Abstract
Background:
Ferula asafoetida is introduced as a valuable remedy for hysteria and some other nervous disorders in Iranian traditional medicine. Asafoetida is an oleo-gum-resin obtained from the exudates of the roots of the Ferula asafoetida. Previous studies have shown that this oleo gum resin has antioxidant, anti-apoptosis, and differentiation properties in the nervous system. The aim of this study was to evaluate the effect of asafoetida on the death of oligodendrocytes and demyelination in male C57BL/6 mice in cuprizone (CPZ)-induced animal model of multiple sclerosis.
Methods:
Demyelination was induced by oral administration of rats with the 0.2% CPZ that was added to the usual diet for 8 weeks. Animals intraperitoneally received daily asafoetida at doses of 25 or 50 mg/kg of bodyweight simultaneously. At the end of the weeks, animal brains were removed and fixed to histological studies using Luxol fast blue staining. Asafoetida was screened for its antioxidant activity using 2, 2-diphenyl-1-picylhydrazyl free radical scavenging assay and for its inhibitory activity against lipid peroxidation catalyzed by soybean lipoxygenase.
Results:
The results of this study showed that asafoetida significantly decreased infiltration rate in both groups of asafoetida 25 and 50 mg/kg, respectively (P < 0.01). Histological evaluations showed the lower demyelination in LFB in the group treated with asafoetida.
Conclusions:
The results of this study showed that asafoetida plays a neuro protective role in CPZ models of multiple sclerosis by reducing neuronal demyelination and oligodendrocytes death.
Keywords: Antioxidant, asafoetida, cuprizone, multiple sclerosis
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS) characterized by recurrent and progressive demyelination/remyelination cycles, resulting in the development of scleroses in both the white and gray matter of the CNS, axonal damage, neuroinflammation, and neuronal loss.[1] Demyelination is accompanied by depletion of oligodendrocyte precursor cells, loss of mature oligodendrocytes, astrogliosis, and infiltration of macrophages/microglia and T lymphocytes.[2] Although MS was first described in the 18th century, the development of efficacious therapy has always being a challenge. This is perhaps because the exact etiopathological mechanism underlying MS has remained elusive. Cuprizone (CPZ) is a copper chelating agent and is frequently used to study factors that affect oligodendrocytes death and myelin loss.[3] The CPZ model of demyelination is characterized by apoptotic death of mature oligodendrocytes and is accompanied by neuroinflammation and motor dysfunction.[4] Mice show progressive demyelination when they are kept on a 0.2% cuprizone diet, with a peak in demyelination observed after 5 weeks of CPZ.[5] In the recent years, the tendency to herbal medicine has been increased and people have recognized and used of many cultivated or wild plants and its products have less toxic effects than synthetic drugs and are a good source for novel therapeutic agents.[6] Plants of the genus Ferula belongs to the family of Apiaceae include about 130 species that distributed throughout central Asia and Mediterranean area.[7] Ferula asafoetida L. is one the species that wildly grows in the central area of Iran and important part of this plant and several other species of Ferula is an oleo gum resin (asafoetida) yielded from incisions in the stem and/or roots of these plants.[8] In Iranian folk medicine, asafoetida is used as an anticonvulsant[7] as well as it is chewed as an antiepileptic in Morocco.[8] In Ayurveda, asafoetida is introduced as a valuable remedy for hysteria and nervous disorders.[9] In Afghanistan, hot water extract of the dried gum is also taken orally for hysteria. Nepalese use it as a sedative and a diuretic agent.[10] In Iranian folk medicine, asafoetida is used as an anticonvulsant agent as well as it is chewed as an antiepileptic in Morocco.[11] Recent pharmacological and biological studies have also shown several pharmacological activities such as antioxidant,[12] antileishmanial,[13] cancer chemopreventive,[14] anticonvulsant[15] anti-diabetic,[16] antispasmodic,[17] hypotensive,[18] and antinociceptive.[19] Asafoetida consists of three main fractions, including resin (40–64%), gum (25%), and essential oil (10–17%). The resin fraction contains ferulic acid and its esters, coumarins, sesquiterpene coumarins, and other terpenoids.[20] The gum includes glucose, galactose, l-arabinose, rhamnose, glucuronic acid, polysaccharides, and glycoproteins, and the volatile fraction contains sulfur-containing compounds, monoterpenes, and other volatile terpenoids. Although some interesting results have been obtained from their potential therapeutic usefulness of asafoetida on neural disorders, there is no study evaluating the neuroprotective effect against demyelination of this herb have been reported yet. In this work, preventive effect of asafoetida was evaluated on demyelination induced by administration of cuprizone in male C57BL/6 mice.
Methods
Animals
Twenty male C57BL/6 mice (7 weeks old, five mice in each group) were purchased from the animal house of Ahvaz University of M edical S ciences. Animals were divided into four groups and there were five mice in each group. The first group (normal control group) received standard rodent chow without CPZ, the second group (CPZ control group) received 0.2% CPZ diet, the third group received 0.2% CPZ + asafoetida 25 mg/kg i.p., and the fourth group received 0.2% CPZ + 50 mg/kg i.p. simultaneously for 8 weeks. At the end of 42-day extract exposure, the animals were sacrificed by cervical dislocation under ether anesthesia, and brains were removed and fixed to histological studies using Luxol fast blue (LFB) staining. The animal studies were performed within the guidelines set by the Shahid Sadoughi University of Medical Sciences.
Preparation of plant oleo-gum resin
Ferula assafoetida oleo-gum-resin was collected from Tabas region (Yazd province, Iran) during the summer, and the plant species was botanically identified by Dr. Abbas Zarezadeh in Yazd Agricultural Research Center. The dried powder of asafoetida was soaked in distilled water overnight at room temperature, and the yielded suspension was used intraperitoneally. Concentrations and dosages of the suspension were expressed as a crude amount of the dried oleo-gum-resin used in preparing the stock solution.[21]
Histopathological assessment
To evaluate histopathological assessment, the right corpus callosum of all sacrificed mice was removed and immediately fixed in neutral 10% formalin and then embedded in paraffin and was sectioned (5–6 μm). Then sections were examined for cellular infiltration and density of myelin with LFB. The stained sections were examined by Olympus light microscopy (Olympus, Japan). Infiltration leucocytes rate in the neural tissue of the different groups assessed: (0 = No infiltration, 1 = Low infiltration, 2 = Medium infiltration, and 3 = High Infiltration). The cell numbers were counted in a blind manner under a light microscope using a 100× magnification of 12 sections examined per animal and at least 25–30 fields, and the mean score was calculated.[22]
Quantification of myelination by measuring the density of LFB staining. Photomicrographs (200×) were taken of12 sections of each animal. The extent of demyelination was measured using the Micrometrics SE Premium 4, and in each image, the ratio of the extent of the demyelination region to the total area of the target area was measured.[23]
Lipoxygenase inhibition activity of asafoetida
The soybean 15-lipoxygenase was used to test the 15-lox inhibitory activity of asafoetida. For this purpose, 50 mL of extract solution was added to test solution containing: 3 ml of phosphate buffer (0.1 M, pH = 8), 50 mL enzyme solution (final concentration: 167 U/ml) to achieve the enzyme inhibition between 20 and 80%. After 4 min incubation of test solution, the substrate (Linoleic acid, final concentration: 134 mM) was added and the change in absorbance was measured for 60 s at 234 nm. The IC50 value was calculated graphically using the slopes of absorbance curves. The enzyme solution was kept in ice and tested at intervals to ensure that the enzyme activity was constant. All experiments were performed by UV/Vis Unico Double Beam Spectrophotometer at 25 °C in triplicate.[24]
Antioxidant activity assay of asafoetida
The antioxidant activity of asafoetida was evaluated spectrophotometrically following the diphenyl-1-picrylhydrazy (DPPH) method. Asafoetida was evaluated at 100 mg/L, by mixing 0.75 mL with 1.5 mL of a freshly prepared DPPH solution (20 mg/L); then, the sample was mixed thoroughly and kept in the dark for 30 min, at room temperature. After that, each mixture was tested for the DPPH radical-scavenging activity by reading the absorbance at 517 nm on a spectrophotometer. As blank was used, a solution prepared by mixing 0.75 mL of ultra-pure water with 1.5 mL of the DPPH solution (20 mg/L) and reading at the same wavelength. The antioxidant activity percentage was calculated following the formula:
Antioxidant activity (%) = [(A Control - A Extract)/A Control] × 100
Where A Control is the absorbance of a DPPH solution without extract, A Extract is the absorbance of the tested extract, which is equal to the absorbance of the plant extract and the DPPH (20 mg/L) minus the blank extract absorbance. The samples were run in triplicate and the mean value of three of them was recorded.[24]
Statistical analysis
Statistical data were assessed with one-way ANOVA, followed by post hoc Tukey test using Graph pad prism version 5. Results were expressed as mean ± standard error (S.E.M). A value of P < 0.05 was considered significant.
Results
Histopathological findings
We found a massive leukocyte infiltration into the brain sections in the MS control group [Figures 1 and 2]. Myelination and demyelination were measured by the density of LFB staining. In the MS group, we observed a considerable reduction in LFB staining as compared to a normal control group. Moderate demyelination was observed in the treatment groups (asafoetida 25 and 50 mg/kg) compared to the MS group indicated that remyelination in the treatment groups. A reduction in the oligodendrocytes was also observed in the MS group. There was a significant reduction of the myelin density in the MS group (MS group 17.71 ± 0.2 versus control 93.17 ± 0.2, P < 0.01). An increase in the density of the myelin was seen in treatment group asafoetida 25 (88.08 ± 0.2) and asafoetida 50 mg/kg (86.12 ± 0.1), P < 0.05 [Figure 3].
Figure 1.
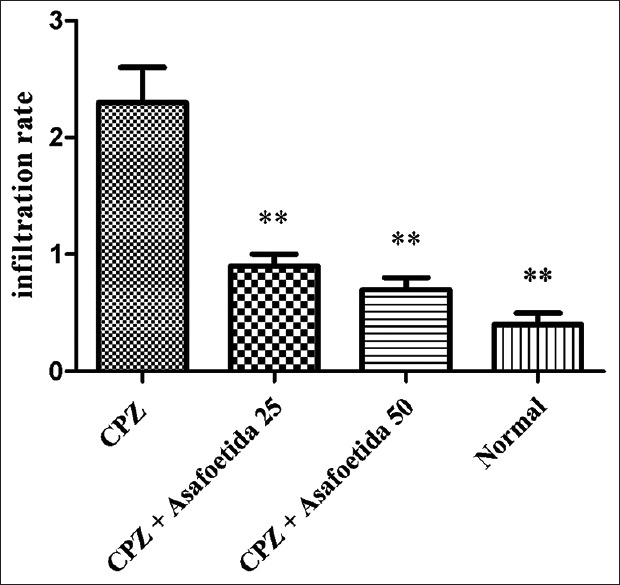
Leucocytes infiltration in different groups. There was a significant increase in the infiltrated leucocytes in the MS group versus normal control and treatment groups. ** Statistically significant at P < 0.01
Figure 2.
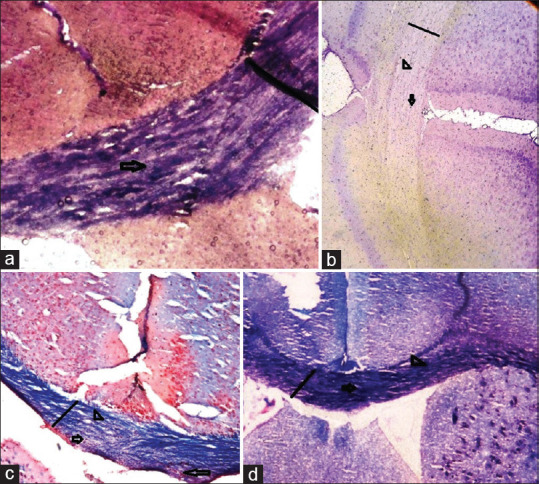
Luxol fast blue staining on tissue sections from different groups. (a) normal control group, showed normal blue staining of LFB. (b) MS group, blue color of LFB was significantly reduced, which indicated a high amount of demyelination in this group. (c and d) treatment groups, demyelination is considerably lower than the MS group. Myelin density is shown by a straight line, myelin loss is shown by arrowhead, and oligodendrocytes are shown by an arrow. (Magnification 200×)
Figure 3.
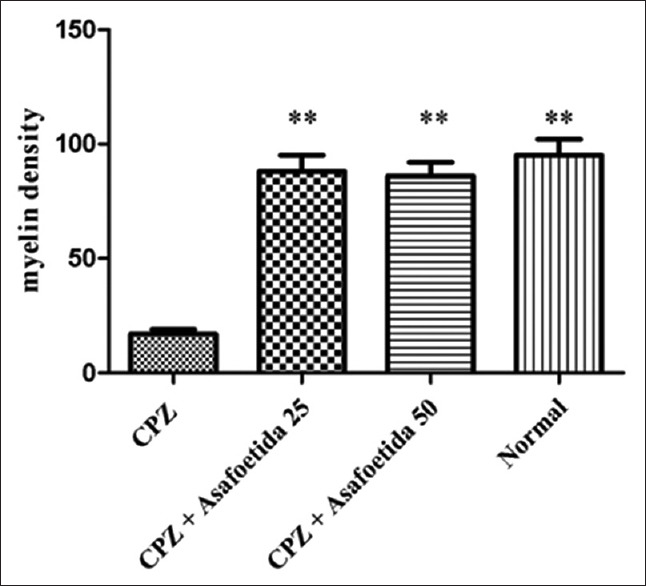
The myelin density in different groups. There was a significant decrease in the myelin density in MS group versus normal control and treatment groups. **Statistically significant at P<0.01
Lipoxygenase inhibitory and radical scavenging activity
The LOX activity of asafoetida was measured as an increase in the absorbance at 234 nm, which reflects the formation of hydroperoxylinoleic acid. The IC50 asafoetida highest inhibitory effect obtained 23 μg/ml. Our results also showed that the IC50 of antioxidant activity of the asafoetida was 109 μg/ml [Table 1].
Table 1.
Antioxidant and lipoxygenase inhibitory activities of asafoetida
| DPPH(IC50) | Lipoxygenase Inhibition(IC50) |
|---|---|
| 109 µg/ml | 23 mg/mL |
Discussion
The CPZ-induced model of demyelination has been extensively used to study the process of remyelination and has provided invaluable information regarding the effectiveness of interventions. CPZ is a white powder that is soluble slightly in water by forming a complex with two copper ions, generate Cu-CPZ complex to reduce free Cu.[25] Therefore, enzymes (e.g., superoxide dismutase-1) use copper as cofactor cannot work properly because of the fact that CPZ will reduce the amount of free Cu.[26] Consequently, it results in reversible demyelination and oligodendrocyte apoptosis during CPZ consumption that these changes are apparent in the corpus callosum.[27] Traditional usages and some recent findings suggested that Ferula asafoetida can exert some effects on the function of the nervous system particularly in neuro protective and nerve stimulating effects.[8]Ferula asafoetida extracts treatment on glutamate-induced cell damaged in primary culture of rat cerebellar granule neurons was investigated by Tayeboon et al.[28] Neuro protective effects of extracts of Ferula asafoetida against glutamate-induced neurotoxicity confirmed by an increased glutamate-induced reduction in cellular viability and attenuated glutamate-induced apoptotic/necrotic cell death. The extract exerted antiapoptotic activity in cerebellar granule neurons owing to cell cycle arrest in G0G1 phase, which explains the beneficial effects of Ferula asafoetida extract as therapies for neurologic disorders. In vitro studies were carried out by Moghadam et al. to identify the response of isolated sciatic nerves to various concentrations of asafoetida solved in Lock's solution.[29] In vivo studies were also conducted to evaluate its effect on amelioration of peripheral neuropathy in mice.[30] These experiments indicated that incubating the nerves in aqueous extract of the asafoetida increased the amplitude and decreased the latent period of nerve compound action potential. The ability of asafoetida to facilitate the healing process in peripheral nerves is also confirmed by the histological and behavioral studies. In vitro experiments showed that asafoetida is a nerve stimulant and its management in neuropathic mice exerted neuroprotection effects through stimulating axonal regeneration and remyelination and decrement of lymphocyte infiltration.[30] Other components that exist in asafoetida are hinesol and mmbelliferone with anti-inflammatory and anti-coagulative effects.[8] Besides, ferulic acid is one of the most bioactive coumarins of asafoetida with variety of known actions such as anti-coagulatory, vasodilatory, and antioxidative effects.[8] The results of HPLC analysis also showed that there was a high concentration of ferulic acid and umbelliferone in ashke asafoetida. It was reported recently by Lee et al. that ferulic acid improves peripheral nerve regeneration by the induction of Schwann cell proliferation.[31] They also found that the NCV of injured rat sciatic nerves improved after ferulic acid treatment. These data support the point that asafoetida can be at least effective on treatment of neuropathy because of its ferulic acid. However, arachidonic acid cascade is suggested to become activated during demyelination.[32] Asafoetida is full of sulfurous compounds and surviving effect of polysulfide compounds on neurons derived from mouse embryo were reported previously.[33] Sulfur-containing nutraceuticals, having neuro protective effects, may exert some direct antioxidative effects; their principal mode of neuro protection is through activation of endogenous antioxidant systems, including gene targets of the Nrf2/ARE transcription factor pathway.[34] In addition, some other components such as sesquiterpene coumarins, sodium ferulate, and ferulic acid are also neuro protective.[29] Ferulic acid could improve the survival rate of neurons through inhibiting ICAM-1 mRNA expression.[35] The sesquiterpene coumarins such as fukane furomarin B, E, F, and G could suppress No production.[36] We found a massive leucocyte infiltration into the brain sections in the MS control group [Figures 1 and 2b]. In the MS group, we observed a considerable reduction in LFB staining as compared to the normal control group. Moderate demyelination was observed in the treatment group (asafoetida 25 and 50 mg/kg) compared to MS group indicated that remyelination happened in the treatment groups. According to the histological assays, we found that asafoetida treatment increased the axonal regeneration and remyelination, and conversely reduced the rate of lymphocyte infiltration in the neuropathic tissue. We proposed that asafoetida could reverse the damage to the nerves by using some multiple actions: anti coagulating and muscle-relaxing effects by increasing blood flow, neuro protecting, and nerve stimulating effects through increasing nerve growth, anti-oxidative, and anti-apoptotic factors by prevention of cell death, and anti-inflammatory effect by reducing the acute immune responses within the injured tissue. Different mechanisms seem to impact on this activity such as radical scavenging activity of sulfur-containing compounds, lipoxygenase inhibition by umbelliprenin and its derivatives, increase in the activity of endogenous antioxidants, and decrease in oxidative parameters. The LOX activity was measured as an increase in the absorbance at 234 nm, which reflects the formation of hydroperoxylinoleic acid. The IC50 asafoetida highest inhibitory effect obtained 23 μg/ml. In this work, our results also showed that the IC50 of the antioxidant activity of asafoetida was 109 μg/ml. Increased expression of 5-lipoxygenase (5-LO) in lesions and of 5-LO-derived leukotriene (LT) products in the cerebrospinal fluid have been reported in patients with MS.[1] These data suggest that the 5-LO pathway is involved in microglial activation and neuroinflammation independently of the demyelination process.
Conclusions
In summary, our results present the first evidence protective effect asafoetida on CPZ-induced demyelination and lipoxygenase inhibitory activity as demonstrated by in vivo male C57BL/6 mice. More detailed molecular mechanisms, for instance, genomic and proteomic responses underlying the asafoetida remain to be elucidated. Besides, further investigation is needed to determine the clinical efficacy and safety of asafoetida in human subjects with MS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank all people who have assisted the experimental procedure and to the research deputy of Yazd Shahid Sadughi Medical University as the sponsor of this research.
References
- 1.Yoshikawa K, Palumbo S, Toscano C, Bosetti F. Inhibition of 5-lipoxygenase activity in mice during CPZ-induced demyelination attenuates neuroinflammation, motor dysfunction and axonal damage. Prostaglandins Leukot Essent Fatty Acids. 2011;85:43–52. doi: 10.1016/j.plefa.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: Pathology of the newly forming lesion. Ann Neurol. 2004;55:458–68. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 3.Abakumova T, Kuz'kina A, Zharova M, Pozdeeva D, Gubskii I, Shepeleva I, et al. Cuprizone model as a tool for preclinical studies of the efficacy of multiple sclerosis diagnosis and therapy. Bull Exp Biol Med. 2015;159:111–5. doi: 10.1007/s10517-015-2903-z. [DOI] [PubMed] [Google Scholar]
- 4.Franco-Pons N, Torrente M, Colomina MT, Vilella E. Behavioral deficits in the cuprizone-induced murine model of demyelination/remyelination. Toxicol Lett. 2007;169:205–13. doi: 10.1016/j.toxlet.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Hiremath M, Saito Y, Knapp G, Ting JY, Suzuki K, Matsushima G. Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol. 1998;92:38–49. doi: 10.1016/s0165-5728(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 6.Bagheri SM, Dashti-R MH. Influence of asafoetida on prevention and treatment of memory impairment induced by d-galactose and NaNO2 in mice. AmJ Alzheimer's DisOther Demen. 2015;30:607–12. doi: 10.1177/1533317515576388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagheri SM, Sahebkar A, Gohari AR, Saeidnia S, Malmir M, Iranshahi M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm Biol. 2010;48:242–6. doi: 10.3109/13880200903081796. [DOI] [PubMed] [Google Scholar]
- 8.Iranshahy M, Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)—A review. J Ethnopharmacol. 2011;134:1–10. doi: 10.1016/j.jep.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 9.Bagheri SM, Abdian-Asl A, Moghadam MT, Yadegari M, Mirjalili A, Zare-Mohazabieh F, et al. Antitumor effect of Ferula assa foetida oleo gum resin against breast cancer induced by 4T1 cells in BALB/c mice. J Ayurveda Integr Med. 2017;8:152–8. doi: 10.1016/j.jaim.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandyopadhyay D, Basak B, Chatterjee A, Lai TK, Banerji A, Banerji J, et al. Saradaferin, a new sesquiterpenoid coumarin from Ferula assafoetida. Nat Prod Res. 2006;20:961–5. doi: 10.1080/14786410600823431. [DOI] [PubMed] [Google Scholar]
- 11.Kiasalari Z, Khalili M, Roghani M, Heidari H, Azizi Y. Antiepileptic and antioxidant effect of hydroalcoholic extract of ferula assa foetida gum on pentylentetrazole-induced kindling in male mice. Basic Clin Neurosci. 2013;4:299–306. [PMC free article] [PubMed] [Google Scholar]
- 12.Dehpour AA, Ebrahimzadeh MA, Seyed Fazel N, Seyed Mohammad N. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas Aceites. 2009;60:405–12. [Google Scholar]
- 13.Bafghi AF, Bagheri SM, Hejazian SH. Antileishmanial activity of Ferula assa-foetida oleo gum resin against Leishmania major: An in vitro study. J Ayurveda Integr Med. 2014;5:223–6. doi: 10.4103/0975-9476.146567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saleem M, Alam A, Sultana S. Asafoetida inhibits early events of carcinogenesis: Achemopreventive study. Life Sci. 2001;68:1913–21. doi: 10.1016/s0024-3205(01)00977-8. [DOI] [PubMed] [Google Scholar]
- 15.Bagheri SM, Rezvani ME, Vahidi AR, Esmaili M. Anticonvulsant effect of ferula assa-foetida oleo gum resin on chemical and amygdala-kindled rats. N Am J Med Sci. 2014;6:408–12. doi: 10.4103/1947-2714.139296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu-Zaiton AS. Anti-diabetic activity of Ferula assafoetida extract in normal and alloxan-induced diabetic rats. Pak J Biol Sci. 2010;13:97–100. doi: 10.3923/pjbs.2010.97.100. [DOI] [PubMed] [Google Scholar]
- 17.Bagheri S, Hejazian S, Dashti-R M. The relaxant effect of seed fs essential oil and oleo-gum-resin of ferula assa-foetida on isolatedrat's ileum. Ann Med Health Sci Res. 2015;4:238–41. doi: 10.4103/2141-9248.129050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fatehi M, Farifteh F, Fatehi-Hassanabad Z. Antispasmodic and hypotensive effects of Ferula asafoetida gum extract. J Ethnopharmacol. 2004;91:321–4. doi: 10.1016/j.jep.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Bagheri S, Dashti-R M, Morshedi A. Antinociceptive effect of Ferula assa-foetida oleo-gum-resin in mice. Res Pharm Sci. 2014;9:207–12. [PMC free article] [PubMed] [Google Scholar]
- 20.Azizian H, Ebrahim Rezvani M, Esmaeilidehaj M, Bagheri SM. Anti-obesity, fat lowering and liver steatosis protective effects of Ferula asafoetida gum in type 2 diabetic rats: Possible involvement of leptin. IJDO. 2012;4:120–6. [Google Scholar]
- 21.Bagheri SM, Yadegari M, Mirjalily A, Rezvani ME. Evaluation of toxicity effects of asafetida on biochemical, hematological, and histological parameters in male wistar rats. Int J Toxicol. 2015;22:61–5. doi: 10.4103/0971-6580.172258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haghmorad D, Mahmoudi MB, Mahmoudi M, Rab SZ, Rastin M, Shegarfi H, et al. Calcium intervention ameliorates experimental model of multiple sclerosis. Oman Med J. 2014;29:185–9. doi: 10.5001/omj.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chitnis T, Imitola J, Wang Y, Elyaman W, Chawla P, Sharuk M, et al. Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. Am J Pathol. 2007;170:1695–712. doi: 10.2353/ajpath.2007.060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagheri SM, Hedesh ST, Mirjalili A, Dashti-R MH. Evaluation of anti-inflammatory and some possible mechanisms of antinociceptive effect of ferula assa foetida oleo gum resin. J Evid Based Complementary Altern Med. 2015;21:271–6. doi: 10.1177/2156587215605903. [DOI] [PubMed] [Google Scholar]
- 25.Manto M. Abnormal copper homeostasis: Mechanisms and roles in neurodegeneration. Toxics. 2014;2:327–45. [Google Scholar]
- 26.Omotoso GO, Gbadamosi IT, Afolabi TT, Abdulwahab AB, Akinlolu AA. Ameliorative effects of Moringa on cuprizone-induced memory decline in rat model of multiple sclerosis. Anat Cell Biol. 2018;51:119–27. doi: 10.5115/acb.2018.51.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acs P, Kipp M, Norkute A, Johann S, Clarner T, Braun A, et al. 17β-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia. 2009;57:807–14. doi: 10.1002/glia.20806. [DOI] [PubMed] [Google Scholar]
- 28.Tayeboon GS, Tavakoli F, Hassani S, Khanavi M, Sabzevari O, Ostad SN. Effects of cymbopogon citratus and Ferula assa-foetida extracts on glutamate-induced neurotoxicity. In Vitro Cell Dev Biol Anim. 2013;49:706–15. doi: 10.1007/s11626-013-9656-7. [DOI] [PubMed] [Google Scholar]
- 29.Moghadam FH, Zarch BV, Shafiei M. Double edged effect of gum-resin of ferula assa-foetida on lifespan of neurons. Iran J Basic Med Sci. 2013;16:668–71. [PMC free article] [PubMed] [Google Scholar]
- 30.Moghadam FH, Dehghan M, Zarepur E, Dehlavi R, Ghaseminia F, Ehsani S, et al. Oleo gum resin of Ferula assa-foetida L.ameliorates peripheral neuropathy in mice. J Ethnopharmacol. 2014;154:183–9. doi: 10.1016/j.jep.2014.03.069. [DOI] [PubMed] [Google Scholar]
- 31.Lee SC, Tsai CC, Yao CH, Chen YS, Wu MC. Ferulic acid enhances peripheral nerve regeneration across long gaps. Evid Based Complement Alternat Med. 2013;2013:876327. doi: 10.1155/2013/876327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palumbo S, Toscano C, Parente L, Weigert R, Bosetti F. Time-dependent changes in the brain arachidonic acid cascade during cuprizone-induced demyelination and remyelination. Prostaglandins Leukot Essent Fatty Acids. 2011;85:29–35. doi: 10.1016/j.plefa.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010;15:7792–814. doi: 10.3390/molecules15117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C, Kong A-NT. Dietary chemopreventive compounds and ARE/EpRE signaling. Free Radic Res. 2004;36:1505–16. doi: 10.1016/j.freeradbiomed.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Cheng CY, Su SY, Tang NY, Ho TY, Chiang SY, Hsieh CL. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008;1209:136–50. doi: 10.1016/j.brainres.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 36.Motai T, Kitanaka S. Sesquiterpene phenylpropanoids from Ferula fukanensis and their nitric oxide production inhibitory effects. J Nat Prod. 2005;68:365–8. doi: 10.1021/np040215c. [DOI] [PubMed] [Google Scholar]


