients gave written informed consent in accordance with the NCI institutional review board regulations.
2.2 Tumor Pathology Evaluation
Tumor samples consisting of biopsy or resection specimens were obtained either from referring institutions or from procedures performed at the NCI and evaluated by the Laboratory of Pathology, NCI. Routine histopathological analysis was performed and tumors were subtyped according to the World Health Organization histological classification of TETs.1 For the purposes of this analysis tumors were categorized into three groups: thymoma, thymic carcinoma and thymic neuroendocrine tumors (NETs). Although thymic NETs are included under the category of thymic carcinomas in the WHO classification1 we separated them into an independent category based on previous literature showing an absence of mesothelin expression in small cell carcinoma and carcinoids.8, 20 All but one of the thymic carcinomas tested (19/20) were positive for CD5, or KIT (15/16), with all tested cases positive for at least one of these markers.
2.3 Tumor Mesothelin Expression
Mesothelin IHC was performed on tumor samples using monoclonal antibody 5B2 (Novocastra/Leica, Bannockburn, IL) at a dilution of 1:40. Incubation with primary antibody was preceded by 20 minutes heat-induced epitope retrieval in citrate buffer, pH 6.0. The detection was performed with Ventana Ultra View detection kit with DAB as the chromogen.
Immunohistochemical staining was evaluated by a pathologist (MM) with expertise in IHC who was blinded to the clinical data. The positivity (strength of labeling) was assessed as negative (no labeling), or positive, and the percentage of positive cells was also estimated.
2.4 Serum Mesothelin
Mesothelin (nmol/L) was measured in serum using the MesomarkTM (Fujirebio Diagnostics, Inc., Malvern, PA). Assays were run according to manufacturer’s instructions, blinded to patient data. The normal level of serum mesothelin is ≤ 1.5nM/L.
2.5 Statistical Methods
The association of gender with mesothelin positivity was determined using Fisher’s exact test. The association of race and histology with mesothelin positivity was determined by Mehta’s modification to Fisher’s exact test21, while a Cochran-Armitage test for trend was used to assess the association between stage and mesothelin positivity.22 The Kruskal-Wallis test was used to compare distributions of mesothelin expression (%) according to histology. Overall survival was defined as the time from date of diagnosis of metastatic cancer to date of death or last follow-up. The association between mesothelin expression or histology and survival was estimated using the Kaplan-Meier method, with curves compared using a log-rank test. Hazard ratios and associated confidence intervals were determined using a Cox proportional hazards model. All p-values are two-tailed and presented without adjustment for multiple comparisons. Univariate survival analyses were performed on the following prognostic factors: age, sex, race, stage and histology. Log-rank test and trend test p-values were calculated as appropriate. Prognostic factors associated with survival were subsequently included in a multiple proportional hazards regression analysis. Details of statistical methods used for univariate survival and multiple proportional hazards (PH) regression analyses are provided in the supplemental appendix.
3. Results
Seventy-one patients with TETs were included in this study. The clinico-pathological characteristics of patients are summarized in Table 1. Thymic carcinoma, thymoma, and thymic neuroendocrine tumors (NETs) accounted for 34 (48%), 29 (41%), and 8 (11%) cases respectively. Most thymic carcinomas (n = 29) were non-keratinizing squamous cell carcinomas. In addition, there was 1 keratinizing squamous cell carcinoma, 1 basaloid carcinoma and 3 unclassified carcinomas. Thymic NETs consisted of 2 atypical carcinoids and 6 neuroendocrine carcinomas. The thymoma group comprised of 3 AB, 2 B1, 13 B2, and 11 B3 thymomas. Median age of patients was 51 years (range, 20–86 years); 38 (54%) patients were male; 52 (73%) were white; Asians and blacks constituted 11% and 10% respectively. Tumor samples were obtained at advanced stages (Masaoka stages III and IV) in 70 of 71 (99%) of patients.
Table 1.
Patient Characteristics
| N (%) | |
|---|---|
| Age, Median (range) | 51 (20–86) |
| Sex | |
| Male | 38 (54) |
| Female | 33 (46) |
| Race | |
| White | 52 (73) |
| Asian | 8 (11) |
| Black | 7 (10) |
| Hispanic | 3 (4) |
| Mixed | 1 (1) |
| Histology | |
| Thymoma | 29 (41) |
| AB | 3 |
| B1 | 2 |
| B2 | 13 |
| B3 | 11 |
| Thymic carcinoma | 34 (48) |
| Squamous, non-keratinizing | 29 |
| Squamous, keratinizing | 1 |
| Basaloid | 1 |
| Uncategorized | 3 |
| Thymic neuroendocrine tumors | 8 (11) |
| Atypical carcinoid | 2 |
| Large cell neuroendocrine carcinoma | 6 |
| Stage at presentation | |
| II | 1 (1) |
| III | 4 (6) |
| IVA | 15 (21) |
| IVB | 51 (72) |
3.1 Mesothelin expression in thymic epithelial tumors and association with clinico-pathological data
Characteristics of mesothelin expression in TETs are detailed in Table 2, and Figure 1 shows representative images of mesothelin expression in TETs. Of the 34 thymic carcinomas assessed, mesothelin expression was observed in 27 (79%) samples. Among these samples, immunoreaction was membranous, strong, and homogenous, with staining observable in more than 50% of tumor cells in 19 (70%) cases. In thirteen of 27 (48%) cases of mesothelin-expressing thymic carcinoma, nearly all viable tumor cells expressed mesothelin. Among thymomas (n=29), mesothelin expression was infrequent; only 3 (10%) cases (2 WHO subtype B3, and 1 WHO subtype B2) expressed mesothelin. Mesothelin expression was absent in thymic NETs (Figure 2).
Table 2.
Mesothelin expression in thymic epithelial tumors (n=71)
| Histology | Number of samples | Positive N (%) | Percentage of mesothelin
positive cells |
Negative N (%) | ||
|---|---|---|---|---|---|---|
| < 1% | 1–50% | 51–100% | ||||
|
| ||||||
| Thymic carcinoma | 34 | 27 (79) | 0 | 8 | 19* | 7 (21) |
| Squamous | 30 | 25 (83) | 0 | 8 | 17 | 5 (17) |
| Basaloid | 1 | 0 | 0 | 0 | 0 | 1 (100) |
| Uncategorized | 3 | 2 (67) | 0 | 0 | 2 | 1 (33) |
|
| ||||||
| Thymoma | 29 | 3 (10) | 1 | 1 | 1 | 26 (90) |
|
| ||||||
| Thymic neuroendocrine tumors^ | 8 | 0 | 0 | 0 | 0 | 8 (100) |
Mesothelin expression was detected in 60% of tumor cells in 2 (SCC and uncategorized), 70% in 1 (SCC), 80% in 2 (both SCC), 95% in 1 (SCC) and 100% in 13 cases (12 SCC, 1 uncategorized) respectively.
Thymic neuroendocrine tumors consisted of 2 cases of atypical carcinoid and 6 cases of large cell neuroendocrine carcinoma.
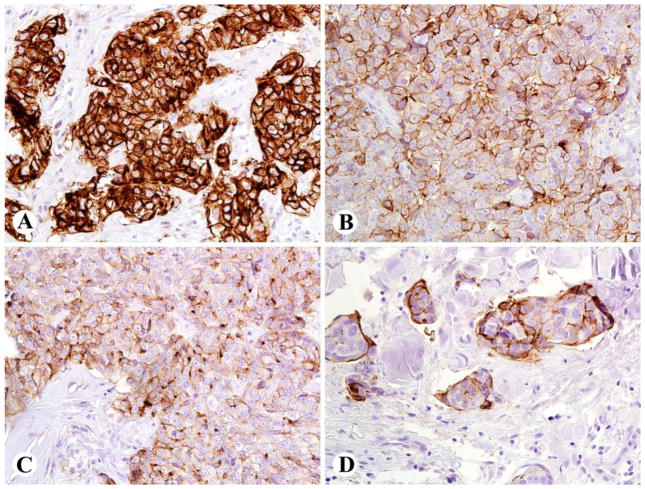
Figure 1.
Patterns of mesothelin immunostaining in thymic non-keratinizing squamous cell carcinomas (SCC) [using monoclonal antibody 5B2 (Novocastra/Leica, Bannockburn, IL) at a dilution of 1:40]. A: Thymic SCC with strong 3+ staining in 100% of tumor cells, with membrane and cytoplasmic labeling in 100% of tumor cells, B: Thymic SCC with strong 3+ staining essentially restricted to cell membranes, in 80% of cells, C: Thymic SCC with moderate to strong 2–3+ staining in cell membrane and cytoplasmic dots, in 70% of cells, D: Thymic SCC with strong membrane staining in 50% of tumor cells.
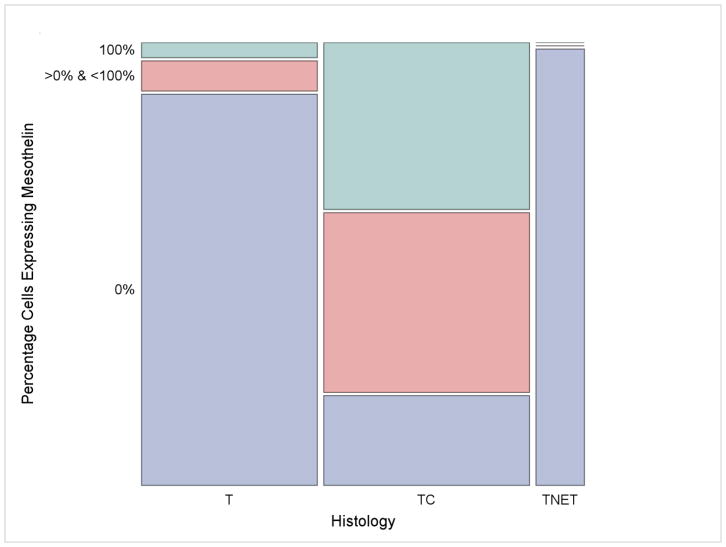
Figure 2.
Mesothelin expression based on histology. Any level of mesothelin expression was seen in a significantly larger proportion of thymic carcinoma (n=27, 79%; middle column) than thymoma (n=3, 10%; left column) (P<0.0001). Mesothelin expression was absent in thymic neuroendocrine tumors (right column). Tumors showing 100% mesothelin expression included 12 cases of squamous cell carcinoma, 1 uncategorized case of thymic carcinoma and 1 case of B3 thymoma. T: thymoma; TC: thymic carcinoma; TNET: thymic neuroendocrine tumors.
The association between mesothelin expression and clinico-pathological characteristics is shown in Table 3. A strong association was observed between mesothelin expression and TET histology: among samples expressing mesothelin 90% were thymic carcinomas compared with only 10% of thymoma (P<0.0001); mesothelin expression was absent in all eight thymic NET samples evaluated. The degree of mesothelin expression within each histologic subgroup and comparison between histologic subgroups is presented in Supplemental Tables 1 and 2. There was no evidence for an association between mesothelin expression and sex, race or stage of disease.
Table 3.
Association of mesothelin expression in thymic epithelial tumors with clinico-pathological data
| Mesothelin expression | P value | ||
|---|---|---|---|
| Negative | Positive | ||
| N (%) | N (%) | ||
| Sex | 0.47 | ||
| Male | 20(49) | 18(60) | |
| Female | 21(51) | 12(40) | |
| Race | 0.43 | ||
| White | 30(73) | 22(73) | |
| Black | 3(7) | 4(13) | |
| Hispanic | 3(7) | 0(0) | |
| Asian | 5(12) | 3(10) | |
| Mixed | 0(0) | 1(3) | |
| Histology | <0.0001 | ||
| Thymic carcinoma | 7(17) | 27(90) | |
| Thymoma | 26(63) | 3(10) | |
| Thymic neuroendocrine tumor | 8(20) | 0 | |
| Stage | 0.49 | ||
| II | 0(0) | 1(3) | |
| III | 2(5) | 2(7) | |
| IV | 39(95) | 27(90) | |
3.2 Association of mesothelin expression with survival of patients with thymic epithelial tumors
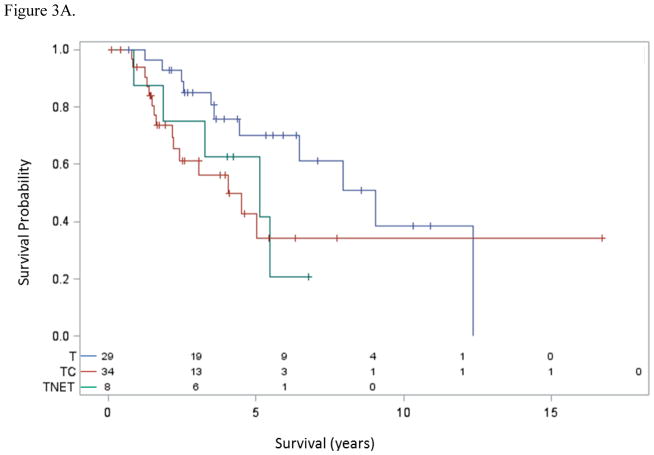
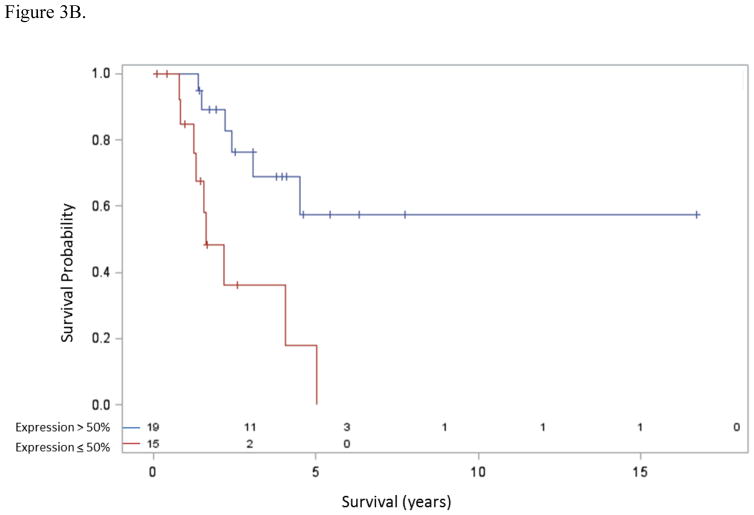
Patients with thymic carcinoma had inferior overall survival compared to patients with thymoma [median 3.99 years (95% confidence interval (CI): 2.15 years to undefined) vs. 8.93 years (95% CI: 4.38 to 12.18 years); hazard ratio (HR)=2.18, 95% CI 0.98 4.85; p=0.051] (Figure 3A). Also, the 8 patients with thymic NETs had similar survival (median 5.06 years, 95% CI: 0.87 years to undefined) to those with thymic carcinoma (p=0.42) and also somewhat inferior overall survival to patients with thymoma (p=0.068). Among patients with thymic carcinoma (n=34), those with high mesothelin expression, defined as mesothelin expression in > 50% cells (n=19), had a significantly improved overall survival over patients with either no or low mesothelin expression (n=15) [median not reached vs. 1.60 years (95% CI: 1.24–4.94 years); HR=4.46, 95% CI: 1.55–12.80; P=0.0026] (Figure 3B).
Figure 3.
Figure 3A. Survival and histology. Median overall survival was significantly longer for patients with thymoma compared to other histologies. T: thymoma, TC: thymic carcinoma, TNET: thymic neuroendocrine tumor.
Figure 3B. Survival and mesothelin expression in thymic carcinoma. Median overall survival was significantly longer for patients with >50% expression of mesothelin (not reached) versus ≤50% expression (1.60 years, 95% CI: 1.24–4.94 years), HR 4.46 (95% CI: 1.55–12.80), p=0.0026.
3.3 Univariate survival and multiple PH regression analyses
Univariate and multiple regression results are presented in Table 4 (all patients, n=71) and Table 5 (patients with thymic carcinoma, n=34). We found no evidence that age, race, or mesothelin expression was related to survival using all of the data in univariate analysis (Table 4). Histology was weakly associated with survival (p=0.11). Males had a slightly lower survival rate than females (log-rank p=0.073, HR = 1.94). Patients with stage IVB TETs had lower survival rates than stage IIB/II/IVA TETs (log-rank p=0.052, HR = 2.59). Sex, stage and histology were included in the model and the hazard ratios changed little between the univariate and regression models.
Table 4.
Univariate and multiple proportional hazards regression analyses using data from all patients (n=71)
| Univariate | Multiple Regression | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Stratum Compared* (N) | P (Log-rank) | P (Trend) | Hazard Ratio (HR) | 95% CI | Hazard Ratio (HR) | 95% CI |
| Age | 0.37 | 0.68 | |||||
| <40 (16) vs. 40–51 (19) | 0.54 | 0.20, 1.41 | |||||
| <40 vs. 51–62 (18) | 0.46 | 0.17, 1.26 | |||||
| <40 vs. >62 (18) | 0.91 | 0.33, 2.47 | |||||
| Sex | Female (33) vs. Male (38) | 0.073 | 1.94 | 0.93, 4.06 | 1.81 | 0.87, 3.81 | |
| Race | Asian (8), Black (7), Hispanic (3), Mixed (1) | 0.15 | |||||
| Other (19) vs. White (52) | 0.56 | 1.27 | 0.56, 2.88 | ||||
| Stage | IIB/III (5), IVA (15), IVB (51) | 0.075 | 0.081 | ||||
| IIB/III/IVA (20) vs. IVB (51) | 0.052 | 2.59 | 0.99, 6.77 | 2.65 | 0.91, 7.17 | ||
| Histology | 0.11 | ||||||
| T (29) vs. TC (34) | 2.18 | 0.98, 4.85 | 2.07 | 0.91, 4.69 | |||
| T vs. TNET (8) | 2.13 | 0.73, 6.26 | 1.75 | 0.59, 5.19 | |||
| Mesothelin expression | >50 (20) vs. ≤50 (51) | 0.53 | 1.31 | 0.56, 3.06 | |||
T: thymoma; TC: thymic carcinoma; TNET: thymic neuroendocrine tumors;
The reference group used to calculate the hazard ratio is indicated first.
Table 5.
Univariate and multiple proportional hazards regression analyses for patients with thymic carcinoma (n=34)
| Univariate | Multiple Regression | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Comparison* (N) | P (Log-rank) | P (Trend) | Hazard Ratio (HR) | 95% CI | Hazard Ratio (HR) | 95% CI |
| Age | 0.17 | 0.31 | |||||
| <40 (6) vs. 40–51 (6) | 0.15 | 0.02, 1.29 | |||||
| <40 vs. 51–62 (9) | 0.29 | 0.07, 1.13 | |||||
| <40 vs. >62 (13) | 0.39 | 0.11, 1.38 | |||||
| Sex | Female (13) vs. Male (21) | 0.32 | 1.72 | 0.59, 5.04 | 2.32 | 0.74, 7.22 | |
| Race | |||||||
| Other (8) vs. White (26) | 0.83 | 1.15 | 0.32, 4.09 | ||||
| Stage | IIB/III (4), IVA (4), IVB (26) | 0.87 | 0.62 | ||||
| IIB/III/IVA (8) vs. IVB (26) | 0.59 | 1.40 | 0.39, 4.99 | 1.93 | 0.51, 7.38 | ||
| Mesothelin expression | >50 (19) vs. <50 (15) | 0.003 | 4.46 | 1.55, 12.80 | 5.39 | 1.77, 16.40 | |
The reference group used to calculate the hazard ratio is indicated first.
For patients with thymic carcinoma (n=34), only mesothelin expression was associated with survival in either univariate or PH model analysis (Table 5). However, these results should be viewed cautiously since the sample sizes in some of the strata are very small.
3.3 Tumor mesothelin expression and serum mesothelin levels
The median serum mesothelin levels in thymic carcinoma and thymoma were 0.77 nM/L (range 0.09–2.53) and 0.90 nM/L (range 0.55–2.65) respectively. Only three patients with thymoma (subtypes AB, B2 and B3 respectively) and two patients with thymic carcinoma had serum mesothelin levels above the normal range.
4. Discussion
We believe this is the first study to systematically characterize mesothelin expression in TETs and evaluate its association with clinico-pathologic variables and survival. This analysis of a comprehensive TET cohort demonstrates that mesothelin expression is seen in a majority of cases of thymic carcinoma: strong membranous expression of mesothelin (staining in greater than 50% tumor cells) was observed in 70% cases and nearly all tumor cells expressed mesothelin in 38%. These results are comparable to observations in epithelioid mesothelioma where 73% cases show strong mesothelin expression.9 Furthermore, mesothelin expression was prognostic in thymic carcinoma with high mesothelin expression being associated with longer survival. Mesothelin expression was infrequent in thymoma and absent in thymic NETs.
To our knowledge, only two previous studies have assessed mesothelin expression in TETs. Pan et al found mesothelin immunoreactivity in 36% (8 of 22) thymic carcinomas and Khoury et al reported an expression rate of 42% (5 of 12).14, 15 The cause of discrepancy between rates of expression of mesothelin in patients with thymic carcinoma in our study compared with prior reports remains uncertain. The predominance of patients with stage IV thymic carcinoma (29 out of 34 cases, 85%) in our series might have influenced the results since higher rates of mesothelin expression have been observed in other advanced-stage tumors compared to early-stage tumors.23 Other factors such as differences in IHC technique could also potentially explain the discrepancy in results compared to previously published studies. Among thymomas, our finding of infrequent mesothelin expression is in line with prior studies, which found no mesothelin immunoreactivity in 79 cumulative patients.14, 15 Our observation of mesothelin expression in 3 patients with thymoma (including two cases of B3 thymoma) could possibly reflect the histological heterogeneity of TETs and its impact on sampling and subtype classification.24
Our finding of a high frequency and strong membranous expression of mesothelin in advanced thymic carcinoma raises the possibility of using mesothelin-directed immunotherapy to treat these tumors. Mesothelin expression in normal human tissues is observed only in a single layer of mesothelial cells lining the pleura, peritoneum and pericardium, surface epithelial cells of the ovary, tunica vaginalis, rete testis and the tonsillar and fallopian tube epithelial cells.8 Previous studies by our group and others have demonstrated high mesothelin expression in a number of other cancers, including epithelioid mesotheliomas, pancreatic and biliary adenocarcinomas, gastric and ovarian cancers.7–10 In several of these cancers, mesothelin-targeted therapies are being evaluated in clinical trials.25–27 We recently demonstrated major tumor regressions that were durable in chemotherapy-refractory patients with advanced epithelioid mesothelioma using the anti-mesothelin immunotoxin, SS1P.25 Partial responses to DMOT4039A, an antibody-drug conjugate targeting mesothelin have also been observed in advanced pancreatic cancer and platinum-resistant ovarian cancer.28
Despite strong tumoral expression of mesothelin, serum mesothelin levels were low in thymic carcinoma. Low serum mesothelin levels despite tumoral overexpression have been described previously in pancreatic carcinoma.29 This is in contrast to mesothelioma, another mesothelin-expressing tumor where serum mesothelin is increased in 80% of cases where tumors express mesothelin.30 Serum mesothelin detected in the sera of cancer patients consists predominantly of the extracellular domain of the membrane bound mesothelin, which is shed into circulation.31 Although the mechanisms underlying mesothelin shedding is not fully understood32, it is conceivable that these mechanisms are modulated differently in thymic carcinoma compared to other mesothelin-expressing tumors.
To our knowledge, this is the largest series of TETs to be systematically assessed for mesothelin expression. The strengths of our study include tumor samples derived from a uniform patient population consisting of patients with advanced TETs who enrolled on phase I/II clinical trials, and availability of long-term follow-up data.
5. Conclusions
We demonstrate that mesothelin is expressed in a majority of advanced thymic carcinomas, whereas it is infrequent in thymoma and thymic NETs. Mesothelin expression was prognostic in thymic carcinoma with high mesothelin expression being associated with significantly prolonged survival. Despite strong tumoral expression of mesothelin, serum mesothelin levels were low. The functional role of mesothelin in thymic carcinoma and its potential role as a target for therapeutic interventions need further investigation.
Supplementary Material
Highlights.
Mesothelin, a cell surface antigen is a target for tumor-directed therapy.
Thymic carcinomas frequently demonstrate strong mesothelin expression.
High mesothelin expression in thymic carcinoma is associated with longer survival.
Mesothelin-directed therapy should be evaluated in advanced thymic carcinoma.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Disclosures: None of the authors have any conflicts of interest to disclose.
Conflict of Interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. International Agency for Research on Cancer; Lyon: 2015. pp. 183–243. [DOI] [PubMed] [Google Scholar]
- 2.Quintanilla-Martinez L, Wilkins EW, Jr, Choi N, Efird J, Hug E, Harris NL. Thymoma. Histologic subclassification is an independent prognostic factor. Cancer. 1994;74:606–617. doi: 10.1002/1097-0142(19940715)74:2<606::aid-cncr2820740212>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.Rajan A, Carter CA, Berman A, Cao L, Kelly RJ, Thomas A, Khozin S, Chavez AL, Bergagnini I, Scepura B, Szabo E, Lee MJ, Trepel JB, Browne SK, Rosen LB, Yu Y, Steinberg SM, Chen HX, Riely GJ, Giaccone G. Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: a multicentre, open-label, phase 2 trial. Lancet Oncol. 2014;15:191–200. doi: 10.1016/S1470-2045(13)70596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: Apr, 2013. http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site. [Google Scholar]
- 5.Chen Y, Gharwan H, Thomas A. Novel biologic therapies for thymic epithelial tumors. Front Oncol. 2014;4:103. doi: 10.3389/fonc.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: Identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 8.Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Ordonez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003;16:192–197. doi: 10.1097/01.MP.0000056981.16578.C3. [DOI] [PubMed] [Google Scholar]
- 10.Hassan R, Kreitman RJ, Pastan I, Willingham MC. Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol. 2005;13:243–247. doi: 10.1097/01.pai.00000141545.36485.d6. [DOI] [PubMed] [Google Scholar]
- 11.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly RJ, Sharon E, Pastan I, Hassan R. Mesothelin-Targeted Agents in Clinical Trials and in Preclinical Development. Mol Cancer Ther. 2012;11:517–525. doi: 10.1158/1535-7163.MCT-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol. 2012;13:e301–e310. doi: 10.1016/S1470-2045(12)70126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan CC, Chen PC, Chou TY, Chiang H. Expression of calretinin and other mesothelioma-related markers in thymic carcinoma and thymoma. Hum Pathol. 2003;34:1155–1162. doi: 10.1053/j.humpath.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Khoury T, Chandrasekhar R, Wilding G, Tan D, Cheney RT. Tumour eosinophilia combined with an immunohistochemistry panel is useful in the differentiation of type B3 thymoma from thymic carcinoma. Int J Exp Pathol. 2011;92:87–96. doi: 10.1111/j.1365-2613.2010.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–2492. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Giaccone G, Rajan A, Berman A, Kelly RJ, Szabo E, Lopez-Chavez A, Trepel J, Lee MJ, Cao L, Espinoza-Delgado I, Spittler J, Loehrer PJ., Sr Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J Clin Oncol. 2011;29:2052–2059. doi: 10.1200/JCO.2010.32.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas A, Rajan A, Szabo E, Tomita Y, Carter CA, Scepura B, Lopez-Chavez A, Lee MJ, Redon CE, Frosch A, Peer CJ, Chen Y, Piekarz R, Steinberg SM, Trepel JB, Figg WD, Schrump DS, Giaccone G. A phase I/II trial of belinostat in combination with cisplatin doxorubicin, and cyclophospahmide in thymic epithelial tumors: a clinical and translational study. Clin Cancer Res. 2014;20:5392–5402. doi: 10.1158/1078-0432.CCR-14-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas A, Rajan A, Berman A, Tomita Y, Brzezniak C, Lee MJ, Lee S, Ling A, Spittler AJ, Carter CA, Guha U, Wang Y, Szabo E, Meltzer P, Steinberg SM, Trepel JB, Loehrer PJ, Giaccone G. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol. 2015;16:177–186. doi: 10.1016/S1470-2045(14)71181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miettinen M, Sarlomo-Rikala M. Expression of calretinin, thrombomodulin, keratin 5, and mesothelin in lung carcinomas of different types: an immunohistochemical analysis of 596 tumors in comparison with epithelioid mesotheliomas of the pleura. Am J Surg Pathol. 2003;27:150–158. doi: 10.1097/00000478-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Mehta CR, Patel NR. A network algorithm for performing Fisher’s exact test in rXc contingency tables. J Am Stat Assoc. 1983;78:427–434. [Google Scholar]
- 22.Agresti A. Categorical Data Analysis. New York: John Wiley and Sons Inc; 1990. pp. 79–129. [Google Scholar]
- 23.Cheng WF, Huang CY, Chang MC, Hu YH, Chiang YC, Chen YL, Hsieh CY, Chen CA. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br J Cancer. 2009;100:1144–1153. doi: 10.1038/sj.bjc.6604964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran CA, Suster S. On the histologic heterogeneity of thymic epithelial neoplasms. Impact of sampling in subtyping and classification of thymomas. Am J Clin Pathol. 2000;114:760–766. doi: 10.1309/CYJH-9RXM-P2PK-120J. [DOI] [PubMed] [Google Scholar]
- 25.Hassan R, Miller AC, Sharon E, Thomas A, Reynolds JC, Ling A, Kreitman RJ, Miettinen MM, Steinberg SM, Fowler DH, Pastan I. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5:208ra147. doi: 10.1126/scitranslmed.3006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, Schweizer C, Weil S, Laheru D. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res. 2010;16:6132–6138. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, Sterman DH, Hassan R, Lutz E, Moyer B, Giedlin M, Louis JL, Sugar EA, Pons A, Cox AL, Levine J, Murphy AL, Illei P, Dubensky TW, Jr, Eiden JE, Jaffee EM, Laheru DA. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–868. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weekes CD, Lamberts LE, Borad MJ, Voortman J, McWilliams RR, Diamond JR, de Vries EG, Verheul HM, Lieu CH, Kim GP, Wang Y, Scales SJ, Samineni D, Brunstein F, Choi Y, Maslyar DJ, Colon-Otero G. Phase I study of DMOT4039A, an antibody-drug conjugate targeting mesothelin, in patients with unresectable pancreaticor platinum-resistant ovarian cancer. Mol Cancer Ther. 2016 doi: 10.1158/1535-7163.MCT-15-0693. pii: molcanther.0693.2015. [DOI] [PubMed] [Google Scholar]
- 29.Sharon E, Zhang J, Hollevoet K, Steinberg SM, Pastan I, Onda M, Gaedcke J, Ghadimi BM, Ried T, Hassan R. Serum mesothelin and megakaryocyte potentiating factor in pancreatic and biliary cancers. Clin Chem Lab Med. 2012;50:721–725. doi: 10.1515/CCLM.2011.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassan R, Remaley AT, Sampson ML, Zhang J, Cox DD, Pingpank J, Alexander R, Willingham M, Pastan I, Onda M. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res. 2006;12:447–453. doi: 10.1158/1078-0432.CCR-05-1477. [DOI] [PubMed] [Google Scholar]
- 31.Hellstrom I, Raycraft J, Kanan S, Sardesai NY, Verch T, Yang Y, Hellstrom KE. Mesothelin variant 1 is released from tumor cells as a diagnostic marker. Cancer Epidemiol Biomarkers Prev. 2006;15:1014–1020. doi: 10.1158/1055-9965.EPI-05-0334. [DOI] [PubMed] [Google Scholar]
- 32.Ho M, Onda M, Wang QC, Hassan R, Pastan I, Lively MO. Mesothelin is shed from tumor cells. Cancer Epidemiol Biomarkers Prev. 2006;15:1751. doi: 10.1158/1055-9965.EPI-06-0479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.