Abstract
Objective
To evaluate sodium-glucose cotransporter-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists in patients with type 2 diabetes at varying cardiovascular and renal risk.
Design
Network meta-analysis.
Data sources
Medline, Embase, and Cochrane CENTRAL up to 11 August 2020.
Eligibility criteria for selecting studies
Randomised controlled trials comparing SGLT-2 inhibitors or GLP-1 receptor agonists with placebo, standard care, or other glucose lowering treatment in adults with type 2 diabetes with follow up of 24 weeks or longer. Studies were screened independently by two reviewers for eligibility, extracted data, and assessed risk of bias.
Main outcome measures
Frequentist random effects network meta-analysis was carried out and GRADE (grading of recommendations assessment, development, and evaluation) used to assess evidence certainty. Results included estimated absolute effects of treatment per 1000 patients treated for five years for patients at very low risk (no cardiovascular risk factors), low risk (three or more cardiovascular risk factors), moderate risk (cardiovascular disease), high risk (chronic kidney disease), and very high risk (cardiovascular disease and kidney disease). A guideline panel provided oversight of the systematic review.
Results
764 trials including 421 346 patients proved eligible. All results refer to the addition of SGLT-2 inhibitors and GLP-1 receptor agonists to existing diabetes treatment. Both classes of drugs lowered all cause mortality, cardiovascular mortality, non-fatal myocardial infarction, and kidney failure (high certainty evidence). Notable differences were found between the two agents: SGLT-2 inhibitors reduced admission to hospital for heart failure more than GLP-1 receptor agonists, and GLP-1 receptor agonists reduced non-fatal stroke more than SGLT-2 inhibitors (which appeared to have no effect). SGLT-2 inhibitors caused genital infection (high certainty), whereas GLP-1 receptor agonists might cause severe gastrointestinal events (low certainty). Low certainty evidence suggested that SGLT-2 inhibitors and GLP-1 receptor agonists might lower body weight. Little or no evidence was found for the effect of SGLT-2 inhibitors or GLP-1 receptor agonists on limb amputation, blindness, eye disease, neuropathic pain, or health related quality of life. The absolute benefits of these drugs vary substantially across patients from low to very high risk of cardiovascular and renal outcomes (eg, SGLT-2 inhibitors resulted in 3 to 40 fewer deaths in 1000 patients over five years; see interactive decision support tool (https://magicevidence.org/match-it/200820dist/#!/) for all outcomes.
Conclusions
In patients with type 2 diabetes, SGLT-2 inhibitors and GLP-1 receptor agonists reduced cardiovascular and renal outcomes, with some differences in benefits and harms. Absolute benefits are determined by individual risk profiles of patients, with clear implications for clinical practice, as reflected in the BMJ Rapid Recommendations directly informed by this systematic review.
Systematic review registration
PROSPERO CRD42019153180.
Introduction
Diabetes affects half a billion people worldwide and accounted for 1.5 million deaths in 2016.1 Glucose lowering is a mainstay of treatment.2 In people with type 2 diabetes and higher risks of cardiovascular disease, several large scale randomised controlled trials have investigated sodium-glucose cotransporter-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists and reported reductions in cardiovascular mortality and non-fatal cardiovascular complications with both classes of drug.3 4 The mortality reductions have not, however, proved consistent across trials, leaving clinicians and patients uncertain of the magnitude of benefits.5 6 7 8
Recommendations released in 2019 by the American Diabetes Association include using SGLT-2 inhibitors and GLP-1 receptor agonists in the management of diabetes for people with cardiovascular disease or kidney disease who have not reached their glycaemic target goals.2 European Society of Cardiology guidelines in 2019 recommended SGLT-2 inhibitors and GLP-1 receptor agonists in people with type 2 diabetes and cardiovascular disease or a high risk of cardiovascular disease.9 National Institute for Health and Care Excellence (NICE) guidance, updated in 2019, suggested a stepped approach to intensification of treatment with diabetes drugs, considering metformin as the first treatment with additions of dual and triple treatment from several drug classes, including SGLT-2 inhibitors.10
Several published systematic reviews and meta-analyses have summarised glucose lowering treatments for people with type 2 diabetes, including a network meta-analysis of stepped intensification of treatment added to metformin and sulfonylurea.11 Results from more recent meta-analyses report reductions in mortality and selected cardiovascular and renal events with both SGLT-2 inhibitors and GLP-1 receptor agonists.12 13 14 15 A network meta-analysis including all available glucose lowering treatments, the full range of important outcomes, GRADE (grading of recommendations assessment, development, and evaluation) certainty ratings, and estimates of absolute benefits and harms in patients with varying risks of cardiovascular and kidney disease remains unavailable. Moreover, several large scale trials published recently require updated evidence synthesis.5 7 8 16
We therefore conducted a systematic review and network meta-analysis evaluating the benefits and harms of SGLT-2 inhibitors and GLP-1 receptor agonists in adults with type 2 diabetes. This review is conducted as part of the BMJ Rapid Recommendations project, a collaborative initiative from the MAGIC (Making GRADE the Irresistible Choice) Evidence Ecosystem Foundation (https://magicevidence.org/) and The BMJ. The aim of the initiative is to provide trustworthy practice guidelines within months of newly released evidence, underpinned by rigorous evidence summaries. This systematic review is part of a forthcoming BMJ Rapid Recommendations cluster and a fuller version on MAGICapp (www.magicapp.org). Although the network meta-analysis includes all classes of diabetes drugs, it focuses on these SGLT-2 inhibitors and GLP-1 receptor agonists added to other diabetes drugs, and in relation to one another.17
Methods
Protocol registration
We registered the protocol for this systematic review with PROSPERO (CRD42019153180).
Guideline panel involvement
In accordance with the BMJ Rapid Recommendations process, a guideline panel that comprised content experts, diabetologists, nephrologists, internal medicine physicians, primary care physicians, methodologists, and patients provided critical oversight of the review. The panel reviewed the protocol, identified the population, selected and ranked important patient outcomes, and recommended baseline risks on which absolute treatment effects were calculated.
Search strategy
The literature search designed by an information specialist was conducted in Medline, Embase, and the Cochrane Central Register of Controlled Trials from March 2016 to 11 August 2020 without language restriction (appendix 1). The search from a previous network meta-analysis provided records from database inception to March 2016.11
Study selection
Two reviewers, working independently, screened citations and evaluated full text records for eligible studies using Covidence systematic review software (Veritas Health Innovation, Melbourne, VIC, Australia; https://www.covidence.org). A third reviewer (BT or SCP) resolved disagreements by consensus.
Parallel group randomised controlled trials were eligible if they compared SGLT-2 inhibitors or GLP-1 receptor agonists with one another or with other glucose lowering treatments, placebo, or standard care in adults with type 2 diabetes. We included studies reporting outcomes at 24 weeks or longer. Treatment was given either as monotherapy or added to non-randomised background glucose lowering management and other treatments.
Using definitions of outcomes specified in individual randomised controlled trials, the review examined all patient-important outcomes as defined by the guideline panel, regardless of whether evidence was available in the eligible trials: all cause mortality, cardiovascular mortality, non-fatal myocardial infarction, non-fatal stroke, kidney failure, admission to hospital for heart failure, severe hypoglycaemia, blindness, eye disease requiring intervention, health related quality of life, body weight, amputation, neuropathic pain, diabetic ketoacidosis, serious hyperglycaemia, genital infection, Fournier gangrene, severe gastrointestinal events, pancreatic cancer, and pancreatitis. Glycated haemoglobin A1c was not identified as an outcome important to patients by the guideline panel, although it was included in the review because it is prioritised by some decision makers.
Data extraction
For each eligible study, two reviewers independently extracted the following: study characteristics (year of publication, country or countries, funding, duration), population (setting, sample size, patient demographics, coexisting illnesses), description of interventions (drug class, name, dose), and outcomes. Reviewers resolved disagreements by discussion or, if necessary, consultation with a third reviewer.
Risk of bias assessment
Two reviewers independently assessed risk of bias, with adjudication by a third reviewer (SCP) using the Cochrane tool for assessing risk of bias in randomised trials, which includes random sequence generation, allocation concealment, blinding, missing outcome data, and selective reporting of outcomes.18 Each domain was judged as low, unclear, or high risk of bias. Reviewers considered allocation concealment as low risk if there were no reported methods for allocation concealment and participants and investigators were blind to treatment allocation.
Data synthesis and analysis
For each direct comparison of two treatments, we conducted a frequentist pairwise meta-analysis using a restricted maximum likelihood estimation and reported, with corresponding 95% confidence intervals, odds ratios for dichotomous outcomes, mean differences for continuous outcomes (body weight and glycated haemoglobin A1c), and standardised mean difference for health related quality of life.19 We assessed statistical heterogeneity using the I2 statistic and funnel plots for evidence of small study effects in analyses including 10 or more studies.
We conducted network meta-analysis using frequentist methods with restricted maximum likelihood estimation to quantify network heterogeneity, assuming a common heterogeneity estimate within a network. We assessed agreement between direct and indirect estimates in every closed loop of evidence using node splitting approaches, and for the entire network using a design-by-treatment interaction model.20 We imputed missing standard deviations for continuous variables when absent using standard deviations borrowed from other similar randomised controlled trials.21 22
The guideline panel recommended five baseline risk categories to estimate absolute effects of treatment on cardiovascular and kidney outcomes in different categories of patients, reflecting typical clinical scenarios in practice. Patient categories were defined as very low risk (no or few (<3) cardiovascular risk factors), low risk (three or more cardiovascular risk factors), moderate risk (cardiovascular disease), high risk (chronic kidney disease (estimated glomerular filtration rate 45-75 mL/min per 1.73 m2 with albuminuria >300 mg/g (30 mg/mmol) or estimated glomerular filtration rate 15-45 mL/min per 1.73 m2)), and very high risk (cardiovascular disease and chronic kidney disease)). The panel calculated absolute treatment effects of the network estimates based on the event rates from the best sources of evidence, including cohort studies, risk prediction equations, or the placebo arm in available trials.23 24 25 26 27 28 We estimated baseline absolute risk per 1000 patients treated for five years, extrapolating from shorter term data when necessary and assuming a constant annual risk of the selected outcome for each year up to five years. A single estimate was obtained for all outcomes and treatment comparisons, which was used in combination with the baseline risk estimates to present results as absolute effects. We planned subgroup analysis if sufficient data were available according to trial duration, BMI, presence of high cardiovascular risk, and presence of chronic kidney disease. All pairwise and network meta-analyses were performed with Stata 13 MP (StataCorp, College Station, TX) using published routines.29
Assessment of evidence certainty
The GRADE approach provided the methods to assess the certainty of the evidence for direct and network comparisons, using a non-contextualised approach.30 31 Ratings of evidence certainty for direct estimates included considerations of risk of bias, inconsistency, indirectness, imprecision, and publication bias.
The lower of the ratings from the two direct estimates forming the dominant first order loop provided the starting point for certainty ratings for each indirect estimate, with further downward rating for intransitivity,32 if present. The estimate that provided the most information—direct or indirect—was the basis for the certainty of the network estimates. If these estimates contributed similar amounts of information, we chose the higher of the two certainty judgments. If evidence of incoherence between direct and indirect estimates existed, the certainty of the network estimate was rated down, and we used the estimate with the highest certainty—direct or indirect—as the best estimate of the treatment effect. MAGICapp (https://www.magicapp.org/) provided the platform to develop tables of the GRADE summary of findings. We also provide the absolute estimates of effect and associated uncertainties across risk groups, derived from best relative estimates of effect from the network meta-analysis. These results are presented in evidence summaries and decision aids developed in MAGICapp, including an interactive decision support tool for multiple treatment choices (https://magicevidence.org/match-it/200820dist/#!/).
Patient and public involvement
Four patient partners living with type 2 diabetes were included in the guideline panel that oversaw the generation of this paper. Patient partners were recruited from patient advocacy organisations and Cochrane Task Exchange. They were involved as full panel members in modifying the research question and identifying and prioritising patient-important outcomes. There was no public participation in this project.
Results
Description of included studies
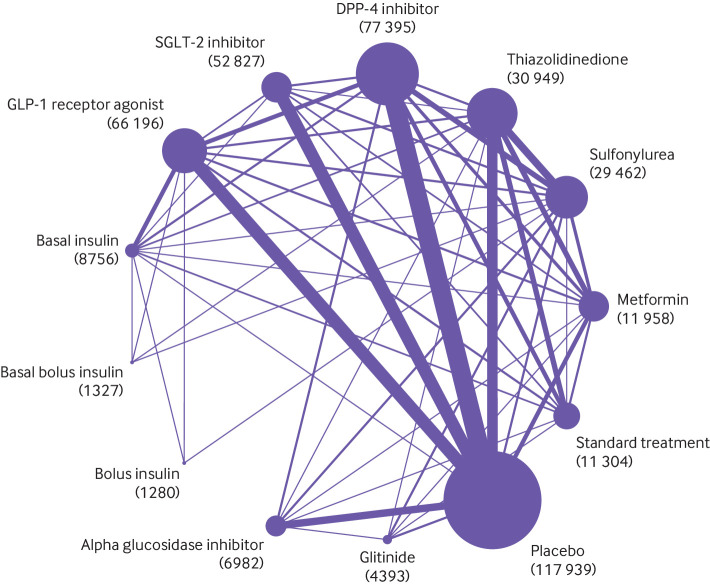
The electronic search yielded 23 185 unique records. Screening and full text article analysis identified 764 trials including 421 346 patients (fig 1, appendix 2) comparing 11 glucose lowering drugs, placebo, or standard care. Figure 2 shows the network of treatment comparisons in available trials. The trial sample size ranged from 16 to 17 160. The median trial mean age was 57.1 years, and the median proportion of men was 55.6%. At baseline, the median trial mean glycated haemoglobin A1c was 8.1% and BMI was 30.1. Eligibility criteria included coronary artery disease or macrovascular disease in 35 trials,3 6 7 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 atrial fibrillation in 1 trial,65 heart failure in 9 trials,66 67 68 69 70 71 72 73 74 chronic kidney disease and/or albuminuria in 37 trials,8 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 and high risk of cardiovascular or kidney disease in 10 trials.4 5 16 111 112 113 114 115 116 117 Treatment with SGLT-2 inhibitors or GLP-1 receptor agonists was generally added to background glucose lowering treatment.
Fig 1.
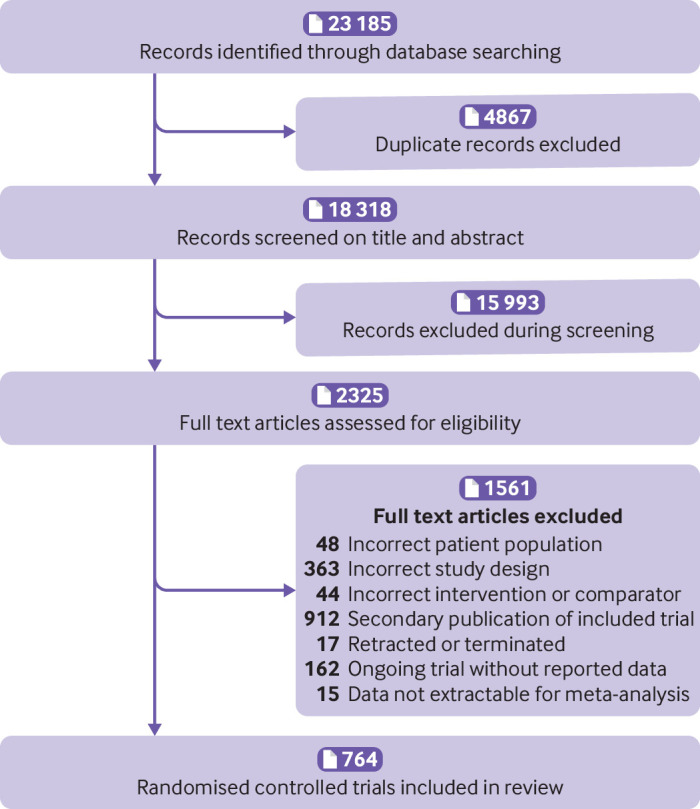
PRISMA flow diagram of studies included in the review of glucose lowering treatments for type 2 diabetes
Fig 2.
Network plot of trials evaluating glucose lowering treatments for type 2 diabetes. Network shows the number of participants assigned to each glucose lowering class with the size of each circle proportional to the number of randomly assigned participants in the treatment comparisons (sample size for the specific treatment shown in brackets). Line widths are proportional to the number of trials comparing the corresponding pair of treatments. The most frequent drug comparison was DPP-4 inhibitors compared with placebo. DPP-4= dipeptidyl peptidase-4 inhibitors; GLP-1=glucagon-like peptide-1; SGLP-1=sodium-glucose cotransporter-2
Risk of bias
Appendix 3 presents the risk of bias in each trial. The key limitation was low levels of reported blinding for participants, investigators, and outcome assessors. Three hundred and seven (40.2%) of 764 trials were at low risk of bias in random sequence generation and 529 (69.2%) were at low risk of bias in allocation concealment. Four hundred and sixty two trials (60.5%) reported blinding for participants and investigators, and 105 trials (13.7%) reported blinding for outcome assessment. Three hundred and twenty eight trials (42.9%) were adjudicated as being at low risk of attrition bias and 355 trials (46.5%) were at low risk of bias from selective outcome reporting.
Outcomes
Appendix 4 presents the network plot for each outcome. No evidence of global network inconsistency or heterogeneity was found except for health related quality of life (appendix 5), and no serious concerns of incoherence between direct and indirect evidence (appendix 6). Appendix 7 presents network estimates for each drug comparison for all outcomes. The expected absolute differences in treatment with SGLT-2 inhibitors or GLP-1 receptor agonists compared with placebo and each other are shown in table 1, table 2, table 3, table 4, and appendix 8. The interactive decision support tool at https://magicevidence.org/match-it/200820dist/#!/ shows absolute estimates of effect for key outcomes in patients with type 2 diabetes, from very low to very high risk of cardiovascular and renal outcomes.
Table 1.
Summary of anticipated absolute differences comparing sodium-glucose cotransporter-2 inhibitor treatment with placebo treatment per 1000 patients with diabetes type 2 and with very low to very high cardiovascular risk, treated for five years
| Risk* | All cause mortality | Cardiovascular mortality | Non-fatal myocardial infarction | Non-fatal stroke | Kidney failure | Hospital admission for heart failure | Diabetic ketoacidosis | Genital infection | Body weight |
|---|---|---|---|---|---|---|---|---|---|
| Very low | 3 fewer (4 fewer to 2 fewer) ⊕⊕⊕ |
2 fewer (3 fewer to 1 fewer) ⊕⊕⊕ |
4 fewer (6 fewer to 1 fewer) ⊕⊕⊕ |
0 more (3 fewer to 4 more) ⊕⊕⊕ |
1 fewer (1 fewer to 0) ⊕⊕⊕ |
2 fewer (2 fewer to 1 fewer) ⊕⊕⊕ |
0 (1 fewer to 2 more) ⊕⊕⊕ |
143 more (119 more to 170 more) ⊕⊕⊕⊕ |
1.92 kg lower (2.23 lower to 1.62 lower) over 6 months ⊕⊕ |
| Low | 10 fewer (15 fewer to 6 fewer) ⊕⊕⊕⊕ |
7 fewer (11 fewer to 4 fewer) ⊕⊕⊕⊕ |
7 fewer (12 fewer to 2 fewer) ⊕⊕⊕⊕ |
1 more (6 fewer to 8 more) ⊕⊕⊕⊕ |
3 fewer (4 fewer to 1 fewer) ⊕⊕⊕⊕ |
9 fewer (11 fewer to 7 fewer) ⊕⊕⊕⊕ |
|||
| Moderate | 18 fewer (25 fewer to 10 fewer) ⊕⊕⊕⊕ |
12 fewer (18 fewer to 6 fewer) ⊕⊕⊕⊕ |
13 fewer (21 fewer to 3 fewer) ⊕⊕⊕⊕ |
1 more (11 fewer to 13 more) ⊕⊕⊕⊕ |
6 fewer (9 fewer to 2 fewer) ⊕⊕⊕⊕ |
23 fewer (28 fewer to 17 fewer) ⊕⊕⊕⊕ |
|||
| High | 26 fewer (36 fewer to 14 fewer) ⊕⊕⊕⊕ |
16 fewer (25 fewer to 8 fewer) ⊕⊕⊕⊕ |
14 fewer (23 fewer to 3 fewer) ⊕⊕⊕⊕ |
1 more (12 fewer to 15 more) ⊕⊕⊕⊕ |
25 fewer (37 fewer to 9 fewer) ⊕⊕⊕⊕ |
29 fewer (36 fewer to 22 fewer) ⊕⊕⊕⊕ |
|||
| Very high | 40 fewer (56 fewer to 21 fewer) ⊕⊕⊕⊕ |
24 fewer (36 fewer to 12 fewer) ⊕⊕⊕⊕ |
21 fewer (34 fewer to 5 fewer) ⊕⊕⊕⊕ |
2 more (17 fewer to 21 more) ⊕⊕⊕⊕ |
38 fewer (58 fewer to 14 fewer) ⊕⊕⊕⊕ |
58 fewer (73 fewer to 44 fewer) ⊕⊕⊕⊕ |
Risk categories represent the following patient populations: very low=no or less than three cardiovascular risk factors; low=three or more cardiovascular risk factors; moderate=cardiovascular disease; high=chronic kidney disease (reduced glomerular filtration rate or macroalbuminuria); very high=cardiovascular disease and chronic kidney disease.
Certainty of the evidence for each estimate is shown: high certainty ⊕⊕⊕⊕; moderate certainty ⊕⊕⊕; low certainty ⊕⊕; very low certainty ⊕.
Table 2.
Summary of anticipated absolute differences comparing glucagon-like peptide-1 receptor agonist treatment with placebo treatment per 1000 patients with diabetes type 2 and with very low to very high cardiovascular risk, treated for five years
| Risk* | All cause mortality | Cardiovascular mortality | Non-fatal myocardial infarction | Non-fatal stroke | Kidney failure | Hospital admission for heart failure | Severe gastrointestinal events | Body weight |
|---|---|---|---|---|---|---|---|---|
| Very low | 2 fewer (3 fewer to 1 fewer) ⊕⊕⊕ |
2 fewer (3 fewer to 1 fewer) ⊕⊕⊕ |
2 fewer (4 fewer to 0) ⊕⊕⊕ |
5 fewer (7 fewer to 2 fewer) ⊕⊕⊕ |
0 (1 fewer to 0) ⊕⊕⊕ |
0 (1 fewer to 0) ⊕⊕⊕ |
58 more (9 more to 142 more) ⊕⊕ |
145 kg lower (1.72 lower to 1.18 lower) over 6 months ⊕⊕ |
| Low cardiovascular risk factors | 8 fewer (11 fewer to 4 fewer) ⊕⊕⊕⊕ |
5 fewer (9 fewer to 2 fewer) ⊕⊕⊕⊕ |
4 fewer (8 fewer to 1 fewer) ⊕⊕⊕⊕ |
9 fewer (13 fewer to 4 fewer) ⊕⊕⊕⊕ |
2 fewer (1 fewer to 3 fewer) ⊕⊕⊕⊕ |
2 fewer (4 fewer to 1 more) ⊕⊕⊕⊕ |
||
| Moderate | 13 fewer (18 fewer to 6 fewer) ⊕⊕⊕⊕ |
9 fewer (15 fewer to 1 fewer) ⊕⊕⊕⊕ |
8 fewer (15 fewer to 1 fewer) ⊕⊕⊕⊕ |
16 fewer (24 fewer to 7 fewer) ⊕⊕⊕⊕ |
4 fewer (7 fewer to 2 fewer) ⊕⊕⊕⊕ |
4 fewer (11 fewer to 2 more) ⊕⊕⊕⊕ |
||
| High | 17 fewer (25 fewer to 9 fewer) ⊕⊕⊕⊕ |
12 fewer (20 fewer to 4 fewer) ⊕⊕⊕⊕ |
9 fewer (16 fewer to 1 fewer) ⊕⊕⊕⊕ |
17 fewer (26 fewer to 7 fewer) ⊕⊕⊕⊕ |
19 fewer (28 fewer to 7 fewer) ⊕⊕⊕⊕ |
6 fewer (14 fewer to 3 more) ⊕⊕⊕⊕ |
||
| Very high | 24 fewer (35 fewer to 12 fewer) ⊕⊕⊕⊕ |
18 fewer (30 fewer to 6 fewer) ⊕⊕⊕⊕ |
13 fewer (24 fewer to 2 fewer) ⊕⊕⊕⊕ |
25 fewer (39 fewer to 11 fewer) ⊕⊕⊕⊕ |
29 fewer (44 fewer to 10 fewer) ⊕⊕⊕⊕ |
11 fewer (28 fewer to 5 more) ⊕⊕⊕⊕ |
Risk categories represent the following patient populations: very low=no or less than three cardiovascular risk factors; low=three or more cardiovascular risk factors; moderate=cardiovascular disease; high=chronic kidney disease (reduced glomerular filtration rate or macroalbuminuria); very high=cardiovascular disease and chronic kidney disease.
Certainty of the evidence for each estimate is shown: high certainty ⊕⊕⊕⊕; moderate certainty ⊕⊕⊕; low certainty ⊕⊕; very low certainty ⊕.
Table 3.
Summary of anticipated absolute differences comparing sodium-glucose cotransporter-2 inhibitor treatment with glucagon-like peptide-1 receptor agonist treatment per 1000 patients with diabetes type 2 and with very low to very high cardiovascular risk, treated for five years
| Risk* | All cause mortality | Cardiovascular mortality | Non-fatal myocardial infarction | Non-fatal stroke | Kidney failure | Hospital admission for heart failure | Body weight | Diabetic ketoacidosis | Serious hyperglycaemia | Genital infection |
|---|---|---|---|---|---|---|---|---|---|---|
| Very low | 1 fewer (3 fewer to 1 more) ⊕⊕⊕ |
0 fewer (2 fewer to 1 more) ⊕⊕⊕ |
1 fewer (4 fewer to 2 more) ⊕⊕⊕ |
5 more (1 more to 10 more) ⊕⊕⊕ |
0 (1 fewer to 0) ⊕⊕ |
1 fewer (2 fewer to 1 fewer) ⊕⊕⊕ |
0.47 kg lower (0.09 lower to 0.85 lower) over 6 months ⊕⊕⊕ |
1 more (0 to 3 more) ⊕⊕⊕ |
5 more (2 fewer to 19 more) ⊕⊕⊕ |
158 more (64 more to 299 more) ⊕⊕⊕⊕ |
| Low | 4 fewer (10 fewer to 4 more) ⊕⊕⊕⊕ |
2 fewer (6 fewer to 4 more) ⊕⊕⊕⊕ |
3 fewer (8 fewer to 4 more) ⊕⊕⊕⊕ |
9 more (1 more to 19 more) ⊕⊕⊕⊕ |
1 fewer (2 fewer to 2 more) ⊕⊕⊕ |
7 fewer (10 fewer to 4 fewer) ⊕⊕⊕⊕ |
||||
| Moderate | 6 fewer (17 fewer to 7 more) ⊕⊕⊕⊕ |
3 fewer (11 fewer to 6 more) ⊕⊕⊕⊕ |
5 fewer (15 fewer to 7 more) ⊕⊕⊕⊕ |
16 more (2 more to 33 more) ⊕⊕⊕⊕ |
1 fewer (5 fewer to 3 more) ⊕⊕⊕ |
18 fewer (25 fewer to 11 fewer) ⊕⊕⊕⊕ |
||||
| High | 9 fewer (24 fewer to 10 more) ⊕⊕⊕⊕ |
4 fewer (15 fewer to 8 more) ⊕⊕⊕⊕ |
5 fewer (16 fewer to 8 more) ⊕⊕⊕⊕ |
18 more (3 more to 36 more) ⊕⊕⊕⊕ |
6 fewer (21 fewer to 13 more) ⊕⊕⊕ |
24 fewer (32 fewer to 13 fewer) ⊕⊕⊕⊕ |
||||
| Very high | 13 fewer (37 fewer to 16 more) |
5 fewer (22 fewer to 12 more) |
7 fewer (24 fewer to 11 more) |
27 more (4 more to 53 more) |
10 fewer (34 fewer to 20 more) |
48 fewer (66 fewer to 27 fewer) |
Risk categories represent the following patient populations: very low=no or less than three cardiovascular risk factors; low=three or more cardiovascular risk factors; moderate=cardiovascular disease; high=chronic kidney disease (reduced glomerular filtration rate or macroalbuminuria); very high=cardiovascular disease and chronic kidney disease.
Certainty of the evidence for each estimate is shown: high certainty ⊕⊕⊕⊕; moderate certainty ⊕⊕⊕; low certainty ⊕⊕; very low certainty ⊕.
Table 4.
GRADE summary of findings to illustrate absolute effects based on cardiovascular and renal risk, for all cause mortality for sodium-glucose cotransporter-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists compared with placebo or each other
| Comparison | Relative effect (odds ratio (95% CI)) | Anticipated absolute effects over five years | Anticipated absolute effects (95% CI) over five years | Certainty in treatment effects (GRADE) | Plain text summary | ||
|---|---|---|---|---|---|---|---|
| Baseline risk* | Risk with control | Risk with intervention | |||||
| SGLT-2 inhibitor v placebo | 0.85 (0.79 to 0.92) | Very low | Placebo: 20 per 1000 | SGLT-2 inhibitor: 17 per 1000 | 3 fewer per 1000 (from 2 fewer to 4 fewer) |
Moderate due to indirectness | SGLT-2 inhibitor treatment probably reduces all cause mortality in people with diabetes and few or no cardiovascular risk factors |
| Low | Placebo: 70 per 1000 | SGLT-2 inhibitor: 60 per 1000 | 10 fewer per 1000 (from 6 fewer to 15 fewer) |
High | SGLT-2 inhibitor treatment reduces all cause mortality in people with diabetes and cardiovascular risk factors | ||
| Moderate | Placebo: 120 per 1000 | SGLT-2 inhibitor: 102 per 1000 | 18 fewer per 1000 (from 10 fewer to 25 fewer) |
High | SGLT-2 inhibitor treatment reduces all cause mortality in people with diabetes and established cardiovascular disease | ||
| High | Placebo: 170 per 1000 | SGLT-2 inhibitor: 144 per 1000 | 26 fewer per 1000 (from 14 fewer to 36 fewer) |
High | SGLT-2 inhibitor treatment reduces all cause mortality in people with diabetes and chronic kidney disease | ||
| Very high | Placebo: 265 per 1000 | SGLT-2 inhibitor: 225 per 1000 | 40 fewer per 1000 (from 21 fewer to 56 fewer) |
High | SGLT-2 inhibitor treatment reduces all cause mortality in people with diabetes and established cardiovascular disease and chronic kidney disease | ||
| GLP-1 receptor agonist v placebo | 0.88 (0.83 to 0.94) | Very low | Placebo: 20 per 1000 | GLP-1 receptor agonist: 18 per 1000 | 2 fewer per 1000 (from 1 fewer to 3 fewer) | Moderate due to indirectness | GLP-1 receptor agonist treatment probably reduces all cause mortality in people with diabetes and few or no cardiovascular risk factors |
| Low | Placebo: 70 per 1000 | GLP-1 receptor agonist: 62 per 1000 | 8 fewer per 1000 (from 4 fewer to 11 fewer) |
High | GLP-1 receptor agonist treatment reduces all cause mortality in people with diabetes and cardiovascular risk factors | ||
| Moderate | Placebo: 120 per 1000 | GLP-1 receptor agonist: 107 per 1000 | 13 fewer per 1000 (from 6 fewer to 18 fewer) |
High | GLP-1 receptor agonist treatment reduces all cause mortality in people with diabetes and established cardiovascular disease | ||
| High | Placebo: 170 per 1000 | GLP-1 receptor agonist: 153 per 1000 | 17 fewer per 1000 (from 9 fewer to 25 fewer) |
High | GLP-1 receptor agonist treatment reduces all cause mortality in people with diabetes and chronic kidney disease | ||
| Very high | Placebo: 265 per 1000 | GLP-1 receptor agonist: 241 per 1000 | 24 fewer per 1000 (from 12 fewer to 35 fewer) |
High | GLP-1 receptor agonist treatment reduces all cause mortality in people with diabetes and established cardiovascular disease and chronic kidney disease | ||
| SGLT-2 inhibitor v GLP-1 receptor agonist | 0.95 (0.86 to 1.06) | Very low | GLP-1 receptor agonist: 18 per 1000 | SGLT-2 inhibitor: 17 per 1000 | 1 fewer per 1000 (from 1 more to 3 fewer) |
Moderate due to indirectness | SGLT-2 inhibitor treatment and GLP-1 receptor agonist treatment probably have similar effects on all cause mortality in people with diabetes and few or no cardiovascular risk factors |
| Low | GLP-1 receptor agonist: 62 per 1000 | SGLT-2 inhibitor: 58 per 1000 | 4 fewer per 1000 (from 4 more to 10 fewer) |
High | SGLT-2 inhibitor treatment and GLP-1 receptor agonist treatment have similar effects on all cause mortality in people with diabetes and cardiovascular risk factors | ||
| Moderate | GLP-1 receptor agonist: 107 per 1000 | SGLT-2 inhibitor: 101 per 1000 | 6 fewer per 1000 (from 7 more to 17 fewer) |
High | SGLT-2 inhibitor treatment and GLP-1 receptor agonist treatment have similar effects on all cause mortality in people with diabetes and established cardiovascular disease | ||
| High | GLP-1 receptor agonist: 153 per 1000 | SGLT-2 inhibitor: 144 per 1000 | 9 fewer per 1000 (from 10 more to 24 fewer) |
High | SGLT-2 inhibitor treatment and GLP-1 receptor agonist treatment have similar effects on all cause mortality in people with diabetes and chronic kidney disease | ||
| Very high | GLP-1 receptor agonist: 241 per 1000 | SGLT-2 inhibitor: 228 per 1000 | 13 fewer per 1000 (from 16 more to 37 fewer) |
High | SGLT-2 inhibitor treatment and GLP-1 receptor agonist treatment have similar effects on all cause mortality in people with diabetes and established cardiovascular disease and chronic kidney disease | ||
GRADE=grading of recommendations assessment, development, and evaluation.
Risk categories represent the following patient populations: very low=no or less than three cardiovascular risk factors; low=three or more cardiovascular risk factors; moderate=cardiovascular disease; high=chronic kidney disease (reduced glomerular filtration rate or macroalbuminuria); very high=cardiovascular disease and chronic kidney disease.
The point estimate of the absolute effect for GLP-1 receptor agonist treatment, obtained from GLP-1 receptor agonist treatment versus placebo, was used to calculate the absolute effect for SGLT-2 inhibitors versus GLP-1 receptor agonists.
All cause and cardiovascular mortality
Two hundred and thirty eight trials including 290 662 patients reported all cause mortality. SGLT-2 inhibitors lowered all cause mortality compared with placebo (odds ratio 0.85 (95% confidence interval 0.79 to 0.92); 3 fewer per 1000 in five years for very low risk patients (moderate certainty); 10 fewer for low risk patients (high certainty); 18 fewer for moderate risk patients (high certainty); 26 fewer for high risk patients (high certainty); and 40 fewer for very high risk patients (high certainty); table 4). GLP-1 receptor agonists lowered all cause mortality compared with placebo (0.88 (0.83 to 0.94); 2, 8, 13, 17, and 24 fewer per 1000 in five years for very low, low, moderate, high, and very high risk patients respectively, moderate to high certainty; table 4).
SGLT-2 inhibitors and GLP-1 receptor agonists had similar effects on all cause mortality (0.95 (0.86 to 1.06, moderate to high certainty; table 3).
One hundred and thirty five trials including 226 701 patients reported cardiovascular mortality. SGLT-2 inhibitors lowered cardiovascular mortality compared with placebo (0.84 (0.76 to 0.92); 2, 7, 12, 16, and 24 fewer per 1000 in five years for very low, low, moderate, high, and very high risk patients respectively, moderate to high certainty; table 1), as did GLP-1 receptor agonists (0.88 (0.80 to 0.96); 2, 5, 9, 12, and 18 fewer per 1000 in five years for very low, low, moderate, high, and very high risk patients respectively, moderate to high certainty; table 2). SGLT-2 inhibitors and GLP-1 receptor agonists did not have different effects on cardiovascular mortality (0.96 (0.84 to 1.09, moderate to high certainty; appendix 8).
Non-fatal myocardial infarction
Two hundred and eight trials including 265 921 patients reported non-fatal myocardial infarction. SGLT-2 inhibitors lowered odds of non-fatal myocardial infarction compared with placebo (odds ratio 0.87 (95% confidence interval 0.79 to 0.97); 4, 7, 13, 14, and 21 fewer per 1000 in five years for very low, low, moderate, high, and very high risk patients respectively, moderate to high certainty; appendix 8), as did GLP-1 receptor agonists (0.92 (0.85 to 0.99); 2, 4, 8, 9, 13 fewer per 1000 in five years for very low, low, moderate, high, and very high risk patients respectively, moderate to high certainty; appendix 8). SGLT-2 inhibitors and GLP-1 receptor agonists did not have different effects on non-fatal myocardial infarction (0.95 (0.84 to 1.08); moderate to high certainty; appendix 8).
Non-fatal stroke
One hundred and seventy six trials including 261 434 patients reported non-fatal stroke. SGLT-2 inhibitors had little or no effect on non-fatal stroke (odds ratio 1.01 (95% confidence interval 0.89 to 1.14); moderate to high certainty; appendix 8). GLP-1 receptor agonists reduced non-fatal stroke (0.84 (0.76 to 0.93); 5, 9, 16, 17, and 25 fewer per 1000 in five years for very low, low, moderate, high, and very high risk patients respectively, moderate to high certainty; appendix 8). SGLT-2 inhibitors had higher odds of non-fatal stroke than GLP-1 receptor agonists (1.20 (1.03 to 1.41); moderate to high certainty; appendix 8).
Kidney failure
Thirty three trials including 98 284 patients reported kidney failure, defined generally as estimated glomerular filtration rate below 15 ml/min per 1.73 m2 or start of kidney replacement treatment. SGLT-2 inhibitors reduced kidney failure (odds ratio 0.71 (95% confidence interval 0.57 to 0.89); 1,3, 6, 25, and 38 fewer per 1000 in five years for very low, low, moderate, high, and very high risk patients respectively, moderate to high certainty; appendix 8). GLP-1 receptor agonists also reduced kidney failure (0.78 (0.67 to 0.92); 0, 2, 4, 19, and 29fewer per 1000 in five years for very low, low, moderate, high, and very high risk patients respectively, moderate to high certainty; appendix 8). SGLT-2 inhibitors and GLP-1 receptor agonists probably did not have different effects on kidney failure (0.91 (0.69 to 1.20); low to moderate certainty).
Admission to hospital for heart failure
One hundred and forty nine trials including 242 361 patients reported admission to hospital for heart failure. SGLT-2 inhibitors reduced admission for heart failure (odds ratio 0.70 (95% confidence interval 0.63 to 0.77); 2, 9, 23, 29, and 58 fewer per 1000 in five years for very low, low, moderate, high, and very high risk patients respectively, moderate to high certainty; appendix 8). GLP-1 receptor agonists had little or no effect on hospitalisation for heart failure (0.94 (0.85 to 1.03), moderate to high certainty; appendix 8). SGLT-2 inhibitors reduced hospitalisation for heart failure compared with GLP-1 receptor agonists (0.74 (0.65 to 0.85); 1, 7, 18, 24, and 48 fewer per 1000 in five years for very low, low, moderate, high, and very high risk patients respectively, moderate to high certainty; table 3).
Blindness and eye disease requiring intervention
Seven trials including 68 221 patients reported blindness. The results of the network meta-analysis were uninformative as blindness was rarely reported and the confidence intervals for estimated treatment effects were very wide (appendix 7). The outcome of eye disease requiring intervention was not specifically reported in any trial.
Health related quality of life
Twenty five trials including 11 912 patients reported health related quality of life during a median follow-up of six months. The reported instruments included the Diabetes Treatment Satisfaction Questionnaire, the Short Form 12 and 36, the EuroQoL five dimensions (EQ-5D), the Diabetes Quality of Life, Treatment Satisfaction Status Scale, and the Impact of Weight on Quality of Life. SGLT-2 inhibitors had uncertain effects on health related quality of life (standardised mean difference 0.14 (95% confidence interval −0.08 to 0.36); low certainty; appendix 8). GLP-1 receptor agonists might increase health related quality of life (0.13 (0.03 to 0.23); low certainty; appendix 8). No evidence was found that SGLT-2 inhibitors and GLP-1 receptor agonists had different effects on health related quality of life (0.01 (−0.19 to 0.20); low certainty; appendix 8).
Body weight
Four hundred and sixty nine trials including 226 361 patients reported body weight during a median follow-up of six months. SGLT-2 inhibitor treatment and GLP-1 receptor agonist treatment might lower body weight (mean difference for SGLT-2 inhibitors −1.92 kg (95% confidence interval −2.23 to −1.62); low certainty; mean difference for GLP-1 receptor agonists −1.45 kg (−1.72 to −1.18); low certainty; appendix 8). SGLT-2 inhibitors appeared to lower body weight to a greater extent than GLP-1 receptor agonists (−0.47 kg (−0.85 to −0.09); moderate certainty).
Glycated haemoglobin A1c
Six hundred and four trials including 242 745 patients reported glycated haemoglobin A1c during a median follow-up of six months. SGLT-2 inhibitors (mean difference −0.60% (95% confidence interval −0.67 to −0.54); low certainty) and GLP-1 receptor agonists (−0.89% (−0.95 to −0.82); low certainty) might lower glycated haemoglobin A1c levels more than placebo (low certainty). GLP-1 receptor agonists reduced glycated haemoglobin A1c levels to a greater extent than SGLT-2 inhibitors (mean difference −0.28% (95% CI −0.37 to −0.19); high certainty).
Other outcomes
GLP-1 receptor agonists reduced serious hyperglycaemia with moderate to high certainty (15 fewer events in 1000 patients treated with GLP-1 receptor agonists over five years). Results showed the effects of treatment on amputation or neuropathic pain were very uncertain (appendix 8).
Harms
No difference in the odds of serious hypoglycaemia was found in a comparison of SGLT-2 inhibitor or GLP-1 receptor agonist treatment with placebo or with each other in high or moderate certainty evidence (appendix 8). SGLT-2 inhibitor treatment and GLP-1 receptor agonist treatment probably did not increase diabetic ketoacidosis compared with placebo or each other (moderate certainty). SGLT-2 inhibitors increased genital infection compared with placebo and GLP-1 receptor agonists (high certainty). The effects of treatments on Fournier gangrene were uncertain. GLP-1 receptor agonists might incur severe gastrointestinal events (low certainty). An increase in pancreatic cancer or pancreatitis is probably not likely with SGLT-2 inhibitor or GLP-1 receptor agonist treatment compared with placebo (low to moderate certainty), and there might be no difference between SGLT-2 inhibitor and GLP-1 receptor agonist treatment for pancreatic cancer (low certainty). SGLT-2 inhibitor treatment might incur a lower risk of pancreatitis than GLP-1 receptor agonist treatment (low certainty).
No evidence was found that treatment effects differed according to trial level characteristics, including trial duration, BMI, cardiovascular risk, and presence of chronic kidney disease (appendix 9).
Discussion
Principal findings
This network meta-analysis provides high certainty evidence that SGLT-2 inhibitors and GLP-1 receptor agonists, when added to other diabetes treatment, reduce mortality, non-fatal myocardial infarction, kidney failure, and serious hyperglycaemia. In the absence of head-to-head trials, we also found high certainty evidence for notable differences between SGLT-2 inhibitors and GLP-1 receptor agonists; SGLT-2 inhibitors reduced admission to hospital for heart failure more than GLP-1 receptor agonists, whereas GLP-1 receptor agonists reduced non-fatal stroke more than SGLT-2 inhibitors (which appeared to have no effect). Differential effects of SGLT-2 inhibitors and GLP-1 receptor agonists on other patient important outcomes included lower body weight and increased quality of life, albeit with small absolute differences. Harms also differed. SGLT-2 inhibitors caused genital infection, and GLP-1 receptor agonists might increase severe gastrointestinal events. Importantly, the absolute benefits for cardiovascular and renal outcomes varied substantially for patients, depending on their absolute cardiovascular risk (very low to very high; https://magicevidence.org/match-it/200820dist/#!/) Taken together, the results have clear implications for clinical practice, as reflected in the BMJ Rapid Recommendations directly informed by our systematic review.
Strength and limitations of this study
The strengths of the review include a sensitive search to identify eligible trials, and independent study identification, selection, data extraction, and adjudication of risk of bias by two reviewers. The review used the GRADE approach to report the certainty of available evidence and reported estimated absolute risks for all outcomes, including patients with varying risks of cardiovascular and kidney disease. Limitations include the heterogeneity in clinical settings of the included trials, although the consistency of results across studies diminishes this concern. Some outcomes resulted in imprecise estimates of effects and low certainty evidence.
Clinical uncertainties
SGLT-2 inhibitors failed to reduce non-fatal stroke in the same way that they reduced other cardiovascular endpoints, a finding not scientifically intuitive. Trials generally did not include patients with lowest cardiovascular risk. Accordingly, the certainty of evidence was graded down for the lowest risk patients owing to indirectness. The panel agreed that a non-contextualised approach was appropriate to assess imprecision in estimates of effects to inform judgments about the certainty of evidence in the network meta-analysis. We therefore did not adjudicate whether the range of treatment effects for each outcome was trivial, small, moderate, or large; assess critical outcomes simultaneously; or make inferences about their value relative to each other.31 The related BMJ Rapid Recommendation will adjudicate the importance of the absolute effects of treatment and rate certainty accordingly. This approach might lower the adjudicated evidence certainty for several outcomes that inform the BMJ Rapid Recommendations. Whether SGLT-2 inhibitors and GLP-1 receptor agonists given together provide additional benefits compared with each treatment alone is not answered by this review. Notably, the effects of treatment on glycated haemoglobin A1c and body weight were provided in trials with an average follow up of six months. Possibly, the relative effects of the SGLT-2 inhibitor and GLP-1 receptor agonist treatment on these outcomes might have been different in shorter duration trials than in the longer therapeutic evaluations of cardiovascular outcomes.
Comparisons with other studies
This systematic review included substantially more trials—including large and recently published trials—and patients than previously reported reviews and is based on priority setting and received oversight by a panel with a range of clinical and lived experiences. SGLT-2 inhibitors and GLP-1 receptor agonists had similar treatment effects on mortality as observed by a previous network meta-analysis published in 2016, although precision in the treatment estimates has increased substantially.11 The results provide evidence to support guideline recommendations made in 2019 that patients at highest risk of cardiovascular disease and kidney disease are likely to have important benefits on risks of cardiovascular events and heart failure with SGLT-2 inhibitors.2 9 This network meta-analysis aims to provide a broader representation of both relative and absolute estimates of a wide range of important clinical outcomes.
The analysis is consistent with large scale observational analyses of SGLT-2 inhibitor treatment in a range of countries and settings, which suggest beneficial effects on a range of clinical outcomes, including reduced mortality and heart failure in people with diabetes at low cardiovascular risk and those with atherosclerotic cardiovascular disease.118 119 The effects of treatment in the available randomised trials in our analyses are smaller than seen with observational data, as the trial data enable analyses that reduce the risks of confounding by treatment indication; in other words, in real world datasets, people who receive drug treatment might be healthier or have other characteristics that lead to better outcomes than people who are not prescribed treatment. The present review can discern important differences between the effect of SGLT-2 inhibitors and GLP-1 receptor agonists on mortality and heart failure, not identified in large scale non-randomised studies.120
Policy implications
This systematic review of 764 clinical trials provides detailed information for decision makers about the benefits and harms of SGLT-2 inhibitors and GLP-1 receptor agonists on important outcomes in adults with type 2 diabetes. It includes trials with publicly available information up to August 2020. A core finding suggests that there is high certainty that SGLT-2 inhibitors reduce all cause and cardiovascular mortality, non-fatal myocardial infarction, kidney failure, and admission to hospital for heart failure. GLP-1 receptor agonist treatment reduces all cause and cardiovascular mortality, non-fatal myocardial infarction, non-fatal stroke, and kidney failure. SGLT-2 inhibitor treatment reduces admission to hospital for heart failure to a greater extent than GLP-1 receptor agonist treatment, whereas GLP-1 receptor agonist treatment reduces non-fatal stroke more than SGLT-2 inhibitors. The effect of SGLT-2 inhibitors and GLP-1 receptor agonists on many other important patient outcomes is uncertain. Glycated haemoglobin A1c was not chosen as an important outcome in the review by the oversight panel, which included patients and clinicians, but it was reported as it is considered by some decision makers to be a measure of glycaemic control and indicative of a more immediate measure of diabetes control.
Conclusions
Trials of SGLT-2 inhibitors in people with existing cardiovascular disease and chronic kidney disease with or without diabetes are emerging or ongoing,7 69 121 indicating that SGLT-2 treatment decreases heart failure in the absence of type 2 diabetes. The findings of the Dapagliflozin and Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomised controlled trial are awaited to identify whether dapagliflozin decreases kidney and cardiovascular events in people regardless of the presence of diabetes.121
In conclusion, this network meta-analysis provides evidence of the absolute benefits and harms of SGLT-2 inhibitors and GLP-1 receptor agonists. The balance of benefits and harms depends on individual risk profiles of patients, and thus the results support a risk stratified approach for provision of SGLT-2 inhibitors and GLP-1 receptor agonists to patients with type 2 diabetes. Accordingly, the associated BMJ Rapid Recommendations will provide risk stratified recommendations and highlight the need for shared decision making to allow patients and clinicians to make well informed decisions together.
These data provide direct evidence for the absolute benefits and harms of SGLT-2 inhibitors and GLP-1 receptor agonists. The findings of this research paper raise questions about how they should be used in clinical practice. In the linked article, readers will find recommendations on SGLT-2 inhibitors and GLP-1 receptor agonists based on data from this paper. To do this a guideline panel has considered how direct this evidence is and using GRADE methodology has integrated this with, for example, the values and preferences of patients and resource implications. To read more about the guidelines please see the guideline article in this package.
What is already known on this topic
Although trial results conflict, sodium-glucose transporter 2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists probably reduce cardiovascular and kidney disease when added to other glucose lowering treatment in adults with type 2 diabetes
Uncertainty remains about the relative and absolute benefits and harms of these drugs across all important outcomes in patients with type 2 diabetes at varying levels of cardiovascular and kidney disease risk
What this study adds
Benefits shared by SGLT-2 inhibitors and GLP-1 receptor agonists include reductions in all cause and cardiovascular mortality, myocardial infarction, kidney failure, and serious hyperglycaemia (high certainty evidence) and lower body weight (low certainty) without incurring severe hypoglycaemia (high certainty)
The two drug classes appear to have similar relative effects on all cause and cardiovascular mortality, myocardial infarction, kidney failure, health related quality of life, and serious hyperglycaemia
Absolute benefits of these drugs vary substantially based on cardiovascular and renal disease risk profiles of patients with type 2 diabetes, as reflected in risk stratified BMJ Rapid Recommendations directly informed by this systematic review
Acknowledgments
We thank members of the Rapid Recommendations panel for critical feedback on the review question and outcome selection, GRADE judgments, and manuscript. We thank Ruth Mitchell for developing the search strategy.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributors: SCP, AN, and GFMS conceived the study. SCP and GFMS designed the search strategy. SCP performed the literature search. SCP, BT, MR, PN, VS, DWJ, MCR, SVB, YC, ACNF, MB, LIF, AL, NA, YL, ST, TM, LG, NK, RDB, RM, AKRC, HW, XYC, XZ, JL, AFR, ADGC, YW, LL, SS, RCS, and FDG screened studies for eligibility; SCP, MR, and PN assessed the risk of bias; and SCP, BT, MR, PN, VS, DWJ, SVB, YC, ACNF, MB, LIF, AL, NA, YL, ST, TM, LG, NK, RB, RM, AKRC, HW, XC, XZ, JL, AFR, ADGC, YW, LL, SS, RCS, and FDG performed data extraction. SCP, BT, RAM, POV, SL, QH, AN, DT, DWJ, MT, MW, GG, and GFMS interpreted the data analysis; SCP, MR, PN, and GFMS assessed the certainty of the evidence; SCP wrote the first draft of the manuscript; and all other authors revised the manuscript. SCP and GFMS are guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This systematic review did not receive any funding.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: SCP, BT, RAM, POV, SL, QH, DT, MR, PN, VS, YC, ACNF, MB, LIF, AL, NA, YL, ST, TM, NK, RDB, RM, AKRC, HW, XC, XZ, JL, AFR, ADGC, YW, LL, SS, RCS, FDG, RRG, MW, GG, GFMS: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years. SL was supported by grants from the National Natural Science Foundation of China (grant number 21534008), Sichuan Science and Technology Programme (grant number 2019YFH0150), and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (grant numbers ZYGD18022 and 2020HXF011). None of these grants contribute to this work. AN reports grants and personal fees from Novo Nordisk, grants from Sanofi, grants and personal fees from Astra Zeneca, grants from Pikdare, grants from AlfaSigma, outside the submitted work. DWJ reports grants and personal fees from Baxter Healthcare, grants and personal fees from Fresenius Medical Care, other from Amgen, personal fees from Astra Zeneca, personal fees from AWAK, grants from National Health and Medical Research Council of Australia, personal fees from Ono, outside the submitted work. MT reports a grant to the institution by Daichi Sankyo in lieu of a personal honorarium. MCR reports grants from Sanofi, grants from NovoNordisk, grants from AstraZeneca, grants from Pikdare, during the conduct of the study. SVB reports advisory board membership to Bayer Australia and AstraZeneca, speaking honoraria from Bayer Australia and Pfizer Australia; non-financial research support from Bayer AG. LG reports personal fees from Boehringer-Engelheim, personal fees from Lilly, personal fees from Astra Zeneca, non-financial support from Novo Nordisk, outside the submitted work.
The manuscript’s guarantors (SCP and GFMS) affirm that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
Dissemination to participants and related patient and public communities: The paper informs an upcoming BMJ Rapid Recommendation (https://www.bmj.com/rapid-recommendations) on the use of SGLT-2 inhibitors and GLP-1 receptor agonists, also available in MAGICapp (https://www.magicapp.org/) for organisations to reuse or adapt for their own materials and purposes.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
No additional data available.
References
- 1.World Health Organization. Global Health Observatory (GHO) data. https://www.who.int/data/gho/data/themes/topics/causes-of-death. World Health Organization, Geneva, Switzerland.
- 2. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care 2019;42(Suppl 1):S90-102. 10.2337/dc19-S009 [DOI] [PubMed] [Google Scholar]
- 3. Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117-28. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 4. Marso SP, Daniels GH, Brown-Frandsen K, et al. LEADER Steering Committee. LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311-22. 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wiviott SD, Raz I, Bonaca MP, et al. DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347-57. 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 6. Hernandez AF, Green JB, Janmohamed S, et al. Harmony Outcomes committees and investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519-29. 10.1016/S0140-6736(18)32261-X [DOI] [PubMed] [Google Scholar]
- 7. McGuire DK, Cannon CP, Pratley R, et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV). Diabetologia 2018;61(Supplement 1):S305-6. [DOI] [PubMed] [Google Scholar]
- 8. Perkovic V, Jardine MJ, Neal B, et al. CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295-306. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 9. Cosentino F, Grant PJ, Aboyans V, et al. ESC Scientific Document Group . 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255-323. 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence. Type 2 diabetes in adults: management (NICE Guideline No. 28). Retrieved from https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#drug-treatment-2. 2019.
- 11. Palmer SC, Mavridis D, Nicolucci A, et al. Comparison of clinical outcomes and adverse events associated with glucose lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA 2016;316:313-24. 10.1001/jama.2016.9400 [DOI] [PubMed] [Google Scholar]
- 12. Zheng SL, Roddick AJ, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA 2018;319:1580-91. 10.1001/jama.2018.3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019;7:845-54. 10.1016/S2213-8587(19)30256-6 [DOI] [PubMed] [Google Scholar]
- 14. Hussein H, Zaccardi F, Khunti K, Seidu S, Davies MJ, Gray LJ. Cardiovascular efficacy and safety of sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists: a systematic review and network meta-analysis. Diabet Med 2019;36:444-52. 10.1111/dme.13898 [DOI] [PubMed] [Google Scholar]
- 15. Arnott C, Li Q, Kang A, et al. Sodium‐glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta‐analysis. J Am Heart Assoc 2020;9:e014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenstock J, Perkovic V, Johansen OE, et al. CARMELINA Investigators . Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA 2019;321:69-79. 10.1001/jama.2018.18269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siemieniuk RA, Agoritsas T, Macdonald H, Guyatt GH, Brandt L, Vandvik PO. Introduction to BMJ Rapid Recommendations. BMJ 2016;354:i5191. 10.1136/bmj.i5191 [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group. Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y, Wang W, Zhang AB, Bai X, Zhang S. Epley and Semont maneuvers for posterior canal benign paroxysmal positional vertigo: A network meta-analysis. Laryngoscope 2016;126:951-5. 10.1002/lary.25688 [DOI] [PubMed] [Google Scholar]
- 20. Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol 2013;42:332-45. 10.1093/ije/dys222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44. 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- 22. Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 2006;59:7-10. 10.1016/j.jclinepi.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 23. Hirji I, Andersson SW, Guo Z, Hammar N, Gomez-Caminero A. Incidence of genital infection among patients with type 2 diabetes in the UK General Practice Research Database. J Diabetes Complications 2012;26:501-5. 10.1016/j.jdiacomp.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 24. Dieleman JP, Kerklaan J, Huygen FJ, Bouma PA, Sturkenboom MC. Incidence rates and treatment of neuropathic pain conditions in the general population. Pain 2008;137:681-8. 10.1016/j.pain.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 25. Takeuchi M, Kawamura T, Sato I, Kawakami K. Population-based incidence of diabetic ketoacidosis in type 2 diabetes: medical claims data analysis in Japan. Pharmacoepidemiol Drug Saf 2018;27:123-6. 10.1002/pds.4271 [DOI] [PubMed] [Google Scholar]
- 26. Hippisley-Cox J, Coupland C. Development and validation of risk prediction equations to estimate future risk of blindness and lower limb amputation in patients with diabetes: cohort study. BMJ 2015;351:h5441. 10.1136/bmj.h5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Basu S, Sussman JB, Berkowitz SA, Hayward RA, Yudkin JS. Development and validation of Risk Equations for Complications Of type 2 Diabetes (RECODe) using individual participant data from randomised trials. Lancet Diabetes Endocrinol 2017;5:788-98. 10.1016/S2213-8587(17)30221-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elley CR, Robinson T, Moyes SA, et al. Derivation and validation of a renal risk score for people with type 2 diabetes. Diabetes Care 2013;36:3113-20. 10.2337/dc13-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brignardello-Petersen R, Bonner A, Alexander PE, et al. GRADE Working Group . Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol 2018;93:36-44. 10.1016/j.jclinepi.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 31. Hultcrantz M, Rind D, Akl EA, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol 2017;87:4-13. 10.1016/j.jclinepi.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaimai A, Caldwell DM, Li T, Higgins J, Salanti G. Chapter 11: Undertaking network meta-analyses. In: Higgins J, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions version 61. 2020. www.training.cochrane.org/handbook2020.
- 33. Gerstein HC, Ratner RE, Cannon CP, et al. APPROACH Study Group . Effect of rosiglitazone on progression of coronary atherosclerosis in patients with type 2 diabetes mellitus and coronary artery disease: the assessment on the prevention of progression by rosiglitazone on atherosclerosis in diabetes patients with cardiovascular history trial. Circulation 2010;121:1176-87. 10.1161/CIRCULATIONAHA.109.881003 [DOI] [PubMed] [Google Scholar]
- 34. Hedblad B, Zambanini A, Nilsson P, Janzon L, Berglund G. Rosiglitazone and carotid IMT progression rate in a mixed cohort of patients with type 2 diabetes and the insulin resistance syndrome: main results from the Rosiglitazone Atherosclerosis Study. J Intern Med 2007;261:293-305. 10.1111/j.1365-2796.2007.01767.x [DOI] [PubMed] [Google Scholar]
- 35. Cefalu WT, Leiter LA, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin’s effects on glycemia and cardiovascular risk factors in high-risk patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. Diabetes Care 2015;38:1218-27. 10.2337/dc14-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choi D, Kim SK, Choi SH, et al. Preventative effects of rosiglitazone on restenosis after coronary stent implantation in patients with type 2 diabetes. Diabetes Care 2004;27:2654-60. 10.2337/diacare.27.11.2654 [DOI] [PubMed] [Google Scholar]
- 37. Pfeffer MA, Claggett B, Diaz R, et al. ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247-57. 10.1056/NEJMoa1509225 [DOI] [PubMed] [Google Scholar]
- 38. Tanaka A, Shimabukuro M, Machii N, et al. EMBLEM Investigators . Effect of empagliflozin on endothelial function in patients with type 2 diabetes and cardiovascular disease: results from the multicenter, randomized, placebo- controlled, double-blind EMBLEM trial. Diabetes Care 2019;42:e159-61. 10.2337/dc19-1177 [DOI] [PubMed] [Google Scholar]
- 39. Verma S, Mazer CD, Yan AT, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation 2019;140:1693-702. 10.1161/CIRCULATIONAHA.119.042375 [DOI] [PubMed] [Google Scholar]
- 40. Kuramitsu S, Miyauchi K, Yokoi H, et al. Effect of sitagliptin on plaque changes in coronary artery following acute coronary syndrome in diabetic patients: the ESPECIAL-ACS study. J Cardiol 2017;69:369-76. 10.1016/j.jjcc.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 41. White WB, Cannon CP, Heller SR, et al. EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327-35. 10.1056/NEJMoa1305889 [DOI] [PubMed] [Google Scholar]
- 42. Finn AV, Oh JS, Hendricks M, et al. Predictive factors for in-stent late loss and coronary lesion progression in patients with type 2 diabetes mellitus randomized to rosiglitazone or placebo. Am Heart J 2009;157:383.e1-8. 10.1016/j.ahj.2008.11.013 [DOI] [PubMed] [Google Scholar]
- 43. Gantz I, Chen M, Suryawanshi S, et al. A randomized, placebo-controlled study of the cardiovascular safety of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2017;16:112. 10.1186/s12933-017-0593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hirano M, Nakamura T, Kitta Y, et al. Rapid improvement of carotid plaque echogenicity within 1 month of pioglitazone treatment in patients with acute coronary syndrome. Atherosclerosis 2009;203:483-8. 10.1016/j.atherosclerosis.2008.07.023 [DOI] [PubMed] [Google Scholar]
- 45. Hirano M, Nakamura T, Obata JE, et al. Early improvement in carotid plaque echogenicity by acarbose in patients with acute coronary syndromes. Circ J 2012;76:1452-60. 10.1253/circj.CJ-11-1524 [DOI] [PubMed] [Google Scholar]
- 46. Hong SJ, Choi SC, Cho JY, et al. Pioglitazone increases circulating microRNA-24 with decrease in coronary neointimal hyperplasia in type 2 diabetic patients- optical coherence tomography analysis. Circ J 2015;79:880-8. 10.1253/circj.CJ-14-0964 [DOI] [PubMed] [Google Scholar]
- 47. Kaku K, Daida H, Kashiwagi A, et al. Long-term effects of pioglitazone in Japanese patients with type 2 diabetes without a recent history of macrovascular morbidity. Curr Med Res Opin 2009;25:2925-32. 10.1185/03007990903328124 [DOI] [PubMed] [Google Scholar]
- 48. Laberge A, Brassard P, Arsenault B, et al. Positive effect of the PPAR-gamma agonist rosiglitazone on hemodynamic response to exercise in type 2 diabetic men after coronary artery bypass graft surgery: a 1-yr randomized study. Can J Cardiol 2016;32(Supplement 1):S223-4 10.1016/j.cjca.2016.07.356 . [DOI] [Google Scholar]
- 49. Lee HW, Lee HC, Kim BW, et al. Effects of low dose pioglitazone on restenosis and coronary atherosclerosis in diabetic patients undergoing drug eluting stent implantation. Yonsei Med J 2013;54:1313-20. 10.3349/ymj.2013.54.6.1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moriwaki K, Takeuchi T, Fujimoto N, et al. Effect of sitagliptin on coronary flow reserve assessed by magnetic resonance imaging in type 2 diabetic patients with coronary artery disease. Circ J 2018;82:2119-27. 10.1253/circj.CJ-18-0083 [DOI] [PubMed] [Google Scholar]
- 51. Nishio K, Sakurai M, Kusuyama T, et al. A randomized comparison of pioglitazone to inhibit restenosis after coronary stenting in patients with type 2 diabetes. Diabetes Care 2006;29:101-6. 10.2337/diacare.29.01.06.dc05-1170 [DOI] [PubMed] [Google Scholar]
- 52. Ogasawara D, Shite J, Shinke T, et al. Pioglitazone reduces the necrotic-core component in coronary plaque in association with enhanced plasma adiponectin in patients with type 2 diabetes mellitus. Circ J 2009;73:343-51. 10.1253/circj.CJ-08-0699 [DOI] [PubMed] [Google Scholar]
- 53. Osman A, Otero J, Brizolara A, et al. Effect of rosiglitazone on restenosis after coronary stenting in patients with type 2 diabetes. Am Heart J 2004;147:e23. 10.1016/j.ahj.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 54. Nissen SE, Nicholls SJ, Wolski K, et al. PERISCOPE Investigators . Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 2008;299:1561-73. 10.1001/jama.299.13.1561 [DOI] [PubMed] [Google Scholar]
- 55. Phrommintikul A, Wongcharoen W, Kumfu S, et al. Effects of dapagliflozin vs vildagliptin on cardiometabolic parameters in diabetic patients with coronary artery disease: a randomised study. Br J Clin Pharmacol 2019;85:1337-47. 10.1111/bcp.13903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Takagi T, Okura H, Kobayashi Y, et al. POPPS Investigators . A prospective, multicenter, randomized trial to assess efficacy of pioglitazone on in-stent neointimal suppression in type 2 diabetes: POPPS (Prevention of In-Stent Neointimal Proliferation by Pioglitazone Study). JACC Cardiovasc Interv 2009;2:524-31. 10.1016/j.jcin.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 57. Tanaka A, Komukai S, Shibata Y, et al. Pioglitazone Reduce Inflammation and Restenosis with and without Drug Eluting Stent (PRIDE) Study Investigators . Effect of pioglitazone on cardiometabolic profiles and safety in patients with type 2 diabetes undergoing percutaneous coronary artery intervention: a prospective, multicenter, randomized trial. Heart Vessels 2018;33:965-77. 10.1007/s00380-018-1143-3 [DOI] [PubMed] [Google Scholar]
- 58. Dormandy JA, Charbonnel B, Eckland DJ, et al. PROactive Investigators . Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279-89. 10.1016/S0140-6736(05)67528-9 [DOI] [PubMed] [Google Scholar]
- 59. Hong J, Zhang Y, Lai S, et al. SPREAD-DIMCAD Investigators . Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013;36:1304-11. 10.2337/dc12-0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suzuki K, Tanaka S, Aoki C, Kato K, Jojima T, Aso Y. Greater efficacy and improved endothelial dysfunction in untreated type 2 diabetes with liraglutide versus sitagliptin. Dokkyo J Med Sci 2014;41:211-20. [Google Scholar]
- 61. Kato Y, Iwata A, Zhang B, et al. Effects of dipeptidyl peptidase-4 inhibitor sitagliptin on coronary atherosclerosis as assessed by intravascular ultrasound in type 2 diabetes mellitus with coronary artery disease. IJC Metab Endocr 2017;16:1-9 10.1016/j.ijcme.2017.06.005 . [DOI] [Google Scholar]
- 62. Varghese A, Yee MS, Chan CF, et al. Effect of rosiglitazone on progression of atherosclerosis: insights using 3D carotid cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2009;11:24. 10.1186/1532-429X-11-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang G, Wei J, Guan Y, Jin N, Mao J, Wang X. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reduces clinical inflammatory responses in type 2 diabetes with coronary artery disease after coronary angioplasty. Metabolism 2005;54:590-7. 10.1016/j.metabol.2004.11.017 [DOI] [PubMed] [Google Scholar]
- 64. Wang Q, Wang D, Cheng A, Sun FY, Li Z. Comparison between the effects of sitagliptin and liraglutide on blood glucose and cognitive function of patients with both type 2 diabetes mellitus and post-stroke mild cognitive impairment. Int J Clin Exp Med 2020;13:1219-27. [Google Scholar]
- 65. Liu B, Wang J, Wang G. Beneficial effects of pioglitazone on retardation of persistent atrial fibrillation progression in diabetes mellitus patients. Int Heart J 2014;55:499-505. 10.1536/ihj.14-107 [DOI] [PubMed] [Google Scholar]
- 66. Oe H, Nakamura K, Kihara H, et al. FESC, for Effect of a DPP-4 inhibitor on left ventricular diastolic dysfunction in patients with type 2 diabetes and diabetic cardiomyopathy (3D) study investigators . Comparison of effects of sitagliptin and voglibose on left ventricular diastolic dysfunction in patients with type 2 diabetes: results of the 3D trial. Cardiovasc Diabetol 2015;14:83. 10.1186/s12933-015-0242-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Arturi F, Succurro E, Miceli S, et al. Liraglutide improves cardiac function in patients with type 2 diabetes and chronic heart failure. Endocrine 2017;57:464-73. 10.1007/s12020-016-1166-4 [DOI] [PubMed] [Google Scholar]
- 68. Tanaka A, Hisauchi I, Taguchi I, et al. CANDLE Trial Investigators . Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: a randomized trial (CANDLE). ESC Heart Fail 2020;7:1585-94. 10.1002/ehf2.12707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McMurray JJV, Solomon SD, Inzucchi SE, et al. DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995-2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 70. Dargie HJ, Hildebrandt PR, Riegger GA, et al. A randomized, placebo-controlled trial assessing the effects of rosiglitazone on echocardiographic function and cardiac status in type 2 diabetic patients with New York Heart Association functional class I or II heart failure. J Am Coll Cardiol 2007;49:1696-704. 10.1016/j.jacc.2006.10.077 [DOI] [PubMed] [Google Scholar]
- 71. Sharma A, Ambrosy AP, DeVore AD, et al. Liraglutide and weight loss among patients with advanced heart failure and a reduced ejection fraction: insights from the FIGHT trial. ESC Heart Fail 2018;5:1035-43. 10.1002/ehf2.12334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Giles TD, Miller AB, Elkayam U, Bhattacharya M, Perez A. Pioglitazone and heart failure: results from a controlled study in patients with type 2 diabetes mellitus and systolic dysfunction. J Card Fail 2008;14:445-52. 10.1016/j.cardfail.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 73. Singh JSS, Mordi IR, Vickneson K, et al. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the REFORM Trial. Diabetes Care 2020;43:1356-9. 10.2337/dc19-2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McMurray JJV, Ponikowski P, Bolli GB, et al. VIVIDD Trial Committees and Investigators . Effects of vildagliptin on ventricular function in patients with type 2 diabetes mellitus and heart failure: a randomized placebo-controlled trial. JACC Heart Fail 2018;6:8-17. 10.1016/j.jchf.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 75. Abe M, Okada K, Maruyama T, Maruyama N, Soma M, Matsumoto K. Clinical effectiveness and safety evaluation of long-term pioglitazone treatment for erythropoietin responsiveness and insulin resistance in type 2 diabetic patients on hemodialysis. Expert Opin Pharmacother 2010;11:1611-20. 10.1517/14656566.2010.495119 [DOI] [PubMed] [Google Scholar]
- 76. Abe M, Higuchi T, Moriuchi M, et al. Efficacy and safety of saxagliptin, a dipeptidyl peptidase-4 inhibitor, in hemodialysis patients with diabetic nephropathy: a randomized open-label prospective trial. Diabetes Res Clin Pract 2016;116:244-52. 10.1016/j.diabres.2016.04.034 [DOI] [PubMed] [Google Scholar]
- 77. Allegretti AS, Zhang W, Zhou W, et al. Safety and effectiveness of bexagliflozin in patients with type 2 diabetes mellitus and stage 3a/3b CKD. Am J Kidney Dis 2019;74:328-37. 10.1053/j.ajkd.2019.03.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Morikawa A, Ishizeki K, Iwashima Y, et al. Pioglitazone reduces urinary albumin excretion in renin-angiotensin system inhibitor-treated type 2 diabetic patients with hypertension and microalbuminuria: the APRIME study. Clin Exp Nephrol 2011;15:848-53. 10.1007/s10157-011-0512-3 [DOI] [PubMed] [Google Scholar]
- 79. Arjona Ferreira JC, Corry D, Mogensen CE, et al. Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial. Am J Kidney Dis 2013;61:579-87. 10.1053/j.ajkd.2012.11.043 [DOI] [PubMed] [Google Scholar]
- 80. Arjona Ferreira JC, Marre M, Barzilai N, et al. Efficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiency. Diabetes Care 2013;36:1067-73. 10.2337/dc12-1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 2018;6:605-17. 10.1016/S2213-8587(18)30104-9 [DOI] [PubMed] [Google Scholar]
- 82. Bakris GL, Ruilope LM, McMorn SO, et al. Rosiglitazone reduces microalbuminuria and blood pressure independently of glycemia in type 2 diabetes patients with microalbuminuria. J Hypertens 2006;24:2047-55. 10.1097/01.hjh.0000244955.39491.88 [DOI] [PubMed] [Google Scholar]
- 83. Chacra A, Gantz I, Mendizabal G, et al. A randomised, double-blind, trial of the safety and efficacy of omarigliptin (a once-weekly DPP-4 inhibitor) in subjects with type 2 diabetes and renal impairment. Int J Clin Pract 2017;71:e12955. 10.1111/ijcp.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pollock C, Stefánsson B, Reyner D, et al. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2019;7:429-41. 10.1016/S2213-8587(19)30086-5 [DOI] [PubMed] [Google Scholar]
- 85. Fioretto P, Del Prato S, Buse JB, et al. DERIVE Study Investigators . Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): the DERIVE Study. Diabetes Obes Metab 2018;20:2532-40. 10.1111/dom.13413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yale JF, Bakris G, Cariou B, et al. DIA3004 Study Group . Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 2014;16:1016-27. 10.1111/dom.12348 [DOI] [PubMed] [Google Scholar]
- 87. Barnett AH, Mithal A, Manassie J, et al. EMPA-REG RENAL trial investigators . Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2014;2:369-84. 10.1016/S2213-8587(13)70208-0 [DOI] [PubMed] [Google Scholar]
- 88. Hiramatsu T, Asano Y, Mabuchi M, Imai K, Iguchi D, Furuta S. Liraglutide relieves cardiac dilated function than DPP-4 inhibitors. Eur J Clin Invest 2018;48:e13007. 10.1111/eci.13007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hu ZY, Zhao JW. Effect of rosiglitazone on serum CRP and plasma PAI-1 in patients with early type 2 diabetic nephropathy. Zhongyuan Yikan 2007;34:27-8. [Google Scholar]
- 90. Ito M, Abe M, Okada K, et al. The dipeptidyl peptidase-4 (DPP-4) inhibitor vildagliptin improves glycemic control in type 2 diabetic patients undergoing hemodialysis. Endocr J 2011;58:979-87. 10.1507/endocrj.EJ11-0025 [DOI] [PubMed] [Google Scholar]
- 91. Jerums G, Murray RM, Seeman E, et al. Lack of effect of gliclazide on early diabetic nephropathy and retinopathy: a two-year controlled study. Diabetes Res Clin Pract 1987;3:71-80. 10.1016/S0168-8227(87)80010-4 [DOI] [PubMed] [Google Scholar]
- 92. Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014;85:962-71. 10.1038/ki.2013.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kashiwagi A, Takahashi H, Ishikawa H, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab 2015;17:152-60. 10.1111/dom.12403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Davies MJ, Bain SC, Atkin SL, et al. Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care 2016;39:222-30. [DOI] [PubMed] [Google Scholar]
- 95. Lukashevich V, Schweizer A, Shao Q, Groop PH, Kothny W. Safety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24-week randomized placebo-controlled trial. Diabetes Obes Metab 2011;13:947-54. 10.1111/j.1463-1326.2011.01467.x [DOI] [PubMed] [Google Scholar]
- 96. Groop PH, Cooper ME, Perkovic V, et al. Linagliptin and its effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: the randomized MARLINA-T2D trial. Diabetes Obes Metab 2017;19:1610-9. 10.1111/dom.13041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Maruyama T, Takashima H, Tei R, Furukawa T, Maruyama N, Abe M. MON-300 Efficacy and safety of canagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in diabetic kidney disease: a randomized open-label prospective trial. Kidney Int Rep 2019;4(Supplement):S422-3 10.1016/j.ekir.2019.05.1109 . [DOI] [Google Scholar]
- 98. McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care 2013;36:237-44. 10.2337/dc12-0706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Milovanova L, Taranova M, Milovanova S, et al. DPP-4 inhibitors (linagliptin) impact on klotho serum level in patients with type 2 diabetes. Diabetes Technol Ther 2019;21(Supplement 1):A147. [Google Scholar]
- 100. Nakamura T, Ushiyama C, Osada S, Hara M, Shimada N, Koide H. Pioglitazone reduces urinary podocyte excretion in type 2 diabetes patients with microalbuminuria. Metabolism 2001;50:1193-6. 10.1053/meta.2001.26703 [DOI] [PubMed] [Google Scholar]
- 101. Nakamura T, Matsuda T, Kawagoe Y, et al. Effect of pioglitazone on carotid intima-media thickness and arterial stiffness in type 2 diabetic nephropathy patients. Metabolism 2004;53:1382-6. 10.1016/j.metabol.2004.05.013 [DOI] [PubMed] [Google Scholar]
- 102. Nakamura T, Sugaya T, Kawagoe Y, Ueda Y, Koide H. Effect of pioglitazone on urinary liver-type fatty acid-binding protein concentrations in diabetes patients with microalbuminuria. Diabetes Metab Res Rev 2006;22:385-9. 10.1002/dmrr.633 [DOI] [PubMed] [Google Scholar]
- 103. Neff KJ, Tobin LM, Hogan AE, Docherty NG, Le Roux CW, O’Shea D. The effect of low dose liraglutide on renal inflammation in type 2 diabetic kidney disease: a randomised controlled study. Diabet Med 2016;33(Suppl 1):64. [Google Scholar]
- 104. Nowicki M, Rychlik I, Haller H, et al. Long-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety study. Int J Clin Pract 2011;65:1230-9. 10.1111/j.1742-1241.2011.02812.x [DOI] [PubMed] [Google Scholar]
- 105. Pistrosch F, Passauer J, Herbrig K, Schwanebeck U, Gross P, Bornstein SR. Effect of thiazolidinedione treatment on proteinuria and renal hemodynamic in type 2 diabetic patients with overt nephropathy. Horm Metab Res 2012;44:914-8. 10.1055/s-0032-1314836 [DOI] [PubMed] [Google Scholar]
- 106. Raji A, Scott R, Morgan J, et al. Safety and efficacy of sitagliptin compared with dapagliflozin in patients with type 2 diabetes, mild renal impairment, and inadequate glycaemic control on metformin +/− a sulfonylurea. Diabetologia 2018;61(Supplement 1):S379-80. [Google Scholar]
- 107. Halden TAS, Kvitne KE, Midtvedt K, et al. Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care 2019;42:1067-74. 10.2337/dc19-0093 [DOI] [PubMed] [Google Scholar]
- 108. Takashima H, Yoshida Y, Nagura C, et al. Renoprotective effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, in type 2 diabetes patients with chronic kidney disease: a randomized open-label prospective trial. Diab Vasc Dis Res 2018;15:469-72. 10.1177/1479164118782872 [DOI] [PubMed] [Google Scholar]
- 109. Grunberger G, Camp S, Johnson J, et al. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the VERTIS RENAL randomized study. Diabetes Ther 2018;9:49-66. 10.1007/s13300-017-0337-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 2013;15:463-73. 10.1111/dom.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Neal B, Perkovic V, Mahaffey KW, et al. CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644-57. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 112. Rosenstock J, Kahn SE, Johansen OE, et al. CAROLINA Investigators . Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA 2019;322:1155-66. 10.1001/jama.2019.13772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ikonomidis I, Pavlidis G, Thymis J, et al. Effects of glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with type 2 diabetes mellitus after 12-month treatment. J Am Heart Assoc 2020;9:e015716. 10.1161/JAHA.119.015716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Li J, Feng Z, Li Q, He Y, Zhao C, He J. Insulin glargine effectively achieves glycemic control and improves insulin resistance in patients with early type 2 diabetes that exhibit a high risk for cardiovascular disease. Exp Ther Med 2014;8:147-52. 10.3892/etm.2014.1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. McGuire DK, Abdullah SM, See R, et al. Randomized comparison of the effects of rosiglitazone vs. placebo on peak integrated cardiovascular performance, cardiac structure, and function. Eur Heart J 2010;31:2262-70. 10.1093/eurheartj/ehq228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Scirica BM, Bhatt DL, Braunwald E, et al. SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317-26. 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- 117. Yee MS, Pavitt DV, Dhanjil S, Godsland IF, Richmond W, Johnston DG. The effects of rosiglitazone on atherosclerotic progression in patients with type 2 diabetes at high cardiovascular risk. Diabet Med 2010;27:1392-400. 10.1111/j.1464-5491.2010.03089.x [DOI] [PubMed] [Google Scholar]
- 118. Birkeland KI, Jørgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol 2017;5:709-17. 10.1016/S2213-8587(17)30258-9 [DOI] [PubMed] [Google Scholar]
- 119. Kosiborod M, Cavender MA, Fu AZ, et al. CVD-REAL Investigators and Study Group* . Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs. Circulation 2017;136:249-59. 10.1161/CIRCULATIONAHA.117.029190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Poonawalla I, Bowe A, Tindal M, Meah Y, Schwab P. 36-OR: Comparative effectiveness of SGLT2 inhibitors vs. GLP-1 agonists. Diabetes 2020;69(Supplement 1) 10.2337/db20-36-OR. [DOI] [Google Scholar]
- 121. Heerspink HJL, Stefansson BV, Chertow GM, et al. DAPA-CKD Investigators . Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 2020;35:274-82. 10.1093/ndt/gfz290 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material
Data Availability Statement
No additional data available.