Abstract
Objective:
Research on attention-deficit/hyperactivity disorder (ADHD) points to the possibility that contextual factors (e.g., time of day, school vs. home) may be related to symptoms and impairment. This prior research has relied on laboratory-based or retrospective, global approaches which has limited ecological validity. The present study substantively contributes to the extant literature by examining adolescents’ ADHD symptoms in the real world across the day on both school and non-school days to test whether symptoms worsened throughout the day and were higher on school days relative to non-school days.
Method:
As part of a larger study, 83 adolescents taking stimulant medication for ADHD (Mage = 14.7, 66% identified as boys/men, 78% White) completed a 17-day ecological momentary assessment protocol that included wake-up and bedtime reports and two reports in the afternoon and evening. These assessments asked about ADHD symptoms and stimulant medication usage since the last report. Hypotheses were tested using multilevel modeling.
Results:
Accounting for demographic covariates and medication usage, ADHD symptoms worsened quadratically, peaking at the afternoon report and subsequently declining, across school days but not non-school days. Mean-level ADHD symptoms were also worse on school days relative to non-school days. Results did not differ across gender.
Conclusions:
Our study is the first to examine important environmental factors (school, time of day) in real time in relation to level of naturalistically occurring ADHD symptoms. Our findings highlight the importance of advancing treatments to support adolescents with ADHD on school days and in the afternoon.
Keywords: attention-deficit/hyperactivity disorder, ADHD, ecological momentary assessment, adolescent, real-world
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder with an estimated prevalence of 5-7% among children and adolescents (Thomas, Sanders, Doust, Beller, & Glasziou, 2015; Willcutt, 2012). The core symptoms of ADHD include inattention, hyperactivity and impulsivity (American Psychiatric Association, 2013). Symptoms of ADHD are associated with significant social, educational, and health impairments in childhood through adulthood (Barkley, Murphy, & Fischer, 2010; Hechtman, 2016), including significant impairments in adolescence (Sibley, Kuriyan, Evans, Waxmonsky, & Smith, 2014). ADHD symptoms are typically assessed with global “trait”-like measurements (e.g., past six months). Research that has examined ADHD in association with outcomes has largely taken this “trait-like” approach which may obscure important changes in ADHD symptoms throughout the day that could be affected by the immediate environment or individual circumstances (e.g., sleep disturbances; see Lunsford-Avery, Krystal, & Kollins, 2016 for a review of ADHD symptoms and sleep in adolescence). The current study examined the possibility that adolescents’ ADHD symptoms systematically change throughout the day and vary across school and non-school days. Focusing on variation in symptoms may elucidate important environmental treatment targets that could further reduce impairments for adolescents with ADHD.
ADHD Symptoms Across Time and Setting
Although research has not directly examined whether ADHD symptoms vary over the course of the day and week in the naturalistic environment, some preliminary evidence supports this possibility. For example, a study of 43 children with ADHD (Mean age=10) found better cognitive task performance in the morning than later in the day and more disruptive behaviors in the afternoon (Zagar & Bowers, 1983), suggesting that symptoms may worsen over the course of the day. Further, in analog classroom studies ADHD symptoms worsen over the course of the day in children (e.g., Pelham et al., 2001). Additionally, a large body of literature has found only modest associations between parent and teacher ratings of inattention, hyperactivity, and impulsivity (e.g., Antrop, Roeyers, Oosterlaan, & Van Oost, 2002; Gomez, 2007; Mitsis, McKay, Schulz, Newcorn, & Halperin, 2000), which could in part result from differences in actual behavior across context (e.g., school versus home). In adolescence, students typically have multiple teachers, and the differences in symptom ratings across teachers could also indicate within-day variability in symptoms for teenagers with ADHD (Molina, Pelham, Blumenthal, & Galiszewski, 1998). Taken together, these findings suggest that ADHD symptoms and symptom severity may fluctuate across settings and over the course of the day. Naturalistic or ecological momentary assessment (EMA) that measures ADHD symptoms several times throughout the day and across real-world contexts is needed to directly examine this possibility.
EMA and ADHD
EMA has numerous strengths over traditional questionnaire-based assessments (Shiffman, Stone, & Hufford, 2008). Particularly germane to the current study is the ability to capture near real-time changes (e.g., Carpenter, Wycoff, & Trull, 2016), decreased reliance on retrospective report (e.g., reflecting over several hours versus two weeks or months), and ability to examine temporal processes and variation in symptoms (Maes et al., 2015). Despite these strengths, research has not leveraged EMA to examine ADHD symptoms in adolescent samples and relatively few studies have examined real-world ADHD symptoms in children or adults (Miguelez-Fernandez et al., 2018).
While studies have not examined fluctuation of ADHD symptoms over the day and across days (in particular, comparing school and non-school days), research has demonstrated the validity of EMA in ADHD samples. A meta-analysis of 23 studies, of which 15 included children and young adolescents with ADHD (Miguelez-Fernandez et al., 2018), demonstrated the value of leveraging EMA to study ADHD. Individual studies (e.g., Whalen et al., 2010; Rosen et al., 2013) support the notion that ADHD symptoms and related impairments may vary meaningfully throughout the day, differ across the week, and can be validly measured via self- and parent-report. For example, children with ADHD (ages 7-12; n = 25) have been found to have higher mean levels of EMA mother-reported impatience, restlessness, talking too loud and too much, anger and sadness compared to children without ADHD (n = 27; (Whalen et al., 2006). In this same study, child self-report was also examined, and these results showed that the ADHD group experienced more stress on the weekends compared to the nonADHD group. A separate study, utilizing parent report across a 28-day period, (ages 8–12) found that children with ADHD and higher emotional impulsivity demonstrated greater overall levels of functional impairment and more variability in their impairment (Walerius, Reyes, Rosen, & Factor, 2018). While EMA research on ADHD symptoms in adults is even more sparse and has smaller samples (N’s = 10-15 participants) it also demonstrated the utility of capturing ADHD symptoms in naturalistic settings and the validity of real-world self-report of ADHD symptoms (Gehricke, Hong, Wigal, Chan, & Doan, 2011; Gehricke, Whalen, Jamner, Wigal, & Steinhoff, 2006).
However, there are also significant gaps in the existing literature that limit understanding on naturalistic changes in ADHD symptoms. For example, the studies in the recent meta-analysis on this topic (Miguelez-Fernandez et al., 2018) relied largely on parent report, focused on childhood or very early adolescence (< age 13), and examined emotion regulation processes as opposed to inattention, impulsivity, and hyperactivity. The authors of this meta-analysis concluded that context-specific evaluation of ADHD symptoms is needed and research on adolescents may be particularly important.
Research assessing fluctuation in ADHD symptoms in naturalistic contexts, with EMA or other related method (e.g., daily diaries), has not been conducted for the period of mid- to late-adolescence. However, examination of ADHD symptoms in the real world may be particularly important given the unique demands of this developmental stage. Increased autonomy from parents, expanded peer influence and stressors, earlier school start times and changing academic demands, and shifted sleep schedules all may contribute to increased fluctuation in ADHD symptoms across the day relative to childhood; in turn, such changes in symptoms may result in subsequent impairment unique to adolescence (Sibley et al., 2014). Self-perception of ADHD symptom fluctuation and their impact on functioning is also increasingly important in adolescence as teens assume increasing responsibility for their behavior, and treatment (Wolraich et al., 2019; Brinkman et al., 2012). Assessing adolescent report of ADHD symptoms throughout and across days has the additional advantage of tracking how experiences change across context (home vs. school) without being confounded by also changing the reporter (e.g., parent vs. teacher) or missing significant parts of the day (e.g., parents are not present at school to see adolescent symptoms). Understanding adolescent perception of when symptoms may peak during the day, as well as how symptoms vary across days as a function of school attendance, can inform intervention efforts or facilitate increased support during those times.
Gender Differences in ADHD
Findings on gender differences in ADHD suggest that boys and girls with ADHD may have different clinical presentations. For example, research has shown that girls with ADHD may have fewer hyperactive/impulsive symptoms compared to boys with ADHD (Hinshaw, Owens, Sami, & Fargeon, 2006). Additionally, in a community-based sample of children and adolescents, boys met criteria for ADHD more often than girls with a ratio of 3:1 (Willcutt, 2012). The difference in referral rates are even more disparate with boys referred more frequently for ADHD treatment than girls (Nøvik et al., 2006). This research may indicate that boys experience more impairment from ADHD than girls. However, in a study examining the effects of methylphenidate on ADHD symptoms in girls and boys in a laboratory classroom, girls had a more positive response to the medication after 1.5 hours but then had a poorer response after 12 hours compared to boys (Sonuga-Barke et al., 2007). These findings raise the possibility that ADHD symptoms may worsen more for girls throughout the day compared to boys. Understanding whether symptoms fluctuate differently and are differentially affected by context for boys and girls with ADHD could point to more personalized medicine and may identify strategies that could be time-and/or context specific for boys or girls. As research has not examined gender differences in fluctuations in ADHD symptoms in the real world, the current study sought to begin to address this current limitation.
Current Study
The current study examined if and how ADHD symptoms change over the course of the day and across school and non-school days for a sample of adolescents with ADHD who completed a 17 day EMA protocol. Participants were from a larger study of adolescents recruited on the basis of being stimulant-treated for ADHD in pediatric primary care. We hypothesized that ADHD symptoms would worsen throughout the day from wake-up to bedtime. Second, we hypothesized that ADHD symptoms would be worse on school days compared to non-school days and that they would worsen more rapidly over the course of the school day (versus non-school day). Post-hoc analyses were conducted to examine if these findings differed for days participants were medicated (versus unmedicated). Lastly, given the inconsistent findings on gender differences and lack of research on real-world symptoms comparing girls and boys, we explored possible gender differences in our main hypotheses but did not hold a priori hypotheses.
Method
Participants
Participants were recruited from an ongoing intervention study of stimulant diversion risk in adolescence. To be enrolled in the larger study, participants were required to be prescribed stimulant medication for ADHD by their pediatrician. Their pediatricians were at one of seven practices that were randomized to either receive training in assessing and preventing stimulant diversion during primary care visits or to continue their practice as usual. Additionally, given the focus on stimulant diversion risk in adolescence, participants were required to be between the ages of 13-18 and attending school (homeschoolers excluded).
The subsample for the current study consisted of 94 adolescents recruited to participate in a 17-day EMA study upon completion of their baseline participation and prior to deployment of the practice-based intervention in the second cohort of the larger ongoing study (n = 172; n = 158 adolescents consented to be contacted for the EMA sub-study). At baseline, participants and their parents completed online questionnaires assessing sociodemographic characteristics, global ADHD symptoms, stimulant medication use, and other information. Participants for the EMA sub-study were contacted an average of 2 weeks (M = 13.6 days) after completing the baseline survey (range: 1-6 weeks; n = 4 did not complete the full baseline assessment). Participants more than 60 days out from their baseline survey were excluded to ensure that baseline information remained accurate (n = 4 participants timed out of this window). Additionally, upon phone contact to confirm interest in the sub-study (n = 8 declined to participate), all participants confirmed that they were currently taking ADHD stimulant medication (n = 2 were no longer taking stimulant medication and were excluded from EMA). Enrollment continued on a rolling basis as participants completed their baseline surveys until we reached our target N of 80 participants (46 participants were not contacted because EMA recruitment was completed). Recruitment for this sub-study oversampled girls to permit exploration of gender differences.
To ensure sufficient data for the present analyses, only cases that completed the morning assessment and at least one other assessment on at least two school days and two non-school days were included, resulting in a final sample of 83 adolescents (Mage = 14.7; 66% boys, 78% White). Average EMA compliance for our final sample across the 17 days was high (85%: an average of 58 of the possible 68 prompts were completed). The final subsample did not significantly differ from either the remainder of the cohort 2 participants or the entire larger study sample (cohort 1 and cohort 2) on age, race, self-reported global ADHD symptoms or parent-reported global ADHD symptoms.
Study Design
Study procedures were approved by the University of Pittsburgh’s Institutional Review Board. Participants who met eligibility criteria for the larger study were contacted via phone upon completion of their baseline survey which was timed to occur during the fall-early winter to ensure all participants were in school. During the baseline survey participants provided consent electronically to participate in the EMA study. Study staff then contacted interested participants via phone and provided additional study details and asked questions to confirm eligibility and to set up the 17-day EMA prompt schedule.
Participants provided study staff with their typical bed and wake time for both school days and non-school days to anchor the last and first assessment point of each day. All prompts were sent via text message containing a direct link to a password-protected web-based questionnaire. The first assessment prompt was sent 15 minutes after reported wake time and the last assessment was sent to participants 15 minutes prior to reported bedtime. An additional 2 assessments were sent during the afternoon post school day (between 2:50 pm - 4:20 pm) and during the evening (between 6:30 pm - 8:00 pm). No assessments were delivered during the school day. Participants were instructed that they had 40 minutes to respond to the prompt and that they would receive 3 reminder texts in 10-minute intervals during this 40-minute window. Participants chose whether to use their personal smartphone or a study issued smartphone. All participants were provided instruction on how to ensure safe and accurate completion of the assessments (e.g., avoid answering prompts when driving, keep password secure). Participants were scheduled by study staff at the end of this phone call to begin the EMA protocol on the following Friday to maximally capture both school day and non-school day experiences. Participants could earn up to $10 per day if they completed 75% or more of the assessments daily. Additionally, a $25 bonus was provided if a participant answered questions for at least 85% of all EMA prompts across the 17-day study period.
Measures
Demographics.
Participants self-reported gender identity, age, and race at the baseline assessment. Gender was dummy-coded so that 0 = girl and 1 = boy. Age was centered at the sample mean (14.7). Race was dummy coded so that 0 = White and 1 = other race (Black or African American and more than one race).
Global retrospective ADHD symptoms.
The Disruptive Behaviors Disorders scale (DBD; Pelham, Gnagy, Greenslade, & Milich, 1992; Sibley et al., 2012) was adapted for adolescents and administered to both the adolescent participant and the parent that was primarily in charge of the adolescent’s pediatric healthcare. Both the adolescent and the parent were instructed to rate the adolescent’s behavior over the past 6 months when the adolescent had not taken stimulant medication. Item response options ranged from 0 = “not at all” to 3 = “very much.” The modified version of this scale contained 9 symptoms of inattention (e.g., easily distracted, not listening, careless mistakes; adolescent alpha = .92; parent alpha = .92) and 9 symptoms of hyperactivity/impulsivity (e.g., interrupts/intrudes, talks too much, fidgeting, blurts out answers; adolescent alpha = .92; parent alpha = .92). An inattention subscale and separate hyperactivity/impulsivity subscale were computed by averaging the symptoms for each domain. We also created a total ADHD scale that was the average response across all 18 questions (adolescent alpha = .95; parent alpha = .95).
Momentary ADHD symptoms.
At each of the four daily assessments, participants self-reported about their ADHD symptoms (4 inattention items, 3 impulsivity items, and 3 hyperactivity items) since the last assessment prompt (e.g., since the last assessment prompt… “I forgot things,” “I said things without thinking,” “People said I was hyper”). Anchors ranged from 0 = “not at all” to 3 = “very much.” Total ADHD symptoms was calculated as the mean of the 10 items at each assessment. Inattention, impulsivity, and hyperactivity domains were also examined separately by computing the means of these subscales. These items were adapted from the disruptive behavior disorders scale (Pelham et al., 1992), the momentary impulsivity scale (Tomko et al., 2014), and prior EMA assessments of ADHD (Gehricke et al., 2011).
Daily medication usage.
Participants reported at each assessment whether they had taken ADHD medication since the last assessment. For the current analyses, a participant was coded as being medicated that day if they endorsed this item as “yes” at any assessment point during the day. Whether the participant was medicated that day was coded so that 0 = unmedicated and 1 = medicated. Medication was usually (81% of the time) taken in the morning across the 17-days (Mean time = 8:28 am; medication was taken prior to completion of the morning report 54.4% of the time).
School day versus non-school day.
School day and non-school day were coded from the data in a two-step process. First, all weekends were coded as non-school days. Study staff also asked participants to identify known holiday breaks from school (e.g., Thanksgiving, winter break), and these days were coded as non-school days. All remaining days were coded as school days. School day was coded so that 0=school day and 1=non-school day so that the main effect of the time terms in the presence of the interaction reflected change in ADHD symptoms over time on school days.
Analytic Plan
To test our hypotheses, we conducted a series of multilevel regression analyses predicting naturalistic ADHD symptoms from both a linear and quadratic term for time over the course of the day to determine whether ADHD symptoms worsened over the course of the day (Hypothesis 1), whether the participant attended school that day (to determine whether ADHD symptoms were worse on school days than on non-school days; Hypothesis 2a), school day*time interactions (to determine whether ADHD symptoms worsened more on school days than on non-school days; Hypothesis 2b), gender (to explore whether ADHD symptoms differed for boys and girls), and covariates (age, race, and whether the participant was medicated that day). Lastly, gender*time interactions were added to explore whether ADHD symptoms changed differently over the course of the day for boys and girls; however, they were nonsignificant and were therefore removed from the model. These procedures were repeated separately for each ADHD symptom cluster (inattentive, hyperactivity, and impulsivity symptoms) to probe for potential differences by ADHD symptom domain. Post-hoc analyses were conducted within medicated days and unmedicated days to further disentangle school and medication effects. All models included a random intercept and random effects of both linear and quadratic time. Time across the day was centered at the first random prompt (during the after-school hours) to facilitate interpretation of the main effect of school day in the presence of the school day*time interaction as the difference in ADHD symptoms on school days versus non-school days reported at the afternoon prompt. Analyses were conducted in RStudio Version 1.1.383.
Results
Descriptive Results
On average, participants started the EMA study 22 days (range 2–59 days) after they completed their baseline assessment for the larger study. Most participants (77%) met DSM-5 ADHD symptom threshold (based on parent and self-report from the baseline assessment, taking the higher rating per symptom). Participants’ grade in school ranged from 7th to 12th, with the largest proportion (34%) in 8th grade, and the median grade point average was a B+ per self-report (B per parent report). At the time of the baseline assessment, participants had been taking stimulant medication for ADHD for an average of 5.8 years (per parent report). Parent-reported median household income was $75,000 - $99,999, and 58% of parents were college graduates.
On average across the 17-days, participants reported low levels of total ADHD symptoms in EMA (M = .34, SD = .42), along with low levels of inattentive symptoms (M = .41, SD = .54), hyperactivity symptoms (M = .32, SD = .48), and impulsivity symptoms (M = .27, SD = .45). EMA-rated total ADHD symptoms were significantly correlated with self-reported global retrospective (but not parent-rated) total ADHD symptoms at baseline (r = .31, p < .01; Mean self-report baseline symptoms = 1.26; Mean parent-report baseline symptoms = 1.58) at baseline.
Participants attended school on 51% of the days during the 17-day EMA protocol, and they reported taking stimulant medication for ADHD on 56% of all days (72% of school days, 39% of non-school days). Bivariate correlations indicated that participants were more likely to take their medication on school days (r = .34, p < .001) and boys were more likely to report taking medication than were girls (X 2 = 6.46, p = .012).
Primary Results
Table 1 displays the results of each multilevel regression analysis. To test our first hypothesis, we examined the effects of both linear and quadratic time. The main effects of both were significant, such that symptoms tended to worsen over the course of the day quadratically, first worsening and subsequently improving in the late afternoon/evening hours. This pattern was consistent for total ADHD symptoms and all three symptom clusters. To test our second hypothesis, we next examined the effect of school day on ADHD symptoms. As shown in the first column of Table 1, total ADHD symptoms (as measured at the first random prompt in the after-school hours) were significantly worse on school days compared to non-school days. This finding applied to both inattentive and hyperactivity symptoms but not impulsivity symptoms. (A post-hoc analysis excluding time from the model showed that mean-level total ADHD symptoms, averaging across all time points within each day, likewise tended to be worse on school days than on non-school days, B = −.02, p < .05, as did mean-level inattentive symptoms, B = −.05, p < .001). Additionally, there were significant school day*time interactions for both linear and quadratic time, such that symptoms worsened even more on school days than non-school days, and in a more quadratic than linear form (see Figure 1). This pattern was again consistent for total ADHD symptoms and for all three symptom clusters. The only covariate that was significant in analyses was medication. ADHD symptoms (across all symptom clusters) were worse on days when participants were medicated.
Table 1:
Summary of Multilevel Regression Analyses Predicting ADHD Symptoms Across the Day
| ADHD Symptoms | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Total ADHD |
Inattentive |
Hyperactivity |
Impulsivity |
|||||
| Predictor | B | SE B | B | SE B | B | SE B | B | SE B |
| Intercept | .388*** | .061 | .482*** | .079 | .389*** | .064 | .256*** | .059 |
| Race | .012 | .080 | −.009 | .011 | .006 | .082 | .022 | .074 |
| Age | −.004 | .022 | .018 | .029 | −.013 | .023 | −.022 | .020 |
| Medicated | .029** | .010 | .027* | .014 | .025* | .013 | .034** | .012 |
| School day | −.048*** | .012 | −.083*** | .017 | −.042** | .015 | −.007 | .015 |
| Gender | −.046 | .070 | −.041 | .093 | −.081 | .072 | −.004 | .065 |
| Time | .052*** | .011 | .054*** | .013 | .057*** | .012 | .044*** | .012 |
| Time2 | −.037*** | .007 | −.046*** | .009 | −.036*** | .008 | −.027*** | .008 |
| School day * Time | −.048*** | .010 | −.056*** | .014 | −.048*** | .013 | −.038** | .013 |
| School day * Time2 | .032*** | .008 | .038*** | .011 | .033*** | .010 | .023* | .010 |
Note. All models include person-level random intercepts and time slopes (linear and quadratic).
0 = White, 1 = Other race;
Age centered at the sample mean (14.7);
0 = unmedicated day, 1 = medicated day;
0 = school day, 1 = non-school day;
0=girl, 1 = boy;
EMA time point across the day, centered at afternoon (after school) prompt.
p < .05.
p < .01.
p < .001.
Figure 1.
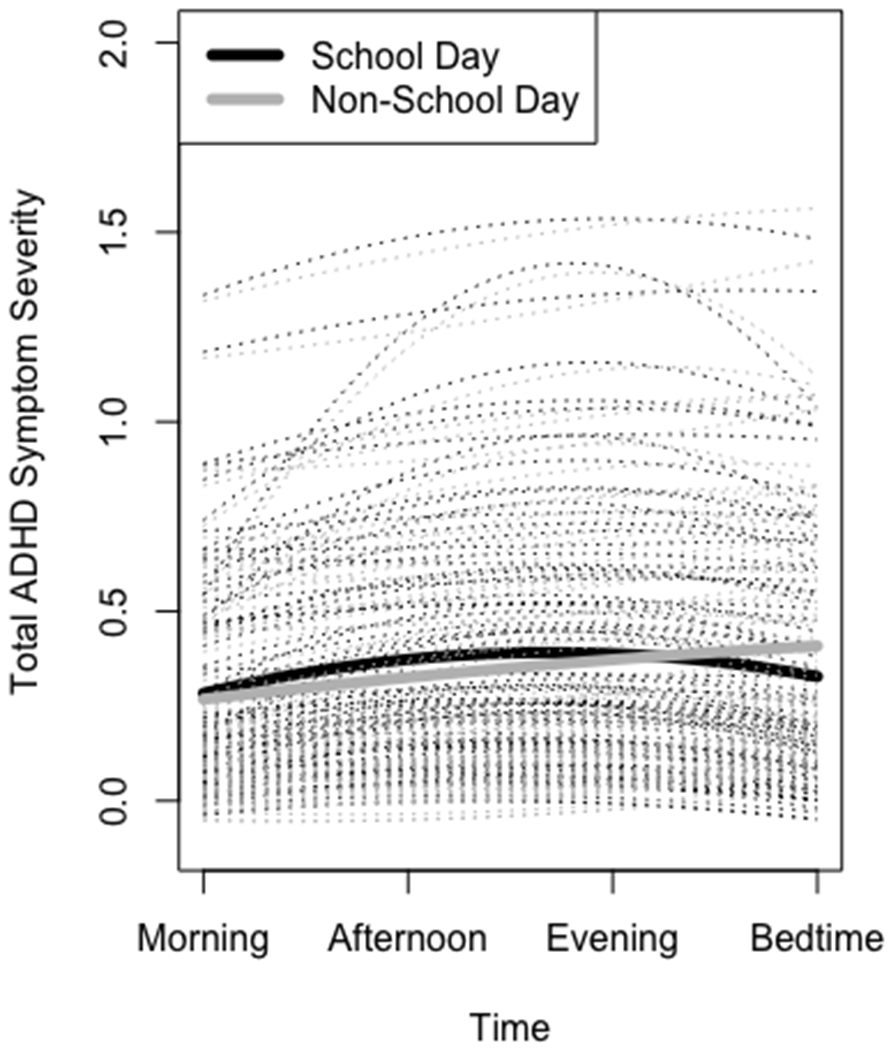
Model-implied quadratic worsening of ADHD symptom severity across school days vs. non-school days on average (solid lines) and for individual participants (dashed lines).
Post-hoc Secondary Results
To further probe the effect of worsening ADHD symptoms across the day, we conducted post-hoc multilevel regression analyses in which three dummy-coded time variables comparing the second, third, and fourth assessment points of each day to the morning (first) assessment point predicted ADHD symptoms. The same covariates described above were included, along with interaction terms between each of the assessment points and day type (school vs. non-school day). On school days, total ADHD symptoms were worse at the second (B = .134), third (B = .079), and fourth (B = .066) assessment points than at the morning assessment point (ps < .001). However, on non-school days, total ADHD symptoms did not significantly differ from the morning assessment point at any of the assessment points. This pattern of results was the same across all symptom domains (inattention, impulsivity, hyperactivity). Additionally, a multilevel regression analysis adjusting for the same covariates showed that total ADHD symptoms increased significantly more from the morning assessment point to the second assessment point on school days compared to non-school days (B = .11, p < .001). These results provide further support that the primary findings are being driven by school attendance as opposed to a longer amount of time that passes between the morning and second assessment point relative to timing of the other assessments. The pattern of results was the same across all symptom domains.
Next, because of our unexpected medication effect in the primary analyses and to disentangle medication effects from school attendance, we conducted analyses within medicated days and unmedicated days separately. Results for the medicated days paralleled our primary findings: ADHD symptoms worsened in a quadratic fashion, peaking at the afternoon report (B = −.04, p < .001. The main and interaction effects of school also remained significant, with symptoms being worse overall (B = −.06, p < .001) and having a higher peak on medicated school days compared to medicated non-school days (B = .04, p < .001). However, results for unmedicated days showed a different pattern. ADHD symptoms worsened in a linear fashion throughout the day (B = .04, p < .01), but the quadratic effect only approached statistical significance (B = −.02, p = .085). Additionally, ADHD symptoms marginally worsened in a linear fashion on unmedicated school days relative to unmedicated non-school days (B = −.03, p = .10), and the quadratic form showing a worsening peak in symptoms by the afternoon report on school days was no longer significant (B = −.03, p = .585).
Exploratory Gender Differences
There was no significant main effect of gender for total ADHD symptoms or for any of the symptom clusters (see Table 1). Further, as noted above, gender*time interactions were not significant for total ADHD symptoms or for any of the symptom clusters (linear time: Bs = .01 to.02, ps = .277 to .449; quadratic time: Bs = −.009 to −.01, ps = .479 to .602), so they were excluded from the models.
Discussion
Traditional, global assessment of ADHD symptoms and analog classroom studies have limited ecological validity and are unable to directly examine naturally occurring contextual factors that are likely to increase or decrease inattention, hyperactivity, or impulsivity. The current study is the first to examine real-world changes in adolescents’ ADHD symptoms across the day and week as a function of school attendance. We found that symptoms worsened throughout the day and peaked in the late afternoon on school days but did not significantly change throughout the day on non-school days. Results also showed that symptoms were higher on school days than on non-school days and were similar for boys and girls.
Our school day versus non-school day naturalistic findings are novel and build substantively on existing literature. Our results showed that adolescents experienced higher inattentive and hyperactivity symptoms on school days compared to non-school days, and all three symptom domains worsened more from the morning to early afternoon on school days. A variety of possibilities may contribute to worsening symptoms on school days compared to non-school days. First, increased cognitive demand on school days may exacerbate ADHD symptoms. While this has been shown in classroom analog studies with children (Pelham et al., 2001), our study is the first to demonstrate this in the real world. Follow-up analyses designed to isolate the change in symptoms from the morning to the afternoon provide further evidence for school demands increasing ADHD symptoms as opposed to simply the passage of time or timing of medication wearing off, as ADHD symptoms only significantly worsened on school days. In fact, when examined within medicated days only, ADHD symptoms still worsened on school days. These post-hoc findings are especially informative because they allowed us to disentangle medication effects from the influence of school. Taken together, our results indicate the importance of developing additional supports besides medication for adolescents with ADHD to manage symptoms on school days.
In addition to elevated cognitive load, another possible contributor to increased ADHD symptoms on school days is diminished sleep. Prior research has shown that a significant portion (~1/3) of adolescents sleep fewer than the recommended 8-10 hours per night (Basch, Basch, Ruggles, & Rajan, 2014) and that the early start times of school fuel these deficits (Wittmann, Dinich, Merrow, & Roenneberg, 2006). Consistent with this possibility, in our study, participants on average started their morning assessment at 6:44am on school days versus 10:08am on non-school days -- a difference of over 3 hours. Adolescents with ADHD have also been found to have higher rates of insomnia and delayed sleep phase disorder compared to adolescents without ADHD (Hysing, Lundervold, Posserud, & Sivertsen, 2016). Additionally, greater sleep duration for adolescents with ADHD is associated with fewer parent-reported daytime ADHD symptoms (Becker et al., 2019; Corkum et al., 2016). Taken together, these findings underscore the possibility that early wake-up times on school days may negatively affect adolescents with ADHD more than adolescents without ADHD. Research integrating different domains of sleep disturbances and objective sleep measurements via actigraphy data in relation to school attendance and ADHD symptoms is needed, as sleep duration and quality on school nights may be modifiable treatment targets to reduce school-related ADHD impairments.
A counterintuitive finding of the current study that warrants mention is that ADHD symptoms were worse on days when participants took medication compared to when they did not. Since this research was not a medication trial and we did not systemically vary what days medication was taken or when it was taken during the day we interpret these results within the contextual factors that may be influencing why participants take their medication. For example, participants may be more likely to take their medication when they perceive their ADHD symptoms to be higher in the morning (which could also reflect poor sleep). Similarly, they may have taken medication prior to school days that they recognized as likely to be particularly challenging as an attempt to manage symptoms proactively. Additional research designed to experimentally manipulate medication usage across school and non-school days and also directly asking participants why they took (or did not take) their medication can help further answer these interesting possibilities.
An exploratory goal of the current study was to examine differences in real-world ADHD symptoms across gender. We over-sampled girls from the larger study to facilitate examination of ADHD symptoms in this understudied group. Our findings failed to show any significant differences in changes in ADHD symptoms for boys and girls. These results preliminarily suggest a similar pattern of worsening symptoms across school days for boys and girls with ADHD. However, these null findings should be interpreted cautiously given that our sample only included 28 girls. Boys in our sample were also more likely to report taking their medication on a daily basis, which could diminish possible mean level differences in symptoms for boys and girls. Further examination of gender differences with larger samples of girls and examination of contextual factors (e.g., peers) that may affect symptoms differently for boys and girls are needed.
Our study provides novel information regarding real-world ADHD symptoms, including how attention, impulsivity, and hyperactivity symptoms vary as a function of time of day and across school and non-school days. The design of the study is a notable strength; however, several limitations warrant acknowledgement. All participants in the current sample were prescribed and reported taking stimulant medication as a requirement for enrollment into the larger intervention study. Further, at baseline, the majority of participants (84.3%) were prescribed one stimulant medication and reported taking this medication once daily (79.5%; 9.6% reported less than once a day/as needed) in the morning. This homogeneity of stimulant medication regimens reduced our ability to directly examine the effects of a booster medication in the afternoon or symptom fluctuation when unmedicated (although our analyses provided limited support for symptom worsening throughout school days when unmedicated). Power to detect a moderating effect of school attendance within unmedicated days was reduced, since the majority of participants took their medication the majority of school days. Recruitment of a sample of adolescents who meet criteria for ADHD but range in their prescribed medication regimen (e.g., currently unmedicated, prescribed one stimulant once daily, prescribed one stimulant and an afternoon booster) may be an important next step to understand symptom fluctuation across contexts and medication usage and timing.
Additionally, all participants were initially recruited from pediatric primary care offices, which may have contributed to low overall reported ADHD symptoms and decreased generalizability to other ADHD populations such as specialty care, clinic-referred populations. However, pediatric primary care physicians are currently the most common providers of ADHD treatment (Goodwin, Gould, Blanco, & Olfson, 2001; Howie, Pastor, & Lukacs, 2014), and therefore this sample reflects an important, prevalent group of adolescent patients with ADHD.
Another limitation is that parents did not complete the EMA protocol along with their children, so under-reporting of symptoms by adolescents is a potential concern. Interestingly, EMA-measured ADHD symptoms were correlated with global self-report but not with global parent-report of ADHD symptoms. Although symptoms may be overall under-reported, our primary concern in this study was relative fluctuation within individuals, and this was detected. Moreover, adolescence involves increased autonomy compared with childhood and increasing demand for self-monitoring to accomplish daily goals and participate increasingly in treatment (Brinkman et al., 2011; Wolraich et al., 2019). In addition, proportion of time spent with peers versus parents (Barnes, Hoffman, Welte, Farrell, & Dintcheff, 2007) reduces parents’ abilities to accurately report on their teens’ in-the-moment ADHD symptoms. A future dyadic study that includes both parent and adolescent report of real-time symptoms, particularly during periods of homework completion and other significant contexts such as weekend activities, might lead to higher real-world symptom reporting and aid identification of periods of elevated symptoms and impairment.
Lastly, by design, adolescents were not prompted to answer questions during school hours to avoid disrupting their focus or disciplinary action for using their cellphone. However, this approach does not allow for capture of momentary changes that may have occurred during mid-morning, lunchtime, or early afternoon hours, as it relies on participants to reflect on and average over these time periods. ADHD symptoms may have peaked earlier in the afternoon, and our design may have missed this peak. We also do not have objective confirmation that an adolescent actually attended school on a given day which may have resulted in a small number of days being miscoded as school days when participants were sick or stayed home. Future research examining ADHD symptoms more frequently throughout the entire day, including typical “school day” hours in conjunction with passive assessment of location (e.g., GPS coordinates) or school attendance records, is needed to more fully understand points of elevated symptoms at school.
Despite these limitations, our findings significantly advance understanding of the dynamic and changing nature of ADHD symptoms over the course of the day and as a function of school attendance and medication usage. Understanding how symptoms vary throughout the day and across settings (e.g., school vs. home) for adolescents with ADHD could inform treatment strategies to optimize outcomes for this population. Potential treatment targets could include behavioral strategies during the school day or the period of time after school is dismissed (Evans, Owens, & Bunford, 2014; Sibley et al., 2014), or increasing sleep duration on school nights. Further research is warranted to examine the impact of these factors, on non-school days as well when homework needs to be completed and symptom-exacerbated risky behaviors may be increasingly important for adolescents with ADHD (e.g., driving).
Acknowledgments
Funding: Funding for this study was provided by the National Institute on Drug Abuse DA040213
References
- Antrop I, Roeyers H, Oosterlaan J, & Van Oost P (2002). Agreement between parent and teacher ratings of disruptive behavior disorders in children with clinically diagnosed ADHD. Journal of Psychopathology and Behavioral Assessment, 24(1), 67–73. [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: Author. [Google Scholar]
- Barkley RA, Murphy KR, & Fischer M (2010). ADHD in adults: What the science says: Guilford Press. [Google Scholar]
- Barnes GM, Hoffman JH, Welte JW, Farrell MP, & Dintcheff BA (2007). Adolescents’ time use: Effects on substance use, delinquency and sexual activity. Journal of Youth and Adolescence, 36(5), 697–710. [Google Scholar]
- Basch CE, Basch CH, Ruggles KV, & Rajan S (2014). Prevalence of sleep duration on an average school night among 4 nationally representative successive samples of American high school students, 2007-2013. Preventing Chronic Disease, 11, E216–E216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP, Epstein JN, Tamm L, Tilford AA, Tischner CM, Isaacson PA, … Beebe DW (2019). Shortened sleep duration causes sleepiness, inattention, and oppositionality in adolescents with attention-deficit/hyperactivity disorder: Findings from a crossover sleep restriction/extension study. Journal of the American Academy of Child and Adolescent Psychiatry, 58(4), 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman WB, Sherman SN, Zmitrovich AR, Visscher MO, Crosby LE, Phelan K,J, Donovan EF (2012). In their own words: adolescent views on ADHD and their evolving role managing medication. Academic Pediatrics, 12(1), 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RW, Wycoff AM, & Trull TJ (2016). Ambulatory assessment: New adventures in characterizing dynamic processes. Assessment, 23(4), 414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkum P, Lingley-Pottie P, Davidson F, McGrath P, Chambers CT, Mullane J, … Weiss SK (2016). Better nights/better days—distance intervention for insomnia in school-aged children with/without ADHD: a randomized controlled trial. Journal of Pediatric Psychology, 41(6), 701–713. [DOI] [PubMed] [Google Scholar]
- Evans SW, Owens JS, & Bunford N (2014). Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology, 43(4), 527–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehricke J-G, Hong N, Wigal TL, Chan V, & Doan A (2011). ADHD medication reduces cotinine levels and withdrawal in smokers with ADHD. Pharmacology Biochemistry and Behavior, 98(3), 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehricke J-G, Whalen CK, Jamner LD, Wigal TL, & Steinhoff K (2006). The reinforcing effects of nicotine and stimulant medication in the everyday lives of adult smokers with ADHD: A preliminary examination. Nicotine & Tobacco Research, 8(1), 37–47. [DOI] [PubMed] [Google Scholar]
- Gomez R (2007). Australian parent and teacher ratings of the DSM-IV ADHD symptoms: differential symptom functioning and parent-teacher agreement and differences. Journal of Attention Disorders, 11(1), 17–27. [DOI] [PubMed] [Google Scholar]
- Goodwin R, Gould MS, Blanco C, & Olfson M (2001). Prescription of psychotropic medications to youths in office-based practice. Psychiatric Services, 52(8), 1081–1087. [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Swanson JM, Steinhoff K, Fried J, Posner K, Lerner M, … Tulloch S (2003). A pharmacokinetic/pharmacodynamic study comparing a single morning dose of Adderall to twice-daily dosing in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry, 42(10), 1234–1241. [DOI] [PubMed] [Google Scholar]
- Hechtman L (2016). Attention Deficit Hyperactivity Disorder: Adult Outcome and Its Predictors: Oxford University Press. [Google Scholar]
- Hinshaw SP, Owens EB, Sami N, & Fargeon S (2006). Prospective follow-up of girls with attention-deficit/hyperactivity disorder into adolescence: evidence for continuing cross-domain impairment. Journal of Consulting and Clinical Psychology, 74(3), 489. [DOI] [PubMed] [Google Scholar]
- Howie LD, Pastor PN, & Lukacs S (2014). Use of medication prescribed for emotional or behavioral difficulties among children aged 6-17 years in the United States, 2011-2012: Citeseer. [PubMed] [Google Scholar]
- Hysing M, Lundervold AJ, Posserud M-B, & Sivertsen B (2016). Association between sleep problems and symptoms of attention deficit hyperactivity disorder in adolescence: results from a large population-based study. Behavioral Sleep Medicine, 14(5), 550–564. [DOI] [PubMed] [Google Scholar]
- Lunsford-Avery JR, Krystal AD, & Kollins SH (2016). Sleep disturbances in adolescents with ADHD: A systematic review and framework for future research. Clinical Psychology Review, 50, 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes IH, Delespaul PA, Peters ML, White MP, van Horn Y, Schruers K, … Joore M (2015). Measuring health-related quality of life by experiences: the experience sampling method. Value in Health, 18(1), 44–51. [DOI] [PubMed] [Google Scholar]
- Miguelez-Fernandez C, de Leon SJ, Baltasar-Tello I, Peñuelas-Calvo I, Barrigon ML, Capdevila AS, … Carballo JJ (2018). Evaluating attention-deficit/hyperactivity disorder using ecological momentary assessment: a systematic review. ADHD Attention Deficit and Hyperactivity Disorders, 10(4), 247–265. [DOI] [PubMed] [Google Scholar]
- Mitsis EM, McKAY KE, Schulz KP, Newcorn JH, & Halperin JM (2000). Parent–teacher concordance for DSM-IV attention-deficit/hyperactivity disorder in a clinic-referred sample. Journal of the American Academy of Child and Adolescent Psychiatry, 39(3), 308–313. [DOI] [PubMed] [Google Scholar]
- Molina BS, Pelham WE, Blumenthal J, & Galiszewski E (1998). Agreement among teachers’ behavior ratings of adolescents with a childhood history of attention deficit hyperactivity disorder. Journal of Clinical Child Psychology, 27(3), 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nøvik TS, Hervas A, Ralston SJ, Dalsgaard S, Pereira RR, Lorenzo MJ, & Group AS (2006). Influence of gender on attention-deficit/hyperactivity disorder in Europe–ADORE. European Child and Adolescent Psychiatry, 15(1), i15–i24. [DOI] [PubMed] [Google Scholar]
- Owens JS, Goldfine ME, Evangelista NM, Hoza B, & Kaiser NM (2007). A critical review of self-perceptions and the positive illusory bias in children with ADHD. Clinical Child and Family Psychology Review, 10(4), 335–351. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Burrows-Maclean L, Williams A, Fabiano GA, Morrisey SM, … Hoffman MT (2001). Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics, 107(6), e105–e105. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, & Milich R (1992). Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 31(2), 210–218. [DOI] [PubMed] [Google Scholar]
- Pliszka S (2007). Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 46(7), 894–921. [DOI] [PubMed] [Google Scholar]
- Rosen PJ, Epstein JN, & Van Orden G (2013). I know it when I quantify it: Ecological momentary assessment and recurrence quantification analysis of emotion dysregulation in children with ADHD. ADHD Attention Deficit and Hyperactivity Disorders, 5(3), 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Collette F, Cajochen C, & Peigneux P (2007). A time to think: circadian rhythms in human cognition. Cognitive Neuropsychology, 24(7), 755–789. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, & Hufford MR (2008). Ecological momentary assessment. Annual Review of Clinical Psychology, 4, 1–32. [DOI] [PubMed] [Google Scholar]
- Sibley MH, Kuriyan AB, Evans SW, Waxmonsky JG, & Smith BH (2014). Pharmacological and psychosocial treatments for adolescents with ADHD: An updated systematic review of the literature. Clinical Psychology Review, 34(3), 218–232. [DOI] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE Jr, Molina BS, Gnagy EM, Waschbusch DA, Garefino AC, … Karch KM (2012). Diagnosing ADHD in adolescence. Journal of Consulting and Clinical Psychology, 80(1), 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Coghill D, Markowitz JS, Swanson JM, Vandenberghe M, & Hatch SJ (2007). Sex differences in the response of children with ADHD to once-daily formulations of methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry, 46(6), 701–710. [DOI] [PubMed] [Google Scholar]
- Team, R. (2017). RStudio: Integrated Development for R (version 1.1. 383)[Computer software]. In. [Google Scholar]
- Thomas R, Sanders S, Doust J, Beller E, & Glasziou P (2015). Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics, 135(4), e994–e1001. [DOI] [PubMed] [Google Scholar]
- Tomko RL, Solhan MB, Carpenter RW, Brown WC, Jahng S, Wood PK, & Trull TJ (2014). Measuring impulsivity in daily life: the momentary impulsivity scale. Psychological Assessment, 26(2), 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walerius DM, Reyes RA, Rosen PJ, & Factor PI (2018). Functional impairment variability in children with ADHD due to emotional impulsivity. Journal of Attention Disorders, 22(8), 724–737. [DOI] [PubMed] [Google Scholar]
- Whalen CK, Henker B, Ishikawa SS, Emmerson NA, Swindle R, & Johnston JA (2010). Atomoxetine versus stimulants in the community treatment of children with ADHD: an electronic diary study. Journal of Attention Disorders, 13(4), 391–400. [DOI] [PubMed] [Google Scholar]
- Whalen CK, Henker B, Jamner LD, Ishikawa SS, Floro JN, Swindle R, … Johnston JA (2006). Toward mapping daily challenges of living with ADHD: Maternal and child perspectives using electronic diaries. Journal of Abnormal Child Psychology, 34(1), 111–126. [DOI] [PubMed] [Google Scholar]
- Willcutt EG (2012). The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics, 9(3), 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, & Roenneberg T (2006). Social jetlag: misalignment of biological and social time. Chronobiology International, 23(1-2), 497–509. [DOI] [PubMed] [Google Scholar]
- Wolraich ML, Hagan JF, Allan C, et al. ; Subcommittee on Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. (2019). Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics, 144(4):e20192528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagar R, & Bowers ND (1983). The effect of time of day on problem solving and classroom behavior. Psychology in the Schools, 20(3), 337–345. [Google Scholar]


