Key Points
Question
Does exposure to electronic cigarettes (e-cigarettes) in salt vs free-base nicotine formulations improve the appeal and sensory experience of vaping?
Findings
In this randomized clinical trial, 119 adult nicotine or tobacco product users rated puffs from e-cigarettes in nicotine salt (benzoic acid added) and nicotine free-base (no benzoic acid) formulations. Salt vs free-base nicotine formulations resulted in statistically significant higher ratings of appeal, sweetness, and smoothness, and lower ratings of bitterness and harshness.
Meaning
In this study, acid additives in e-cigarettes that change nicotine from free base to salt appeared to enhance the appeal and sensory experience of vaping and merit consideration in e-cigarette regulation.
This randomized clinical trial assessed whether controlled exposure to e-cigarette puffs with salt vs free-base nicotine formulations improved the appeal and sensory experience of vaping among adult current nicotine or tobacco product users.
Abstract
Importance
Alkaline free-base nicotine is bitter and a respiratory irritant. High-nicotine electronic cigarette (e-cigarette) products contain acid additives that change nicotine from a free-base to a protonated salt chemical form, which could improve the sensory experience of vaping, particularly among never smokers unaccustomed to inhaling free-base nicotine.
Objective
To determine whether exposure to e-cigarettes with salt vs free-base nicotine formulations improves the appeal and sensory experience of vaping e-cigarettes and whether nicotine formulation effects differ by e-cigarette flavor and ever combustible cigarette smoking status.
Design, Setting, and Participants
Single-visit double-blind within-participant randomized clinical trial was conducted in an academic medical center outpatient clinical research facility in Southern California. Participants were 119 individuals with past 30-day e-cigarette or combustible cigarette use aged 21 years or older recruited from November 2019 to March 2020.
Interventions
Participants self-administered standardized puffs of each 10 differently flavored e-cigarette solutions using a pod-style device. Each flavor was administered in salt (benzoic acid added) and free-base (no benzoic acid) nicotine formulations with commensurate nicotine concentrations (mean, 23.6 mg/mL). The 20 solutions were administered in randomly assigned sequences. Immediately after puffing each solution, participants rated appeal and sensory attributes.
Main Outcomes and Measures
Self-reported appeal (mean of like, dislike [reverse-scored], and willingness to use again ratings) and 4 sensory attributes (sweetness, smoothness, bitterness, and harshness; analyzed individually) on visual analog scales with not at all and extremely anchors (range, 0-100).
Results
Of the 119 participants; 39 (32.8%) were female. The mean (SD) age was 42.1 (14.4) years; 105 (88.2%) were ever combustible cigarette smokers, and 66 (55.5%) were current e-cigarette users. Salt vs free-base nicotine formulations produced higher ratings of appeal (salt vs free-base mean difference effect estimate: b = 12.0; 95% CI, 9.9-14.1; P < .001), sweetness (b = 9.3; 95% CI, 7.1-11.4; P < .001), and smoothness (b = 17.4; 95% CI, 15.2-19.6; P < .001) and lower ratings of bitterness (b = −13.3; 95% CI, −15.4 to −11.2; P < .001) and harshness (b = −21.0; 95% CI, −23.2 to −18.7; P < .001). Nicotine formulation effects largely generalized across different flavors and the smoothness-enhancing and harshness-reducing effects of nicotine salt were stronger in never vs ever cigarette smokers.
Conclusions and Relevance
In this randomized clinical trial of adult current nicotine or tobacco product users, controlled exposure to e-cigarette puffs with salt vs free-base nicotine formulations appeared to increase product appeal and improve the sensory experience of vaping, particularly among never smokers. Regulatory policies limiting acid additives in e-cigarettes might reduce the appeal of high-nicotine e-cigarettes among populations deterred from vaping e-cigarettes that emit harsh aerosol.
Trial Registration
ClinicalTrials.gov Identifier: NCT04399031
Introduction
Electronic cigarettes (e-cigarettes) have evolved over time. Between 2013 and 2018, there was a 6-fold increase in the proportion of total US e-cigarette sales consisting of products with high nicotine concentrations,1 including the JUUL e-cigarette brand, which has been widely used by adolescents and young adults.2,3
Although nicotine has neuropharmacologically mediated reinforcing effects once absorbed into the bloodstream, alkaline free-base nicotine is bitter and irritates the airways.4,5 Before the entry of the JUUL e-cigarette brand into the market, e-cigarettes contained nicotine in its alkaline free-base chemical form, and free-base nicotine products with higher nicotine concentrations produced aerosol that was perceived by users as harsh, bitter, and less appealing4 and were infrequently sold.1 JUUL and other manufacturers of high-nicotine e-cigarettes have begun adding organic acids to their products, which changes nicotine from a free base to a protonated salt.6 It has been hypothesized that e-cigarettes with high nicotine concentrations in salt vs free-base nicotine formulations produce less aversive sensory effects, which might make e-cigarettes easier to inhale, more appealing, and more addictive.7 To date, this hypothesis has gone untested. Evidence that nicotine salt formulations enhance the appeal and sensory qualities of vaping might suggest that new regulations limiting sales of e-cigarettes with acid additives might benefit public health for populations who do not use e-cigarettes to quit smoking.
This trial tested the hypothesis that exposure to e-cigarettes with salt vs free-base nicotine formulations would increase user-reported appeal and improve the sensory attributes of vaping. Additional objectives were to determine the generalizability of the results across different e-cigarette flavors and populations by examining whether nicotine formulation effects differed by flavor and ever combustible cigarette smoking status.
Methods
Participants
Individuals from the Los Angeles area were recruited to participate in a single-visit randomized clinical trial conducted in an academic medical center outpatient research facility in southern California. Inclusion criteria required past 30-day nicotine or tobacco product use of either e-cigarettes (e-cigarette use ≥3-day/week over the past 30 days; lifetime vaping duration ≥2 months; used nicotine-containing e-cigarettes) or combustible cigarettes (cigarette use ≥3-day/week for ≥2 years; interest in trying e-cigarettes if not also using e-cigarettes). Current smokers who also used e-cigarettes were eligible. Exclusion criteria were planning to quit using nicotine or tobacco products, currently or planning to become pregnant/breastfeeding, current daily use of tobacco products other than combustible cigarettes or e-cigarettes, and positive results of breath alcohol test at study visit. Participants provided written informed consent and were enrolled from November 2019 to March 2020. Participant accrual was halted prematurely due to coronavirus disease 2019 and trial registry information was updated on ClinicalTrials.gov in May 2020. The University of Southern California Institutional Review Board approved the study. The trial protocol is available in Supplement 1. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.8
Design and Materials
A within-participant randomized double-blind design was used. The procedure used custom e-cigarette solutions in 10 flavors (green apple, strawberry, chocolate, vanilla, menthol, cool menthol, peppermint, spearmint, subtle tobacco, and full-flavored tobacco) each in nicotine salt and free-base formulations with 50/50 propylene glycol/vegetable glycerin vehicle (Avail Vapors). For each pair of solutions for a respective flavor, the constituents were equivalent with the exception that the nicotine salt formulation included benzoic acid at a 1:1 molar ratio to nicotine and the free-base formulation did not. Nicotine concentration, density, propylene glycol/vegetable glycerin vehicle, and pH tests of each solution were conducted by the Roswell Park Comprehensive Cancer Center Nicotine and Tobacco Product Assessment Core (eMethods in Supplement 2) and used to calculate the proportion of nicotine in protonated (salt) form. Each solution was administered via a pod-style e-cigarette device (Suorin iShare Pod System Device; 7W; 2.0-Ω resistance; 130-milliamp hour built-in battery), which resembles JUUL and includes pod cartridge inserts that can be filled with custom solutions.
Procedure
After a telephone eligibility screen, participants were invited to a 3-hour study visit and instructed to abstain from using nicotine or tobacco products for 2 hours before arrival.9,10 During informed consent, participants were instructed that the study investigated the effects of e-cigarettes with different nicotine and flavorings. After informed consent, participants provided carbon monoxide and alcohol breath samples (BACtrack S80, BACtrack). Female participants provided urine samples for pregnancy tests.
Participants then completed the standardized e-cigarette appeal and sensory rating procedure, which has shown sensitivity to nicotine, device power, and flavor manipulations,9,10,11,12 in a ventilated testing room. The 20 trials (10 flavors presented in 2 nicotine formulations [free-base and salt]) followed a practice trial with flavorless nicotine-free solution. Each participant received a randomized ordering of the 20 e-cigarette solutions developed using a random sequence generator by staff who did not interact with participants and prepared products in the randomized order sequence before the visits. Participants and data collection staff were blinded to the solutions administered at each trial. During each administration, participants viewed a tutorial video with instructions directing them through the controlled guided puffing procedure, which involved a 1-puff cycle (10-second preparation, 4-second inhalation, 1-second hold, and 2-second exhale interval) for each product immediately followed by appeal and sensory attribute ratings. After rating each product, participants were given water before the next trial to minimize sensory carryover. The procedure was separated into four 5-trial blocks spaced at least 10 minutes apart. Participant characteristics questionnaires were completed between blocks.
Measures
Study Outcomes
After each single-puff trial, participants rated the product they just vaped on visual analog scales (range, 0-100) with answers to the following questions: (1) “How much did you like the e-cigarette?”; (2) “How much did you dislike the e-cigarette?”; (3) “Would you use this e-cigarette again?”; (4) “How sweet was the e-cigarette?”; (5) “How smooth was the e-cigarette?”; (6) “How bitter was the e-cigarette?”; and (7) “How harsh was the e-cigarette?” Rating anchors were not at all and extremely for each measure, except use again (not at all and definitely). The ratings liking, disliking (reverse-scored), and willingness to use again represent related but nonredundant measures of appeal (eg, individuals may feel ambivalent, expressing both liking and disliking of a product or they can dislike a product but be willing to try it again).9,13 Thus, we calculated an appeal composite score based on the mean of these 3 ratings for each trial (Cronbach α = .93) per prior work.9,10,11,12 Sensory ratings were analyzed separately per previous factor analyses indicating that appeal items shared a common factor, whereas sensory attributes loaded onto distinct item-specific factors.9
Participant Characteristics
Questionnaires assessing demographic and tobacco product use history characteristics included a question assessing ever combustible cigarette smoking status, defined as lifetime smoking 100 or more cigarettes.14,15,16 The question responses are shown in the Table. The Penn State Electronic Cigarette Dependence Index,17 an e-cigarette dependence severity measure (range, 0-20), and the Fagerström Test for Cigarette Dependence,18 a cigarette dependence severity questionnaire (range, 0-10), were also administered. Test strips (NicAlert; Jant Pharmacal Corporation) that provide a semiquantitative index of salivary or urine cotinine and exhaled carbon monoxide (Vitalograph) were collected to provide descriptive data on nicotine and combustible tobacco exposure, respectively.
Table. Participant Characteristics.
| Variable | No. (%)a |
|---|---|
| Female sex | 39 (32.8) |
| Age, mean (SD), y | 42.1 (14.4) |
| Race/ethnicity | |
| White | 35 (30.2) |
| Black | 46 (39.7) |
| Asian | 9 (7.8) |
| Multi-racial | 12 (10.3) |
| Otherb | 14 (12.1) |
| Hispanic | 23 (19.8) |
| Tobacco product use characteristics | |
| Ever combustible cigarette smokingc | 105 (88.2) |
| Current e-cigarette vaping statusd | 66 (55.5) |
| Current other tobacco product usee | 27 (25.0) |
| Biomarkers, mean (SD) | |
| Carbon monoxide, ppm | 6.4 (5.9) |
| Cotinine semiquantitative levelf | 4.0 (1.6) |
| Combustible cigarettes | |
| Age started smoking regularly, mean (SD), yg | 18.7 (6.9) |
| Current cigarettes/d, mean (SD)h | 11.0 (6.8) |
| Cigarettes/d when smoking heaviest, mean (SD)g | 17.8 (10.9) |
| No. of days smoked in past 30 dh | 23.4 (10.7) |
| FTCD, mean (SD)h | 4.4 (2.2) |
| Usually smoke(d) menthol cigarettesg | 47 (53.4) |
| e-Cigarettes | |
| PSECD, mean (SD)i | 10.1 (4.9) |
| Puffs per day, mean (SD)i | 85.5 (90.4) |
| Nicotine concentration typically used, mean (SD), mg/mLi | 29.3 (20.6) |
| Duration of e-cigarette use, mean (SD), moj | 21 (16.2) |
| No. of days vaped in past 30 di | 23.4 (8.6) |
| e-Cigarette device type typically usedj | |
| Cig-a-like | 2 (3.3) |
| Tank/pen | 5 (8.2) |
| Advanced personal vaporizer/mod | 11 (18.0) |
| Pod-based | 34 (55.7) |
| Other | 9 (14.8) |
| Preferred e-cigarette flavorj | |
| Fruit | 28 (45.9) |
| Dessert | 7 (11.5) |
| Mint | 6 (9.8) |
| Menthol | 9 (14.8) |
| Tobacco | 9 (14.8) |
| Other | 2 (3.3) |
Abbreviations: e-cigarette, electronic cigarette; FTCD, Fagerström Test for Cigarette Dependence; PSECD, Penn State Electronic Cigarette Dependence Index.
Overall N = 119. Sample size ranges from 110-119 across variables due to differential patterns of missing data across variables.
Includes American Indian or Alaskan Native, Middle Eastern, Pacific Islander (including Hawaii), and other.
Smoked ≥100 cigarettes lifetime and in the past 30 days (n = 22 were former smokers who did not smoke at all in past month, n = 83 were current [past-month] smokers).
Vaped ≥3 days per week for past ≥2 months.
Past 30-day use of “chewing tobacco, snuff or dip,” “dissolvable tobacco product,” “bidis,” “kreteks,” “regular pipe tobacco,” “snus,” “big cigars,” “little cigars or cigarillos,” or “hookah water pipe.”
Test strip (range, 1–6; 0 = 0-10, 1 = 10-30, 2 = 30-100, 3 = 100-200, 4 = 200-500, 5 = 500-1000, 6 = >1000 ng/mL).
Former or current smokers only (n = 105). Numbers range from 83-105 due to missing data across variables.
Current smokers only (n = 83). Numbers range from 80-83 due to missing data across variables.
Current users of e-cigarettes only (n = 66). Numbers range from 54-66 due to missing data across variables.
Ever users of e-cigarettes only (n = 64). Numbers range from 54-64 due to missing data across variable.
Statistical Analysis
After descriptive analyses of the study sample and materials, the primary analysis used multilevel models (MLMs). MLMs modeled rating outcomes from each trial as separate data points (20 total observations per participant; 10 per nicotine formulation condition) with participant-level random effects. The MLMs generate results that can be interpreted as mean effects collapsed across all trials within each condition. Primary models tested the fixed-effects nicotine formulation (salt vs free-base) on each outcome with and without adjusting for trial order (range, 1-20). Secondary models examined the generalizability of nicotine formulation effects across flavors and smoking status by testing the within-by-within interaction for nicotine formulation and flavor (10-level categorical variable) and the between-by-within interaction for never vs ever smoking status and nicotine formulation on each outcome, respectively. The MLMs yielded unstandardized effect estimates with standard errors (B [SE]), which reflect the difference in mean ratings between conditions. Of the analytic sample (N = 119), there were 5 participants with trial-level missing data (range, 4-16 trials); MLMs used all available data for participants with 1 or more observations. Smoothness ratings were introduced after the first 8 participants, resulting in an analytic sample of 111 for this outcome. P values were 2-tailed with .05 significance levels. Benjamini-Hochberg multiple test corrections were used to maintain a .05 studywise false discovery rate.19 Analyses were conducted using Stata version 16 (StataCorp LLC).20 Additional sensitivity analyses are detailed in the Results section.
Results
Descriptive Results
Of the 119 participants; 39 (32.8%) were female. The mean (SD) age was 42.1 (14.4) years; 105 (88.2%) (22 former and 83 current smokers) were ever combustible cigarette smokers, and 66 (55.5%) were current e-cigarette users (Table; Figure 1). Ever combustible cigarette smokers reported on average to have been moderate smokers during their heaviest smoking period (mean [SD] number of cigarettes smoked/d = 17.8 [10.9]). Current smokers reported having medium current cigarette dependence severity (mean [SD] Fagerström Test for Cigarette Dependence score, 4.4 [2.2]) and included 53 smokers who never regularly vaped e-cigarettes. Among the 66 (55.5%) current e-cigarette users, the modal e-cigarette device (55.7% pod style) and mean (SD) nicotine concentration typically used (29.3 [20.6] mg/mL) were similar to the products used in this study protocol, and 30 participants were current dual users of both combustible and e-cigarettes (Table).
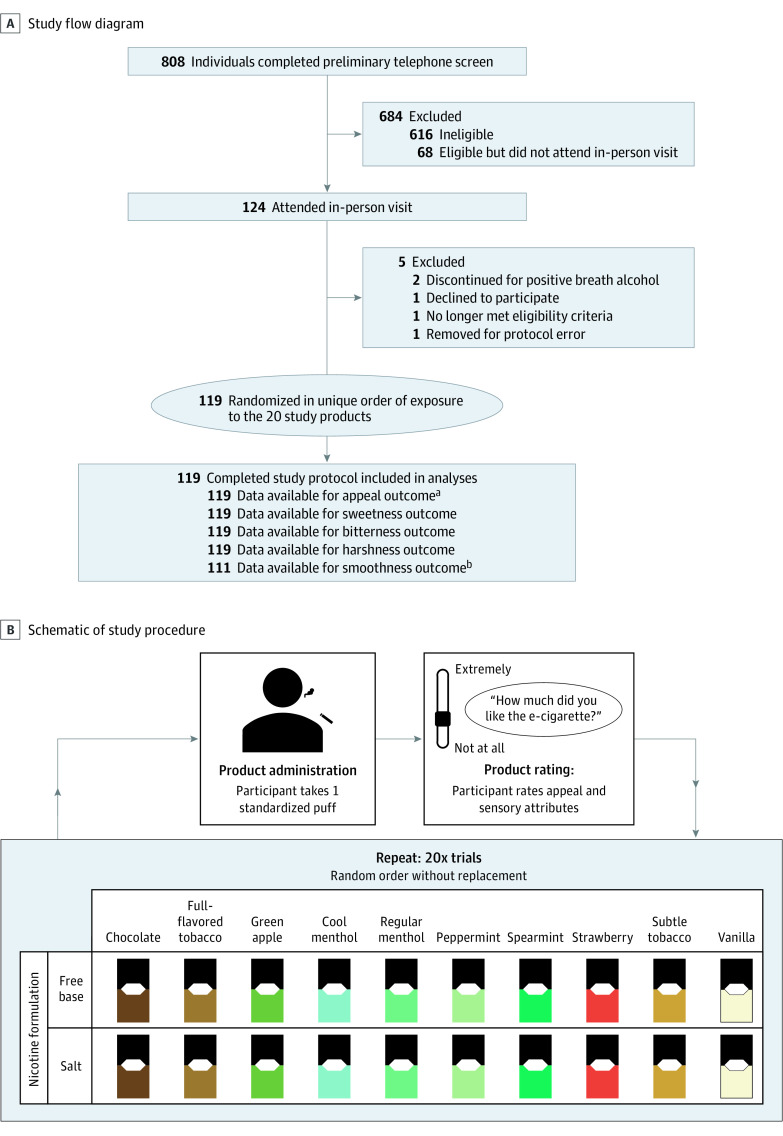
Figure 1. Study Flow Diagram and Schematic.
eCigarette indicates electronic cigarette.
aMean of like, dislike (reverse-scored), and willingness to use again ratings on visual analog scale.
bSmoothness measure introduced into the study after the first 8 participants had already completed the protocol.
In the 10 salt nicotine solutions, the mean (SD) nicotine concentration value was 23.4 (0.9) mg/mL, pH was 6.6 (1.1), propylene glycol/vegetable glycerin vehicle proportion was 52.5 (2.6)/47.5 (2.6), density was 1.1 (0.02) g/mL, and estimated protonated nicotine was 97.8% (2.3%). The free-base nicotine solutions had a mean (SD) nicotine concentration value of 23.8 (1.7) mg/mL, pH of 8.9 (1.1), propylene glycol/vegetable glycerin vehicle proportion of 52.5 (2.6)/47.5 (2.6), density of 1.1 (0.03) g/mL, and estimated protonated nicotine of 17.5% (25.7%) (eTable 1 in Supplement 2).
Primary Results
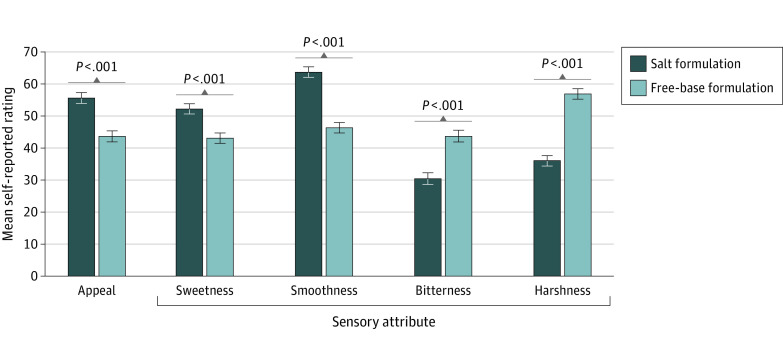
The MLMs revealed that salt vs free-base nicotine formulations produced significantly higher ratings of appeal (mean [SE]: 55.6 [1.7] vs 43.6 [1.7]; difference in means effect estimate b = 12.0; 95% CI, 9.9-14.1), sweetness (mean [SE]: 52.2 [1.6] vs 43.0 [1.6]; b = 9.3; 95% CI, 7.1-11.4), and smoothness (mean [SE]: 63.7 [1.6] vs 46.3 [1.6]; b = 17.4; 95% CI, 15.2-19.6). Salt vs free-base nicotine produced significantly lower ratings of bitterness (mean [SE]: 30.4 [1.8] vs 43.7 [1.8];b = −13.3; 95% CI, −15.4 to −11.2) and harshness (mean [SE]: 36.0 [1.6] vs 56.9 [1.6]; b = −21.0; 95% CI, −23.2 to −18.7) (Figure 2). To determine whether sensitization or habituation during the procedure affected the results, analyses were retested after adjusting for trial number (1-20), which produced the same pattern of nicotine formulation effects across all outcomes (eTable 2 in Supplement 2).
Figure 2. Mean (SE) Appeal and Sensory Attribute Ratings, by Nicotine Formulation of Electronic Cigarettes.
The number of participants was 119 for all outcomes except harshness (n = 111). Appeal refers to the mean of liking, willingness-to-use-again, and disliking (reverse-scored) (range, 0-100) scores.
Secondary Results
Stratified by Flavor
The interactions between nicotine formulation and flavor were nonsignificant for all outcomes, except harshness (F = 3.1; P = .001). (Figure 3; eTables 2 and 3 in Supplement 2). The harshness-reducing effect of nicotine salt was significant for all flavors except chocolate (b = −5.6; 95% CI, −13.0 to 1.8) (Figure 3). Chocolate appeared to be an outlying flavor driving the interaction, as post-hoc analyses excluding chocolate flavor trials found nonsignificant interaction between flavor and nicotine formulation effects on all outcomes (eTable 2 in Supplement 2).
Figure 3. Nicotine Formulation Effect Estimates, Stratified by Flavor.
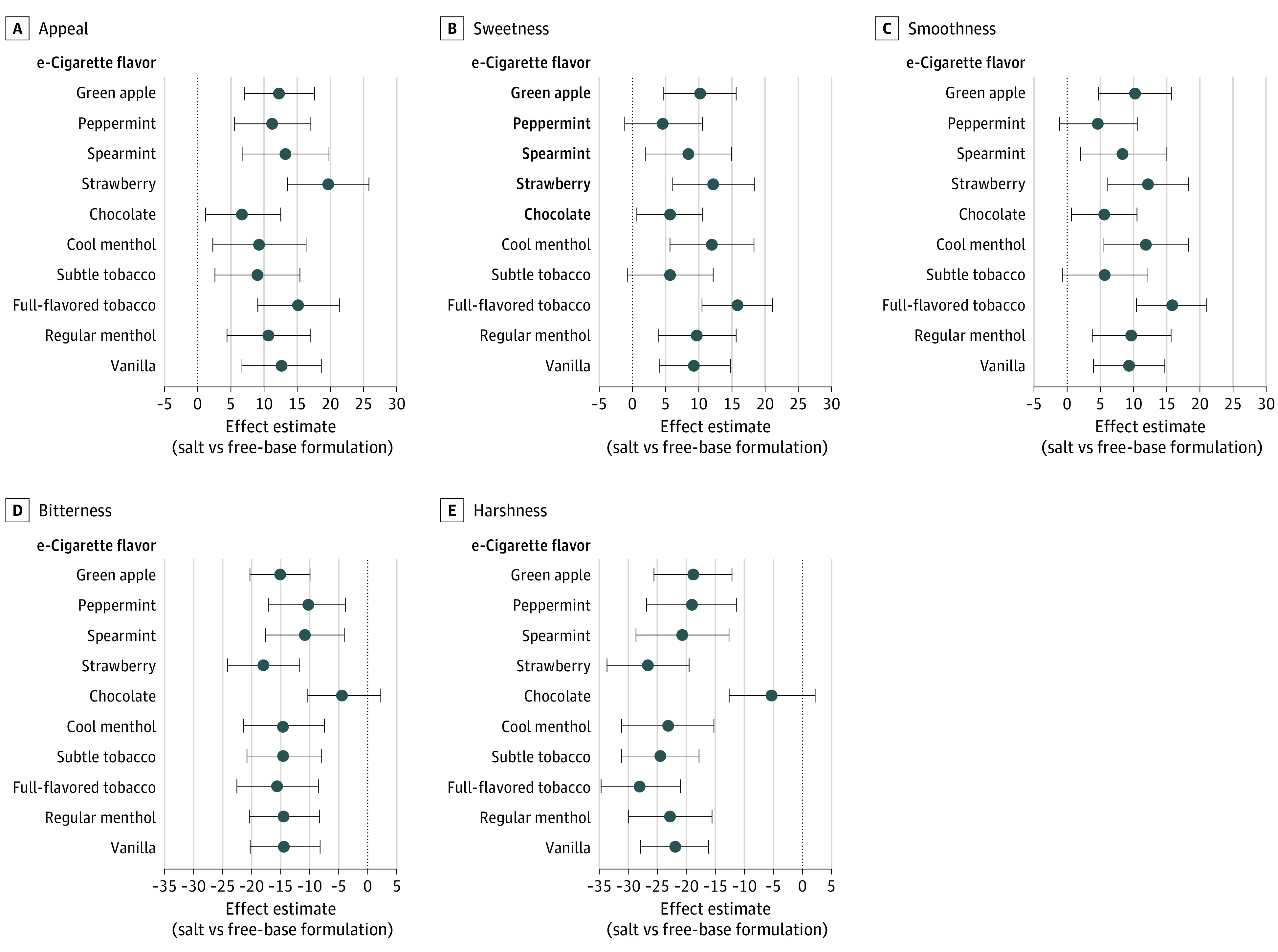
The number of participants was 119 for all outcomes except harshness (n = 111). Effect estimate (salt vs free-base). Error bars are 95% CIs. Appeal refers to the mean of liking, willingness-to-use-again, and disliking (reverse-scored) (range, 0-100) scores. e-Cigarette indicates electronic cigarettes.
Stratified by Smoking Status
Participants classified as never smoking e-cigarette users (n = 14; mean [SD] age, 22.3 [1.9] years) or ever smokers (n = 105; mean [SD] age, 44.8 [13.2] years) were compared in tests of interactions between nicotine formulation and smoking status. Interactions were significant for smoothness (B, 10.4; 95% CI, 3.4-17.5; P = .004) and harshness (b: −12.1; 95% CI, −19.0 to −5.2; P = .001) and nonsignificant for other outcomes (eTable 3 in Supplement 2; Figure 4). The smoothness-enhancing (b: 26.7; 95% CI, 20.2-33.2 vs B: 16.3; 95% CI, 13.9-18.6) and harshness-reducing (b: −31.7; 95% CI, −37.4 to −25.9 vs b: −19.5; 95% CI, −22.0 to −17.1) effects of nicotine salt were stronger in never vs ever smokers.
Figure 4. Mean (SE) Appeal and Sensory Attribute Ratings of Electronic Cigarettes, by Nicotine Formulation in Ever and Never Smokers.
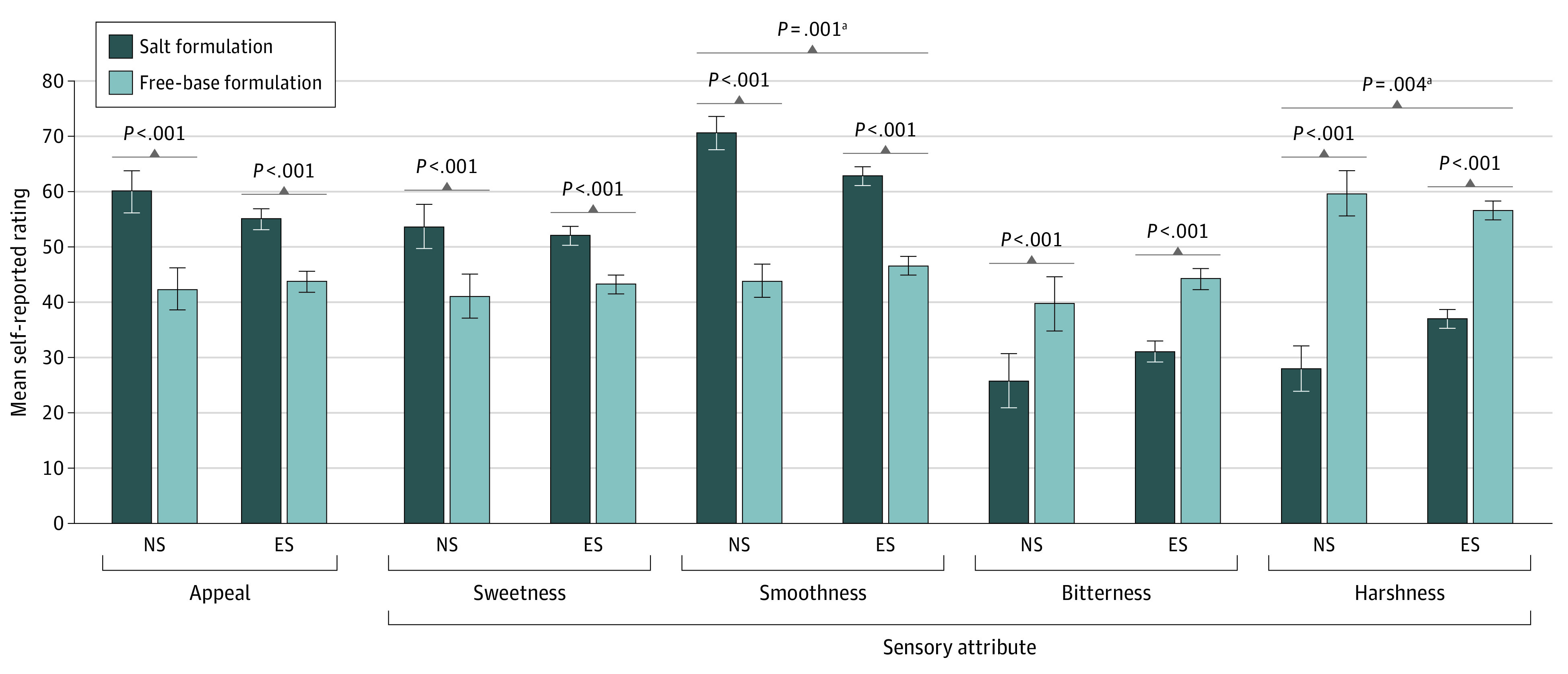
Never smokers (n = 14): smoked <100 combustible cigarettes in lifetime. Ever smokers (n = 105): smoked ≥100 combustible cigarettes in lifetime. Appeal refers to the mean of liking, willingness-to-use-again, and disliking (reverse-scored) (range, 0-100) scores. ES indicates ever smoking participants; NS, never smoking participants.
aP value for test of interaction between nicotine formulation and ever smoking status.
Sensitivity Analysis
Sensitivity analyses tested the generalizability and robustness of the findings. Consistent with the primary results, salt vs free-base nicotine increased appeal, sweetness, and smoothness and reduced bitterness and harshness in participants without significant previous experience using e-cigarettes (n = 53) (eTable 4 in Supplement 2), after adjusting for variability in the nicotine concentration of e-cigarette solutions (eTable 5 in Supplement 2), and regardless of whether participants used pod-style e-cigarettes (n = 34) or other types of e-cigarettes (n = 27) (eTable 6 in Supplement 2).
Discussion
The results of this study indicate that the addition of an acidic compound to e-cigarettes, which changes nicotine from free-base to salt,6 improves the appeal and sensory experience of vaping. Surveillance and market research indicate national sales of high-nicotine pod-style e-cigarette products that contain nicotine salt formulations, daily use prevalence, and addiction have increased since 2013, particularly in young people.1,2,3,21,22,23,24 Research on the reasons high-nicotine pod-style e-cigarette products are widely used have predominately focused on their marketing, design, flavors, and cultural trends,3,25,26 without systematically investigating the effects of nicotine formulation.
The present study addresses this gap using a tightly controlled double-blind trial designed to experimentally control for variation in marketing, device design, flavors, cultural trends, preexisting user preferences, and other factors in product choice. The use of e-cigarette solutions that were custom-designed to match nicotine concentration level, flavors, and other constituents across nicotine salt and free-base conditions permitted isolation of nicotine formulation effects. There was specificity in the results: nicotine salt enhanced desirable sensory attributes and suppressed undesirable sensory attributes of e-cigarette aerosol and did so independently of nicotine concentration.
Previous studies applying methods similar to the present study found that increasing nicotine concentration in e-cigarettes with free-base nicotine formulations generated aerosol that was perceived by users as harsher, more bitter, less sweet, less smooth, and less appealing overall.7,9,27,28 Prior to the advent of nicotine salt formulations, e-cigarette solutions predominately contained nicotine concentrations 2 to 10 times lower than JUUL1 and were often paired with high-wattage devices to obtain aerosol that was both satisfying and capable of delivering high nicotine levels.4 Based on the current findings, it is plausible that nicotine salt formulations make modern diminutive pod-style e-cigarette devices capable of producing aerosol that is rich with nicotine and enjoyable to the user despite containing small batteries with modest electrical output. By improving the sensory experience of vaping, individuals who first try e-cigarettes with nicotine salt formulations might find the nicotine-rich aerosol they emit palatable and easy to inhale. Thus, the likelihood of use continuation (instead of desistance) might be higher with nicotine salt than free-base e-cigarettes, which may prolong exposure to nicotine’s addictive properties and encourage long-term vaping patterns. This continuation could be a factor in the rise in e-cigarette use prevalence, frequency, and dependence symptoms in the US since 2015, assuming our findings may be generalizable outside of Los Angeles and to youths, new users unaccustomed to vaping, and across different products. While generalizability remains a question, secondary analyses suggest the results extend to smokers without significant prior experience vaping, across various flavored products, and to young adult never smokers.
Tobacco companies have historically used acid additives in combustible cigarettes based on industry research demonstrating that lowering pH increases the smoothness and palatability of tobacco smoke.29 Increasing the alkalinity of cigarette smoke increases perceived bitterness,30 and lowering pH can suppress the bitter-enhancing effects of some alkaline compounds.31 The proportion of free-base to salt nicotine in tobacco aerosol increases volatility, oral and upper airway deposition, stimulation of pharynx nicotinic receptors, and sensations of harshness.5 Long-term smokers may be familiar with such effects due to experience inhaling cigarette smoke, which might explain why the relative differences in perceived harshness and smoothness between salt and free-base formulations were less pronounced in ever vs never smokers in this study.
The health implications of vaping vary by age and smoking status. For older adult smokers who are unable to quit smoking, having nicotine salt e-cigarettes on the market might be advantageous if the sensory properties of these products facilitate transition from cigarettes to e-cigarettes.32 Both ever and never smokers in this study found the nicotine salt products significantly more appealing than free-base nicotine products, although young adult never smokers were more sensitive to the harshness-reducing effects of nicotine salt formulations than ever smokers. For never smokers and young people, having palatable and smooth nicotine salt e-cigarettes on the market that encourage chronic vaping patterns might be disadvantageous. Risks from long-term e-cigarette use include exposure to respiratory and cardiovascular toxins, potential for disrupted growth of brain pathways underlying mood and attention regulation, and nicotine dependence.32,33 Regulations reducing the availability of nicotine salt e-cigarettes may benefit the health of youths and never smoker populations who are unaccustomed to inhaling free-base nicotine. Because never smokers and youths might be deterred by the bitterness and harshness of e-cigarettes with free-base nicotine formulations that lack acid additives, they might be less likely to become long-term users of e-cigarettes if free-base nicotine products were the only e-cigarettes on the market. Regulatory agencies could decline to authorize (or unilaterally prohibit) sales of new or existing e-cigarette products that contain benzoic acid or other acid additives demonstrated to change nicotine from free base to protonated nicotine salt.6
The current findings merit consideration alongside accumulating evidence on the direct biological effects of exposure to protonated (salt) vs unprotonated (free-base) nicotine. Nicotine salt might be more likely to alter lung epithelium inflammatory responses, which could increase risk of respiratory illness.34 Protonated nicotine is less likely to diffuse across membranes, which could reduce nicotine absorption rate and other health effects.34 e-Cigarette devices with nicotine salt vs free base tend to have lower power output,1,35 which could reduce emissions of toxins caused by overheating that occurs with high-powered devices.32 On the whole, acid additives may increase the appeal and regular use of e-cigarettes while also having nuanced effects on the inherent harms of e-cigarette exposure.
Limitations
This study has limitations. First, this study’s methods assess immediate sensory reactions to aerosol in a controlled setting and do not address product appeal due to neuropharmacologically mediated nicotine reinforcement, marketing, and other factors. Second, while nicotine formulation effects largely generalized across differently flavored products in this study, the extent to which these results generalize to other e-cigarette products is unclear, including nicotine salt products with acids other than benzoic acid. In addition, the ratio of free-base to salt nicotine in e-cigarettes lies on a continuum,36 and further studies of dose-response effects of free-base to salt nicotine are warranted. Third, it is unknown whether these results in adults will generalize to adolescents. Last, because there is no consensus on the optimal measurement of e-cigarette product appeal, different measurement strategies and rating scale anchors merit inclusion in further research.
Conclusions
In this randomized clinical trial of adult current nicotine or tobacco product users, controlled exposure to e-cigarette puffs with salt vs free-base nicotine formulations increased product appeal and improved the sensory experience of vaping, particularly among never smokers. Regulatory policies limiting acid additives in e-cigarettes might reduce the appeal of high-nicotine e-cigarettes among populations deterred from vaping e-cigarettes that emit harsh aerosol.
Trial Protocol
eMethods
eTable 1. Constituents of e-Cigarette Solutions
eTable 2. Effects of Nicotine Formulation on Appeal and Sensory Attributes in Sensitivity Analyses
eTable 3. Effects of Nicotine Formulation in Overall Sample and Stratified by Smoking Use Status and Interaction Effects
eTable 4. Effects of Nicotine Formulation in Current Smokers without Significant Previous Experience Using e-Cigarettes
eTable 5. Effects of Nicotine Formulation With and Without Adjusting for Nicotine concentration
eTable 6. Effects of Nicotine Formulation in e-Cigarette Users, Stratified by e-Cigarette Device Type Used
eReferences
Data Sharing Statement
References
- 1.Romberg AR, Miller Lo EJ, Cuccia AF, et al. Patterns of nicotine concentrations in electronic cigarettes sold in the United States, 2013-2018. Drug Alcohol Depend. 2019;203:1-7. doi: 10.1016/j.drugalcdep.2019.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallone DM, Cuccia AF, Briggs J, Xiao H, Schillo BA, Hair EC. Electronic cigarette and JUUL use among adolescents and young adults. JAMA Pediatr. 2020;174(3):277-286. doi: 10.1001/jamapediatrics.2019.5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leventhal AM, Miech R, Barrington-Trimis J, Johnston LD, O’Malley PM, Patrick ME. Flavors of e-cigarettes used by youths in the United States. JAMA. 2019;322(21):2132-2134. doi: 10.1001/jama.2019.17968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeVito EE, Krishnan-Sarin S. e-Cigarettes: impact of e-liquid components and device characteristics on nicotine exposure. Curr Neuropharmacol. 2018;16(4):438-459. doi: 10.2174/1570159X15666171016164430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pankow JF. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem Res Toxicol. 2001;14(11):1465-1481. doi: 10.1021/tx0100901 [DOI] [PubMed] [Google Scholar]
- 6.Harvanko AM, Havel CM, Jacob P, Benowitz NL. Characterization of nicotine salts in 23 electronic cigarette refill liquids. Nicotine Tob Res. 2020;22(7):1239-1243. doi: 10.1093/ntr/ntz232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrington-Trimis JL, Leventhal AM. Adolescents’ use of “pod mod” e-cigarettes: urgent concerns. N Engl J Med. 2018;379(12):1099-1102. doi: 10.1056/NEJMp1805758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dwan K, Li T, Altman DG, Elbourne D. CONSORT 2010 statement: extension to randomised crossover trials. BMJ. 2019;366:l4378. doi: 10.1136/bmj.l4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leventhal A, Cho J, Barrington-Trimis J, Pang R, Schiff S, Kirkpatrick M. Sensory attributes of e-cigarette flavours and nicotine as mediators of interproduct differences in appeal among young adults. Tob Control. 2020;29(6):679-686. doi: 10.1136/tobaccocontrol-2019-055172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leventhal AM, Mason TB, Kirkpatrick MG, Anderson MK, Levine MD. E-cigarette device power moderates the effects of non-tobacco flavors and nicotine on product appeal in young adults. Addict Behav. 2020;107:106403. doi: 10.1016/j.addbeh.2020.106403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leventhal AM, Goldenson NI, Barrington-Trimis JL, Pang RD, Kirkpatrick MG. Effects of non-tobacco flavors and nicotine on e-cigarette product appeal among young adult never, former, and current smokers. Drug Alcohol Depend. 2019;203:99-106. doi: 10.1016/j.drugalcdep.2019.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenson NI, Kirkpatrick MG, Barrington-Trimis JL, et al. Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: application of a novel methodology. Drug Alcohol Depend. 2016;168:176-180. doi: 10.1016/j.drugalcdep.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwak HS, Anh BH, Lee Y, et al. . Comparison of bipolar and bivariate measurements of liking and disliking percepts in novel products. Food Qual Prefer. 2013; 30(2):328-335. doi: 10.1016/j.foodqual.2013.07.002 [DOI] [Google Scholar]
- 14.Villanti AC, Johnson AL, Ambrose BK, et al. Flavored tobacco product use in youth and adults: findings from the first wave of the PATH study (2013-2014). Am J Prev Med. 2017;53(2):139-151. doi: 10.1016/j.amepre.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg CJ. Preferred flavors and reasons for e-cigarette use and discontinued use among never, current, and former smokers. Int J Public Health. 2016;61(2):225-236. doi: 10.1007/s00038-015-0764-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodu B, Plurphanswat N. e-Cigarette use among US adults: Population Assessment of Tobacco and Health (PATH) study. Nicotine Tob Res. 2018;20(8):940-948. doi: 10.1093/ntr/ntx194 [DOI] [PubMed] [Google Scholar]
- 17.Foulds J, Veldheer S, Yingst J, et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking e-cigarette users. Nicotine Tob Res. 2015;17(2):186-192. doi: 10.1093/ntr/ntu204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127. doi: 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- 19.Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B (Methodological). 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 20.StataCorp , Stata statistical software: Release 16. StataCorp LLC. 2019. [Google Scholar]
- 21.Hammond D, Wackowski OA, Reid JL, O’Connor RJ. Use of JUUL e-cigarettes among youth in the United States. Nicotine Tob Res. 2020;22(5):827-832. doi: 10.1093/ntr/nty237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hrywna M, Bover Manderski MT, Delnevo CD. Prevalence of electronic cigarette use among adolescents in New Jersey and association with social factors. JAMA Netw Open. 2020;3(2):e1920961-e1920961. doi: 10.1001/jamanetworkopen.2019.20961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackler RK, Ramamurthi D. Nicotine arms race: JUUL and the high-nicotine product market. Tob Control. 2019;28(6):623-628. doi: 10.1136/tobaccocontrol-2018-054796 [DOI] [PubMed] [Google Scholar]
- 24.Dai H, Leventhal AM. Prevalence of e-cigarette use among adults in the United States, 2014-2018. JAMA. 2019. Published online September 16, 2019. doi: 10.1001/jama.2019.15331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allem J-P, Dharmapuri L, Unger JB, Cruz TB. Characterizing JUUL-related posts on Twitter. Drug Alcohol Depend. 2018;190:1-5. doi: 10.1016/j.drugalcdep.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keamy-Minor E, McQuoid J, Ling PM. Young adult perceptions of JUUL and other pod electronic cigarette devices in California: a qualitative study. BMJ Open. 2019;9(4):e026306. doi: 10.1136/bmjopen-2018-026306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pullicin AJ, Kim H, Brinkman MC, Buehler SS, Clark PI, Lim J. Impacts of nicotine and flavoring on the sensory perception of e-cigarette aerosol. Nicotine Tob Res. 2020;22(5):806-813. doi: 10.1093/ntr/ntz058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosbrook K, Green BG. Sensory effects of menthol and nicotine in an e-cigarette. Nicotine Tob Res. 2016;18(7):1588-1595. doi: 10.1093/ntr/ntw019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keithly L, Ferris Wayne G, Cullen DM, Connolly GN. Industry research on the use and effects of levulinic acid: a case study in cigarette additives. Nicotine Tob Res. 2005;7(5):761-771. doi: 10.1080/14622200500259820 [DOI] [PubMed] [Google Scholar]
- 30.Kozlowski LT, Kleiman RM. Effects of oral pH on cigarette smoking. Pharmacol Biochem Behav. 1978;9(4):477-480. doi: 10.1016/0091-3057(78)90045-X [DOI] [PubMed] [Google Scholar]
- 31.Sakurai T, Misaka T, Nagai T, et al. pH-Dependent inhibition of the human bitter taste receptor hTAS2R16 by a variety of acidic substances. J Agric Food Chem. 2009;57(6):2508-2514. doi: 10.1021/jf8040148 [DOI] [PubMed] [Google Scholar]
- 32.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems . Public Health Consequences of E-Cigarettes. National Academies Press; 2018. [Google Scholar]
- 33.US Department of Health and Human Services . E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 34.Shao XM, Friedman TC. Pod-mod vs conventional e-cigarettes: nicotine chemistry, pH, and health effects. J Appl Physiol (1985). 2020;128(4):1056-1058. doi: 10.1152/japplphysiol.00717.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voos N, Goniewicz ML, Eissenberg T. What is the nicotine delivery profile of electronic cigarettes? Expert Opin Drug Deliv. 2019;16(11):1193-1203. doi: 10.1080/17425247.2019.1665647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duell AK, Pankow JF, Peyton DH. Nicotine in tobacco product aerosols: ‘It’s déjà vu all over again’. Tob Control. 2020;29(6):656-662. doi: 10.1136/tobaccocontrol-2019-055275 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods
eTable 1. Constituents of e-Cigarette Solutions
eTable 2. Effects of Nicotine Formulation on Appeal and Sensory Attributes in Sensitivity Analyses
eTable 3. Effects of Nicotine Formulation in Overall Sample and Stratified by Smoking Use Status and Interaction Effects
eTable 4. Effects of Nicotine Formulation in Current Smokers without Significant Previous Experience Using e-Cigarettes
eTable 5. Effects of Nicotine Formulation With and Without Adjusting for Nicotine concentration
eTable 6. Effects of Nicotine Formulation in e-Cigarette Users, Stratified by e-Cigarette Device Type Used
eReferences
Data Sharing Statement