Abstract
As individuals age, they experience increased difficulties producing speech, especially with infrequent words. Older adults report that word retrieval difficulties frequently occur and are highly frustrating. However, little is known about how age affects the neural basis of language production. Moreover, age-related increases in brain activation are often observed, yet there is disagreement about whether such increases represent a form of neural compensation or dedifferentiation. We used fMRI to determine if there are age-related differences in functional activation during picture naming, and whether such differences are consistent with a compensatory, dedifferentiation, or hybrid account that factors in difficulty. Healthy younger and older adults performed a picture-naming task with stimuli that varied in lexical frequency—our proxy for difficulty. Both younger and older adults were sensitive to lexical frequency behaviorally and neurally. However, younger adults performed more accurately overall, and engaged both language (bilateral insula and temporal pole) and cognitive control (bilateral superior frontal gyri and left cingulate) regions to a greater extent than older adults when processing lower frequency items. In both groups, poorer performance was associated with increases in functional activation consistent with dedifferentiation. Moreover, there were age-related differences in the strength of these correlations, with better performing younger adults modulating the bilateral insula and temporal pole and better performing older adults modulating bilateral frontal pole and precuneus. Overall, these findings highlight the influence of task difficulty on fMRI activation in older adults and suggest that as task difficulty increases, older and younger adults rely on different neural resources.
Keywords: fMRI, Language Production, Lexical Frequency, Neural Dedifferentiation, Aging
Healthy aging is characterized by language production difficulties, including increased word retrieval failures, slower speech rates, and increased use of filler words (Burke & Shafto, 2004; Burke & Shafto, 2008; Horton, Spieler, & Shriberg, 2010). Older adults report that increased word retrieval failures and tip-of-the-tongue (TOT) states are common and frustrating experiences that hinder their communication abilities (Burke & Shafto, 2004). Further, these language production difficulties may prompt interlocutors to make accommodations when communicating with older adults, often causing older adults to withdraw from social interactions and have lowered self-esteem (Ryan, Giles, Bartolucci, & Henwood, 1986; Ryan, Hummert, & Boich, 1995).
Many behavioral studies have demonstrated age differences in speech production including decreased speech rate, increased speech disfluencies, and decreased accuracy and speed in producing long and complex nonwords (Duchin & Mysak, 1987; Sadagopan & Smith, 2013; Searl, Gabel, & Fulks, 2002). These increased language production difficulties in older adults are a combined result of cognitive (Burke & Shafto, 2008; Shafto & Tyler, 2014), as well as motoric declines (Bilodeau-Mercure et al., 2015; Bilodeau‐Mercure & Tremblay, 2016; McKetton et al., 2018). For example, older adults experience more variability in their speech production, including a slower speaking rate and increased difficulty with articulatory demands compared to younger adults (Bilodeau‐Mercure & Tremblay, 2016) which may reflect age-related differences in word retrieval and speech planning or decreased stability in speech motor control (Wohlert & Smith, 1998).
Age-related speech production deficits may also arise from phonological deficits. For example, word retrieval failures, or TOT states, in which individuals are able to describe the concept they wish to convey but are temporarily unable to produce the corresponding word, may arise from phonological processing difficulties (Burke & Shafto, 2004). TOT states occur more often with low-frequency words, which is consistent with general lexical frequency effects, in which less frequent words are recalled more slowly (Jescheniak & Levelt, 1994; Oldfield & Wingfield, 1965). One theoretical framework proposed to account for these age-related deficits in language production is the Transmission Deficit Hypothesis (TDH), which suggests that age-related deficits in word retrieval result from declines in connection strength within phonological representations (Burke, MacKay, Worthley, & Wade, 1991). The hypothesis suggests that although all connections weaken with age, phonological processes are particularly vulnerable to the effects of decline due to a sparser, non-redundant organization; whereas, the semantic system’s redundancy and relatively increased interconnectedness helps to preserve semantic abilities despite concomitant age-related decline (Burke et al., 1991). These factors may lead to greater age-related phonological, compared to semantic, declines in production.
Age-related language production declines in phonological processes may be intertwined with item frequency effects, as word retrieval failures occur most often with low-frequency items, such as proper names and uncommon words (Burke et al., 2004; Burke et al., 1991). However, prior research has also demonstrated behavioral frequency effects in word retrieval and production in both younger and older adults (Gollan, Montoya, Cera, & Sandoval, 2008; LaGrone & Spieler, 2006; Newman & German, 2005). A study by Newman and German (2005) which examined the influence of lexical factors on picture naming found similar effects of frequency on accuracy across age. Similarly, Gollan et al. (2008) found similar magnitudes of slowing for older and younger adults when comparing high and low-frequency items (15% vs. 12% slowing for older and younger adults respectively). Additionally, a study by LaGrone and Spieler (2006) found that both younger and older adults had more difficulty naming low-frequency items. However, LaGrone and Spieler also found that older adults were slower to name items with lower name agreement, suggesting that older adults may be more susceptible to lexical competition. Collectively, although older adults often have more difficulty naming in general (i.e., lower accuracies, slower reaction times), these studies suggest that we should expect similar behavioral frequency patterns in younger and older adults.
Although the pattern of frequency effects may be similar behaviorally, the neural resources that support such processes may change with age. In healthy younger adults, language production is largely left lateralized. Critical regions supporting language production include the left inferior frontal gyrus and insula which support executive aspects of language such as word selection, the left precentral gyrus which supports articulatory and speech planning processes, temporal cortices which support both phonological aspects (superior temporal gyrus), as well as lexical and semantic processes (middle and inferior temporal gyri), and parietal regions such as the angular gyrus (semantics) and the supramarginal gyrus (phonological processes). For an outline of the brain regions involved in language processing, please see Figure 1. However, older adults often exhibit differences in neural activity compared to younger adults (Huettel, Singerman, & McCarthy, 2001). A common observation in the aging literature across numerous cognitive domains is an increase in functional activation for older adults, particularly in right frontal regions, which have been associated with executive function and memory recall (Cabeza, 2002; Li & Lindenberger, 1999; Reuter-Lorenz & Cappell, 2008). However, interpretation of such activation increases hinges on their relationship with behavioral performance. If increases in functional activation correspond with maintained or improved behavioral performance, then the overactivation may be compensatory (e.g., Daselaar et al., 2015). Others have suggested that overactivation in older adults is a consequence of neural dedifferentiation, wherein highly specialized neural networks become less specific and less efficient with age, resulting in declines in task performance (Li & Lindenberger, 1999). This account is consistent with increases in fMRI activation that correlate with poorer behavioral performance or when fMRI activation does not correlate with behavior. Additionally, the compensation-related utilization of neural circuits hypothesis (CRUNCH), proposes that at lower task demands, older adults may exhibit similar levels of activation or compensatory patterns of overactivation; however, as task demands increase, a resource ceiling is reached, brain-behavior relations deteriorate, and behavioral performance declines (Reuter-Lorenz & Cappell, 2008). Such performance declines may be due to task disengagement or an inability to effectively switch strategies to maintain performance (e.g., Cabeza & Dennis, 2012).
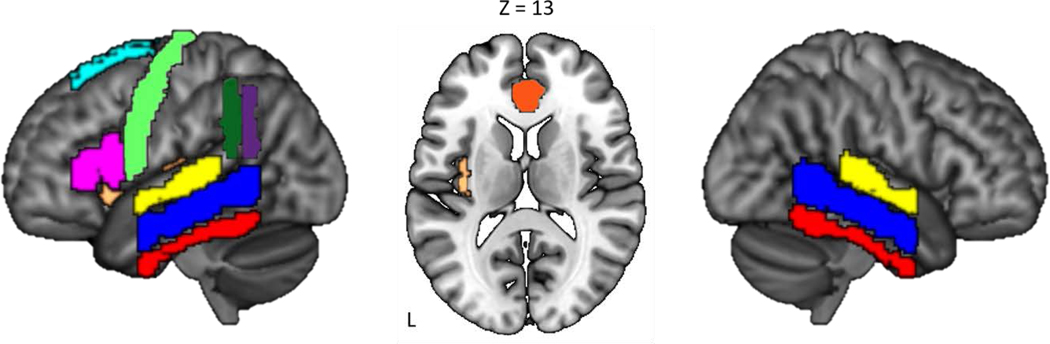
Figure 1.
Brain Regions Which Support Language Production. Precentral Gyrus (light green), Inferior Frontal Gyrus (pink), Insula (tan), Superior Temporal Gyrus (yellow), Middle Temporal Gyrus (blue), Inferior Temporal Gyrus (red), Supramarginal Gyrus (green), and the Angular Gyrus (purple). The Superior Frontal Gyrus (light blue) and Cingulate Gyrus (orange) are not necessarily involved in language processing but are cognitive control regions that are relevant to the current study.
In the context of the neural theories of aging introduced above, there have been several fMRI studies conducted specifically on language production that show the general trend of older adults eliciting increased bilateral and frontal activation compared to younger adults (Diaz, Johnson, Burke, & Madden, 2014; Diaz, Johnson, Burke, Truong, & Madden, 2018; Geva et al., 2014; Meinzer et al., 2009; Obler et al., 2010; Rizio, Moyer, & Diaz, 2017; Wierenga et al., 2008; Zhang et al., 2013). However, the function of such increases during language production is still debated because there has been support for both neural dedifferentiation and compensation accounts and increases in activation often occur outside of core language regions (Diaz et al., 2014; Tremblay, Sato, & Deschamps, 2017; Wierenga et al., 2008). For instance, Wierenga and colleagues (2008) reported compensatory patterns in which high-performing older adults elicited increased activation in the right inferior frontal gyrus, a region associated with executive function in general, relative to low-performing older adults during picture naming. However, activation increases in the right precentral gyrus, a motor control region, correlated with worse behavioral performance in older adults compared to younger adults suggesting dedifferentiation. These results suggest that not all right hemisphere activation is compensatory, and the particular brain region in which overactivation is found may be important for determining its relationship to behavioral performance (Diaz et al., 2014; Tremblay & Deschamps, 2016; Tremblay et al., 2017; Wierenga et al., 2008).
Similar to Wierenga and colleagues, others examining motor control of speech have found evidence of compensation and dedifferentiation. Performance on more demanding and complex speech tasks resulted in increased activation in intrinsic control and attentional networks (right posterior cingulate and right middle frontal gyrus, respectively) for older adults compared to younger adults, and such overactivation was associated with better performance (Tremblay et al., 2017). Because these compensatory activations were found outside of core language regions, the improved performance in older adults may be due to the increased allocation of neural cognitive control resources. In contrast, increased functional activation in the right precentral gyrus was associated with longer speech movement time, supporting a dedifferentiation account (Tremblay et al., 2017).
Additional evidence supporting dedifferentiation comes from Diaz and colleagues (2014), who used a covert picture-naming task with preceding phonological and semantic cues. Specifically, participants saw either a semantic cue such as “grooved?” (meaning is it grooved?) or a phonological cue, such as “Starts with L?” followed by two pictures presented side by side to which participants indicated whether they matched the cue. Older adults performed less accurately than younger adults during the phonological, but not the semantic condition and exhibited increased activation to all conditions in numerous brain regions compared to younger adults; however, the increased activation did not relate to behavioral performance in this or a second similar study (Diaz et al., 2018), which may be explained by weaker brain-behavior relations in healthy older adults during language production. Therefore, we see some evidence of compensation (e.g., Tremblay et al., 2017; Wierenga et al., 2008); however, with increased task complexity, these brain–behavior relations may become weaker (e.g., Diaz et al., 2014; Diaz et al., 2018) or require engagement of cognitive control regions outside of the typical language network (e.g., Tremblay et al., 2017).
The aim of the current study was to investigate the neural bases of frequency effects in picture naming in younger and older adults. First, with respect to frequency effects we hypothesized that picture naming performance would differ as a function of item frequency in both younger and older adults—accuracy would be positively correlated with frequency because frequent words are activated more often and thus are easier to retrieve (Burke et al., 1991). Likewise, for both younger and older adults, as frequency increases, we expected to see decreased functional activation in language areas involved in lexical selection and phonological retrieval and encoding, such as the left inferior frontal gyrus, left insula, left temporal cortex, and left supramarginal gyrus. Generally, reductions in functional activation that correspond to improved behavioral performance suggest that less neural recruitment is required for successful performance and are often interpreted as facilitation.
Second, we were interested in age-related differences in the patterns of functional activation and if age-related differences exist, whether functional activation patterns support a compensatory, dedifferentiation, or hybrid account that factors in task difficulty (i.e., CRUNCH). We hypothesized that older adults would elicit increased activation compared to younger adults, particularly in prefrontal regions that are involved with cognitive control, such as the middle and superior frontal gyri (i.e., a main effect of Age Group; Sowell, Thompson, Tessner, & Toga, 2001; Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997; Tremblay et al., 2017), and also within language regions such as temporal and parietal cortices. We were also interested in whether there was an interaction between frequency and age groups in terms of behavior and activation. If changes in frequency modulate brain activation differently in younger and older adults this would imply that older and younger adults rely on different neural mechanisms during word retrieval, and the regions which are differentially engaged would speak to the potential mechanism behind neural frequency effects (i.e., increased recruitment of core language regions vs. recruitment of cognitive control regions). Finally, we looked at brain-behavior correlations to characterize age-related differences in activation. If such age-related increases are compensatory, then increased functional activation should be positively correlated with task accuracy; however, if the increases in functional activation are due to neural dedifferentiation, then overactivation should either relate to worse picture-naming performance or not relate to performance at all. If brain-behavior relations vary as a function of task difficulty, using frequency as a proxy for difficulty, then this would provide evidence for the CRUNCH model.
Methods
Participants
Thirty-one older adults and 30 younger adults participated in the study. One older participant was excluded due to an incidental finding (i.e., a non-neurotypical mass or lesion, which was discovered unintentionally). For our older adult group (age range = 60 – 79, M = 69.5, SD = 6.13, females = 17), we targeted adults aged 60 years and older because age-related cognitive deficits typically manifest by this age. Thirty younger adults (age range = 18 – 34, M = 22.37, SD = 3.21, females = 16) recruited from the Pennsylvania State University campus also participated in the study as a comparison group. A detailed characterization of participants is provided in Table 1 and 2. All participants were healthy, right-handed, Native English speakers with normal or corrected-to-normal vision, and no self-reported neurological, psychological, or major medical conditions (e.g., diabetes, heart disease). All participants scored at least a 27 (Younger: M = 29.13, SD = 0.90; Older: M = 28.87, SD = 1.04; range for both groups = 27 – 30) on the Mini Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975), and were required to have at least 12 years of education (Younger: M = 16.03, SD = 2.44; Older: M = 17.44 years, SD = 6.13; range = 12 – 25 years1). Participants were excluded if they scored above 5 on the Geriatric Depression Scale as this can be suggestive of depression (Younger: M = 1, SD = 1.26, range = 0 – 5; Older: M = 0.43, SD = 0.68, range = 0 – 2; Yesavage et al., 1982). No participants were taking any psychotropic medications that might affect the brain or cerebral blood flow, including those classified as anti-depressants or anti-anxiety medications. All participants provided written informed consent and all experimental procedures were approved by the Institutional Review Board of The Pennsylvania State University.
Table 1.
Neuropsychological Testing Information
| Neuropsychological Information | Younger | Older |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (years)* | 22.37 (3.21) | 69.5 (6.13) |
| Education (years)* | 16.03 (2.44) | 17.44 (6.13) |
| Stroop Effect* | 19.22 (37.85) | 51.56 (58.85) |
| Total Verbal Fluency* | 74.17 (14.44) | 63.07 (15.43) |
| FAS Total* | 48.47 (10.38) | 40.3 (14.12) |
| Animals* | 25.70 (6.29) | 22.43 (6.17) |
| Vocabulary | 51.00 (7.11) | 52.83 (6.80) |
| Simple Speed (ms) | 272.66 (23.31) | 273.70 (38.42) |
| Complex Speed (ms)* | 284.25 (24.26) | 341.14 (47.86) |
| Immediate Recall | 11.80 (1.86) | 10.87 (2.37) |
| Delayed Recall | 10.30 (2.34) | 9.23 (2.87) |
| Recognition (accuracy) | 0.89 (0.08) | 0.89 (0.06) |
| Nonverbal Working Memory (accuracy)* | 0.79 (0.08) | 0.67 (0.09) |
Note. indicates significant differences between younger and older adults, p < .05
Table 2.
Neuropsychological Correlations with Age, collapsed across both groups
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 1 | |||||||||||||
| 2. Education | 0.34** | 1 | ||||||||||||
| 3. Stroop Effect | 0.31* | 0.19 | 1 | |||||||||||
| 4. Total Verbal Fluency | −0.33** | 0.13 | −0.24* | 1 | ||||||||||
| 5. FAS Total | −0.29** | 0.14 | −0.23* | 0.91*** | 1 | |||||||||
| 6. Animals Total | −0.23 | 0.04 | −0.16 | 0.59*** | 0.23 | 1 | ||||||||
| 7. Vocabulary | 0.21 | 0.56*** | 0.11 | 0.20* | 0.16 | 0.15 | 1 | |||||||
| 8. Simple Speed (ms) | 0.02 | −0.08 | 0.06 | 0.02 | 0.00 | 0.05 | 0.12 | 1 | ||||||
| 9. Complex Speed (ms) | 0.66*** | 0.15 | 0.34** | −0.30* | −0.31** | −0.1 | 0.23 | 0.45*** | 1 | |||||
| 10. Immediate Recall | −0.24 | 0.05 | −0.04 | 0.47*** | 0.38** | 0.38** | 0.34** | 0.15 | −0.20 | 1 | ||||
| 11. Delayed Recall | −0.21 | 0.09 | −0.03 | 0.45*** | 0.32** | 0.40** | 0.33** | 0.05 | −0.13 | 0.85*** | 1 | |||
| 12. Recognition | 0.04 | 0.19 | −0.03 | 0.14 | 0.06 | 0.20 | 0.28* | 0.07 | 0.09 | 0.38** | 0.47*** | 1 | ||
| 13. Nonverbal Working Memory | −0.58*** | −0.23 | −0.14 | 0.20 | 0.22 | 0.01 | −0.11 | 0.05 | −0.45*** | 0.17 | 0.23 | 0.02 | 1 | |
| 14. Naming Accuracy | −0.22* | 0.20 | −0.06 | 0.37*** | 0.32** | 0.27* | 0.41*** | 0.17 | −0.28* | 0.44*** | 0.46*** | 0.22 | 0.41*** | 1 |
p < .001,
p < .01,
p < .05
Cognitive Assessment
Participants completed a neuropsychological test battery to provide a broad characterization of their cognitive abilities prior to the MRI scanning session. The assessment included measures of inhibitory control, language, processing speed, and working memory. The results from the neuropsychological battery are summarized in Table 1 and the correlation matrices across groups are provided in Table 2. To see the correlation matrices within groups, please see the Online Supplement. Inhibitory control was evaluated via a computerized color Stroop Task presented in E-Prime (E-Prime 2.0, Psychology Software Tools, Inc., Pittsburgh, PA). Language ability was assessed using phonemic and categorical verbal fluency, as well as the Wechsler Adult Intelligence Scale (WAIS-III) vocabulary subtest (Tombaugh, Kozak, & Rees, 1999; Wechsler, 1997). Processing speed was assessed with a simple reaction time task (shape detection) and a choice reaction time task (i.e., whether an arrow pointed to the left or to the right). Verbal memory was assessed using the immediate and delayed recall tasks and a word recognition task from the Califorina Verbal Learning Test (Alexander, Stuss, & Fansabedian, 2003). Nonverbal working memory was assessed using a task adapted from Saults and Cowan (2007) that involved pattern comparison of colored squares.
Stimulus Materials and Procedure
For the main picture naming task, stimuli included 180 photographs, taken from two normed databases (Brodeur, Dionne-Dostie, Montreuil, & Lepage, 2010; Brodeur, Guérard, & Bouras, 2014; Moreno-Martínez & Montoro, 2012; see the Online Supplement for the full list of picture names along with their lexical characteristics, name agreement, age of acquisition, familiarity, and visual complexity information). Based on naming data provided by the two databases, the average name agreement of the selected images was 74% and the average H-index value was 1.04. H-index is a measure of name agreement that accounts for variability in the number of responses given by participants for a particular image (i.e., higher name agreement corresponds with a lower H-index value; Britt, Ferrara, & Mirman, 2016; Snodgrass & Vanderwart, 1980). Sixty unique items were presented in a blocked fMRI design for each of the three conditions: low-, medium-, and high-frequency. Frequencies, word lengths, and number of phonemes were obtained from the English Lexicon Project (Balota et al., 2007). Visual complexity, familiarity, and name agreement data were obtained from the two normed databases from which the images were drawn (Brodeur et al., 2010; Brodeur et al., 2014; Moreno-Martínez & Montoro, 2012). Age of acquisition ratings were available for items from the Moreno-Martínez and Montoro (2012) database. Because the Brodeur databases did not provide age of acquisition ratings, age of acquisition ratings for these items were obtained from Kuperman, Stadthagen-Gonzalez, and Brysbaert (2012). Because frequency is nonlinearly distributed, we used the logged HAL frequency values. Low-frequency items were defined as log frequency less than 6.8 (mean frequency = 5.45, SD = 0.86, range = 2.3 – 6.7, e.g., thimble, gavel); medium frequency items were defined as log frequency 7.3 – 8.2 (mean frequency = 7.68, SD = 0.25, e.g., chalk, lime); and high-frequency items were defined as log frequency greater than 8.8 (mean frequency = 9.97, SD = 0.80, range = 8.8 – 12.44, e.g., shirt, glasses). Word length (number of letters) was matched across conditions (range = 3–10; average length: low = 5.91 (SD = 1.05), medium = 5.80 (SD = 1.64), high = 5.93 (SD = 1.10); F(2, 177) = 0.19, n.s.). Overall, the number of phonemes did not significantly vary across conditions (range = 2–9; average number of phonemes: low = 4.93 (SD = 1.02), medium = 4.82 (SD = 1.63), high = 4.40 (SD = 1.22), F(2, 177) = 2.74, n.s.). Additionally, visual complexity of the images did not significantly vary across conditions (range = 1.18–4.22, average visual complexity: low = 2.55 (SD = 0.56), medium = 2.54 (SD = 0.74), high = 2.47 (SD = 0.67), F(2, 177) = 0.29, n.s.). Examining the other lexical characteristics revealed that age of acquisition and familiarity differed across conditions (AoA: range = 2.33–11.37, average AoA: low = 6.45 (SD = 2.20), medium = 5.08 (SD = 1.84), high = 4.61 (SD = 2.16), F(2, 177) = 12.53, p < .001); familiarity range = 1.64–4.91, average familiarity: low = 3.77 (SD = 0.64), medium = 4.07 (SD = 0.66), high = 4.21 (SD = 0.57), F(2, 177) = 7.86, p = .001). This is not surprising given that frequency was used as the manipulation and high-frequency items tend to be acquired earlier and are encountered more often. Name agreement for the low-frequency items was significantly lower than name agreement of both the medium- and high-frequency items (range = 8–100, average name agreement: low = 70.13 (SD = 22.21), medium = 81.22 (SD = 19.01), high = 80.38 (SD = 20.89), F(2, 177) = 5.31, SD = 21.24, p = .006).
Immediately prior to scanning, participants practiced overt picture naming in a mock scanner while minimizing head motion, using photographs comparable to those used in the main experiment (please see the Online Supplement). The picture-naming task incorporated a blocked fMRI design which maximizes detection of the hemodynamic response (15 items per block, block duration = 30s, 4 blocks per condition, totaling 12 blocks). Each trial consisted of a single target picture on a white background (picture duration = 1500 ms, inter-trial interval = 500 ms). A fixation cross was presented between pictures and during rest blocks. All 12 blocks were presented during two, 4.5-minute runs (six blocks per run). The order of blocks was randomized within each run for all participants. Thus, condition was not confounded with time or age group. In addition, the order of runs was counterbalanced for younger adults, however this was not done for older adults, due to an oversight2. Picture blocks alternated with 15s blocks in which a fixation cross was presented to allow for recovery of the hemodynamic signal. During rest blocks, participants were instructed to look at the fixation cross. Pictures were constrained to be 7” wide or 5.5” tall to standardize the presentation size without distorting the picture’s aspect ratio. Participants were instructed to name pictures overtly, as quickly and accurately as possible, to use only one word in their responses, and to speak in a normal conversation voice. Overt verbal responses were recorded and filtered using a dual-channel, MR-compatible fiber optic microphone (Optoacoustics Ltd., Or-Yehuda, Israel) for offline accuracy analyses.
Acquisition of MRI Data
Imaging data were acquired using a 3T Siemens Prisma Fit MRI Scanner. T-1 weighted anatomical images were collected (voxel size = 1.0 mm3, FoV = 256 mm, TR = 2300 ms, TE = 2.28 ms, TI = 900 ms, flip angle = 8°, number of slices = 192), as were functional images (voxel size = 3.0 mm3, FoV = 240 mm, TR = 2500 ms, TE = 25 ms, flip angle = 90°, interleaved slice acquisition, 114 volumes per run, number of slices = 41).
Behavioral Data Analysis
Recordings from the scanner session were transcribed for accuracy after the scanning session was completed by listening to the filtered microphone recordings. An explicit classification rubric (See Online Supplement) was devised for accuracy coding. Naming trials were marked as correct if the participant’s response matched the target name exactly (e.g., shirt for shirt), was the plural form of the target (e.g., shirts for shirt), was an abbreviated form of the target (e.g., T.V. for television), or if an acceptable synonym was given (e.g., slacks for pants). Naming trials were marked as incorrect if any other answers were provided or if no response was given. Inter-rater reliability calculated on a subset of the data revealed 97.6% agreement, κ = .94, between the two raters, suggesting good adherence to the scoring rubric.
We employed a logistic mixed-effect regression analysis, using the glmer function in the lme4 package in R (version 3.5.1) to examine the effects of frequency, age, and their interaction (Bates, Mächler, Bolker, & Walker, 2014; Venables & Smith, 2008). This approach has the advantage of considering individual data points (rather than data averaged over participants or items) and controls for random variations across participants and items simultaneously, producing more generalizable results. Following Barr, Levy, Scheepers and Tily (2013), we always started with the “full” random effects structure. If this model failed to converge, we systematically simplified the random effects structure based on Barr et al.’s (2013) recommendations until convergence was achieved. We report the R syntax used for the model in the Online Supplement3.
We conducted a between-groups analysis to assess if there were significant main effects of frequency and age group or an interaction between these two factors. Age group was a categorical variable that assessed between group variability (age group was centered to achieve convergence: Younger = −.5, Older = .5) and frequency was re-coded to numerical values to convert it to a continuous variable (low = −1, medium = 0, high = 1). Accuracies of correct responses were coded as 1s and accuracies of incorrect responses were coded as 0s. Random intercepts of participant and item, and random slopes of frequency for participants were included in the model.
fMRI Data Analysis
All functional and anatomical images were visually inspected to ensure data quality, specifically looking for the presence of artifacts or signal dropout. All images were assessed for the number of potentially clipped voxels, mean signal fluctuation to noise ratio (SFNR) and per-slice variation (Glover et al., 2012). After ensuring data quality, all non-brain tissue in the anatomical images was removed using Optimized Brain Extraction for Pathological Brains (optiBET: Lutkenhoff et al., 2014). Preprocessing and analyses were conducted through FSL version 5.0.9, with FEAT (fMRI expert analysis tool; Smith et al., 2004; Woolrich et al., 2009). Preprocessing steps included motion correction (FSL MCFLIRT), slice timing correction (interleaved), spatial smoothing (FWHM = 5mm), high-pass filtering, linear registration, and normalization. Participants moved, on average, 0.27 mm (range: 0.07 mm – 0.82 mm), which is within the typical recommendation of less than half a voxel of motion for task-based fMRI (Poldrack, Mumford, & Nichols, 2011). Functional images were coregistered to the participant’s own anatomical image and then registered to MNI space using an FSL template brain. We used a double-gamma hemodynamic response function to model the BOLD signal for each block. First level analyses were conducted on each participant’s individual runs, including the standard motion parameters as nuisance covariates. Analyses from previous steps were combined across participants in group-level analyses using FMRIB’s local analysis of mixed effects (FLAME 1+2, Beckmann, Jenkinson, & Smith, 2003; Woolrich et al., 2004). All analyses used a whole-brain approach with significant activations determined in a two-step process. Statistically significant voxels were identified using a voxel-wise Z threshold of 2.3, p < .01. Then, identified clusters were corrected for multiple comparisons based on Gaussian random field theory such that only clusters with a significance of p < .05 were retained (Hayasaka & Nichols, 2003; Worsley, 2001). All reported results and figures reflect these corrections for multiple comparisons.
Initially, we collapsed across the frequency conditions to look for a main effect of picture naming relative to a fixation baseline and examined functional activation to determine which brain regions were sensitive to picture naming in the two groups. We conducted an ANOVA to examine the effects of Frequency (high < medium < low), Age Group, and the interaction of these two factors. When effects were found, follow-up contrast analyses were conducted to determine the direction of the effect. For example, for a significant main effect of Age Group we examined the data to see which, if any, regions showed more functional activation in younger adults (younger > older) and which, if any, regions showed more activation in older adults (older > younger). Next, we conducted a brain-behavior correlation to examine the relationship between picture naming accuracy and functional activation across all participants. This analysis was conducted by using the individual participant block-level accuracies as the regressor. We calculated accuracy by condition and run (i.e., average accuracy for each frequency condition within each run for each participant) and correlated these values with the corresponding fMRI activation maps. These values were combined into single cross-condition accuracy-fMRI analysis that reflected a combination of within- and between-participant effects. These accuracy-fMRI analyses were run across all participants, and an interaction analysis was run to determine if there were significant differences in these brain-behavior relations between older and younger adults. Significant regions of activation were identified using the Harvard-Oxford atlas (Desikan et al., 2006). Activation peaks as well as sub-peaks are provided in the tables to provide a more detailed characterization of the activations.
fMRI-based power analyses were implemented using fMRIpower software (fmripower.org; Mumford & Nichols, 2008). Based on data from a previous picture naming study (Rizio et al., 2017), 30 participants should provide greater than 90% power to detect significant effects in frontal, occipital, and inferior and superior temporal regions (p < .01).
Results
Behavior
Logistic mixed-effects regression analysis4 revealed a main effect of Frequency, with increasing frequency being associated with increasing accuracy, z = 6.73, p < .001, eta squared = .67. We also found a main effect of Age Group, with older adults having lower accuracy than younger adults overall (mean accuracies Older: low = 49.31%, medium = 69.44%, high = 81.40%; Younger: low = 51.78%, medium = 77.11%, high = 83.78%, eta squared = .04), z = 2.11, p = .035. As noted in the methods section, although condition was randomized within each run for all participants, run order was only counterbalanced for younger but not older adults due to an oversight. Analyses to determine the effect of order on behavioral performance revealed that there were significant group differences in accuracy performance in run one but not in run two. The older adults’ accuracy significantly improved during run two and no longer differed significantly from the younger adults’ accuracy.
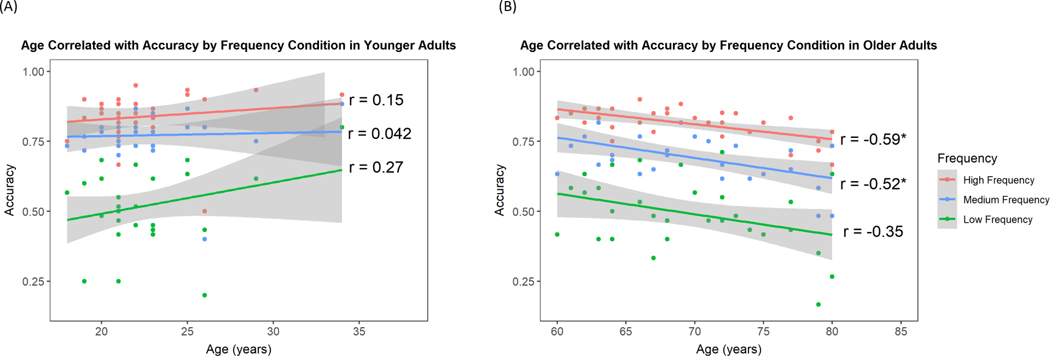
The interaction between Frequency and Age Group was not significant, z = −1.39, p = .17, eta squared = .02. However, within older adults, subsequent correlations between age and accuracy for each of the three frequency conditions (see Figure 2) revealed significant correlations between age and accuracy in the high- and medium-frequency conditions, F(1, 28) = 15.15, r = −0.59, r2 = 0.35, p < .001, 95% CI [−0.79, −0.30] and F(1, 28) = 10.12, r = −0.52, r2 = 0.27, p < .01, 95% CI [−0.74, −0.19], respectively. The correlation for age and accuracy in the low-frequency condition trended towards significance, F(1, 28) = 4.04, r = −0.35, r2 = 0.13, p = .054, 95% CI [−0.63, −0.006]. The correlations between age and accuracy were not significant in the younger adults, likely due to their lack of variability in age, although this could also be due to an undetected nonlinearity.
Figure 2.
Age Correlated with Accuracy by Frequency Condition. (A) Age correlations with accuracy across frequency condition were not significant in younger adults in any of the three frequency conditions. There was likely not enough variability in age in the younger adult group to detect any relationship. (B) In the older adults, there were significant negative correlations between accuracy and age on the high- and medium-frequency conditions. The correlation between the low-frequency condition and age only trended towards significance.
fMRI Results
Picture-Naming Activation
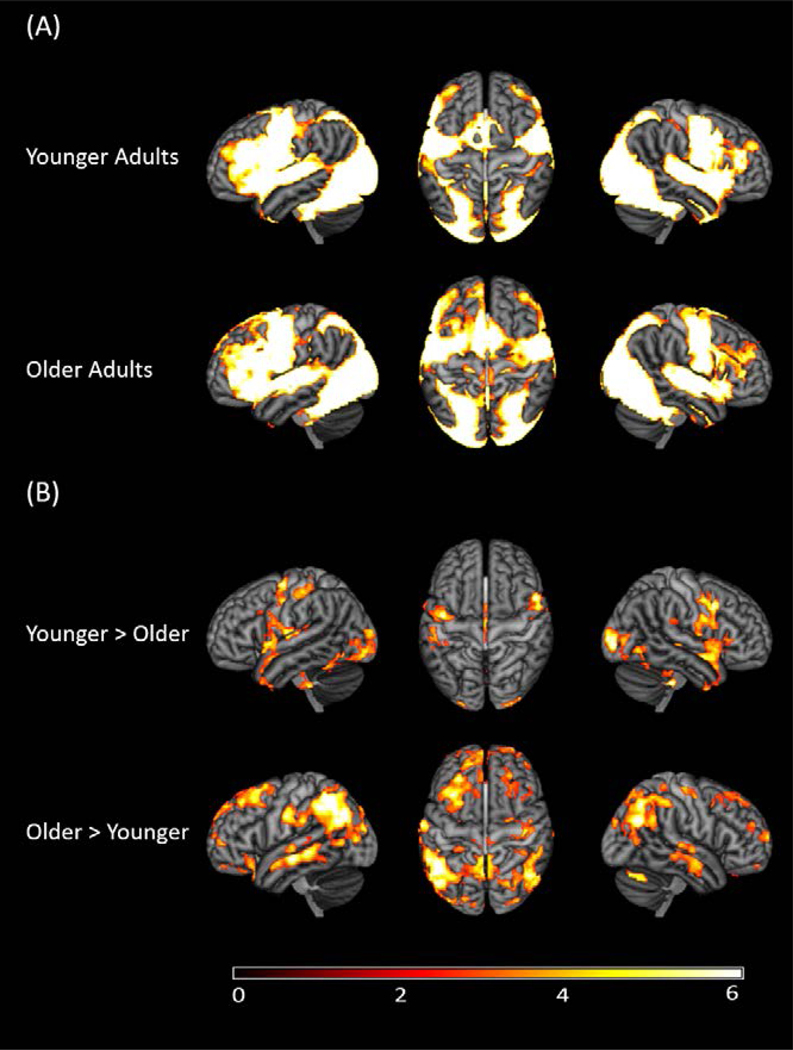
To examine functional activation to picture-naming in general, we first collapsed across the low-, medium-, and high-frequency conditions. As expected, there was widespread functional activation for pictures relative to fixation (see Figure 3A, Table 3) in both younger and older adults. Regions included typical language regions, such as the left inferior frontal gyrus and bilateral superior temporal gyri, extending into premotor regions.
Figure 3.
Functional activation during the picture-naming task after correcting for multiple comparisons. (A) Significant functional activation to picture naming (collapsed across the low-, medium-, and high-frequency conditions) in younger and older adults. (B) Contrast analyses showing greater functional activation in younger versus older adults and older versus younger adults. Results indicated that there was one significant cluster in the younger versus older adults comparison and four significant clusters in the older versus younger adults comparisons. See Table 3 and 4 for additional details. The color bar reflects the Z-scores.
Table 3.
Coordinates for Peak and Sub-Peaks of Activation to Picture Naming
| Peak MNI |
||||||
|---|---|---|---|---|---|---|
| Region | Hemisphere | Voxels | Max Z | X | Y | Z |
| Picture-Naming Activation Younger Adults | ||||||
| Lateral occipital cortex | Right | 126829 | 21.1 | 32 | −88 | 0 |
| Inferior frontal gyrus | Left | — | 10.9 | −53 | 10 | 20 |
| Superior parietal lobule | Right | — | 12.0 | 30 | −48 | 51 |
| Superior temporal gyrus | Left | — | 14.4 | −66 | −20 | 6 |
| Lateral occipital cortex | Left | — | 17.5 | −34 | −90 | 0 |
| Older Adults | ||||||
| Occipital fusiform gyrus | Right | 120586 | 19.4 | 34 | −80 | −20 |
| Inferior frontal gyrus | Left | — | 11.7 | −56 | 20 | 24 |
| Superior parietal lobule | Right | — | 12.1 | 30 | −56 | 56 |
| Superior temporal gyrus | Left | — | 11.4 | −66 | −22 | 6 |
| Lateral occipital cortex | Left | — | 19.4 | −42 | −86 | −6 |
Note. The voxel-wise Z threshold = 2.3, p < .01. Clusters with a corrected significance of p < .05 were retained. Sub-peaks are included to provide a more thorough characterization of the functional activation.
Age and Frequency Effects
We conducted a 2 × 3 ANOVA to assess differences in functional activation between Age Groups, parametric differences in brain activation across conditions (high- < medium- < low-frequency), and whether these two factors interacted. There was a significant main effect of Age Group across several regions (Figure 3B, Table 4). Results of the main effect and planned contrasts indicated that younger adults had significantly more functional activation in one large, connected cluster (see Table 4). This cluster was centered in a visual object processing region— the left temporal occipital fusiform cortex, which spread to other visual regions including bilateral occipital cortex. This cluster also included language-relevant regions such as the left precentral gyrus, bilateral precentral gyri, bilateral temporal poles, bilateral superior temporal gyri, and left inferior temporal gyrus (temporo-occipital part). Cognitive control regions, including the right inferior and middle frontal gyri, were also engaged. Older adults elicited greater functional activation than younger adults in left hemisphere cognitive control regions such as the middle and superior frontal gyri, which spread to a region that has been noted as a semantic hub—left temporal pole. Additionally, older adults elicited more activation in typical left hemisphere language regions including the bilateral superior temporal gyri (auditory and phonological processing), which extended to the bilateral middle temporal gyri (lexical and semantic processing), as well as the bilateral angular gyri, and left supramarginal gyrus. The bilateral lateral occipital cortex and bilateral precuneus, which are known for their involvement in vision, and recall, visual imagery, and internal monitoring respectively, were also engaged. Effect sizes for the main effect of Age Group ranged between .36 to 1, reflecting small (cerebellum), to medium (superior temporal gyrus and temporal occipital fusiform gyrus) to large effects (middle frontal gyrus and angular gyrus).
Table 4.
Coordinates for Peak and Sub-Peaks of Activation for the Main Effect of Age Group
| Peak MNI |
||||||
|---|---|---|---|---|---|---|
| Region | Hemisphere | Voxels | Max Z | X | Y | Z |
| Younger > Older | ||||||
| Temporal occipital fusiform cortex | Left | 36184 | 9.81 | −26 | −50 | −16 |
| Inferior frontal gyrus | Right | — | 8.77 | 40 | 14 | 8 |
| Middle frontal gyrus | Right | — | 7.24 | 52 | 11 | 44 |
| Precentral gyrus | Left | — | 6.34 | −50 | 2 | 22 |
| Precentral gyrus | Right | — | 5.97 | 56 | 2 | 22 |
| Temporal pole | Right | — | 6.13 | 49 | 9 | −13 |
| Temporal pole | Left | — | 5.42 | −49 | 5 | −15 |
| Superior temporal gyrus | Right | — | 6.70 | 48 | −2 | −12 |
| Superior temporal gyrus (Heschl’s gyrus) | Left | — | 6.25 | −49 | −22 | 11 |
| Precentral gyrus | Left | — | 7.48 | −57 | −13 | 13 |
| Temporal fusiform cortex | Right | — | 5.99 | 28 | −30 | −25 |
| Inferior temporal gyrus (temporo-occipital part) | Left | — | 5.35 | −46 | −53 | −22 |
| Lateral occipital cortex | Left | — | 6.06 | −34 | −88 | −14 |
| Occipital pole | Right | — | 9.43 | 30 | −92 | −2 |
| Older > Younger | ||||||
| Middle frontal gyrus | Left | 14809 | 8.25 | −32 | 20 | 60 |
| Frontal pole | — | — | 7.07 | 0 | 58 | 0 |
| Superior frontal gyrus | Left | — | 6.47 | −26 | 34 | 48 |
| Temporal pole | Left | — | 7.73 | −42 | 24 | −22 |
| Superior temporal gyrus | Right | 1101 | 5.56 | 72 | −24 | 2 |
| Middle temporal gyrus | Right | — | 5.37 | 62 | −8 | −16 |
| Angular gyrus | Left | 21216 | 9.03 | −48 | −58 | 44 |
| Angular gyrus | Right | — | 5.55 | 45 | −55 | 44 |
| Supramarginal gyrus | Left | — | 7.99 | −56 | −48 | 42 |
| Superior temporal gyrus | Left | — | 5.61 | −68 | −18 | −2 |
| Middle temporal gyrus | Left | — | 8.80 | −68 | −18 | −12 |
| Temporal pole | Left | — | 4.93 | −60 | 4 | −20 |
| Precuneus | Left | — | 6.29 | −9 | −49 | 36 |
| Precuneus | Right | — | 6.23 | 10 | −45 | 36 |
| Lateral occipital cortex | Right | — | 8.50 | 50 | −64 | 28 |
| Lateral occipital cortex | Left | — | 8.84 | −48 | −72 | 34 |
| Cerebellum | Right | 945 | 7.08 | 44 | −76 | −28 |
Note. The voxel-wise Z threshold = 2.3, p < .01. Clusters with a corrected significance of p < .05 were retained. Sub-peaks are included to provide a more thorough characterization of the functional activation.
In addition to significant main effects of Age Group, there was also a significant main effect of Frequency (shown in younger and older adults in Figure 4, Table 5), in which there was more activation for low-frequency words. Effect sizes for this main effect were greater than 1 in all regions, indicating large effect sizes. These effects were found in cognitive control regions including the bilateral frontal poles, bilateral anterior- and para- cingulate gyri, as well as within language regions such as the bilateral inferior frontal gyri, which spread to the insula, and left supramarginal gyrus. Visual processing regions (bilateral lateral occipital cortex) were also engaged more when processing lower frequency items. There were no regions in which high-frequency words elicited greater activation than low-frequency words.
Figure 4.
Functional activation during the picture-naming task after correcting for multiple comparisons. Colored areas reflect a parametric analysis (high < medium < low) where lower frequency items elicited more functional activation compared to medium and higher frequency items in younger and older adults and the interaction, which shows regions were younger adults elicited more activation than older adults to lower frequency items. The color bar reflects the Z-scores.
Table 5.
Coordinates for Peak and Sub-Peaks of Activation for the Main Effect of Frequency and the interaction between Age Group and Frequency
| Peak MNI |
||||||
|---|---|---|---|---|---|---|
| Region | Hemisphere | Voxels | Max Z | X | Y | Z |
| Frequency Effects (Low > High) | ||||||
| Frontal pole | Left | 3514 | 5.68 | −36 | 56 | 16 |
| Frontal pole | Right | — | 4.73 | 28 | 58 | 26 |
| Inferior frontal gyrus | Left | 1234 | 5.25 | −36 | 24 | −2 |
| Orbital frontal cortex | Left | — | 4.52 | −32 | 20 | −12 |
| Insula | Left | — | 4.46 | −42 | 18 | −6 |
| Temporal pole | Left | — | 4.62 | −56 | 16 | −6 |
| Inferior frontal gyrus | Right | 1196 | 4.73 | 46 | 22 | 0 |
| Orbital frontal cortex | Right | — | 4.06 | 42 | 24 | −10 |
| Insula | Right | — | 4.58 | 34 | 22 | 4 |
| Paracingulate gyrus | Left | 2797 | 6.61 | −2 | 24 | 34 |
| Paracingulate gyrus | Right | — | 5.30 | 6 | 24 | 36 |
| Cingulate gyrus | Left | — | 4.68 | −8 | 26 | 24 |
| Cingulate gyrus | Right | — | 4.77 | 6 | 32 | 24 |
| Superior parietal lobe | Left | 1099 | 3.97 | −32 | −54 | 52 |
| Supramarginal gyrus | Left | — | 3.73 | −50 | −30 | 44 |
| Lateral occipital cortex | Right | 13907 | 7 | 50 | −74 | −4 |
| Lateral occipital cortex | Left | — | 6.78 | −48 | −78 | −4 |
| Fusiform gyrus | Right | — | 5.12 | 42 | −61 | −18 |
| Fusiform gyrus | Left | — | 6.90 | −44 | −54 | −20 |
| Frequency Effects (High>Low) | ||||||
| No significant effects | ||||||
| Interaction between Frequency and Age Group | ||||||
| Insula | Left | 7324 | 4.33 | −30 | 14 | −14 |
| Insula | Right | 3.91 | 31 | 17 | −9 | |
| Orbital frontal cortex | Right | — | 3.99 | 28 | 12 | −22 |
| Temporal pole | Left | — | 4.10 | −38 | 12 | −38 |
| Temporal pole | Right | 3.69 | 38 | 18 | −35 | |
| Temporal fusiform cortex | Left | — | 4.33 | −34 | −4 | −30 |
| Superior frontal gyrus | 1305 | 3.88 | 0 | 14 | 54 | |
| Paracingulate gyrus | Left | — | 3.64 | −2 | 14 | 42 |
| Cingulate gyrus | Left | — | 3.55 | −8 | 22 | 26 |
Note. The voxel-wise Z threshold = 2.3, p < .01. Clusters with a corrected significance of p < .05 were retained. Sub-peaks are included to provide a more thorough characterization of the functional activation.
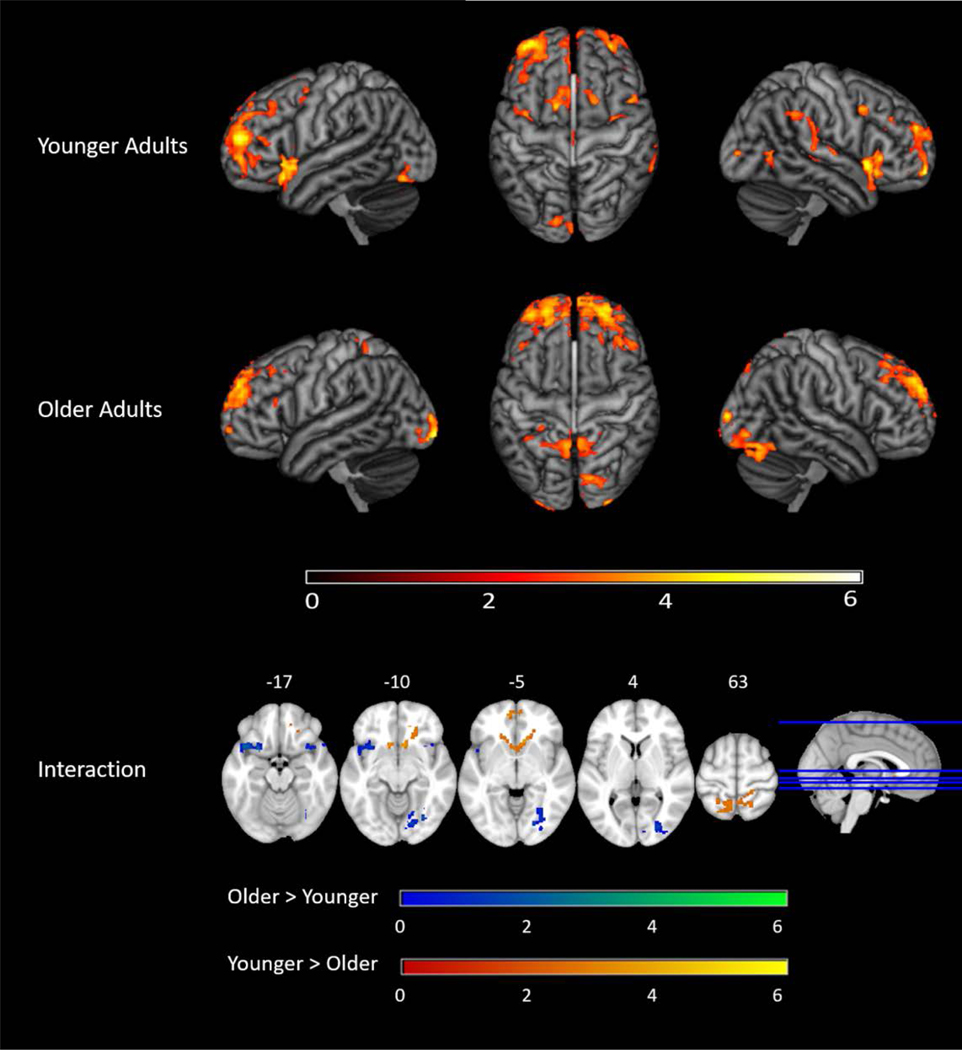
Of greatest interest, there was also a significant interaction between Frequency and Age Group (Figure 4, Table 5 and 6), demonstrating that younger and older adults had different neural frequency effects. Effect sizes for the interaction were greater than 1 in all regions, indicating large effect sizes. Specifically, younger adults had larger differences in activation as a function of frequency compared to older adults and this was driven by older adults eliciting less activation compared to younger adults during the low-frequency condition (Table 6). Significant interaction effects were found in language relevant regions including the bilateral insula, which extended to the bilateral temporal poles, as well as cognitive control regions such as the bilateral superior frontal gyri, which extended to the left anterior cingulate and paracingulate gyri.
Table 6.
Average Activation in Significant Clusters for Younger and Older Adults
| Younger Adults | Older Adults | |
|---|---|---|
| Region | Mean Z-Score | Mean Z-Score |
| Insula Cluster | ||
| High Frequency Condition | 2.39 | 2.52 |
| Medium Frequency Condition | 3.53 | 1.90 |
| Low Frequency Condition | 4.28 | 0.98 |
| SFG Cluster | ||
| High Frequency Condition | 3.46 | 4.08 |
| Medium Frequency Condition | 4.55 | 3.51 |
| Low Frequency Condition | 6.22 | 3.82 |
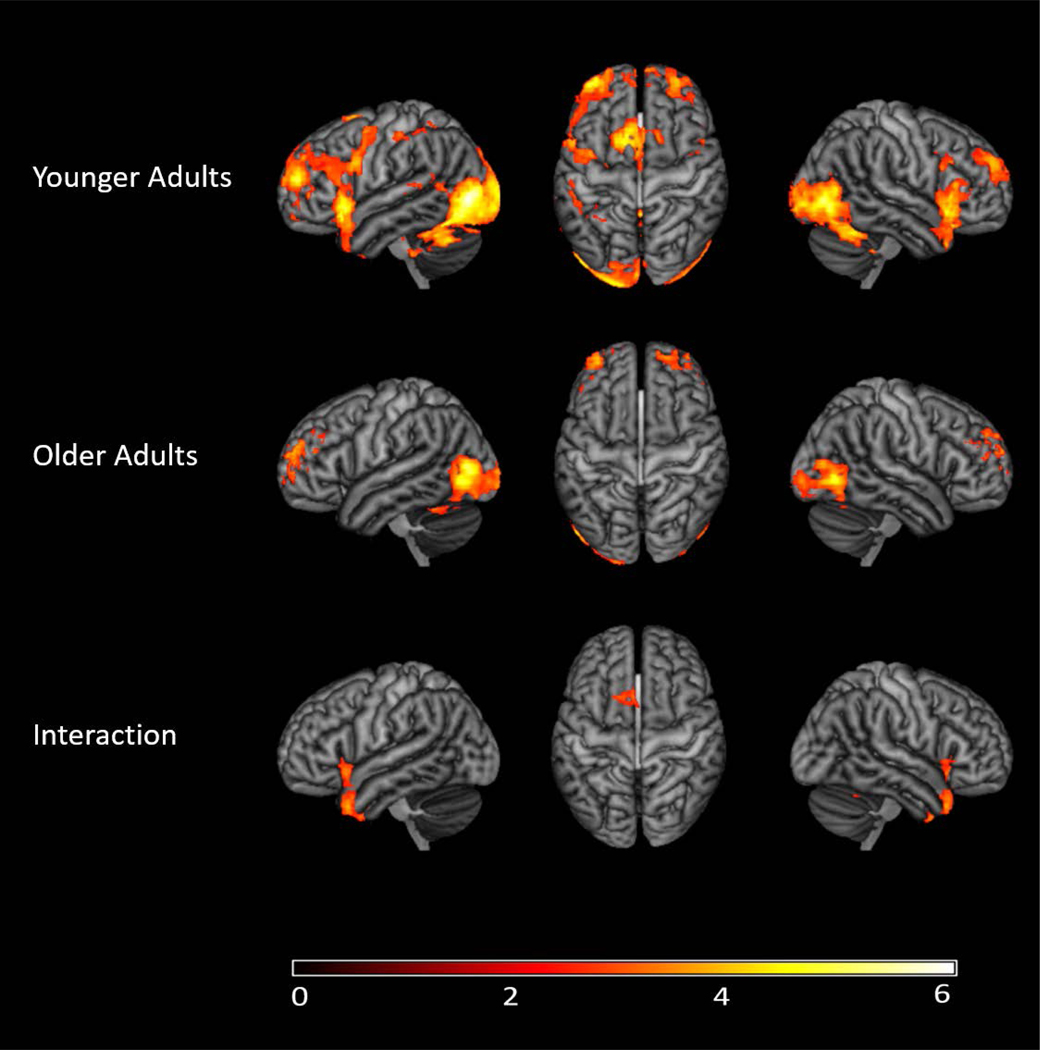
Functional Activation Correlations with Behavior
We were also interested in the relationship between behavioral performance and brain activation to better understand the behavioral consequences of changes in activation (see Table 7). Here we examined the relationship between accuracy on the picture-naming task and brain activation across and within groups. Across both groups, there was a negative correlation between accuracy and functional activation, that is, as accuracy increased there were significant decreases in activation, consistent with behavioral facilitation. These were found in regions implicated in cognitive control such as the left frontal pole, left orbital frontal cortex, and bilateral cingulate gyri, as well as language regions and right-hemisphere language homologues including the right temporal pole, right postcentral gyrus, and left supramarginal gyrus. Finally, visual processing regions, such as the bilateral lateral occipital cortex were also engaged. We also examined the relationship between accuracy and brain activation within each group to see if there were any age-related differences in these effects. As shown in Figure 5, both groups had negative correlations between accuracy and functional activation, suggesting that across the lifespan improvements in behavioral performance are associated with smaller regions of activation. However, there were also significant group differences in these brain-behavior relationships. Younger adults elicited stronger negative correlations between accuracy and activation, compared with older adults, in the bilateral orbital frontal cortex, bilateral temporal poles, as well as the right occipital fusiform gyrus, which extended to lateral occipital cortex. Older adults elicited stronger negative accuracy-activation correlations than younger adults in largely non-language regions including the bilateral frontal pole, right postcentral gyrus, bilateral superior parietal lobule, and bilateral precuneus. There were no significant positive accuracy-activation correlations in any analysis.
Table 7.
Coordinates for Peak and Sub-Peaks of Activation for the Accuracy-fMRI Correlations
| Peak MNI |
||||||
|---|---|---|---|---|---|---|
| Region | Hemisphere | Voxels | Max Z | X | Y | Z |
| Negative Accuracy Correlation Across Groups | ||||||
| Frontal pole | Left | 7699 | 5.25 | −36 | 56 | 16 |
| Orbital frontal cortex | Left | 1195 | 5.10 | −32 | 26 | −6 |
| Insula | Left | — | 4.99 | −32 | 22 | −2 |
| Temporal pole | Left | — | 3.94 | −54 | 16 | −10 |
| Temporal pole | Right | 1217 | 5.31 | 50 | 16 | −8 |
| Insula | Right | — | 4.73 | 34 | 20 | 6 |
| Orbital frontal cortex | Right | — | 4.55 | 36 | 28 | −8 |
| Inferior frontal gyrus | Right | — | 3.42 | 56 | 28 | 0 |
| Postcentral gyrus | Right | 640 | 3.74 | 40 | −36 | 52 |
| Supramarginal gyrus | Right | — | 3.30 | 64 | −44 | 36 |
| Superior parietal lobule | Right | — | 3.07 | 34 | −50 | 48 |
| Cingulate gyrus | — | 2737 | 4.65 | 0 | −44 | 8 |
| Supramarginal gyrus | Left | 748 | 5.40 | −34 | −44 | 36 |
| Superior parietal lobule | Left | — | 3.66 | −30 | −56 | 54 |
| Lateral occipital cortex | Right | 8424 | 4.76 | 44 | −74 | −16 |
| Lateral occipital cortex | Left | — | 4.57 | −38 | −90 | −6 |
| Occipital pole | Right | — | 4.64 | 8 | −98 | −4 |
| Occipital fusiform gyrus | Right | — | 4.44 | 34 | −70 | −20 |
| Younger Adults | ||||||
| Frontal pole | Left | 2260 | 5.61 | −46 | 54 | −16 |
| Precentral gyrus | Left | — | 4.42 | −34 | −4 | 42 |
| Orbital frontal cortex | Left | 1447 | 5.43 | −32 | 24 | −6 |
| Inferior frontal gyrus | Left | — | 4.54 | −44 | 10 | −2 |
| Temporal pole | Left | — | 4.52 | −48 | 12 | −6 |
| Inferior frontal gyrus | Right | 1526 | 5.21 | 48 | 16 | −6 |
| Insula | Right | — | 5.14 | 32 | 18 | 6 |
| Orbital frontal cortex | Right | — | 5.10 | 34 | 28 | −6 |
| Paracingulate gyrus | Left | 5449 | 5.36 | −4 | 16 | 44 |
| Cingulate gyrus | Right | — | 5.04 | 10 | 20 | 30 |
| Superior frontal gyrus | Right | — | 4.49 | 26 | −2 | 42 |
| Superior temporal gyrus | Right | 700 | 3.74 | 70 | −12 | 6 |
| Supramarginal gyrus | Right | — | 3.60 | 62 | −44 | 36 |
| Cingulate gyrus | — | 596 | 3.42 | 0 | −18 | 40 |
| Precuneus Cortex | Right | — | 3.41 | 12 | −36 | 46 |
| Lateral occipital cortex | Left | 704 | 4.06 | −40 | −78 | −18 |
| Occipital fusiform gyrus | Left | — | 3.47 | −30 | −76 | −14 |
| Lateral occipital cortex | Right | 4110 | 5.16 | 30 | −80 | 2 |
| Middle temporal gyrus | Right | — | 4.13 | 44 | −58 | 6 |
| Occipital fusiform gyrus | Right | — | 3.93 | 30 | −72 | −4 |
| Occipital pole | — | — | 3.89 | 0 | −94 | 6 |
| Older Adults | ||||||
| Frontal pole | Right | 4182 | 5.01 | 34 | 56 | 30 |
| Frontal pole | Left | — | 4.75 | −22 | 56 | 32 |
| Precuneus | Left | 1458 | 3.94 | −6 | −54 | 68 |
| Precuneus | Right | — | 3.93 | 2 | −48 | 70 |
| Occipital pole | Left | 3055 | 5.63 | −20 | −104 | −8 |
| Occipital pole | Right | — | 4.58 | 26 | −98 | 6 |
| Positive Accuracy Correlation | ||||||
| No significant correlations | ||||||
| Interaction between accuracy and age group | ||||||
| Stronger negative correlations in Younger Adults | ||||||
| Orbital frontal cortex | Left | 295 | 4.03 | −24 | 14 | −14 |
| Temporal pole | Left | — | 3.71 | −45 | 16 | −18 |
| Insula | Left | — | 3.35 | −38 | 15 | −14 |
| Orbital frontal cortex | Right | 163 | 4.10 | 30 | 12 | −22 |
| Temporal pole | Right | — | 3.27 | 51 | 18 | −22 |
| Intracalcarine cortex/Lingual gyrus | Right | 17 | 2.62 | 10 | −86 | −2 |
| Occipital fusiform gyrus | Right | 446 | 3.87 | 32 | −70 | −12 |
| Lateral occipital cortex | Right | — | 3.77 | 28 | −82 | 3 |
| Stronger negative correlations in Older Adults | ||||||
| Frontal pole | Left | 59 | 3.72 | −6 | 56 | −6 |
| Frontal pole | Right | — | 2.49 | 5 | 57 | −2 |
| Frontal medial cortex | Left | — | 3.26 | −6 | 54 | −8 |
| Subcallosal cortex | Right | 370 | 4.2 | 8 | 18 | −8 |
| Precuneus | Left | 386 | 3.73 | −14 | −62 | 44 |
| Postcentral gyrus | Right | — | 2.70 | 18 | −40 | 68 |
| Precuneus | Right | — | 3.23 | 4 | −51 | 66 |
| Superior parietal lobule | Right | — | 2.82 | 18 | −53 | 62 |
| Superior parietal lobule | Left | — | 3.15 | −10 | −54 | 66 |
| Lateral occipital cortex | Left | — | 3.01 | −20 | −61 | 64 |
Note. The voxel-wise Z threshold = 2.3, p < .01. Clusters with a corrected significance of p < .05 were retained. Sub-peaks are included to provide a more thorough characterization of the functional activation.
Figure 5.
Functional activation correlations after correcting for multiple comparisons. Negative correlations between functional activation to each condition and accuracy, as well as the interaction between accuracy and age group. As accuracy increased during picture naming, activation in these areas decreased in younger and older adults. The interaction illustrates regions in which younger adults showed stronger negative accuracy-activation correlations compared to older adults (blue-green color bar, i.e., less negative is more positive) as well as regions in which older adults showed stronger negative accuracy-activation correlations compared to younger adults (red-yellow color bar). The color bars reflect the Z-scores.
Discussion
Older adults often report language production difficulties, particularly for low-frequency words. In this study, we examined how frequency affects functional activation by manipulating picture-naming difficulty—older and younger adults named pictures with target names that had low-, medium-, and high-frequencies. We expected both groups to show behavioral frequency effects and that there would be increased functional activation while naming lower frequency items compared to higher frequency items to offset the increased linguistic demand of low-frequency items. Consistent with these hypotheses, both younger and older adults showed behavioral frequency effects, in which lower frequency words were responded to less accurately, suggesting that behavioral frequency effects are present across the lifespan in healthy adults. The stability of frequency effects across the lifespan is consistent with prior behavioral research. For example, LaGrone and Spieler (2006) found that both younger and older adults had more difficulty naming low-frequency items compared to high-frequency items. Gollan et al. (2008) showed that older and younger adults had similar magnitudes of slowing to low-frequency items, and Newman and German (2005) found similar frequency effects across the lifespan. Although older adults showed similar frequency effects, they were also less accurate in their responses overall compared to younger adults, consistent with previous reports of age-related decline in picture naming and language production more broadly. However, these age-related behavioral differences, particularly for accuracy, may be less robust. Analyses of run order showed that older adults’ performance improved over time, resulting in no significant age-group differences in accuracy during the second run. This suggests that older adults may have needed more time to adjust to the task, and this may be particularly true for the oldest, older adults. Follow-up correlations on age and accuracy within the older adult group showed that there were significant negative correlations between age and accuracy on the high- and medium-frequency conditions, and that the correlation for the low-frequency condition trended towards significance5 (See Figure 2), suggesting that increased age within the older adult group was associated with worse performance.
Patterns of functional activation were partially consistent with our behavioral effects. Both groups showed significant neural effects of frequency (Figure 4, Table 5), particularly in frontal regions such as the bilateral frontal pole, bilateral inferior frontal gyri, and bilateral insula, as well as in cingulate and occipital-fusiform regions. The prefrontal cortex is involved in a variety of executive functions, as well as working memory, and maintaining multiple tasks (Gilbert et al., 2006). Both groups also elicited increases in activation for low-frequency words in the bilateral cingulate, known for its involvement in conflict monitoring and inhibition, and in lateral occipital cortex, which is a region that is involved in object perception and object recognition (Grill-Spector, Kourtzi, & Kanwisher, 2001). These results suggest that less frequent items required increased cognitive-control and perceptual resources6 for both younger and older adults.
Prior studies have examined neural frequency effects in younger adults (Graves et al., 2007; Wilson et al., 2009). For example, Graves at al. (2007) found that activity in the posterior superior temporal gyrus was modulated by word frequency due to its role in accessing lexical phonology. Relatedly, Wilson et al. (2009) found increased functional activation for lower frequency words in the left inferior temporal cortex and temporoparietal junction. Although we did not find frequency effects in posterior STG as Graves et al. (2007) did, the occipital-fusiform activation that we observed extended into the temporo-occipital portion of left inferior temporal gyrus which appears to overlap with the same region observed by Wilson et al. (2009).
We also observed a significant group by condition interaction in the bilateral insula which extended into the bilateral temporal poles, and in the bilateral superior frontal gyri (SFG) which extended into the bilateral cingulate gyri, in which younger adults engaged these regions to a greater extent than older adults. Planned comparisons showed that older adults elicited similar levels of activation to high-frequency items as younger adults, but older adults engaged key language (insula) and cognitive control regions (SFG, cingulate) less as lexical demands increased when processing lower frequency items (See Tables 5 & 6 for details). Of particular relevance, the bilateral insula is involved in articulatory control and coordination of speech production (e.g., Oh, Duerden, & Pang, 2014), while superior frontal and cingulate regions have been implicated in inhibition and conflict monitoring, both generally (e.g., Botvinick, Cohen, & Carter, 2004; MacDonald, Cohen, Stenger, & Carter, 2000) and during language production (Abutalebi & Green, 2007; Piai, Roelofs, Acheson, & Takashima, 2013). Overall, these findings suggest that older adults were equally sensitive to high-frequency items in terms of brain activation, but when processing lower frequency items older adults engaged these language and cognitive control regions to a lesser extent (see Table 6). Although we did not observe compensatory age-related increases in activation for high-frequency words, these findings are theoretically consistent with the CRUNCH model in that they suggest that patterns of brain activation may vary as a function of task, or in this case, lexical difficulty. Moreover, these findings are consistent with the Transmission Deficit Hypothesis in that age-related neural processing differences were specifically associated with lower frequency items, which due to their infrequent use may have weaker lexical and phonological links. One may wonder if these results demonstrating that older adults engaged the brain less while processing lower frequency items reflects enhanced neural efficiency. We do not believe this is the case because older adults had poorer naming performance compared to younger adults overall and relative to one another among older adults. Although the Age Group × Frequency interaction was not significant in the behavioral analyses, older adults were less neurally engaged when processing lower frequency items. This discrepancy between the behavioral and neural results may also reflect a discrepancy between the sensitivity of our measures, in that accuracy is less sensitive compared to reaction time, whereas blocked fMRI designs robustly track hemodynamic changes.
In addition to our main effect of frequency and our interaction of age and frequency, we also observed significant effects of age on fMRI activation generally, with younger adults engaging several language relevant regions more than older adults, including the bilateral frontal cortex, bilateral superior temporal gyri, and bilateral inferior temporal regions (see Figure 3B). As we hypothesized, older adults elicited more activation than younger adults in control regions such as the left middle frontal gyrus. We also observed age-related increases in the bilateral precuneus which has been implicated in visual imagery and monitoring internal states (Raichle, 2010; Raichle et al., 2001). Specifically, this region is part of the default mode network, which has been implicated in mind wandering, recalling memories, internal dialogue, and interoception. Lastly, we also observed age-related increases in core language regions including the left angular gyrus, left supramarginal gyrus, and bilateral superior and middle temporal gyri. These age differences suggest that while younger adults continue to rely on core language regions, older adults recruit both language-relevant regions, as well as regions outside of the language network. This age-related increased recruitment of non-language regions may also reflect reduced network segregation (e.g., Chan et al., 2014), a form of dedifferentiation, which has been associated with poorer cognitive performance.
To more fully understand what these increases in brain activation mean, we examined the relationship between functional activation and task performance. Across both groups, there was a negative correlation between accuracy and activation in frontal regions, including left frontal pole, left orbital frontal cortex, and left insula (Figure 5), as well as bilateral supramarginal gyri, bilateral cingulate gyri, and bilateral occipital cortex. The negative accuracy-activation patterns are consistent with a dedifferentiation account and because the pattern of negative correlations between accuracy and functional activation was seen in both younger and older adults, this suggests that individuals who performed more poorly had more extensive patterns of activation regardless of age. Moreover, we found that these negative accuracy-activation correlations significantly differed between younger and older adults. Younger adults had stronger brain-behavior correlations in the bilateral insula, a region that was also significant in our Age × Frequency interaction, as well as in the bilateral temporal poles and right occipital-fusiform gyrus. In contrast, older adults had stronger brain-behavior correlations in the bilateral frontal pole and bilateral precuneus, which was a region that older adults engaged more than younger adults in our main effect of Age Group. These combined results suggest that older adults who recruited non-language regions less, were able to name pictures more accurately.
Although our results support age-related decline when processing low-frequency words, one limitation of this study is that it did not employ a sparse-sampling method7 during fMRI data collection. However, there were several reasons for not using this particular data collection technique. While a sparse sampling method can be beneficial when collecting overt production data to minimize the head motion inherent to speaking, and subsequent motion artifacts, the amount of data collected in the same scan time would be halved. Additionally, while images are not acquired during overt production, residual effects of head-motion distortions on the magnetic field can still be present when the scanner resumes acquiring images. An alternative approach would have been to use a covert production task in the scanner to avoid motion artifacts; however, covert and overt language production result in disparate BOLD responses, with overt production eliciting more activation in premotor cortex, left insula, and left superior temporal gyrus (Shuster & Lemieux, 2005). Another limitation inherent to the scanning environment is the presence of noise, which may reduce the amount of auditory feedback available to the participants and increases the ambient noise in general. Another procedural limitation is that since a block design was used, we were unable to perform any analyses examining frequency as an item-level, continuous variable.
In addition to the procedural limitations, another limitation is that the age of acquisition (AoA), familiarity, and name agreement for each item differed significantly across conditions. This may be expected because each of these variables tends to be correlated with lexical frequency. Some studies have found that frequency effects disappear when controlling for age of acquisition (Carroll & White, 1973a, 1973b; Garlock, Walley, & Metsala, 2001), however, the opposite finding (i.e., AoA effects disappear when controlling for frequency) would also be true due to the shared variance (Brysbaert & Ghyselinck, 2006; Meschyan & Hernandez, 2002). Additionally, as discussed in Newman and German (2005), the effects of frequency and AoA are less well understood in older adults.
In conclusion, our results indicate that both behavioral and neural frequency effects are found in healthy younger and older adults. Although similar frequency effects in behavior were observed in both the younger and older adults, there were age-related differences in the neural bases of these effects, with younger adults engaging key language and cognitive control regions to a greater extent, compared with older adults. Critically, these age-related differences were most apparent for lower frequency items, suggesting that lower frequency words elicit less activation in older adults compared to younger adults. We also observed brain–behavior correlations that were consistent with a neural dedifferentiation account. In general, increases in activation corresponded with declines in behavioral performance, for both younger and older adults. Moreover, age group differences in these brain-behavior relations suggest that while younger adults continue to effectively modulate language-relevant regions, such as the insula and temporal pole, older adults’ performance is most influenced by down-regulation of regions outside of the language network, such as the frontal pole and precuneus. Overall, these results suggest that the frequency with which an item is encountered influences retrieval across the lifespan, that older adults show a decline in the recruitment of language and cognitive control regions, particularly for lower frequency words, and that increases in activation were associated with worse performance, consistent with a dedifferentiation account.
Supplementary Material
Acknowledgments
This publication was supported by a grant from the Penn State Social Sciences Research Institute to Nancy A. Dennis, Michele T. Diaz, and Kristina A. Neely and, in part, by NIH UL1 TR002014 and KL2 TR002015 to Kristina A. Neely, and by NIH R01 AG034138 to Michele T. Diaz. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. We thank the staff and scientists at the Social, Life, & Engineering Sciences Imaging Center, where the experiment was conducted, for their support. The information presented in this manuscript has not previously been disseminated in other forms, including conference presentations. Behavioral and fMRI data and analysis scripts are available online at https://osf.io/ae69q/?view_only=fa18519067a54d08b862846a97e8b61d
Footnotes
Education was measured in years, and one participant had multiple advanced degrees. Although groups significantly differed in years of education, there were no significant effects of education on accuracy or fMRI activation.
Because of this difference between participant groups, we conducted additional analyses to examine the potential effects of run order on behavior and functional activation. Potential effects of these analyses are discussed in the results section when they are relevant. Full details on these analyses and results can be found in the Online Supplement.
A second model was run in which the lexical properties that significantly differed across frequency conditions (i.e., name agreement, age of acquisition, and familiarity) were included as fixed effects to determine if the frequency effects were explained by these variables. The R syntax for this model can be found in the Online Supplement. R files for these analyses are available in the Open Science Framework.
Results from a follow-up analysis including name agreement, age of acquisition, and familiarity as fixed effects still revealed a significant main effect of frequency when controlling for the previously mentioned lexical properties. We also found significant main effects of name agreement and age of acquisition, which were expected because these properties tend to be correlated with frequency. There were no significant effects of familiarity. These findings suggest that our current results cannot be explained by the differences in name agreement, age of acquisition, or familiarity.
It is possible that this correlation did not reach significance due to the low-frequency condition being the most variable, which could have contributed to weaker statistical effects.
However, because items were matched on visual complexity, it is unlikely that differences in perceptual features per se are driving these effects.
A sparse sampling method introduces silent delays during data acquisition, allowing for overt responses to be made by participants without introducing motion artifacts (Perrachione & Ghosh, 2013).
Contributor Information
Victoria H. Gertel, Department of Psychology, The Pennsylvania State University
Hossein Karimi, Department of Psychology, The Pennsylvania State University.
Nancy A. Dennis, Department of Psychology, The Pennsylvania State University
Kristina A. Neely, School of Kinesiology, Auburn University.
Michele T. Diaz, Department of Psychology, The Pennsylvania State University
References
- Abutalebi J, & Green D. (2007). Bilingual language production: The neurocognition of language representation and control. Journal of Neurolinguistics, 20, 242–275. [Google Scholar]
- Alexander M, Stuss D, & Fansabedian N. (2003). California Verbal Learning Test: Performance by patients with focal frontal and non‐frontal lesions. Brain, 126(6), 1493–1503. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Hutchison KA, Cortese MJ, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, & Treiman R. (2007). The English lexicon project. Behavior Research Methods, 39(3), 445–459. [DOI] [PubMed] [Google Scholar]
- Barr DJ, Levy R, Scheepers C, & Tily HJ. (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language, 68(3), 255–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S. (2014). Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823. [Google Scholar]
- Bilodeau-Mercure M, Kirouac V, Langlois N, Ouellet C, Gasse I, & Tremblay P. (2015). Movement sequencing in normal aging: Speech, oro-facial, and finger movements (Vol. 37). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau‐Mercure M, & Tremblay P. (2016). Age differences in sequential speech production: articulatory and physiological factors. Journal of the American Geriatrics Society, 64(11), e177–e182. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, & Carter CS. (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences, 8(12), 539–546. [DOI] [PubMed] [Google Scholar]
- Britt AE, Ferrara C, & Mirman D. (2016). Distinct effects of lexical and semantic competition during picture naming in younger adults, older adults, and people with aphasia. Frontiers in Psychology, 7(813). 10.3389/fpsyg.2016.00813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur Dionne-Dostie E., Montreuil T, & Lepage M (2010). The Bank of Standardized Stimuli (BOSS), a new set of 480 normative photos of objects to be used as visual stimuli in cognitive research. PloS One, 5(5), e10773. 10.1371/journal.pone.0010773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur Guérard K, .& Bouras M. (2014). Bank of Standardized Stimuli (BOSS) phase II: 930 new normative photos. PloS One, 9(9), e106953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brysbaert M, & Ghyselinck M. (2006). The effect of age of acquisition: Partly frequency related, partly frequency independent. Visual Cognition, 13(7–8), 992–1011. [Google Scholar]
- Burke DM, Locantore JK, Austin AA, & Chae B. (2004). Cherry pit primes Brad Pitt. Psychological Science, 15(3), 164–170. 10.1111/j.09567976.2004.01503004.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, MacKay DG, Worthley JS, & Wade E. (1991). On the tip of the tongue: What causes word finding failures in young and older adults? Journal of Memory and Language, 30(5), 542–579. 10.1016/0749-596X(91)90026-G [DOI] [Google Scholar]
- Burke DM, & Shafto MA. (2004). Aging and language production. Current Directions in Psychological Science, 13(1), 21–24. 10.1111/j.09637214.2004.01301006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, & Shafto MA. (2008). Language and aging In Craik F & Salthouse T (Eds.), The Handbook of Aging and Cognition (Vol. 3, pp. 373–443). [Google Scholar]
- Cabeza R. (2002). Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging, 17(1), 85–100. 10.1037/0882-7974.17.1.85 [DOI] [PubMed] [Google Scholar]
- Cabeza R, & Dennis NA. (2012). Frontal lobes and aging: Deterioration and compensation In Stuss DT & Knight RT (Eds.), Principles of Frontal Lobe Function. (2nd ed, pp. 628–652). New York: Oxford University Press. [Google Scholar]
- Carp J, Park J, Polk TA, & Park DC. (2011). Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. Neuroimage, 56(2), 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JB, & White MN. (1973a). Age-of-acquisition norms for 220 picturable nouns. Journal of Verbal Learning and Verbal Behavior, 12(5), 563–576. [Google Scholar]
- Carroll JB, & White MN. (1973b). Word frequency and age of acquisition as determiners of picture-naming latency. The Quarterly Journal of Experimental Psychology, 25(1), 85–95. [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, & Wig GS. (2014). Decreased segregation of brain systems across the healthy adult lifespan. Proceedings of the National Academy of Sciences of the United States of America, 111(46), E4997–5006. 10.1073/pnas.1415122111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Schafer G, & Akyürek EG. (2010). Name agreement in picture naming: An ERP study. International Journal of Psychophysiology, 76(3), 130–141. 10.1016/j.ijpsycho.2010.03.003 [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Iyengar V, Davis SW, Eklund K, Hayes SM, & Cabeza RE. (2015). Less wiring, more firing: Low-performing older adults compensate for impaired white matter with greater neural activity. Cerebral Cortex, 25(4), 983–990. 10.1093/cercor/bht289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, & Cabeza R. (2011). Age-related dedifferentiation of learning systems: An fMRI study of implicit and explicit learning. Neurobiology of Aging, 32(12), 2318e2317–2330. 10.1016/j.neurobiolaging.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, & Killiany RJ. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Diaz MT, Johnson MA, Burke DM, & Madden DJ. (2014). Age-related differences in the neural bases of phonological and semantic processes. Journal of Cognitive Neuroscience, 26(12), 2798–2811. 10.1162/jocn_a_00665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, Johnson MA, Burke DM, Truong T-K, & Madden DJ. (2018). Age-related differences in the neural bases of phonological and semantic processes in the context of task-irrelevant information. Cognitive, Affective, & Behavioral Neuroscience. 10.3758/s13415-018-00671-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchin SW, & Mysak ED. (1987). Disfluency and rate characteristics of young adult, middle-aged, and older males. Journal of Communication Disorders, 20(3), 245–257. 10.1016/0021-9924(87)90022-0 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiaticr Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Garlock VM, Walley AC, & Metsala JL. (2001). Age-of-acquisition, word frequency, and neighborhood density effects on spoken word recognition by children and adults. Journal of Memory and Language, 45(3), 468–492. [Google Scholar]
- Geva S, Jones P, Crinion J, J Price C, Baron J-C, & A Warburton E. (2014). The Effect of Aging on the Neural Correlates of Phonological Word Retrieval (Vol. 24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, & Burgess PW. (2006). Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. Journal of Cognitive Neuroscience, 18(6), 932–948. 10.1162/jocn.2006.18.6.932 [DOI] [PubMed] [Google Scholar]
- Glover GH, Mueller BA, Turner JA, Van Erp TG, Liu TT, Greve DN, Voyvodic JT, Rasmussen J, Brown GG, & Keator DB. (2012). Function biomedical informatics research network recommendations for prospective multi-center functional MRI studies. Journal of Magnetic Resonance Imaging, 36(1), 39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Montoya RI, Cera C, & Sandoval TC. (2008). More use almost always means a smaller frequency effect: Aging, bilingualism, and the weaker links hypothesis. Journal of Memory and Language, 58(3), 787–814. 10.1016/j.jml.2007.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, & Gordon JK. (2007). A neural signature of phonological access: Distinguishing the effects of word frequency from familiarity and length in overt picture naming. Journal of Cognitive Neuroscience, 19(4), 617–631. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, & Kanwisher N. (2001). The lateral occipital complex and its role in object recognition. Vision Research, 41(10), 1409–1422. 10.1016/S0042-6989(01)00073-6 [DOI] [PubMed] [Google Scholar]
- Hayasaka S, & Nichols TE. (2003). Validating cluster size inference: random field and permutation methods. Neuroimage, 20(4), 2343–2356. [DOI] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393–402. [DOI] [PubMed] [Google Scholar]
- Horton WS, Spieler DH, & Shriberg E. (2010). A corpus analysis of patterns of age-related change in conversational speech. Psychology and Aging, 25(3), 708–713. 10.1037/a0019424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Singerman JD, & McCarthy G. (2001). The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage, 13(1), 161–175. [DOI] [PubMed] [Google Scholar]
- Jescheniak JD, & Levelt WJM (1994). Word frequency effects in speech production: Retrieval of syntactic information and of phonological form. Journal of Experimental Psychology: Learning, Memory, and Cognition, 20(4), 824–843. 10.1037/0278-7393.20.4.824 [DOI] [Google Scholar]
- Kuperman V, Stadthagen-Gonzalez H, & Brysbaert M. (2012). Age-of-acquisition ratings for 30,000 English words. Behavior Research Methods, 44(4), 978–990. [DOI] [PubMed] [Google Scholar]
- LaGrone S, & Spieler DH. (2006). Lexical competition and phonological encoding in young and older speakers. Psychology and Aging, 21(4), 804. [DOI] [PubMed] [Google Scholar]
- Li S-C, & Lindenberger U. (1999). Cross-level unification: A computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age Cognitive Neuroscience of Memory (pp. 103–146): Hogrefe & Huber. [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, & Buckner RL. (2002). Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron, 33(5), 827–840. [DOI] [PubMed] [Google Scholar]
- Loibl M, Beutling W, Kaza E, & Lotze M. (2011). Non-effective increase of fMRI-activation for motor performance in elder individuals. Behavioural Brain Research, 223(2), 280–286. [DOI] [PubMed] [Google Scholar]
- Lutkenhoff ES, Rosenberg M, Chiang J, Zhang K, Pickard JD, Owen AM, & Monti MM. (2014). Optimized brain extraction for pathological brains (optiBET). PloS One, 9(12), e115551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, & Carter CS. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288(5472), 1835–1838. [DOI] [PubMed] [Google Scholar]
- McKetton L, Sobczyk O, Duffin J, Poublanc J, Sam K, Crawley AP, Venkatraghavan L, Fisher JA, & Mikulis DJ. (2018). The aging brain and cerebrovascular reactivity. Neuroimage, 181, 132–141. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Wilser L, Eulitz C, Rockstroh B, Conway T, Gonzalez-Rothi L, & Crosson B. (2009). Neural signatures of semantic and phonemic fluency in young and old adults. Journal of Cognitive Neuroscience, 21(10), 2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschyan G, & Hernandez A. (2002). Age of acquisition and word frequency: Determinants of object-naming speed and accuracy. Memory & Cognition, 30(2), 262–269. [DOI] [PubMed] [Google Scholar]
- Moreno-Martínez FJ, & Montoro PR. (2012). An ecological alternative to Snodgrass & Vanderwart: 360 high quality colour images with norms for seven psycholinguistic variables. PloS One, 7(5), e37527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, & Nichols TE. (2008). Power calculation for group fMRI studies accounting for arbitrary design and temporal autocorrelation. Neuroimage, 39(1), 261–268. 10.1016/j.neuroimage.2007.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RS, & German DJ. (2005). Life Span Effects of Lexical Factors on Oral Naming. Language and Speech, 48(2), 123–156. 10.1177/00238309050480020101 [DOI] [PubMed] [Google Scholar]
- Obler LK, Rykhlevskaia E, Schnyer D, Clark-Cotton MR, Spiro III A, Hyun J, Kim D-S, Goral M, & Albert ML. (2010). Bilateral brain regions associated with naming in older adults. Brain and Language, 113(3), 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh A, Duerden EG, & Pang EW. (2014). The role of the insula in speech and language processing. Brain and Language, 135, 96–103. 10.1016/j.bandl.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC, & Wingfield A. (1965). Response latencies in naming objects. Quarterly Journal of Experimental Psychology, 17(4), 273–281. 10.1080/17470216508416445 [DOI] [PubMed] [Google Scholar]
- Perrachione TK, & Ghosh SS. (2013). Optimized design and analysis of sparse-sampling fMRI experiments. Frontiers in Neuroscience, 7, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piai V, Roelofs A, Acheson DJ, & Takashima A. (2013). Attention for speaking: Domain-general control from the anterior cingulate cortex in spoken word production. Frontiers in Human Neuroscience, 7(321), 1–14. 10.3389/fnhum.2013.00832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA, & Nichols TE. (2011). Handbook of functional MRI data analysis: Cambridge University Press. [Google Scholar]
- Psychology Software Tools, I. (2012). E-Prime (Version 2.0).
- Raichle ME. (2010). Two views of brain function. Trends in Cognitive Sciences, 14(4), 180–190. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, & Cappell KA. (2008). Neurocognitive Aging and the Compensation Hypothesis. Current Directions in Psychological Science, 17(3), 177–182. 10.1111/j.1467-8721.2008.00570.x [DOI] [Google Scholar]
- Riecker A, Gröschel K, Ackermann H, Steinbrink C, Witte O, & Kastrup A. (2006). Functional significance of age-related differences in motor activation patterns. Neuroimage, 32(3), 1345–1354. [DOI] [PubMed] [Google Scholar]
- Rizio AA, Moyer KJ, & Diaz MT. (2017). Neural evidence for phonologically based language production deficits in older adults: An fMRI investigation of age-related differences in picture-word interference. Brain and Behavior, 7(4), e00660. 10.1002/brb3.660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan Giles H., Bartolucci G, & Henwood K (1986). Psycholinguistic and social psychological components of communication by and with the elderly. Language & Communication, 6(1–2), 1–24. [Google Scholar]
- Ryan Hummert M. L, & Boich LH. (1995). Communication predicaments of aging: Patronizing behavior toward older adults. Journal of Language and Social Psychology, 14(1–2), 144–166. [Google Scholar]
- Sadagopan N, & Smith A. (2013). Age differences in speech motor performance on a novel speech task. Journal of Speech, Language, and Hearing Research, 56(5), 1552–1566. [DOI] [PubMed] [Google Scholar]
- Saults JS, & Cowan N. (2007). A central capacity limit to the simultaneous storage of visual and auditory arrays in working memory. Journal of Experimental Psychology: General, 136(4), 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searl JP, Gabel RM, & Fulks JS. (2002). Speech disfluency in centenarians. Journal of Communication Disorders, 35(5), 383–392. 10.1016/S00219924(02)00084-9 [DOI] [PubMed] [Google Scholar]
- Shafto MA, & Tyler LK. (2014). Language in the aging brain: The network dynamics of cognitive decline and preservation. Science, 346(6209), 583–587. 10.1126/science.1254404 [DOI] [PubMed] [Google Scholar]
- Shuster LI, & Lemieux SK. (2005). An fMRI investigation of covertly and overtly produced mono-and multisyllabic words. Brain and Language, 93(1), 20–31. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, & Flitney DE. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, & Vanderwart M. (1980). A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory, 6(2), 174. [DOI] [PubMed] [Google Scholar]
- Sowell Thompson P. M., Tessner KD, & Toga AW (2001). Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. Journal of Neuroscience, 21(22), 8819–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, & Farah MJ. (1997). Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences, 94(26), 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, & Rees L. (1999). Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of Clinical Neuropsychology, 14(2), 167–177. 10.1093/arclin/14.2.167 [DOI] [PubMed] [Google Scholar]
- Tremblay P, & Deschamps I. (2016). Structural brain aging and speech production: a surface-based brain morphometry study. Brain Structure and Function, 221(6), 3275–3299. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Sato M, & Deschamps I. (2017). Age differences in the motor control of speech: An fMRI study of healthy aging. Human Brain Mapping, 38(5), 2751–2771. 10.1002/hbm.23558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables W, & Smith D. (2008). An introduction to R: a programming environment for data analysis and graphics. [Google Scholar]
- Vitkovitch M, & Tyrrell L. (1995). Sources of disagreement in object naming. The Quarterly Journal of Experimental Psychology Section A, 48(4), 822–848. 10.1080/14640749508401419 [DOI] [Google Scholar]
- Wechsler D. (1997) Wechsler adult intelligence scale - III. New York: Psychological Corporation. [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJ, Conway T, Cato MA, Briggs R, & Crosson B. (2008). Age-related changes in word retrieval: role of bilateral frontal and subcortical networks. Neurobiology of Aging, 29(3), 436–451. 10.1016/j.neurobiolaging.2006.10.024 [DOI] [PubMed] [Google Scholar]
- Wilson SM, Isenberg AL, & Hickok G. (2009). Neural correlates of word production stages delineated by parametric modulation of psycholinguistic variables. Human Brain Mapping, 30(11), 3596–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlert AB, & Smith A. (1998). Spatiotemporal stability of lip movements in older adult speakers. Journal of Speech, Language, and Hearing Research, 41(1), 41–50. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, & Smith SM. (2009). Bayesian analysis of neuroimaging data in FSL. Neuroimage, 45(1 Suppl), S173–186. 10.1016/j.neuroimage.2008.10.055 [DOI] [PubMed] [Google Scholar]
- Worsley K. (2001). Statistical analysis of activation images. Functional MRI: an introduction to methods, 14, 251–270. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, & Leirer VO. (1982). Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatr Research, 17(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sachdev PS, Wen W, Kochan NA, Crawford JD, Brodaty H, Slavin MJ, Reppermund S, Kang K, & Trollor JN. (2013). Grey matter correlates of three language tests in non-demented older adults. PloS One, 8(11), e80215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.