Abstract
Ischemic stroke (IS), a common cerebrovascular disease, results from a sudden blockage of a blood vessel in the brain, thereby restricting blood supply to the area in question, and making a significantly negative impact on human health. Unfortunately, current treatments, that are mainly based on a recanalization of occluded blood vessels, are insufficient or inaccessible to many stroke patients. Recently, the profound influence of the neurovascular unit (NVU) on recanalization and the prognosis of IS have become better understood; in‐depth studies of the NVU have also provided novel approaches for IS treatment. In this article, we review the intimate connections between the changes in the NVU and IS outcomes, and discuss possible new management strategies having practical significance to IS. We discuss the concept of the NVU, as well as its roles in IS blood‐brain barrier regulation, cell preservation, inflammatory immune response, and neurovascular repair. Besides, we also summarize the influence of noncoding RNAs in NVU, and IS therapies targeting the NVU. We conclude that both the pathophysiological and neurovascular repair processes of IS are strongly associated with the homeostatic state of the NVU and that further research into therapies directed at the NVU could expand the range of treatments available for IS.
Keywords: Ischemia, MicroRNAs, Neurovascular unit, Stroke, Therapy
Neurovascular unit plays a leading role in the pathophysiological process of ischemic stroke. Both cell‐based therapies and drugs targeting the neurovascular unit can fight against ischemic stroke. The entire multiple cells’ interaction framework of the neurovascular unit are worth to further study to explore the therapeutic potential of the neurovascular unit in clinical.
1. BACKGROUND
Stroke is the leading cause of disability, as well as the second leading cause of death worldwide. 1 , 2 , 3 , 4 , 5 Ischemic stroke (IS), in particular, accounts for 85% of strokes. 6 Due to the death of brain cells following the permanent or transient blockage of blood vessels, IS imposes a heavy economic and health burden on society. 4 , 7 Following the failure of several decades of large scale neuroprotective clinical trials, the focus of stroke treatment shifted from a neuroprotective approach to neurovascular protection. 8 , 9 The concept of the neurovascular unit (NVU), comprised of neurons, astrocytes, smooth muscle cells (SMCs), endothelial cells (ECs), pericytes (PCs), and the basal lamina matrix, emphasizes the unique cross talk between neurons and the cerebral vasculature, and ultimately, the pivotal role the NVU plays in IS progression. 10 With effective reperfusion strategies implemented, intravenous thrombolysis and thrombectomy have become the most common treatments administered to IS patients. 11 Nowadays, the usage of tissue‐type plasminogen activator (tPA) consists a widely accepted treatment, that is most effective when administered within 4.5 h after acute ischemic stroke (AIS). 12 , 13 , 14 Unfortunately, it is only applicable to a limited number of patients because of the strict time window of tPA treatment. Thus, the provision of other effective treatments is urgently required. 13
In this review, we discuss the effects of the NVU on blood‐brain barrier (BBB) regulation, cell preservation, inflammatory immune response, and neurovascular repair during or after IS, as well as the regulation of the NVU by noncoding RNAs (ncRNAs). Lastly, we review existing therapeutic approaches and prospects for IS treatments targeting the NVU.
2. THE CONCEPT OF THE NEUROVASCULAR UNIT
The neurovascular unit (NVU), a groundbreaking concept consisting of multiple components, includes neurons, glial cells, vascular cells (endothelial cells or ECs, pericytes or PCs, and smooth muscle cells or SMCs), and the basal lamina matrix within the brain vasculature. 15 , 16 The concept emerged from the first Stroke Progress Review Group meeting of the National Institute of Neurological Disorders and Stroke of the National Institutes of Health represents a conceptual framework incorporating neurons and the adjacent vasculature. 10
It is now recognized that the interactions between various components of the NVU are highly important. 15 The BBB and cerebral blood flow (CBF) are precisely controlled by the NVU, thus maintaining a homeostatic brain microenvironment. 16 , 17 Endothelial cells form a highly specialized membrane around blood vessels. 17 Pericytes in the central nervous system (CNS) contribute to both neurogenesis and vasculogenesis, and PCs localized within blood vessels may act as multipotent vascular stem cells. 17 , 18 , 19 , 20 , 21 , 22 The loss of PCs leads to reduced expression of specific tight junction (TJ) proteins and subsequent BBB disruption. 23 Astrocytes extend end feet to PCs and SMCs to regulate their constriction and relaxation, thereby adjusting CBF. 24 , 25 Astrocytes also regulate the balance of synaptic glutamate partly via Ca2+ oscillations as a timely response to changes in ions and metabolism in neuronal cells. 25 Although the classic definition of NVU does not include microglia and oligodendrocytes, structurally and functionally they are closely related to the NVU. Oligodendrocytes not only produce neurotrophic factors, but also form myelin sheaths that support the transmission of action potentials. 26 Furthermore, they may also serve as antigen‐presenting cells. 27 As immune cells in the CNS, microglia can modulate the innate immunity of astrocytes by releasing various signaling molecules. 28 , 29 In summary, all the NVU components are closely related in structure, and integral in function to preserve brain homeostasis.
3. THE ROLES OF THE NVU IN IS
The pathophysiological process of IS consists of three stages in time and space: a) the hyperacute phase (minutes to 6 h); b) the acute and subacute phase (hours to 7 d); and c) the chronic phase. During the course of injury and inflammation, endogenous protective and repair mechanisms are activated simultaneously, and the ratio of these activities determines the outcome of IS. 30 The roles NVU plays in IS are crucial, which we summarize in four parts: BBB regulation, cell preservation, inflammatory immune response, and repair during or after IS.
3.1. BBB regulation during IS
The function of the BBB depends on the TJs between ECs and the perivascular microenvironment. In the acute phase following the initiation of ischemia, NVU dysfunction directly promotes the breakdown of the BBB. For example, the reduction of expression of certain proteins (such as occludin, claudin‐5, and ZO‐1) enhances BBB permeability and increases the risk of inducing vasogenic cerebral edema. 30 , 31 In addition, PCs further promote the development of cerebral edema by transforming into a proinflammatory phenotype. 30 Glial cells may contribute to BBB destruction via matrix metalloproteinases (MMPs), such as MMP‐9, which digest BBB matrix proteins. 32 , 33 To date, tPA is the only therapeutic agent that has been approved for the treatment of patients with AIS. 12 , 34 , 35 However, tPA itself activates MMPs, further exacerbating the destruction of the BBB, which not only promotes the development of neuroinflammation and edema, but also increases the risk of cerebral hemorrhage in patients treated with thrombolysis. 36 , 37
Therefore, in order to prevent the further development of IS, BBB protection must be a top priority; BBB repair can assist with the treatment of IS. Perlecan is a major protein of the basement membrane, with upregulated expression after IS in mice. The core protein of Perlecan called DV attaches to PC and EC, and promotes pericyte migration through the integral protein α5β1 via PDGFRβ signaling, subsequently regulating BBB repair. 38 The permeability of the BBB is increased in mice through CLEC14A knockdown ECs, in which TJ proteins are attenuated. 39 The PDGFR‐β signaling also regulates the recruitment of PCs into injury lesions to promote BBB recovery. 40
3.2. Cell preservation
Endothelial cells are the first to be damaged in ischemic brain regions. The integrity of the TJs between ECs can be enhanced by PCs via the secretion of glial cell‐derived neurotrophic factor (GDNF) and angiopoietin‐1 (Ang‐1), which ultimately protects ECs from necrosis. 41 Pretreatment with neutralizing antibodies of Ang‐1 blocks the PC‐induced upregulation of TJ proteins. 41
A variety of neurotrophic factors are expressed by pericytes, including the brain‐derived neurotrophic factor, nerve growth factor, and neurotrophin‐3, which provide neuroprotective effects and facilitate neuronal and axonal regeneration in response to IS. 42 , 43 After pericyte ablation with diphtheria toxin, the loss of pleiotrophin, a pericyte‐secreted growth factor enriched in PCs to provide neurotrophic support, leads to both rapid neuron and CBF loss, and results in BBB damage in mice. 44 Astrocyte‐specific Swell1 deletion mice exhibited remissive glutamate‐dependent neuronal excitability and brain damage after IS. 45 Additionally, reactive astrocytes restrict neuronal migration toward the IS brain lesion through direct contact, while new neurons positioned close to the lesion promote functional recovery via increased Slit‐Robo signaling. 46
3.3. Inflammatory immune response
Glial cells are key components of the CNS. 47 , 48 At the onset of stroke, astrocytes are activated immediately by molecules released from the site of injury. 49 They secrete proinflammatory factors and MMPs that destroy the BBB, as well as neurotrophic factors that protect ischemic sites. 49 In cerebral ischemia‐reperfusion mouse models, the overexpression of IL‐15 in astrocytes enhances the effector functions of CD8 + T and NK cells, and thus aggravates ischemic brain injury. 50 In response to stroke, the number of Treg cells and astrocyte‐derived levels of IL‐33 and CCL1 increase. Elevated levels of amphiregulin secreted by Treg cells further regulate the IL‐6 and STAT3 pathways, thereby improving neurological functional defects. 51 , 52
Microglia also display both pro‐ and anti‐inflammatory phenotypes (named M1 and M2, respectively) and respond rapidly to ischemia during IS. 53 , 54 Within one day after IS, the proliferation and activation of microglia induced a strong inflammatory response (upregulation of TNF, IL‐1β, and IL‐6), causing severe damage to the CNS. 51 , 55 Protective cytokines, such as neurotrophic IGF‐1, are secreted by microglia cells several days after the onset of IS, contributing to nerve repair and survival. 56 The inhibition of microglia activation by complement inhibitors can protect stressed neurons and reduce neuroinflammation in a mice model. 57 On the contrary, if treatment with CSF1R antagonist reduces microglia and increases the number of neutrophils, the brain damage in mice IS brain tissue becomes even more serious. 58 Interferon regulatory factors (IRF) are regulators of macrophage activation. The downregulation of IRF4 leads to the increased expression of IRF5, which in turn enhances the activation of M1, leading to enhanced proinflammatory response and poor stroke prognosis. On the contrary, the decrease of IRF5 helps to enhance M2 activation, inhibits the proinflammatory response, and aids functional recovery. 59 Activated microglia and their fragmented mitochondria induce astrocyte transformation into reactive A1 astrocytes. 60 , 61 These lack most of the normal astrocyte functions and are neurotoxic to neurons and maturely differentiated oligodendrocytes. 60 Interestingly, astrocytes also secrete the cytokine interleukin‐33, which in turn promotes microglial synaptic remodeling. 62
3.4. Neurovascular repair
Following the acute phase of IS, the inflammatory response in the infarcted area starts to decrease and tissue repair begins to intensify. Although reactive glial cells are harmful in the early stage, reactive astrocytes also play a role as a phagocytic phenotype in engulfing cell debris to aid the recovery of brain damage via the ABCA1‐mediated pathway. 46 The subsequently formed glial scars may still hinder the axonal bud bulging through the extensive expression of axon regeneration inhibitors (such as chondroitin sulfate proteoglycans). On the other hand, normal brain tissue is isolated from the damaged area to minimize the magnification of lesions and inflammation. 63 The morphology of astrocytes after 2 h of transient middle cerebral artery occlusion in mice showed that the deterioration of astrocyte ultrastructure is much slower than that of neurons, indicating that, under ischemic conditions, astrocytes are more resistant to injury than adjacent neurons. 64 In the absence of astrocytes, glutamate neurotoxicity occurs at lower concentrations in the cerebral cortex of mice. 65
Pericytes serve as critical regulators during angiogenesis after IS via various signaling pathways, including the Ang/Tie system, VEGF signaling, the PDGF‐β/PDGFR‐β system, and RGS5 signaling. 66 The upregulation of ephrinB2 acted beneficially on neurovascular repair after IS by increasing pericyte recruitment and endothelial‐pericyte cell interaction. On the contrary, the inhibition of ephrinB2 expression in ECs or PCs leads to worse outcomes. 67 Furthermore, the vascular endothelial growth factor isoform‐B also stimulates neurovascular repair following IS by promoting the function of PCs via VEGFR‐1. 68 After a reasonable decline of pericyte‐derived fibrosis, the number of raphespinal and corticospinal tract axons was increased, which was proportional to the degree of functional recovery after CNS injury. 69 (Figure 1).
FIGURE 1.
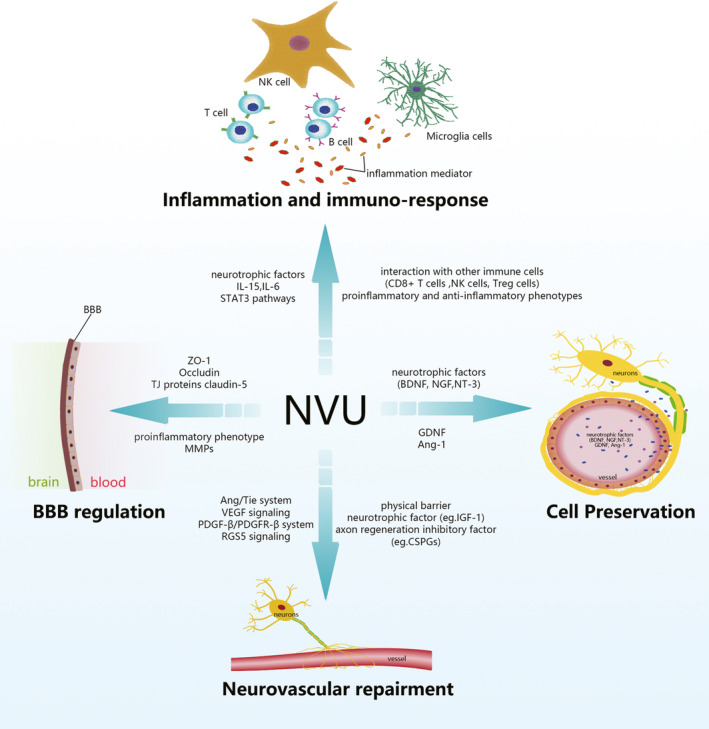
The roles of the neurovascular unit in ischemic stroke
In summary, the NVU is able to regulate BBB integrity, cell preservation, inflammatory immune response, and repair during or after IS. It secrets a variety of proteins to prevent BBB from breakdown and to promote its functional recovery. The integrity of the NVU provides strong support to cell preservation through a subtle regulation of neurotrophic factors and subsequent signaling pathways. Interestingly, it plays its critical role as a two‐edged sword in inflammatory immune responses to IS, with both the pro‐ and anti‐inflammatory phenotypes of the NVU responding rapidly to ischemia. The inhibition of microglia activation can protect the CNS from microglia related inflammatory immune response; however, the use of antagonists inhibiting microglia activation will block subsequent functional recovery. The increased recruitment of PCs and ECs is beneficial to neurovascular repair after IS, while a reasonable decrease of pericyte‐derived fibrosis promotes the outcome of CNS injury.
4. REGULATION OF THE NVU BY NCRNAS IN IS
Mechanisms through which NVU plays a role in IS are highly complicated, with several genes involved in relevant regulatory pathways. In addition to the large number of immuno‐inflammatory molecules discussed above, a growing number of studies have begun to recognize the important roles ncRNAs play in NVU, though no relevant work to summarize these roles exists in this regard. Therefore, a discussion on the involvement of ncRNAs in IS through NVU is detailed herein, which helps to deepen the understanding of the pathophysiological process of IS, thereby opening up possible new directions for treatment. 30
Firstly, microRNAs (miRNAs) regulate gene or protein expression by inhibiting translation. 70 In an established ischemia/reperfusion (I/R) rat model, exosomal mir‐26b‐5p inhibits M1 polarization of microglia via targeting CH25H and inactivating the TLR pathway, leading to reduced nerve injury after cerebral I/R. A reduction of exosomal mir‐26b‐5p has the opposite effect. 71 Recently, it was shown that expression levels of inflammatory cytokines elevate, whereas anti‐inflammatory cytokines (IL‐4, IL‐10) and mir‐30d‐5p decrease in AIS patients compared with normal control. In addition, adipose‐derived stem cell‐derived exosomes enriched with mir‐30d‐5p have a protective effect on AIS via autophagy‐mediated microglia M1 polarization reduction. 72
Long noncoding RNAs (lncRNAs) serve as miRNA sponges or antagomirs. 73 They contribute greatly to the pathophysiological process of IS, for example, the lncRNA Malat1 is capable of sponging mir‐26b, mir‐30a, mir‐145, mir‐205‐3p, and mir‐200c‐3p from protecting cerebral microvascular endothelial cells, and attenuating neuronal cell death following IS. 74 , 75 , 76 The expression of lncRNA Nespas is significantly upregulated after IS in an MCAO mice model. While the silencing of Nespas accelerates the apoptosis of microglia, increased Nespas expression alleviates ischemic brain lesion by inhibiting NF‐κB activation and TRIM8‐induced K63‐linked polyubiquitination of TAK1 in microglia. 77
Emerging evidence suggests that multiple circular RNAs (circRNAs) serve as novel biomarkers and important regulators, or even triggers in various cancers. 78 , 79 , 80 , 81 Using circRNA microarray and genome‐wide bioinformatic analysis to study ischemic responses in mice subjected to transient middle cerebral artery occlusion (tMCAO) and to plasma samples from AIS patients, Han et al reported that circhectd1, a mir‐142 sponge, downregulates mir‐142 activity and thus leads to lower TCDD inducible poly[ADP‐ribose] polymerase (TIPARP) expression. This subsequently results in the inhibition of astrocyte activation via autophagy, while the downregulation of circHectd1 expression decreases infarct areas. In addition, circhectd1 is expressed at higher levels in AIS tissues and plasma than in control samples. These findings indicate that circhectd1 could be used as a novel type of biomarker and potential therapeutic target for IS. 82 Additional, relevant studies are summarized in Table 1.
TABLE 1.
The regulation of the NVU by ncRNAs in IS
| NVU components | ncRNAs | Regulated Molecules /Pathways | Effect | References |
|---|---|---|---|---|
| Astrocytes | Mir‑29b | AQP4 | Protection against ischemia‐reperfusion injury | 112 |
| Mir‐133b | TGF‐β (mir‐206/RABEPK) | Regulation of neurovascular plasticity | 113 | |
| Endothelial cells (ECs) | Mir‐27b | AMPK | Regulation of tube formation and migration | 114 |
| Mir‐383 | Peroxisome proliferator‐activated receptor gamma | Promotion of neurotrophy and inhibition of abnormal apoptosis | 115 | |
| Mir‐140‐5p | Vascular endothelial growth factor A (VEGFA) | Cell proliferation, migration and tube formation | 116 | |
| Mir‐155 | TGF‐β/BMP, SMAD5, mTOR, NO | Improvement of CBF and supporting microvasculature | 117 | |
| Mir‐107 | Dicer‐1 | Angiogenesis | 118 | |
| Mir‐24‐1‐5p | HIF‐1α | Angiogenesis | 119 | |
| Mir‐191 | NF‐Κb | Angiogenesis | 120 | |
| Mir‐181a | IL‐6/TNF‐α | Inhibition of the oxidized low‐density lipoprotein (ox‐LDL)‐induced immune inflammatory response | 121 | |
| Mir‐126‐3p/‐5p | IL‐1β, TNF‐α, VCAM‐1, E‐selectin | Maintenance of BBB integrity | 122 | |
| Mir‐194‐ 1 | TGF‐β/SMAD | Reduction of the inflammatory response and EC permeability | 123 | |
| Neurons | Mir‐106b‐5p | Mcl‐1/Bcl‐2 | Inhibition of apoptosis and oxidative stress | 124 |
| Mir‐149‐5p | P53/Caspase‐3 | Regulation of cell survival and apoptosis | 125 | |
| Mir‐455 | TRAF3 | Inhibition of neuronal cell death | 126 | |
| Mir‐365 | OXR1 | Activation of antioxidant signals | 127 | |
| Neurons, Astrocytes | Mir‐19a‐3p | Polyclonal Antibody to Adiponectin Receptor 2 (ADIPOR2) | Modulation of glucose metabolism and neuronal apoptosis | 128 |
| Microglia | Mir‐124 | Increase of M2‐like polarized microglia number | Neuroprotection and functional improvement | 129 |
| Mir‐26b‐5p | TLR | Regulation of microglia M1 polarization | 71 | |
| Mir‐30d‐5p | Autophagy | Regulation of microglia M1 polarization | 72 | |
| Endothelial cells (ECs) | LncRNA‐H19 | NF‐κB | Inhibition of EC apoptosis in the ASO model | 130 |
| Malat1 | Mir‐26b, mir‐30a, mir‐145, mir‐205‐3p and mir‐200c‐3p sponge | Protection of the NVU | 74, 75, 76 | |
| Vascular smooth muscle cells (SMCs) | LncRNA‐MEG3 | ABCA1 | Regulation of proliferation and apoptosis in VSMCs | 131 |
| LncRNA‐BANCR | NK | Facilitation of SMC proliferation and migration | 132 | |
| Astrocytes | CircHECTD1 | Mir‐142 sponge | Inhibition of astrocytic activation via autophagy | 82 |
| Pericytes (PCs) | CPWWP2A | Mir‐579 sponge, angiopoietin 1, occludin and SIRT1 | Decrease in vascular dysfunction | 133 |
5. IS THERAPIES TARGETING THE NVU
The aims of the current review are to overcome the limitations of the existing treatment strategies for IS and pursue faster recovery times and better recovery results. Three treatment methods targeting the NVU are summarized here cell‐based therapies, neuronal regeneration, and NVU protection.
5.1. Cell‐based therapies
Cell‐based therapy is an exciting emergent approach. Results of studies demonstrating that bone marrow stromal cells (MSCs) work well for promoting positive outcomes in IS models are promising. 83 Indeed, the exogenous transplantation of MSCs initiates the repair steps of angiogenesis, axonal remodeling, and synaptic formation. The expression of neurotrophic factors is stimulated by MSCs in astrocytes, thereby enhancing neuron survival and the expression of Cx43, which promotes the gap junction of astrocytes. 84 Furthermore, the inhibition of nerve scar formation by MSC transplantation after stroke may also promote axonal regeneration, thus enhancing the capacity for nerve repair. 85 MicroRNAs in the miR‐17‐92 cluster, which are enriched in exosomes derived from MSCs, accelerate the reconstruction of axon‐myelin and thus recovery from IS. 86 Many types of stem cells, including but not limited to adipose stem cells (ADSC), MSCs and pluripotent stem cells, can differentiate into functional PCs. 87 , 88 , 89 However, the issue of how to transfer sufficient numbers of functional transplanted cells to specific sites remains to be addressed. In this aspect, a scaffold‐free cell sheet has been used to transplant sufficient numbers of allogeneic adipose‐derived mesenchymal stem cells in a rat stroke model. 90 Transplanted stem cells not only replace dead neurons, but also secrete a variety of nutritional and growth factors to promote NVU regeneration and repair. 91 Unfortunately, even though the transplantation of the stem cells has been achieved successfully, their subsequent survival, proliferation, migration, and differentiation still encounter a series of challenges. 91
5.2. Neuronal regeneration
Neuroplasticity influences rehabilitation and recovery of the injury site affected by stroke. Cultured human cortical astrocytes transplanted into mice have been reprogrammed into functional neurons through retroviral expression of NeuroD1. 92 Endothelial progenitor cells (EPCs) secrete growth factors, including FGF‐b, VEGF, and PDGF‐BB, into cell‐free conditioned media (CM). This was utilized in an IS mouse model, where both angiogenesis and neurogenesis are enhanced in mice treated with CM rich in growth factors from EPC cultures. 93 In the infarct region of brain, Caveolin‐1 (Cav‐1) upregulates to accelerate neovascularization in wild‐type mice, while Cav‐1 knockout mice display the inverse effects. 94 Electrical stimulation (ES) based on nanomaterials has a positive effect on the fate of neural stem cells (NSCs) in vitro. Hence, ES treatment could be a potential complementary noninvasive therapy during NSCs transplantation. 95 , 96 Moreover, the combination of ADSC, sodium ferulate, and n‐butylidenephthalide yields improve neovascularization and neurogenesis compared with single stem cell treatment. 97
5.3. NVU protection
Reperfusion injury further leads to deterioration following IS. 98 With pretreatment of 4‐methoxybenzyl alcohol in rats subjected to reperfusion injury, the ratio of surviving neurons increases compared with controlled groups via the regulation of Bcl‐2, caspase‐3, and Bax, while the ultrastructure of glial cells is significantly protected. 99 β‐Caryophyllene maintains BBB integrity and prevents neuronal apoptosis by reducing proinflammatory factors and oxidative stress damage. 100 Pericytes, though, are more vulnerable than neurons in an ischemic environment. 101 Mitochondrial metabolism in astrocytes is enhanced by the purinergic ligand 2‐methylthioladenosine 5’ diphosphate via increased inositol trisphosphate‐dependent Ca2+ release, which provides protective benefits from IS. 102 After tert‐butylhydroquinone treatment following permanent distal middle cerebral artery occlusion in mice, the activation of astrocytes, and angiogenesis are significantly enhanced. 103 Cilostazol is a commonly used antiplatelet drug, which prevents the pathological detachment of astrocyte foot processes or PCs and also stimulates VEGFR2 expression and PC proliferation, thereby protecting the NVU integrity and promoting neurovascular protection. 104 Notch‐Jagged signaling in astrocytes is increased in selegiline‐treated MCAO rats compared with control, helping to preserve the capillary network after IS. 105 Treatment with tPA inhibits the secretion of glial cell‐derived trophic factors and damages PCs, but edaravone can reverse the damage, and maintains NVU integrity after tPA treatment. 106 In a mouse model where ECs, SMCs, and PCs partly lack guanylyl cyclase B, the endothelial C‐type natriuretic peptide acts on PCs, thereby regulating microcirculatory flow and blood pressure. 107 Additionally, teriflunomide improves pericyte coverage and survival, resulting in decreased TJ protein breakdown and BBB leakage. 108 A recent study has shown that novel interpericyte tunneling nanotubes could build a functional network to regulate neurovascular coupling through linking two separate PCs on different capillaries in the mouse retina. 109
Under hypothermic conditions, the isolation between basement membrane and pericytes is not observable at the ultrastructural level, indicating a well‐preserved BBB. 110 Hypothermia can be attained in mice by HPI‐201 injection. Following severe stroke, HPI‐201 treated C57BL/6 mice recover with lower neurological severity scores, decreased expression levels of inflammatory factors, higher BBB integrity, and more complete conservation of the NVU compared with the controls. 111 Thus, hypothermic protection could be a potential method to protect the NVU from IS.
6. FINAL REMARKS AND CONCLUSION
Undoubtedly, the NVU plays a leading role in the pathophysiological process of IS, with profound effects on the BBB, cell preservation, inflammatory immune response, and neurovascular repair. Both cell‐based and pharmacological therapies targeting the NVU can fight against deleterious outcomes following an ischemic stroke. The IS therapeutic philosophy has moved from the neuronal era to the neurovascular era; therefore, we must consider the entire framework of the NVU and conduct thorough investigations on the multiple interactions between its cells to further explore the therapeutic potential of the NVU in clinical settings.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHORS’ CONTRIBUTIONS
LY Wang and LY Zhang read literatures and prepare the manuscript; Chao Zhang collect literatures; XX Xiong and Jian Shen prepare the manuscript.
Wang L, Xiong X, Zhang L, Shen J. Neurovascular Unit: A critical role in ischemic stroke. CNS Neurosci Ther. 2021;27:7–16. 10.1111/cns.13561
Wang and Xiong are contributed equally to this work.
Contributor Information
Xiaoxing Xiong, Email: xiaoxingxiong@whu.edu.cn.
Jian Shen, Email: 1314006@zju.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Owolabi MO, Akarolo‐Anthony S, Akinyemi R, et al. The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc J Afr. 2015;26(2 Suppl 1):S27‐S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith M, Reddy U, Robba C, Sharma D, Citerio G. Acute ischaemic stroke: challenges for the intensivist. Intensive Care Med. 2019;45(9):1177‐1189. [DOI] [PubMed] [Google Scholar]
- 3. Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. 2015;35(6):888‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson W, Onuma O, Owolabi M, Sachdev S. Stroke: a global response is needed. Bull World Health Organ. 2016;94(9):634‐634A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun Z, Gu L, Wu KE, et al. VX‐765 enhances autophagy of human umbilical cord mesenchymal stem cells against stroke‐induced apoptosis and inflammatory responses via AMPK/mTOR signaling pathway. CNS Neurosci Ther. 2020;26(9):952‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarvari S, Moakedi F, Hone E, Simpkins JW, Ren X. Mechanisms in blood‐brain barrier opening and metabolism‐challenged cerebrovascular ischemia with emphasis on ischemic stroke. Metab Brain Dis. 2020;35(6):851‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zorowitz RD, Chen E, Tong KB, Laouri M. Costs and rehabilitation use of stroke survivors: a retrospective study of Medicare beneficiaries. Top Stroke Rehabil. 2009;16(5):309‐320. [DOI] [PubMed] [Google Scholar]
- 8. Ayer A, Hwang BY, Appelboom G, Connolly ES Jr. Clinical trials for neuroprotective therapies in intracerebral hemorrhage: a new roadmap from bench to bedside. Transl Stroke Res. 2012;3(4):409‐417. [DOI] [PubMed] [Google Scholar]
- 9. Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke. 2004;35(2):354‐356. [DOI] [PubMed] [Google Scholar]
- 10. Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leigh R, Knutsson L, Zhou J, van Zijl PC. Imaging the physiological evolution of the ischemic penumbra in acute ischemic stroke. J Cereb Blood Flow Metab. 2018;38(9):1500‐1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317‐1329. [DOI] [PubMed] [Google Scholar]
- 13. Lambertsen KL, Finsen B, Clausen BH. Post‐stroke inflammation‐target or tool for therapy? Acta Neuropathol. 2019;137(5):693‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun M, Chen X, Yin Y‐X, et al. Role of pericyte‐derived SENP1 in neuronal injury after brain ischemia. CNS Neurosci Ther. 2020;26(8):815‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lo EH, Rosenberg GA. The neurovascular unit in health and disease: introduction. Stroke. 2009;40(3 Suppl):S2‐S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12(12):723‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Armulik A, Genové G, Mäe M, et al. Pericytes regulate the blood‐brain barrier. Nature. 2010;468(7323):557‐561. [DOI] [PubMed] [Google Scholar]
- 18. Nakagomi T, Molnár Z, Nakano‐Doi A, et al. Ischemia‐induced neural stem/progenitor cells in the pia mater following cortical infarction. Stem Cells Dev. 2011;20(12):2037‐2051. [DOI] [PubMed] [Google Scholar]
- 19. Karow M, Sánchez R, Schichor C, et al. Reprogramming of pericyte‐derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11(4):471‐476. [DOI] [PubMed] [Google Scholar]
- 20. Ahmed TA, Shousha WG, Abdo SM, Mohamed IK, El‐Badri N. Human adipose‐derived pericytes: biological characterization and reprogramming into induced pluripotent stem cells. Cell Physiol Biochem. 2020;54(2):271‐286. [DOI] [PubMed] [Google Scholar]
- 21. Tachibana M, Yamazaki Y, Liu CC, Bu G, Kanekiyo T. Pericyte implantation in the brain enhances cerebral blood flow and reduces amyloid‐β pathology in amyloid model mice. Exp Neurol. 2018;300:13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakagomi T, Kubo S, Nakano‐Doi A, et al. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells. 2015;33(6):1962‐1974. [DOI] [PubMed] [Google Scholar]
- 23. Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janzer RC, Raff MC. Astrocytes induce blood‐brain barrier properties in endothelial cells. Nature. 1987;325(6101):253‐257. [DOI] [PubMed] [Google Scholar]
- 25. Zonta M, Angulo MC, Gobbo S, et al. Neuron‐to‐astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6(1):43‐50. [DOI] [PubMed] [Google Scholar]
- 26. Kuhn S, Gritti L, Crooks D, Dombrowski Y. Oligodendrocytes in development, myelin generation and beyond. Cells. 2019;8(11):1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harrington EP, Bergles DE, Calabresi PA. Immune cell modulation of oligodendrocyte lineage cells. Neurosci Lett. 2020;715:134601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirkley KS, Popichak KA, Afzali MF, Legare ME, Tjalkens RB. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J Neuroinflammation. 2017;14(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu LR, Liu JC, Bao JS, Bai QQ, Wang GQ. Interaction of microglia and astrocytes in the neurovascular Unit. Front Immunol. 2020;11:1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stamatovic SM, Phillips CM, Martinez‐Revollar G, Keep RF, Andjelkovic AV. Involvement of epigenetic mechanisms and non‐coding RNAs in blood‐brain barrier and neurovascular unit injury and recovery after stroke. Front Neurosci. 2019;13:864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sladojevic N, Stamatovic SM, Johnson AM, et al. Claudin‐1‐dependent destabilization of the blood‐brain barrier in chronic stroke. J Neurosci. 2019;39(4):743‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mun‐Bryce S, Rosenberg GA. Matrix metalloproteinases in cerebrovascular disease. J Cereb Blood Flow Metab. 1998;18(11):1163‐1172. [DOI] [PubMed] [Google Scholar]
- 33. Seo JH, Miyamoto N, Hayakawa K, et al. Oligodendrocyte precursors induce early blood‐brain barrier opening after white matter injury. J Clin Invest. 2013;123(2):782‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta‐analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929‐1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Y, Zhu Z‐Y, Lu B‐W, et al. Rosiglitazone ameliorates tissue plasminogen activator‐induced brain hemorrhage after stroke. CNS Neurosci Ther. 2019;25(12):1343‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi Z‐S, Duckwiler GR, Jahan R, et al. Early blood‐brain barrier disruption after mechanical thrombectomy in acute ischemic stroke. J Neuroimaging. 2018;28(3):283‐288. [DOI] [PubMed] [Google Scholar]
- 37. Wang W, Li M, Chen Q, Wang J. Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke: mechanisms, models, and biomarkers. Mol Neurobiol. 2015;52(3):1572‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakamura K, Ikeuchi T, Nara K, et al. Perlecan regulates pericyte dynamics in the maintenance and repair of the blood‐brain barrier. J Cell Biol. 2019;218(10):3506‐3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim Y, Lee S, Zhang H, et al. CLEC14A deficiency exacerbates neuronal loss by increasing blood‐brain barrier permeability and inflammation. J Neuroinflammation. 2020;17(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shen J, Xu G, Zhu R, et al. PDGFR‐β restores blood‐brain barrier functions in a mouse model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2019;39(8):1501‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang YL, Hui YN, Guo B, Ma JX. Strengthening tight junctions of retinal microvascular endothelial cells by pericytes under normoxia and hypoxia involving angiopoietin‐1 signal way. Eye (Lond). 2007;21(12):1501‐1510. [DOI] [PubMed] [Google Scholar]
- 42. Shimizu F, Sano Y, Saito K, et al. Pericyte‐derived glial cell line‐derived neurotrophic factor increase the expression of claudin‐5 in the blood‐brain barrier and the blood‐nerve barrier. Neurochem Res. 2012;37(2):401‐409. [DOI] [PubMed] [Google Scholar]
- 43. Arimura K, Ago T, Kamouchi M, et al. PDGF receptor β signaling in pericytes following ischemic brain injury. Curr Neurovasc Res. 2012;9(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 44. Nikolakopoulou AM, Montagne A, Kisler K, et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci. 2019;22(7):1089‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang J, Vitery MDC, Chen J, Osei‐Owusu J, Chu J, Qiu Z. Glutamate‐releasing SWELL1 channel in astrocytes modulates synaptic transmission and promotes brain damage in stroke. Neuron. 2019;102(4):813‐27.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaneko N, Herranz‐Pérez V, Otsuka T, et al. New neurons use Slit‐Robo signaling to migrate through the glial meshwork and approach a lesion for functional regeneration. Sci Adv. 2018;4(12):eaav0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. von Bartheld CS, Bahney J, Herculano‐Houzel S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J Comp Neurol. 2016;524(18):3865‐3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging. 2008;29(11):1754‐1762. [DOI] [PubMed] [Google Scholar]
- 49. Amantea D, Micieli G, Tassorelli C, et al. Rational modulation of the innate immune system for neuroprotection in ischemic stroke. Front Neurosci. 2015;9:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee GA, Lin T‐N, Chen C‐Y, et al. Interleukin 15 blockade protects the brain from cerebral ischemia‐reperfusion injury. Brain Behav Immun. 2018;73:562‐570. [DOI] [PubMed] [Google Scholar]
- 51. Xu S, Lu J, Shao A, Zhang JH, Zhang J. Glial cells: role of the immune response in ischemic stroke. Front Immunol. 2020;11:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ito M, Komai K, Mise‐Omata S, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565(7738):246‐250. [DOI] [PubMed] [Google Scholar]
- 53. Wen R‐X, Shen H, Huang S‐X, et al. P2Y6 receptor inhibition aggravates ischemic brain injury by reducing microglial phagocytosis. CNS Neurosci Ther. 2020;26(4):416‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu Z‐J, Ran Y‐Y, Qie S‐Y, et al. Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti‐inflammatory phenotype through STAT3 pathway. CNS Neurosci Ther. 2019;25(12):1353‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Q‐S, Ding H‐G, Chen S‐L, et al. Hypertonic saline mediates the NLRP3/IL‐1β signaling axis in microglia to alleviate ischemic blood‐brain barrier permeability by downregulating astrocyte‐derived VEGF in rats. CNS Neurosci Ther. 2020;26(10):1045‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lalancette‐Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27(10):2596‐2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alawieh A, Langley EF, Tomlinson S. Targeted complement inhibition salvages stressed neurons and inhibits neuroinflammation after stroke in mice. Sci Transl Med. 2018;10(441). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Otxoa‐de‐Amezaga A, Miró‐Mur F, Pedragosa J, et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol. 2019;137(2):321‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Al Mamun A, Chauhan A, Qi S, et al. Microglial IRF5‐IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes. Proc Natl Acad Sci U S A. 2020;117(3):1742‐1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Joshi AU, Minhas PS, Liddelow SA, et al. Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat Neurosci. 2019;22(10):1635‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vainchtein ID, Chin G, Cho FS, et al. Astrocyte‐derived interleukin‐33 promotes microglial synapse engulfment and neural circuit development. Science. 2018;359(6381):1269‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24(9):2143‐2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gürer G, Gursoy‐Ozdemir Y, Erdemli E, Can A, Dalkara T. Astrocytes are more resistant to focal cerebral ischemia than neurons and die by a delayed necrosis. Brain Pathol. 2009;19(4):630‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rosenberg PA, Aizenman E. Hundred‐fold increase in neuronal vulnerability to glutamate toxicity in astrocyte‐poor cultures of rat cerebral cortex. Neurosci Lett. 1989;103(2):162‐168. [DOI] [PubMed] [Google Scholar]
- 66. Cai W, Liu H, Zhao J, et al. Pericytes in brain injury and repair after ischemic stroke. Transl Stroke Res. 2017;8(2):107‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ghori A, Freimann FB, Nieminen‐Kelhä M, et al. EphrinB2 activation enhances vascular repair mechanisms and reduces brain swelling after mild cerebral ischemia. Arterioscler Thromb Vasc Biol. 2017;37(5):867‐878. [DOI] [PubMed] [Google Scholar]
- 68. Jean LeBlanc N, Guruswamy R, ElAli A. Vascular endothelial growth factor isoform‐B stimulates neurovascular repair after ischemic stroke by promoting the function of pericytes via vascular endothelial growth factor receptor‐1. Mol Neurobiol. 2018;55(5):3611‐3626. [DOI] [PubMed] [Google Scholar]
- 69. Dias DO, Kim H, Holl D, et al. Reducing pericyte‐derived scarring promotes recovery after spinal cord injury. Cell. 2018;173(1):153‐65.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci U S A. 2006;103(11):4034‐4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li G, Xiao L, Qin H, et al. Exosomes‐carried microRNA‐26b‐5p regulates microglia M1 polarization after cerebral ischemia/reperfusion. Cell Cycle. 2020;19(9):1022‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72. Jiang M, Wang H, Jin M, et al. Exosomes from MiR‐30d‐5p‐ADSCs reverse acute ischemic stroke‐induced, autophagy‐mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem. 2018;47(2):864‐878. [DOI] [PubMed] [Google Scholar]
- 73. Alishahi M, Ghaedrahmati F, Kolagar TA, et al. Long non‐coding RNAs and cell death following ischemic stroke. Metab Brain Dis. 2019;34(5):1243‐1251. [DOI] [PubMed] [Google Scholar]
- 74. Li Z, Li J, Tang N. Long noncoding RNA Malat1 is a potent autophagy inducer protecting brain microvascular endothelial cells against oxygen‐glucose deprivation/reoxygenation‐induced injury by sponging miR‐26b and upregulating ULK2 expression. Neuroscience. 2017;354:1‐10. [DOI] [PubMed] [Google Scholar]
- 75. Wang S, Han X, Mao Z, Xin Y, Maharjan S, Zhang B. MALAT1 lncRNA induces autophagy and protects brain microvascular endothelial cells against oxygen‐glucose deprivation by binding to miR‐200c‐3p and upregulating SIRT1 expression. Neuroscience. 2019;397:116‐126. [DOI] [PubMed] [Google Scholar]
- 76. Guo D, Ma JI, Yan L, et al. Down‐regulation of Lncrna MALAT1 attenuates neuronal cell death through suppressing beclin1‐dependent autophagy by regulating Mir‐30a in cerebral ischemic stroke. Cell Physiol Biochem. 2017;43(1):182‐194. [DOI] [PubMed] [Google Scholar]
- 77. Deng Y, Chen D, Wang L, et al. Silencing of long noncoding RNA nespas aggravates microglial cell death and neuroinflammation in ischemic stroke. Stroke. 2019;50(7):1850‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jeck WR, Sorrentino JA, Wang K, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19(2):141‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang S, Zhang JY, Lu LJ, Wang CH, Wang LH. MiR‐630 promotes epithelial ovarian cancer proliferation and invasion via targeting KLF6. Eur Rev Med Pharmacol Sci. 2017;21(20):4542‐4547. [PubMed] [Google Scholar]
- 80. Chen Q, Liu T, Bao YI, et al. CircRNA cRAPGEF5 inhibits the growth and metastasis of renal cell carcinoma via the miR‐27a‐3p/TXNIP pathway. Cancer Lett. 2020;469:68‐77. [DOI] [PubMed] [Google Scholar]
- 81. Shen Z, Zhou L, Zhang C, Xu J. Reduction of circular RNA Foxo3 promotes prostate cancer progression and chemoresistance to docetaxel. Cancer Lett. 2020;468:88‐101. [DOI] [PubMed] [Google Scholar]
- 82. Han B, Zhang Y, Zhang Y, et al. Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142‐TIPARP: implications for cerebral ischemic stroke. Autophagy. 2018;14(7):1164‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jendelová P, Kubinová Š, Sandvig I, Erceg S, Sandvig A, Syková E. Current developments in cell‐ and biomaterial‐based approaches for stroke repair. Expert Opin Biol Ther. 2016;16(1):43‐56. [DOI] [PubMed] [Google Scholar]
- 84. Gao Q, Li Y, Chopp M. Bone marrow stromal cells increase astrocyte survival via upregulation of phosphoinositide 3‐kinase/threonine protein kinase and mitogen‐activated protein kinase kinase/extracellular signal‐regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult. Neuroscience. 2005;136(1):123‐134. [DOI] [PubMed] [Google Scholar]
- 85. Shen LH, Li Y, Gao Q, Savant‐Bhonsale S, Chopp M. Down‐regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain. Glia. 2008;56(16):1747‐1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xin H, Liu Z, Buller B, et al. MiR‐17‐92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon‐myelin remodeling and motor electrophysiological recovery after stroke. J Cereb Blood Flow Metab. 2020;17‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mendel TA, Clabough EBD, Kao DS, et al. Pericytes derived from adipose‐derived stem cells protect against retinal vasculopathy. PLoS One. 2013;8(5):e65691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang H‐H, Cui Y‐L, Zaorsky NG, et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett. 2016;375(2):349‐359. [DOI] [PubMed] [Google Scholar]
- 89. Greenwood‐Goodwin M, Yang J, Hassanipour M, Larocca D. A novel lineage restricted, pericyte‐like cell line isolated from human embryonic stem cells. Sci Rep. 2016;6:24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ryu B, Sekine H, Homma J, et al. Allogeneic adipose‐derived mesenchymal stem cell sheet that produces neurological improvement with angiogenesis and neurogenesis in a rat stroke model. J Neurosurg. 2019;132(2):442‐455. [DOI] [PubMed] [Google Scholar]
- 91. Chen J, Leak RK, Yang GY. Perspective for stroke and brain injury research: mechanisms and potential therapeutic targets. CNS Neurosci Ther. 2015;21(4):301‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell. 2014;14(2):188‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rosell A, Morancho A, Navarro‐Sobrino M, et al. Factors secreted by endothelial progenitor cells enhance neurorepair responses after cerebral ischemia in mice. PLoS One. 2013;8(9):e73244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Blochet C, Buscemi L, Clément T, Gehri S, Badaut J, Hirt L. Involvement of caveolin‐1 in neurovascular unit remodeling after stroke: Effects on neovascularization and astrogliosis. J Cereb Blood Flow Metab. 2020;40(1):163‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhu R, Sun Z, Li C, Ramakrishna S, Chiu K, He L. Electrical stimulation affects neural stem cell fate and function in vitro. Exp Neurol. 2019;319:112963. [DOI] [PubMed] [Google Scholar]
- 96. Cho YW, Kim DS, Suhito IR, Han DK, Lee T, Kim TH. Enhancing neurogenesis of neural stem cells using homogeneous nanohole pattern‐modified conductive platform. Int J Mol Sci. 2019;21(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhao YH, Liu NW, Ke CC, et al. Combined treatment of sodium ferulate, n‐butylidenephthalide, and ADSCs rehabilitates neurovascular unit in rats after photothrombotic stroke. J Cell Mol Med. 2019;23(1):126‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yamashita T, Kamiya T, Deguchi K, et al. Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain. J Cereb Blood Flow Metab. 2009;29(4):715‐725. [DOI] [PubMed] [Google Scholar]
- 99. He F, Dai R, Zhou X, et al. Protective effect of 4‐Methoxy benzyl alcohol on the neurovascular unit after cerebral ischemia reperfusion injury. Biomed Pharmacother. 2019;118:109260. [DOI] [PubMed] [Google Scholar]
- 100. Tian X, Peng J, Zhong J, et al. β‐Caryophyllene protects in vitro neurovascular unit against oxygen‐glucose deprivation and re‐oxygenation‐induced injury. J Neurochem. 2016;139(5):757‐768. [DOI] [PubMed] [Google Scholar]
- 101. Tachibana M, Ago T, Wakisaka Y, et al. Early reperfusion after brain ischemia has beneficial effects beyond rescuing neurons. Stroke. 2017;48(8):2222‐2230. [DOI] [PubMed] [Google Scholar]
- 102. Zheng W, Watts LT, Holstein DM, et al. Purinergic receptor stimulation reduces cytotoxic edema and brain infarcts in mouse induced by photothrombosis by energizing glial mitochondria. PLoS One. 2010;5(12):e14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Luo C, Yi B, Fan W, et al. Enhanced angiogenesis and astrocyte activation by ecdysterone treatment in a focal cerebral ischemia rat model. Acta Neurochir Suppl. 2011;110(Pt 1):151‐155. [DOI] [PubMed] [Google Scholar]
- 104. Omote Y, Deguchi K, Kono S, et al. Neurovascular protection of cilostazol in stroke‐prone spontaneous hypertensive rats associated with angiogenesis and pericyte proliferation. J Neurosci Res. 2014;92(3):369‐374. [DOI] [PubMed] [Google Scholar]
- 105. Nardai S, Dobolyi A, Pál G, et al. Selegiline promotes NOTCH‐JAGGED signaling in astrocytes of the peri‐infarct region and improves the functional integrity of the neurovascular unit in a rat model of focal ischemia. Restor Neurol Neurosci. 2015;33(1):1‐14. [DOI] [PubMed] [Google Scholar]
- 106. Deguchi K, Liu N, Liu W, et al. Pericyte protection by edaravone after tissue plasminogen activator treatment in rat cerebral ischemia. J Neurosci Res. 2014;92(11):1509‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Špiranec K, Chen W, Werner F, et al. Endothelial C‐type natriuretic peptide acts on pericytes to regulate microcirculatory flow and blood pressure. Circulation. 2018;138(5):494‐508. [DOI] [PubMed] [Google Scholar]
- 108. Lu Z, Zhang D, Cui K, et al. Neuroprotective action of teriflunomide in a mouse model of transient middle cerebral artery occlusion. Neuroscience. 2020;428:228‐241. [DOI] [PubMed] [Google Scholar]
- 109. Alarcon‐Martinez L, Villafranca‐Baughman D, Quintero H, et al. Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature. 2020;585(7823):91‐95. [DOI] [PubMed] [Google Scholar]
- 110. Duz B, Oztas E, Erginay T, Erdogan E, Gonul E. The effect of moderate hypothermia in acute ischemic stroke on pericyte migration: an ultrastructural study. Cryobiology. 2007;55(3):279‐284. [DOI] [PubMed] [Google Scholar]
- 111. Zhao Y, Wei ZZ, Lee JH, et al. Pharmacological hypothermia induced neurovascular protection after severe stroke of transient middle cerebral artery occlusion in mice. Exp Neurol. 2020;325:113133. [DOI] [PubMed] [Google Scholar]
- 112. Zheng Y, Pan C, Chen M, Pei A, Xie L, Zhu S. miR‐29a ameliorates ischemic injury of astrocytes in vitro by targeting the water channel protein aquaporin 4. Oncol Rep. 2019;41(3):1707‐1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Xin H, Wang F, Li Y, et al. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from MicroRNA 133b‐overexpressing multipotent mesenchymal stromal cells. Cell Transplant. 2017;26(2):243‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yuan Y, Zhang Z, Wang Z, Liu J. MiRNA‐27b regulates angiogenesis by targeting AMPK in mouse ischemic stroke model. Neuroscience. 2019;398:12‐22. [DOI] [PubMed] [Google Scholar]
- 115. Pei L, Meng S, Yu W, Wang Q, Song F, Ma L. Inhibition of microRNA‐383 ameliorates injury after focal cerebral ischemia via targeting PPARγ. Cell Physiol Biochem. 2016;39(4):1339‐1346. [DOI] [PubMed] [Google Scholar]
- 116. Sun J, Tao S, Liu L, Guo D, Xia Z, Huang M. miR‐140‐5p regulates angiogenesis following ischemic stroke by targeting VEGFA. Mol Med Rep. 2016;13(5):4499‐4505. [DOI] [PubMed] [Google Scholar]
- 117. Caballero‐Garrido E, Pena‐Philippides JC, Lordkipanidze T, et al. In vivo inhibition of miR‐155 promotes recovery after experimental mouse stroke. J Neurosci. 2015;35(36):12446‐12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Li Y, Mao L, Gao Y, Baral S, Zhou Y, Hu B. MicroRNA‐107 contributes to post‐stroke angiogenesis by targeting Dicer‐1. Sci Rep. 2015;5:13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cui H, Yang A, Zhou H, et al. Thrombin‐induced miRNA‐24‐1‐5p upregulation promotes angiogenesis by targeting prolyl hydroxylase domain 1 in intracerebral hemorrhagic rats. J Neurosurg. 2020:1‐12. [DOI] [PubMed] [Google Scholar]
- 120. Gu Y, Ampofo E, Menger MD, Laschke MW. miR‐191 suppresses angiogenesis by activation of NF‐κB signaling. Faseb j. 2017;31(8):3321‐3333. [DOI] [PubMed] [Google Scholar]
- 121. Zhu J, Yao K, Wang Q, et al. Circulating miR‐181a as a potential novel biomarker for diagnosis of acute myocardial infarction. Cell Physiol Biochem. 2016;40(6):1591‐1602. [DOI] [PubMed] [Google Scholar]
- 122. Pan J, Qu M, Li Y, et al. MicroRNA‐126‐3p/‐5p overexpression attenuates blood‐brain barrier disruption in a mouse model of middle cerebral artery occlusion. Stroke. 2020;51(2):619‐627. [DOI] [PubMed] [Google Scholar]
- 123. Qu S, Yang L, Liu Z. MicroRNA‐194 reduces inflammatory response and human dermal microvascular endothelial cells permeability through suppression of TGF‐β/SMAD pathway by inhibiting THBS1 in chronic idiopathic urticaria. J Cell Biochem. 2020;121(1):111‐124. [DOI] [PubMed] [Google Scholar]
- 124. Li P, Shen M, Gao F, et al. An antagomir to microRNA‐106b‐5p ameliorates cerebral ischemia and reperfusion injury in rats via inhibiting apoptosis and oxidative stress. Mol Neurobiol. 2017;54(4):2901‐2921. [DOI] [PubMed] [Google Scholar]
- 125. Teertam SK, Jha S, Prakash BP. Up‐regulation of Sirt1/miR‐149‐5p signaling may play a role in resveratrol induced protection against ischemia via p53 in rat brain. J Clin Neurosci. 2020;72:402‐411. [DOI] [PubMed] [Google Scholar]
- 126. Yao S, Tang B, Li G, Fan R, Cao F. miR‐455 inhibits neuronal cell death by targeting TRAF3 in cerebral ischemic stroke. Neuropsychiatr Dis Treat. 2016;12:3083‐3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mo J‐L, Pan Z‐G, Chen X, et al. MicroRNA‐365 knockdown prevents ischemic neuronal injury by activating oxidation resistance 1‐mediated antioxidant signals. Neurosci Bull. 2019;35(5):815‐825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ge XL, Wang JL, Liu X, Zhang J, Liu C, Guo L. Inhibition of miR‐19a protects neurons against ischemic stroke through modulating glucose metabolism and neuronal apoptosis. Cell Mol Biol Lett. 2019;24:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hamzei Taj S, Kho W, Riou A, Wiedermann D, Hoehn M. MiRNA‐124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials. 2016;91:151‐165. [DOI] [PubMed] [Google Scholar]
- 130. Li ZF, Shu XJ, Chang YW, Liu SY, Wang WH. Effect of lncRNA H19 on the apoptosis of vascular endothelial cells in arteriosclerosis obliterans via the NF‐κB pathway. Eur Rev Med Pharmacol Sci. 2019;23(10):4491‐4497. [DOI] [PubMed] [Google Scholar]
- 131. Wang M, Li C, Zhang Y, Zhou X, Liu Y, Lu C. LncRNA MEG3‐derived miR‐361‐5p regulate vascular smooth muscle cells proliferation and apoptosis by targeting ABCA1. Am J Transl Res. 2019;11(6):3600‐3609. [PMC free article] [PubMed] [Google Scholar]
- 132. Li H, Liu X, Zhang L, Li X. LncRNA BANCR facilitates vascular smooth muscle cell proliferation and migration through JNK pathway. Oncotarget. 2017;8(70):114568‐114575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Liu C, Ge H‐M, Liu B‐H, et al. Targeting pericyte‐endothelial cell crosstalk by circular RNA‐cPWWP2A inhibition aggravates diabetes‐induced microvascular dysfunction. Proc Natl Acad Sci U S A. 2019;116(15):7455‐7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


