Abstract
The blood–brain barrier (BBB) is an important physiological barrier that separates the central nervous system (CNS) from the peripheral circulation, which contains inflammatory mediators and immune cells. The BBB regulates cellular and molecular exchange between the blood vessels and brain parenchyma. Normal functioning of the BBB is crucial for the homeostasis and proper function of the brain. It has been demonstrated that peripheral inflammation can disrupt the BBB by various pathways, resulting in different CNS diseases. Recently, clinical research also showed CNS complications following SARS‐CoV‐2 infection and chimeric antigen receptor (CAR)‐T cell therapy, which both lead to a cytokine storm in the circulation. Therefore, elucidation of the mechanisms underlying the BBB disruption induced by peripheral inflammation will provide an important basis for protecting the CNS in the context of exacerbated peripheral inflammatory diseases. In the present review, we first summarize the physiological properties of the BBB that makes the CNS an immune‐privileged organ. We then discuss the relevance of peripheral inflammation‐induced BBB disruption to various CNS diseases. Finally, we elaborate various factors and mechanisms of peripheral inflammation that disrupt the BBB.
Keywords: blood–brain barrier, central nervous system, inflammation, inflammatory factors, molecular mechanism
1. INTRODUCTION
The circulatory system contains blood vessels that distribute blood with nutrients and oxygen and remove waste products and CO2 from the tissue. Its normal function is essential for maintaining homeostasis of the organism. The inner layer of blood vessels is made of vascular endothelial cells (ECs). 1 The endothelium is distinct in structure and function, and can be continuous non‐fenestrated, continuous fenestrated, or discontinuous dependent on the organ requirements. 2 The brain and spinal cord comprise central nervous system (CNS) that controls critical functions of the body. CNS vasculature has a unique anatomy and physiology making the CNS a so‐called “immune‐privileged” organ, although this idea was challenged in the past several decades. 3 Located between the CNS tissue and peripheral blood circulation, the blood–brain barrier (BBB) regulates cellular and molecular exchange between the blood vessels and brain parenchyma. ECs, pericytes, and astrocytes are the major components of the BBB, and basement membrane between them is also required for the BBB function and integrity. 1
An important function of the BBB is to maintain the homeostasis of the central nervous system (CNS). BBB dysfunction is implicated in various neurological diseases, such as Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and stroke. 4 It was recently reported that in patients with autoimmune diseases such as rheumatoid arthritis, treatment with tumor necrosis factor (TNF) inhibitor increased the risk of CNS inflammation and subsequent BBB breakdown. 5 In addition, patient infected with SARS‐CoV‐2 or those using chimeric antigen receptor (CAR)‐T cell therapy for lymphocytoma could also develop CNS complications, probably due to BBB disruption induced by peripheral inflammation, although definitive conclusion is not drawn yet. Peripheral inflammation refers to the activation of the innate or adaptive immune system and release of proinflammatory cytokines against various pathological stimuli outside of the CNS. It is normally a kind of protective response of the body against multiple insults. Since the BBB is highly susceptible to the inflammatory stimuli, inappropriate peripheral inflammation such as lipopolysaccharide (LPS) can impact the BBB function via different pathways. 6 , 7 , 8
In this review, we briefly describe current understandings on BBB structure and functions. Particularly, we elaborate the most recent advances in mechanisms of BBB disruption secondary to peripheral inflammatory conditions, which have been largely overlooked in the research of CNS diseases.
2. STRUCTURE AND CONSTITUENTS OF THE BBB
The neurovascular unit (NVU) usually consists of endothelial cells, mural cells (i.e., vascular smooth muscle cells and pericytes), basement membrane, glia cells (astrocytes and microglia cells), and neurons, which collectively contribute to BBB integrity. 9 ECs form the inner lining of all blood vessels. BBB ECs are quite different in structure and function from those in other tissues, and the typical characteristics that distinguish them from other ECs include the following: (a) paracellular transport of solutes is blocked due to tight junctions, (b) fenestrations are absent and transcytosis are reduced, limiting transcellular transport of solutes, (c) for transfer of required solutes from the blood to parenchymal cells of the brain, specific transporters, such as GLUT1 (glucose transporter 1), are expressed, (d) to remove toxic substances from CNS parenchymal cells, specific pumps, such as P‐glycoprotein (P‐gp), are expressed, (e) the low expression level of leukocyte adhesion molecules (LAMs) in BBB endothelial cells helps restrict entry of immune cells into the CNS, and (f) ECs of the BBB harbor more mitochondria than of other tissues, which might be related to providing the energy that ionic transport requires (Figure 1). 4 , 10 , 11 , 12 , 13 These features of the CNS ECs contribute to highly selected movement of solutes in and out of the CNS parenchyma, maintaining a stable microenvironment for proper neuronal function. 14
FIGURE 1.
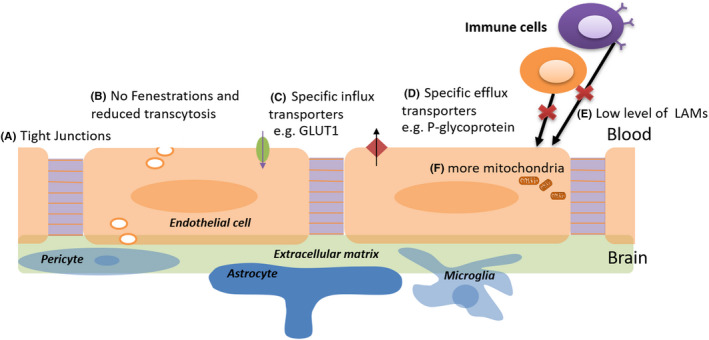
Schematic diagram of the physiological characteristics of the BBB. GLUT1, glucose transporter 1; LAMs, leukocyte adhesion molecules
Pericytes cover the CNS capillaries and regulate vascular stability, diameter, cerebral blood flow, and extracellular membrane protein secretion. 15 Astrocytes span around the vascular endothelium and pericytes via end‐feet, and they are in contact with neurons and regulate BBB permeability. 16 , 17 Microglial cells are a component of the NVU, but the link between microglial cells and BBB ECs and its effect on formation and regulation of BBB properties remains to be fully explored. 18 Furthermore, the BBB is composed of non‐cellular elements, the extracellular membrane (ECM). All of these cellular and non‐cellular components together maintain the BBB structural and functional integrity (Figure 1).
3. BBB FUNCTION AND ITS REGULATION
The complex cellular and non‐cellular components of the BBB collaboratively maintain the BBB function, and impairment in any one of these elements can lead to BBB disruption. 3 For example, the endothelial tight junctions (TJs) and lack of fenestrae contribute to a physical barrier, leaving carriers and/or receptors the only means for protein transportation into the CNS. 19 , 20 ECs play an important role in vascular biology, such as maintaining permeability, homeostasis, and vessel wall integrity and preventing thrombosis. 3 Pericytes help preserve the TJs of ECs (e.g., Claudin‐5, Occludin, and ZO‐1) and regulate transcytosis in ECs, maintaining the integrity and normal permeability of the BBB. 19 , 21 , 22 Astrocytes secrete factors that are key to maintain BBB properties, including sonic hedgehog (Shh), vascular endothelial growth factor (VEGF), angiopoietins‐1 (Ang‐1), angiotensin‐converting enzyme‐1 (ACE‐1), glial‐derived neurotrophic factor (GDNF), and apolipoprotein E (ApoE). 7 ECM is a dynamic component of the BBB, and regulates BBB structure and function by impacting cell–cell and cell–matrix interaction within the NVU. 23 Microglia cells are known as the immune cells in the CNS. They can be activated and categorized into two opposite types: M1 and M2, which produce either cytotoxic or neuroprotective effects. 18 Under inflammatory conditions, they can be activated to M1 or M2 phenotype, thus damaging or protecting the BBB integrity. 24 , 25 , 26 These cellular and non‐cellular components of NVU are affected directly or indirectly by peripheral inflammation, resulting in disruption of the BBB and CNS alterations.
4. PERIPHERAL INFLAMMATION AND CNS DISEASES
4.1. Role of BBB disruption in the effects of peripheral inflammation on CNS diseases
Both preclinical and clinical studies have found that peripheral inflammation in the form of infection is a common contributing factor for the development and deterioration of CNS diseases, such as neurodegenerative diseases AD, PD, MS, and stroke. A possible explanation is that BBB disruption in infections increases the susceptibility to CNS diseases. 27 In AD patients, peripheral inflammation increases the level of amyloid beta (β‐amyloid) in the brain. 28 In amyloid precursor protein (APP) transgenic mice, peripheral injection of LPS increased BBB permeability, allowing for infiltration of peripheral proinflammatory factors such as IL‐6 and TNF‐α, and promoting neurological inflammation and disease progression. 29 , 30 LPS‐induced BBB disruption also plays an important role in the transmission of Tau, probably in a non‐microglia‐dependent pathway. 31 Aside from AD, evidence also showed peripheral inflammation as a potential risk factor for PD and other neurodegenerative disease. 32 , 33 Likewise, dysregulated systemic inflammation is present in PD, as evidenced by high levels of IL‐1β, IL‐2, TNF‐α, CD4+ and CD8+ T lymphocytes in both serum and cerebrospinal fluid. 34 In the pathogenesis of MS, one of the most important mechanisms is the infiltration of autoreactive CD4+ T cells and other white cells into the CNS, whereas the degree of BBB destruction in experimental autoimmune encephalomyelitis (EAE) model is strongly correlated with disease severity. 35
Ischemic and hemorrhagic stroke also presents with BBB disruption, and experimental models and clinical observations together have shown that peripheral inflammation (e.g., LPS, anaphylaxis, and infection) is more likely to aggravate BBB disruption and even worsen the outcome of stroke. 36 , 37 , 38 , 39 For example, the adaptive immune system is activated following cerebral ischemia, and the peripheral immune cells, such as T cells and B cells, rapidly infiltrate the diseased brain and release various cytokines, including pro‐inflammatory cytokines (TNF‐α, IL‐1β, IL‐6) leading to blood vessels and BBB damage, and anti‐inflammatory cytokines (IL‐13, IL‐10, IL‐4, TGF‐β) extenuating the ischemic injury. 40 , 41 , 42 Moreover, both immune cells and cytokines induce immunodepression after stroke, which leads to an increased incidence of infections such as pneumonia. 43 , 44 , 45 It is not exactly clear whether BBB dysfunction induced by inflammation is the cause or complication of CNS disease, and further study to understand the role of peripheral inflammation on BBB function and the influence on CNS disease can provide a basis for clinical treatment of the disease to a certain extent.
4.2. BBB disruption in COVID‐19‐related CNS symptoms
In December of 2019, a case of pneumonia caused by a novel coronavirus, SARS‐CoV‐2, emerged in Wuhan, China, and rapidly spread around the world. This new disease is termed coronavirus disease 2019 (COVID‐19) by the World Health Organization (WHO). The most common symptoms of COVID‐19 are fever, cough, and tiredness. 46 As for its CNS symptoms, according to a retrospective, observational case series of 214 patients, 24.8% of them had CNS manifestations, including ataxia, impaired consciousness, dizziness, and headache. 47 The most severe cases were 4 with ischemic stroke and 1 with cerebral hemorrhage who died of respiratory failure. 47 Inflammatory storm is considered one of the causes of death in severe and critical COVID‐19 cases, with over half of which have lymphopenia and a cytokine storm. 48 Consistently, an increased release of cytokines (IL‐1β, IL1RA, IL‐6, TNF‐α) and chemokines (CCL2, CCL3, CCL5) occurred after infection. 49 , 50 , 51 Conceivably, anakinra (IL‐1 blockade) and tocilizumab (IL‐6 receptor blockade) are showing significant survival benefits in COVID‐19 patients with hyperinflammation. 52
Although the incidence of CNS complications is high in SARS‐CoV‐2 infections, the pathogenesis is barely known for now. Some researchers believe the cytokine storm during infection persistently affects the CNS. 53 It is highly likely that BBB disruption might play an important role in the CNS complications associated with COVID‐19. 54 However, more solid and direct evidence is needed to prove it.
4.3. BBB disruption in CAR‐T therapy‐associated neurotoxicity
Chimeric antigen receptor (CAR)‐T cell therapy is a rapidly developing novel strategy for acute lymphoblastic leukemia (ALL) or chronic lymphocytic leukemia (CLL). 55 , 56 , 57 , 58 Currently approved CAR‐T therapies targeting CD19 showed profound therapeutic effects in ALL. 59
However, the toxic effects of CAR‐T cells are worrying. The most important and common toxic effects are cytokine release syndrome (CRS) and the associated neurotoxicity, with the most severe of which being lethal cerebral edema. 59 , 60 , 61 , 62 , 63 , 64 CD3+ T cells, CD19+ B cells, and high levels of cytokines (IFN‐γ, IL‐6) were detectable in the cerebrospinal fluid (CSF) in ALL patients complicated with cerebral edema as soon as a few hours after CD19 CAR‐T cell infusion. This was accompanied by cerebral CRS, probably due to the cytokines produced by BBB‐penetrating CAR‐T cells. 65 Moreover, cytokines such as IL‐6, IFN‐γ, and TNF‐α were known to directly activate endothelial cells. Patients with severe neurotoxicity showed evidence of endothelial activation, characterized by increased BBB permeability, serving as another important mechanism for neurotoxicity in CAR‐T cell treatment. 66 , 67 More recently, researchers reported high CD19 expression in human brain mural cells, but not in mouse mural cells, and that is a possible on‐target mechanism for CD19 CAR‐T cell‐mediated neurotoxicity, meanwhile suggesting limitations in preclinical animal models of neurotoxicity. 68
Thus far, the mechanisms through which CAR‐T cells cause BBB dysfunction and neurotoxicity remain enigmatic. Nevertheless, it is believed to be closely related to the peripheral inflammatory responses. More in‐depth studies are needed to increase the safety of CAR‐T cell therapy in clinical applications.
5. MECHANISMS OF PERIPHERAL INFLAMMATION‐INDUCED BBB DISRUPTION
Peripheral inflammation is basically a protective response for the organism. However, excessive and dysregulated inflammation leads to adverse effects. For example, various non‐neurological systemic infections often come with CNS dysfunction, such as pneumonia and urinary systemic infection, which may be a result of chronic CNS disease. 69 , 70 The BBB protects the CNS from potential peripheral insults; therefore, damaging the BBB is considerably harmful to the CNS. Discussed below are mechanisms on how peripheral inflammation impacts the BBB (Figure 2).
FIGURE 2.
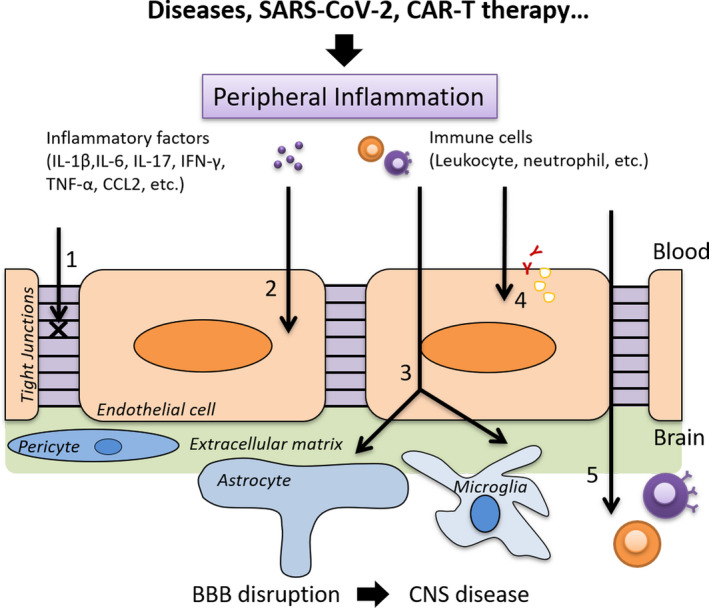
Mechanisms of BBB disruption induced by peripheral inflammation. 1 Changes in tight junctions; 2 damage to endothelial cells; 3 activation of astrocytes and microglia; 4 alteration of multiple transport pathways and receptors; 5 penetration of peripheral immune cells
5.1. Changes in tight junctions (TJs)
TJs are vital components that maintain BBB integrity and normal functioning, such that TJ changes directly lead to BBB disruption. Lots of bacterial and viral infections cause degradation or disorganization of TJs indirectly through diverse pathways. 71 , 72 , 73 For example, cytokines including IL‐1β, IL‐6, IL‐9, IL‐17, IFN‐γ, TNF‐α, and CCL2, can lead to reduced TJ expression or false TJ allocation. 74 , 75 , 76 , 77 , 78 , 79 Claudin‐5 among others is the most important TJ protein responsible for selective permeability of the BBB, and inflammation leads to its downregulation and BBB disruption. 80 In old mice, LPS‐mediated peripheral inflammation resulted in the degeneration of TJ proteins, including claudin‐5. 81 On the other hand, IL‐1β led to a discontinuous distribution of claudin‐5 along the plasma membrane of brain endothelial cells. 75 Apart from claudin‐5, LPS‐induced systemic inflammation was also associated with degradation of occludin. 82 Another recent study showed that peripheral cytokines reduced expression of ZO‐1 in mice with pre‐existing tumors. 83
Nowadays, changes in TJs are usually used as indicators of BBB dysfunction. However, there are indirect causes for changes in TJs, such as MMPs, nitric oxide (NO), reactive oxygen species (ROS), Rho‐kinase (ROCK), and NF‐κB signaling pathways. 84 , 85 , 86 , 87 , 88 The specific mechanisms will be discussed below.
5.2. Damage to endothelial cells
As the primary component of BBB, EC is another important target of peripheral inflammation. Research has shown that LPS has a direct toxic effect on the BBB endothelium by inhibiting P‐gp activity and inducing secretion of MMPs, resulting in membrane abnormalities, endoplasmic reticulum (ER) stress, and mitochondrial damage, and eventually, cell apoptosis. 89 , 90 MAPK signaling also contributes to LPS‐induced EC apoptosis. 91 EC is breakdown, and BBB impairment further facilitates the introduction of neurotoxic substances into the CNS, increasing the risk of other diseases. 89 , 92 Another consequence of peripheral inflammation is the upregulated expression of adhesive molecules on ECs, such as Vascular Cell Adhesion Molecule 1 (VCAM‐1), Intercellular Adhesion Molecule 1 (ICAM‐1), and E‐selectin. This allows for trafficking of peripheral immune cells into the CNS, seen in aging and chronic inflammation. 71 , 93 , 94 , 95 In addition, IL‐1β is found to induced upregulation of α5 integrin‐dependent adhesion of EC, which then disrupts the integrity of BBB through altering cell–cell junctions and cell–matrix adhesion. 75
5.3. Activation of astrocytes and microglia
Astrocytes play a vital role in maintaining BBB integrity and regulating its function. Depending on the immune trigger or the phase of inflammation, they produce either pro‐ or antiinflammatory mediators that affect BBB permeability and infiltration of peripheral immune cells. 96 Under an inflammatory condition, astrocytes secrete VEGF‐A, which activates the eNOS signaling in ECs and downregulates the expression of occludin and claudin‐5, resulting in easy entry of peripheral lymphocytes into the CNS. 97 , 98 It has been reported that during inflammation astrocytes altered claudin‐5 expression likely by upregulating the immune‐related GTPase family M‐1 protein (IRGM‐1) in the EAE mouse model. 99 In middle cerebral artery occlusion (MCAO) model, researchers found high IL‐9 expression in peripheral blood and IL‐9 receptors on astrocytes, and further study revealed that IL‐9 enhances the permeability of the BBB by promoting the secretion of VEGF‐A from astrocytes. 76 Peripheral inflammation induced by LPS can cause proliferation and activation of astrocytes, changes in the end‐feet structure and altered expression of other related gene, which collectively and indirectly lead to destruction of the BBB. 100 , 101
Microglia cells are part of the NVU, but their interaction with BBB ECs and effects on BBB properties are not well known as mentioned above. Even so, there is evidence that shows inflammation‐activated microglia contribute to BBB disruption. 102 There are two pathways for microglia activation: the M1 proinflammatory pathway and M2 antiinflammatory pathway. 103 M1 microglia contribute to BBB dysfunction and vascular “leak” mainly through production of proinflammatory mediators, promotion of immune cell trafficking, and oxidative stress. 18 , 104 The proinflammatory signaling in M1 microglia involves toll‐like receptor (TLR)‐4, 25 , 105 the IFN‐γ receptor complex, 106 the granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) receptor, 107 and COX2. 108 , 109 , 110 The secretion of TNF‐α, IL‐1β, IL‐6, IL‐12, CCL2, and CXCL10 is shown to change TJs (claudin‐5, occludin, ZO‐1, and ZO‐2) and critical BBB transporters like P‐gp proteins. 18 , 26 , 102 , 111 , 112 , 113 , 114 Besides, the chemokines CCL2 and CXCL10 promote trafficking of immune cells cross the BBB, including monocytes and macrophages, which is observed in stroke. 115 , 116 In addition to cytokines and chemokines, there is ROS production and oxidative stress in M1 microglia, and it is related to increased expression of iNOS during peripheral inflammation induced by LPS, and also stroke. 117 , 118 Different from M1 microglia, M2 microglia play protective roles in BBB disruption, including immune regulation, inflammation dampening, and repair/injury resolution. 119 , 120 The polarization to M2 microglia is mediated by IL‐4 receptor, FCγ receptor, IL‐10 receptor, and VEGFR2 signaling. 119 , 121 M2 microglia mainly produce antiinflammatory mediators such as TGF‐β1, IL‐10, and IL‐4 122 , 123 Receptors for TGF‐β1 are expressed at BBB and TGF‐β1 may have significant positive effects on BBB integrity via activin receptor‐like kinase (ALK)‐1 and −5 signaling. 124 IL‐10 secretion and IL‐10 receptor promote inflammation suppression and migration of regulatory T cells that alleviate brain injury. 119 , 125 In fact, microglia are highly dynamic and their transition from M1 to M2 is complicated and not known clearly. A latest research gave more profound evidence that microglia play a dual role in maintaining BBB integrity in distinct time course of peripheral inflammation. 24 Initially, brain microglia migrate to cerebral vessels in response to CCL5 and express claudin‐5, thus maintain BBB integrity, whereafter they transform into another phenotype that contributes to BBB leakage. 24
5.4. Effects of peripheral immune cells
Under normal physiological conditions, the BBB restricts the entry of peripheral immune cells into the CNS through low expression of LAMs. However, this is interrupted in pathological conditions. Indeed, peripheral immune cells can have dual roles in BBB integrity depending on different microenvironment and their subtypes, but more evidence show that the infiltration of peripheral immune cells contributes to the disruption of BBB in several neurodegenerative disorders, and even that BBB damage may occur before effector immune cells infiltrate at local sites due to peripheral inflammation‐related or irrelated reasons. 126 , 127 , 128 , 129 For example, the inflammatory factors secreted by immune cells, such ROS, and MMPs (MMP‐1 and MMP‐2), promote their own migration into the CNS and increase BBB permeability simultaneously, forming a vicious cycle. 74 , 130 , 131
Lymphocytes including T cells (CD4+ T helper cells, γδT cells, CD8+ cytotoxic T cells), B cells, and NK cells are detrimental to BBB integrity. For T lymphocytes, the interaction of myelin‐reactive CD4+ T cells and cerebrovascular ECs plays an important role in regulating BBB integrity. Decisive events in MS and EAE include activation of myelin‐reactive CD4+ T cells which then differentiate into effector (Th1 and Th17) and regulatory (Treg) at peripheral tissues, and subsequently transmigrate across the BBB. 132 , 133 Th1 and Th17 cells play proinflammatory roles through distinct pathways, while Th2 cells perform antiinflammatory function in stroke (reviewed in Ref. [38]). Th1 cells mainly release proinflammatory cytokines (IL‐2, IFN‐γ, and TNF‐α), promote the transformation of microglia to M1, and mediate cellular immune response. 134 , 135 Th17 cells secret IL‐17, IL‐21, and IL‐22 and promote the recruitment of CD4+ T cells. 136 , 137 IL‐17A (a member of IL‐17 cytokines) activation contributes to BBB disruption by inducing oxidative stress, which then activates the endothelium and downregulates TJ protein occludin. 137 In addition, peripheral CD8+ T cells activation and brain infiltration are detrimental to neural tissue after stroke, in which IL‐2 plays a role. 138 CD4+ and CD8+ T cells are found in the brain up to one month post ischemic stroke, and their prolonged activation may affect the outcome of stroke. 139 But Tregs, as an important subtype of Th2 cells, may be neuroprotective and protect BBB integrity by maintaining immune tolerance, suppressing the activation of other immune cells, or regulating cerebral endothelial function. 39 Peripheral B cells are a key player in MS on both sides of BBB. They upregulate the activated leukocyte cell adhesion molecule (ALCAM) expression in MS patients and promote the CNS recruitment of monocytes and CD4+ T cells, as ALCAM plays a role in BBB integrity for its cell surface localization and association with junctional proteins. 140 , 141 , 142 NK cells are a type of innate immune cells and cytotoxic lymphocytes. It is reported that in cerebral ischemia NK cells produce cytokines such as IFN‐γ, IP‐10, and cause BBB disruption. 71 , 143
Other type of immune cells—myeloid cells such as neutrophils, monocytes, dendritic cells (DCs), and mast cells, also influence BBB function via distinct mechanisms. Neutrophils produce a variety of proinflammatory cytokines that affect BBB function, including IL‐1β, TNF‐α, IL‐6, IL‐12, and IFN‐γ, whereas TNF‐α can further induce the recruitment of neutrophils to the CNS. 144 , 145 , 146 Additionally, when neutrophils transmigrate into the CNS, they secret IL‐1 and activate the antigen‐presenting cells (APC) locally, which subsequently activate endothelial IL‐1R1 signaling that induces T cell recruitment and exacerbates CNS inflammation. 147 Monocytes may migrate across the BBB depending on the upregulation of cytokines (IL‐1) and junction molecules (ALCAM, JAM‐A, PECAM‐1, and CD99). 148 , 149 As a result, HIV‐infected monocytes with upregulation of ALCAM, JAM‐A, and CCR2 on their surface are more likely to cross the endothelium monolayer than noninfected monocytes in response to CCL2, a chemokine, which is elevated in the CNS and CSF of HIV‐infected people. 150 , 151 The bone marrow‐derived monocytes (BMDMs) can affect the BBB integrity and control immune infiltration by releasing related cytokines during stroke, whereby exacerbating BBB injury. 127 Macrophages and DCs are found in the perivascular space between the endothelial and parenchymal basement membranes under inflammation, then help to activate lymphocytes that subsequently breach the BBB. 152 , 153 , 154 The activated mast cells can also produce various proinflammatory mediators, such as histamine, chymase, tryptase, TNF‐α, IL‐6, and IL‐13, which activate MMP‐2 and MMP‐9, thus altering BBB permeability. 155 , 156 Actually, myeloid cells, including monocytes, neutrophils, macrophages, and activated microglia, are highly plastic depending on the environment such as interaction with ischemic neurovascular unit during late repair phase of stroke, which can be potential immunotherapeutic targets. 154 , 157 , 158
5.5. Other changes in the BBB
In addition to the above pathways in which peripheral inflammation affects the BBB, it is demonstrated that morphological changes may not necessarily occur when peripheral inflammation impacts BBB integrity. For instance, TJs may remain intact during inflammation while the functional integrity of the BBB is impaired. 27
Multiple transport pathways are altered by peripheral inflammation. Efflux transporters are downregulated, including P‐gp on the astrocytic end‐feet, along with those for anions, amino acids, and β‐amyloid. Meanwhile, influx transporters are upregulated, including those for insulin, monoamine, and lysosomal enzymes. 27 In addition, the cerebral endothelium expresses IL‐1, IL‐6, and TNF‐α receptors; thus, these circulating cytokines can directly activate the endothelium, causing BBB dysfunction. 159 This may be associated with nuclear transcription factor IκB. 160 What is more, LPS, TNF‐α, and IL‐1β can enhance the expression of cyclooxygenase (COX) in the cerebral endothelium. 159 It has been reported that a high dose of LPS causes BBB damage through COX‐dependent pathways. 161 Recently, it was identified that dynamic changes of CD antigens, such as CD54 and CD106 in brain vessels, allowed for leukocyte migration with and without alterations of other major functional molecules after LPS injection. 162
6. CONCLUSION AND FUTURE PERSPECTIVE
The BBB is a complex CNS structure that precisely regulates the transport of ions, molecules, and cells between the CNS and periphery. It protects the brain from damage and maintains the normal biochemical microenvironment. Peripheral inflammation is one of the comorbid conditions that is involved in BBB breakage and its dysfunction, and its mechanisms are extremely complicated (Table 1). Take SARS‐CoV‐2, for example, the recent COVID‐19 patients showed ischemic stroke and cerebral hemorrhage, highlighting the potentially critical role of BBB disruption by peripheral inflammation. Our lacking in knowledge in understanding it makes it even harder for treatment and prevention of serious CNS complications in COVID‐19 patients. More work is needed to understand the heterogeneity and signaling mechanisms intrinsic to BBB development, maintenance, disruption, and repair. Although some of the molecular and cellular pathways have been reported, it is crucial to identify how these different signaling pathways collaborate with one another during the development and maintenance of the BBB. Answers to these questions could help tell the exact mechanisms of BBB disruption that lead to various neurological diseases.
TABLE 1.
Mechanisms of peripheral inflammation causing BBB disruption, and the corresponding examples and references.
| Mechanisms of peripheral inflammation‐induced BBB disruption | Examples | References |
|---|---|---|
| Changes in TJs | Expression and/or location changes in claudin−5, occludin, ZO−1, etc. | 71,72,73,74,75,76,77,78,79,80,81,82,84,83,85,86,87,88,92,97,98,99,102 |
| Damage to ECs | ECs apoptosis, membrane abnormalities, ER stress, and mitochondrial damage. | 89,90,91 |
| Upregulation of VCAM−1, ICAM−1, and E‐selectin expression in ECs. | 93,94,71,95 | |
| Upregulation of α5 integrin‐dependent adhesion. | 75 | |
| Activation of astrocytes and microglia | Astrocytes: increased secretion of VEGF‐A. | 97,98,99,76 |
| Astrocytes: proliferation, activation, and changes in the end‐feet structure. | 100,101 | |
|
Microglia: M1 pro‐inflammatory microglia; M2 antiinflammatory microglia. |
24,26,102,105,25,106,107,108,109,110,111,112,113,114,115,116,117,118,119, 163 |
|
| Effects of peripheral immune cells | Migration of peripheral immune cells to CNS promoted by inflammatory mediators (ROS, MMP, etc.). | 74,126,130,131 |
|
Effects of lymphocytes on BBB: myelin‐specific CD4+ T cells, Th1, Th17 cells, CD8+ T cells, Th2 cells (Tregs), B cells, NK cells, etc. |
||
|
Effects of myeloid cells on BBB: neutrophils, monocytes, macrophages, DCs, mast cells, etc. |
||
| Others | Changes in transport pathways: efflux and influx transporters. | 27,159,160,161,162 |
| Peripheral inflammation in CNS diseases (AD, PD, MS, stroke, etc.) | 28,29,30,31,32,33,34,35,36,37,40,41,43,44,45,132,133,138 | |
| SARS‐CoV−2 virus infection‐induced peripheral inflammation affecting BBB. | 46,47,48,49,50,51,52,53, 54 | |
| Neurotoxic effects of CAR‐T therapy. | 59,60,61,62,63,64,65,67,66,68 164 |
Although it is almost certain that peripheral inflammation can induce BBB dysfunction, it is not clear whether this serves as an etiology for the development of various CNS diseases. Future research to study on this attribute could provide a basis for clinical treatment of the disease. It will also hint the identifications of new therapeutic strategies in various CNS diseases targeting BBB repair. On top of that, it may also shed lights to develop effective strategies for the CNS delivery of drugs to treat a wide range of neurological diseases.
CONFLICTS OF INTEREST
The author declares no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (81771293), Science and Technology Innovation Commission of Shenzhen Municipality (JCYJ20170413165705083, JCYJ20200109114608075, SGLH20180625142404672), International collaboration project of Chinese Academy of Sciences (172644KYSB20200045), CAS‐Croucher Funding Scheme for Joint Laboratories and Guangdong Innovation Platform of Translational Research for Cerebrovascular Diseases. B. H. is supported by Chinese Government Scholarship (CSC) for International Students.
Huang X, Hussain B, Chang J. Peripheral inflammation and blood–brain barrier disruption: effects and mechanisms. CNS Neurosci Ther.2021;27:36–47. 10.1111/cns.13569
Huang and Hussain are contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Daneman R, Prat A. The blood–brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffman GS, Calabrese LH. Vasculitis: determinants of disease patterns. Nat Rev Rheumatol. 2014;10:454‐462. [DOI] [PubMed] [Google Scholar]
- 3. Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood‐brain barrier. Nat Med. 2013;19:1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zlokovic BV. The blood‐brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178‐201. [DOI] [PubMed] [Google Scholar]
- 5. Kunchok A, Aksamit AJ Jr, Davis JM 3rd, et al. Association between tumor necrosis factor inhibitor exposure and inflammatory central nervous system events. JAMA Neurol. 2020;77:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu WY, Wang ZB, Wang Y, et al. Increasing the permeability of the blood‐brain barrier in three different models in vivo. CNS Neurosci Ther. 2015;21:568‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sonar SA, Lal G. Blood‐brain barrier and its function during inflammation and autoimmunity. J Leukoc Biol. 2018;103:839‐853. [DOI] [PubMed] [Google Scholar]
- 8. Logsdon AF, Erickson MA, Chen X, et al. Inter‐alpha inhibitor proteins attenuate lipopolysaccharide‐induced blood‐brain barrier disruption and downregulate circulating interleukin 6 in mice. J Cereb Blood Flow Metab. 2020;40:1090‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vallon M, Chang J, Zhang H, Kuo CJ. Developmental and pathological angiogenesis in the central nervous system. Cell Mol Life Sci. 2014;71:3489‐3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aird WC. Phenotypic Heterogeneity of the Endothelium: II. Representative vascular beds. Circ Res. 2007;100:158‐173. [DOI] [PubMed] [Google Scholar]
- 11. Correale J, Villa A. Cellular elements of the blood‐brain barrier. Neurochem Res. 2009;34:2067‐2077. [DOI] [PubMed] [Google Scholar]
- 12. Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223‐236. [DOI] [PubMed] [Google Scholar]
- 13. Lee MJ, Jang Y, Han J, et al. Endothelial‐specific Crif1 deletion induces BBB maturation and disruption via the alteration of actin dynamics by impaired mitochondrial respiration. J Cereb Blood Flow Metab. 2020;40:1546‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chow BW, Gu C. The molecular constituents of the blood‐brain barrier. Trends Neurosci. 2015;38:598‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nature Neurosci. 2007;10:1369‐1376. [DOI] [PubMed] [Google Scholar]
- 17. Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41‐53. [DOI] [PubMed] [Google Scholar]
- 18. Ronaldson PT, Davis TP. Regulation of blood–brain barrier integrity by microglia in health and disease: a therapeutic opportunity. J Cereb Blood Flow Metab. 2020;40:S6‐S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19:771‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood‐Brain Barrier. Cell. 2015;163:1064‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood‐brain barrier integrity during embryogenesis. Nature. 2010;468:562‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng Z, Chopp M, Chen J. Multifaceted roles of pericytes in central nervous system homeostasis and disease. J Cereb Blood Flow Metab. 2020;40:1381‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reed MJ, Damodarasamy M, Banks WA. The extracellular matrix of the blood‐brain barrier: structural and functional roles in health, aging, and Alzheimer's disease. Tissue Barriers. 2019;7:1651157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haruwaka K, Ikegami A, Tachibana Y, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nature Commun. 2019;10:5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sumi N, Nishioku T, Takata F, et al. Lipopolysaccharide‐activated microglia induce dysfunction of the blood‐brain barrier in rat microvascular endothelial cells co‐cultured with microglia. Cell Mol Neurobiol. 2010;30:247‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishioku T, Matsumoto J, Dohgu S, et al. Tumor necrosis factor‐α mediates the blood‐brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J Pharmacol Sci. 2010;112:251‐254. [DOI] [PubMed] [Google Scholar]
- 27. Varatharaj A, Galea I. The blood‐brain barrier in systemic inflammation. Brain Behav Immun. 2017;60:1‐12. [DOI] [PubMed] [Google Scholar]
- 28. Le Page A, Dupuis G, Frost EH, et al. Role of the peripheral innate immune system in the development of Alzheimer's disease. Exp Gerontol. 2018;107:59‐66. [DOI] [PubMed] [Google Scholar]
- 29. Jaeger LB, Dohgu S, Sultana R, et al. Lipopolysaccharide alters the blood‐brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer's disease. Brain Behav Immun. 2009;23:507‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takeda S, Sato N, Ikimura K, Nishino H, Rakugi H, Morishita R. Increased blood‐brain barrier vulnerability to systemic inflammation in an Alzheimer disease mouse model. Neurobiol Aging. 2013;34:2064‐2070. [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Zhang S, Li X, et al. Peripheral inflammation promotes brain tau transmission via disrupting blood–brain barrier. Biosci Rep. 2020;40:BSR20193629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. García‐Domínguez I, Veselá K, García‐Revilla J, et al. Peripheral inflammation enhances microglia response and nigral dopaminergic cell death in an in vivo MPTP model of parkinson's disease. Front Cell Neurosci. 2018;12:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Herrera AJ, Espinosa‐Oliva AM, Oliva‐Martin MJ, Carrillo‐Jimenez A, Venero JL, de Pablos RM. Collateral damage: contribution of peripheral inflammation to neurodegenerative diseases. Curr Top Med Chem. 2015;15:2193‐2210. [DOI] [PubMed] [Google Scholar]
- 34. Mei M, Zhou Y, Liu M, et al. Antioxidant and anti‐inflammatory effects of dexrazoxane on dopaminergic neuron degeneration in rodent models of Parkinson's disease. Neuropharmacology. 2019;160:107758. [DOI] [PubMed] [Google Scholar]
- 35. Fabis MJ, Scott GS, Kean RB, Koprowski H, Hooper DC. Loss of blood–brain barrier integrity in the spinal cord is common to experimental allergic encephalomyelitis in knockout mouse models. Proc Natl Acad Sci U S A. 2007;104:5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dénes Á, Ferenczi S, Kovács KJ. Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood‐ brain barrier damage and brain oedema independently of infarct size. J Neuroinflammation. 2011;8:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Palasik W, Fiszer U, Lechowicz W, Czartoryska B, Krzesiewicz M, Lugowska A. Assessment of relations between clinical outcome of ischemic stroke and activity of inflammatory processes in the acute phase based on examination of selected parameters. Eur Neurol. 2005;53:188‐193. [DOI] [PubMed] [Google Scholar]
- 38. Li Y, Zhu Z‐Y, Huang T‐T, et al. The peripheral immune response after stroke‐A double edge sword for blood‐brain barrier integrity. CNS Neurosci Ther. 2018;24:1115‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saand AR, Yu F, Chen J, Chou SHY. Systemic inflammation in hemorrhagic strokes – A novel neurological sign and therapeutic target? J Cereb Blood Flow Metab. 2019;39:959‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qin X, Akter F, Qin L, et al. Adaptive immunity regulation and cerebral ischemia. Front Immunol. 2020;11:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ao L‐Y, Yan Y‐Y, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66:342‐355. [DOI] [PubMed] [Google Scholar]
- 42. Bernstein DL, Zuluaga‐Ramirez V, Gajghate S, et al. miR‐98 reduces endothelial dysfunction by protecting blood‐brain barrier (BBB) and improves neurological outcomes in mouse ischemia/reperfusion stroke model. J Cereb Blood Flow Metab. 2020;40:1953‐1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jin R, Liu S, Wang M, Zhong W, Li G. Inhibition of CD147 attenuates stroke‐associated pneumonia through modulating lung immune response in mice. Front Neurol. 2019;10:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ordovas‐Montanes J, Rakoff‐Nahoum S, Huang S, Riol‐Blanco L, Barreiro O, von Andrian UH. The regulation of immunological processes by peripheral neurons in homeostasis and disease. Trends Immunol. 2015;36:578‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Prass K, Meisel C, Höflich C, et al. Stroke‐induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1‐like immunostimulation. J Exp Med. 2003;198:725‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology. 2019;77:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blanco‐Melo D, Nilsson‐Payant BE, Liu WC, et al. Imbalanced host response to SARS‐CoV‐2 drives development of COVID‐19. Cell. 2020;181:1036.e9‐1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Serrano‐Castro PJ, Estivill‐Torrus G, Cabezudo‐Garcia P, et al. Impact of SARS‐CoV‐2 infection on neurodegenerative and neuropsychiatric diseases: a delayed pandemic? Neurologia. 2020;Influencia, de la infeccion SARS‐CoV‐2 sobre enfermedades neurodegenerativas y neuropsiquiatricas: inverted question markuna pandemia demorada? 35:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Najjar S, Najjar A, Chong DJ, et al. Central nervous system complications associated with SARS‐CoV‐2 infection: integrative concepts of pathophysiology and case reports. J Neuroinflammation. 2020;17:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. 2013;14:e205‐e217. [DOI] [PubMed] [Google Scholar]
- 56. Forman SJ, Rowe JM. The myth of the second remission of acute leukemia in the adult. Blood. 2013;121:1077‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor‐modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee DW, Kochenderfer JN, Stetler‐Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose‐escalation trial. Lancet. 2015;385:517‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA‐1: a multicenter study of KTE‐C19 Anti‐CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25:285‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Turtle CJ, Hanafi L‐A, Berger C, et al. CD19 CAR‐T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity associated with CD19‐targeted CAR‐T cell therapies. CNS Drugs. 2018;32:1091‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T‐cell therapy in patients with B‐cell acute lymphoblastic leukemia. Cancer Discov. 2018;8:958‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hu Y, Sun J, Wu Z, et al. Predominant cerebral cytokine release syndrome in CD19‐directed chimeric antigen receptor‐modified T cell therapy. J Hematol Oncol. 2016;9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gust J, Hay KA, Hanafi L‐A, et al. Endothelial activation and blood‐brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR‐T cells. Cancer Discov. 2017;7:1404‐1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rice J, Nagle S, Randall J, Hinson HE. Chimeric antigen receptor T cell‐related neurotoxicity: mechanisms, clinical presentation, and approach to treatment. Curr Treat Options Neurol. 2019;21:40. [DOI] [PubMed] [Google Scholar]
- 68. Parker KR, Migliorini D, Perkey E, et al. Single‐cell analyses identify brain mural cells expressing CD19 as potential off‐tumor targets for CAR‐T immunotherapies. Cell. 2020;183:126 e17‐142 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Buljevac D, Flach HZ, Hop WCJ, et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125:952‐960. [DOI] [PubMed] [Google Scholar]
- 70. Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Presta I, Vismara M, Novellino F, et al. Innate immunity cells and the neurovascular unit. Int J Mol Sci. 2018;19:3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nakamuta S, Endo H, Higashi Y, et al. Human immunodeficiency virus type 1 gp120‐mediated disruption of tight junction proteins by induction of proteasome‐mediated degradation of zonula occludens‐1 and ‐2 in human brain microvascular endothelial cells. J Neurovirol. 2008;14:186‐195. [DOI] [PubMed] [Google Scholar]
- 73. Coureuil M, Mikaty G, Miller F, et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood‐brain barrier in multiple sclerosis? FEBS Lett. 2011;585:3770‐3780. [DOI] [PubMed] [Google Scholar]
- 75. Labus J, Woltje K, Stolte KN, et al. IL‐1beta promotes transendothelial migration of PBMCs by upregulation of the FN/alpha5beta1 signalling pathway in immortalised human brain microvascular endothelial cells. Exp Cell Res. 2018;373:99‐111. [DOI] [PubMed] [Google Scholar]
- 76. Tan S, Shan Y, Lin Y, et al. Neutralization of interleukin‐9 ameliorates experimental stroke by repairing the blood‐brain barrier via down‐regulation of astrocyte‐derived vascular endothelial growth factor‐A. FASEB J. 2019;33:4376‐4387. [DOI] [PubMed] [Google Scholar]
- 77. Labus J, Hackel S, Lucka L, Danker K. Interleukin‐1beta induces an inflammatory response and the breakdown of the endothelial cell layer in an improved human THBMEC‐based in vitro blood‐brain barrier model. J Neurosci Methods. 2014;228:35‐45. [DOI] [PubMed] [Google Scholar]
- 78. Menard C, Pfau ML, Hodes GE, et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. 2017;20:1752‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen W, Ju X‐Z, Lu Y, Ding X‐W, Miao C‐H, Chen J‐W. Propofol improved hypoxia‐impaired integrity of blood‐brain barrier via modulating the expression and phosphorylation of zonula occludens‐1. CNS Neurosci Ther. 2019;25:704‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nitta T, Hata M, Gotoh S, et al. Size‐selective loosening of the blood‐brain barrier in claudin‐5‐deficient mice. J Cell Biol. 2003;161:653‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang X, Xue G‐X, Liu W‐C, et al. Melatonin alleviates lipopolysaccharide‐compromised integrity of blood‐brain barrier through activating AMP‐activated protein kinase in old mice. Aging Cell. 2017;16:414‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Piro JR, Suidan GL, Quan J, et al. Inhibition of 2‐AG hydrolysis differentially regulates blood brain barrier permeability after injury. J Neuroinflammation. 2018;15:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wu T, Wang X, Zhang R, et al. Mice with pre‐existing tumors are vulnerable to postoperative cognitive dysfunction. Brain Res. 2020;1732:146650. [DOI] [PubMed] [Google Scholar]
- 84. Qin LH, Huang W, Mo XA, Chen YL, Wu XH. LPS induces occludin dysregulation in cerebral microvascular endothelial cells via MAPK signaling and augmenting MMP‐2 Levels. Oxid Med Cell Longev. 2015;2015:120641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wong D, Dorovini‐Zis K, Vincent SR. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood–brain barrier. Exp Neurol. 2004;190:446‐455. [DOI] [PubMed] [Google Scholar]
- 86. Han D, Fang W, Zhang R, et al. Clematichinenoside protects blood brain barrier against ischemic stroke superimposed on systemic inflammatory challenges through up‐regulating A20. Brain Behav Immun. 2016;51:56‐69. [DOI] [PubMed] [Google Scholar]
- 87. Liu X, Sui B, Sun J. Blood‐brain barrier dysfunction induced by silica NPs in vitro and in vivo: involvement of oxidative stress and Rho‐kinase/JNK signaling pathways. Biomaterials. 2017;121:64‐82. [DOI] [PubMed] [Google Scholar]
- 88. Gao M, Lu W, Shu Y, et al. Poldip2 mediates blood‐brain barrier disruption and cerebral edema by inducing AQP4 polarity loss in mouse bacterial meningitis model. CNS Neurosci Ther. 2020;26:1288‐1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cardoso FL, Kittel Á, Veszelka S, et al. Exposure to lipopolysaccharide and/or unconjugated bilirubin impair the integrity and function of brain microvascular endothelial cells. PLoS One. 2012;7:e35919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bernhart E, Kogelnik N, Prasch J, et al. 2‐Chlorohexadecanoic acid induces ER stress and mitochondrial dysfunction in brain microvascular endothelial cells. Redox Biol. 2018;15:441‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Karahashi H, Michelsen KS, Arditi M. Lipopolysaccharide‐induced apoptosis in transformed bovine brain endothelial cells and human dermal microvessel endothelial cells: the role of JNK. J Immunol. 2009;182:7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mayerhofer R, Fröhlich EE, Reichmann F, et al. Diverse action of lipoteichoic acid and lipopolysaccharide on neuroinflammation, blood‐brain barrier disruption, and anxiety in mice. Brain Behav Immun. 2017;60:174‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yu C‐C, Chen H‐L, Chen M‐H, et al. Vascular inflammation is a risk factor associated with brain atrophy and disease severity in parkinson's disease: a case‐control study. Oxid Med Cell Longev. 2020;2020:2591248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yousef H, Czupalla CJ, Lee D, et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat Med. 2019;25:988‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Souza PS, Gonçalves ED, Pedroso GS, et al. Physical exercise attenuates experimental autoimmune encephalomyelitis by inhibiting peripheral immune response and blood‐brain barrier disruption. Mol Neurobiol. 2017;54:4723‐4737. [DOI] [PubMed] [Google Scholar]
- 96. Farfara D, Feierman E, Richards A, et al. Knockdown of circulating C1 inhibitor induces neurovascular impairment, glial cell activation, neuroinflammation, and behavioral deficits. Glia. 2019;67:1359‐1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Argaw AT, Asp L, Zhang J, et al. Astrocyte‐derived VEGF‐A drives blood‐brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122:2454‐2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF‐mediated disruption of endothelial CLN‐5 promotes blood‐brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang C, Wang C, Dong H, et al. Immune‐related GTPase Irgm1 exacerbates experimental auto‐immune encephalomyelitis by promoting the disruption of blood‐brain barrier and blood‐cerebrospinal fluid barrier. Mol Immunol. 2013;53:43‐51. [DOI] [PubMed] [Google Scholar]
- 100. Biesmans S, Meert TF, Bouwknecht JA, et al. Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediators Inflamm. 2013;2013:271359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zamanian JL, Xu L, Foo LC, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. da Fonseca ACC, Matias D, Garcia C, et al. The impact of microglial activation on blood‐brain barrier in brain diseases. Front Cell Neurosci. 2014;8:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang J, Xing H, Wan L, Jiang X, Wang C, Wu Y. Treatment targets for M2 microglia polarization in ischemic stroke. Biomed Pharmacother. 2018;105:518‐525. [DOI] [PubMed] [Google Scholar]
- 104. Zhang ZJ, Zheng XX, Zhang XY, Zhang Y, Huang BY, Luo T. Aging alters Hv1‐mediated microglial polarization and enhances neuroinflammation after peripheral surgery. CNS Neurosci Ther. 2020;26:374‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yang X, Zhang JD, Duan L, Xiong HG, Jiang YP, Liang HC. Microglia activation mediated by toll‐like receptor‐4 impairs brain white matter tracts in rats. J Biomed Res. 2018;32:136‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Moritz KE, McCormack NM, Abera MB, et al. The role of the immunoproteasome in interferon‐γ‐mediated microglial activation. Sci Rep. 2017;7:9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ponomarev ED, Shriver LP, Maresz K, Pedras‐Vasconcelos J, Verthelyi D, Dittel BN. GM‐CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:39‐48. [DOI] [PubMed] [Google Scholar]
- 108. Yang Y, Rosenberg GA. Blood‐brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323‐3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Atallah A, Mhaouty‐Kodja S, Grange‐Messent V. Chronic depletion of gonadal testosterone leads to blood‐brain barrier dysfunction and inflammation in male mice. J Cereb Blood Flow Metab. 2017;37:3161‐3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Akundi RS, Candelario‐Jalil E, Hess S, et al. Signal transduction pathways regulating cyclooxygenase‐2 in lipopolysaccharide‐activated primary rat microglia. Glia. 2005;51:199‐208. [DOI] [PubMed] [Google Scholar]
- 111. Wang Q‐S, Ding H‐G, Chen S‐L, et al. Hypertonic saline mediates the NLRP3/IL‐1β signaling axis in microglia to alleviate ischemic blood‐brain barrier permeability by downregulating astrocyte‐derived VEGF in rats. CNS Neurosci Ther. 2020;26:1045‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kuno R, Wang J, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Autocrine activation of microglia by tumor necrosis factor‐α. J Neuroimmunol. 2005;162:89‐96. [DOI] [PubMed] [Google Scholar]
- 113. Liu Y‐H, Wu P‐H, Kang C‐C, et al. Group A streptococcus subcutaneous infection‐induced central nervous system inflammation is attenuated by blocking peripheral TNF. Front Microbiol. 2019;10:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Poller B, Drewe J, Krähenbühl S, Huwyler J, Gutmann H. Regulation of BCRP (ABCG2) and P‐glycoprotein (ABCB1) by cytokines in a model of the human blood‐brain barrier. Cell Mol Neurobiol. 2010;30:63‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Huang M, Wan Y, Mao L, et al. Inhibiting the migration of M1 microglia at hyperacute period could improve outcome of tMCAO rats. CNS Neurosci Ther. 2017;23:222‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Faustino J, Chip S, Derugin N, et al. CX3CR1‐CCR2‐dependent monocyte‐microglial signaling modulates neurovascular leakage and acute injury in a mouse model of childhood stroke. J Cereb Blood Flow Metab. 2019;39:1919‐1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Moreno B, Jukes J‐P, Vergara‐Irigaray N, et al. Systemic inflammation induces axon injury during brain inflammation. Ann Neurol. 2011;70:932‐942. [DOI] [PubMed] [Google Scholar]
- 118. Li Y, Zhu Z‐Y, Lu B‐W, et al. Rosiglitazone ameliorates tissue plasminogen activator‐induced brain hemorrhage after stroke. CNS Neurosci Ther. 2019;25:1343‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Subramaniam SR, Federoff HJ. Targeting microglial activation states as a therapeutic avenue in Parkinson's disease. Front Aging Neurosci. 2017;9:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Liu Z‐J, Ran Y‐Y, Qie S‐Y, et al. Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti‐inflammatory phenotype through STAT3 pathway. CNS Neurosci Ther. 2019;25:1353‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Esposito E, Hayakawa K, Ahn BJ, et al. Effects of ischemic post‐conditioning on neuronal VEGF regulation and microglial polarization in a rat model of focal cerebral ischemia. J Neurochem.;146:160‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Butovsky O, Talpalar AE, Ben‐Yaakov K, Schwartz M. Activation of microglia by aggregated beta‐amyloid or lipopolysaccharide impairs MHC‐II expression and renders them cytotoxic whereas IFN‐gamma and IL‐4 render them protective. Mol Cell Neurosci. 2005;29:381‐393. [DOI] [PubMed] [Google Scholar]
- 123. Zhou X, Spittau B, Krieglstein K. TGFβ signalling plays an important role in IL4‐induced alternative activation of microglia. J Neuroinflamm. 2012;9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Abdullahi W, Davis TP, Ronaldson PT. Functional expression of P‐glycoprotein and organic anion transporting polypeptides at the blood‐brain barrier: understanding transport mechanisms for improved cns drug delivery? AAPS J. 2017;19:931‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhou K, Zhong QI, Wang Y‐C, et al. Regulatory T cells ameliorate intracerebral hemorrhage‐induced inflammatory injury by modulating microglia/macrophage polarization through the IL‐10/GSK3β/PTEN axis. J Cereb Blood Flow Metab. 2017;37:967‐979. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126. Alvarez JI, Saint‐Laurent O, Godschalk A, et al. Focal disturbances in the blood‐brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol Dis. 2015;74:14‐24. [DOI] [PubMed] [Google Scholar]
- 127. Srivastava A, Srivastava P, Verma R. Role of bone marrow‐derived macrophages (BMDMs) in neurovascular interactions during stroke. Neurochem Int. 2019;129:104480. [DOI] [PubMed] [Google Scholar]
- 128. Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154:204‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Takarada‐Iemata M, Yoshikawa A, Ta HM, et al. N‐myc downstream‐regulated gene 2 protects blood‐brain barrier integrity following cerebral ischemia. Glia. 2018;66:1432‐1446. [DOI] [PubMed] [Google Scholar]
- 130. Victoria ECG, Toscano ECdB, Oliveira FMS, et al. Up‐regulation of brain cytokines and metalloproteinases 1 and 2 contributes to neurological deficit and brain damage in transient ischemic stroke. Microvasc Res. 2020;129:103973. [DOI] [PubMed] [Google Scholar]
- 131. Zhang C, Brandon NR, Koper K, Tang P, Xu Y, Dou H. Invasion of peripheral immune cells into brain parenchyma after cardiac arrest and resuscitation. Aging Dis. 2018;9:412‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sonar SA, Lal G. Differentiation and transmigration of CD4 T cells in neuroinflammation and autoimmunity. Front Immunol. 2017;8:1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kuo PC, Weng WT, Scofield BA, et al. Dimethyl itaconate, an itaconate derivative, exhibits immunomodulatory effects on neuroinflammation in experimental autoimmune encephalomyelitis. J Neuroinflammation. 2020;17:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Arumugam TV, Granger DN, Mattson MP. Stroke and T‐cells. Neuromolecular Med. 2005;7:229‐242. [DOI] [PubMed] [Google Scholar]
- 135. Wang S, Zhang H, Xu Y. Crosstalk between microglia and T cells contributes to brain damage and recovery after ischemic stroke. Neurol Res. 2016;38:495‐503. [DOI] [PubMed] [Google Scholar]
- 136. Brait VH, Arumugam TV, Drummond GR, Sobey CG. Importance of T lymphocytes in brain injury, immunodeficiency, and recovery after cerebral ischemia. J Cereb Blood Flow Metab. 2012;32:598‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Huppert J, Closhen D, Croxford A, et al. Cellular mechanisms of IL‐17‐induced blood‐brain barrier disruption. Faseb J. 2010;24:1023‐1034. [DOI] [PubMed] [Google Scholar]
- 138. Zhou YX, Wang X, Tang D, et al. IL‐2mAb reduces demyelination after focal cerebral ischemia by suppressing CD8(+) T cells. CNS Neurosci Ther. 2019;25:532‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Xie L, Li W, Hersh J, Liu R, Yang SH. Experimental ischemic stroke induces long‐term T cell activation in the brain. J Cereb Blood Flow Metab. 2019;39:2268‐2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Michel L, Grasmuck C, Charabati M, et al. Activated leukocyte cell adhesion molecule regulates B lymphocyte migration across central nervous system barriers. Sci Transl Med. 2019;11:eaaw0475. [DOI] [PubMed] [Google Scholar]
- 141. Cayrol R, Wosik K, Berard JL, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137‐145. [DOI] [PubMed] [Google Scholar]
- 142. Lécuyer M‐A, Saint‐Laurent O, Bourbonnière L, et al. Dual role of ALCAM in neuroinflammation and blood‐brain barrier homeostasis. Proc Natl Acad Sci U S A. 2017;114:E524‐E533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zhang Y, Gao Z, Wang D, et al. Accumulation of natural killer cells in ischemic brain tissues and the chemotactic effect of IP‐10. J Neuroinflamm. 2014;11:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Aubé B, Lévesque SA, Paré A, et al. Neutrophils mediate blood‐spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J Immunol. 2014;193:2438‐2454. [DOI] [PubMed] [Google Scholar]
- 145. McKittrick CM, Lawrence CE, Carswell HV. Mast cells promote blood brain barrier breakdown and neutrophil infiltration in a mouse model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2015;35:638‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Carmona‐Mora P, Ander BP, Jickling GC, et al. Distinct peripheral blood monocyte and neutrophil transcriptional programs following intracerebral hemorrhage and different etiologies of ischemic stroke. J Cereb Blood Flow Metab. 2020:271678X20953912.Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Levesque SA, Pare A, Mailhot B, et al. Myeloid cell transmigration across the CNS vasculature triggers IL‐1beta‐driven neuroinflammation during autoimmune encephalomyelitis in mice. J Exp Med. 2016;213:929‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Stranahan AM, Hao S, Dey A, Yu X, Baban B. Blood‐brain barrier breakdown promotes macrophage infiltration and cognitive impairment in leptin receptor‐deficient mice. J Cereb Blood Flow Metab. 2016;36:2108‐2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012;91:401‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Williams DW, Anastos K, Morgello S, Berman JW. JAM‐A and ALCAM are therapeutic targets to inhibit diapedesis across the BBB of CD14+CD16+ monocytes in HIV‐infected individuals. J Leukoc Biol. 2015;97:401‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Jaureguiberry‐Bravo M, Lopez L, Berman JW. Frontline Science: buprenorphine decreases CCL2‐mediated migration of CD14(+) CD16(+) monocytes. J Leukoc Biol. 2018;104:1049‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Agrawal SM, Williamson J, Sharma R, et al. Extracellular matrix metalloproteinase inducer shows active perivascular cuffs in multiple sclerosis. Brain. 2013;136:1760‐1777. [DOI] [PubMed] [Google Scholar]
- 153. Mishra MK, Yong VW. Myeloid cells — targets of medication in multiple sclerosis. Nat Rev Neurol. 2016;12:539‐551. [DOI] [PubMed] [Google Scholar]
- 154. Yang T, Guo R, Zhang F. Brain perivascular macrophages: Recent advances and implications in health and diseases. CNS Neurosci Ther. 2019;25:1318‐1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tchougounova E, Lundequist A, Fajardo I, Winberg J‐O, Åbrink M, Pejler G. A key role for mast cell chymase in the activation of pro‐matrix metalloprotease‐9 and pro‐matrix metalloprotease‐2. J Biol Chem. 2005;280:9291‐9296. [DOI] [PubMed] [Google Scholar]
- 156. Dong H, Zhang W, Zeng X, et al. Histamine induces upregulated expression of histamine receptors and increases release of inflammatory mediators from microglia. Mol Neurobiol. 2014;49:1487‐1500. [DOI] [PubMed] [Google Scholar]
- 157. Zhu Z, Zheng LI, Li Y, et al. Potential immunotherapeutic targets on myeloid cells for neurovascular repair after ischemic stroke. Front Neurosci. 2019;13:758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wang X, Xuan W, Zhu Z‐Y, et al. The evolving role of neuro‐immune interaction in brain repair after cerebral ischemic stroke. CNS Neurosci Ther. 2018;24:1100‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Skelly DT, Hennessy E, Dansereau M‐A, Cunningham C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL‐1Β, TNF‐α and IL‐6 challenges in C57BL/6 mice. PLoS One. 2013;8:e69123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Quan N, He L, Lai W. Endothelial activation is an intermediate step for peripheral lipopolysaccharide induced activation of paraventricular nucleus. Brain Res Bull. 2003;59:447‐452. [DOI] [PubMed] [Google Scholar]
- 161. Banks WA, Gray AM, Erickson MA, et al. Lipopolysaccharide‐induced blood‐brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation. 2015;12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Sato K, Tachikawa M, Watanabe M, Uchida Y, Terasaki T. Selective protein expression changes of leukocyte‐migration‐associated cluster of differentiation antigens at the blood‐brain barrier in a lipopolysaccharide‐induced systemic inflammation mouse model without alteration of transporters, receptors or tight junction‐related protein. Biol Pharm Bull. 2019;42:944‐953. [DOI] [PubMed] [Google Scholar]
- 163. Haley MJ, Brough D, Quintin J, Allan SM. Microglial priming as trained immunity in the brain. Neuroscience. 2019;405:47‐54. [DOI] [PubMed] [Google Scholar]
- 164. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T‐cell therapy in refractory large B‐Cell Lymphoma. N Engl J Med. 2017;377:2531‐2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


