Abstract
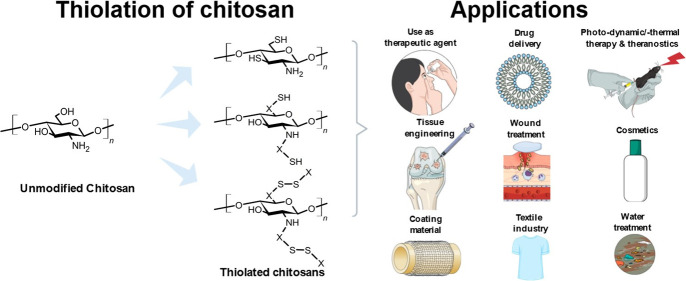
Various properties of chitosan can be customized by thiolation for very specific needs in a wide range of application areas. Since the discovery of thiolated chitosans, many studies have proven their advantageous characteristics, such as adhesion to biological surfaces, adjustable cross-linking and swelling behavior, controllable drug release, permeation as well as cellular uptake enhancement, inhibition of efflux pumps and enzymes, complexation of metal ions, antioxidative properties, and radical scavenging activity. Simultaneously, these polymers remain biodegradable without increased toxicity. Within this Review, an overview about the different possibilities to covalently attach sulfhydryl ligands to the polymeric backbone of chitosan is given, and the resulting versatile physiochemical properties are discussed in detail. Furthermore, the broad spectrum of applications for thiolated chitosans in science and industry, ranging from their most advanced use in pharmaceutical and medical science over wastewater treatment to the impregnation of textiles, is addressed.
1. Introduction
Derived from chitin via deacetylation, chitosan displays a cationic charge, differentiating it from other polymers and making it a unique polysaccharide with properties like biocompatibility, biodegradability, and antimicrobial activity. Various derivatives as well as forms of chitosan have been developed to further adjust the properties of this polymer for different applications.1−10 Among these derivatives, thiolated chitosans, obtained by the covalent attachment of different −SH group-bearing ligands mainly to the primary amino but also to the hydroxyl groups of the polymer, might be the most auspicious ones. As these kind of chitosans were shown to exhibit substantially improved properties over the unmodified polymer, the variety of thiolated chitosans and the number of sound applications has strongly increased since their discovery in 1998.11−13 Having the great potential of these polymers in mind, and taking into consideration all the opportunities offered by more and more new types of thiolated chitosans exhibiting additional functions, we are certain that they will further alter the landscape of biomaterial sciences and engineering.
This Review should encourage and motivate scientists in academia and industry to move in or intensify their activities in this promising research field. It provides an overview of the various thiolated chitosans and the chemistry behind them. The basic characteristics of unmodified chitosan and its thiolation are described, followed by the properties gained by the addition of immobilized thiol groups. Furthermore, applications of thiolated chitosans in science and industry ranging from their pharmaceutical and biomedical use over cosmetics and wastewater treatment to the impregnation of textiles are discussed.
2. Chitosan: Its Chemistry and Thiolation
Chitosan, as displayed in Figure 1, is a copolymer consisting of N-acetyl-d-glucosamine and d-glucosamine connected by linear β-(1→4) glycosidic bonds.14 It is obtained via partial deacetylation of chitin, a polysaccharide that is a main part of the exoskeleton of fungi, insects, and crustaceans and, after cellulose, the most abundant natural polymer.15 The acetyl groups are cleaved off either enzymatically by chitin deacetylase or in alkaline conditions using concentrated sodium hydroxide, resulting in different degrees of deacetylation (DD). Together with the molecular weight of chitosan, the DD determines the physicochemical characteristics, such as solubility, of chitosan. As the resulting amino groups show a pKa value of 6.5, chitosan is protonated in acidic surroundings and therefore displays water solubility as well as a cationic charge.16
Figure 1.
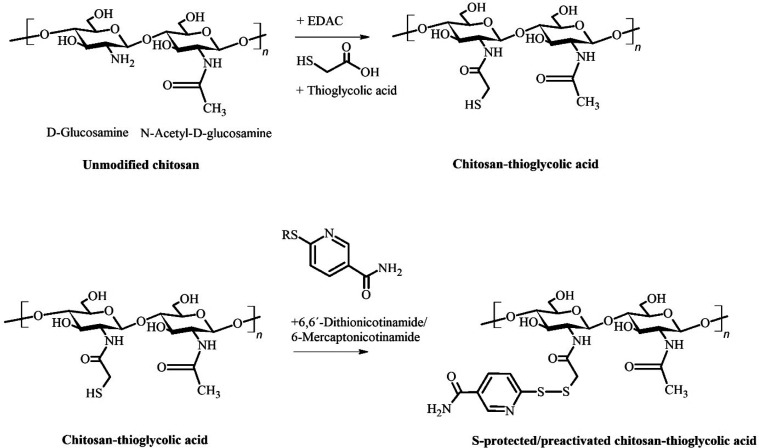
Example of the synthesis of an S-protected thiolated chitosan derivative, displaying the initial structure of chitosan, an amidation with thioglycolic acid mediated by 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDAC), and the protection/preactivation step with 6-mercaptonicotinamide (6-MNA; R = H) or the dimer form of this reagent, namely 6,6′-dithionicotinamide (R = 6-MNA).
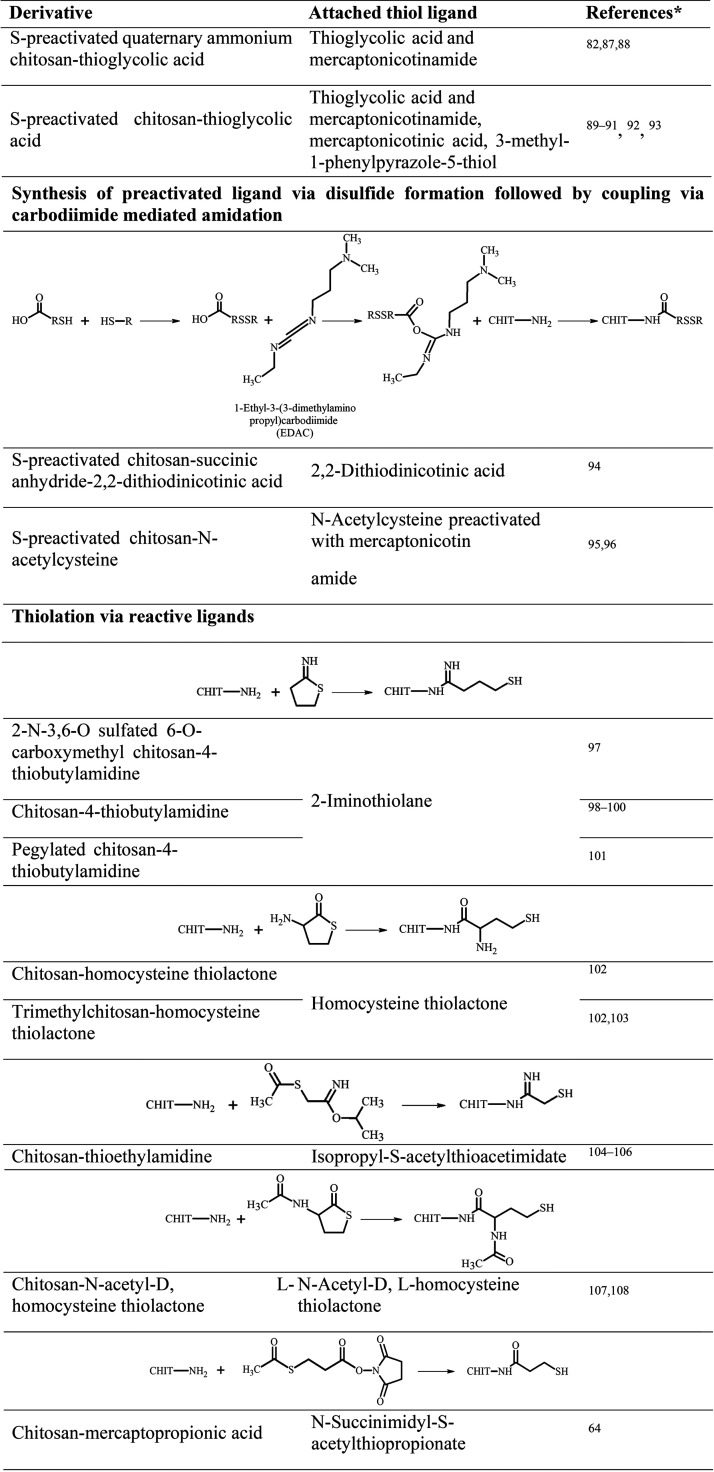
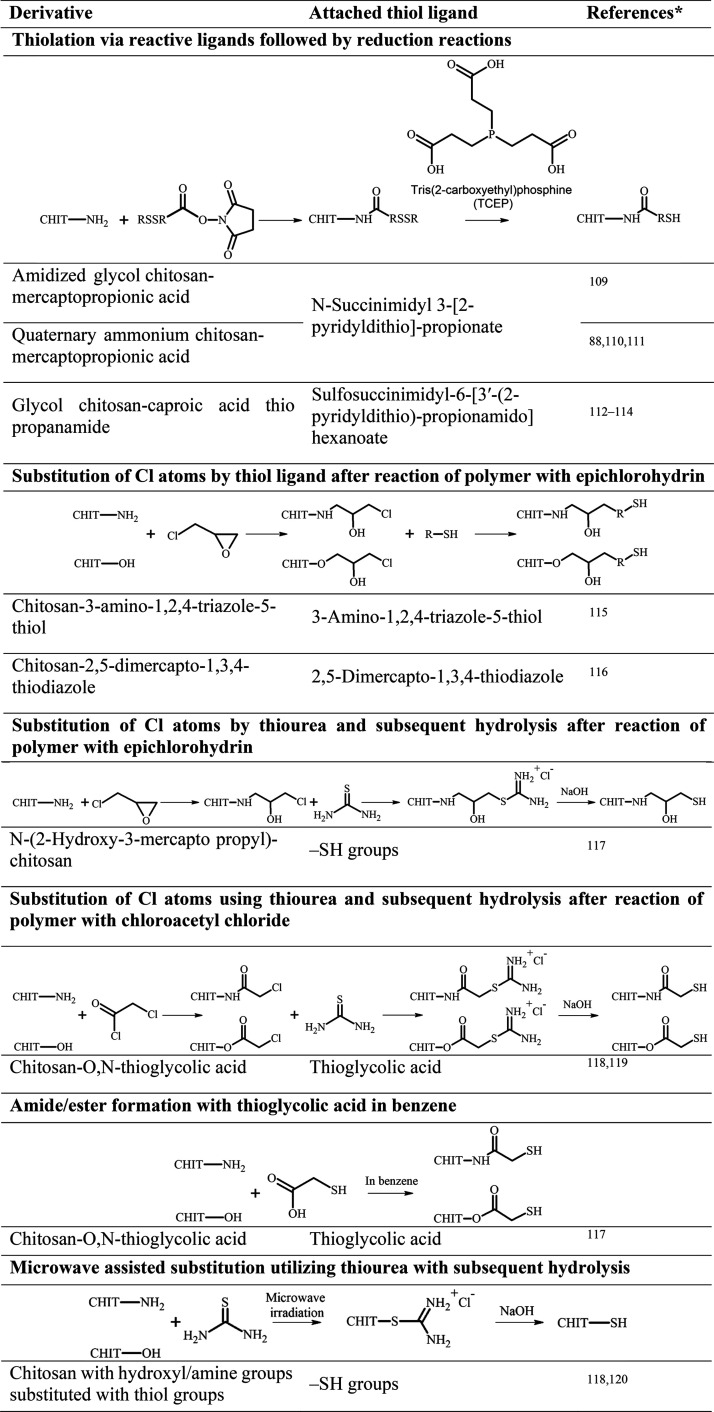
In general, the syntheses of thiolated chitosans can be divided into methods affecting the hydroxy moieties, the amino groups, or both. Subsequently, these methods can be further categorized according to the way thiol groups are introduced, either by direct substitution or by attaching a −SH-bearing ligand. The thiol groups of these ligands directly exist as such or need to be first disclosed via additional chemical treatment. Furthermore, the resulting −SH moieties can be S-protected/preactivated via disulfide formation with another thiol-bearing ligand. Thereby, the free thiol groups are protected from oxidation and show an increased reactivity over a broader pH range.17 Attaching an already S-protected/preactivated ligand is another way to synthesize these derivatives. As an example, the synthesis of S-protected/preactivated chitosan thioglycolic acid is depicted in Figure 1. In a first step, thioglycolic acid is attached to the amino group of pristine chitosan via carbodiimide-mediated amidation, and afterward, the resulting free −SH moieties are S-protected/preactivated by a disulfide exchange reaction with 6-mercaptonicotinamide or 6,6′-dithionicotinamide.
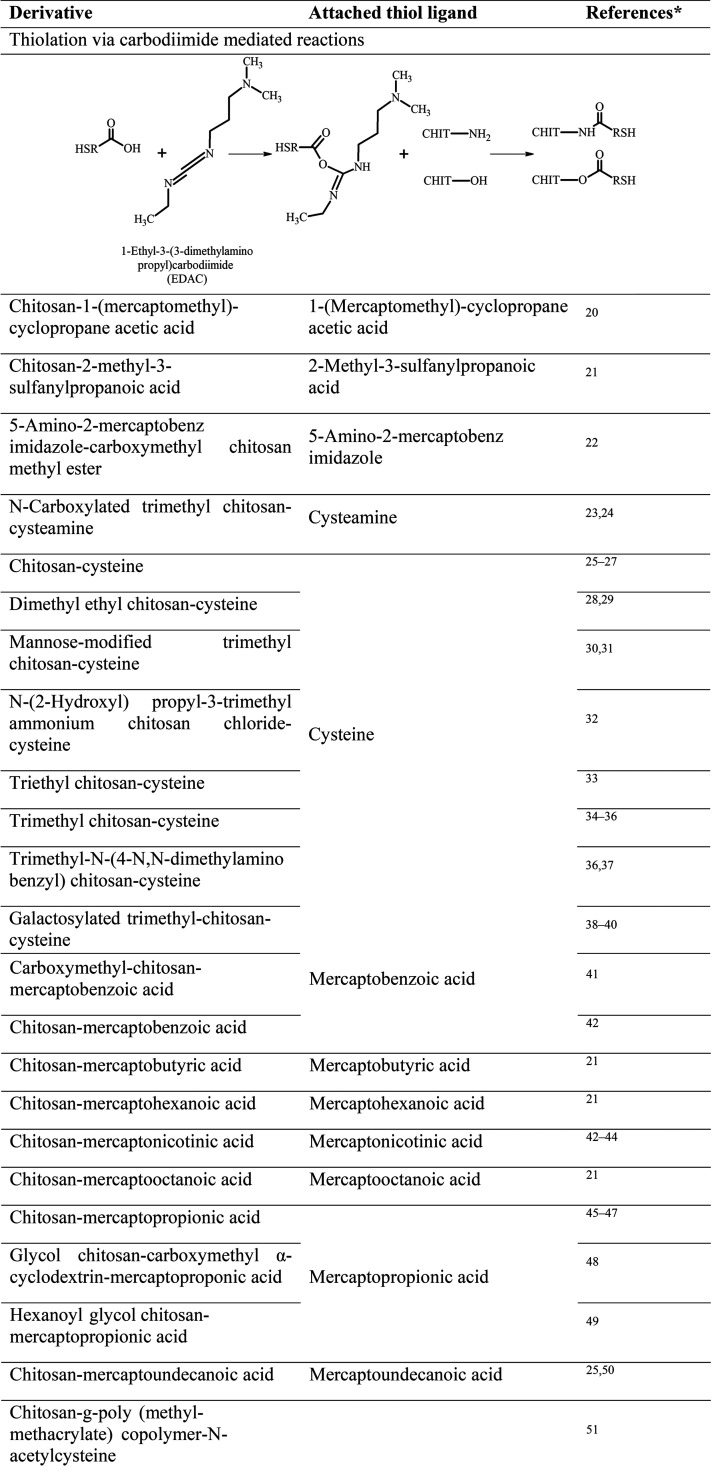
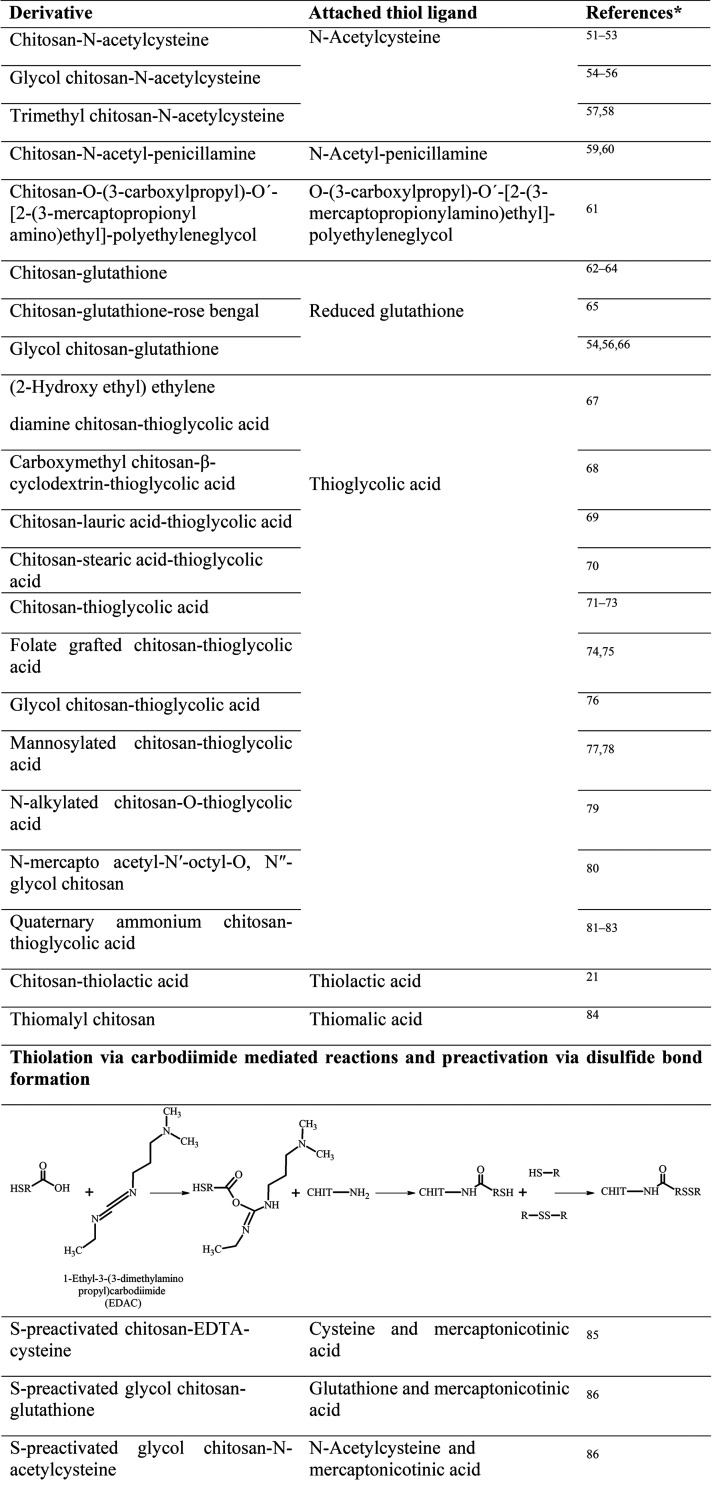
Since the synthesis of the first thiolated chitosan,12 numerous derivatives with various ligands, illustrated in Table 1, have been synthesized. For the analysis of the amount of immobilized −SH groups of these thiolated chitosans, Ellman’s reagent optionally with previous reduction of disulfide bonds with borohydride or iodometric titration can be used.18 Phadungcharoen et al., for instance, have conducted the Ellman’s assay via smartphone, providing an easy, fast, and reliable analysis of thiolated polymers without access to sophisticated equipment.19
Table 1. Overview of Available Thiolated Chitosans with the Schematically Depicted Utilized Synthesis Method and Attached Thiol Ligand (CHIT = Chitosan Polymer.
Where applicable limited to three references; for further references please contact the corresponding author.
3. Properties of Thiolated Chitosans
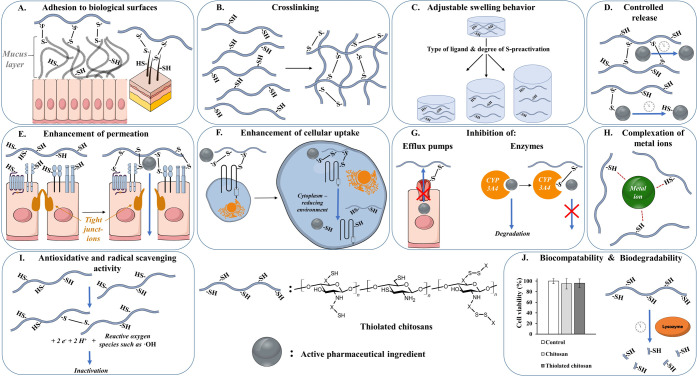
Due to the covalent attachment of thiol groups to chitosan, various properties of this polysaccharide, as outlined in Figure 2, can be gained. These properties are discussed in detail within this section.
Figure 2.
Properties gained by the covalent attachment of thiol groups to chitosan. (A) Adhesion of thiolated chitosans to biological surfaces such as mucins or keratins. (B) Cross-linking of thiolated chitosans due to disulfide formation, improving in situ gelling properties and mechanical stability. (C) Adjustable swelling behavior using different ligands and degrees of S-preactivation. (D) Controlled release of covalently bound active pharmaceutical ingredients (APIs) or prolonged API release out of cross-linked polymers. (E) Enhanced API permeation due to opened tight junctions caused by the interaction of thiolated chitosans with cysteine-bearing membrane receptors and enzymes. (F) Increased absorptive endocytosis of API-loaded thiolated chitosan carriers by disulfide formation with exofacial thiols of transmembrane proteins. (G) Inhibition of efflux pumps and enzymes due to the formation of disulfide bonds with thiolated chitosans. (H) Complexation of metal ions by sulfhydryl groups of thiolated chitosans. (I) Disulfide formation of thiolated chitosans, causing inactivation of reactive oxygen species. (J) Proven biocompatibility of thiolated chitosans in comparison to unmodified chitosan and customizable degradation rate of the thiolated polymer utilizing different ligands.
3.1. Adhesion to Biological Surfaces
Adhesion to substructures of biological surfaces like mucins or keratins is a key parameter of thiolated chitosans, as for instance in drug delivery a prolonged residence time on mucosal membranes is essential for local therapy or for non-invasive systemic delivery.121 To understand the underlying mechanism of mucoadhesion, the composition of the mucus has to be considered. It mainly consists of water, inorganic salts, lipids, and mucin glycoproteins, whereby its composition as well as thickness varies at different application sites.15 As mucin glycoproteins bear negatively charged sialic and sulfonic acid moieties, mucoadhesive properties of pristine chitosan mainly result from ionic interactions of its positively charged amino groups with these sialic and sulfonic acids.15,122 Besides these ionic interactions also van der Waals, hydrogen, and hydrophobic bonds take part in the mucoadhesion of unmodified chitosan.123,124
By introducing thiol-bearing ligands to chitosan, covalent bonds are added to the so far mentioned interactions with mucosal surfaces due to the formation of disulfides with cysteine residues of mucins.125,126 This covalent bond formation is also responsible for the adhesion of thiolated chitosans to keratinous surfaces, as keratins display a high cysteine content of 7–20%.127 Thus, thiolated chitosan exhibits an enhanced mucosal as well as keratinous adhesion compared to the unmodified polymer, whereby a lower pH of the surrounding medium intensifies this effect due to a reduced reactivity of the thiol groups, resulting in a lower extent of oxidation prior to surface contact.42,89 Therefore, preactivation of the attached −SH groups can further increase adhesiveness of thiolated chitosans, as these preactivated forms display on the one hand an improved reactivity over a wide pH range and are, on the other hand, less prone to oxidization.17,89,125
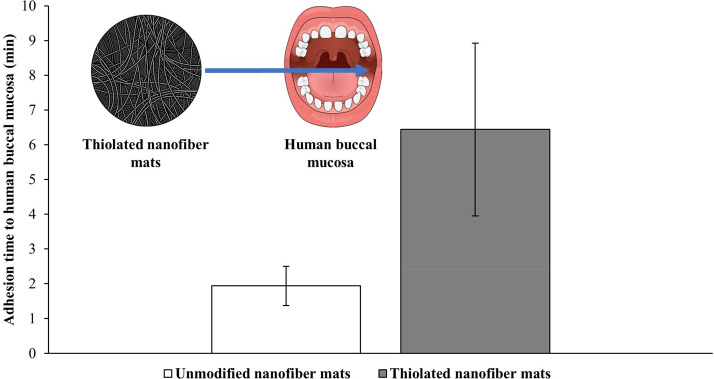
Samprasit et al., for example, showed the enhanced mucoadhesive properties of thiolated chitosans by an in vivo study with human volunteers using cysteine thiolated nanofiber mats. As illustrated in Figure 3, an over 3-fold prolonged adhesion time to buccal mucosa compared to unmodified nanofiber mats was observed, in good accordance with in vitro data acquired also within this study on porcine buccal mucosa.128
Figure 3.
Adhesion time of thiolated nanofiber mats to human buccal mucosa determined in human volunteers. Indicated values are mean (n = 3) ± SD. According to Samprasit et al.128
3.2. Cross-Linking
Thiolated chitosans display enhanced in situ gelling properties compared to pristine chitosan, caused by cross-linking due to the formation of intra- as well as interchain disulfide bridges via oxidation.129−131 This cross-linking process can be accelerated by the addition of different oxidizing agents such as hydrogen peroxide, leading, for example, to a dynamic viscosity increase for chitosan–thioglycolic acid of up to 16 500-fold within 20 min.132
Furthermore, Hintzen et al. showed an impact of the thiolated chitosan’s molecular weight on in situ gelation. A lower molecular weight resulted in a distinct increase in viscosity due to a higher flexibility as well as mobility of polymer chains, leading to a higher degree of cross-linking.133 Moreover, as with an increasing hydroxide ion concentration more negatively charged thiolate anions are available, the tendency for disulfide bond formation increases. Therefore, thiolated chitosans like chitosan–thioglycolic acid do not form disulfides at pH < 5, and so they are not useful as in situ gelling systems for drug delivery at applications sites with acidic pH.
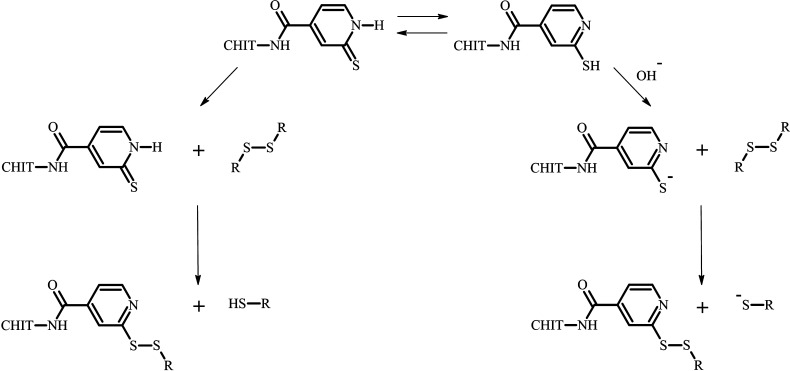
Based on these considerations, Millotti et al. synthesized a thiolated chitosan, namely chitosan–mercaptonicotinic acid, with pH-independent cross-linking properties. This thiolated chitosan bears two tautomeric forms (Figure 4) and, thus, can also react in a protonated state, as one of its forms displays a nucleophile (C=S) as well as proton donor (N–H) within its structure, enabling the formation of disulfides.134 In addition to the mentioned gelling process via oxidation, thiol–ene reactions between thiolated chitosans and alkenes can be utilized to generate in situ gelling systems.135 The gelling characteristics of such systems can be altered by the concentration of available thiol groups on chitosan and by the type of reaction partner.72,136,137
Figure 4.
Illustration of the tautomeric forms of chitosan–mercaptonicotinic acid and possible disulfide formation reactions of these forms. CHIT = chitosan polymer.
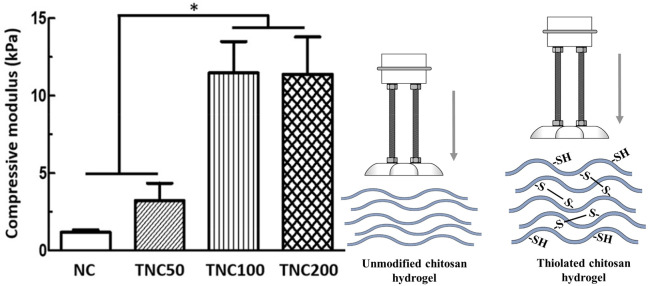
Apart from in situ gelation, cross-linking is also used to improve the mechanical properties of chitosan. For example, Wu et al. increased the physical strength of a biodegradable and biocompatible hydrogel composed of an N-isopropylacrylamide–chitosan copolymer via coupling of N-acetylcysteine. The compressive modulus, as highlighted in Figure 5, of a thiolated chitosan copolymer gel was over 9-fold improved compared to that of the unmodified copolymer gel.138 Furthermore, Miles et al. investigated the mechanical properties of chitosan films in order to construct a robust material. A 4-fold higher tensile strength, 6-fold higher breaking strain, and 14-fold increased average toughness were observed for chitosan–N-acetylcysteine with a 20% degree of substitution compared to the unmodified polymer. Additionally, the resilience of the prepared thiolated polymer films, characterizing the ability to deform without energy dissipation, was significantly enhanced. In contrast, the crystallinity of the films decreased due to the attachment of thiol groups. Usually, a higher crystallinity results in stronger mechanical properties. Cross-linking within the polymer was compensating this behavior, leading to a material with lower crystallinity and, at the same time, enhanced mechanical properties.139
Figure 5.
Average compressive modulus of hydrogels composed of N-isopropylacrylamide and chitosan or chitosan–N-acetylcysteine calculated from the slope of the stress–strain curve in a range of 10–20% strain (toe region). NC = N-isopropylacrylamide-g-chitosan copolymer; TNC = thiol-modified N-isopropylacrylamide-g-chitosan with different amounts of free thiol groups (TNC50: 101.84 ± 18.35 μmol/g, TNC100: 141.91 ± 27.15 μmol/g, TNC200: 299.39 ± 8.11 μmol/g). Significantly higher moduli were observed for TNC100 as well as TNC200 compared to NC and TNC50 (p < 0.05). Indicated values are means (n = 3) ± SD. Reprinted with permission from ref (138). Copyright 2018 Elsevier.
3.3. Swelling Behavior
Introduced −SH groups have an impact on the swelling characteristics of chitosan, as these groups display a lower hydrophilicity compared to hydroxyl and amino groups and can additionally cross-link the polymer chains via disulfide bridges.25,90 Thus, the swelling of thiolated chitosans is in many cases slower and less pronounced than that of unmodified chitosan. A slow and stable swelling process resulting in robust and firm networks is desired for applications such as wound healing, where high amounts of exudate can occur over an extended time period.44,137
Apart from −SH groups, the type of attached ligand has also a substantial impact on the swelling behavior of the polymer. Medeiros et al., for example, observed a 20% and 30% minor swelling capacity of chitosan–mercaptoundecanoic acid in comparison to chitosan–cysteine and unmodified chitosan, respectively, caused by the hydrophobicity of the attached acyl chain.25 For a thiolated chitosan with a hydrophilic ligand like chitosan–homocysteine thiolactone, however, an up to 5-fold increased swelling ratio was determined in comparison to unmodified chitosan.102 Furthermore, preactivating thiolated chitosans with hydrophobic ligands such as 6-mercaptonicotinamide can result in a decrease in swelling capacity, as a 40% lower weight gain of S-preactivated chitosan–thioglycolic acid compared to chitosan–thioglycolic acid without preactivation was observed.17
Accordingly, the swelling capacity of a thiolated chitosan can be adjusted to match the intended area of application by using an appropriate ligand and degree of S-preactivation.
3.4. Controlled Release
A sustained release of an active pharmaceutical ingredient (API) can be essential for drug delivery, as drug concentrations remaining within the therapeutic window for an extended period of time can reduce side effects and increase treatment efficacy. Moreover, patient compliance and convenience can be improved with controlled drug delivery systems.140
The release of APIs is influenced by the cross-linking density of a hydrogel, as thereby, the swelling behavior of the hydrogel is altered, resulting in a minor drug diffusion.89,141 For instance, Moreno et al. obtained differently swelling gels based on increasing amounts of 6-mercaptonicotinic acid and cysteine attached to chitosan and could modulate the release of ranibizumab and aflibercept with these gels over 7 days. As the release profile and retention of these two drugs out of the hydrogels were comparable to those of fluorescein isothiocyanate–dextran, with an average molecular weight of 40 kDa, physical entrapment in the gel systems was identified as the main cause for sustained drug release.44
For formulations like liposomes, micro- or nanoparticles composed of or coated with thiolated chitosans, various studies have shown a sustained release of encapsulated drugs resulting from disulfide bridge formation within and between the polymer chains controlling API liberation.75,142−146 For example, Trapani et al. could sustain the release of dopamine from liposomes coated with chitosan–glutathione. In comparison to uncoated liposomes, displaying a release of 10% within 48 h, about 1% of the neurotransmitter was released from the coated liposomes. Due to this release behavior, dopamine could be protected from autoxidation.66
Furthermore, polymer tablets based on thiolated chitosans display longer disintegration times in comparison to tablets comprising just unmodified chitosan due to a higher mechanical stability caused by formed disulfide bridges. Consequently, drug release can be sustained. Millotti et al., for instance, showed that 25% of insulin is released from chitosan–6-mercaptonicotinic acid tablets within 180 min, whereas at this time point already 80% of the APIs are released from unmodified chitosan tablets.43
Additionally, it is possible to delay API release by utilizing non-covalent drug–thiol ligand interactions. Due to these interactions, a significantly different liberation of metronidazole was observed out of tablets consisting of chitosan–N-acetylcysteine entirely S-protected with 6-mercaptonicotinamide with 320 μmol ligand/gram polymer, in comparison to tablets composed of the thiolated chitosan with 160 μmol of ligand/gram polymer.96
Another way to achieve a sustained release is based on binding drugs covalently to carrier systems via disulfide bridges with the thiol ligands. Via this mechanism, Chen et al. as well as Liu et al. achieved a controlled release for the BMP2-derived peptide P24. In both studies the peptide was coupled to chitosan–4-thiobutylamidine, which was confirmed via FT-IR147 or by X-ray photoelectron spectroscopy.148 As shown in Figure 6, the hydrogel prepared by Liu et al. displayed a zero-order release of the peptide over 16 days.148 Chen et al., however, extended this time frame to 90 days with their hydrogel formulation and observed an enhanced performance regarding the repair of bone defects in vivo in rats.147
Figure 6.
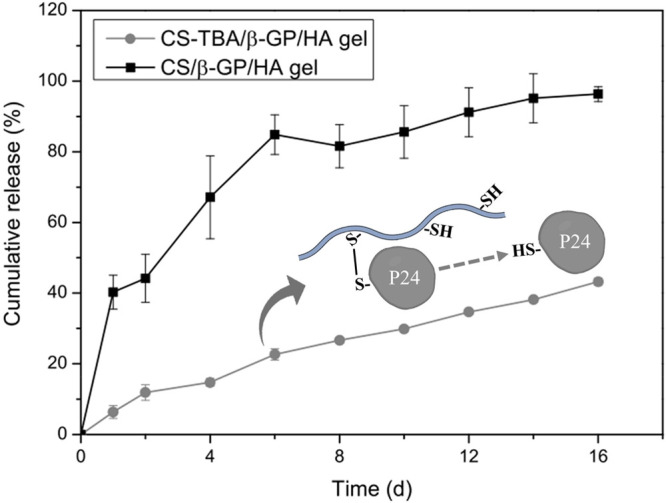
In vitro release profiles of BMP2-derived peptide P24 (P24) from chitosan–4-thio-butylamidine/β-glycerophosphate disodium/hydroxyapatite (CS-TBA/β-GP/HA) as well as from chitosan/β-glycerophosphate disodium/hydroxyapatite (CS/β-GP/HA) hydrogels. Indicated values are means (n = 4) ± SD. Reprinted with permission from ref (148). Copyright 2016 Elsevier.
3.5. Enhancement of Permeation
A major reason for the ability of thiolated chitosans to enhance the permeation of incorporated APIs is based on the opening of tight junctions by interacting with thiol groups of cysteine-bearing membrane receptors and enzymes.92,149 In detail, different mechanisms are claimed to contribute to this tight junction opening.
On the one hand, thiolated chitosan could inhibit protein tyrosine phosphatase dephosphorylating tyrosine subunits on occludin, thus opening tight junctions.149 On the other hand, as membrane receptors such as epidermal growth factor and insulin-like growth factor contain high amounts of cysteine, thiolated chitosans could interact with these and cause the activation of the intracellular protein tyrosine kinase Src due to phosphorylation. As a result, claudin-4 proteins are disrupted, leading to tight junction opening.92 The structure and pKa of attached ligands have substantial impacts on the permeation-enhancing effect, whereby for 6-mercaptonicotinic acid the most pronounced effect was observed.150 Responsible for this superior effect are likely the two tautomeric forms of 6-mercaptonicotinic acid, leading to more reactive thiol groups under physiological pH conditions, as described in section 3.2.134 Other attached thiol ligands can be oxidized, especially at pH values ≥5, causing an impaired permeation enhancing effect. As a result, S-protected forms display a more pronounced permeation enhancement due to their higher pH stability.151,152
Moreover, in the case of thiolated chitosans, the remaining cationic amino groups can electrostatically interact with anionic carboxyl groups of transmembrane receptors like integrin αvβ3, thereby inducing claudin-4 down-regulation and opening of tight junctions.92 Additionally, a prolonged mucosal residence time provided by the mucoadhesive properties of thiolated chitosans, as well as their inhibiting effect on API degrading enzymes as described in section 3.8, may contribute to the permeation enhancing effect. Another advantage of thiolated chitosans is their high molecular weight, avoiding their absorption and consequently preventing systemic toxicity and prolonging their permeation enhancing effect.153 Furthermore, as the permeation enhancing mechanism of thiolated chitosans differs from those of other permeation enhancers, a combination can lead to an additive effect.154
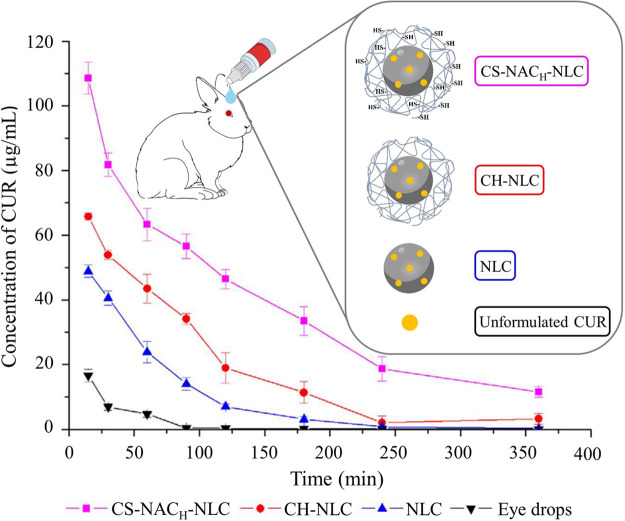
The permeation enhancement of thiolated chitosan for different APIs could be observed in various in vivo studies.43,143,155−161 Liu et al., for example, observed a 2.4-fold increased area under the curve (AUC) for curcumin in New Zealand albino rabbit tears after ocular administration of nanostructured lipid carriers coated with chitosan–N-acetylcysteine compared to a coating with unmodified chitosan (Figure 7).143
Figure 7.
Concentration of curcumin (CUR) in New Zealand albino rabbit tears after ocular administration of CUR embedded in eye drops, nanostructured lipid carriers (NLCs), NLCs coated with chitosan (CH-NLC), or NLCs coated with chitosan–N-acetylcysteine (CS-NACH-NLC). Indicated values are means (n = 6) ± SD. Reprinted with permission from ref (143), licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).
3.6. Enhancement of Cellular Uptake
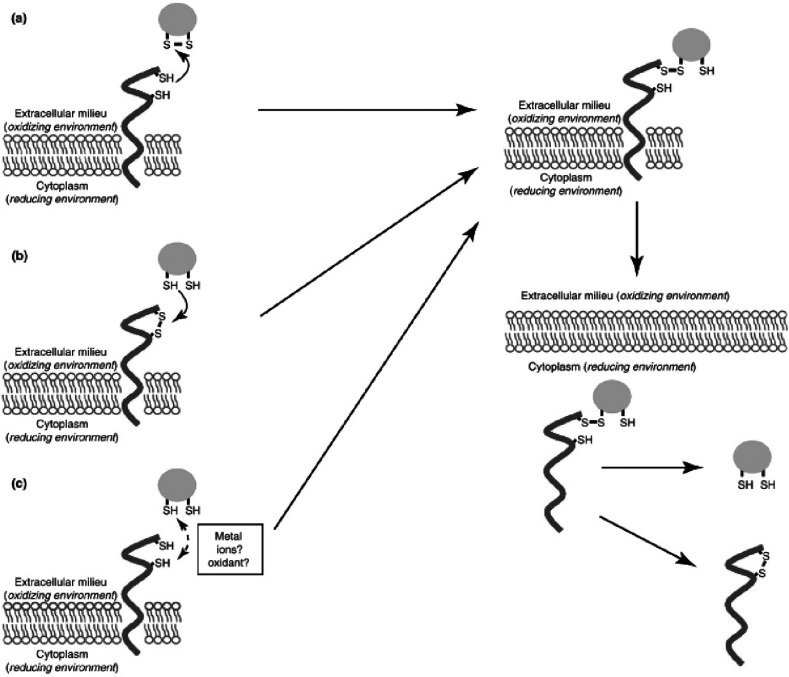
Thiolated chitosans increase cellular uptake by different mechanisms.162 The first mechanism is based on reactions of thiol functions present on the surface of the polymeric carrier with exofacial thiols of transmembrane proteins such as glycosylphosphatidylinositol-linked proteins or proteins non-covalently bound to the plasma membrane, leading to a more efficient absorptive endocytosis. As shown in Figure 8, three different types of these reactions can be assumed. First, an exofacial thiol group can react with a disulfide bond within the cargo-bearing vector, leading to the formation of a mixed disulfide bond. Second, a disulfide exchange reaction between a −SH group of the carrier and a disulfide bond within a cell surface protein may take place. Finally, the third possibility involves the formation of an intermolecular disulfide bond between a thiol group of the carrier and one of a cell surface protein. In each case, the resulting complex is internalized, and the cargo is released after internal cleavage of the previously formed disulfide bond.163 This cleavage mainly takes place within the reducing environment of the cytosol where there is a higher glutathione content compared the extracellular space. Thus, thiolated carrier systems can be designed for targeted intracellular release which is, for instance, important for gene therapy.164,165
Figure 8.
Potential mechanisms of thiolated chitosan drug carriers reacting with proteins displaying exofacial thiol groups. (a) A disulfide bond exchange reaction between a reactive thiol group at the cell surface and a disulfide bond of the vector takes place. (b) Again a disulfide bond exchange reaction occurs whereby this time a thiol group of the carrier attacks a disulfide bond of the protein. (c) Formation of a disulfide bridge between a thiol group of the vector and an exofacial thiol group of a protein. Metal ions or oxidizing agents can enhance this reaction. Each time a mixed disulfide complex emerges, it is internalized and subsequently reduced within the endosome or cytoplasm, resulting in release of the carrier. Reprinted with permission from ref (163). Copyright 2012 Elsevier.
Consequently, the cellular uptake decreases when the thiol groups of a thiolated chitosan carrier are oxidized to a large extent, as only the reaction of an exofacial thiol group with a disulfide bond of the carrier can take place. Additionally, overstabilization of the carrier surface due to the oxidization process can further decrease the uptake.166
The second mechanism contributing to enhanced cellular uptake is based on the stability of thiolated chitosan vectors during incubation with nucleases. Within the uptake process, the cargo needs to be protected from enzymatic degradation inside and outside the cells, for instance, in the small-intestinal fluid. On the one hand, the steric access of the nucleases is hampered to thiolated chitosan carriers with a cross-linked surface. On the other hand, thiolated chitosans form complexes with divalent cations (see section 3.9) necessary for the activity of nucleases.167 Third, opening of tight junctions increases the total cell surface area available for interacting with carrier systems. This opening of tight junctions may also enable the access of carriers to the basolateral surface of cells, from which uptake may occur. Moreover, as mentioned for the ability of thiolated chitosan to enhance the permeation, the increased mucoadhesion of thiolated carriers may contribute to the cellular uptake efficiency.
He et al., for instance, observed a 74% reduced serum TNF-α production after oral administration of siRNA encapsulated in trimethyl chitosan–cysteine nanoparticles to mice, whereas the naked administered TNF-α siRNA showed no effect.35
3.7. Inhibition of Efflux Pumps
As multi-drug-resistant proteins like P-gp and MRP1, being responsible for a reduced bioavailability of various APIs and resistance of tumors to chemotherapy as well as of bacteria to antibiotics, exhibit cysteine subunits within their transmembrane-channel-forming structures, thiolated chitosans can form disulfide bonds with these efflux pumps and thereby inhibit the activity of these transporters.56,168−170
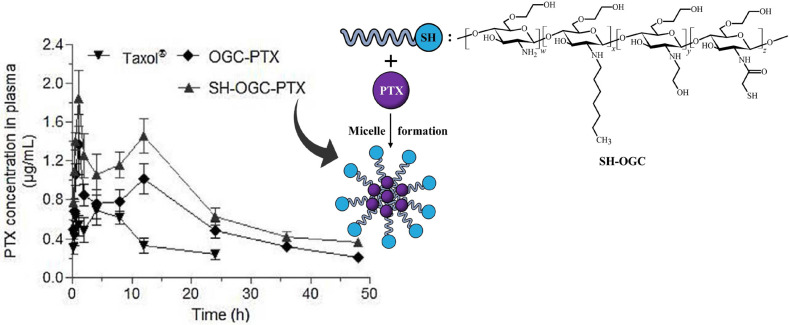
Sakloetsakun et al. confirmed this mechanism by analyzing the influence of the amount of thiol groups attached to chitosan on P-gp inhibition. The most pronounced effect was found for the highest concentration of −SH groups.150 Moreover, by attaching pyridyl substructures to thiolated chitosans and thereby forming S-protected derivatives with a higher reactivity of thiol groups, a more distinct P-gp inhibition could be achieved.152 Huo et al. utilized this efflux pump inhibiting effect by first confirming a P-gp inhibition of N-mercaptoacetyl-N′-octyl-O,N″-glycol chitosan using the P-gp substrate Rhodamine-123. Afterward, this thiolated polymer was used to prepare micelles loaded with the P-gp substrate paclitaxel, an API for cancer treatment. With this carrier, a 3.8-fold as well as 1.4-fold increased bioavailability of paclitaxel, as illustrated in Figure 9, in rats compared to the market product Taxol and the non-sulfhydrylated micelles, respectively, was achieved.80
Figure 9.
Concentration–time curve of paclitaxel (PTX) in rat plasma. PTX was orally administered via the reference market product Taxol, micelles based on N-octyl-O,N′-glycol chitosan (OGC-PTX), or micelles prepared with N-mercaptoacetyl-N′-octyl-O,N″-glycol chitosan (SH-OGC-PTX). Indicated values are means (n = 3) ± SD. Reprinted with permission from ref (80). Copyright 2018 Elsevier.
3.8. Inhibition of Enzymes
Thiolated chitosans have also shown to impede various enzymes. Again, disulfide bridge formation between the thiol groups of chitosan and cysteine substructures of enzymes seems to be responsible for this effect. The ability of thiolated chitosans to chelate divalent cations (see section 3.9, representing essential cofactors for most enzymes to maintain their activity, further contributes to this effect. Accordingly, chitosan–thioglycolic acid could be identified as an inhibitor of different enzymes, namely the drug-metabolizing CYP3A4 and CYP2A6, which together participate in the metabolism of over 60% of administered APIs, and of trypanothione reductase, a vital enzyme for parasitic protozoa such as leishmania and trypanosomes.171,172 Moreover, for chitosan–4-thiobutylamidine, an inhibitory activity was found against myeloperoxidase and metalloproteinases, enzymes important for wound healing, whereas overexpression of these enzymes can interfere with the healing process and lead to chronic wounds.99,173
3.9. Complexation of Metal Ions
Thiolated chitosans have the ability to form complexes with different metal ions, especially divalent metal ions, due to their −SH groups. As depicted in Figure 10, for example, nanoparticles made from N-(2-hydroxyl)propyl-3-trimethylammonium chitosan chloride–cysteine showed an improved removal of Hg2+, Cu2+, Zn2+, Cd2+, as well as Pb2+ from aqueous solutions compared to non-thiolated nanoparticles.32 Cd2+ was also more efficiently adsorbed from dithiocarbamate–chitosan beads in comparison to beads prepared with the unmodified polymer, and nanoparticles coated with chitosan–4-thiobutylamidine showed stronger Ca2+ binding compared to unmodified polymer-coated nanoparticles.174
Figure 10.
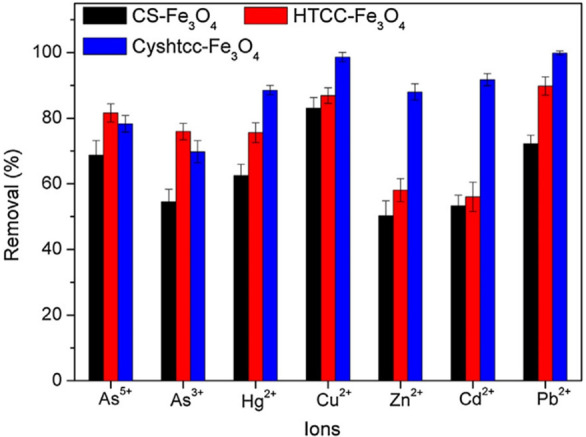
Removal efficiencies of nanoparticles prepared with chitosan–Fe3O4 (CS-Fe3O4), N-(2-hydroxyl)propyl-3-trimethylammonium chitosan chloride–Fe3O4 (HTCC-Fe3O4), and N-(2-hydroxyl)propyl-3-trimethylammonium chitosan chloride–cysteine–Fe3O4 (Cyshtcc-Fe3O4). Reprinted with permission from ref (32). Copyright 2018 Elsevier.
Furthermore, chitosan–thioglycolic acid could be identified as an effective Ni2+ adsorbent, as it displays a 40% higher binding capacity than the unmodified polymer.175 Additionally, a thiolated chitosan prepared by microwave-assisted substitution of the amine/hydroxyl groups with thiol groups was able to bind 85.4% of As3+ as well as 87.0% of As5+ within sorption studies and was utilized as a Hg2+ sorbent.118,120 The described complex formation with metal ions contributes to the ability of thiolated chitosans to enhance the permeation of different substances and to inhibit various enzymes.
3.10. Antioxidative and Radical Scavenging Activity
The antioxidative and radical scavenging activity of thiols results from their ability to form disulfides according to the following equation:
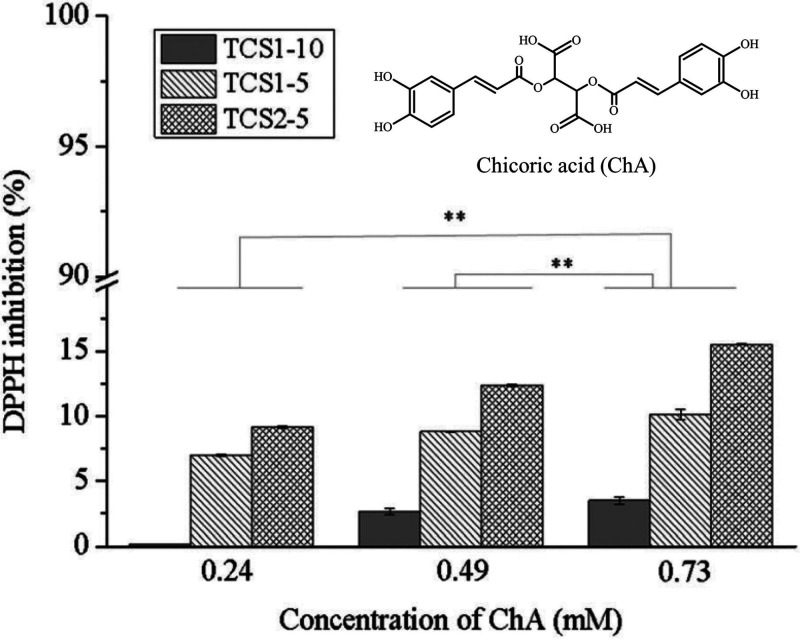
Due to this equilibrium reaction, thiols can take part in many biological processes and are able, via the emerging electrons and protons, to inactivate toxic radicals, leading, for example, to insufficient wound healing.176,177 In the case of chitosan, even the non-thiolated polymer displays an antioxidative effect caused by the available amino groups. A higher degree of deacetylation consequently results in an increased antioxidative activity, whereby this effect can be strongly improved due to thiolation.99,119,178,179 Chauhan et al. showed a radical scavenging activity of up to 82% for chitosan modified by thioglycolic acid at the amino as well as hydroxyl groups, compared to an activity of about 20% for the unmodified polymer, whereby the antioxidative activity increased with the amount of attached −SH groups.119 This correlation was also observed by Stefanov et al., as shown in Figure 11, utilizing chitosan–4-thiobutylamidine hydrogels for application on chronic wounds. As these hydrogels were prepared using the antioxidative active chicoric acid as a cross-linker, higher applied concentrations of this acid contributed to the antioxidative activity of resulting gels due to an increased amount of released chicoric acid.99
Figure 11.
Antioxidative activity expressed as percentage amount of 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) inhibition of different chitosan hydrogels, depending on the degree of chitosan thiolation as well as the concentration of chicoric acid (ChA) used for cross-linking and gelation of the thiolated chitosans. Thiolated chitosans used for hydrogel preparation displayed 212.5 μmol (TCS1-10), 372.5 μmol (TCS1-5), or 502.7 μmol (TCS2-5) of free thiol groups per gram of polymer. Indicated values are means (n = 3) ± SD. Statistical significance was calculated using one-way ANOVA (p < 0.05). Reprinted with permission from ref (99). Published by The Royal Society of Chemistry and licensed under a Creative Commons Attribution 3.0 Unported Licence (CC BY 3.0).
3.11. Safety Profile of Thiolated Chitosans
Despite the fact that unmodified chitosan is a biocompatible polymer, each thiolated chitosan has to be regarded as a novel compound with specific properties and with its own safety profile.180 As highlighted in Table 2, various studies have shown the biocompatibility of different thiolated chitosans. Among them, in vivo studies and in particular clinical investigations in humans are of most importance to determine the safety profile of thiolated chitosans. For instance, Schmidl et al. observed no serious adverse events within a controlled randomized double-blind study including 38 patients for chitosan–N-acetylcysteine administered as eye drops.181 These results were confirmed by Lorenz et al. via an investigation with 102 patients and within further clinical trials.182−186 Moreover, nanofiber mats based on chitosan–cysteine were proven to be non-toxic when applied in the human oral cavity for dental caries prevention.128
Table 2. Overview of Various Thiolated Chitosans Displaying Biocompatibility within the Listed Studies.
| derivative | utilized cytotoxicity tests | references | |
|---|---|---|---|
| (2-hydroxyethyl)ethylenediamine chitosan–thioglycolic acid | cell assay | MTT assay on Calu-3 and A549 cells | (67) |
| chitosan–4-thiobutylamidine | cell assays | viability assay on human dermal fibroblasts utilizing Presto Blue | (72) |
| cell count and morphology investigation of human dermal fibroblasts after staining with Hoechst and phalloidin conjugated to Alexa Fluor 488 dye | (72) | ||
| effect of polymer gel formulations on ciliary beat frequency of human nasal epithelial cells | (188) | ||
| Alamar Blue assay with human skin fibroblasts using hydrogel formulations | (173) | ||
| direct contact assay on HeLa, Caco-2/TC7, and HT-29/MTX cells | (189) | ||
| red blood cell lysis test | (190) | ||
| BrdU-based enzyme-linked immunosorbent assay on L-929 mouse fibroblast cells | (190) | ||
| MTT assay on L-929 mouse fibroblast cell | (190) | ||
| in rats | polymer hydrogel injected into the spinal cord of male Wistar rats | (72) | |
| chitosan–cysteine | cell assays | cell counting kit-8 assay on HaCaT and MCF-7 cells | (191) |
| MTT assay on human stomach carcinoma epithelial cells | (192) | ||
| MTT assay on human osteosarcoma and HEK 293 T cells | (25) | ||
| live/dead assay on human osteosarcoma, HEK 293 T and MCF-7 cells | (25, 191) | ||
| in humans | nanofiber mats were applied on buccal mucosa | (128) | |
| chitosan–lauric acid–thioglycolic acid | cell assay | MTT assay on human gingiva cells | (69) |
| chitosan–mercaptoundecanoic acid | cell assays | MTT assay on human osteosarcoma and HEK 293 T cells | (25, 50) |
| live/dead assay on human osteosarcoma and HEK 293 T cells | (25, 50) | ||
| chitosan–N-acetylcysteine | cell assay | LDH assay on human conjunctival cells | (193) |
| in rabbits | polymer microspheres investigated in eyes of albino New Zealand rabbits | (145) | |
| eye drops applied in albino New Zealand rabbits | (194) | ||
| in humans | clinical investigations using eye drops | (181−184, 186, 195) | |
| chitosan–thioglycolic acid | cell assays | MTT assay on bone-marrow-derived macrophages | (78) |
| MTT assay on human corneal epithelium cells | (161) | ||
| in rats | Draize skin irritation method with microneedle patches | (196) | |
| glycol chitosan–glutathione | cell assay | MTT assay on CaCo-2 cells | (86) |
| glycol chitosan–N-acetylcysteine | cell assay | MTT assay on CaCo-2 cells | (55, 86) |
| hexanoyl glycol chitosan–mercaptopropionic acid | cell assays | MTT assay on HeLa cells and human lung fibroblasts | (49) |
| direct contact test with human conjunctiva epithelial cells | (49) | ||
| live/dead assay with human conjunctiva epithelial cell aggregates | (49) | ||
| N-carboxylated trimethyl chitosan–reduced cystamine | cell assays | LDH and XTT assay on Calu-3 cells | (24) |
| XTT assay on H1299 cells | (23) | ||
| S-protected chitosan–EDTA–cysteine | cell assay | Resazurin assay on CaCo-2 cells | (85) |
| S-protected chitosan–N-acetylcysteine | cell assay | Resazurin assay on bladder mucosa | (95) |
| S-protected glycol chitosan–glutathione | cell assay | MTT assay on CaCo-2 cells | (86) |
| S-protected glycol chitosan–N-acetylcysteine | cell assay | MTT assay on CaCo-2 cells | (86) |
| trimethyl-chitosan–cysteine | cell assay | XTT assay on HEK293T and MCF-7 as well as SKOV-3 cells | (36) |
| trimethyl chitosan–N-acetylcysteine | cell assay | MTT assay on HeLa cells | (57) |
To prevent long-term toxic effects when thiolated chitosans are used, for instance, in drug delivery or tissue engineering, biodegradability has to be ensured. In general, as chitosan itself is a polysaccharide exhibiting cleavable glycosidic bonds, different enzymes such as lysozyme, cellulose, and pectinase are able to degrade the polymeric chain to non-toxic oligosaccharides.180,187 A decelerated degradation, however, can be of interest, depending on the application of the thiolated chitosan. For example, in tissue engineering, the formulation, on the one hand, has to display stability to substitute damaged or missing tissue for a sufficiently long time period to promote cell growth and, on the other hand, has to be biodegradable to enable its replacement by the cured tissue, requiring no further surgical intervention to change or remove the applied system.71,180
In the case of thiolated chitosan, the extent and rate of degradation can be adjusted by the attachment of a ligand, whereby a more hydrophobic ligand seems to increase the affinity for lysozyme, cellulase, and pectinase, resulting in a fast and more pronounced degradation in comparison to that of chitosan itself. In contrast, when hydrophilic ligands like glutathione are used, a slower degradation compared to the unmodified polymer is achieved.187 As enzymes like lysozyme used for biodegradability studies exhibit disulfide bridges within their structure, being essential to preserve their active center, thiolated chitosans can react with these substructures, leading to conformational alterations and consequently an inactivation of such enzymes. Moreover, cross-linking of thiolated chitosans via oxidation results in a lower degradation due to an impeded access of enzymes to cleavable sites.99,106,187
4. Applications of Thiolated Chitosans
As described in section 3, thiolated chitosans exhibit versatile properties enabling their use in different application areas. These applications are summarized in Table 3 as well as Table 4 and discussed in detail in the following.
Table 3. Overview of Dosage Forms Based on Thiolated Chitosans.
| dosage form | active pharmaceutical ingredient | references |
|---|---|---|
| Used as Therapeutic Agent | ||
| eye drops | chitosan–N-acetylcysteine | (181, 182, 185, 194, 195) |
| chitosan–cysteine | (197) | |
| Drug Delivery Systems | ||
| Oral Delivery | ||
| hydrogel | leuprolide | (158) |
| liposomes | calcitonin | (91) |
| docetaxel | (75) | |
| micelles | paclitaxel | (80) |
| microparticles | acyclovir | (198) |
| nanoemulsion | curcumin | (55) |
| nanoparticles | amoxicillin | (192) |
| cherry extract delivery | (87) | |
| docetaxel | (63, 74, 146, 199) | |
| fluorescein diacetate as model compound | (82) | |
| insulin | (84, 200, 201) | |
| low-molecular-weight heparin | (160) | |
| Map4k4 siRNA | (38) | |
| paromomycin | (78) | |
| sitagliptin | (202) | |
| TNF-α siRNA | (31, 112) | |
| polymer solution | Rhodamine-123 as model P-gp substrate | (203) |
| polymer tablets | antide | (151, 155) |
| calcitonin | (204) | |
| insulin | (43, 205) | |
| naproxen | (86) | |
| Rhodamine-123 as model P-gp substrate | (203) | |
| self-emulsifying drug delivery system | insulin | (206) |
| Ocular Delivery | ||
| nanoparticles | curcumin | (143, 144) |
| polymer solution | dexamethasone | (111) |
| Nasal Delivery | ||
| hydrogel containing proniosomes | duloxetine | (207) |
| microparticles | insulin | (208, 209) |
| paliperidone | (210) | |
| nanoparticles | bovine serum albumin as model compound for vaccination | (67) |
| galantamine | (211) | |
| insulin | (156) | |
| leuprolide | (212) | |
| pDNA encoding for green fluorescent protein | (213) | |
| selegiline | (157) | |
| theophylline | (214) | |
| tizanidine | (215) | |
| Buccal delivery | ||
| freeze-dried hydrogels | bovine serum albumin as model macromolecular compound | (216, 217) |
| insulin | (218) | |
| nanofiber mats | Garcinia mangostana extract | (128) |
| α-mangostin | (219) | |
| polymer films | calcium fluoride | (220) |
| fluconazole | (221) | |
| lignocaine | (220) | |
| risedronate | (73) | |
| polymer films containing nanoparticles | insulin | (28, 33) |
| polymer solution | pituitarity adenlyate cyclase-activating polypeptide | (222) |
| polymer tablets | pituitarity adenlyate cyclase-activating polypeptide | (159) |
| Vaginal Delivery | ||
| microparticles | tenofovir | (223) |
| nanofibers | tenofovir | (224) |
| nanoparticles | tenofovir | (225) |
| polymer tablets | metronidazole | (96) |
| Intravesical Delivery | ||
| microparticles | fluorescein diacetate as model compound | (226) |
| nanoparticles | fluorescein diacetate as model compound | (226) |
| gemcitabine | (227) | |
| Pulmonary Delivery | ||
| Nanoparticles | calcitonin | (76) |
| Transdermal Delivery | ||
| polymer film | carvedilol | (228) |
| microneedle patch | tacrolimus | (196) |
| Colonic Delivery | ||
| microcapsules | probiotic bacteria | (229) |
| Parenteral delivery | ||
| hydrogels | bendamustine | (136) |
| curcumin | (191, 230, 231) | |
| nanoparticles | 5-fluorouracil | (232) |
| curcumin | (232) | |
| meglumine antimoniate | (172) | |
| pDNA encoding for green fluorescent protein | (34, 79) | |
| TNF-α siRNA | (112) | |
| VEGF siRNA | (233) | |
Table 4. Overview of Application Forms of Thiolated Chitosans.
| application form | function | references |
|---|---|---|
| Diagnostics, Theranostics, and Photothermal/Photodynamic Therapy | ||
| cell chip | cell-mediated cytotoxicity assay, disease diagnosis, and anticancer drug assessments | (234) |
| nanoparticles | intravascular optical imaging of high risk plaques | (235) |
| long-time imaging of HeLa cells | (53) | |
| theranostic agent for tumors | (236) | |
| nanorods | photothermic agent | (237) |
| superparamagnetic iron oxide nanoparticles | in vivo tracking of stem cells | (238) |
| Tissue Engineering | ||
| electrospun membranes | delivery of VEGF and PDGF for blood vessel regeneration | (239) |
| electrospun membranes | delivery of QK peptide for blood vessel regeneration | (240) |
| hydrogels | carrier for cell-specific bioactive extracellular matrices | (241) |
| support of chondrocyte growth and matrix deposition to promote cartilage repair | (242) | |
| thermosensitive cell carrier/scaffold for tissue regeneration | (138) | |
| polyelectrolyte multilayers | redox-mediated fibronectin and fibroblast adhesion | (45, 46) |
| free-standing membranes or coatings of implants and tissue engineering scaffolds | (243) | |
| scaffold | biomimetic scaffold for the controlled and sustained delivery of BMP-derived peptide P24 to promote osteogenesis and bone repair | (147) |
| Wound Treatment | ||
| bandage | biodegradable bandage for the treatment of surgical site infections | (71) |
| freeze-dried hydrogel | hemostatic dressing | (27) |
| hydrogels | dressing for chronic wound management | (173) |
| gel formulation to accelerate wound closure and promote angiogenic markers, alignment of collagen fibers, and blood vessel formation | (244) | |
| Coating and Material Science | ||
| polymer films | antibacterial additives for the plastic industry | (101) |
| bacterial anti-adhesive coating | (245) | |
| high-performance composite for functional devices or fuel cells | (246) | |
| polymer solution | development of antimicrobial coatings | (64) |
| polymer grafted textile | biocidal finishing agent in textile production | (52) |
| Water Treatment | ||
| 3D sponge | wastewater treatment of organic dye pollution and bacteria contamination | (50) |
| chitosan beads | adsorption of precious metals | (116) |
| magnetic composite | metal remediation under neutral conditions | (32) |
| multilayer immunosensor | biocompatible and sensitive immunosensor for detecting Escherichia coli | (247) |
| nanoceria | photo-inactivation of bacteria in hospital effluent | (168) |
| polymer film | adsorption of Ni2+ from aqueous solutions | (175) |
| polymer solutions | As3+/As5+ removal in groundwater | (118) |
| selective and sensitive Hg2+ colorimetric sensor | (120) | |
| Cosmetics | ||
| creams | preventing the permeation of heavy metals ion into the skin to inhibit contact dermatitis | (175) |
4.1. Use as Therapeutic Agents
Topical therapy with biopolymers such as carboxymethylcellulose or hyaluronic acids is commonly used to treat dry eye syndrome (DES) symptoms. Due to short resident times of these lubricants, frequent instillation is necessary. As thiolated chitosans display pronounced adhesion to biological surfaces (see section 3.1), chitosan–N-acetylcysteine (C-NAC) was evaluated within various clinical studies for the treatment of DES within the past decade.181−185
Within these studies, substantial improvements in symptoms of DES were achieved. A controlled randomized double-blind study, for instance, demonstrated a significant increase in tear film thickness, lasting for 24 h after one single instillation, and corneal damage could be reduced in >60% of patients.181 As a result, C-NAC-based eye drops have been available in European pharmacies since 2019 under the trade name Lacrimera.
However, as several authors of the aforementioned study stated a conflict of interest (the study was sponsored by Lacrimera’s producer Croma-Pharma GmbH), the results must be treated with some reservation. Therefore, the effect of C-NAC on DES was independently evaluated by Messina and Dua, applying Lacrimera once a day in 18 patients suffering from moderate to severe dry eye disease with superficial punctate keratitis. Patients applied one eye drop in the morning for 5 days, and slit-lamp examinations were conducted prior to treatment and at 1 week as well as 3 weeks after treatment. Additionally, images were taken at these time points to evaluate the impact of C-NAC on DES. Based on the statistically significant improvement within two different scoring systems (subjective: Ocular Surface Disease Index, OSDI; objective: Oxford Grading System, OGS), the authors of the study not only confirmed the outcome of the aforementioned study181 but also suggested that the indications for the use of Lacrimera may be extended to patients suffering from other ocular surface pathology associated with dry eyes. Diagnosis and severity of dry eye disease pre and post treatment evaluated within this study showing the effectiveness of C-NAC-based eye drops are listed Table 5.195 The efficacy of Lacrimera in the treatment of corneal epithelial defects was also observed by Fischak et al. in an in vivo study in rabbits, as applying Lacrimera two times daily resulted in a significantly faster corneal wound healing compared to placebo. These outcomes further underlined the aforementioned recommendation to extend the application area of Lacrimera.194
Table 5. Diagnosis and Severity of Dry Eye Disease Symptoms of 18 Patients Pre and Post (3 Weeks) Treatment with One Eye Drop of Lacrimera in the Morning for 5 Daysa.
| OSDI (subjective) |
OGS (objective) |
|||
|---|---|---|---|---|
| associated condition | pre | post | pre | post |
| granular dystrophy, corneal grafts | 62.5 | 31.3 | III | I |
| persistent epithelial defect, glaucoma | 55.6 | 41.7 | II | I |
| ocular cicatricial pemphigoid, persistent epithelial defect | 53.6 | 35.7 | III | II |
| glaucoma | 46.9 | 31.3 | II | I |
| lasik | 71.4 | 17.9 | IV | 0 |
| rheumatoid arthritis | 35.7 | 17.9 | II | I |
| epithelial defect, neurotrophic keratopathy | 25 | 12.5 | I | 0 |
| superior limbic keratitis | 22.7 | 11.4 | II | I |
| rheumatoid arthritis | 50 | 25 | III | I |
| dry eyes | 45.5 | 11.4 | III | 0 |
| ocular cicatricial pemphigoid, glaucoma, dry eyes | 55.6 | 41.7 | II | I |
| corneal graft, dry eyes | 46.9 | 31.3 | III | II |
| corneal decompensation | 37.5 | 25 | II | I |
| glaucoma | 46.9 | 15.6 | III | 0 |
| neurotrophic keratopathy | 41.7 | 21.8 | II | I |
In addition to eye drops based on C-NAC, the corneal wound healing capacity of nanoparticles composed of chitosan–cysteine was evaluated by Zahir-Jouzdani et al. in vivo. After 21 days, no visual difference in corneal appearance was found between mice with induced corneal damage treated with chitosan–cysteine nanoparticles and animals without inflicted corneal wounds. As these results were confirmed by histological investigations, further products for ocular treatment containing thiolated chitosans will enter the global market within the next years.197
4.2. Drug Delivery Systems
The application of thiolated chitosans within drug delivery systems has already been described in detail within numerous reviews.1,2,248−250 Furthermore, a summary of dosage forms, APIs, and routes of administration, and results of in vivo studies, are listed in Table 3 and Table 6, respectively.
Table 6. Overview of In Vivo Studies with Drug Delivery Systems Comprising Thiolated Chitosans (TC; CS = Pristine Chitosan or Corresponding Mother Polymer).
| derivative | species | application form | application | results | references |
|---|---|---|---|---|---|
| chitosan–4-thiobutylamidine | mice | nanoparticles | intravenous delivery of 5-fluorouracil and curcumin | A sustained release over 72 h of curcumin and 5-fluorouracil was achieved by incorporating these APIs in TC nanoparticles. Furthermore, an 18.8-fold higher AUC was analyzed for 5-fluorouracil compared to the API solution, and for curcumin a 6.5-fold increased AUC was obtained. | (232) |
| rats | microparticles | nasal insulin delivery | A bioavailability of 7% and a calculated absolute pharmacological efficacy of 5% were obtained for TC. CS displayed a bioavailability of 4% and a pharmacological efficacy of 0.7%. | (209) | |
| oral acyclovir delivery | Mean residence time of TC microparticles was 17.9 h. CS particles showed only a mean residence time of 12.4 h. Furthermore, a 1.2-fold higher AUC was obtained for TC microparticles in relation to CS particles. | (198) | |||
| polymer tablets | oral calcitonin delivery | Delivery system based on TC decreased the plasma calcium concentration to 91%, whereas control tablets based on CS had no impact on plasma calcium level. | (204) | ||
| oral delivery of P-gp substrates | Tablets based on TC increased the AUC of Rhodamine-123 by 217% in comparison to buffer control and by 58% compared to CS. | (203) | |||
| pigs | polymer tablets | buccal pituitary adenylate cyclase-activating polypeptide delivery | Delivery system based on TC led to a bioavailability of 1%, whereas no API was detected in plasma using CS. | (159) | |
| oral antide delivery | For the administered solution, no API was analyzed in plasma. In contrast, for TC tablets, an absolute bioavailability of 1.1% was obtained. | (155) | |||
| chitosan–cysteine | mice | hydrogel | curcumin-containing formulations were injected into the breast fat pad | For the hydrogel composed of TC-coated liposomes, no tumor recurrence was observed, whereas unmodified liposomes displayed a recurrence rate of 50%. | (191) |
| chitosan–glutathione | rats | nanoparticles | oral docetaxel delivery | Oral bioavailability of the API was increased to 68.9% for TC nanoparticles compared to 6.5% for the commercially available reference. Furthermore, for TC nanoparticles, a drug release for 216 h was observed, whereas for the commercially available reference product, the release lasted only for 24 h. | (63) |
| chitosan–mercaptonicotinic acid | mice | nanoparticles | oral insulin delivery | The AUC after oral administration of TC nanoparticles was 4-fold improved compared to that of CS nanoparticles. | (201) |
| nanoparticles | intramuscular delivery of pDNA encoding for green fluorescent protein | Gene expression persisted up to 60 days. | (79) | ||
| rats | polymer tablets | oral insulin delivery | For tablets based on TC, a 4.8-fold higher AUC was observed in comparison to those based on CS. | (43) | |
| chitosan–mercaptopropionic acid | rats | nanoparticles | oral insulin delivery | An increased insulin concentration and a decreased glucose level were analyzed for streptozotocin-induced diabetic rats. | (200) |
| chitosan–N-acetylcysteine | rats | nanoparticles | nasal insulin delivery | Intranasal administration of API-loaded nanoparticles based on TC enhanced the relative bioavailability of the API (12%) compared with CS nanoparticles (7%) and control insulin solution (1%). | (156) |
| rabbits | nanoparticles | ocular curcumin delivery | For TC-coated nanoparticles, the significantly highest ocular retention was observed by fluorescence imaging, and a 29.9-fold increased AUC was obtained compared to that with curcumin eye drops. Uncoated nanoparticles displayed a 6.0-fold higher AUC, and for CS-coated nanoparticles a 12.3-fold increased AUC was detected. | (143) | |
| humans | nanofiber mats | local oral delivery of Garcinia mangostana extract or α-mangostin for caries prevention | API-loaded nanofiber mats based on TC achieved a ≥70% reduction in Streptococcus spp. and Lactobacillus spp. | (128, 219) | |
| chitosan–thioglycolic acid | mice | nanoparticles | nasal theophylline delivery | Theophylline administered via TC nanoparticles more strongly attenuated pulmonary inflammation and epithelial damage as well as goblet cell hyperplasia and resulted in a lower amount of infiltrated inflammatory cells compared to API delivery by CS nanoparticles. | (214) |
| nasal vaccination with bovine serum albumin (proof of concept) | High levels of IgG, IgG1, and IgG2a antibodies were found within the animals, demonstrating the potential of TC-based carriers for nanovaccines. | (67) | |||
| nasal delivery of selegiline | Animals treated with a system based on TC showed a significantly reduced immobility time, increased sucrose water intake, and higher locomotor activity compared to the group receiving a formulation with unmodified polymer. | (157) | |||
| intranasal delivery of plasmid DNA encoding for green fluorescent protein | Cross-linked TC/pDNA nanoparticles displayed a significantly higher transfection efficacy (47%) after 14 days in comparison to particles based on CS (21%). | (213) | |||
| rats | hydrogels | oral leuprolide delivery | Gel formulation based on TC and CS led to an absolute bioavailability of 283% and 43%, respectively. | (158) | |
| nanoparticles | oral low-molecular-weight heparin delivery | Compared with nanoparticles based on CS, the anticoagulant effect was significantly longer (maximal activated partial thromboplastin time was 2-fold increased) for nanoparticles based on TC. | (160) | ||
| oral docetaxel delivery | Oral bioavailability was 7.5-fold improved in comparison to DTX suspension. | (146) | |||
| oral sitagliptin delivery | A 4.7-fold increased efficacy in lowering plasma glucose concentration was achieved for TC nanoparticles compared to the API solution. | (202) | |||
| nasal leuprolide delivery | An absolute bioavailability of 2.6%, 4.3%, or 18.5% was observed by administering the API in solution or via nanoparticles based on CS or TC, respectively. | (212) | |||
| pulmonary calcitonin delivery | For calcitonin-loaded nanoparticles based on TC, the hypocalcemic effect lasted for 24 h and a pharmacological availability of 40% was analyzed, whereas for CS nanoparticles, a hypocalcemic effect of 12 h and pharmacological availability of 27% were obtained. | (76) | |||
| intravesical delivery | More than 50% of nanoparticles based on TC remained in the bladder after 6 h, resulting in a 4-fold higher bioadhesion compared to unmodified CS nanoparticles. | (226) | |||
| self-emulsifying drug delivery system | oral insulin delivery | TC formulation displayed a 3.3-fold higher AUC compared to oral insulin solution | (206) | ||
| chitosan–thioglycolic acid–6-mercaptonicotinamide | rats | liposomes | oral salmon calcitonin delivery | Liposomes coated with TC and S-preactivated TC achieved 5.7- and 8.2-fold improved decreases in blood calcium level, respectively, in comparison to the API administered in solution. | (91) |
| polymer tablets | oral antide delivery | An absolute bioavailability of 0.03% was observed for CS tablets, which could be increased to 1.4% using TC tablets. | (151) | ||
| dimethyl ethyl chitosan–mercaptopropionic acid | rabbit | polymer solution | ocular dexamethasone delivery | CS-API solution showed a 3.4-fold higher AUC in comparison to the API solution without chitosan. For the TC-API solution, however, a 5.7-fold higher AUC was found. | (111) |
| galactosylated trimethyl-chitosan–cysteine | mice | nanoparticles | oral delivery of Map4k4 siRNA | Daily oral administration of galactolsylated TC nanoparticles containing siMap4k4 significantly improved dextrane sulfate sodium-induced ulcerative colitis body weight loss, colon length shortening, and increase of myeloperoxidase activity. | (38) |
| hexanoic acid, 6-[(mercapto-1-oxopropyl)amino]chitosan | mice | nanoparticles | oral delivery of TNF-α siRNA | TC particles showed high accumulation at the arthritic joint sites in collagen-induced arthritis mice, significantly inhibiting inflammation and bone erosion comparable to methotrexate (5 mg/kg). | (112) |
| intravenous administration of VEGF siRNA | A 34.4% decreased VEGF expression in extracted tumor tissue was analyzed for TC nanoparticles in reference to the control. Moreover, a synergistic effect was obtained by administering TC nanoparticles together with bevacizumab, as thereby VEGF expression was reduced by 43.5%. | (233) | |||
| mannosylated trimethyl-chitosan–cysteine | mice | nanoparticles | oral delivery of TNF-α siRNA | Orally delivered TC nanoparticles inhibited TNF-α production in macrophages, protecting mice with acute hepatic injury from inflammation-induced liver damage and lethality. | (31) |
| N-mercaptoacetyl-N′-octyl-O,N″-glycol chitosan | rats | micelles | oral paclitaxel delivery | TC micelles increased the bioavailability of paclitaxel to 78%, being 3.8-fold higher compared to the marketed reference product and 1.4-fold higher in relation to micelles based on CS. | (80) |
| thiomalylchitosan | rats | nanoparticles | oral insulin delivery | For insulin-loaded TC nanoparticles, a 35% reduced blood glucose level was observed, whereas for CS nanoparticles blood glucose level decreased by 17%. | (84) |
| trimethyl-chitosan–cysteine | mice | nanoparticles | intramuscular delivery of pDNA encoding for green fluorescent protein | Transfection with TC achieved a 2.3-fold and 4.1-fold higher efficiency than CS and Lipofectamine2000, respectively. | (34) |
In the following, results of a study conducted with docetaxel are described in detail to illustrate the advantages of thiolated chitosans for drug delivery. For this API, increased intestinal retention through thiol-mediated mucoadhesion, enhancement in paracellular transport through the opening of tight junctions, protection from P-gp recognition, P-gp-independent transcytosis across the intestinal endothelium, and inhibition of P-gp efflux were identified using thiolated chitosan delivery systems.63,146,199 Accordingly, Saremi et al., as displayed in Figure 12, achieved a 10.6-fold higher oral bioavailability of docetaxel administered to rats via nanoparticles coated with chitosan–glutathione in comparison to the market formulation. These results exemplify the potential of thiolated chitosans for drug delivery.63
Figure 12.
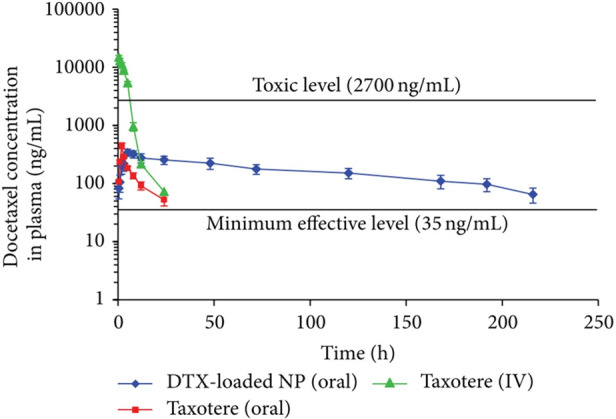
Docetaxel plasma concentration in rats after intravenous injection of the market formulation Taxotere (red ■), oral administration of Taxotere (green ▲), and oral administration of docetaxel-loaded nanoparticles coated with chitosan–glutathione (DTX-loaded NP, blue ◆). Indicated values are means (n = 5) ± SD. Reprinted with permission from ref (63). Copyright 2013 Saremi et al., distributed under the Creative Commons Attribution License (CC BY 3.0).
4.3. Theranostics: Photodynamic and Photothermal Therapy
Photodynamic and photothermal therapy are known as therapies involving electromagnetic energy as the trigger and either a photosensitizer, leading to the formation of reactive oxygen species (ROS), or a metal, converting electromagnetic energy into heat (via the surface plasmon resonance phenomena). In both ways, ROS or heat is used to selectively kill cells. Gold nanorods (GNRs), synthesized via seed-mediated growth assisted by cetyltrimethylammonium bromide (CTAB), represent the most studied nanoparticular system for these therapies. Due to a high unspecific cytotoxicity, the use of CTAB-linked GNR is limited.
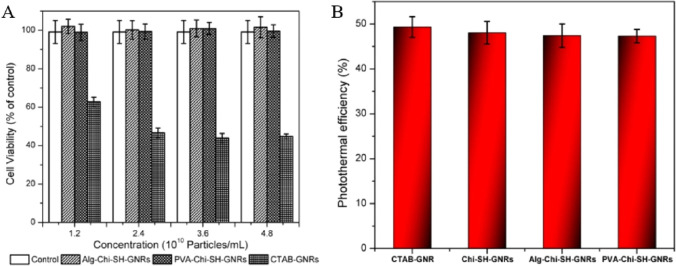
Almada et al. evaluated the cytotoxicity and photothermal efficiency of GNRs prepared with a chitosan–3-mercaptopropionic acid conjugate. Thereby, the substitution of CTAB with thiolated chitosan significantly increased the viability of MDA-MB-231 cells from 40% to 100%. However, to overcome a nearly neutral zeta potential at physiological pH, GNRs based on thiolated chitosan were coated with an additional polymer to prevent aggregation. GNRs formed with thiolated chitosan and coated with either poly(vinyl alcohol) or alginate achieved an equivalent photothermal efficiency compared to CTAB-GNR, as illustrated in Figure 13.237
Figure 13.
Cell viability of MDA-MB-231 line treated with GNRs. Photothermal efficiency (η) of GNRs covered with different polymers. Chi–SH-GNR = chitosan-coated GNRs; Alg-Chi-SH-GNR = chitosan-coated GNRs covered with alginate; PVA-Chi-SH-GNR = chitosan-coated GNRs covered with poly(vinyl alcohol). Reprinted with permissions from ref (237). Copyright 2017 Elsevier.
Similar results were obtained by Iqbal et al., who showed the photothermal and photodynamic activity of cobalt-dotted zinc oxide particles coated with thiolated chitosan in sunlight. Thereby, an antibacterial activity against methicillin-resistant Staphylococcus aureus of 3–5% in the dark and 100% after activation in sunlight for 15 min was observed. Moreover, nanoparticles coated with thiolated chitosan displayed a significantly enhanced antimicrobial activity upon photo-inactivation of methicillin-resistant S. aureus compared to uncoated nanoparticles.62 This synergistic effect of ROS production and antimicrobial activity was also found for iron-doped nanocerias (nanoparticles formed with cerium) that were utilized to eradicate antibiotic-resistant bacteria prevalent in hospital wastewater in sunlight.168
However, a major drawback of photodynamic therapy is toxicity due to a broad distribution of the utilized reagent in the body. Therefore, chitosan glutathione was used to develop multifunctional photosensitive selenium nanoparticles with the potential to target activated macrophages via CD44 as well as FR-β receptors and convert H2O2 to singlet oxygen (1O2) to specifically eradicate inflammatory macrophages. To generate such a carrier, the −SH groups of the thiolated chitosan were utilized to couple the photosensitizer Rose Bengal via amide bond formation, and catalase was conjugated by disulfide bond formation. After internalization, the intracellular reduction of disulfide bonds between catalase and the nanoparticles triggered the release of the enzyme, causing the degradation of H2O2 to O2. The resulting O2 was converted to 1O2 by Rose Bengal, leading to a cytotoxic effect on proinflammatory-activated macrophages. In addition, quenching of intracellular H2O2 allowed for better fluorescence imaging, as well as inhibiting inflammation-associated nitric oxide production. In conclusion, more specific imaging and stronger photodynamic therapy effects for detecting and killing activated macrophages make this system highly attractive for theranostic applications.65
Additionally, Bharathiraja et al. successfully demonstrated the applicability of chitosan thioglycolic acid in the field of theranostics in vivo. Tumors, induced by injecting MDA-MB-231 cells into mice, were relapsed and undetectable after 20 days following the administration of nanocomposites composed of thiolated chitosan, palladium nanoparticles, and RGD peptide (for MDA-MB-231 targeted delivery) and laser irradiation.236 Thus, the lower cytotoxicity of thiolated chitosan while maintaining sufficient photothermal efficiency and the redox-responsive release of S–S coupled enzymes make thiolated chitosans highly interesting for theranostics research and development.
4.4. Tissue Engineering
In addition to transplantation, tissue engineering provides another way to treat tissue and organ loss or damage. Thereby, cells from the patient, from another genetically non-identical individual, or from animal species are seeded to a biocompatible 3D matrix combined with the possibility to incorporate bioactive molecules within this scaffold to improve cellular function and desired tissue formation.
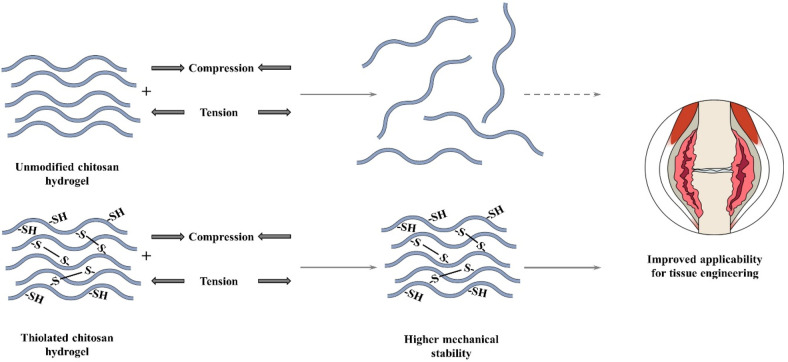
The polymer matrix should exhibit hydration characteristics similar to those of the tissue and an interconnected microstructure to promote the transport of nutrients as well as oxygen, and should enable an easy modification of the chemical ligand for cell attachment, while maintaining its mechanical structure until the tissue has been completely formed.138,251 Among others, chitosan-based hydrogels exhibit such properties; however, they also show several common shortcomings, such as easy breakability, low strength, and poor elasticity.242,252,253 In order to overcome these issues, Wu et al. attached N-acetylcysteine to an N-isopropylacrylamide–chitosan-based hydrogel and observed improved mechanical properties, such as an over 9-fold improvement of the compressive modulus and a correlation between storage modulus and cross-linking density, as demonstrated in Figure 14. Simultaneously, the hydrogel facilitated adequate cell proliferation of mesenchymal stem cells and fibroblasts as well as osteoblasts.138
Figure 14.
Illustrative comparison of the mechanical characteristics of an unmodified chitosan hydrogel and a thiolated chitosan hydrogel intended for use in tissue engineering.
Liu et al. utilized a similar method to establish a stable dual network gel formulation by coupling chitosan–N-acetylcysteine to silk fibroin. The introduced thiolated chitosan enhanced the strength and stiffness of the gel, whereas the silk fibroin component primarily contributed to its elasticity.
The resulting scaffold facilitated a three-dimensional growth of chondrocytes, making the composite highly interesting for cartilage repair.242 Moreover, Chen et al. demonstrated the applicability of thiolated chitosan for bone tissue engineering in vivo. The osteoinductive protein P24 was coupled via disulfide bond formation on a biomimetic scaffold based on thiolated chitosan and hydroxyapatite. Compared to the control (scaffold without P24), a significantly higher ectopic osteogenesis level in rat dorsal muscle pockets as well as superior performance in the reconstruction of calvarial bone defects were observed for the P24-loaded scaffold.147
4.5. Wound Treatment
Treatment of chronic wounds requires intensive medical intervention at huge healthcare costs, and the incidence of such wounds increases as more people get older and suffer from diabetes.173 Different strategies have been developed to make the wound healing process faster and less painful.
For instance, the wound healing properties of chitosan–N-acetylcysteine on corneal tissue were assessed in rabbits with monocular epithelial debridement. Time for wound healing was significantly reduced in the thiolated chitosan group compared to the placebo group treated with phosphate buffered saline. The authors of the study stated that the underlying mechanism is not entirely clear but may be at least partially related to well-known effects of unmodified chitosan on wound healing.194In vitro studies conducted on fibroblasts revealed no statistically significant difference in migration and viability of cells treated with thiolated chitosan and its parent polymer.88 In a similar approach, Zahir-Jouzdani et al. demonstrated in vitro that the thiolation of chitosan through cysteine conjugation had no effect on the anti-fibrotic and anti-angiogenic properties compared to treatment with the polymer. However, thiolated chitosan was preferred for the following in vivo studies in mice, due to the increased mucoadhesion to the cornea compared to pristine chitosan. For assessing the wound healing capacity of chitosan–cysteine, corneal wounds were induced by applying NaOH to the right eye of the animals. After 21 days, the corneal appearance of the group treated with thiolated chitosan was similar to that of healthy animals, and only minor inflammation was observed. The treatment could also successfully prevent hyphema, a usually painful collection of blood inside the anterior chamber of the eye that can cause permanent vision problems if left untreated.197
Moreover, sustained antibacterial activity against infection is of great importance for wound healing. Therefore, Trp-rich peptide (PSI) was coupled via disulfide formation to chitosan cysteine to provide a sustained in vitro release of this polypeptide with a broad-spectrum antimicrobial activity over 20 days. However, no significant difference in wound closure was observed in vivo in mice, as hydrogels loaded with PSI based on thiolated chitosan as well as pristine chitosan had formed normal epidermal–dermal layer structure after 21 days.254 A more pronounced influence of chitosan–thioglycolic acid on wound closure rate in vivo in mice was observed by Arshad et al., underlined by results shown in Figure 15. The wound healing capacity and wound closure rate of a thiolated chitosan alginate zinc oxide nanoparticles bandage were more enhanced compared to those of the reference groups treated with either the unmodified chitosan alginate bandage with incorporated zinc oxide nanoparticles or a marketed product. The authors stated that enhanced mucoadhesion and retention of the bandage ensured proper dosing and enhanced antimicrobial activity, and resulted in an increased healing capacity and wound closure.71 Nevertheless, inhibitory activity of sulfhydryl groups against the chronic wound enzymes observed in vitro and ex vivo might also be responsible for improved wound closure.99,173
Figure 15.
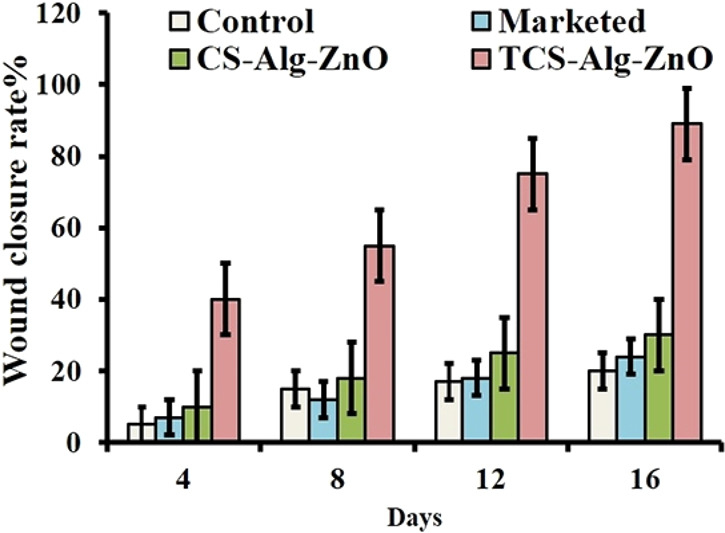
In vivo analysis of wound healing ability of bandages in mice wounded by sterilized needles near the hind limb. Graph showing speed of wound closure in terms of reduction in wound size after application of bandages. CS-Alg-ZnO = chitosan alginate zinc oxide nanoparticles; TCS-Alg-ZnO = thiolated alginate zinc oxide nanoparticles. Reprinted with permission from ref (71). Copyright 2019 Arshad et al., distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
4.6. Coating Material
Coating with thiolated chitosan to alter surface characteristics is a promising approach to inhibit bacterial adhesion and biofilm formation on materials prone to fouling. For instance, carboxymethyl chitosan–4-thiobutylamidine was coupled via Michael addition on maleimido-containing tannic acid anchored on stainless steel. Thereby, the surface coated with thiolated chitosan exhibited a 70% reduced protein adsorption and 91% decreased adhesion of Escherichia coli compared to stainless steel modified with maleimido-containing tannic acid.255 The reduced antifouling can be attributed to the increased hydrophilicity of the surface, since protein adsorption and bacterial adhesion correlate with the hydrophobicity of the surface.256,257 However, no conclusion based on these results could be made on the anti-adhesive effect of the thiol moiety.
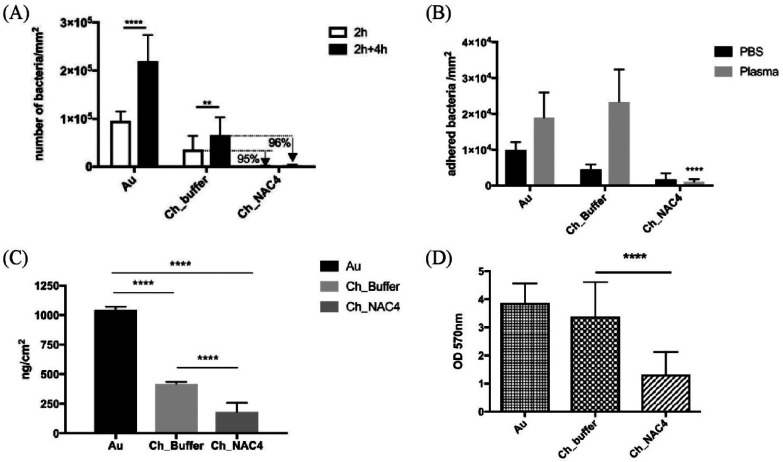
In another study conducted by Costa et al., a bacterial anti-adhesive coating was developed to avoid Staphylococcus aureus adhesion and biofilm formation. Biological studies confirmed chitosan–N-acetylcysteine to be a promising material, as it promoted a ∼95% decrease in bacterial adhesion compared to unmodified chitosan coating (Figure 16a). The authors stated that the reduction of free available amino groups of chitosan as well as the increase of surface hydrophilicity might decrease bacterial adhesion directly or indirectly through the decrease of protein adsorption. This theory was underlined by higher adherence of bacteria on a gold surface and a gold surface coated with pristine chitosan in the presence of protein from human plasma (Figure 16b). Moreover, thiolation of chitosan promoted a 5.5- and 2.2-fold reduction of protein adsorption from human plasma compared to gold and pristine chitosan, respectively (Figure 16c). Anti-adherence properties of thiolated chitosan were further proven through the quantification of the total biofilm mass, as this parameter was efficiently reduced using this thiolated chitosan for surface modification (Figure 16d). As declared by the authors, it was unclear if the exposed thiol groups had any direct contribution to preventing specific adhesion (e.g., degrading relevant disulfide bridges of bacterial adhesins), or if the overall mechanism was purely based on non-specific anti-adhesive effects.245 Therefore, protein as well as bacterial adhesion studies performed with coatings of chitosan with different amounts of free thiol groups might be helpful to assess the influence of the thiol moiety.
Figure 16.
Evaluation of anti-adhesive properties of films based on gold (Au), Chitosan (Ch_Buffer), and chitosan–N-acetylcysteine (Ch_NAC4). (A) S. aureus adhesion on Au, Ch_Buffer, and Ch_NAC4 films after 2 h incubation in growth medium (white bars) and 4 h re-incubation on fresh growth medium after the 2 h pre-incubation period (black bars). (B) S. aureus adhesion on Au, Ch_Buffer, and Ch_NAC4 after 2 h incubation in phosphate-buffered saline (PBS) or PBS supplemented with 1% human plasma. (C) Mass of proteins from 1% (v/v) human plasma adsorbed on Au, Ch_Buffer, and Ch_NAC4 surfaces. (D) Effect of Au, chitosan, and thiolated chitosan on S. aureus total biofilm biomass formation. Data represented as means (n = 3) ± SD. Reprinted with permission from ref (245), licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0).
Furthermore, pegylated chitosan–4-thiobutylamidine was investigated within the development of a polymer film intended for use as a food-packaging material. The thiol-modified film exhibited antibacterial activity, which was absent for native chitosan, and displayed higher compatibility with polyethylene, as a more transparent film was obtained.101
4.7. Textile Industry
From yarn formation to a finished evening dress, industrial textile manufacturing includes several processing steps that end in a final finishing operation, where biocidal finishing agents are used to provide antimicrobial characteristics to shield the end commodities from microbial degradation.258 For such biocidal finishing agents, not only the antimicrobial efficiencies but also environmental, health, and safety aspects must be considered. Its biocompatibility and biodegradability make chitosan a highly promising agent for this application. However, its weak adhesion to fibers results in a gradual leaching from the textile with repetitive washing.
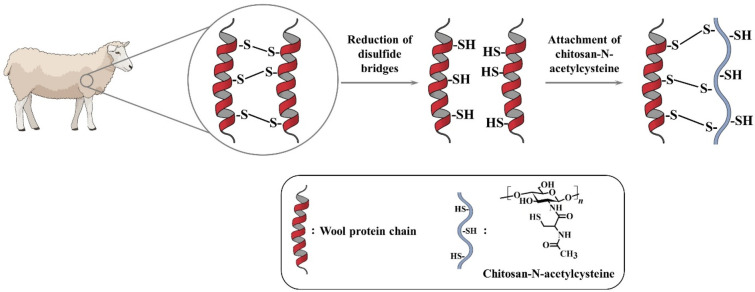
In order to enforce the adhesion of chitosan, several methods were developed using cross-linking agents, combinations of physical treatments with UV or plasma sources, as well as biological treatments like enzymatic methods.259 More recently, Zhang et al. investigated a novel approach, illustrated in Figure 17, for grafting of thiolated chitosan onto wool via disulfide bond formation between the thiol-bearing side chain N-acetylcysteine and sulfhydryl groups of the wool proteins (keratins).
Figure 17.
Schematic depiction of grafting chitosan–N-acetylcysteine onto wool fibers.
Besides the improvement of several characteristics important for textile manufacturing (shrink-resistance, dyeing ability), bacteriostatic rates up to 58.32% and 63.96% were achieved for disulfide-linked chitosan and adsorbed unmodified chitosan, respectively. Thiolation of chitosan decreased the number of free amino groups, causing a slight weakening of the antibacterial properties. However, considering that the greatest disadvantage of chitosan as a biocidal is its weak adhesion,260 disulfide cross-linking seems to be a novel, highly promising approach for the use of thiolated chitosans within the textile industry.52
4.8. Water Treatment
Pollution of drinking water is an issue that has been increasing over the past few years. Due to their physiochemical, mechanical, and biological properties, thiolated chitosans offer a great potential for applications within water treatment by complexing and adsorbing inorganic pollutants.116−118 However, a comparison of different studies is delicate, since experimental conditions are not always the same (temperature, pH, concentration of metal as well as adsorbent, incubation time, and interfering ions/metal). Nevertheless, the outcomes summarized in Table 7 underline the potential of thiolated chitosans as adsorbents in wastewater treatment.
Table 7. Adsorption Capacities of Thiolated Chitosans Evaluated for Heavy Metal Removal.
| species | adsorption (milligrams of heavy metal ion per gram polymer) | removal efficiency (%) | commentary | references |
|---|---|---|---|---|
| As3+ | 15.0–17.08 | 85.4 | Thiolated chitosan (amine and hydroxyl groups were substituted with −SH groups using thiourea and microwave irradiation) showed high adsorption ability for As3+ and As5+ at different pH ranges. Arsenic concentrations were reduced below the limit determined by the WHO for drinking water. Moreover, thiolated chitosan also efficiently adsorbed As5+ and As3+ in solutions with high concentrations of further ions. | (118)a |
| As5+ | 15.4–17.70 | 87.0 | ||
| Pd2+ | 175.4 | 83.58–99.08 | Adsorption and desorption efficiencies for Pd2+ comparable to those of commercially available resins like Lewatit TP214 were achieved using chitosan 3-amino-1,2,4-triazole-5-thiol as adsorbent. | (115)a |
| As5+ | 66.27 | 70–80 | Maximum adsorption capacity of N-(2-hydroxyl)propyl-3-trimethylammonium chitosan–cysteine for Pb2+ was higher compared to most of the reported materials. Adsorption kinetic studies indicated that the adsorption was mainly promoted by a chemical process. Furthermore, chemisorption, ion exchange, surface complexation, physical adsorption, electrostatic interaction, and precipitation improved the adsorption capacity of the composite for the tested metal ions. Additionally, a sufficient regeneration performance was observed. | (32)a |
| As3+ | 67.69 | 60–70 | ||
| Hg2+ | 28.00 | 70–90 | ||
| Cu2+ | 33.99 | 90–100 | ||
| Zn2+ | 13.63 | 90 | ||
| Cd2+ | 16.34 | 90 | ||
| Pb2+ | 235.63 | 90–100 | ||
| Au3+ | 198.5 | 80 | The hydrogel based on chitosan–2,5-dimercapto-1,3,4-thiodiazole was efficient in removing Au3+, Pd2+, and Pt4+ from dilute solutions. Sorption studies revealed a considerable capacity for Au3+ ions, which might be useful in the removal of gold from ores. | (116) |
| Pd2+ | 17.0 | 100 | ||
| Pt4+ | 15.3 | >80 | ||
| Ni2+ | 0.131 (mole of metal ion per mole polymer unit) | 83 | Chitosan-glutathione displayed a 40% higher adsorption capacity for Ni2+ ions from aqueous solution compared to the unmodified polymer. | (175) |
| Cu2+ | 208–238 | n.d. | N-(2-Hydroxy-3-mercaptopropyl)-chitosan exhibited an up to 1.6-fold improved retention capacity for mercury ions compared to unmodified chitosan. | (117) |
| Hg2+ | 556–588 | n.d. | ||
Publication displays tables summarizing adsorption data of the corresponding pollutant published by other research groups.
Moreover, the complexation capability of chitosan-SH was also utilized for colorimetric determination of Hg2+. The developed sensor showed a quick response, good selectivity, and high sensitivity, with a detection limit of 0.465 ppb by the naked eye, below the upper limit of Hg2+ in drinking water of 6 ppb according to the WHO.120 Besides inorganic pollutants, thiolated chitosans are also under investigation for treatment of organic pollutants within wastewater. Recently, an Fe3O4–thiolated chitosan hybrid composite was synthesized for laccase immobilization to decolorize the textile dyes Reactive Blue 171 and Acid Blue 74. The enzyme was covalently attached via disulfide bond formation onto the composite and retained its high decolorization activity during repeated use.261
In a different approach, Carvalho et al. evaluated 3D sponges based on a chitosan–mercaptoundecanoic acid conjugate as adsorbent. Their study revealed a high adsorption capacity of 388 mg/g and a removal efficiency greater than 90% over a broad range of concentration from 20 to 1200 mg/L for the model organic pollutant Methyl Orange.50 Furthermore, thiolated chitosans were applied within the removal of antimicrobial contaminations. Khan et al. developed iron-doped nanoceria (nanoparticles consisting of cerium) coated with folate-grafted chitosan–thioglycolic acid. Metal-doped nanoceria are generally used due to their ability to generate ROS by photocatalytic activity. In their study, they effectively demonstrated a total eradication of all bacteria present in hospital wastewater by applying nanoparticles in a concentration of 100 μg/mL.168 Overall, these studies underline the potential of thiolated chitosans for application in water treatment by removing organic as well as inorganic pollutants, immobilizing enzymes utilized to degrade specific substances, and for developing composites to eliminate antimicrobial contaminations.
4.9. Cosmetics
The application of cosmetics based on thiolated chitosans for hair, eyelashes, and eyebrows treatments (styling gels, repairing agents, coloring agents, detergents, and coating), nail polishes, make-up, and antiperspirants is highly interesting, due to the thiol-bearing proteins of hair, nails, and skin.262 Thus, thiolated chitosans exhibit excellent adhesion via covalent disulfide bond formation with cysteine subunits of these surface proteins, stabilizing the product after application without smearing and dissolution.263 As a result, several patents regarding the use of thiolated chitosans as cosmetic ingredients were filed.262−264
However, the European Commission database for information on cosmetic substances and ingredients (CosIng) has currently listed only one thiolated chitosan applied as a film-forming, skin-conditioning, and skin-protecting agent within cosmetic products, namely chitosan–glutathione. According to a patent filed for this thiolated chitosan, it displays a 40% higher binding capacity for Ni2+ ions in comparison to unmodified chitosan.175 As heavy metals and in particular Ni2+ from, for instance, jewelry are common causes of contact dermatitis, chitosan–glutathione, so-called Chitatione, is utilized in the cosmetic lines Nidiesque and Nuev to coat the skin with a film and thereby prevent the permeation of metals ions, inhibiting allergy symptoms, as illustrated in Figure 18.265,266
Figure 18.
Schematic illustration of contact dermatitis prevention by cosmetics containing chitosan–glutathione.
5. Future Perspectives
The great future potential of thiolated chitosans lies in the broad diversity of sulfhydryl ligands that can be covalently attached to the polymeric backbone. Due to this diversity, specific properties can be adjusted on demand, and additional functions can be introduced. As thiol/disulfide exchange reactions and the formation of disulfides are directed by opposite charges in their surroundings attracting each other and bringing the reactive sulfur species close to each other, in particular charged ligands will likely be used to a higher extent in the years to come. Such ligands will allow the formation of more targeted disulfide bonds between thiol or disulfide substructures of interest. Another category of sulfhydryl ligands that will definitely shape the future landscape of thiolated chitosans are less reactive S-protected thiols. So far, thiol groups on chitosan were S-protected by the formation of disulfide bonds with mercaptopyridine analogues, as illustrated in Figure 1. Due to the electron-withdrawing effect of the π-system of the pyridine, the disulfide bond is highly reactive toward free thiols. In contrast, thiol groups on chitosan, being S-protected by the formation of disulfide bonds with sulfhydryl ligands that do not exhibit such an electron-withdrawing effect like cysteine or N-acetylcysteine, are less reactive. Very recently, Netsomboon et al. demonstrated that, when thiol groups are S-protected with N-acetylcysteine instead of with a highly reactive mercaptopyridine analogue, even higher mucoadhesive properties can be achieved. This effect was explained by a more pronounced interpenetration of the less reactive thiolated chitosan into deeper regions of the mucus gel layer, which is more tightly anchored there than highly reactive S-protected thiolated chitosans reacting already with just loose mucus on the surface of the gel layer.267 Furthermore, having the booming thiol–ene cross-linking of biopolymers in mind,268 we expect the development of numerous potential hydrogels containing thiolated chitosans that are cross-linked by this type of reaction.
On the application side, further product developments will follow, as the success of the first products containing thiolated chitosans on the global market sends out a strong positive signal. For example, Lacrimera eye drops showed impressive improvements in late-stage clinical trials in treatment of dry eye syndrome. Accordingly, the same thiolated chitosan will likely show similar potential for the treatment of dry mouth and vaginal syndromes. In the case of drug delivery, thiolated chitosans are already in clinical trials. Other areas of applications, such as the use of thiolated chitosans in the textile industry, are comparatively young, but there is no doubt that sound products based on thiolated chitosans will also emerge from there.
Glossary
Abbreviations
- DD
degree of deacetylation
- API
active pharmaceutical ingredient
- DES
dry eye syndrome
- C-NAC
chitosan–N-acetylcysteine
- ROS
reactive oxygen species
- GNR
gold nanorod
- CTAB
cetyltrimethylammonium bromide
- PSI
Trp-rich-peptide
The authors declare no competing financial interest.
References
- Xing L.; Du L.; Luo C.-Q.; Zhou T.-J.; Zhu Y.; Gong J.-H.; Jin Y.; Jiang H.-L. Chitosan and Its Derivatives as Chemical Drug Delivery Carriers. Curr. Org. Chem. 2018, 22 (7), 690–707. 10.2174/1385272821666170818160236. [DOI] [Google Scholar]
- Ways T. M.; Lau W.; Khutoryanskiy V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers (Basel, Switz.) 2018, 10 (3), 267. 10.3390/polym10030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T.; Tanaka M.; Huang Y.-Y.; Hamblin M. R. Chitosan Preparations for Wounds and Burns: Antimicrobial and Wound-Healing Effects. Expert Rev. Anti-Infect. Ther. 2011, 9 (7), 857–879. 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boamah P. O.; Huang Y.; Hua M.; Zhang Q.; Wu J.; Onumah J.; Sam-Amoah L. K.; Boamah P. O. Sorption of Heavy Metal Ions onto Carboxylate Chitosan Derivatives—A Mini-Review. Ecotoxicol. Environ. Saf. 2015, 116, 113–120. 10.1016/j.ecoenv.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Şenel S.; McClure S. J. Potential Applications of Chitosan in Veterinary Medicine. Adv. Drug Delivery Rev. 2004, 56 (10), 1467–1480. 10.1016/j.addr.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Alves N. M.; Mano J. F. Chitosan Derivatives Obtained by Chemical Modifications for Biomedical and Environmental Applications. Int. J. Biol. Macromol. 2008, 43 (5), 401–414. 10.1016/j.ijbiomac.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Choi C.; Nam J.-P.; Nah J.-W. Application of Chitosan and Chitosan Derivatives as Biomaterials. J. Ind. Eng. Chem. 2016, 33, 1–10. 10.1016/j.jiec.2015.10.028. [DOI] [Google Scholar]
- Miretzky P.; Cirelli A. F. Hg(II) Removal from Water by Chitosan and Chitosan Derivatives: A Review. J. Hazard. Mater. 2009, 167 (1–3), 10–23. 10.1016/j.jhazmat.2009.01.060. [DOI] [PubMed] [Google Scholar]
- Ahmed T.; Aljaeid B. Preparation, Characterization, and Potential Application of Chitosan, Chitosan Derivatives, and Chitosan Metal Nanoparticles in Pharmaceutical Drug Delivery. Drug Des., Dev. Ther. 2016, 10, 483. 10.2147/DDDT.S99651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arca H. Ç.; Günbeyaz M.; Şenel S. Chitosan-Based Systems for the Delivery of Vaccine Antigens. Expert Rev. Vaccines 2009, 8 (7), 937–953. 10.1586/erv.09.47. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnürch A.Mucoadhesive Polymers, Their Use and Their Production Method. Austria Patent AT269105T, 1998.
- Bernkop-Schnürch A.; Brandt U. M.; Clausen A. E. Synthesis and in Vitro Evaluation of Chitosan-Cysteine Conjugates. Sci. Pharm. 1999, 67, 196–208. [Google Scholar]
- Leichner C.; Jelkmann M.; Bernkop-Schnürch A. Thiolated Polymers: Bioinspired Polymers Utilizing One of the Most Important Bridging Structures in Nature. Adv. Drug Delivery Rev. 2019, 151–152, 191–221. 10.1016/j.addr.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Thakur V. K.; Thakur M. K. Recent Advances in Graft Copolymerization and Applications of Chitosan: A Review. ACS Sustainable Chem. Eng. 2014, 2 (12), 2637–2652. 10.1021/sc500634p. [DOI] [Google Scholar]
- Kumar A.; Vimal A.; Kumar A. Why Chitosan? From Properties to Perspective of Mucosal Drug Delivery. Int. J. Biol. Macromol. 2016, 91, 615–622. 10.1016/j.ijbiomac.2016.05.054. [DOI] [PubMed] [Google Scholar]
- Younes I.; Rinaudo M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13 (3), 1133–1174. 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dünnhaupt S.; Barthelmes J.; Thurner C. C.; Waldner C.; Sakloetsakun D.; Bernkop-Schnürch A. Protected Thiolated Chitosan: Synthesis and in Vitro Characterization. Carbohydr. Polym. 2012, 90 (2), 765–772. 10.1016/j.carbpol.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werle M.; Bernkop-Schnürch A. Thiolated Chitosans: Useful Excipients for Oral Drug Delivery. J. Pharm. Pharmacol. 2008, 60 (3), 273–281. 10.1211/jpp.60.3.3001. [DOI] [PubMed] [Google Scholar]
- Phadungcharoen N.; Patrojanasophon P.; Opanasopit P.; Ngawhirunpat T.; Chinsriwongkul A.; Rojanarata T. Smartphone-Based Ellman’s Colourimetric Methods for the Analysis of d-Penicillamine Formulation and Thiolated Polymer. Int. J. Pharm. (Amsterdam, Neth.) 2019, 558, 120–127. 10.1016/j.ijpharm.2018.12.078. [DOI] [PubMed] [Google Scholar]
- Luo Q.; Han Q.; Wang Y.; Zhang H.; Fei Z.; Wang Y. The Thiolated Chitosan: Synthesis, Gelling and Antibacterial Capability. Int. J. Biol. Macromol. 2019, 139, 521–530. 10.1016/j.ijbiomac.2019.08.001. [DOI] [PubMed] [Google Scholar]
- Croce M.; Conti S.; Maake C.; Patzke G. R. Synthesis and Screening of N-Acyl Thiolated Chitosans for Antibacterial Applications. Carbohydr. Polym. 2016, 151, 1184–1192. 10.1016/j.carbpol.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Kongsong M.; Songsurang K.; Sangvanich P.; Siralertmukul K.; Muangsin N. Design, Synthesis, Fabrication and in Vitro Evalution of Mucoadhesive 5-Amino-2-Mercaptobenzimidazole Chitosan as Low Water Soluble Drug Carriers. Eur. J. Pharm. Biopharm. 2014, 88 (3), 986–997. 10.1016/j.ejpb.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Varkouhi A. K.; Verheul R. J.; Schiffelers R. M.; Lammers T.; Storm G.; Hennink W. E. Gene Silencing Activity of SiRNA Polyplexes Based on Thiolated N, N, N -Trimethylated Chitosan. Bioconjugate Chem. 2010, 21 (12), 2339–2346. 10.1021/bc1003789. [DOI] [PubMed] [Google Scholar]
- Verheul R. J.; Van Der Wal S.; Hennink W. E. Tailorable Thiolated Trimethyl Chitosans for Covalently Stabilized Nanoparticles. Biomacromolecules 2010, 11 (8), 1965–1971. 10.1021/bm1002784. [DOI] [PubMed] [Google Scholar]
- Medeiros Borsagli F. G. L.; Carvalho I. C.; Mansur H. S. Amino Acid-Grafted and N-Acylated Chitosan Thiomers: Construction of 3D Bio-Scaffolds for Potential Cartilage Repair Applications. Int. J. Biol. Macromol. 2018, 114 (2017), 270–282. 10.1016/j.ijbiomac.2018.03.133. [DOI] [PubMed] [Google Scholar]
- Li R.; Liu Q.; Wu H.; Wang K.; Li L.; Zhou C.; Ao N. Preparation and Characterization of In-Situ Formable Liposome/Chitosan Composite Hydrogels. Mater. Lett. 2018, 220, 289–292. 10.1016/j.matlet.2018.03.052. [DOI] [Google Scholar]
- Meena L. K.; Raval P.; Kedaria D.; Vasita R. Study of Locust Bean Gum Reinforced Cyst-Chitosan and Oxidized Dextran Based Semi-IPN Cryogel Dressing for Hemostatic Application. Bioact. Mater. 2018, 3 (3), 370–384. 10.1016/j.bioactmat.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavian E.; Dorkoosh F. A.; Rafiee-Tehrani M. Design, Characterization and Ex Vivo Evaluation of Chitosan Film Integrating of Insulin Nanoparticles Composed of Thiolated Chitosan Derivative for Buccal Delivery of Insulin. Drug Dev. Ind. Pharm. 2014, 40 (5), 691–698. 10.3109/03639045.2014.886590. [DOI] [PubMed] [Google Scholar]
- Mortazavian E.; Amini M.; Dorkoosh F. A.; Amini H.; Khoshayand M. R.; Amini T.; Rafiee-Tehrani M. Preparation, Design for Optimization and in Vitro Evaluation of Insulin Nanoparticles Integrating Thiolated Chitosan Derivatives. J. Drug Delivery Sci. Technol. 2014, 24 (1), 40–49. 10.1016/S1773-2247(14)50006-8. [DOI] [Google Scholar]
- He C.; Yin L.; Song Y.; Tang C.; Yin C. Optimization of Multifunctional Chitosan–SiRNA Nanoparticles for Oral Delivery Applications, Targeting TNF-α Silencing in Rats. Acta Biomater. 2015, 17, 98–106. 10.1016/j.actbio.2015.01.041. [DOI] [PubMed] [Google Scholar]
- He C.; Yin L.; Tang C.; Yin C. Multifunctional Polymeric Nanoparticles for Oral Delivery of TNF-α SiRNA to Macrophages. Biomaterials 2013, 34 (11), 2843–2854. 10.1016/j.biomaterials.2013.01.033. [DOI] [PubMed] [Google Scholar]
- Song X.; Li L.; Zhou L.; Chen P. Magnetic Thiolated/Quaternized-Chitosan Composites Design and Application for Various Heavy Metal Ions Removal, Including Cation and Anion. Chem. Eng. Res. Des. 2018, 136 (iii), 581–592. 10.1016/j.cherd.2018.06.025. [DOI] [Google Scholar]
- Rahbarian M.; Mortazavian E.; Dorkoosh F. A.; Rafiee Tehrani M. Preparation, Evaluation and Optimization of Nanoparticles Composed of Thiolated Triethyl Chitosan: A Potential Approach for Buccal Delivery of Insulin. J. Drug Delivery Sci. Technol. 2018, 44 (2018), 254–263. 10.1016/j.jddst.2017.12.016. [DOI] [Google Scholar]
- Zhao X.; Yin L.; Ding J.; Tang C.; Gu S.; Yin C.; Mao Y. Thiolated Trimethyl Chitosan Nanocomplexes as Gene Carriers with High in Vitro and in Vivo Transfection Efficiency. J. Controlled Release 2010, 144 (1), 46–54. 10.1016/j.jconrel.2010.01.022. [DOI] [PubMed] [Google Scholar]
- He C.; Yin L.; Tang C.; Yin C. Trimethyl Chitosan-Cysteine Nanoparticles for Systemic Delivery of TNF-α SiRNA via Oral and Intraperitoneal Routes. Pharm. Res. 2013, 30 (10), 2596–2606. 10.1007/s11095-013-1086-4. [DOI] [PubMed] [Google Scholar]
- Rahmani S.; Hakimi S.; Esmaeily A.; Samadi F. Y.; Mortazavian E.; Nazari M.; Mohammadi Z.; Tehrani N. R.; Tehrani M. R. Novel Chitosan Based Nanoparticles as Gene Delivery Systems to Cancerous and Noncancerous Cells. Int. J. Pharm. (Amsterdam, Neth.) 2019, 560, 306–314. 10.1016/j.ijpharm.2019.02.016. [DOI] [PubMed] [Google Scholar]
- Hakimi S.; Mortazavian E.; Mohammadi Z.; Samadi F. Y.; Samadikhah H.; Taheritarigh S.; Tehrani N. R.; Rafiee-Tehrani M. Thiolated Methylated Dimethylaminobenzyl Chitosan: A Novel Chitosan Derivative as a Potential Delivery Vehicle. Int. J. Biol. Macromol. 2017, 95, 574–581. 10.1016/j.ijbiomac.2016.10.094. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Tang C.; Yin C. Galactosylated Trimethyl Chitosan–Cysteine Nanoparticles Loaded with Map4k4 SiRNA for Targeting Activated Macrophages. Biomaterials 2013, 34 (14), 3667–3677. 10.1016/j.biomaterials.2013.01.079. [DOI] [PubMed] [Google Scholar]
- Han L.; Tang C.; Yin C. Dual-Targeting and PH/Redox-Responsive Multi-Layered Nanocomplexes for Smart Co-Delivery of Doxorubicin and SiRNA. Biomaterials 2015, 60, 42–52. 10.1016/j.biomaterials.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Cheng W.; Tang C.; Yin C. Effects of Particle Size and Binding Affinity for Small Interfering RNA on the Cellular Processing, Intestinal Permeation and Anti-Inflammatory Efficacy of Polymeric Nanoparticles. J. Gene Med. 2015, 17 (10–12), 244–256. 10.1002/jgm.2866. [DOI] [PubMed] [Google Scholar]
- Gao C.; Liu T.; Dang Y.; Yu Z.; Wang W.; Guo J.; Zhang X.; He G.; Zheng H.; Yin Y.; Kong X. PH/Redox Responsive Core Cross-Linked Nanoparticles from Thiolated Carboxymethyl Chitosan for in Vitro Release Study of Methotrexate. Carbohydr. Polym. 2014, 111, 964–970. 10.1016/j.carbpol.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Mueller C.; Verroken A.; Iqbal J.; Bernkop-Schnuerch A. Thiolated Chitosans: In Vitro Comparison of Mucoadhesive Properties. J. Appl. Polym. Sci. 2012, 124 (6), 5046–5055. 10.1002/app.35622. [DOI] [Google Scholar]
- Millotti G.; Laffleur F.; Perera G.; Vigl C.; Pickl K.; Sinner F.; Bernkop-Schnürch A. In Vivo Evaluation of Thiolated Chitosan Tablets for Oral Insulin Delivery. J. Pharm. Sci. 2014, 103 (10), 3165–3170. 10.1002/jps.24102. [DOI] [PubMed] [Google Scholar]
- Moreno M.; Pow P. Y.; Tabitha T. S. T.; Nirmal S.; Larsson A.; Radhakrishnan K.; Nirmal J.; Quah S. T.; Geifman Shochat S.; Agrawal R.; Venkatraman S. Modulating Release of Ranibizumab and Aflibercept from Thiolated Chitosan-Based Hydrogels for Potential Treatment of Ocular Neovascularization. Expert Opin. Drug Delivery 2017, 14 (8), 913–925. 10.1080/17425247.2017.1343297. [DOI] [PubMed] [Google Scholar]
- Esmaeilzadeh P.; Menzel M.; Groth T. Cyclic Redox-Mediated Switching of Surface Properties of Thiolated Polysaccharide Multilayers and Its Effect on Fibroblast Adhesion. ACS Appl. Mater. Interfaces 2018, 10 (37), 31168–31177. 10.1021/acsami.8b12259. [DOI] [PubMed] [Google Scholar]
- Esmaeilzadeh P.; Köwitsch A.; Liedmann A.; Menzel M.; Fuhrmann B.; Schmidt G.; Klehm J.; Groth T. Stimuli-Responsive Multilayers Based on Thiolated Polysaccharides That Affect Fibroblast Cell Adhesion. ACS Appl. Mater. Interfaces 2018, 10 (10), 8507–8518. 10.1021/acsami.7b19022. [DOI] [PubMed] [Google Scholar]
- Esquivel R.; Juárez J.; Almada M.; Ibarra J.; Valdez M. A. Synthesis and Characterization of New Thiolated Chitosan Nanoparticles Obtained by Ionic Gelation Method. Int. J. Polym. Sci. 2015, 2015, 1–18. 10.1155/2015/502058. [DOI] [Google Scholar]
- Sivasubramanian M.; Kim Y.-J.; Chae S. Y.; Son S.; Jo D.-G.; Lee K. C.; Yoo C. K.; Park J. H. Thiolated Glycol Chitosan Bearing α -Cyclodextrin for Sustained Delivery of PEGylated Human Growth Hormone. J. Biomater. Sci., Polym. Ed. 2012, 23 (15), 1995–2005. 10.1163/092050611X603700. [DOI] [PubMed] [Google Scholar]
- Cho I. S.; Oh H. M.; Cho M. O.; Jang B. S.; Cho J.; Park K. H.; Kang S.-W.; Huh K. M. Synthesis and Characterization of Thiolated Hexanoyl Glycol Chitosan as a Mucoadhesive Thermogelling Polymer. Biomater. Res. 2018, 22 (1), 30. 10.1186/s40824-018-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho I. C.; Medeiros Borsagli F. G. L.; Mansur A. A. P.; Caldeira C. L.; Haas D. J.; Lage A. P.; Ciminelli V. S. T.; Mansur H. S. 3D Sponges of Chemically Functionalized Chitosan for Potential Environmental Pollution Remediation: Biosorbents for Anionic Dye Adsorption and ‘Antibiotic-Free’ Antibacterial Activity. Environ. Technol. 2019, 0 (0), 1–21. 10.1080/09593330.2019.1689302. [DOI] [PubMed] [Google Scholar]
- Noi I.; Schlachet I.; Kumarasamy M.; Sosnik A. Permeability of Novel Chitosan-g-Poly(Methyl Methacrylate) Amphiphilic Nanoparticles in a Model of Small Intestine In Vitro. Polymers (Basel, Switz.) 2018, 10 (5), 478. 10.3390/polym10050478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Zhang N.; Wang Q.; Wang P.; Yuan J.; Shen J.; Fan X. Disulfide Bond Reconstruction: A Novel Approach for Grafting of Thiolated Chitosan onto Wool. Carbohydr. Polym. 2019, 203, 369–377. 10.1016/j.carbpol.2018.09.074. [DOI] [PubMed] [Google Scholar]
- Duan Y.; Duan R.; Liu R.; Guan M.; Chen W.; Ma J.; Chen M.; Du B.; Zhang Q. Chitosan-Stabilized Self-Assembled Fluorescent Gold Nanoclusters for Cell Imaging and Biodistribution in Vivo. ACS Biomater. Sci. Eng. 2018, 4 (3), 1055–1063. 10.1021/acsbiomaterials.7b00975. [DOI] [PubMed] [Google Scholar]
- Denora N.; Lopedota A.; Perrone M.; Laquintana V.; Iacobazzi R. M.; Milella A.; Fanizza E.; Depalo N.; Cutrignelli A.; Lopalco A.; Franco M. Spray-Dried Mucoadhesives for Intravesical Drug Delivery Using N -Acetylcysteine- and Glutathione-Glycol Chitosan Conjugates. Acta Biomater. 2016, 43, 170–184. 10.1016/j.actbio.2016.07.025. [DOI] [PubMed] [Google Scholar]
- Langella A.; Calcagno V.; De Gregorio V.; Urciuolo F.; Imparato G.; Vecchione R.; Netti P. A. In Vitro Study of Intestinal Epithelial Interaction with Engineered Oil in Water Nanoemulsions Conveying Curcumin. Colloids Surf., B 2018, 164, 232–239. 10.1016/j.colsurfb.2018.01.028. [DOI] [PubMed] [Google Scholar]
- Trapani A.; Palazzo C.; Contino M.; Perrone M. G.; Cioffi N.; Ditaranto N.; Colabufo N. A.; Conese M.; Trapani G.; Puglisi G. Mucoadhesive Properties and Interaction with P-Glycoprotein (P-Gp) of Thiolated-Chitosans and -Glycol Chitosans and Corresponding Parent Polymers: A Comparative Study. Biomacromolecules 2014, 15 (3), 882–893. 10.1021/bm401733p. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Jin Z.; Huang W.; Sheng Y.; Wang Z.; Guo S. Tailor-Made Ternary Nanopolyplexes of Thiolated Trimethylated Chitosan with PDNA and Folate Conjugated Cis-Aconitic Amide-Polyethylenimine for Efficient Gene Delivery. Int. J. Biol. Macromol. 2020, 152, 948–956. 10.1016/j.ijbiomac.2019.10.212. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Wang J.; Li J.; Huang S.; Sheng Y. Thiolated Trimethylated Chitosan for Efficient Gene Delivery. Prog. Mod. Biomed. 2016, 33, 6414–6417. 10.13241/j.cnki.pmb.2016.33.004. [DOI] [Google Scholar]
- Atyabi F.; Talaie F. Thiolated Chitosan Nanoparticles as a Delivery System for Antisense Therapy: Evaluation against EGFR in T47D Breast Cancer Cells. Int. J. Nanomed. 2011, 6, 1963. 10.2147/IJN.S22731. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Atyabi F.; Talaie F.; Dinarvand R. Thiolated Chitosan Nanoparticles as an Oral Delivery System for Amikacin: In Vitro and Ex Vivo Evaluations. J. Nanosci. Nanotechnol. 2009, 9 (8), 4593–4603. 10.1166/jnn.2009.1090. [DOI] [PubMed] [Google Scholar]
- Hauptstein S.; Bonengel S.; Griessinger J.; Bernkop-Schnürch A. Synthesis and Characterization of PH Tolerant and Mucoadhesive (Thiol–Polyethylene Glycol) Chitosan Graft Polymer for Drug Delivery. J. Pharm. Sci. 2014, 103 (2), 594–601. 10.1002/jps.23832. [DOI] [PubMed] [Google Scholar]
- Iqbal G.; Faisal S.; Khan S.; Shams D. F.; Nadhman A. Photo-Inactivation and Efflux Pump Inhibition of Methicillin Resistant Staphylococcus Aureus Using Thiolated Cobalt Doped ZnO Nanoparticles. J. Photochem. Photobiol., B 2019, 192, 141–146. 10.1016/j.jphotobiol.2019.01.021. [DOI] [PubMed] [Google Scholar]
- Saremi S.; Dinarvand R.; Kebriaeezadeh A.; Ostad S. N.; Atyabi F. Enhanced Oral Delivery of Docetaxel Using Thiolated Chitosan Nanoparticles: Preparation, In Vitro and In Vivo Studies. BioMed Res. Int. 2013, 2013, 1–8. 10.1155/2013/150478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrin T. H. C.; Fadel V.; Martins D. B.; Dias S. A.; Cruz A.; Sergio L. M.; Arcisio-Miranda M.; Castanho M. A. R. B.; dos Santos Cabrera M. P. Synthesis and Characterization of Peptide–Chitosan Conjugates (PepChis) with Lipid Bilayer Affinity and Antibacterial Activity. Biomacromolecules 2019, 20 (7), 2743–2753. 10.1021/acs.biomac.9b00501. [DOI] [PubMed] [Google Scholar]
- Lu K.-Y.; Lin P.-Y.; Chuang E.-Y.; Shih C.-M.; Cheng T.-M.; Lin T.-Y.; Sung H.-W.; Mi F.-L. H. 2 O 2 -Depleting and O 2 -Generating Selenium Nanoparticles for Fluorescence Imaging and Photodynamic Treatment of Proinflammatory-Activated Macrophages. ACS Appl. Mater. Interfaces 2017, 9 (6), 5158–5172. 10.1021/acsami.6b15515. [DOI] [PubMed] [Google Scholar]
- Trapani A.; Mandracchia D.; Tripodo G.; Cometa S.; Cellamare S.; De Giglio E.; Klepetsanis P.; Antimisiaris S. G. Protection of Dopamine towards Autoxidation Reaction by Encapsulation into Non-Coated- or Chitosan- or Thiolated Chitosan-Coated-Liposomes. Colloids Surf., B 2018, 170, 11–19. 10.1016/j.colsurfb.2018.05.049. [DOI] [PubMed] [Google Scholar]
- Sinani G.; Sessevmez M.; Gök M. K.; Özgümüş S.; Alpar H. O.; Cevher E. Modified Chitosan-Based Nanoadjuvants Enhance Immunogenicity of Protein Antigens after Mucosal Vaccination. Int. J. Pharm. (Amsterdam, Neth.) 2019, 569, 118592. 10.1016/j.ijpharm.2019.118592. [DOI] [PubMed] [Google Scholar]
- Alamdarnejad G.; Sharif A.; Taranejoo S.; Janmaleki M.; Kalaee M. R.; Dadgar M.; Khakpour M. Synthesis and Characterization of Thiolated Carboxymethyl Chitosan-Graft-Cyclodextrin Nanoparticles as a Drug Delivery Vehicle for Albendazole. J. Mater. Sci.: Mater. Med. 2013, 24 (8), 1939–1949. 10.1007/s10856-013-4947-9. [DOI] [PubMed] [Google Scholar]
- Kazemi M. S.; Mohammadi Z.; Amini M.; Yousefi M.; Tarighi P.; Eftekhari S.; Rafiee Tehrani M. Thiolated Chitosan-Lauric Acid as a New Chitosan Derivative: Synthesis, Characterization and Cytotoxicity. Int. J. Biol. Macromol. 2019, 136, 823–830. 10.1016/j.ijbiomac.2019.06.132. [DOI] [PubMed] [Google Scholar]
- Mahmood A.; Lanthaler M.; Laffleur F.; Huck C. W.; Bernkop-Schnürch A. Thiolated Chitosan Micelles: Highly Mucoadhesive Drug Carriers. Carbohydr. Polym. 2017, 167 (31), 250–258. 10.1016/j.carbpol.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Arshad R.; Sohail M. F.; Sarwar H. S.; Saeed H.; Ali I.; Akhtar S.; Hussain S. Z.; Afzal I.; Jahan S.; Anees-ur-Rehman; Shahnaz G. ZnO-NPs Embedded Biodegradable Thiolated Bandage for Postoperative Surgical Site Infection: In Vitro and in Vivo Evaluation. PLoS One 2019, 14 (6), e0217079 10.1371/journal.pone.0217079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrman A. E.; Farrag M.; Grimm R. K.; Leipzig N. D. Evaluation of in Situ Gelling Chitosan-PEG Copolymer for Use in the Spinal Cord. J. Biomater. Appl. 2018, 33 (3), 435–446. 10.1177/0885328218792824. [DOI] [PubMed] [Google Scholar]
- Mukherjee D.; Srinivasan B.; Anbu J.; Azamthulla M.; Banala V. T.; Ramachandra S. G. Improvement of Bone Microarchitecture in Methylprednisolone Induced Rat Model of Osteoporosis by Using Thiolated Chitosan-Based Risedronate Mucoadhesive Film. Drug Dev. Ind. Pharm. 2018, 44 (11), 1845–1856. 10.1080/03639045.2018.1503297. [DOI] [PubMed] [Google Scholar]
- Sohail M. F.; Hussain S. Z.; Saeed H.; Javed I.; Sarwar H. S.; Nadhman A.; Huma Z.; Rehman M.; Jahan S.; Hussain I.; Shahnaz G. Polymeric Nanocapsules Embedded with Ultra-Small Silver Nanoclusters for Synergistic Pharmacology and Improved Oral Delivery of Docetaxel. Sci. Rep. 2018, 8 (1), 13304. 10.1038/s41598-018-30749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohail M. F.; Javed I.; Hussain S. Z.; Sarwar S.; Akhtar S.; Nadhman A.; Batool S.; Irfan Bukhari N.; Saleem R. S. Z.; Hussain I.; Shahnaz G. Folate Grafted Thiolated Chitosan Enveloped Nanoliposomes with Enhanced Oral Bioavailability and Anticancer Activity of Docetaxel. J. Mater. Chem. B 2016, 4 (37), 6240–6248. 10.1039/C6TB01348A. [DOI] [PubMed] [Google Scholar]
- Makhlof A.; Werle M.; Tozuka Y.; Takeuchi H. Nanoparticles of Glycol Chitosan and Its Thiolated Derivative Significantly Improved the Pulmonary Delivery of Calcitonin. Int. J. Pharm. (Amsterdam, Neth.) 2010, 397 (1–2), 92–95. 10.1016/j.ijpharm.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Shahnaz G.; Edagwa B. J.; McMillan J.; Akhtar S.; Raza A.; Qureshi N. A.; Yasinzai M.; Gendelman H. E. Development of Mannose-Anchored Thiolated Amphotericin B Nanocarriers for Treatment of Visceral Leishmaniasis. Nanomedicine 2017, 12 (2), 99–115. 10.2217/nnm-2016-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal I.; Sarwar H. S.; Sohail M. F.; Varikuti S.; Jahan S.; Akhtar S.; Yasinzai M.; Satoskar A. R.; Shahnaz G. Mannosylated Thiolated Paromomycin-Loaded PLGA Nanoparticles for the Oral Therapy of Visceral Leishmaniasis. Nanomedicine 2019, 14 (4), 387–406. 10.2217/nnm-2018-0038. [DOI] [PubMed] [Google Scholar]
- Lin F.; Rong J.; Wang M.; Bao D.; Wang Y.; Gong Z.; Gu Y.; Zhao Y.; Ge X. Chitosan-Based Core–Shell Structured Particles for in Vivo Sustainable Gene Transfection. J. Mater. Chem. B 2016, 4 (5), 893–901. 10.1039/C5TB02074C. [DOI] [PubMed] [Google Scholar]
- Huo M.; Fu Y.; Liu Y.; Chen Q.; Mu Y.; Zhou J.; Li L.; Xu W.; Yin T. N-Mercapto Acetyl-N′-Octyl-O, N″-Glycol Chitosan as an Efficiency Oral Delivery System of Paclitaxel. Carbohydr. Polym. 2018, 181, 477–488. 10.1016/j.carbpol.2017.10.066. [DOI] [PubMed] [Google Scholar]
- Zambito Y.; Felice F.; Fabiano A.; Di Stefano R.; Di Colo G. Mucoadhesive Nanoparticles Made of Thiolated Quaternary Chitosan Crosslinked with Hyaluronan. Carbohydr. Polym. 2013, 92 (1), 33–39. 10.1016/j.carbpol.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Fabiano A.; Piras A. M.; Uccello-Barretta G.; Balzano F.; Cesari A.; Testai L.; Citi V.; Zambito Y. Impact of Mucoadhesive Polymeric Nanoparticulate Systems on Oral Bioavailability of a Macromolecular Model Drug. Eur. J. Pharm. Biopharm. 2018, 130 (March), 281–289. 10.1016/j.ejpb.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Fabiano A.; Chetoni P.; Zambito Y. Mucoadhesive Nano-Sized Supramolecular Assemblies for Improved Pre-Corneal Drug Residence Time. Drug Dev. Ind. Pharm. 2015, 41 (12), 2069–2076. 10.3109/03639045.2015.1066798. [DOI] [PubMed] [Google Scholar]
- Rekha M. R.; Sharma C. P. Simultaneous Effect of Thiolation and Carboxylation of Chitosan Particles Towards Mucoadhesive Oral Insulin Delivery Applications: An In Vitro and In Vivo Evaluation. J. Biomed. Nanotechnol. 2015, 11 (1), 165–176. 10.1166/jbn.2015.1904. [DOI] [PubMed] [Google Scholar]
- Netsomboon K.; Suchaoin W.; Laffleur F.; Prüfert F.; Bernkop-Schnürch A. Multifunctional Adhesive Polymers: Preactivated Thiolated Chitosan-EDTA Conjugates. Eur. J. Pharm. Biopharm. 2017, 111, 26–32. 10.1016/j.ejpb.2016.10.029. [DOI] [PubMed] [Google Scholar]
- Perrone M.; Lopalco A.; Lopedota A.; Cutrignelli A.; Laquintana V.; Franco M.; Bernkop-Schnürch A.; Denora N. S-Preactivated Thiolated Glycol Chitosan Useful to Combine Mucoadhesion and Drug Delivery. Eur. J. Pharm. Biopharm. 2018, 132, 103–111. 10.1016/j.ejpb.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Beconcini D.; Fabiano A.; Zambito Y.; Berni R.; Santoni T.; Piras A.; Di Stefano R. Chitosan-Based Nanoparticles Containing Cherry Extract from Prunus Avium L. to Improve the Resistance of Endothelial Cells to Oxidative Stress. Nutrients 2018, 10 (11), 1598. 10.3390/nu10111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beconcini D.; Fabiano A.; Di Stefano R.; Macedo M. H.; Felice F.; Zambito Y.; Sarmento B. Cherry Extract from Prunus Avium L. to Improve the Resistance of Endothelial Cells to Oxidative Stress: Mucoadhesive Chitosan vs. Poly(Lactic-Co-Glycolic Acid) Nanoparticles. Int. J. Mol. Sci. 2019, 20 (7), 1759. 10.3390/ijms20071759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffleur F. Evaluation of Chemical Modified Hydrogel Formulation for Topical Suitability. Int. J. Biol. Macromol. 2017, 105, 1310–1314. 10.1016/j.ijbiomac.2017.07.152. [DOI] [PubMed] [Google Scholar]
- Laffleur F.; Fischer A.; Schmutzler M.; Hintzen F.; Bernkop-Schnürch A. Evaluation of Functional Characteristics of Preactivated Thiolated Chitosan as Potential Therapeutic Agent for Dry Mouth Syndrome. Acta Biomater. 2015, 21, 123–131. 10.1016/j.actbio.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Gradauer K.; Barthelmes J.; Vonach C.; Almer G.; Mangge H.; Teubl B.; Roblegg E.; Dünnhaupt S.; Fröhlich E.; Bernkop-Schnürch A.; Prassl R. Liposomes Coated with Thiolated Chitosan Enhance Oral Peptide Delivery to Rats. J. Controlled Release 2013, 172 (3), 872–878. 10.1016/j.jconrel.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Zhou S.; Deng F.; Chen X.; Wang X.; Wang Y.; Zhang H.; Dai W.; He B.; Zhang Q.; Wang X. The Function and Mechanism of Preactivated Thiomers in Triggering Epithelial Tight Junctions Opening. Eur. J. Pharm. Biopharm. 2018, 133, 188–199. 10.1016/j.ejpb.2018.10.014. [DOI] [PubMed] [Google Scholar]
- Müller C.; Ma B. N.; Gust R.; Bernkop-Schnürch A. Thiopyrazole Preactivated Chitosan: Combining Mucoadhesion and Drug Delivery. Acta Biomater. 2013, 9 (5), 6585–6593. 10.1016/j.actbio.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Menzel C.; Silbernagl J.; Laffleur F.; Leichner C.; Jelkmann M.; Huck C. W.; Hussain S.; Bernkop-Schnürch A. 2,2′Dithiodinicotinyl Ligands: Key to More Reactive Thiomers. Int. J. Pharm. (Amsterdam, Neth.) 2016, 503 (1–2), 199–206. 10.1016/j.ijpharm.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Lupo N.; Jalil A.; Nazir I.; Gust R.; Bernkop-Schnürch A. In Vitro Evaluation of Intravesical Mucoadhesive Self-Emulsifying Drug Delivery Systems. Int. J. Pharm. (Amsterdam, Neth.) 2019, 564, 180–187. 10.1016/j.ijpharm.2019.04.035. [DOI] [PubMed] [Google Scholar]
- Lupo N.; Fodor B.; Muhammad I.; Yaqoob M.; Matuszczak B.; Bernkop-Schnürch A. Entirely S-Protected Chitosan: A Promising Mucoadhesive Excipient for Metronidazole Vaginal Tablets. Acta Biomater. 2017, 64, 106–115. 10.1016/j.actbio.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Ho Y.; Wu S.; Mi F.; Chiu Y.; Yu S.; Panda N.; Sung H.-W. Thiol-Modified Chitosan Sulfate Nanoparticles for Protection and Release of Basic Fibroblast Growth Factor. Bioconjugate Chem. 2010, 21 (1), 28–38. 10.1021/bc900208t. [DOI] [PubMed] [Google Scholar]
- Al Harthi S.; Alavi S. E.; Radwan M. A.; El Khatib M. M.; AlSarra I. A. Nasal Delivery of Donepezil HCl-Loaded Hydrogels for the Treatment of Alzheimer’s Disease. Sci. Rep. 2019, 9 (1), 9563. 10.1038/s41598-019-46032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanov I.; Hinojosa-Caballero D.; Maspoch S.; Hoyo J.; Tzanov T. Enzymatic Synthesis of a Thiolated Chitosan-Based Wound Dressing Crosslinked with Chicoric Acid. J. Mater. Chem. B 2018, 6 (47), 7943–7953. 10.1039/C8TB02483A. [DOI] [PubMed] [Google Scholar]
- Javan B.; Atyabi F.; Shahbazi M. Hypoxia-Inducible Bidirectional ShRNA Expression Vector Delivery Using PEI/Chitosan-TBA Copolymers for Colorectal Cancer Gene Therapy. Life Sci. 2018, 202 (April), 140–151. 10.1016/j.lfs.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Davidovich-Pinhas M.; Danin-Poleg Y.; Kashi Y.; Bianco-Peled H. Modified Chitosan: A Step toward Improving the Properties of Antibacterial Food Packages. Food Packag. Shelf Life 2014, 1 (2), 160–169. 10.1016/j.fpsl.2014.01.007. [DOI] [Google Scholar]
- Juntapram K.; Praphairaksit N.; Siraleartmukul K.; Muangsin N. Synthesis and Characterization of Chitosan-Homocysteine Thiolactone as a Mucoadhesive Polymer. Carbohydr. Polym. 2012, 87 (4), 2399–2408. 10.1016/j.carbpol.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Juntapram K.; Praphairaksit N.; Siraleartmukul K.; Muangsin N. Electrosprayed Polyelectrolyte Complexes between Mucoadhesive N,N,N,-Trimethylchitosan-Homocysteine Thiolactone and Alginate/Carrageenan for Camptothecin Delivery. Carbohydr. Polym. 2012, 90 (4), 1469–1479. 10.1016/j.carbpol.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Kafedjiiski K.; Hoffer M.; Werle M.; Bernkop-Schnürch A. Improved Synthesis and in Vitro Characterization of Chitosan–Thioethylamidine Conjugate. Biomaterials 2006, 27 (1), 127–135. 10.1016/j.biomaterials.2005.05.075. [DOI] [PubMed] [Google Scholar]
- Krauland A. H.; Hoffer M. H.; Bernkop-Schnürch A. Viscoelastic Properties of a New in Situ Gelling Thiolated Chitosan Conjugate. Drug Dev. Ind. Pharm. 2005, 31 (9), 885–893. 10.1080/03639040500271985. [DOI] [PubMed] [Google Scholar]
- Kafedjiiski K.; Föger F.; Hoyer H.; Bernkop-Schnürch A.; Werle M. Evaluation of In Vitro Enzymatic Degradation of Various Thiomers and Cross-Linked Thiomers. Drug Dev. Ind. Pharm. 2007, 33 (2), 199–208. 10.1080/03639040600762651. [DOI] [PubMed] [Google Scholar]
- Ferris C.; Casas M.; Lucero M. J.; de Paz M. V.; Jiménez-Castellanos M. R. Synthesis and Characterization of a Novel Chitosan-N-Acetyl-Homocysteine Thiolactone Polymer Using MES Buffer. Carbohydr. Polym. 2014, 111, 125–132. 10.1016/j.carbpol.2014.03.078. [DOI] [PubMed] [Google Scholar]
- Tan S.; Mettu S.; Biviano M. D.; Zhou M.; Babgi B.; White J.; Dagastine R. R.; Ashokkumar M. Ultrasonic Synthesis of Stable Oil Filled Microcapsules Using Thiolated Chitosan and Their Characterization by AFM and Numerical Simulations. Soft Matter 2016, 12 (34), 7212–7222. 10.1039/C6SM01402J. [DOI] [PubMed] [Google Scholar]
- Cheng B.; Thapa B.; Remant K. C.; Xu P. Dual Secured Nano-Melittin for the Safe and Effective Eradication of Cancer Cells. J. Mater. Chem. B 2015, 3 (1), 25–29. 10.1039/C4TB01401D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambito Y.; Fogli S.; Zaino C.; Stefanelli F.; Breschi M. C.; Di Colo G. Synthesis, Characterization and Evaluation of Thiolated Quaternary Ammonium-Chitosan Conjugates for Enhanced Intestinal Drug Permeation. Eur. J. Pharm. Sci. 2009, 38 (2), 112–120. 10.1016/j.ejps.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Zambito Y.; Colo G. Di. Thiolated Quaternary Ammonium–Chitosan Conjugates for Enhanced Precorneal Retention, Transcorneal Permeation and Intraocular Absorption of Dexamethasone. Eur. J. Pharm. Biopharm. 2010, 75 (2), 194–199. 10.1016/j.ejpb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Lee S. J.; Lee A.; Hwang S. R.; Park J.-S.; Jang J.; Huh M. S.; Jo D.-G.; Yoon S.-Y.; Byun Y.; Kim S. H.; Kwon I. C.; Youn I.; Kim K. TNF-α Gene Silencing Using Polymerized SiRNA/ Thiolated Glycol Chitosan Nanoparticles for Rheumatoid Arthritis. Mol. Ther. 2014, 22 (2), 397–408. 10.1038/mt.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J.; Huh M. S.; Lee S. Y.; Min S.; Lee S.; Koo H.; Chu J.-U.; Lee K. E.; Jeon H.; Choi Y.; Choi K.; Byun Y.; Jeong S. Y.; Park K.; Kim K.; Kwon I. C. Tumor-Homing Poly-SiRNA/Glycol Chitosan Self-Cross-Linked Nanoparticles for Systemic SiRNA Delivery in Cancer Treatment. Angew. Chem., Int. Ed. 2012, 51 (29), 7203–7207. 10.1002/anie.201201390. [DOI] [PubMed] [Google Scholar]
- Yhee J. Y.; Song S.; Lee S. J.; Park S.-G.; Kim K.-S.; Kim M. G.; Son S.; Koo H.; Kwon I. C.; Jeong J. H.; Jeong S. Y.; Kim S. H.; Kim K. Cancer-Targeted MDR-1 SiRNA Delivery Using Self-Cross-Linked Glycol Chitosan Nanoparticles to Overcome Drug Resistance. J. Controlled Release 2015, 198, 1–9. 10.1016/j.jconrel.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Nagireddi S.; Golder A. K.; Uppaluri R. Role of EDTA on the Pd(II) Adsorption Characteristics of Chitosan Cross-Linked 3-Amino-1,2,4-Triazole-5-Thiol Derivative from Synthetic Electroless Plating Solutions. Int. J. Biol. Macromol. 2019, 127, 320–329. 10.1016/j.ijbiomac.2019.01.033. [DOI] [PubMed] [Google Scholar]
- Li F.; Bao C.; Zhang J.; Sun Q.; Kong W.; Han X.; Wang Y. Synthesis of Chemically Modified Chitosan with 2,5-Dimercapto-1,3,4-Thiodiazole and Its Adsorption Abilities for Au(III), Pd(II), and Pt(IV). J. Appl. Polym. Sci. 2009, 113, 1604–1610. 10.1002/app.30068. [DOI] [Google Scholar]
- Cárdenas G.; Orlando P.; Edelio T. Synthesis and Applications of Chitosan Mercaptanes as Heavy Metal Retention Agent. Int. J. Biol. Macromol. 2001, 28 (2), 167–174. 10.1016/S0141-8130(00)00156-2. [DOI] [PubMed] [Google Scholar]
- Singh P.; Chauhan K.; Priya V.; Singhal R. K. A Greener Approach for Impressive Removal of As(III)/As(V) from an Ultra-Low Concentration Using a Highly Efficient Chitosan Thiomer as a New Adsorbent. RSC Adv. 2016, 6 (69), 64946–64961. 10.1039/C6RA10595E. [DOI] [Google Scholar]
- Chauhan K.; Sharma R.; Dharela R.; Chauhan G. S.; Singhal R. K. Chitosan-Thiomer Stabilized Silver Nano-Composites for Antimicrobial and Antioxidant Applications. RSC Adv. 2016, 6 (79), 75453–75464. 10.1039/C6RA13466A. [DOI] [Google Scholar]
- Chauhan K.; Singh P.; Singhal R. K. New Chitosan–Thiomer: An Efficient Colorimetric Sensor and Effective Sorbent for Mercury at Ultralow Concentration. ACS Appl. Mater. Interfaces 2015, 7 (47), 26069–26078. 10.1021/acsami.5b06078. [DOI] [PubMed] [Google Scholar]
- Peppas N. A.; Huang Y. Nanoscale Technology of Mucoadhesive Interactions. Adv. Drug Delivery Rev. 2004, 56 (11), 1675–1687. 10.1016/j.addr.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Baos S. C.; Phillips D. B.; Wildling L.; McMaster T. J.; Berry M. Distribution of Sialic Acids on Mucins and Gels: A Defense Mechanism. Biophys. J. 2012, 102 (1), 176–184. 10.1016/j.bpj.2011.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogias I. A.; Williams A. C.; Khutoryanskiy V. V. Why Is Chitosan Mucoadhesive?. Biomacromolecules 2008, 9 (7), 1837–1842. 10.1021/bm800276d. [DOI] [PubMed] [Google Scholar]
- Smart J. The Basics and Underlying Mechanisms of Mucoadhesion. Adv. Drug Delivery Rev. 2005, 57 (11), 1556–1568. 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Cesari A.; Fabiano A.; Piras A. M.; Zambito Y.; Uccello-Barretta G.; Balzano F. Binding and Mucoadhesion of Sulfurated Derivatives of Quaternary Ammonium-Chitosans and Their Nanoaggregates: An NMR Investigation. J. Pharm. Biomed. Anal. 2020, 177, 112852. 10.1016/j.jpba.2019.112852. [DOI] [PubMed] [Google Scholar]
- Leitner V. M.; Walker G. F.; Bernkop-Schnürch A. Thiolated Polymers: Evidence for the Formation of Disulphide Bonds with Mucus Glycoproteins. Eur. J. Pharm. Biopharm. 2003, 56 (2), 207–214. 10.1016/S0939-6411(03)00061-4. [DOI] [PubMed] [Google Scholar]
- Vasconcelos A.; Freddi G.; Cavaco-Paulo A. Biodegradable Materials Based on Silk Fibroin and Keratin. Biomacromolecules 2008, 9 (4), 1299–1305. 10.1021/bm7012789. [DOI] [PubMed] [Google Scholar]
- Samprasit W.; Kaomongkolgit R.; Sukma M.; Rojanarata T.; Ngawhirunpat T.; Opanasopit P. Mucoadhesive Electrospun Chitosan-Based Nanofibre Mats for Dental Caries Prevention. Carbohydr. Polym. 2015, 117, 933–940. 10.1016/j.carbpol.2014.10.026. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnürch A.; Hornof M.; Zoidl T. Thiolated Polymers—Thiomers: Synthesis and in Vitro Evaluation of Chitosan–2-Iminothiolane Conjugates. Int. J. Pharm. (Amsterdam, Neth.) 2003, 260 (2), 229–237. 10.1016/S0378-5173(03)00271-0. [DOI] [PubMed] [Google Scholar]
- Hornof M. In Vitro Evaluation of the Viscoelastic Properties of Chitosan–Thioglycolic Acid Conjugates. Eur. J. Pharm. Biopharm. 2003, 55 (2), 185–190. 10.1016/S0939-6411(02)00162-5. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Kong M.; Feng C.; Cheng X.; Liu Y.; Chen X. Investigation of Gelling Behavior of Thiolated Chitosan in Alkaline Condition and Its Application in Stent Coating. Carbohydr. Polym. 2016, 136, 307–315. 10.1016/j.carbpol.2015.09.049. [DOI] [PubMed] [Google Scholar]
- Sakloetsakun D.; Hombach J. M. R.; Bernkop-Schnürch A. In Situ Gelling Properties of Chitosan-Thioglycolic Acid Conjugate in the Presence of Oxidizing Agents. Biomaterials 2009, 30 (31), 6151–6157. 10.1016/j.biomaterials.2009.07.060. [DOI] [PubMed] [Google Scholar]
- Hintzen F.; Laffleur F.; Sarti F.; Shahnaz G.; Bernkop-Schnürch A. Thiomers: Influence of Molar Mass on in Situ Gelling Properties. Int. J. Pharm. (Amsterdam, Neth.) 2012, 436 (1–2), 120–126. 10.1016/j.ijpharm.2012.05.073. [DOI] [PubMed] [Google Scholar]
- Millotti G.; Samberger C.; Fröhlich E.; Bernkop-Schnürch A. Chitosan- Graft −6-Mercaptonicotinic Acid: Synthesis, Characterization, and Biocompatibility. Biomacromolecules 2009, 10 (11), 3023–3027. 10.1021/bm9006248. [DOI] [PubMed] [Google Scholar]
- Lowe A. B. Thiol–Ene “Click” Reactions and Recent Applications in Polymer and Materials Synthesis: A First Update. Polym. Chem. 2014, 5 (17), 4820–4870. 10.1039/C4PY00339J. [DOI] [Google Scholar]
- Yang N.; Wang Y.; Zhang Q.; Chen L.; Zhao Y. In Situ Formation of Poly (Thiolated Chitosan-Co-Alkylated β-Cyclodextrin) Hydrogels Using Click Cross-Linking for Sustained Drug Release. J. Mater. Sci. 2019, 54 (2), 1677–1691. 10.1007/s10853-018-2910-3. [DOI] [Google Scholar]
- Zhou Y.; Nie W.; Zhao J.; Yuan X. Rapidly in Situ Forming Adhesive Hydrogel Based on a PEG-Maleimide Modified Polypeptide through Michael Addition. J. Mater. Sci.: Mater. Med. 2013, 24 (10), 2277–2286. 10.1007/s10856-013-4987-1. [DOI] [PubMed] [Google Scholar]
- Wu S.-W.; Liu X.; Miller A. L.; Cheng Y.-S.; Yeh M.-L.; Lu L. Strengthening Injectable Thermo-Sensitive NIPAAm-g-Chitosan Hydrogels Using Chemical Cross-Linking of Disulfide Bonds as Scaffolds for Tissue Engineering. Carbohydr. Polym. 2018, 192 (March), 308–316. 10.1016/j.carbpol.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles K. B.; Ball R. L.; Matthew H. W. T. Chitosan Films with Improved Tensile Strength and Toughness from N-Acetyl-Cysteine Mediated Disulfide Bonds. Carbohydr. Polym. 2016, 139, 1–9. 10.1016/j.carbpol.2015.11.052. [DOI] [PubMed] [Google Scholar]
- Uhrich K. E.; Cannizzaro S. M.; Langer R. S.; Shakesheff K. M. Polymeric Systems for Controlled Drug Release. Chem. Rev. (Washington, DC, U. S.) 1999, 99 (11), 3181–3198. 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- Kiene K.; Porta F.; Topacogullari B.; Detampel P.; Huwyler J. Self-Assembling Chitosan Hydrogel: A Drug-Delivery Device Enabling the Sustained Release of Proteins. J. Appl. Polym. Sci. 2018, 135 (1), 45638. 10.1002/app.45638. [DOI] [Google Scholar]
- Li R.; Deng L.; Cai Z.; Zhang S.; Wang K.; Li L.; Ding S.; Zhou C. Liposomes Coated with Thiolated Chitosan as Drug Carriers of Curcumin. Mater. Sci. Eng., C 2017, 80, 156–164. 10.1016/j.msec.2017.05.136. [DOI] [PubMed] [Google Scholar]
- Liu D.; Li J.; Pan H.; He F.; Liu Z.; Wu Q.; Bai C.; Yu S.; Yang X. Potential Advantages of a Novel Chitosan-N-Acetylcysteine Surface Modified Nanostructured Lipid Carrier on the Performance of Ophthalmic Delivery of Curcumin. Sci. Rep. 2016, 6 (1), 28796. 10.1038/srep28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Liu D.; Tan G.; Zhao Z.; Yang X.; Pan W. A Comparative Study on the Efficiency of Chitosan-N-Acetylcysteine, Chitosan Oligosaccharides or Carboxymethyl Chitosan Surface Modified Nanostructured Lipid Carrier for Ophthalmic Delivery of Curcumin. Carbohydr. Polym. 2016, 146, 435–444. 10.1016/j.carbpol.2016.03.079. [DOI] [PubMed] [Google Scholar]
- Rajawat G. S.; Shinde U. A.; Nair H. A. Chitosan-N-Acetyl Cysteine Microspheres for Ocular Delivery of Acyclovir: Synthesis and in Vitro/in Vivo Evaluation. J. Drug Delivery Sci. Technol. 2016, 35, 333–342. 10.1016/j.jddst.2016.08.006. [DOI] [Google Scholar]
- Sajjad M.; Khan M. I.; Naveed S.; Ijaz S.; Qureshi O. S.; Raza S. A.; Shahnaz G.; Sohail M. F. Folate-Functionalized Thiomeric Nanoparticles for Enhanced Docetaxel Cytotoxicity and Improved Oral Bioavailability. AAPS PharmSciTech 2019, 20 (2), 81. 10.1208/s12249-019-1297-z. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Liu X.; Liu R.; Gong Y.; Wang M.; Huang Q.; Feng Q.; Yu B. Zero-Order Controlled Release of BMP2-Derived Peptide P24 from the Chitosan Scaffold by Chemical Grafting Modification Technique for Promotion of Osteogenesis in Vitro and Enhancement of Bone Repair in Vivo. Theranostics 2017, 7 (5), 1072–1087. 10.7150/thno.18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Yu B.; Huang Q.; Liu R.; Feng Q.; Cai Q.; Mi S. In Vitro BMP-2 Peptide Release from Thiolated Chitosan Based Hydrogel. Int. J. Biol. Macromol. 2016, 93, 314–321. 10.1016/j.ijbiomac.2016.08.048. [DOI] [PubMed] [Google Scholar]
- Clausen A. E.; Kast C. E.; Bernkop-Schnürch A. The Role of Glutathione in the Permeation Enhancing Effect of Thiolated Polymers. Pharm. Res. 2002, 19 (5), 602–608. 10.1023/A:1015345827091. [DOI] [PubMed] [Google Scholar]
- Sakloetsakun D.; Iqbal J.; Millotti G.; Vetter A.; Bernkop-Schnürch A. Thiolated Chitosans: Influence of Various Sulfhydryl Ligands on Permeation-Enhancing and P-Gp Inhibitory Properties. Drug Dev. Ind. Pharm. 2011, 37 (6), 648–655. 10.3109/03639045.2010.534484. [DOI] [PubMed] [Google Scholar]
- Dünnhaupt S.; Barthelmes J.; Iqbal J.; Perera G.; Thurner C. C.; Friedl H.; Bernkop-Schnürch A. In Vivo Evaluation of an Oral Drug Delivery System for Peptides Based on S-Protected Thiolated Chitosan. J. Controlled Release 2012, 160 (3), 477–485. 10.1016/j.jconrel.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Dünnhaupt S.; Barthelmes J.; Rahmat D.; Leithner K.; Thurner C. C.; Friedl H.; Bernkop-Schnürch A. Protected Thiolated Chitosan for Oral Delivery of Hydrophilic Macromolecules: Evaluation of Permeation Enhancing and Efflux Pump Inhibitory Properties. Mol. Pharmaceutics 2012, 9 (5), 1331–1341. 10.1021/mp200598j. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnürch A. Permeation Enhancing Polymers in Oral Delivery of Hydrophilic Macromolecules: Thiomer/GSH Systems. J. Controlled Release 2003, 93 (2), 95–103. 10.1016/j.jconrel.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Guggi D.; Bernkop-Schnürch A. Improved Paracellular Uptake by the Combination of Different Types of Permeation Enhancers. Int. J. Pharm. (Amsterdam, Neth.) 2005, 288 (1), 141–150. 10.1016/j.ijpharm.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnürch A.; Pinter Y.; Guggi D.; Kahlbacher H.; Schöffmann G.; Schuh M.; Schmerold I.; Del Curto M. D.; D’Antonio M.; Esposito P.; Huck C. The Use of Thiolated Polymers as Carrier Matrix in Oral Peptide Delivery—Proof of Concept. J. Controlled Release 2005, 106 (1–2), 26–33. 10.1016/j.jconrel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Wang X.; Zheng C.; Wu Z.; Teng D.; Zhang X.; Wang Z.; Li C. Chitosan-NAC Nanoparticles as a Vehicle for Nasal Absorption Enhancement of Insulin. J. Biomed. Mater. Res., Part B 2009, 88B (1), 150–161. 10.1002/jbm.b.31161. [DOI] [PubMed] [Google Scholar]
- Singh D.; Rashid M.; Hallan S. S.; Mehra N. K.; Prakash A.; Mishra N. Pharmacological Evaluation of Nasal Delivery of Selegiline Hydrochloride-Loaded Thiolated Chitosan Nanoparticles for the Treatment of Depression. Artif. Cells, Nanomed., Biotechnol. 2016, 44 (3), 1–13. 10.3109/21691401.2014.998824. [DOI] [PubMed] [Google Scholar]
- Iqbal J.; Shahnaz G.; Perera G.; Hintzen F.; Sarti F.; Bernkop-Schnürch A. Thiolated Chitosan: Development and in Vivo Evaluation of an Oral Delivery System for Leuprolide. Eur. J. Pharm. Biopharm. 2012, 80 (1), 95–102. 10.1016/j.ejpb.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Langoth N.; Kahlbacher H.; Schöffmann G.; Schmerold I.; Schuh M.; Franz S.; Kurka P.; Bernkop-Schnürch A. Thiolated Chitosans: Design and In Vivo Evaluation of a Mucoadhesive Buccal Peptide Drug Delivery System. Pharm. Res. 2006, 23 (3), 573–579. 10.1007/s11095-005-9533-5. [DOI] [PubMed] [Google Scholar]
- Fan B.; Xing Y.; Zheng Y.; Sun C.; Liang G. PH-Responsive Thiolated Chitosan Nanoparticles for Oral Low-Molecular Weight Heparin Delivery: In Vitro and in Vivo Evaluation. Drug Delivery 2016, 23 (1), 238–247. 10.3109/10717544.2014.909908. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Su M.; Tang S.; Wang L.; Liang X.; Meng F.; Hong Y.; Xu Z. Synthesis of Thiolated Chitosan and Preparation Nanoparticles with Sodium Alginate for Ocular Drug Delivery. Mol. Vision 2012, 18, 1973–1982. [PMC free article] [PubMed] [Google Scholar]
- Martien R.; Loretz B.; Sandbichler A. M.; Schnürch A. B. Thiolated Chitosan Nanoparticles: Transfection Study in the Caco-2 Differentiated Cell Culture. Nanotechnology 2008, 19 (4), 045101. 10.1088/0957-4484/19/04/045101. [DOI] [PubMed] [Google Scholar]
- Torres A. G.; Gait M. J. Exploiting Cell Surface Thiols to Enhance Cellular Uptake. Trends Biotechnol. 2012, 30 (4), 185–190. 10.1016/j.tibtech.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Lee Y. S.; Kim S. W. Bioreducible Polymers for Therapeutic Gene Delivery. J. Controlled Release 2014, 190, 424–439. 10.1016/j.jconrel.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brülisauer L.; Gauthier M. A.; Leroux J.-C. Disulfide-Containing Parenteral Delivery Systems and Their Redox-Biological Fate. J. Controlled Release 2014, 195, 147–154. 10.1016/j.jconrel.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Loretz B.; Thaler M.; Bernkop-Schnürch A. Role of Sulfhydryl Groups in Transfection? A Case Study with Chitosan–NAC Nanoparticles. Bioconjugate Chem. 2007, 18 (4), 1028–1035. 10.1021/bc0603079. [DOI] [PubMed] [Google Scholar]
- Martien R.; Loretz B.; Thaler M.; Majzoob S.; Bernkop-Schnürch A. Chitosan–Thioglycolic Acid Conjugate: An Alternative Carrier for Oral Nonviral Gene Delivery?. J. Biomed. Mater. Res., Part A 2007, 82A (1), 1–9. 10.1002/jbm.a.31135. [DOI] [PubMed] [Google Scholar]
- Khan S.; Faisal S.; Shams D. F.; Zia M.; Nadhman A. Photo-Inactivation of Bacteria in Hospital Effluent via Thiolated Iron-Doped Nanoceria. IET Nanobiotechnol. 2019, 13 (8), 875–879. 10.1049/iet-nbt.2019.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharom F. J. ABC Multidrug Transporters: Structure, Function and Role in Chemoresistance. Pharmacogenomics 2008, 9 (1), 105–127. 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- Schmitz T.; Hombach J.; Bernkop-Schnürch A. Chitosan-N-Acetyl Cysteine Conjugates: In Vitro Evaluation of Permeation Enhancing and P-Glycoprotein Inhibiting Properties. Drug Delivery 2008, 15 (4), 245–252. 10.1080/10717540802006708. [DOI] [PubMed] [Google Scholar]
- Iqbal J.; Sakloetsakun D.; Bernkop-Schnürch A. Thiomers: Inhibition of Cytochrome P450 Activity. Eur. J. Pharm. Biopharm. 2011, 78 (3), 361–365. 10.1016/j.ejpb.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Sarwar H. S.; Ashraf S.; Akhtar S.; Sohail M. F.; Hussain S. Z.; Rafay M.; Yasinzai M.; Hussain I.; Shahnaz G. Mannosylated Thiolated Polyethylenimine Nanoparticles for the Enhanced Efficacy of Antimonial Drug against Leishmaniasis. Nanomedicine 2018, 13 (1), 25–41. 10.2217/nnm-2017-0255. [DOI] [PubMed] [Google Scholar]
- Stefanov I.; Pérez-Rafael S.; Hoyo J.; Cailloux J.; Santana Pérez O. O.; Hinojosa-Caballero D.; Tzanov T. Multifunctional Enzymatically Generated Hydrogels for Chronic Wound Application. Biomacromolecules 2017, 18 (5), 1544–1555. 10.1021/acs.biomac.7b00111. [DOI] [PubMed] [Google Scholar]
- Bravo-Osuna I.; Millotti G.; Vauthier C.; Ponchel G. In Vitro Evaluation of Calcium Binding Capacity of Chitosan and Thiolated Chitosan Poly(Isobutyl Cyanoacrylate) Core–Shell Nanoparticles. Int. J. Pharm. (Amsterdam, Neth.) 2007, 338 (1–2), 284–290. 10.1016/j.ijpharm.2007.01.039. [DOI] [PubMed] [Google Scholar]
- Bal W.; Zawisza I.; Fraczyk T.. Method for Synthesis of a Biopolymer Derivative, a Biopolymer Derivative and Its Use. PCT Patent WO 2015/126269 A4, 2015.
- Deneke S. M. Curr. Top. Cell. Regul. 2001, 36, 151–180. 10.1016/S0070-2137(01)80007-8. [DOI] [PubMed] [Google Scholar]
- Dunnill C.; Patton T.; Brennan J.; Barrett J.; Dryden M.; Cooke J.; Leaper D.; Georgopoulos N. T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-Modulating Technologies for Augmentation of the Healing Process. Int. Wound J. 2017, 14 (1), 89–96. 10.1111/iwj.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M. T.; Yang J. H.; Mau J. L. Antioxidant Properties of Chitosan from Crab Shells. Carbohydr. Polym. 2008, 74 (4), 840–844. 10.1016/j.carbpol.2008.05.003. [DOI] [Google Scholar]
- Song C.; Yu H.; Zhang M.; Yang Y.; Zhang G. Physicochemical Properties and Antioxidant Activity of Chitosan from the Blowfly Chrysomya Megacephala Larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. 10.1016/j.ijbiomac.2013.05.039. [DOI] [PubMed] [Google Scholar]
- Croisier F.; Jérôme C. Chitosan-Based Biomaterials for Tissue Engineering. Eur. Polym. J. 2013, 49 (4), 780–792. 10.1016/j.eurpolymj.2012.12.009. [DOI] [Google Scholar]
- Schmidl D.; Werkmeister R.; Kaya S.; Unterhuber A.; Witkowska K. J.; Baumgartner R.; Höller S.; O’Rourke M.; Peterson W.; Wolter A.; Prinz M.; Schmetterer L.; Garhöfer G. A Controlled, Randomized Double-Blind Study to Evaluate the Safety and Efficacy of Chitosan- N -Acetylcysteine for the Treatment of Dry Eye Syndrome. J. Ocul. Pharmacol. Ther. 2017, 33 (5), 375–382. 10.1089/jop.2016.0123. [DOI] [PubMed] [Google Scholar]
- Lorenz K.; Garhofer G.; Hoeller S.; Peterson W.; Vielnascher R. M.; Schoenfeld Z. I.; Prinz M. Long-Term Management of Dry Eye by Once-Daily Use of Chitosan-N-Acetylcysteine (Lacrimera®) Eye Drops. J. Clin. Ophthalmol. 2018, 2 (01), 47–54. 10.35841/clinical-ophthalmology.2.1.47-54. [DOI] [Google Scholar]
- Garhofer G.Safety and Tolerability of Chitosan-N-acetylcysteine Eye Drops in Healthy Young Volunteers, https://clinicaltrials.gov/ct2/show/NCT01015209 (accessed Mar 30, 2019).
- Garhofer G.Local Tolerability of Chitosan-N-acetylcysteine Eye Drops in Healthy Young Volunteers https://clinicaltrials.gov/ct2/show/NCT01278784 (accessed Mar 30, 2019).
- Garhofer G.Evaluation of the Corneal Residence Time of Chitosan-N-acetylcysteine Eye Drops in Patients With Dry Eye Syndrome After Single and Multiple Instillation https://clinicaltrials.gov/ct2/show/NCT01753752 (accessed Mar 30, 2019).
- Garhofer G.Assessment of Safety and Tolerability of Chitosan-N-acetylcysteine Eye Drops in Subjects While Wearing Contact Lenses and Before Insertion of Contact Lenses https://clinicaltrials.gov/ct2/show/NCT01747616 (accessed Mar 24, 2019).
- Laffleur F.; Hintzen F.; Rahmat D.; Shahnaz G.; Millotti G.; Bernkop-Schnürch A. Enzymatic Degradation of Thiolated Chitosan. Drug Dev. Ind. Pharm. 2013, 39 (10), 1531–1539. 10.3109/03639045.2012.719901. [DOI] [PubMed] [Google Scholar]
- Palmberger T. F.; Augustijns P.; Vetter A.; Bernkop-Schnürch A. Safety Assessment of Thiolated Polymers: Effect on Ciliary Beat Frequency in Human Nasal Epithelial Cells. Drug Dev. Ind. Pharm. 2011, 37 (12), 1455–1462. 10.3109/03639045.2011.584537. [DOI] [PubMed] [Google Scholar]
- Pradines B.; Lievin-Le Moal V.; Vauthier C.; Ponchel G.; Loiseau P. M.; Bouchemal K. Cell Line-Dependent Cytotoxicity of Poly(Isobutylcyanoacrylate) Nanoparticles Coated with Chitosan and Thiolated Chitosan: Insights from Cultured Human Epithelial HeLa, Caco2/TC7 and HT-29/MTX Cells. Int. J. Pharm. (Amsterdam, Neth.) 2015, 491 (1–2), 17–20. 10.1016/j.ijpharm.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Guggi D.; Langoth N.; Hoffer M. H.; Wirth M.; Bernkop-Schnürch A. Comparative Evaluation of Cytotoxicity of a Glucosamine-TBA Conjugate and a Chitosan-TBA Conjugate. Int. J. Pharm. (Amsterdam, Neth.) 2004, 278 (2), 353–360. 10.1016/j.ijpharm.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Li R.; Lin Z.; Zhang Q.; Zhang Y.; Liu Y.; Lyu Y.; Li X.; Zhou C.; Wu G.; Ao N.; Li L. Injectable and In Situ -Formable Thiolated Chitosan-Coated Liposomal Hydrogels as Curcumin Carriers for Prevention of In Vivo Breast Cancer Recurrence. ACS Appl. Mater. Interfaces 2020, 12 (15), 17936–17948. 10.1021/acsami.9b21528. [DOI] [PubMed] [Google Scholar]
- Arif M.; Dong Q. J.; Raja M. A.; Zeenat S.; Chi Z.; Liu C. G. Development of Novel PH-Sensitive Thiolated Chitosan/PMLA Nanoparticles for Amoxicillin Delivery to Treat Helicobacter Pylori. Mater. Sci. Eng., C 2018, 83, 17–24. 10.1016/j.msec.2017.08.038. [DOI] [PubMed] [Google Scholar]
- Schuerer N.; Stein E.; Inic-Kanada A.; Ghasemian E.; Stojanovic M.; Montanaro J.; Bintner N.; Hohenadl C.; Sachsenhofer R.; Barisani-Asenbauer T. Effects of Chitosan and Chitosan N-Acetylcysteine Solutions on Conjunctival Epithelial Cells. J. EuCornea 2018, 1 (1), 12–18. 10.1016/j.xjec.2018.04.002. [DOI] [Google Scholar]
- Fischak C.; Klaus R.; Werkmeister R. M.; Hohenadl C.; Prinz M.; Schmetterer L.; Garhöfer G. Effect of Topically Administered Chitosan- N -Acetylcysteine on Corneal Wound Healing in a Rabbit Model. J. Ophthalmol. 2017, 2017, 1–6. 10.1155/2017/5192924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina M.; Dua H. S. Early Results on the Use of Chitosan-N-Acetylcysteine (Lacrimera®) in the Management of Dry Eye Disease of Varied Etiology. Int. Ophthalmol. 2019, 39 (3), 693–696. 10.1007/s10792-018-0843-0. [DOI] [PubMed] [Google Scholar]
- Ahmad Z.; Khan M. I.; Siddique M. I.; Sarwar H. S.; Shahnaz G.; Hussain S. Z.; Bukhari N. I.; Hussain I.; Sohail M. F. Fabrication and Characterization of Thiolated Chitosan Microneedle Patch for Transdermal Delivery of Tacrolimus. AAPS PharmSciTech 2020, 21 (2), 68. 10.1208/s12249-019-1611-9. [DOI] [PubMed] [Google Scholar]
- Zahir-Jouzdani F.; Mahbod M.; Soleimani M.; Vakhshiteh F.; Arefian E.; Shahosseini S.; Dinarvand R.; Atyabi F. Chitosan and Thiolated Chitosan: Novel Therapeutic Approach for Preventing Corneal Haze after Chemical Injuries. Carbohydr. Polym. 2018, 179, 42–49. 10.1016/j.carbpol.2017.09.062. [DOI] [PubMed] [Google Scholar]
- Dhaliwal S.; Jain S.; Singh H. P.; Tiwary A. K. Mucoadhesive Microspheres for Gastroretentive Delivery of Acyclovir: In Vitro and In Vivo Evaluation. AAPS J. 2008, 10 (2), 322. 10.1208/s12248-008-9039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaferro S.; Bouchemal K.; Skanji R.; Gueutin C.; Chacun H.; Ponchel G. Intestinal Permeation Enhancement of Docetaxel Encapsulated into Methyl-β-Cyclodextrin/Poly(Isobutylcyanoacrylate) Nanoparticles Coated with Thiolated Chitosan. J. Controlled Release 2012, 162 (3), 568–574. 10.1016/j.jconrel.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Sudhakar S.; Chandran S. V.; Selvamurugan N.; Nazeer R. A. Biodistribution and Pharmacokinetics of Thiolated Chitosan Nanoparticles for Oral Delivery of Insulin in Vivo. Int. J. Biol. Macromol. 2020, 150, 281–288. 10.1016/j.ijbiomac.2020.02.079. [DOI] [PubMed] [Google Scholar]
- Millotti G.; Perera G.; Vigl C.; Pickl K.; Sinner F. M.; Bernkop-Schnürch A. The Use of Chitosan-6-Mercaptonicotinic Acid Nanoparticles for Oral Peptide Drug Delivery. Drug Delivery 2011, 18 (3), 190–197. 10.3109/10717544.2010.522611. [DOI] [PubMed] [Google Scholar]
- Prabahar K.; Udhumansha U.; Qushawy M. Optimization of Thiolated Chitosan Nanoparticles for the Enhancement of in Vivo Hypoglycemic Efficacy of Sitagliptin in Streptozotocin-Induced Diabetic Rats. Pharmaceutics 2020, 12 (4), 300. 10.3390/pharmaceutics12040300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Föger F.; Schmitz T.; Bernkop-Schnürch A. In Vivo Evaluation of an Oral Delivery System for P-Gp Substrates Based on Thiolated Chitosan. Biomaterials 2006, 27 (23), 4250–4255. 10.1016/j.biomaterials.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Guggi D.; Kast C. E.; Bernkop-Schnürch A. In Vivo Evaluation of an Oral Salmon Calcitonin-Delivery System Based on a Thiolated Chitosan Carrier Matrix. Pharm. Res. 2003, 20 (12), 1989–1994. 10.1023/B:PHAM.0000008047.82334.7d. [DOI] [PubMed] [Google Scholar]
- Krauland A. H.; Guggi D.; Bernkop-Schnürch A. Oral Insulin Delivery: The Potential of Thiolated Chitosan-Insulin Tablets on Non-Diabetic Rats. J. Controlled Release 2004, 95 (3), 547–555. 10.1016/j.jconrel.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Sakloetsakun D.; Dünnhaupt S.; Barthelmes J.; Perera G.; Bernkop-Schnürch A. Combining Two Technologies: Multifunctional Polymers and Self-Nanoemulsifying Drug Delivery System (SNEDDS) for Oral Insulin Administration. Int. J. Biol. Macromol. 2013, 61, 363–372. 10.1016/j.ijbiomac.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Khatoon M.; Sohail M. F.; Shahnaz G.; ur Rehman F.; Fakhar-ud-Din; ur Rehman A.; Ullah N.; Amin U.; Khan G. M.; Shah K. U. Development and Evaluation of Optimized Thiolated Chitosan Proniosomal Gel Containing Duloxetine for Intranasal Delivery. AAPS PharmSciTech 2019, 20 (7), 288. 10.1208/s12249-019-1484-y. [DOI] [PubMed] [Google Scholar]
- Krauland A. H.; Guggi D.; Bernkop-Schnürch A. Thiolated Chitosan Microparticles: A Vehicle for Nasal Peptide Drug Delivery. Int. J. Pharm. (Amsterdam, Neth.) 2006, 307 (2), 270–277. 10.1016/j.ijpharm.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Krauland A. H.; Leitner V. M.; Grabovac V.; Bernkop-Schnürch A. In Vivo Evaluation of a Nasal Insulin Delivery System Based on Thiolated Chitosan. J. Pharm. Sci. 2006, 95 (11), 2463–2472. 10.1002/jps.20700. [DOI] [PubMed] [Google Scholar]
- Nanaki S.; Tseklima M.; Christodoulou E.; Triantafyllidis K.; Kostoglou M.; Bikiaris D. Thiolated Chitosan Masked Polymeric Microspheres with Incorporated Mesocellular Silica Foam (MCF) for Intranasal Delivery of Paliperidone. Polymers (Basel, Switz.) 2017, 9 (11), 617. 10.3390/polym9110617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunena; Singh S. K.; Mishra D. N. Nose to Brain Delivery of Galantamine Loaded Nanoparticles: In-Vivo Pharmacodynamic and Biochemical Study in Mice. Curr. Drug Delivery 2018, 16 (1), 51–58. 10.2174/1567201815666181004094707. [DOI] [PubMed] [Google Scholar]
- Shahnaz G.; Vetter A.; Barthelmes J.; Rahmat D.; Laffleur F.; Iqbal J.; Perera G.; Schlocker W.; Dünnhaput S.; Augustijns P.; Bernkop-Schnürch A. Thiolated Chitosan Nanoparticles for the Nasal Administration of Leuprolide: Bioavailability and Pharmacokinetic Characterization. Int. J. Pharm. (Amsterdam, Neth.) 2012, 428 (1–2), 164–170. 10.1016/j.ijpharm.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Lee D.; Zhang W.; Shirley S. A.; Kong X.; Hellermann G. R.; Lockey R. F.; Mohapatra S. S. Thiolated Chitosan/DNA Nanocomplexes Exhibit Enhanced and Sustained Gene Delivery. Pharm. Res. 2006, 24 (1), 157–167. 10.1007/s11095-006-9136-9. [DOI] [PubMed] [Google Scholar]
- Lee D.; Shirley S. A.; Lockey R. F.; Mohapatra S. S. Thiolated Chitosan Nanoparticles Enhance Anti-Inflammatory Effects of Intranasally Delivered Theophylline. Respir. Res. 2006, 7 (1), 112. 10.1186/1465-9921-7-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D.; Naik S.; Misra A. Improved Transnasal Transport and Brain Uptake of Tizanidine HCl-Loaded Thiolated Chitosan Nanoparticles for Alleviation of Pain. J. Pharm. Sci. 2012, 101 (2), 690–706. 10.1002/jps.22780. [DOI] [PubMed] [Google Scholar]
- Boateng J. S.; Ayensu I. Preparation and Characterization of Laminated Thiolated Chitosan-Based Freeze-Dried Wafers for Potential Buccal Delivery of Macromolecules. Drug Dev. Ind. Pharm. 2014, 40 (5), 611–618. 10.3109/03639045.2014.884126. [DOI] [PubMed] [Google Scholar]
- Ayensu I.; Mitchell J. C.; Boateng J. S. In Vitro Characterisation of Chitosan Based Xerogels for Potential Buccal Delivery of Proteins. Carbohydr. Polym. 2012, 89 (3), 935–941. 10.1016/j.carbpol.2012.04.039. [DOI] [PubMed] [Google Scholar]
- Boateng J.; Mitchell J.; Pawar H.; Ayensu I. Functional Characterisation and Permeation Studies of Lyophilised Thiolated Chitosan Xerogels for Buccal Delivery of Insulin. Protein Pept. Lett. 2014, 21 (11), 1163–1175. 10.2174/0929866521666140805124403. [DOI] [PubMed] [Google Scholar]
- Samprasit W.; Rojanarata T.; Akkaramongkolporn P.; Ngawhirunpat T.; Kaomongkolgit R.; Opanasopit P. Fabrication and In Vitro/In Vivo Performance of Mucoadhesive Electrospun Nanofiber Mats Containing α-Mangostin. AAPS PharmSciTech 2015, 16 (5), 1140–1152. 10.1208/s12249-015-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafar H.; Khan M. I.; Sarwar H. S.; Yaqoob S.; Hussain S. Z.; Tariq I.; Madni A. U.; Shahnaz G.; Sohail M. F. Development and Characterization of Bioadhesive Film Embedded with Lignocaine and Calcium Fluoride Nanoparticles. AAPS PharmSciTech 2020, 21 (2), 60. 10.1208/s12249-019-1615-5. [DOI] [PubMed] [Google Scholar]
- Naz K.; Shahnaz G.; Ahmed N.; Qureshi N. A.; Sarwar H. S.; Imran M.; Khan G. M. Formulation and In Vitro Characterization of Thiolated Buccoadhesive Film of Fluconazole. AAPS PharmSciTech 2017, 18 (4), 1043–1055. 10.1208/s12249-016-0607-y. [DOI] [PubMed] [Google Scholar]
- Langoth N.; Bernkop-Schnürch A.; Kurka P. In Vitro Evaluation of Various Buccal Permeation Enhancing Systems for PACAP (Pituitary Adenylate Cyclase-Activating Polypeptide). Pharm. Res. 2005, 22 (12), 2045–2050. 10.1007/s11095-005-7894-4. [DOI] [PubMed] [Google Scholar]
- Meng J.; Agrahari V.; Ezoulin M. J.; Purohit S. S.; Zhang T.; Molteni A.; Dim D.; Oyler N. A.; Youan B. C. Spray-Dried Thiolated Chitosan-Coated Sodium Alginate Multilayer Microparticles for Vaginal HIV Microbicide Delivery. AAPS J. 2017, 19 (3), 692–702. 10.1208/s12248-016-0007-y. [DOI] [PubMed] [Google Scholar]
- Meng J.; Agrahari V.; Ezoulin M. J.; Zhang C.; Purohit S. S.; Molteni A.; Dim D.; Oyler N. A.; Youan B.-B. C. Tenofovir Containing Thiolated Chitosan Core/Shell Nanofibers: In Vitro and in Vivo Evaluations. Mol. Pharmaceutics 2016, 13 (12), 4129–4140. 10.1021/acs.molpharmaceut.6b00739. [DOI] [PubMed] [Google Scholar]
- Meng J.; Zhang T.; Agrahari V.; Ezoulin M. J.; Youan B.-B. C. Comparative Biophysical Properties of Tenofovir-Loaded, Thiolated and Nonthiolated Chitosan Nanoparticles Intended for HIV Prevention. Nanomedicine 2014, 9 (11), 1595–1612. 10.2217/nnm.13.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelmes J.; Dünnhaupt S.; Unterhofer S.; Perera G.; Schlocker W.; Bernkop-Schnürch A. Thiolated Particles as Effective Intravesical Drug Delivery Systems for Treatment of Bladder-Related Diseases. Nanomedicine 2013, 8 (1), 65–75. 10.2217/nnm.12.76. [DOI] [PubMed] [Google Scholar]
- Ay Şenyiğit Z.; Karavana S. Y.; Ilem Ozdemir D.; Caliskan C.; Waldner C.; Sen S.; Bernkop-Schnürch A.; Baloglu E. Design and Evaluation of an Intravesical Delivery System for Superficial Bladder Cancer: Preparation of Gemcitabine HCl-Loaded Chitosan-Thioglycolic Acid Nanoparticles and Comparison of Chitosan/Poloxamer Gels as Carriers. Int. J. Nanomed. 2015, 10, 6493. 10.2147/IJN.S93750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satheeshababu B. K.; Shivakumar K. L. Synthesis of Conjugated Chitosan and Its Effect on Drug Permeation from Transdermal Patches. Indian J. Pharm. Sci. 2013, 75, 162–170. 10.4103/0250-474X.115461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.; Cao Y.; Ferguson L. R.; Shu Q.; Garg S. Evaluation of Mucoadhesive Coatings of Chitosan and Thiolated Chitosan for the Colonic Delivery of Microencapsulated Probiotic Bacteria. J. Microencapsulation 2013, 30 (2), 103–115. 10.3109/02652048.2012.700959. [DOI] [PubMed] [Google Scholar]
- Mohandas A.; Sun W.; Nimal T. R.; Shankarappa S. A.; Hwang N. S.; Jayakumar R. Injectable Chitosan-Fibrin/Nanocurcumin Composite Hydrogel for the Enhancement of Angiogenesis. Res. Chem. Intermed. 2018, 44 (8), 4873–4887. 10.1007/s11164-018-3340-1. [DOI] [Google Scholar]
- Ning P.; Lü S.; Bai X.; Wu X.; Gao C.; Wen N.; Liu M. High Encapsulation and Localized Delivery of Curcumin from an Injectable Hydrogel. Mater. Sci. Eng., C 2018, 83, 121–129. 10.1016/j.msec.2017.11.022. [DOI] [PubMed] [Google Scholar]
- Anitha A.; Deepa N.; Chennazhi K. P.; Lakshmanan V.-K.; Jayakumar R. Combinatorial Anticancer Effects of Curcumin and 5-Fluorouracil Loaded Thiolated Chitosan Nanoparticles towards Colon Cancer Treatment. Biochim. Biophys. Acta, Gen. Subj. 2014, 1840 (9), 2730–2743. 10.1016/j.bbagen.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Kim M. G.; Jo S. D.; Yhee J. Y.; Lee B. S.; Lee S. J.; Park S. G.; Kang S.-W.; Kim S. H.; Jeong J. H. Synergistic Anti-Tumor Effects of Bevacizumab and Tumor Targeted Polymerized VEGF SiRNA Nanoparticles. Biochem. Biophys. Res. Commun. 2017, 489 (1), 35–41. 10.1016/j.bbrc.2017.05.103. [DOI] [PubMed] [Google Scholar]
- Ko E.-B.; Cho H.-Y.; Kim T.-H.; Yea C.-H.; Choi J.-W. Cell Chip with a Thiolated Chitosan Self-Assembled Monolayer to Detect the Effects of Anticancer Drugs on Breast Normal and Cancer Cells. Colloids Surf., B 2013, 112, 387–392. 10.1016/j.colsurfb.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Kim J. B.; Park K.; Ryu J.; Lee J. J.; Lee M. W.; Cho H. S.; Nam H. S.; Park O. K.; Song J. W.; Kim T. S.; Oh D. J.; Gweon D.; Oh W.-Y.; Yoo H.; Kim J. W. Intravascular Optical Imaging of High-Risk Plaques in Vivo by Targeting Macrophage Mannose Receptors. Sci. Rep. 2016, 6 (1), 22608. 10.1038/srep22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharathiraja S.; Bui N. Q.; Manivasagan P.; Moorthy M. S.; Mondal S.; Seo H.; Phuoc N. T.; Vy Phan T. T.; Kim H.; Lee K. D.; Oh J. Multimodal Tumor-Homing Chitosan Oligosaccharide-Coated Biocompatible Palladium Nanoparticles for Photo-Based Imaging and Therapy. Sci. Rep. 2018, 8 (1), 500. 10.1038/s41598-017-18966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almada M.; Leal-Martínez B. H.; Hassan N.; Kogan M. J.; Burboa M. G.; Topete A.; Valdez M. A.; Juárez J. Photothermal Conversion Efficiency and Cytotoxic Effect of Gold Nanorods Stabilized with Chitosan, Alginate and Poly(Vinyl Alcohol). Mater. Sci. Eng., C 2017, 77, 583–593. 10.1016/j.msec.2017.03.218. [DOI] [PubMed] [Google Scholar]
- Shahnaz G.; Kremser C.; Reinisch A.; Vetter A.; Laffleur F.; Rahmat D.; Iqbal J.; Dünnhaupt S.; Salvenmoser W.; Tessadri R.; Griesser U.; Bernkop-Schnürch A. Efficient MRI Labeling of Endothelial Progenitor Cells: Design of Thiolated Surface Stabilized Superparamagnetic Iron Oxide Nanoparticles. Eur. J. Pharm. Biopharm. 2013, 85 (3), 346–355. 10.1016/j.ejpb.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Jia X.; Han F.; Zhao J.; Zhao Y.; Fan Y.; Yuan X. Dual-Delivery of VEGF and PDGF by Double-Layered Electrospun Membranes for Blood Vessel Regeneration. Biomaterials 2013, 34 (9), 2202–2212. 10.1016/j.biomaterials.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Yang Q.; Zhou F.; Zhao Y.; Jia X.; Yuan X.; Fan Y. Electrospun PELCL Membranes Loaded with QK Peptide for Enhancement of Vascular Endothelial Cell Growth. J. Mater. Sci.: Mater. Med. 2016, 27 (6), 106. 10.1007/s10856-016-5705-6. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Deng C.; Meng F.; Shi Q.; Feijen J.; Zhong Z. Novel Injectable Biodegradable Glycol Chitosan-Based Hydrogels Crosslinked by Michael-Type Addition Reaction with Oligo(Acryloyl Carbonate)-b-Poly(Ethylene Glycol)-b-Oligo(Acryloyl Carbonate) Copolymers. J. Biomed. Mater. Res., Part A 2011, 99A (2), 316–326. 10.1002/jbm.a.33199. [DOI] [PubMed] [Google Scholar]
- Liu J.; Yang B.; Li M.; Li J.; Wan Y. Enhanced Dual Network Hydrogels Consisting of Thiolated Chitosan and Silk Fibroin for Cartilage Tissue Engineering. Carbohydr. Polym. 2020, 227, 115335. 10.1016/j.carbpol.2019.115335. [DOI] [PubMed] [Google Scholar]
- Esmaeilzadeh P.; Köwitsch A.; Heyroth F.; Schmidt G.; Fischer S.; Richter K.; Groth T. Synthesis of Thiolated Polysaccharides for Formation of Polyelectrolyte Multilayers with Improved Cellular Adhesion. Carbohydr. Polym. 2017, 157, 1205–1214. 10.1016/j.carbpol.2016.10.088. [DOI] [PubMed] [Google Scholar]
- Lin Z.; Li R.; Liu Y.; Zhao Y.; Ao N.; Wang J.; Li L.; Wu G. Histatin1-Modified Thiolated Chitosan Hydrogels Enhance Wound Healing by Accelerating Cell Adhesion, Migration and Angiogenesis. Carbohydr. Polym. 2020, 230, 115710. 10.1016/j.carbpol.2019.115710. [DOI] [PubMed] [Google Scholar]
- Costa F.; Sousa D. M.; Parreira P.; Lamghari M.; Gomes P.; Martins M. C. L. N-Acetylcysteine-Functionalized Coating Avoids Bacterial Adhesion and Biofilm Formation. Sci. Rep. 2017, 7 (1), 17374. 10.1038/s41598-017-17310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X.-F.; Gao H.-L.; Su Y.; Wu Y.-D.; Wang X.-Y.; Xue J.-Z.; He T.; Lu Y.; Liu J.-W.; Yu S.-H. Strong and Stiff Ag Nanowire-Chitosan Composite Films Reinforced by Ag–S Covalent Bonds. Nano Res. 2018, 11 (1), 410–419. 10.1007/s12274-017-1644-x. [DOI] [Google Scholar]
- Li Y.; Fang L.; Cheng P.; Deng J.; Jiang L.; Huang H.; Zheng J. An Electrochemical Immunosensor for Sensitive Detection of Escherichia Coli O157:H7 Using C60 Based Biocompatible Platform and Enzyme Functionalized Pt Nanochains Tracing Tag. Biosens. Bioelectron. 2013, 49, 485–491. 10.1016/j.bios.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Jain A.; Hurkat P.; Jain A.; Jain A.; Jain A.; Jain S. K. Thiolated Polymers: Pharmaceutical Tool in Nasal Drug Delivery of Proteins and Peptides. Int. J. Pept. Res. Ther. 2019, 25 (1), 15–26. 10.1007/s10989-018-9704-y. [DOI] [Google Scholar]
- Shah K. U.; Shah S. U.; Dilawar N.; Khan G. M.; Gibaud S. Thiomers and Their Potential Applications in Drug Delivery. Expert Opin. Drug Delivery 2017, 14 (5), 601–610. 10.1080/17425247.2016.1227787. [DOI] [PubMed] [Google Scholar]
- Duggan S.; Cummins W.; O’ Donovan O.; Hughes H.; Owens E. Thiolated Polymers as Mucoadhesive Drug Delivery Systems. Eur. J. Pharm. Sci. 2017, 100, 64–78. 10.1016/j.ejps.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Jiao J.; Huang J.; Zhang Z. Hydrogels Based on Chitosan in Tissue Regeneration: How Do They Work? A Mini Review. J. Appl. Polym. Sci. 2019, 136, 47235. 10.1002/app.47235. [DOI] [Google Scholar]
- Fakhry A.; Schneider G. B.; Zaharias R.; Şenel S. Chitosan Supports the Initial Attachment and Spreading of Osteoblasts Preferentially over Fibroblasts. Biomaterials 2004, 25 (11), 2075–2079. 10.1016/j.biomaterials.2003.08.068. [DOI] [PubMed] [Google Scholar]
- Caló E.; Khutoryanskiy V. V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. 10.1016/j.eurpolymj.2014.11.024. [DOI] [Google Scholar]
- Huang L.; Zhu Z.; Wu D.; Gan W.; Zhu S.; Li W.; Tian J.; Li L.; Zhou C.; Lu L. Antibacterial Poly (Ethylene Glycol) Diacrylate/Chitosan Hydrogels Enhance Mechanical Adhesiveness and Promote Skin Regeneration. Carbohydr. Polym. 2019, 225 (May), 115110. 10.1016/j.carbpol.2019.115110. [DOI] [PubMed] [Google Scholar]
- Xu L. Q.; Pranantyo D.; Neoh K.; Kang E.; Fu G. D. Thiol Reactive Maleimido-Containing Tannic Acid for the Bioinspired Surface Anchoring and Post-Functionalization of Antifouling Coatings. ACS Sustainable Chem. Eng. 2016, 4 (8), 4264–4272. 10.1021/acssuschemeng.6b00760. [DOI] [Google Scholar]
- Mirani Z. A.; Fatima A.; Urooj S.; Aziz M.; Khan M. N.; Abbas T. Relationshipof Cell Surface hydrophobicity with biofilm formation and Growth Rate: A Study on Pseudomonas Aeruginosa, Staphylococcus Aureus, and Escherichia Coli. Iran. J. Basic Med. Sci. 2018, 21 (7), 760–769. 10.22038/ijbms.2018.28525.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe D.; Smart C. A.; Alexander C.; Vulfson E. N. Bacterial Adhesion at Synthetic Surfaces. Appl. Environ. Microbiol. 1999, 65 (11), 4995–5002. 10.1128/AEM.65.11.4995-5002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhav S.; Ahamad A.; Singh P.; Mishra P. K. A Review of Textile Industry: Wet Processing, Environmental Impacts, and Effluent Treatment Methods. Environ. Qual. Manag. 2018, 27 (3), 31–41. 10.1002/tqem.21538. [DOI] [Google Scholar]
- Simoncic B.; Tomsic B. Structures of Novel Antimicrobial Agents for Textiles - A Review. Text. Res. J. 2010, 80 (16), 1721–1737. 10.1177/0040517510363193. [DOI] [Google Scholar]
- Ranjbar-Mohammadi M.; Arami M.; Bahrami H.; Mazaheri F.; Mahmoodi N. M. Grafting of Chitosan as a Biopolymer onto Wool Fabric Using Anhydride Bridge and Its Antibacterial Property. Colloids Surf., B 2010, 76 (2), 397–403. 10.1016/j.colsurfb.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Ulu A.; Birhanli E.; Boran F.; Köytepe S.; Yesilada O.; Ateş B. Laccase-Conjugated Thiolated Chitosan-Fe3O4 Hybrid Composite for Biocatalytic Degradation of Organic Dyes. Int. J. Biol. Macromol. 2020, 150, 871–884. 10.1016/j.ijbiomac.2020.02.006. [DOI] [PubMed] [Google Scholar]
- Leierer J.Mucoadhesive Polymers Having Vitamin B Partial Structures. European Patent EP 2 482 852 B1,2003.
- Prinz M.Chitosan-Thio-Amidine Conjugates and Their Cosmetic as Wells as Pharmaceutic Use. PCT Patent WO 03/020771 A1, 2003.
- Courtois J.-P. A. R.; Liu W.; Smith I. K.; Wang L.; White M. S.; Zhang Q.. Antiperspirant Compositions. PCT Patent WO2007071375A1, June 29, 2007.
- Oh J. E.; Lee H. J.; Choi Y. W.; Choi H. Y.; Byun J. Y. Metal Allergy in Eyelid Dermatitis and the Evaluation of Metal Contents in Eye Shadows. J. Eur. Acad. Dermatol. Venereol. 2016, 30 (9), 1518–1521. 10.1111/jdv.13646. [DOI] [PubMed] [Google Scholar]
- Ahlström M. G.; Thyssen J. P.; Wennervaldt M.; Menné T.; Johansen J. D. Nickel Allergy and Allergic Contact Dermatitis: A Clinical Review of Immunology, Epidemiology, Exposure, and Treatment. Contact Dermatitis 2019, 81 (4), 227–241. 10.1111/cod.13327. [DOI] [PubMed] [Google Scholar]
- Netsomboon K.; Jalil A.; Laffleur F.; Hupfauf A.; Gust R.; Bernkop-Schnürch A. Thiolated Chitosans: Are Cys-Cys Ligands Key to the next Generation?. Carbohydr. Polym. 2020, 242, 116395. 10.1016/j.carbpol.2020.116395. [DOI] [PubMed] [Google Scholar]
- Su J. Thiol-Mediated Chemoselective Strategies for In Situ Formation of Hydrogels. Gels 2018, 4 (3), 72. 10.3390/gels4030072. [DOI] [PMC free article] [PubMed] [Google Scholar]