Abstract
Aims
The canonical Wnt signaling pathway plays an essential role in blood‐brain barrier integrity and intracerebral hemorrhage in preclinical stroke models. Here, we sought to explore the association between canonical Wnt signaling and hemorrhagic transformation (HT) following intravenous thrombolysis (IVT) in acute ischemic stroke (AIS) patients as well as to determine the underlying cellular mechanisms.
Methods
355 consecutive AIS patients receiving IVT were included. Blood samples were collected on admission, and HT was detected at 24 hours after IVT. 117 single‐nucleotide polymorphisms (SNPs) of 28 Wnt signaling genes and exon sequences of 4 core cerebrovascular Wnt signaling components (GPR124, RECK, FZD4, and CTNNB1) were determined using a customized sequencing chip. The impact of identified genetic variants was further studied in HEK 293T cells using cellular and biochemical assays.
Results
During the study period, 80 patients experienced HT with 27 parenchymal hematoma (PH). Compared to the non‐PH patients, WNT7A SNPs (rs2163910, P = .001, OR 2.727; rs1124480, P = .002, OR 2.404) and GPR124 SNPs (rs61738775, P = .012, OR 4.883; rs146016051, P < .001, OR 7.607; rs75336000, P = .044, OR 2.503) were selectively enriched in the PH patients. Interestingly, a missense variant of GPR124 (rs75336000, c.3587G>A) identified in the PH patients resulted in a single amino acid alteration (p.Cys1196Tyr) in the intracellular domain of GPR124. This variant substantially reduced the activity of WNT7B‐induced canonical Wnt signaling by decreasing the ability of GPR124 to recruit cytoplasmic DVL1 to the cellular membrane.
Conclusion
Variants of WNT7A and GPR124 are associated with increased risk of PH in patients with AIS after intravenous thrombolysis, likely through regulating the activity of canonical Wnt signaling.
Keywords: blood‐brain barrier, intracerebral hemorrhage, ischemic stroke, signaling pathway, single‐nucleotide polymorphism
1. INTRODUCTION
Approximately 80% of strokes are caused by thrombotic occlusion of brain arteries with subsequent cerebral ischemia. 1 , 2 The only pharmacological intervention approved by the US Food and Drug Administration (FDA) for acute ischemic stroke (AIS) is the thrombolytic agent recombinant tissue‐type plasminogen activator (rt‐PA). 3 , 4 , 5 , 6 However, rt‐PA treatment significantly increases the risk of hemorrhagic transformation (HT). 7 , 8 , 9 , 10 As a major complication of thrombolytic therapy, HT especially the parenchymal hematoma (PH) is associated with significantly increased stroke morbidity and mortality. 7 , 11 Currently, the risk factors underlying HT pathogenesis remain unclear and clinically amenable biomarkers are urgently needed to better predict the risk and severity of HT.
Brain endothelial cells (BECs) forming the barrier between brain tissue and peripheral blood circulation are called as blood‐brain barrier (BBB) that regulates the molecule diffusion between the blood vessels and brain parenchyma. 12 , 13 BECs are the main component of BBB but pericytes, astrocytes, and extracellular basement matrix are also required to BBB function and integrity, which collectively are called neurovascular unit (NVU). 12 , 14 , 15 BECs possess firm intercellular tight junctions (TJs) and a low level of transcytosis, which limit both paracellular and transcellular pathways to transport most substances from blood to the brain. 16 BBB disruption has been shown to occur in the early reperfusion phase following intravenous thrombolysis and is closely associated with subsequent HT. 17 , 18 , 19 , 20 Animal and human studies indicate that BBB injury occurs within 1‐2 hours after onset of reperfusion and becomes more severe at 12‐24 hours post‐reperfusion, preceding the appearance of HT. 20 , 21 , 22 Re‐opening of the occluded arteries by rt‐PA or rt‐PA combined with endovascular therapy induces reperfusion injury to microvessels through production of reactive oxygen species (ROS), matrix metalloproteinases (MMPs), as well as other factors from blood and brain cells, which together disrupt the neurovascular unit and cause BBB injury. 7 , 23 , 24 , 25 , 26 In addition, rt‐PA may act directly upon endothelial cells, astrocytes, or neutrophils, leading to the activation of PDGF‐CC and MMP‐2/3/9, and thus disrupting BBB function. 18 , 27 , 28 , 29
We and others have found that canonical Wnt signaling (Wnt/β‐catenin signaling) pathway in brain endothelium is essential to BBB formation and integrity during embryonic development and adulthood in animals. Genetic ablations of canonical Wnt signaling components in mice including the Wnt ligand Wnt7a/7b and agonist Norrin, 30 , 31 , 32 , 33 receptor/co‐receptors Fzd4, Lrp5/6, Gpr124, or Reck, 31 , 41 or effector Ctnnb1 (β‐catenin), 30 , 33 , 39 , 42 , 43 compromises BBB formation or integrity, accompanied by widespread or focal intracerebral hemorrhage (ICH). Recently, we demonstrated that following acute cerebral ischemia/reperfusion injury in mice, decreased canonical Wnt signaling activity in Gpr124 knockout brain endothelium led to exacerbation of BBB disruption and severe ICH, resembling severe PH in human strokes. 21 Furthermore, canonical Wnt signaling activity regulated rt‐PA‐induced HT in rodent ischemic stroke models. 44 , 45 , 46 Nevertheless, the impact of canonical Wnt signaling pathway on human BBB integrity and HT after ischemic stroke remains completely unexplored. In the current study, we aimed to identify the association of the canonical Wnt signaling with risk of HT in AIS patients by assessing sequence variations in Wnt signaling genes and determine their effects on the activity of cellular Wnt signaling.
2. METHODS
The de‐identified participant data generated and analyzed in the current study will be available and shared at request of other investigators for purposes of replicating procedures and results.
2.1. Standard protocol approvals and patient consents
Written informed consent was obtained from each patient included in the study prior to enrollment. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and has been approved by the Ethics Committee of the First Hospital of Jilin University prior to initiation of the study.
2.2. Participants
From June 2015 through May 2018, consecutive AIS patients in the First Hospital of Jilin University receiving intravenous thrombolysis were screened for eligibility and enrolled. Enrollment criteria were as follows: (a) Blood samples were collected at admission prior to rt‐PA administration; (b) CT scans performed both at admission and 24 hours after thrombolysis. Under these selection criteria, 355 thrombolysis patients were included in this study. Due to the lack of previous studies identifying the frequencies of the Wnt signaling genetic variants in ischemic stroke patients, we were not able to perform power analysis to estimate the approximate number of patients needed before the study began. Therefore, we performed an interim analysis using the data of the first group of patients recruited during the study and estimated the total patient number required (Tables S1 and S2). Furthermore, a power analysis was performed after study completion.
2.3. Thrombolytic procedure
Acute ischemic stroke patients were considered for the use of rt‐PA thrombolytic therapy, with the following conditions: (a) age > 18 years; (b) presentation within 4.5 hr of symptom onset; (c) ruled out for intracranial and subarachnoid hemorrhage. Clinical examination, blood and coagulation tests, 12‐lead electrocardiogram (ECG), fingertip blood sugar, and blood biochemistry test were performed at admission prior to rt‐PA administration. Rt‐PA was administered according to the relevant institutional protocols and international recommendations (0.9 mg/kg of estimated or measured body weight, 10% as a bolus and 90% as an infusion over a period of 60 minutes, and a maximal dose of 90 mg). Stroke severity was assessed using National Institute of Health Stroke Scale (NIHSS) score by certified vascular neurologists at admission and at 1, 3, and 7 days post‐rt‐PA administration. We recorded relevant medical history including hypertension, atrial fibrillation, and history of taking antiplatelet drugs. All patients were admitted to an acute stroke unit and monitored continuously.
2.4. Hemorrhagic transformation detection
All thrombolytic patients received CT scans at 24 hr after thrombolysis. The CT scan results were evaluated by a professional neurologist blinded to the clinical characteristics of the stroke and its progress. Hemorrhagic transformation was classified according to the European Cooperative Acute Stroke Study I (ECASS I), 11 including (a) no hemorrhagic transformation; (b) hemorrhagic infarction 1 (HI1): scattered small petechiae, no mass effect; (c) hemorrhagic infarction 2 (HI2): more confluent petechiae, no mass effect; (d) parenchymal hematoma 1 (PH1): hematoma within infarcted tissue, occupying <30% of the infarcted area, mild mass effect; and (e) parenchymal hematoma 2 (PH2): hematoma occupying >30% of the infarcted tissue, with significant mass effect.
2.5. Blood collection and DNA extraction
Venous blood samples were collected at admission from patients prior to rt‐PA administration as a standard of care. After collection, samples were centrifuged at 4°C, 1811 g, for 10 minutes. Serum and leukocytes were immediately frozen and stored at −80°C until further the analyses were performed. Genomic DNA of isolated leukocytes was extracted with the GOMag Blood DNA Kit from GeneOn BioTech (Germany, Cat. #GO‐HYAS) following the manufacturer's instructions.
2.6. Detection of single‐nucleotide polymorphisms and gene sequence variations
In total, 117 single‐nucleotide polymorphisms (SNPs) and genetic variants of 28 Wnt signaling pathway‐related genes were reported by previous studies and thus summarized after searching the genome‐wide association studies (GWAS) Catalog of the European Bioinformatics Institute (https://www.ebi.ac.uk/gwas/) (Figure 1). As the sequencing probes were ~100 bp long, additional SNPs that were proximal to the identified SNPs in location in the DNA sequences were also detected. Full‐length exon sequencing for GPR124, RECK, FZD4, and CTNNB1 (gene for β‐CATENIN) was performed additionally to identify any potential sequence variations or mutations not reported previously. Please see Appendix S1 for detailed protocols.
Figure 1.
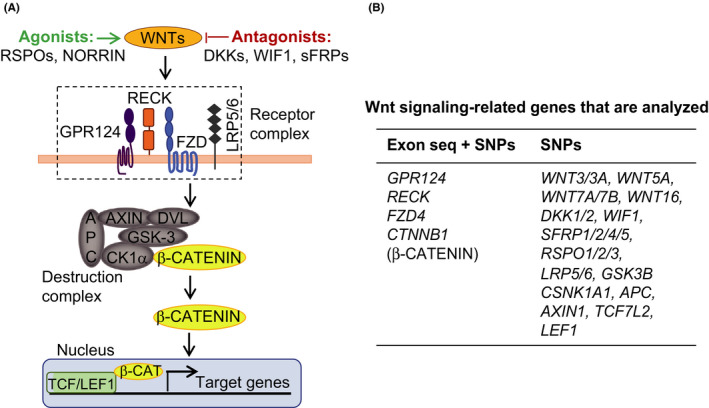
Schematic diagram of the study design. A, Schematic illustration of the canonical Wnt signaling in brain endothelium. B, Summary of Wnt signaling‐related genes assessed in this study. Either both exon sequence variations and known SNPs (left column) or only known SNPs (right column) were determined and analyzed
2.7. Plasmid construction, Western blotting, immunofluorescence, immunoprecipitation, and TOP‐Flash assays
Wild‐type (WT) GPR124, GPR124 with a single‐nucleotide mutation (c.3587G>A, ie, p.Cys1196Tyr), and GPR124 with an intercellular domain deletion (GPR124 △ICD) were cloned into the pcDNA3.1 vector with an N‐terminal 3xFLAG. DVL1/2/3, WNT7B, and FZD4 were cloned into the pcDNA3.1 vector with C‐terminal tags (6xHis tag, HA tag, and Myc tag, respectively). Plasmids were overexpressed in HEK 293T cells. Protein expression was determined by Western blotting, and subcellular location was determined by confocal immunofluorescence staining. Protein interaction between GPR124 and DVL was determined by co‐immunoprecipitation (co‐IP) or co‐immunofluorescence staining (co‐IF). Wnt signaling activity was determined by Axin2 qRT‐PCR or β‐CATENIN‐dependent luciferase transcription assay (TOP‐Flash). Please see Appendix S1 for detailed protocols.
2.8. Statistical analysis
Patient data were analyzed using the Statistical Package for the Social Sciences, version 22.0 (SPSS, IBM Corporation). PLINK1.9 was performed to check principal components analysis (PCA) based on the variance‐standardized relationship matrix for population stratification and Hardy‐Weinberg equilibrium deviation. Single mark association analysis for qualified high‐confidence datasets was performed to compute the odds ratios (ORs) and P‐values in PLINK using Fisher's exact test for dichotomous phenotypes. The continuous variables were presented as mean ± SD or median (interquartile range), depending on the distribution of the variable. The Shapiro‐Wilk test was used to test the normality of the data. The Mann‐Whitney test or Student's t test was used to compare the differences between two independent samples. Comparison of the differences between three independent samples was conducted by Kruskal‐Wallis test. Pairwise comparisons of groups were corrected by the way of Bonferroni correction. Interim analysis to estimate the total patient number and power analysis were done with PASS 11.0. The count data were expressed as the rate (percentage) and analyzed with the chi‐square and Fisher exact tests. All tests were two‐tailed and were considered statistically significant when P < .05 (unless specifically noted).
3. RESULTS
3.1. Patient characteristics and hemorrhagic transformation
During the study period, we consecutively enrolled 355 eligible AIS patients treated with intravenous rt‐PA. Males comprised 74.1% of patients, and the mean age of the patients was 62.88 ± 11.96 years. Median [quartiles] NIHSS score on admission was 9 [5,13], and mean time to treatment from symptoms onset was 188.86 ± 63.16 min. HT was observed in 80 (22.5%) patients, of which there were 53 (14.9%) patients with HI, 27 (7.6%) patients with PH, and 4 (1.1%) patients with symptomatic HT (all PH).
Because only PH but not HI is associated with early neurological deterioration and increased patient mortality, 11 we focused on analyzing the PH versus non‐PH patients. A summary of the demographic and baseline characteristics of these patients is described in Table 1, grouped by PH versus non‐PH.
Table 1.
Demographic and baseline clinical characteristics by parenchymal hematoma (PH) groups
| Factors |
Non‐PH group (n = 328) |
PH group (n = 27) |
χ 2 (t, z) | P |
|---|---|---|---|---|
| Male | 241 (73.5%) | 21 (77.8%) | 0.239 | .661 |
| Age (y) | 62.77 ± 12.01 | 64.00 ± 10.97 | −0.512 | .609 |
| BMI | 24.22 (23.23‐24.69) | 23.87 (22.76‐24.49) | −1.518 | .129 |
| Hypertension | 172 (52.4%) | 15 (55.6%) | 0.097 | .842 |
| Coronary artery disease | 103 (31.4%) | 5 (18.5%) | 1.956 | .195 |
| Atrial fibrillation | 34 (10.4%) | 4 (14.8%) | 0.517 | .512 |
| Antiplatelet therapy | 21 (6.4%) | 4 (14.8%) | .110 | |
| History of stroke | 68 (20.7%) | 4 (14.8%) | 0.540 | .621 |
| Baseline NIHSS | 9 (5‐12) | 10 (5‐15) | −1.178 | .239 |
| Baseline mRS | 4 (3‐4) | 4 (3‐4) | −0.672 | .502 |
| OTT (onset to treatment, min) | 187.13 ± 59.83 | 199.48 ± 62.49 | −1.028 | .305 |
| Platelet count (109/L) | 194.50 (165.00‐229.75) | 201.00 (153.25‐238.25) | −0.177 | .859 |
| INR | 0.94 (0.90‐0.99) | 0.94 (0.91‐0.97) | −0.630 | .529 |
| Baseline left systolic blood pressure (mmHg) | 150.80 ± 21.11 | 154.56 ± 14.79 | −0.905 | .609 |
| Baseline left diastolic blood pressure (mm Hg) | 89.00 (80.00‐97.00) | 88.00 (80.00‐97.00) | −0.330 | .742 |
| Baseline blood glucose (mmol/L) | 7.7 (6.5‐9.4) | 8.9 (6.9‐10.9) | −1.952 | .051 |
| Hyperlipidemia | 26 (7.9%) | 2 (7.4%) | 1.000 | |
| Total cholesterol (mmol/L) | 4.82 (4.19‐5.53) | 4.52 (3.63‐5.76) | −1.262 | .207 |
| LDL (mmol/L) | 2.88 (2.45‐3.48) | 3.20 (2.11‐3.51) | −0.042 | .966 |
| Triglyceride (mmol/L) | 1.35 (0.93‐2.14) | 1.36 (1.05‐1.77) | −0.474 | .635 |
Data were presented as mean ± SD or median (interquartile range).
Abbreviations: BMI, body mass index; HT, hemorrhagic transformation; INR, international normalized ratio; LDL, low‐density lipoprotein; NIHSS, National Institutes of Health Stroke Scale.
3.2. Associations of genetic variations with HT
We thoroughly searched the GWAS Catalog of the European Bioinformatics Institute and identified 117 SNPs after merging the overlapping SNPs for 28 Wnt signaling‐related genes (Figure 1, and full SNP list is available upon request). Additionally, we performed full‐length exon sequencing for GPR124, RECK, FZD4, and CTNNB1 (gene for β‐CATENIN), which represent four core components of the Wnt signaling in the cerebrovasculature, to identify any potential sequence variations or mutations previously unknown to be linked with Wnt signaling‐related phenotypic traits.
Our results showed that compared to the non‐PH patients, five genetic variants belonging to Wnt7A and GPR124 were selectively enriched in the PH patients, including two SNPs of WNT7A (rs2163910, P = .001, odds ratio [OR] 2.727; rs1124480, P = .002, OR 2.404) and three SNPs of GPR124 (rs61738775, P = .012, OR 4.883; rs146016051, P < .001, OR 7.607; rs75336000, P = .044, OR 2.503) (Table 2). Two SNPs of RECK were also found to be enriched in the PH patients but did not reach statistical significance (rs12235235, P = .090, OR 1.882; rs2274522, P = .089, OR 1.936), likely due in part to the limited number of PH patients included in this study, as shown by the interim analysis result during the study (Tables S1 and S2) and power analysis after the study with power values below 0.8 (Table 2). Consistent with the overall benign nature of HI in clinic, we did not identify any genetic variants that were significantly enriched in the HI patients as compared to the non‐HT patients (data not shown).
Table 2.
Association of Wnt signaling genetic variants with HT by PH groups
| Gene name | dbSNP ID | Base change | Variant type or location | Allele Frequency | OR | P‐value | Power | |
|---|---|---|---|---|---|---|---|---|
| Non‐PH (%) | PH (%) | |||||||
| WNT7A | rs2163910 | C>A | 3′‐UTR variant | 36.8 | 61.4 | 2.727 | .0012 | 0.9463 |
| rs1124480 | T>C | 3′‐UTR variant | 23.4 | 42.3 | 2.404 | .0024 | 0.8409 | |
| GPR124 | rs61738775 | G>A | Synonymous variant | 1.2 | 5.8 | 4.883 | .0116 | 0.6488 |
| rs146016051 | G>A | Intron variant | 1.1 | 7.7 | 7.607 | .0002 | 0.8062 | |
| rs75336000 | G>A |
Missense variant (c.3587G>A) |
4.9 | 11.5 | 2.503 | .0441 | 0.5313 | |
| RECK | rs12235235 | G>A | Intron variant | 13.5 | 22.7 | 1.882 | .0903 | 0.4676 |
| rs2274522 | G>A | Intron variant | 11.7 | 20.5 | 1.936 | .0887 | 0.4770 | |
Abbreviations: dbSNP, database single‐nucleotide polymorphism; HT, hemorrhagic transformation; OR, odds ratio; PH, parenchymal hematoma; UTR, untranslated region.
Most of these variants were located in introns or downstream of the gene coding regions, with the exception of a missense variant of GPR124 (rs75336000, c.3587G>A) that is located in exon 19 and yielding an amino acid change (p.Cys1196Tyr) of GPR124 protein. Further analysis showed that 23.1% PH patients carried GPR124 c.3587G>A mutation compared to only 6.8% carriers in non‐PH patients (Figure 2A), suggesting a potential role of this point mutation in the pathogenesis of HT in at least a subset of AIS patients.
Figure 2.
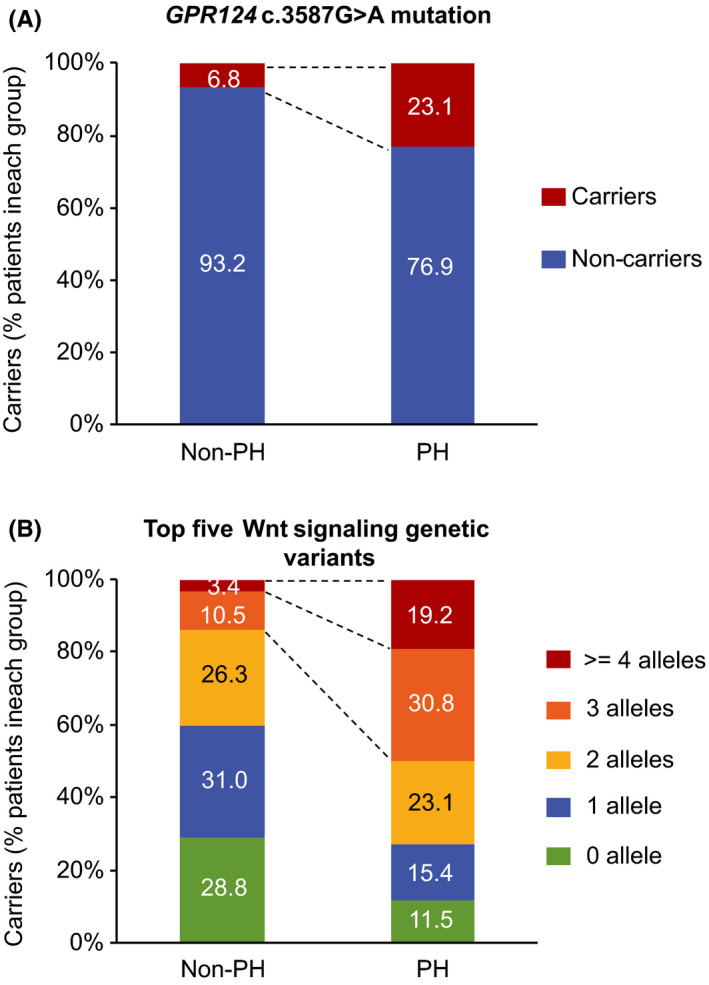
Shift distribution of patient numbers by GPR124 c.3587G>A mutation or by the top five Wnt signaling genetic variants. Number of patients (%) in each group with GPR124 c.3587G>A mutation (A) or with alleles of the top five Wnt signaling genetic variants enriched in the PH patients (WNT7A rs2163910 and rs1124480, GPR124 rs61738775, rs146016051, and rs75336000; 2 alleles/variant, 10 alleles totally) (B) was calculated
In animal genetic studies, Wnt signaling components have been found to act cooperatively or redundantly in the regulation of BBB integrity and ICH. 32 , 39 Therefore, we analyzed the top five genetic variants of Wnt7A and GPR124 genes listed above collectively in order to evaluate their accumulative effects on risk of HT. Compared to the non‐PH group, a significantly higher percentage of PH patients carried either both of the WNT7A variants rs2163910/rs1124480 (63.6% in PH versus 30.7% in non‐PH), or 3 alleles (30.8% in PH versus 10.5% in non‐PH) or >=4 alleles (19.2% in PH versus 3.4% in non‐PH) of the top five Wnt signaling genetic variants (2 alleles/variant, 10 alleles in total; Figure 2B). These data suggest an incremental effect of copy number of these variants on the risk of severe HT.
3.3. GPR124 c.3587G>A mutation reduces Wnt signaling activity by promoting dissociation of cytosolic DVL1 from GPR124
We next examined how the single‐nucleotide mutation (c.3587G>A) of GPR124 affects its protein function and Wnt signaling activity. This nucleotide mutation causes a single amino acid change in the intercellular domain (ICD) of GPR124, that is, p.Cys1196Tyr or C1196Y (Figure 3A). Therefore, we cloned GPR124 wild‐type (WT) and GPR124 C1196Y into pcDNA3.1‐3xFLAG plasmid and overexpressed them in HEK 293T cells. The C1196Y mutation did not change the subcellular location or expression level of GPR124 (Figure 3B‐C). However, this mutation substantially reduced Wnt signaling activity to levels nearly similar to those of empty plasmid control or GPR124 ΔICD, as measured by Wnt target gene Axin2 mRNA levels and β‐catenin‐dependent transcription assay (Figure 3D‐E).
Figure 3.
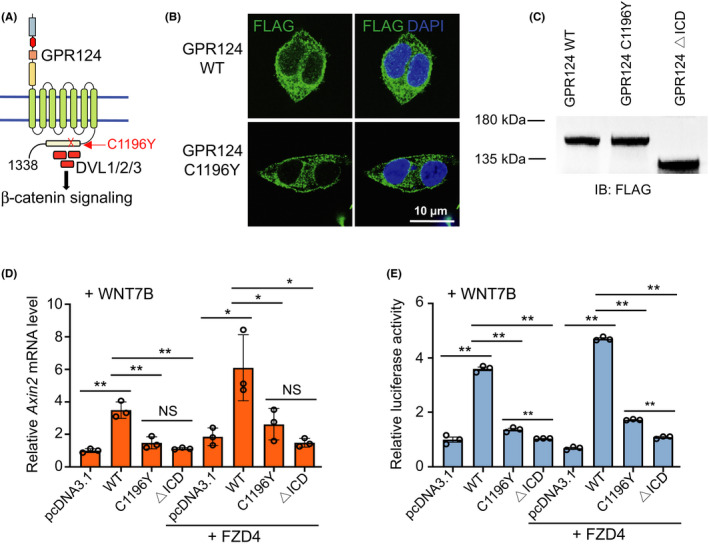
The GPR124 C1196Y mutation reduces Wnt signaling activity. A, Schematic illustration of the C1196Y alteration in the intracellular domain (ICD) of GPR124. B, Wild‐type (WT) GPR124 and GPR124 C1196Y were cloned into pcDNA3.1‐3xFLAG construct and overexpressed in HEK 293T cells. Protein subcellular location was determined by confocal immunofluorescence staining. C, Expression levels of GPR124 WT, GPR124 C1196Y, and GPR124 with ICD deletion (GPR124 △ICD) were determined by Western blotting. D‐E, Wnt signaling activity was measured by AXIN2 qRT‐PCR (D) and β‐catenin‐dependent transcription assay TOP‐Flash (E) after transfecting HEK 293T cells with the indicated plasmids. Data are presented as mean ± SE. *P < .05, **P < .01. NS, not significant, Student's t test
The ICD of GPR124 has been shown to potentiate WNT7 signaling by recruiting cytosolic DVL protein to the cellular membrane and thus increase the interaction between DVL and Wnt receptors FZDs and LRP5/6. 47 Our data showed that GPR124 interacted with DVL1 but not DVL2 or DVL3 in human HEK 293T cells (Figure 4A‐B and Figure S1). Furthermore, the C1196Y mutation of GPR124 significantly decreased the interaction between GPR124 and DVL1, and largely abolished the membrane recruitment of cytosolic DVL1 by GPR124 ICD (Figure 4A‐C). Lastly, we constructed a series of truncated DVL1 mutants and performed co‐IP assays to map the binding sites of DVL1 with GPR124 (Figure 4D). Our data showed that the interaction between DVL1 and GPR124 involved all the three domains (DIX, PDZ, and DEP) of DVL1, wherein the PDZ domain played a more prominent role than the DIX or DEP domain (Figure 4E‐F).
Figure 4.

The GPR124 C1196Y mutation decreases the interaction between DVL1 and GPR124. A, 3xFLAG‐GPR124 WT or 3xFLAG‐GPR124 C1196Y was co‐expressed with DVL1‐His in HEK 293T cells. GPR124 was precipitated with anti‐FLAG antibody, and DVL1 protein bound to GPR124 was detected with anti‐6xHis antibody. This experiment was repeated three times and quantified in (B). Data are presented as mean ± SE. ***P < .01, Student's t test. C, Interaction between GPR124 (WT or C1196Y) and DVL1 was determined by co‐immunofluorescence staining (co‐IF) in HEK 293T cells. D‐F, A series of truncated DVL1 mutants were constructed, and co‐IP was performed to map the binding sites of DVL1 with GPR124 protein. Experiments were repeated three times, and representative results were presented. G, Proposed working model
4. DISCUSSION
In this study, we have extensively examined the association of genetic variants of canonical Wnt signaling pathway with the risk of HT following rt‐PA‐mediated thrombolysis therapy in a cohort of Chinese AIS patients. We identified that several genetic variants of WNT7A and GPR124 are associated with risk of parenchymal hematoma (PH), the clinically detrimental form of HT. We further demonstrated the mechanisms of how these genetic variants may affect cellular canonical Wnt signaling activity, focusing on the GPR124 c.3587G>A mutation. To the best of our knowledge, this is the first report of an association of Wnt signaling pathway variants with human BBB integrity and intracerebral hemorrhage after ischemic stroke, setting an effective example for translating findings of animal studies to human clinical practice.
Previous studies have shown that early HT following a cerebral infarct occurs in as many as 10% to 40% of patients receiving rt‐PA with or without endovascular therapy. 8 , 48 Patients with PH present significantly worse neurological outcomes and a higher mortality rate. 8 , 11 However, the pathogenesis of HT remains unclear and no reliable indicators can predict HT occurrence, which have been profoundly limiting the application of rt‐PA to AIS patients. BBB disruption is reported numerously by many groups following reperfusion therapy in both animal stroke models and AIS patients and is shown to be a major contributing factor of HT. 7 , 17 , 18 , 19 , 20 , 23 , 49 The essential role of cerebrovascular Wnt/β‐catenin signaling in BBB formation and integrity has been well documented by us and others in animal studies. Impaired Wnt/β‐catenin signaling in mouse embryos led to severe BBB disruption and intracerebral hemorrhage by altering expressions of BBB markers Glut‐1, Claudin‐5, and Plvap. 30 , 33 , 34 , 39 , 43 In adult mice, we recently reported that impaired Wnt/β‐catenin signaling adversely impacted BBB integrity was unveiled during cerebral ischemia/reperfusion injury and glioma angiogenesis. 21 , 50 A previous study suggested that disruption of the nuclear localization of endothelial β‐catenin was associated with hemorrhagic lesions in hemorrhagic stroke patients. 42 However, evidence supporting a role for cerebrovascular Wnt signaling in human BBB function and intracerebral hemorrhage during ischemic stroke has been lacking. Our results demonstrated that WNT7A and GPR124 variants were significantly associated with the risk of HT following thrombolytic reperfusion therapy in AIS patients, suggesting that they may be involved in the pathogenesis of HT in humans. Of note, GPR124 variants have a low allele frequency; thus, these variants likely contributed to HT in a subset of AIS patients. Overall, our study is the first to reveal a potential role for the cerebrovascular Wnt signaling in BBB disruption and HT in at least a subset of ischemic stroke patients.
WNT7A and WNT7B are two major WNT ligands expressed in mouse brain and act to maintain the Wnt/β‐catenin signaling in brain endothelium. 30 , 33 In addition, the adhesion G protein‐coupled receptor GPR124 and the membrane‐anchored glycoprotein RECK function cooperatively to specifically potentiate cerebrovascular WNT7 signaling by presenting WNT7 to the FZD receptor extracellularly and recruiting cytosolic DVL to the cell membrane intracellularly. 47 , 51 In mouse embryos, double knockouts (but not single knockouts) of Wnt7a/Wnt7b exhibit CNS‐wide parenchymal hemorrhage, 30 while knockout of either Gpr124 or Reck results in forebrain and spinal cord‐restricted hemorrhage. 34 , 41 Our sequencing data in AIS patients suggest that WNT7 and particularly GPR124 may also play a role in human BBB function regulation. In this study, two variants of WNT7A within the 3′ untranslated region (3′‐UTR variants) were identified to be associated with PH. These two variants may affect the expression levels of WNT7A by regulating its mRNA localization, stability, and translation, 52 which warrant further studies. In our previous study, we have demonstrated a key role of GPR124 in the occurrence of intracerebral hemorrhage following cerebral ischemia/reperfusion injury in mice. 21 Notably, here we identify a missense variant of GPR124 gene (c.3587G>A) that results in a single amino acid change (C1196Y) in the ICD of GPR124 protein, and this variation is significantly enriched in PH patients. Using cellular and biochemical assays, we show that the C1196Y mutation significantly decreases the interaction between GPR124 and DVL1, then almost abolishes the membrane recruitment of cytosolic DVL1 by GPR124, and eventually reduces downstream β‐catenin signaling (Figure 4G). Lastly, we found a copy number incremental effect of different Wnt genetic variants on the risk of HT, suggesting that combined analysis of multiple Wnt signaling genetic variants may better predict the risk of severe PH following thrombolysis therapy in AIS patients.
We acknowledge that there are limitations in this study. First, the study was performed in a single hospital and all patients were Chinese, but doing so may help to ensure the consistency of rt‐PA treatment and diagnosis of HT following thrombolysis and reduce the influence of genetic background differences. Second, the sample size was relatively small, which was due to the low number of AIS patients eligible for rt‐PA therapy in clinic and low occurrence rate of PH in AIS patients. Studies with a larger number of AIS patients from multiple hospitals are needed to further validate our findings. Third, the Wnt SNPs examined in this study were identified using genomic DNA from peripheral blood leukocytes; whether WNT7A and GPR124 are also expressed in these cells and affect HT remains to be determined.
In summary, our findings suggest a role of the canonical Wnt signaling pathway in rt‐PA‐mediated BBB disruption and HT in a subset of AIS patients. The biomarkers reported here may be used in conjunction with other biomarkers reported previously to assist in identifying AIS patients at higher risk for HT and guide the thrombolysis treatment and care of these patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
This study was supported by the National Key R&D Program of China (2016YFC1301600 to Y. Y.), National Natural Science Foundation of China (81771243 to Y. Y., 81771293 to J. C.), Science and Technology Innovation Commission of Shenzhen Municipality (JCYJ20170413165705083, ZDSYS20190902093409851, SGLH20180625142404672), SIAT‐GHMSCB Biomedical Laboratory for Major Diseases, Guangdong Innovation Platform of Translational Research for Cerebrovascular Diseases, and Dongguan Introduction Program of Leading Innovative and Entrepreneurial Talents (to Z. L.). The funders were not involved in study design, data collection, data analysis, manuscript preparation, and/or publication decisions.
Ta S, Rong X, Guo Z‐N, et al. Variants of WNT7A and GPR124 are associated with hemorrhagic transformation following intravenous thrombolysis in ischemic stroke. CNS Neurosci. Ther. 2021;27:71–81. 10.1111/cns.13457
Ta and Rong are contributed equally to this work.
Contributor Information
Yi Yang, Email: doctoryangyi@163.com, Email: yang_yi@jlu.edu.cn.
Junlei Chang, Email: jl.chang@siat.ac.cn.
DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.
REFERENCES
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics‐2017 update: a report from the american heart association. Circulation. 2017;135(10):e146‐e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu S, Wu BO, Liu M, et al. Stroke in china: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394‐405. [DOI] [PubMed] [Google Scholar]
- 3. National Institute of Neurological D, Stroke rt PASSG . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581‐1587. [DOI] [PubMed] [Google Scholar]
- 4. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317‐1329. [DOI] [PubMed] [Google Scholar]
- 5. Zhou Z, Lu J, Liu WW, et al. Advances in stroke pharmacology. Pharmacol Ther. 2018;19123‐19142. [DOI] [PubMed] [Google Scholar]
- 6. Rocha M, Jadhav AP, Jovin TG. Endovascular therapy for large vessel occlusion stroke: An update on the most recent clinical trials. J Cereb Blood Flow Metab. 2019;39(9):1661‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jickling GC, Liu DaZhi, Stamova B, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34(2):185‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khatri P, Wechsler LR, Broderick JP. Intracranial hemorrhage associated with revascularization therapies. Stroke. 2007;38(2):431‐440. [DOI] [PubMed] [Google Scholar]
- 9. Shi L, Rocha M, Leak RK, et al. A new era for stroke therapy: Integrating neurovascular protection with optimal reperfusion. J Cereb Blood Flow Metab. 2018271678X18798162. 2018;38(12):2073–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pang J, Zhang JH, Jiang Y. Delayed recanalization in acute ischemic stroke patients: Late is better than never? J Cereb Blood Flow Metab. 2019;39(12):2536‐2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3‐month outcome in the European cooperative acute stroke study i (ecass i) cohort. Stroke. 1999;30(11):2280‐2284. [DOI] [PubMed] [Google Scholar]
- 12. Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood‐brain barrier. Cell. 2015;163(5):1064‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liebner S, Dijkhuizen RM, Reiss Y, et al. Functional morphology of the blood‐brain barrier in health and disease. Acta Neuropathol. 2018;135(3):311‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng Z, Chopp M, Chen J. Multifaceted roles of pericytes in central nervous system homeostasis and disease. J Cereb Blood Flow Metab. 2020;40(7):1381‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yao Y. Basement membrane and stroke. J Cereb Blood Flow Metab. 2019;39(1):3‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chow BW, Gu C. The molecular constituents of the blood‐brain barrier. Trends Neurosci. 2015;38(10):598‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desilles J‐P, Rouchaud A, Labreuche J, et al. Blood‐brain barrier disruption is associated with increased mortality after endovascular therapy. Neurology. 2013;80(9):844‐851. [DOI] [PubMed] [Google Scholar]
- 18. Yepes M, Sandkvist M, Moore EG, et al. Tissue‐type plasminogen activator induces opening of the blood‐brain barrier via the ldl receptor‐related protein. J Clin Invest. 2003;112(10):1533‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood‐brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56(4):468‐477. [DOI] [PubMed] [Google Scholar]
- 20. Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood‐brain barrier disruption. Stroke. 2004;35(11 Suppl 1):2659‐2661. [DOI] [PubMed] [Google Scholar]
- 21. Chang J, Mancuso MR, Maier C, et al. Gpr124 is essential for blood‐brain barrier integrity in central nervous system disease. Nat Med. 2017;23(4):450‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandoval KE, Witt KA. Blood‐brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32(2):200‐219. [DOI] [PubMed] [Google Scholar]
- 23. Suzuki Y, Nagai N, Umemura K. A review of the mechanisms of blood‐brain barrier permeability by tissue‐type plasminogen activator treatment for cerebral ischemia. Front Cell Neurosci. 2016;102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Savitz SI, Baron JC, Yenari MA, Sanossian N, Fisher M. Reconsidering neuroprotection in the reperfusion era. Stroke. 2017;48(12):3413‐3419. [DOI] [PubMed] [Google Scholar]
- 25. Filchenko I, Blochet C, Buscemi L, et al. Caveolin‐1 regulates perivascular aquaporin‐4 expression after cerebral ischemia. Front Cell Dev Biol. 2020;8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen J, Xu G, Zhu R, et al. Pdgfr‐beta restores blood‐brain barrier functions in a mouse model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2019;39(8):1501‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng T, Petraglia AL, Li Z, et al. Activated protein c inhibits tissue plasminogen activator‐induced brain hemorrhage. Nat Med. 2006;12(11):1278‐1285. [DOI] [PubMed] [Google Scholar]
- 28. Su EJ, Fredriksson L, Geyer M, et al. Activation of pdgf‐cc by tissue plasminogen activator impairs blood‐brain barrier integrity during ischemic stroke. Nat Med. 2008;14(7):731‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cuadrado E, Ortega L, Hernández‐Guillamon M, et al. Tissue plasminogen activator (t‐pa) promotes neutrophil degranulation and mmp‐9 release. J Leukoc Biol. 2008;84(1):207‐214. [DOI] [PubMed] [Google Scholar]
- 30. Stenman JM, Rajagopal J, Carroll TJ, et al. Canonical wnt signaling regulates organ‐specific assembly and differentiation of cns vasculature. Science. 2008;322(5905):1247‐1250. [DOI] [PubMed] [Google Scholar]
- 31. Wang Y, Rattner A, Zhou Y, et al. Norrin/frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151(6):1332‐1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Y, Cho C, Williams J, et al. Interplay of the norrin and wnt7a/wnt7b signaling systems in blood‐brain barrier and blood‐retina barrier development and maintenance. Proc Natl Acad Sci U S A. 2018;115(50):E11827‐E11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Daneman R, Agalliu D, Zhou L, et al. Wnt/beta‐catenin signaling is required for cns, but not non‐cns, angiogenesis. Proc Natl Acad Sci U S A. 2009;106(2):641‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuhnert F, Mancuso MR, Shamloo A, et al. Essential regulation of cns angiogenesis by the orphan g protein‐coupled receptor gpr124. Science. 2010;330(6006):985‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cullen M, Elzarrad MK, Seaman S, et al. Gpr124, an orphan g protein‐coupled receptor, is required for cns‐specific vascularization and establishment of the blood‐brain barrier. Proc Natl Acad Sci U S A. 2011;108(14):5759‐5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson KD, Pan L, Yang X‐M, et al. Angiogenic sprouting into neural tissue requires gpr124, an orphan g protein‐coupled receptor. Proc Natl Acad Sci U S A. 2011;108(7):2807‐2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou Y, Nathans J. Gpr124 controls cns angiogenesis and blood‐brain barrier integrity by promoting ligand‐specific canonical wnt signaling. Dev Cell. 2014;31(2):248‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Posokhova E, Shukla A, Seaman S, et al. Gpr124 functions as a wnt7‐specific coactivator of canonical beta‐catenin signaling. Cell Rep. 2015;10(2):123‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou Y, Wang Y, Tischfield M, et al. Canonical wnt signaling components in vascular development and barrier formation. J Clin Invest. 2014;124(9):3825‐3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vanhollebeke B, Stone OA, Bostaille N, et al. Tip cell‐specific requirement for an atypical gpr124‐ and reck‐dependent wnt/beta‐catenin pathway during brain angiogenesis. Elife. 2015;4:e06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cho C, Smallwood PM, Nathans J. Reck and gpr124 are essential receptor cofactors for wnt7a/wnt7b‐specific signaling in mammalian cns angiogenesis and blood‐brain barrier regulation. Neuron. 2017;95(5):1056‐1073e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tran KA, Zhang X, Predescu D, et al. Endothelial beta‐catenin signaling is required for maintaining adult blood‐brain barrier integrity and central nervous system homeostasis. Circulation. 2016;133(2):177‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liebner S, Corada M, Bangsow T, et al. Wnt/beta‐catenin signaling controls development of the blood‐brain barrier. J Cell Biol. 2008;183(3):409‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang T, Duan YM, Fu Q, et al. Im‐12 activates the wnt‐beta‐catenin signaling pathway and attenuates rtpa‐induced hemorrhagic transformation in rats after acute ischemic stroke. Biochem Cell Biol. 2019;97(6):702‐708. [DOI] [PubMed] [Google Scholar]
- 45. Jean LeBlanc N, Menet R, Picard K, et al. Canonical wnt pathway maintains blood‐brain barrier integrity upon ischemic stroke and its activation ameliorates tissue plasminogen activator therapy. Mol Neurobiol. 2019;56(9):6521‐6538. [DOI] [PubMed] [Google Scholar]
- 46. Wang W, Li M, Wang Y, et al. Gsk‐3beta inhibitor tws119 attenuates rtpa‐induced hemorrhagic transformation and activates the wnt/beta‐catenin signaling pathway after acute ischemic stroke in rats. Mol Neurobiol. 2016;53(10):7028‐7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eubelen M, Bostaille N, Cabochette P, et al. A molecular mechanism for wnt ligand‐specific signaling. Science. 2018;361(6403):1‐13. [DOI] [PubMed] [Google Scholar]
- 48. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723‐1731. [DOI] [PubMed] [Google Scholar]
- 49. Anfray A, Drieu A, Hingot V, et al. Circulating tpa contributes to neurovascular coupling by a mechanism involving the endothelial nmda receptors. J Cereb Blood Flow Metab. 2019. 10.1177/0271678X19883599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reis M, Czupalla CJ, Ziegler N, et al. Endothelial wnt/beta‐catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing pdgf‐b expression. J Exp Med. 2012;209(9):1611‐1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vallon M, Yuki K, Nguyen TD, et al. A reck‐wnt7 receptor‐ligand interaction enables isoform‐specific regulation of wnt bioavailability. Cell Rep. 2018;25(2):339‐349 e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mayr C. What are 3' utrs doing? Cold Spring Harb Perspect Biol. 2019;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.


