Abstract
Background:
Obesity is related to increase in the incidence of morbidity and mortality. Previous studies have led to conflicting results regarding the effect of coenzyme Q10 (CoQ10) supplementation on anthropometric indices. This study aimed to evaluate the efficacy of CoQ10 supplementation on body weight, body mass index (BMI), and waist circumference (WC) through a systematic review and meta-analysis of randomized controlled trials (RCTs).
Methods:
PubMed, Scopus, Web of Science, and Cochrane Library as well as the reference lists of the identified relevant RCTs were searched up to March 2019, and weighted mean differences (WMDs) were pooled by using the random-effects model.
Results:
Twenty RCTs (976 participants) were eligible to be included in the systematic review. The meta-analysis revealed that CoQ10 supplementation had no effect on body weight (WMD = −0.04 kg; 95% confidence interval [CI]: −1.96, 1.6; I2 = 0.0%), BMI (WMD = −0.06 kg/m2; 95% CI: −0.54, 0.42; I2 = 0.0%), and WC (WMD = 0.79 cm; 95% CI: −2.83, 0.04; I2 = 0.0%).
Conclusions:
CoQ10 supplementation might not improve anthropometric indices. Future well-designed trials are still needed to confirm these results.
Keywords: Body mass index, body weight, CoQ10, meta-analysis, ubiquinone, waist circumference
Introduction
The increasing prevalence of obesity and overweight affects different population throughout the world.[1] Obesity can induce several noncommunicable diseases including type 2 diabetes mellitus (T2DM), cardiovascular diseases, stroke, and some types of cancer.[2,3,4] Furthermore, it implied a high economic burden to societies.[5] It results in reduced quality of life.[6] Therefore, it is important to find effective treatment strategies to decrease the risk of obesity-related complications.
Common intervention including restriction on energy intake and increase in energy expenditure by different ways such as exercise for managing body weight has been unsuccessful over a long time period.[7] In addition, use of different ways for beginning or accelerating process of weight loss has been popular among obese and overweight subjects. Antiobesity supplements can be considered as auxiliary treatment to increase compliance and adherence of obese subjects to common intervention for managing weight.[8] Pharmacists have attempted to produce effective weight loss supplement with minimum serious side effects to human health.[9] Because an antiobesity supplement has become increasingly popular among obese subjects, it is important to evaluate the efficacy of available antiobesity products.
Coenzyme Q10 (CoQ10) is fat-soluble and has a vital role in the electron transport chain where energy is obtained by a process called oxidative phosphorylation from dietary intakes. This common supplement has various good health effects such as it reduces blood pressure,[10,11,12] reduces inflammation factors,[13] reduces plasma lipoprotein like Lp(a) concentrations,[14] and plays crucial role in energy expenditure and adenosine triphosphate production.[15]
Animal studies showed that oral administration of CoQ10 led to significant weight loss.[16] Weight loss effects of CoQ10 are modulated by increasing lipid oxidation and energy consumption in adipose tissue and inhibiting adipogenesis through adenosine-monophosphate-activated protein kinase.[17]
Literature review from clinical trials about effects of CoQ10 supplementation on weight and other anthropometric indices have been inconsistence. Izadi et al.[18] investigated the effect of CoQ10 supplementation on hormonal and metabolic indices in patients with polycystic ovary syndrome (PCOS). Intervention resulted in reducing weight and body mass index (BMI) in these patients. Conversely, Moazen et al.[19] observed a slight weight gain after 2 months of consuming CoQ10 supplementation. Thus, to clarify the inconsistences, and for accurate decision-making, a comprehensive systematic review and meta-analysis of all available randomized clinical trials was performed to determine the effect of CoQ10 intervention on anthropometric indices in adults.
Methods
Search strategy
This systematic and meta-analysis was performed based on the Preferred Reporting Item for Systematic Review and meta-analysis (PRISMA) guideline. A systematic research of studies published until March 2019 was conducted on PubMed, Scopus, Web of Science, and Cochrane Library. The search process used the following terms: (“Coenzyme Q10” OR “Co-enzyme Q10” OR CoQ10 OR ubiquinone OR ubiquinol) AND (“Intervention Studies” OR “intervention” OR “controlled trial” OR “randomized” OR “randomised” OR “random” OR “randomly” OR “placebo” OR “assignment”). A manual reference check was performed on pertinent studies to identify further relevant trials. Due to the fact that several studies examined the effect of CoQ10 supplementation on anthropometric indices as the secondary outcome, we did not use anthropometric keywords. The search was performed by two authors (H.M and A.G) without any restrictions. In addition, the reference lists of all eligible articles were checked at the final step to find relevant studies not found from computerized search.
Eligibility criteria
Relevant articles were included if they (1) applied a clinical trial design; (2) examined the effects of CoQ10 on weight, BMI, and waist circumference (WC); (3) provided sufficient information on aforesaid indices in both treatment and control groups; (4) conducted on adults (over 18 years); and (5) administered CoQ10 for at least 4 weeks. Studies were excluded if they (1) were uncontrolled studies; (2) used a mixture of CoQ10 with other substance; (3) reported duplicate data; and (4) were reviews, letters, editorial articles, or case reports. Participants, interventions, comparisons, outcomes, and study design (PICOS) are shown in Table 1.
Table 1.
PICOS criteria used to perform the systematic review and meta-analyses
| Parameter | Criteria |
|---|---|
| Population | Adults |
| Intervention | Q10 |
| Comparator | Matched control group |
| Outcome | Weight, BMI, WC |
| Setting or study design | Randomized controlled trials |
PICOS=Participants, interventions, comparisons, outcomes, and study design; BMI=Body mass index; WC=Waist circumference
Quality assessment
Two authors (A.G and H.M) independently evaluated the quality of the selected articles using Cochrane Collaboration's tool[20] including six domains as follows: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, and (6) selective reporting. Each domain was classified into three categories: low risk of bias, high risk of bias, and unclear risk of bias. Each domain was classified into three categories: low risk of bias, high risk of bias, and unclear risk of bias. According to the mentioned domains, the overall quality of individual study was considered as good (low risk for more than two items), fair (low risk for two items), or weak (low risk for less than two items).
Data extraction
Two independent investigators (H.M and A.H) extracted the relevant data. Any controversy among study selection was discussed and eventually resolved by a third reviewer (A.G). The relevant data were extracted including the following: first author, study location, year of publication, study design, target population, sex, number of participants, intervention duration, outcomes reported in study, and supplement dosage. In addition, we extracted the mean and standard deviation (SD) of anthropometric indices at baseline and end of intervention.
Statistical analysis
The mean change and SD for each anthropometric indices were used to estimate the overall effect size of the intervention. The mean change was calculated as (measure at the end of follow-up in the treatment group − measure at baseline in the treatment group) − (measure at the end of follow-up in the control group − measure at baseline in the control group). The SD of the mean difference for studies that were not reported was calculated by the following formula: SD2 = [(SD baseline2 + SD final2) − (2 × R × SD baseline × SD final)] where correlation coefficient (R) was considered as 0.5.[21] To make sure that our meta-analysis is not sensitive to the selected correlation coefficient (R = 0.5), all the analyses for body indices were repeated by the use of correlation coefficient of 0.2 and 0.8. The random-effects model was used to compute the weighted mean differences (WMDs) with 95% confidence intervals (CIs) for body weight, BMI, and WC. The between-study heterogeneity was evaluated using I-square (I2) test. To elucidate the effects of CoQ10 on anthropometric indices, we carried out a preplanned subgroup analysis based on study duration (≤8 weeks and >8 weeks) and CoQ10 dose (>100 mg/day and ≤100 mg/day). Meta-regression analyses were carried out to examine the effects of CoQ10 dose on anthropometric indices. The proportion of each study in the overall effect was assessed by sensitivity analysis. We used Begg's rank correlation test and Egger's regression asymmetry test to evaluate the publication bias. Statistical analysis was performed using STATA 11 software (Stata Corp., College Station, TX, USA).
Results
Literature search
Overall, 4223 studies were identified in a combined search of electronic databases. Of the 2729 unduplicated papers, 2694 studies were eliminated after primary evaluation of inclusion criteria. After reading full text of the 35 remaining articles, 15 studies were excluded due to inappropriate data (n = 3) and duplicate publication (n = 5), protocol (n = 1), studies with follow-up less than 4 weeks (n = 1), trials on children or adolescence (n = 2), and studies that administrated CoQ10 with other components (n = 3). Finally, 20 records[18,19,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] met the eligibility criteria and were included in the systematic review and meta-analysis. The PRISMA flow diagram for the study selection process is presented in Figure 1.
Figure 1.
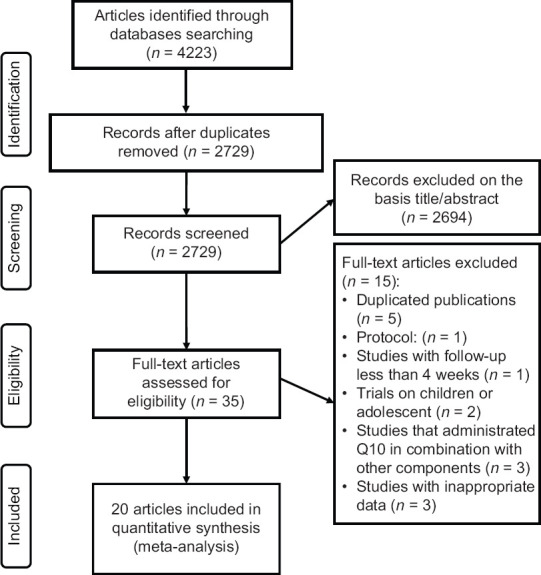
PRISMA flow diagram of study selection process
Study characteristics
A total of 976 participants (490 intervention/486 control) were included in analysis and the mean age of participants ranged from 19 to 60 years. The publication date of articles ranged from 1999 to 2018. Selected studies were conducted in Iran,[18,19,22,25,26,28,30,32,33,34,35,36,37,38] China,[31] Denmark,[27] Hong Kong,[23] Finland,[24] and Australia.[29,39] All included studies used parallel design. The duration of intervention ranged from 4 to 24 weeks. The included trials enrolled participants with T2DM (n = 5),[19,24,26,34,39] nonalcoholic fatty liver disease (n = 2),[25,35] dyslipidemia (n = 1),[31] rheumatoid arthritis (n = 1),[22] type 1 diabetes (n = 1),[27] PCOS (n = 2),[18,30] chronic kidney disease (n = 1),[29] hemodialysis (n = 2),[32,38] ischemic left ventricular systolic dysfunction (n = 1),[23] acute myocardial infarction (n = 1),[28] men with idiopathic oligoasthenoteratozoospermia (n = 1),[36] diabetic nephropathy (n = 1),[33] and metabolic syndrome (n = 1).[37] The dose of CoQ10 ranged from 100 to 300 mg/day. Sixteen trials included both genders,[19,22,23,24,25,27,28,29,31,32,33,34,35,37,38] three trials included woman,[18,26,30] and one trial included man.[36] The characteristics of eligible studies are summarized in Table 2.
Table 2.
Characteristics of eligible studies
| First author (location; year) | RCT design (blinding) | Population | Sex | Sample size (Q10/placebo) | Duration (weeks) | Dose of Q10 (mg/day) | Outcomes |
|---|---|---|---|---|---|---|---|
| Abdollahzad (Iran; 2015) | Parallel (double) | Rheumatoid arthritis | Both | 22/23 | 8 | 100 | Weight, BMI |
| Rahmani (Iran; 2018) | Parallel (double) | Polycystic ovary syndrome | Woman | 20/20 | 12 | 100 | Weight, BMI |
| Fallah (Iran; 2018) | Parallel (double) | Hemodialysis patients | Both | 30/30 | 12 | 120 | Weight, BMI |
| Gholami (Iran; 2018) | Parallel (double) | Type 2 diabetes | Woman | 34/34 | 12 | 100 | Weight, BMI, WC |
| Attar (Iran; 2015) | Parallel (double) | Type 2 diabetes | Both | 31/33 | 12 | 200 | Weight, BMI, WC |
| Jafarvand (Iran; 2016) | Parallel (double) | Nonalcoholic fatty liver disease | Both | 20/21 | 4 | 100 | BMI, WC |
| Zhang (China; 2018) | Parallel (double) | Dyslipidemia | Both | 51/50 | 24 | 120 | WC, weight, BMI |
| Mori (Australia; 2009) | Parallel (double) | Chronic kidney disease | Both | 21/15 | 8 | 200 | Weight |
| Henriksen (Denmark; 1999) | Parallel (double) | Type 1 diabetes mellitus | Both | 17/17 | 12 | 100 | Weight |
| Moazen (Iran; 2015) | Parallel (single) | Type 2 diabetes | Both | 26/26 | 8 | 100 | Weight, BMI |
| Ericsson (Finland; 1999) | Parallel (double) | Type 2 diabetes | Both | 12/11 | 24 | 100 | BMI |
| Dai (Hong Kong; 2011) | Parallel (double) | Ischemic left ventricular systolic dysfunction | Both | 28/28 | 8 | 300 | BMI |
| Farsi (Iran; 2016) | Parallel (double) | Nonalcoholic fatty liver disease | Both | 20/21 | 12 | 100 | Weight, BMI, WC |
| Mohseni (Iran; 2015) | Parallel (double) | Acute myocardial infarction | Both | 26/26 | 12 | 200 | Weight, BMI |
| Gholnari (Iran; 2018) | Parallel (double) | Diabetic nephropathy | Both | 25/25 | 12 | 100 | Weight, BMI |
| Hodgson (Australia; 2002) | Parallel (double) | Type 2 diabetes | Both | 19/18 | 12 | 200 | Weight |
| Izadi (Iran; 2018) | Parallel (double) | Polycystic ovary syndrome | Woman | 22/21 | 8 | 200 | Weight, BMI |
| Shojaei (Iran; 2011) | Parallel (double) | Hemodialysis patients | Both | 13/13 | 12 | 100 | BMI |
| Nadjarzadeh (Iran; 2011) | Parallel (double) | Idiopathic oligoasthenoteratozoospermia | Man | 23/24 | 12 | 200 | BMI |
| Raygan (Iran; 2016) | Parallel (double) | Metabolic syndrome | Both | 30/30 | 8 | 100 | Weight, BMI |
RCT=Randomized controlled trial; BMI=Body mass index; WC=Waist circumference
Risk of bias assessment
Table 3 describes the risk of bias assessment based on different quality domains using Cochrane collaboration tool. From studies included in the systematic review, 11 achieved positive score in more than two main domains and were classified as high quality[18,19,22,23,28,29,30,31,32,33,37] and nine were classified as fair[24,25,26,27,34,35,36,38,39] because they were low risk in two key domains.
Table 3.
Risk of bias assessment for included randomized controlled clinical trails
| First author (publication year) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| Abdollahzad (2015) | + | + | + | + | + | ? |
| Rahmani (2018) | + | ? | + | ? | + | ? |
| Fallah (2018) | + | + | + | - | + | ? |
| Gholami (2018) | ? | ? | + | + | - | ? |
| Attar (2015) | + | ? | - | - | + | ? |
| Zhang (2018) | + | + | + | + | + | ? |
| Mori (2009) | + | + | - | - | + | ? |
| Henriksen (1999) | ? | + | - | - | + | ? |
| Moazen (2015) | + | + | + | - | + | ? |
| Ericsson (1999) | ? | ? | - | - | + | ? |
| Dai (2011) | + | + | + | + | + | ? |
| Farsi (2016) | ? | ? | - | - | + | ? |
| Mohseni (2015) | + | + | + | + | + | ? |
| Jafarvand (2016) | ? | ? | - | - | + | ? |
| Gholnari (2018) | + | + | + | - | + | ? |
| Hodgson (2002) | ? | ? | - | - | + | ? |
| Izadi (2018) | + | + | + | - | + | ? |
| Shojaei (2011) | + | ? | - | - | + | ? |
| Nadjarzadeh (2011) | ? | ? | - | - | + | ? |
| Raygan (2016) | + | + | + | - | + | ? |
Of 20 included studies in the systematic review and meta-analysis, 13 trials used random allocation[18,19,22,23,27,28,29,31,32,33,34,37,38] and mentioned randomization techniques. Eleven studies had a blinded design[18,19,22,23,26,28,30,31,32,33,37] and eleven of them described methods of blinding.[18,19,22,23,26,28,30,31,32,33,37] Nineteen studies had no or few participant's withdrawal and did not describe the reason of excluded subjects.[18,19,22,23,24,25,27,28,29,30,31,32,33,34,35,36,37,38,39] Most of the studies showed low/unclear risk of bias based on incomplete outcome data and selective outcome reporting.
Effects of CoQ10 supplementation on body weight
The effect of the CoQ10 supplementation on weight was examined in 15 clinical.[19,22,25,26,27,28,30,31,32,33,34,37] Pooled effect size indicated a nonsignificant effect of CoQ10 supplementation on body weight (WMD: −0.04 kg; 95% CI: −1.96, 1.60, P = 0.959) [Figure 2]. The effect was homogeneous across the included trials (I2 = 0.0%, P = 1.000). Subgroup analysis based on CoQ10 dose and study duration suggested the nonsignificant effect of CoQ10 on weight [Table 4]. The meta-regression test based on the dosage of CoQ10 did not reveal any dose–response association for weight changes (P = 0.466). In addition, findings from the sensitivity analysis revealed that the exclusion of Moazen (WMD: 0.01 kg; 95% CI: −1.73, 1.76) Attar (WMD: 0.11 kg; 95% CI: −1.69, 1.71), Zhang (WMD: 0.11 kg; 95% CI: −1.73, 1.75), and Izadi (WMD: 0.76 95% CI: −1.65, 1.80) studies from the analysis changed the overall effect.
Figure 2.
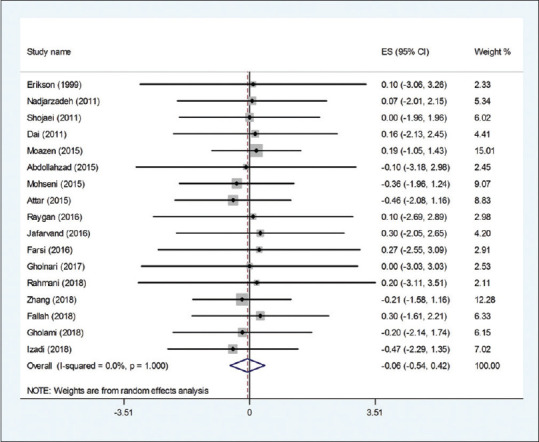
Forest plot of the effect of Q10 supplementation on weight
Table 4.
Subgroup analysis to assess the effect of CoQ10 supplementation on anthropometric indices
| Subgrouped by | No. of trials | Effect size1 | 95% CI | I2 (%) | P for heterogeneity |
|---|---|---|---|---|---|
| Weight | |||||
| Dose | |||||
| Under 100 mg/day | 8 | 0.64 | −1.83, 3.11 | 0.0 | 1.000 |
| Over 100 mg/day | 7 | −0.59 | −2.81, 1.62 | 0.0 | 1.000 |
| Duration | |||||
| Under 8 weeks | 4 | −0.04 | −1.69, 1.60 | 0.0 | 1.000 |
| Over 8 weeks | 11 | 0.1 | −1.75, 1.96 | 0.0 | 1.000 |
| BMI | |||||
| Dose | |||||
| Under 100 mg/day | 10 | 0.09 | −0.69, 0.87 | 0.0 | 1.000 |
| Over 100 mg/day | 7 | −0.15 | −0.76, 0.46 | 0.0 | 1.000 |
| Duration | |||||
| Under 8 weeks | 5 | −0.06 | −1.11, 0.99 | 0.0 | 1.000 |
| Over 8 weeks | 12 | −0.06 | −0.60, 0.48 | 0.0 | 1.000 |
1Calculated by Random-effects model. CI=Confidence interval; BMI=Body mass index
Effects of CoQ10 supplementation on BMI
The effect of the CoQ10 supplementation on BMI was examined in 17 clinical trials.[18,19,22,23,24,25,26,28,30,31,32,33,34,35,36,37,38] Overall, the meta-analysis showed that there was no significant effect of the CoQ10 supplementation on BMI (WMD: −0.06 kg/m2; 95% CI: −0.54., 0.42, P = 0.81) [Figure 3]. There was no evidence of heterogeneity between the effect sizes of included studies (I2 = 0.0%, P = 1.000). The subgroup analysis based on study duration and CoQ10 dose showed that the effect is not statistically significant in all subgroups [Table 4]. The meta-regression test based on the dosage of CoQ10 did not reveal any dose–response association for BMI changes (P = 0.636). In addition, findings from the sensitivity analysis revealed that the exclusion of any single study from the analysis did not alter the overall effect.
Figure 3.
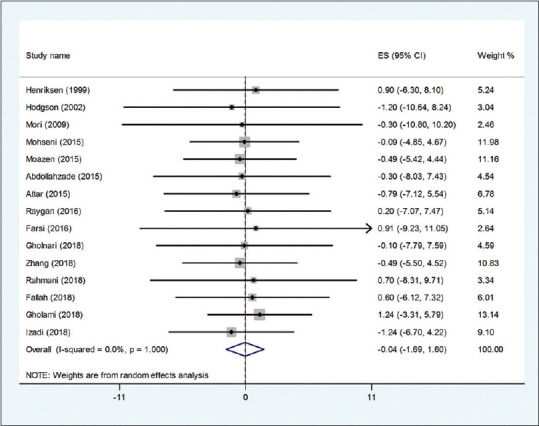
Forest plot of the effect of Q10 supplementation on BMI
Effects of CoQ10 supplementation on WC
The pooled mean difference of five datasets[25,26,31,34,35] for the effects of CoQ10 on WC compared with the placebo group was (WMD: −0.79 cm; 95% CI: −2.83, 0.04, P = 1.25) with no significant heterogeneity (I2 = 0.0 0.873) [Figure 4]. Findings from the sensitivity analysis revealed that the exclusion of any single study from the analysis did not alter the overall effect.
Figure 4.
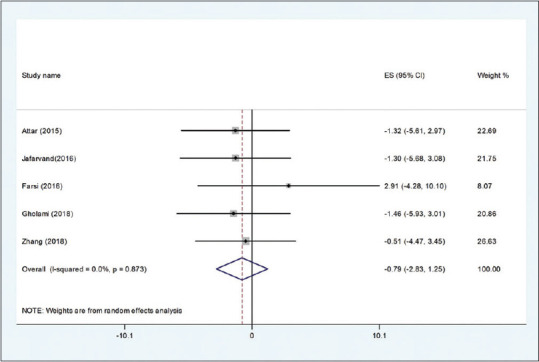
Forest plot of the effect of Q10 supplementation on WC
Publication bias
There was no evidence of publication bias for studies examining the effect of CoQ10on weight (P = 0.96, Begg's test and P = 0.97, Egger's test), BMI (P = 0.36, Begg's test and P = 0.26, Egger's test), and WC (P = 0.99, Begg's test and P = 0.07, Egger's test).
Discussion
Obesity and overweight are one of the major health problems worldwide.[1] Genetic background and environmental factors, that is, excessive energy intake and inactivity, are the main causes of obesity and overweight.[40] The management of this condition is challenging. Nevertheless, common strategies for reducing and managing body weight such as restriction on energy intake and increase in energy expenditure by exercise and other ways have limited success over a long time period.[7] Therefore, antiobesity supplements are very popular among this population. Although there is a lack of conclusive information in their efficacy and possible side effects of these supplements. This issue made it one of the important problems faced by dictations. In this case, a comprehensive systematic review and meta-analysis of available clinical trials can represent the most reliable evidence of CoQ10 supplementation efficacy.
To the best of our knowledge, the current systematic review and meta-analysis examined the efficacy of CoQ10 supplementation on anthropometric indices including body weight, BMI, and WC for the first time. Our meta-analysis results showed that CoQ10 intake is not associated with significant changes in body weight, BMI, and WC. Also, the subgroup analysis based on dose and duration of CoQ10 supplementation had no significant effect on our meta-analysis results.
Recent meta-analyses suggest some beneficial health for CoQ10 supplementation such as reducing the plasma Lp(a) concentrations,[14] partly improving the process of inflammatory state,[41,42] reducing serum triglycerides levels, and helping improve the lipid profiles in patients with metabolic disorders.[43]
Although the exact mechanisms were not clarified yet, the following probable pathways may explain the influence effect of CoQ10 supplementation on body weight: (1) improving cellular bioenergetics because of the roles of CoQ10 in cellular energy processes;[44] (2) inhibition of CoQ10 synthesis strongly triggers adipocyte differentiation while increment of CoQ10 acts in inverse direction;[45] (3) CoQ10 treatment increases fat oxidation and energy consumption in adipose tissue;[46] and (4) CoQ10 inhibits adipogenesis through AMP-activated protein kinase (AMPK). AMPK is an important modulator of energy metabolism that could diminish the expression of important genes involved in adipogenesis such as peroxisome proliferator-activated receptor (PPAR)γ, CCAAT/enhancer binding protein (C/EBP)α, and fatty-acid-binding protein (FABP)[47,48,49,50]
The results showed that CoQ10 supplementation partly decreases the body weight, although these results were not significant. Also, the subgroup analysis based on the dose and duration of CoQ10 supplementation showed a small size and nonsignificant of loss weight. It should be considered that the characteristics of participants have important role in effectiveness of CoQ10. The overall results of this meta-analysis about BMI were not significant. Also, the subgroup analysis could not show any significant effects. It is to be considered that change in BMI is more complex than body weight and other anthropometric indices. Because BMI is depended on height which is fixed in participants. The results showed that CoQ10 supplementation had a nonsignificant effect on WC; the finding should be interpreted with caution because only five studies assessed the WC after CoQ10 supplementation.
Most included studies reported that CoQ10 was well-tolerated and had no adverse side effects. However, some clinical trials with CoQ10 administration found CoQ10 treatment to possibly produce nausea and heartburn. Evidence from well-designed randomized controlled human clinical trials indicates that the upper level for supplements for CoQ10 is 1200 mg/day.[51]
This is the first systematic review and meta-analysis of randomized controlled trials (RCTs) investigating the effect of CoQ10 supplementation on body weight, BMI, and WC. But there are some limitations that must be noted. First, the included trials were performed in subjects with different health condition. So it was so hard find a conclusive result from these trials. Second, usual dietary intakes were not monitored in terms of meat and dairy products in included RCTs which might have an important effect on the results. Third, the results of most of the studies were not adjusted for confounding factors which can affect the weight reduction. There are several evidence regarding the association between anthropometric indices and physical activity,[52] diet,[53] and smoking.[54] Fourth, most of the trials included in this study assessed anthropometric indices as secondary outcomes and there were not much specific trial that designed for evaluation of the effect of CoQ10 on anthropometric indices. This can reduce the accuracy and paresis of data. As the last limitation for our meta-analysis, all trials that were included in the meta-analysis have ignored the measurement of the baseline levels of CoQ10 in participants. It is very important in the interpretation of the effect of CoQ10 on anthropometric indices. The nonsignificant effects of CoQ10 could be related to low level of CoQ10 in participants.
Conclusions
In conclusion, the results of this study suggest that CoQ10 supplementation has no significant beneficial effects on anthropometric indices in adults. However, well-designed clinical trials, particularly in patients with obesity and overweight, are warranted to ultimately assess the effectiveness of this supplementation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Abarca-Gómez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128 9 million children, adolescents, and adults. Lancet. 2017;390:2627–42. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: A meta-analysis of prospective cohort studies. Obes Rev. 2014;15:504–15. doi: 10.1111/obr.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastien M, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56:369–81. doi: 10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2018;92:121–35. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Tremmel M, Gerdtham UG, Nilsson PM, Saha S. Economic burden of obesity: A systematic literature review. Int J Environ Res Public Health. 2017;14:E435. doi: 10.3390/ijerph14040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison KM, Shin S, Tarnopolsky M, Taylor VH. Association of depression & health related quality of life with body composition in children and youth with obesity. J Affect Disord. 2015;172:18–23. doi: 10.1016/j.jad.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Soeliman FA, Azadbakht L. Weight loss maintenance: A review on dietary related strategies. J Res Med Sci. 2014;19:268–75. [PMC free article] [PubMed] [Google Scholar]
- 8.Saltiel AR. New therapeutic approaches for the treatment of obesity. Sci Transl Med. 2016;8:323rv2. doi: 10.1126/scitranslmed.aad1811. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018;14:12–4. doi: 10.1038/nrendo.2017.122. [DOI] [PubMed] [Google Scholar]
- 10.Tabrizi R, Akbari M, Sharifi N, Lankarani KB, Moosazadeh M, Kolahdooz F, et al. The effects of coenzyme Q10 supplementation on blood pressures among patients with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. High Blood Press Cardiovasc Prev. 2018;25:41–50. doi: 10.1007/s40292-018-0247-2. [DOI] [PubMed] [Google Scholar]
- 11.Fallah M, Askari G, Soleimani A, Feizi A, Asemi Z. Clinical trial of the effects of coenzyme q10 supplementation on biomarkers of inflammation and oxidative stress in diabetic hemodialysis patients. Int J Prev Med. 2019;10:12. doi: 10.4103/ijpvm.IJPVM_418_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aslani Z, Shab-Bidar S, Fatahi S, Djafarian K. Effect of coenzyme Q10 supplementation on serum of high sensitivity c-reactive protein level in patients with cardiovascular diseases: A systematic review and meta-analysis of randomized controlled trials. Int J Prev Med. 2018;9:82. doi: 10.4103/ijpvm.IJPVM_263_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan L, Feng Y, Chen GC, Qin LQ, Fu Cl, Chen LH. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2017;119:128–36. doi: 10.1016/j.phrs.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 14.Sahebkar A, Simental-Mendía LE, Stefanutti C, Pirro M. Supplementation with coenzyme Q10 reduces plasma lipoprotein (a) concentrations but not other lipid indices: A systematic review and meta-analysis. Pharmacol Res. 2016;105:198–209. doi: 10.1016/j.phrs.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 15.Alcázar-Fabra M, Navas P, Brea-Calvo G. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim Biophys Acta. 2016;1857:1073–8. doi: 10.1016/j.bbabio.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Shahriary A, Khakzadihe M, Panahi Y. Appraisement function of coenzyme Q10 on body weight changes blood serum biochemical parameters in resembling type 2 diabetic male rats. J Appl Pharm Sci. 2017;7:114–9. [Google Scholar]
- 17.Yuk T, Kim Y, Yang J, Sung J, Jeong HS, Lee J. Nobiletin inhibits hepatic lipogenesis via activation of AMP-activated protein kinase. Evid Based Complement Alternat Med. 2018;2018:7420265. doi: 10.1155/2018/7420265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izadi A, Ebrahimi S, Shirazi S, Taghizadeh S, Parizad M, Farzadi L, et al. Hormonal and metabolic effects of coenzyme q10 and/or vitamin E in patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;104:319–27. doi: 10.1210/jc.2018-01221. [DOI] [PubMed] [Google Scholar]
- 19.Moazen M, Mazloom Z, Ahmadi A, Dabbaghmanesh M, Roosta S. Effect of coenzyme Q10 on glycaemic control, oxidative stress and adiponectin in type 2 diabetes. J Pak Med Assoc. 2015;65:404–8. [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-Analysis. United States: John Wiley & Sons; 2011. [Google Scholar]
- 22.Abdollahzad H, Alipour B, Aghdashi MA, Jafarabadi MA. Coenzyme Q10 supplementation in patients with rheumatoid arthritis: Are there any effects on cardiovascular risk factors? Eur J Integr Med. 2015;7:534–9. [Google Scholar]
- 23.Dai Y-L, Luk T-H, Yiu K-H, Wang M, Yip PM, Lee SW, et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: A randomized controlled trial. Atherosclerosis. 2011;216:395–401. doi: 10.1016/j.atherosclerosis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson J, Forsen T, Mortensen S, Rohde M. The effect of coenzyme Q10 administration on metabolic control in patients with type 2 diabetes mellitus. Biofactors. 1999;9:315–8. doi: 10.1002/biof.5520090229. [DOI] [PubMed] [Google Scholar]
- 25.Farsi F, Mohammadshahi M, Alavinejad P, Rezazadeh A, Zarei M, Engali KA. Functions of coenzyme Q10 supplementation on liver enzymes, markers of systemic inflammation, and adipokines in patients affected by nonalcoholic fatty liver disease: A double-blind, placebo-controlled, randomized clinical trial. J Am Coll Nutr. 2016;35:346–53. doi: 10.1080/07315724.2015.1021057. [DOI] [PubMed] [Google Scholar]
- 26.Gholami M, Rezvanfar MR, Delavar M, Abdollahi M, Khosrowbeygi A. Effects of coenzyme Q10 supplementation on serum values of gamma-glutamyl transferase, pseudocholinesterase, bilirubin, ferritin, and high-sensitivity c-reactive protein in women with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2018 doi: 10.1055/s-0043-124183. [DOI] [PubMed] [Google Scholar]
- 27.Henriksen J, Andersen CB, Hother-Nielsen O, Vaag A, Mortensen SA, Beck-Nielsen H. Impact of ubiquinone (coenzyme Q10) treatment on glycaemic control, insulin requirement and well-being in patients with Type 1 diabetes mellitus. Diabet Med. 1999;16:312–8. doi: 10.1046/j.1464-5491.1999.00064.x. [DOI] [PubMed] [Google Scholar]
- 28.Mohseni M, Vafa M, Zarrati M, Shidfar F, Hajimiresmail SJ, Forushani AR. Beneficial effects of coenzyme Q10 supplementation on lipid profile and intereukin-6 and intercellular adhesion molecule-1 reduction, preliminary results of a double-blind trial in acute myocardial infarction. Int J Prev Med. 2015;6:73. doi: 10.4103/2008-7802.162461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori TA, Burke V, Puddey IB, Irish AB, Cowpland CA, Beilin LJ, et al. The effects of ω3 fatty acids and coenzyme Q10 on blood pressure and heart rate in chronic kidney disease: A randomized controlled trial. J Hypertens. 2009;27:1863–72. doi: 10.1097/hjh.0b013e32832e1bd9. [DOI] [PubMed] [Google Scholar]
- 30.Rahmani E, Jamilian M, Samimi M, Zarezade Mehrizi M, Aghadavod E, Akbari E, et al. The effects of coenzyme Q10 supplementation on gene expression related to insulin, lipid and inflammation in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2018;34:217–22. doi: 10.1080/09513590.2017.1381680. [DOI] [PubMed] [Google Scholar]
- 31.Zhang P, Yang C, Guo H, Wang J, Lin S, Li H, et al. Treatment of coenzyme Q10 for 24 weeks improves lipid and glycemic profile in dyslipidemic individuals. J Clin Lipidol. 2018;12:417–27. doi: 10.1016/j.jacl.2017.12.006. e5. [DOI] [PubMed] [Google Scholar]
- 32.Fallah M, Askari G, Soleimani A, Feizi A, Asemi Z. Clinical trial of the effects of coenzyme Q10 supplementation on glycemic control and markers of lipid profiles in diabetic hemodialysis patients. Int Urol Nephrol. 2018;50:2073–9. doi: 10.1007/s11255-018-1973-z. [DOI] [PubMed] [Google Scholar]
- 33.Gholnari T, Aghadavod E, Soleimani A, Hamidi GA, Sharifi N, Asemi Z. The effects of coenzyme Q10 supplementation on glucose metabolism, lipid profiles, inflammation, and oxidative stress in patients with diabetic nephropathy: A randomized, double-blind, placebo-controlled trial. J Am Coll Nutr. 2018;37:188–93. doi: 10.1080/07315724.2017.1386140. [DOI] [PubMed] [Google Scholar]
- 34.Hosseinzadeh-Attar M, Kolahdouz Mohammadi R, Eshraghian M, Nakhjavani M, Khorrami E, Ebadi M, et al. Reduction in asymmetric dimethylarginine plasma levels by coenzyme Q10 supplementation in patients with type 2 diabetes mellitus. Minerva Endocrinol. 2015;40:259–66. [PubMed] [Google Scholar]
- 35.Jafarvand E, Farhangi MA, Alipour B, Khoshbaten M. Effects of coenzyme Q10 supplementation on the anthropometric variables, lipid profiles and liver enzymes in patients with non-alcoholic fatty liver disease. Bangladesh J Pharmacol. 2016;11:35–42. [Google Scholar]
- 36.Nadjarzadeh A, Sadeghi M, Amirjannati N, Vafa M, Motevalian S, Gohari M, et al. Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in idiopathic oligoasthenoteratozoospermia: A randomized double-blind, placebo controlled trial. J Endocrinol Invest. 2011;34:e224–8. doi: 10.3275/7572. [DOI] [PubMed] [Google Scholar]
- 37.Raygan F, Rezavandi Z, Tehrani SD, Farrokhian A, Asemi Z. The effects of coenzyme Q10 administration on glucose homeostasis parameters, lipid profiles, biomarkers of inflammation and oxidative stress in patients with metabolic syndrome. Eur J Nutr. 2016;55:2357–64. doi: 10.1007/s00394-015-1042-7. [DOI] [PubMed] [Google Scholar]
- 38.Shojaei M, Djalali M, Khatami M, Siassi F, Eshraghian M. Effects of carnitine and coenzyme Q10 on lipid profile and serum levels of lipoprotein (a) in maintenance hemodialysis patients on statin therapy. Iran J Kidney Dis. 2011;5:114–8. [PubMed] [Google Scholar]
- 39.Hodgson J, Watts G, Playford D, Burke V, Croft K. Coenzyme Q 10 improves blood pressure and glycaemic control: A controlled trial in subjects with type 2 diabetes. Eur J Clin Nutr. 2002;56:1137–42. doi: 10.1038/sj.ejcn.1601464. [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, Kelly AS, editors. Review of childhood obesity: From epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92:251–65. doi: 10.1016/j.mayocp.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 41.Mazidi M, Kengne AP, Banach M, Lipid Lipid and Blood Pressure Meta-analysis Collaboration Group. Effects of coenzyme Q10 supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2018;128:130–6. doi: 10.1016/j.phrs.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Zhai J, Bo Y, Lu Y, Liu C, Zhang L. Effects of coenzyme Q10 on markers of inflammation: A systematic review and meta-analysis. PLoS One. 2017;12:e0170172. doi: 10.1371/journal.pone.0170172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharifi N, Tabrizi R, Moosazadeh M, Mirhosseini N, Lankarani KB, Akbari M, et al. The effects of coenzyme Q10 supplementation on lipid profiles among patients with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. Curr Pharm Des. 2018;24:2729–42. doi: 10.2174/1381612824666180406104516. [DOI] [PubMed] [Google Scholar]
- 44.Alam MA, Rahman MM. Mitochondrial dysfunction in obesity: Potential benefit and mechanism of Co-enzyme Q10 supplementation in metabolic syndrome. J Diabetes Metab Disord. 2014;13:60. doi: 10.1186/2251-6581-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carpéné C, Iffiú-Soltész Z. Monoaminergic systems and anti-obesity drug discovery: Chronic administration of sympathicomimetic amines, re-uptake inhibitors, or amine oxidase inhibitors. Anti-Obesity Drug Discov Dev. 2011;1:114–30. [Google Scholar]
- 46.Xu Z, Huo J, Ding X, Yang M, Li L, Dai J, et al. Coenzyme Q10 improves lipid metabolism and ameliorates obesity by regulating CaMKII-mediated PDE4 inhibition. Sci Rep. 2017;7:8253. doi: 10.1038/s41598-017-08899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SK, Lee JO, Kim JH, Kim N, You GY, Moon JW, et al. Coenzyme Q10 increases the fatty acid oxidation through AMPK-mediated PPARα induction in 3T3-L1 preadipocytes. Cell Signall. 2012;24:2329–36. doi: 10.1016/j.cellsig.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 48.Choi Y, Kim Y, Ham H, Park Y, Jeong H-S, Lee J. Nobiletin suppresses adipogenesis by regulating the expression of adipogenic transcription factors and the activation of AMP-activated protein kinase (AMPK) J Agric Food Chem. 2011;59:12843–9. doi: 10.1021/jf2033208. [DOI] [PubMed] [Google Scholar]
- 49.Vingtdeux V, Chandakkar P, Zhao H, Davies P, Marambaud P. Small-molecule activators of AMP-activated protein kinase (AMPK), RSVA314 and RSVA405, inhibit adipogenesis. Mol Med. 2011;17:1022–30. doi: 10.2119/molmed.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zarei P, Rezvanfar MR, Ansarihadipour H, Delavar M, Abdollahi M, Khosrowbeygi A. Effects of coenzyme Q10 supplementation on the serum levels of amylase, adenosine deaminase, catalase, and total antioxidant capacity in women with type 2 diabetes mellitus: A randomized, double-blind placebo-controlled trial. J Res Med Sci. 2018;23:91. doi: 10.4103/jrms.JRMS_970_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hathcock JN, Shao A. Risk assessment for coenzyme Q10 (Ubiquinone) Regul Toxicol Pharmacol. 2006;45:282–8. doi: 10.1016/j.yrtph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Jakicic JM. The role of physical activity in prevention and treatment of body weight gain in adults. J Nutr. 2002;132:3826S–9S. doi: 10.1093/jn/132.12.3826S. [DOI] [PubMed] [Google Scholar]
- 53.Rolls BJ, Drewnowski A, Ledikwe JH. Changing the energy density of the diet as a strategy for weight management. J Am Diet Assoc. 2005;105:98–103. doi: 10.1016/j.jada.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 54.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–9. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]


