Abstract
Background:
Oxidative stress and alteration of lipid profile due to obesity and overweight is a major risk factor for atherosclerotic plaque or coronary artery disease. Because of antioxidant and lipid lowering potential of saffron, this study investigated weight alteration, lipid profiles, and insulin resistance index in high-calorie diet rats treated with aqueous extract of saffron stigma and petal.
Methods:
Forty Sprague-Dawley male rats were randomly divided into 8 groups including healthy control, high-fat diet control, nicotinic acid treated, Anethum graveolens treated, and saffron stigma and petal treated groups. Rats received a high-calorie diet for 16 weeks. For treatment, aqueous extract of saffron stigma (40 and 80 mg/kg) and petal (50 and 100 mg/kg) was used once daily for 4 weeks. Afterward, lipid profile, oxidative stress status, and insulin and adiponectin levels were measured using desired kits.
Results:
There was a significant decrease in the mean weight of the groups receiving saffron stigma and petal compared to control group (P < 0.05). The increased level of insulin hormone in obese group was improved in treated groups especially in the case of saffron stigma. Also, the decreased level of adiponectin was recovered in treated groups. An improvement was seen in oxidative stress markers and lipid profiles in treated groups compared to obesity pair.
Conclusions:
In this study, a remarkable antioxidant and lipid lowering potential was detected for saffron stigma, which could improve insulin resistance in obese rats. Therapeutic and protective effect of saffron is mainly related to its richness in phenolic compounds. Saffron stigma compared with petal had more notable effect, which could and should be mentioned in pharmaceutical studies.
Keywords: Adipocytokine, antioxidants, Crocus sativus, diet, high fat, insulin resistance
Introduction
Nowadays, overweight and obesity are two of the main public health concerns all over the world.[1] At the present time, the prevalence of obesity has steadily increasing, and in the recent decade, about half a billion people have been obese or overweight in the world.[2] In a study conducted in Spain, the prevalence of overweighting and obesity in men and women was 39.8% and 40.8%, respectively.[3] Based on this study, obesity is associated with increased mortality and morbidity in elderly people due to higher levels of circulating lipids that are the main risk factors for heart disease.
Adipose tissue is one of the major endocrine organs in the human body which produces adipocytokines. Adiponectin is one of the most adipocytokines that improves insulin sensitivity in the muscles and liver and has antiinflammatory effects which regulates the body's energy metabolism and prevents the development of atherosclerosis.[4] Obesity and related oxidative stress play an important role in adipose tissue dysregulation which can be involved in suppression of adiponectin production in adipocytes.[5] Because of protective role of adiponectin in this regard, the products with antioxidant agent that restore adiponectin production seem good choice in treatment and prevention of obesity-related complications.
In recent years, several strategies have been proposed to treat obesity, including reduction of energy intake from food, increase in physical activity and aerobic exercise, improving nutritional behavior, use of drugs such as orlistat and sibutramine, surgery, and dietary supplements.[6] Chemical medications have several complications and some limitations. Historically, antiobesity drugs are not safe, and have minimal effect and are not cost-effective. Therefore, there is a need for novel drugs to prevent and treat obesity that are more beneficial and safer than aforementioned drugs.
Today, natural products are considered as high potential organic agents for treatment and prevention of obesity.[7] Different types of herbal and natural products can reduce weight and also help to prevent diet-induced obesity.[8] Saffron (Crocus sativus) is an expensive spice that is cultivated mainly in Iran and on a smaller scale in countries such as India, Greece, and Italy. It is mainly used for aromatizing, flavoring, and food coloring, as well as in traditional medicine including appetite suppression, cancer treatment, asthma, menstrual disorders, liver disease, pain, and mental depression.[9] Numerous human and animal studies have been conducted to examine saffron therapeutic potential in obesity and found favorable effects on antiinflammatory, antioxidative, glucose and lipid lowering properties, and insulin sensitizing potential.[10,11,12] According to the literature, obesity-related inflammation has a key role in promotion of oxidative stress status which promotes metabolic dysfunction.[13] Therefore, it has been hypothesized that agents with antiinflammation and antioxidant potential could be effective in ameliorating obesity-associated oxidative stress and complications.
Antiinflammatory and antioxidant potential of saffron stigma makes it a good choice for diet-induced obesity studies. Due to the presence of flavonoids and anthocyanin in saffron petal composition, use of this by-product of saffron processing in these studies can be noticeable.[14] Therefore, our aim in this study was to evaluate and compare the effect of aqueous extract of saffron stigma and petal on weight changes, lipid profiles, and insulin resistance index in rats fed with high-calorie diet.
Methods
Preparation of aqueous extract
In this study, in order to prepare aqueous extract, saffron (Crocus sativus) was first purchased from South Khorasan, Birjand. The petals and the stigmas were separated and dried in the shade and milled. 10 g of each component was mixed with 90 ml of distilled water as a solvent in a 250 ml Erlenmeyer and then placed in boiling water bath for half an hour. After the mixtures brewed, they were filtered using Whatman filter paper No. 1 and the Buchner Funnel following with freeze drying (DENA VACCUM INDUSTRY, IFD-5012) into a lyophilized dry powder. An extraction yield was 12% and 15% for stigma and petal, respectively.
Animal study
In this experimental study, a total of 40, thirty-day-old Sprague-Dawley male rats, weighing 140 ± 5 g were purchased from the Experimental Medicine Research Center at Birjand University of Medical Sciences and Health Services. The animals were kept in standard laboratory conditions (12 h day/night cycle) with free access to water and food. All animal procedures were approved by the ethical committee of Birjand University of Medical Sciences in accordance with the Institutional Animal Ethics Committee (Ethics code: Ir.bums.REC.1394.309). In this study, 40 rats were divided into 8 groups of 5 as follows:
Group A: healthy control group receiving standard rat chow diet (5% Fat); Groups B-F2: obese groups receiving high-fat diet (60% Fat) for 16 weeks; Group B: obese control group; Group C: obese group receiving nicotinic acid (100 mg/kg); Group D: obese group receiving Anethum graveolens L. (50 mg/kg); Group E1: obese group receiving stigma extract (40 mg/kg); Group E2: obese group receiving stigma extract (80 mg/kg); Group F1: obese group receiving petals extract (50 mg/kg); and Group F2: obese group receiving petals extracts (100 mg/kg). During the study, the animals' weight was measured by a digital scale with a precision of 1 g, with 10 days interval. The oral treatment of the animals was continued daily for 4 weeks. Ingredients of the rat diets are shown in Table 1. Because of antioxidant potential of Anethum graveolens L. (Dill), this extract was used as insulin stimulatory agent and nicotinic acid was used as lipid lowering agent in this study.[15,16]
Table 1.
Formulations of normal and high-fat diets used in this study
| Ingredient | Normal diet (g/kg) | High-fat diet (g/kg) |
|---|---|---|
| Casein | 200 | 200 |
| L-Cystine | 3 | 3 |
| Corn Starch | 650 | 135 |
| Maltodextrin | 65 | 156 |
| Sucrose | 0 | 86 |
| Cellulose | 62.5 | 62.5 |
| Soybean Oil | 50 | 31 |
| Beef tallow | 0 | 357 |
| Mineral Mix | 12.5 | 12.5 |
| Di Calcium phosphate | 16.25 | 16.25 |
| Calcium Carbonate | 6.9 | 6.9 |
| Potassium citrate | 20.6 | 20.6 |
| Vitamin Mix | 12.5 | 12.5 |
| Choline Bitartrate | 2.5 | 2.5 |
| Kcal/100g | 384.5 | 524.8 |
Collection of serum samples and measurement of biochemical parameters
After completion of the treatment period, the animals were fasted for 12 h, and were anesthesia by ether (Merck Company). Then, 5 ml of blood was taken from each rat by cardiac puncture and the serum was isolated by centrifugation. Biochemical factors such as fasting blood glucose (FBG) and lipid profiles such as low-density lipoproteins (LDL-C), high-density lipoproteins (HDL-C), triglycerides (TG), and total cholesterol (TC) were measured by Prestige I24 and Pars Azmun kits (Tehran, Iran). Insulin and adiponectin hormones were measured by ELISA method and relevant kits according to the manufacturer's instruction. Insulin and adiponectin ELISA assay kits were supplied by Glory Science (Thailand). Insulin resistance index calculated using the following formula:
HOMA-IR = {[fasting insulin (μU/ml)] × [fasting glucose (mmol/L)]}/22.5
Evaluation of oxidative stress status
Total antioxidant capacity (TAC) in different groups was measured using ferric reducing antioxidant power (FRAP) method based on Benzie and Strain procedure.[17] The method evaluates the reduction of Fe3+ TPTZ complex to Fe2+-tripyridyltriazine that reflects electron donation of antioxidants by measuring the change in absorbance at 593 nm. For assessing lipid peroxidation, plasma level of malondialdehyde (MDA) was measured using thiobarbituric acid reactive substances (TBARS) method.[18] Changes in absorbance (532 nm) were the criterion for lipid peroxidation.
Statistical analysis
The results were reported as mean ± SD. The data were analyzed using SPSS software version 19 (Chicago: SPSS Inc.), in the significant level α = 0.05 and using One-Way ANOVA and Dunnett post hoc test. The R programming version 3.2.2 was used to draw weight changes graph.
Results
Body weight changes
This study showed that rats receiving normal diet (group A) steadily gained weight from the beginning of week 1 to the end of week 20. However, according to the results of weight changes of rats in different groups (B-F2), which are summarized in Figure 1, it can be mentioned that the groups receiving high-calorie diet had a higher weight gain from the beginning of week 1 to the end of the week 10 compared to the group A (P < 0.05) (P = 0.014).
Figure 1.
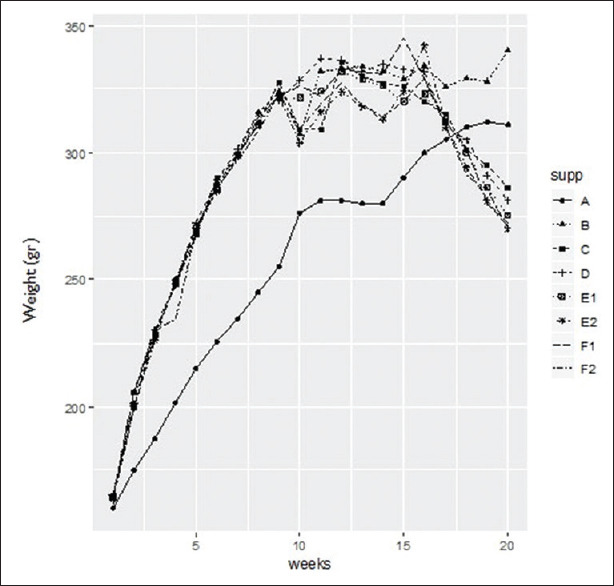
Changes in the weight of diet-induced obese rats in the study groups. Data are expressed as mean ± SD. Group A: Healthy control and normal saline; Group B: High-fat diet obese control and normal saline; Group C: High-fat diet and nicotinic acid; Group D: High-fat diet and Anethum; Group E1: High-fat diet and saffron stigma (40 mg/kg); Group E2: High-fat diet and saffron stigma (80 mg/kg); Group F1: High-fat diet and saffron petal (50 mg/kg); Group F2: High-fat diet and saffron petal (100 mg/kg)
After week 16 and initiation of treatment, the weight gain in group B was higher compared to other groups, while the weight in groups C-F2 gradually decreased and at the end of week 20, the mean weight of the rats in comparison to the group B was changed notably. The effects of nicotinic acid, and stigma and petals' extracts on weight loss of the recipient groups were time-dependent and dose-dependent.
Effects of saffron stigma and petal on serum glucose level and lipid profile
According to the results of the lipid profiles summarized in Table 2, Group B showed an increase in serum levels of TC, TG, and LDL-C and a decrease in HDL-C level compared to the group A. Treatment with nicotinic acid, Anethum and herbal extracts for 4 weeks improved lipid profile in groups C-F2 and decreased serum levels of TC, TG, and LDL-C significantly (P < 0.05) while increased serum levels of HDL-C compared to the group B. The effect of stigma and petals extracts of saffron was dose-dependent.
Table 2.
Effects of saffron stigma and petal extract on biochemical parameters in diet-induced obese rats
| Groups† parametrs | Group A | Group B | Group C | Group D | Group E1 | Group E2 | Group F1 | Group F2 |
|---|---|---|---|---|---|---|---|---|
| TC, mg/dL | 65±14‡* | 117±18 | 80±11* | 67±9.0* | 76±8.7* | 68±9.2* | 84±6.3* | 76±6.3* |
| TG, mg/dL | 45±12* | 125±16 | 78±9.4* | 85±11* | 74±11* | 70±6.7* | 92±12* | 87±7.4* |
| LDL-C, mg/dL | 25±7.5* | 56±5.7 | 24±6.9* | 25±6.2* | 22±7.5* | 28±5.7* | 25±4.6* | 26±4.3* |
| HDL-C, mg/dL | 34±8.4* | 21±6.3 | 27±7.4 | 31±4.4* | 34±5.8* | 38±5.8* | 37±5.9* | 35±6.6* |
| FBG, mg/dL | 74±3.8* | 152±16 | 97±10* | 81±9.5* | 84±6.0* | 79±3.9* | 91±6.8* | 83±7.1* |
†For details of experimental conditions see the text. ‡Data are expressed as mean±SD of 5 rats in each group. In each row, *was considered significant at P<0.05 when compared with the obese control group
As it is shown in Table 2, serum levels of FBG in group A rats were significantly lower than those in group B (P < 0.05) (P = 0.021). The mean serum level of FBG in other groups (C-F2) significantly decreased compared to the group B. The results showed that the effects of stigmata and petals extracts in the groups E-F were dose-dependent.
Effects of saffron stigma and petal on serum level of insulin, adiponectin, and HOMA-IR index
The serum level of insulin hormone was significantly increased in group B compared with group A (P < 0.05) (P = 0.024). The serum level of this hormone in other herbal treated groups (D-F2) showed a significant decrease compared with group B (P < 0.05) [Table 3]. The level of adiponectin in group B displayed a remarkable decline as compared with group A (P < 0.05) (P = 0.019). After treatment with extracts of saffron stigma and petal, serum level of adiponectin significantly increased [Table 3, P < 0.05]. These effects were in dose-dependent manner. Evaluation of HOMA-IR as an insulin resistance index showed increase in group B in compared with group A (P < 0.05) (P = 0.014). A remarkable decrease was seen in insulin resistance after treatment with saffron stigma and petal extracts in groups E1-F2 [Table 3]
Table 3.
Serum levels of insulin and adiponectin hormones in the diet-induced obese rats
| Parameters experimental groups† | Adiponectin (mg/L) | Insulin (ng/mL) | HOMA-IRa |
|---|---|---|---|
| Group A | 5.65±0.21‡* | 4.02±0.05* | 0.7±0.21* |
| Group B | 2.1±0.2 | 6.32±0.11 | 2.4±0.3 |
| Group C | 3.4±0.12 | 5.71±0.09 | 1.4±0.17* |
| Group D | 3.8±0.13 | 5.5±0.17 | 1.1±0.2* |
| Group E1 | 4.2±0.12* | 5.12±0.13* | 1.1±0.18* |
| Group E2 | 4.9±0.32* | 4.5±0.23* | 0.9±0.13* |
| Group F1 | 4.1±0.23* | 5.6±0.45 | 1.3±0.19* |
| Group F2 | 4.5±0.42* | 5.2±0.36* | 1.1±0.11* |
†For details of experimental conditions see the text. ‡Data are expressed as mean±SD of 5 rats in each group. In each column, *was considered significant at P<0.05 when compared with the obese control group. aRefrence Range: <1.0 indicates insulin-sensitive which is optimal. Above 1.9 indicates early insulin resistance. Above 2.9 indicates significant insulin resistance
Effects of saffron stigma and petal on oxidative stress status
Oxidative stress markers are presented in Table 4. The results showed that among treated groups, the highest reduction in total antioxidant capacity was in group B compared to the group A. In groups treated with stigma and petals extracts, nicotinic acid, and Anethum, the total antioxidant capacity increased compared with group B. The increase in total antioxidant capacity in groups treated with saffron extracts was dose-dependent. In addition, groups E1 and E2 showed better effects compared to the groups F1 and F2. The measurement of the MDA levels showed that lipid peroxidation in group B was higher than other groups, and the treatment with the extracts could reduce the level of MDA in a dose-dependent manner [Table 4, P < 0.05].
Table 4.
Effects of saffron stigma and petal on anti-oxidant capacity and lipid peroxidation in diet-induced obese rats
| Paramers Experimental groups† | Total antioxidant (FRAP) (μmol/L) | Malondialdehyde (MDA) (μmol/L) |
|---|---|---|
| Group A | 764±14‡* | 1.67±0.5* |
| Group B | 520±21 | 6.7±1.2 |
| Group C | 655±18* | 4.3±0.09* |
| Group D | 598±11* | 4.9±1.1 |
| Group E1 | 678±22* | 3.8±0.8* |
| Group E2 | 698±13* | 2.9±0.6* |
| Group F1 | 535±21 | 4.6±0.8 |
| Group F2 | 596±16* | 3.9±0.7* |
†For details of experimental conditions see the text. ‡Data are expressed as mean±SD of 5 rats in each group. In each column, * was considered significant at P<0.05 when compared with the obese control group
Discussion
Here, we showed that saffron stigma and petals could improve lipid profile, oxidative stress status, and weight changes in diet-induced obesity.
The results of this study showed that oral treatment with various concentrations of the extracts, nicotinic acid, and Anethum decreased the mean weight of the rats in the treatment groups compared to the obese control group.
During the study period, the weight loss in the groups receiving high dose of petal and stigma were faster than other treatment groups. The results of this study showed that treatment with saffron extract leads to weight loss and improvement of dyslipidemia in the period of treatment. Based on the other studies, the concentration used for herbal plants extract did not impose any toxic effects on the rats.[19,20]
According to the results presented here, saffron petals and stigma caused a decline in serum lipid profile of LDL-C, TG, and TC in groups F2 and E2 and also led to an increase in serum levels of HDL-C in these groups compared to obese group. The highest changes were manifested on LDL-C and HDL-C profiles. Hypolipidemic effects of saffron can be due to the presence of crocin and crocetin compounds in saffron.[21] Further research verified that crocin and crocetin could reduce the absorption of dietary fat and cholesterol.[21,22] In addition, saffron bioactive compounds have pancreatic and gastric lipase inhibitor activity with selectivity for pancreatic lipase, which reduces serum TG and cholesterol levels.[21]
A decrease in serum level of FBG in stigma and petal groups proves hypoglycemic potential of saffron which has also shown in other studies.[11,12,14] The hypoglycemic potential of saffron is due to its bioactive compounds that could improve hyperglycemia via different paths including ameliorating oxidative stress, B cell inflammation, and apoptosis and also through enhancement of pancreatic insulin expression and secretion.[22]
Adiponectin is one of the associated hormones of cardiovascular disease, atherosclerosis, weight gain, and obesity. In the groups received saffron petals and stigma orally (F2, E2), the serum levels of adiponectin hormone were increased compared with the obesity control group. The beneficial effect of herbs in increasing adiponectin was proven in other studies.[23,24]
The increased level of adiponectin in the group treated by saffron stigma (E2) was more than other groups, which indicated the stimulatory effects of saffron stigma on adiponectin synthesis by adipose tissue. Measuring insulin levels and insulin resistance index showed that saffron can decrease the level of this hormone in the E1-F2 groups compared with the healthy control group. This effect was notable for the group treated with saffron stigma 80 mg/kg body mass. Other studies in accordance with the present study showed beneficial effects of saffron on insulin resistance index in metabolic disorders.[25]
Insulin resistance index in obese control group was higher in comparison with other groups, indicating insulin resistance in obese control group. In other groups, this index was low, which was more significant in the group E2 than other groups. Improvement of insulin resistance in treated groups may be due to increased level of adiponectin in these groups. Based on literature, adiponectin increases insulin sensitivity through increasing hepatic insulin receptor substrate 2.[26] According to the results, the highest decrease in HOMA-IR index was seen in group E2 (saffron stigma 80 mg/kg), while the highest increase in adiponectin level also was seen in this group that confirms the association of adiponectin with insulin sensitivity.
According to current results, reduced antioxidant capacity in obese group is improved by saffron stigma and petal treatment, on the other hand lipid peroxidation is decreased by saffron. According to our results, saffron stigma had the highest antioxidant capacity and also the best insulin-sensitizing effect which should be considered in the future studies. Improvement of oxidative stress by saffron can be due to crocin, crocetin, and kaempferol in stigma and petal that scavenge reactive oxygen species and protect pancreatic β cells from inflammation and apoptosis.[14,20,22]
In the present study, Anethum as an herbal-based drug was shown to be more effective than synthetic-based drugs like nicotinic acid. It seems that the use of herbal medicines in comparison to chemical drugs can modify lipid profiles, insulin resistance index, and stress conditions notably. In the current study, we evaluated the effectiveness of stigma and petals in ameliorating oxidative stress and found saffron stigma and petal could be as alternative or supplementary drug in medicine. According to our results, saffron petal which seems to be disposable waste material had notable effects that could be mentioned in pharmaceutical industry. Sample size and lack of clinical trial were our limitations in this study which should be addressed in future with doing more human studies.
Conclusions
The results of this study showed that petal and notably stigma extract of saffron can reduce the serum levels of TC, TG, and LDL-C and increase the serum levels of HDL-C. By mounting the dosage of these extracts, this modifying property increases. On the other hand, the consumption of these extracts increases the serum level of adiponectin hormone and total antioxidant capacity and decreases insulin hormone and MDA. Therefore, it can be concluded that the extract of saffron petals and specially stigma has antiobesity properties. Hence, the use of medicinal herbs, such as saffron, as a complementary treatment, along with conventional treatments for obesity can be proposed to medical community in the near future. However, further studies are required in this regard.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors of this article would like to express their appreciation to the Vice-Chancellor for Research and Technology and the Cardiovascular Disease Research Center of Birjand University of Medical Sciences for funding this study and all those who helped us in performing this study. This article is the result of an approved research project 34/4 by Birjand University of Medical Sciences.
References
- 1.Nematy M, Sakhdari A, Ahmadi-Moghaddam P, Aliabadi M, Kimiagar M, Ilaty A, et al. Prevalence of obesity and its association with socioeconomic factors in elderly Iranians from Razavi-Khorasan province. ScientificWorldJournal. 2009;9:1286–93. doi: 10.1100/tsw.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hug C, Lodish HF. The role of the adipocyte hormone adiponectin in cardiovascular disease. Curr Opin Pharmacol. 2005;5:129–34. doi: 10.1016/j.coph.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world—A growing challenge. N Engl J Med. 2007;356:213–5. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez-Fisac JL, López E, Banegas JR, Graciani A, Rodriguez-Artalejo F. Prevalence of overweight and obesity in elderly people in Spain. Obesity Res. 2004;12:710–5. doi: 10.1038/oby.2004.83. [DOI] [PubMed] [Google Scholar]
- 5.Morihiro M, Shimomura I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev Endocr Metab Dis. 2014;15:1–10. doi: 10.1007/s11154-013-9271-7. [DOI] [PubMed] [Google Scholar]
- 6.Bray GA, Tartaglia LA. Medicinal strategies in the treatment of obesity. Nature. 2000;404:672–7. doi: 10.1038/35007544. [DOI] [PubMed] [Google Scholar]
- 7.Zhang XJ, Deng YX, Shi QZ, He MY, Chen B, Qiu XM. Hypolipidemic effect of the Chinese polyherbal Huanglian Jiedu decoction in type 2 diabetic rats and its possible mechanism. Phytomedicine. 2014;21:615–23. doi: 10.1016/j.phymed.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Jung H, Lim Y, Kim EK. Therapeutic phytogenic compounds for obesity and diabetes. Int J Mol Sci. 2014;15:21505–37. doi: 10.3390/ijms151121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosseinzadeh H, Nassiri-Asl M. Avicenna's (Ibn Sina) the Canon of Medicine and saffron (Crocus sativus): A review. Phytother Res. 2013;27:475–83. doi: 10.1002/ptr.4784. [DOI] [PubMed] [Google Scholar]
- 10.Milajerdi A, Jazayeri S, Hashemzadeh N, Shirzadi E, Derakhshan Z, Djazayeri A, et al. The effect of saffron (Crocus Sativus L.) hydroalcoholic extract on metabolic control in type 2 diabetes mellitus: A triple-blinded randomized clinical trial. J Res Med Sci. 2018;23:16. doi: 10.4103/jrms.JRMS_286_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmati M, Asghari S, Zohoori E. Effects of alcoholic and aqueous extract of barberry, Jujube and saffron petals on serum level of Adiponectin and lipid profile in diabetic rats. Iran J Endocrinol Metab. 2015:16. [Google Scholar]
- 12.Hemmati M, Asghari S, Zohoori E, Karamian M. Hypoglycemic effects of three Iranian edible plants; jujube, barberry and saffron: Correlation with serum adiponectin level. Pak J Pharm Sci. 2015;28:2095–9. [PubMed] [Google Scholar]
- 13.Marseglia L, Manti S, D'Angelo G, Nicotera A, Parisi E, Di Rosa G, et al. Oxidative stress in obesity: A critical component in human diseases. Int J Mol Sci. 2015;16:378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseini A, Razavi BM, Hosseinzadeh H. Saffron (Crocus sativus) petal as a new pharmacological target: A review. Iran J Basic Med Sci. 2018;21:1091–9. doi: 10.22038/IJBMS.2018.31243.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jana S, Shekhawat G. Anethum graveolens: An indian traditional medicinal herb and spice. Pharmacogn Rev. 2010;4:179–84. doi: 10.4103/0973-7847.70915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blond E, Rieusset J, Alligier M, Lambert-Porcheron S, Bendridi N, Gabert L, et al. Nicotinic acid effects on insulin sensitivity and hepatic lipid metabolism: An in vivo to in vitro study. Horm Metab Res. 2014;46:390–6. doi: 10.1055/s-0034-1372600. [DOI] [PubMed] [Google Scholar]
- 17.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 18.Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Markers of oxidative stress during diabetes mellitus. J Biomark. 2013;2013:378790. doi: 10.1155/2013/378790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrasekaran C, Vijayalakshmi M, Prakash K, Bansal V, Meenakshi J, Amit A. Herbal approach for obesity management. Am J Plant Sci. 2012;3:1003. [Google Scholar]
- 20.Hemmati M, Zohoori E, Mehrpour O, Karamian M, Asghari S, Zarban A, et al. Anti-atherogenic potential of jujube, saffron and barberry: Anti-diabetic and antioxidant actions. EXCLI J. 2015;14:908–15. doi: 10.17179/excli2015-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheng L, Qian Z, Zheng S, Xi L. Mechanism of hypolipidemic effect of crocin in rats: Crocin inhibits pancreatic lipase. Eur J Pharmacol. 2006;543:116–22. doi: 10.1016/j.ejphar.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 22.Samaha MM, Said E, Salem HA. A comparative study of the role of crocin and sitagliptin in attenuation of STZ-induced diabetes mellitus and the associated inflammatory and apoptotic changes in pancreatic β-islets. Environ Toxicol Pharm. 2019;72:103238. doi: 10.1016/j.etap.2019.103238. [DOI] [PubMed] [Google Scholar]
- 23.Abo-Raya AO, Alfky NA, Elgazar AF. Anti-obesity and antidiabetic Activities of red ginseng plant extract in obese diabetic male rats. Glob J Pharmacol. 2013;7:390–7. [Google Scholar]
- 24.Sung YY, Kim DS, Choi G, Kim SH, Kim HK. Dohaekseunggi-tang extract inhibits obesity, hyperlipidemia, and hypertension in high-fat diet-induced obese mice. BMC Complement Altern Med. 2014;14:372. doi: 10.1186/1472-6882-14-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mashmoul M, Azlan A, Khazaai H, Yusof BNM, Noor SM. Saffron: A natural potent antioxidant as a promising anti-obesity drug. Antioxidants. 2013;2:293–308. doi: 10.3390/antiox2040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awazawa M, Ueki K, Inabe K, Yamauchi T, Kubota N, Kaneko K, et al. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab. 2011;13:401–12. doi: 10.1016/j.cmet.2011.02.010. [DOI] [PubMed] [Google Scholar]


