Abstract
Background
Episodic flare-ups of fibrodysplasia ossificans progressiva (FOP) are characterized clinically by severe, often posttraumatic, connective tissue swelling and intramuscular edema, followed histologically by an intense and highly angiogenic fibroproliferative reaction. This early inflammatory and angiogenic fibroproliferative response is accompanied by the presence of abundant mast cells far in excess of other reported myopathies.
Results
Using an injury-induced, constitutively-active transgenic mouse model of FOP we show that mast cell inhibition by cromolyn, but not aprepitant, results in a dramatic reduction of heterotopic ossification. Cromolyn, but not aprepitant, significantly decreases the total number of mast cells in FOP lesions. Furthermore, cromolyn specifically diminishes the number of degranulating and resting degranulated mast cells in pre-osseous lesions.
Conclusions
This work demonstrates that consideration of FOP as a type of localized mastocytosis may offer new therapeutic interventions for treatment of this devastating condition.
Keywords: Mast cells, Fibrodyplasia ossificans progressiva (FOP), Heterotopic ossification, ACVR1, Cromolyn
1. Introduction
Mast cells are found within connective tissue and vascularized organs. Their progenitors leave the bone marrow to enter the circulation and mature within target tissue in the presence of the KIT ligand stem cell factor (SCF) [1]. SCF is critical for mast cell expansion,differentiation and survival. Mast cells are activated in response to mechanical stimulation, temperature, snake venom, and antigens via the high affinity receptor for IgE [2]. Their activation results in acute and chronic inflammation, vascular injury, cell recruitment, cell adhesion, and, ultimately, tissue remodeling and angiogenesis [2–4]. Mast cells secrete preformed inflammatory mediators, including serine proteases (i.e. tryptase), histamine, serotonin, proteoglycans (i.e. heparin), and cytokines (e.g., TNF-α), as well as late-phase products such as chemokines and additional cytokines.
Mast cell subtypes cycle among resting granulated (RG), degranulating (DG), and resting degranulated (RD) cells, with RG cells becoming DG cells upon activation and DG cells then becoming RD cells after release of preformed inflammatory and late-phase products [5,6]. Finally, RD cells become RG cells when their inflammatory mediators are reconstituted in cytoplasmic granules [5,6].
In FOP, episodic tissue swelling and intramuscular edema is followed by intense angiogenic and fibroproliferative reactions. The early edematous, preosseous fibroproliferative stages characteristic of FOP flare-ups suggested to Gannon et al. [7] that inflammatory mast cells and their mediators in tissue edema, wound repair, fibrogenesis, angiogenesis, and tumor invasion were potentially involved in the pathology of FOP lesions. They showed that inflammatory mast cells are present at every stage of the development of FOP lesions and are most pronounced at the highly vascular fibroproliferative stage.
Here we test the hypothesis that mast cell inhibitors/stabilizers such as cromolyn (cromogliclic acid) and aprepitant can prevent heterotopic ossification (HO) in a mouse model of FOP. We found that mast cell inhibition by cromolyn, but not aprepitant, results in a dramatic reduction of heterotopic ossification. Cromolyn, but not aprepitant, significantly decreased the total number of mast cells in FOP lesions. In addition, cromolyn specifically diminishes the number of degranulating (“activated”) and resting degranulated mast cells in pre-osseous lesions.
2. Methods
2.1. In vivo testing of mast cell inhibitors
A transgenic mouse model containing a constitutively active (ca)ACVR1 allele flanked by loxP sites (caACVR1 mice) was used in all animal experiments [8,9]. A 50-μL, 0.9% NaCl solution containing adenovirus-Cre (5 × 1010 genome copies per mouse; Penn Vector Core, University of Pennsylvania) to induce expression of caACVR1 and cardiotoxin (10 μM solution; Sigma-Aldrich, St. Louis, MO, USA) to induce an injury/inflammatory response was injected into the hindlimb musculature of mice at 3 weeks of age. Mice were treated via intraperitoneal injection with 800 mg/kg/ day of cromolyn in phosphate-buffered saline(PBS),oral gavage with 20, 100, or 500 mg/kg/day aprepitant in PBS, or vehicle control. For all treatment groups, a minimum of four animals was used per group. Mice were treated for 4 days prior to and 14 days following injection in the left hind limb. Tissues were recovered at 2, 5, 7, 10, or 14 days after injections.
Mice were bred in an animal biosafety level (ABSL) 1 facility then transferred to an ABSL2 facility where procedures were performed. Mice were housed as 1 or 2 mothers with litters and then separated at weaning into cages of no more than 5 males or 5 females. Both males and females were used for experiments. Other aspects of animal care and usage (e.g., light/ dark cycle) were standard and performed by University. Laboratory Animal Resources (ULAR) in accordance with ULAR policies and protocols.
2.2. Micro computerized tomography
Micro computed tomography (μCT) was performed on the injected leg post mortem using a Scanco VivaCT 40 (Bruettisellen, Switzerland) to determine the volume of heterotopic bone and obtain a two-dimensional image of the medial view of the sagittal plane of the limb. Scanning was performed using a source voltage of 55 kV, a source current of 142 μA, and an isotropic voxel size of 10.5 μm. Bone was differentiated from “non-bone” by an upper threshold of 1000 Housfield units and a lower threshold of 150 Housfield units.
2.3. Histology
For histological analyses, animals were euthanized 2, 5 or 7 days post cardiotoxin muscle injury. The left hind limb was removed and placed in 3.7% paraformaldehyde for 72 h. The tissue was decalcified for 3–5days with ImmunoCal™ (Decal Chemical Corporation) and processed for paraffin embedding. Sequential sections were cut at 8 μm and stained with C.E.M. (Combined Eosinophil-Mast Cell) staining kit® (American MasterTech) or Safranin O. C.E.M. staining was performed according to manufacturer’s protocol. Safranin O staining was performed according to standard protocol.
Approximately 10 consecutive sections were alternately stained with C.E.M. or Safranin-O. The region of interest was defined as the entire section above the ankle, not including bone and its articular cartilage. All mast cells within the region of interest were identified and quantified. Mast cell subtypes were characterized as resting granulated (RG), degranulating (DG), or resting degranulated (RD) and distinguished on the basis of major histological features. RG cells have low nuclear to cytoplasmic ratios, dark staining with nuclei less visible, intact membranes, and large sizes. DG cells have varied nuclear to cytoplasmic ratios and variegated staining, are of medium-large sizes, and have broken membranes. RD cells have high nuclear to cytoplasmic ratios, lighter staining with very visible nuclei, intact membranes, and small sizes. Samples were viewed under a Nikon 50i Eclipse microscope and images were captured using Nikon DS-Fi1 camera and NIS elements F version 4.3 software.
2.4. Statistics
Comparison among treatment doses in the aprepitant group was analyzed by one-way analysis of variance (ANOVA), and between cromolyn-treated and control groups by Student t-test (two-tailed). Effects of time and cromolyn treatment were analyzed by two-way ANOVA, followed by the Bonferroni post-test. All statistics were performed using GraphPad Prism 4.0 software (San Diego, CA). Differences were considered statistically significant at a p-value of <0.05, and all data is represented as mean ± SEM.
3. Results
3.1. Cromolyn treatment results in diminished heterotopic bone formation in the caACVR1 animal model
We tested the hypothesis that mast cell inhibition can reduce HO in a mouse model of FOP by using two mast cell antagonists that act by different mechanisms. Aprepitant is a substance P (SP)/neurokinin 1 (NK1) receptor antagonist in mast cells. The SP/NK-1 receptor system affects immune function (especially mast cells) through sensory nerves that modulate neurogenic inflammation. Cromolyn inhibits sensitized mast cell degranulation and indirectly blocks calcium ions from entering the mast cell, thereby preventing mediator release.
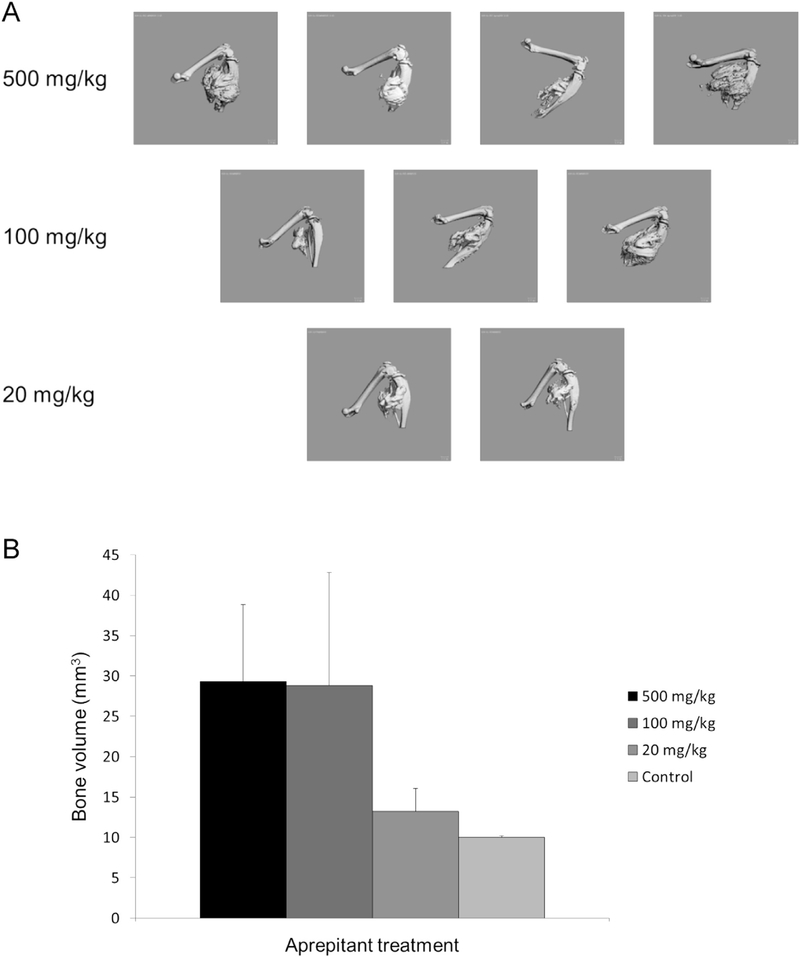
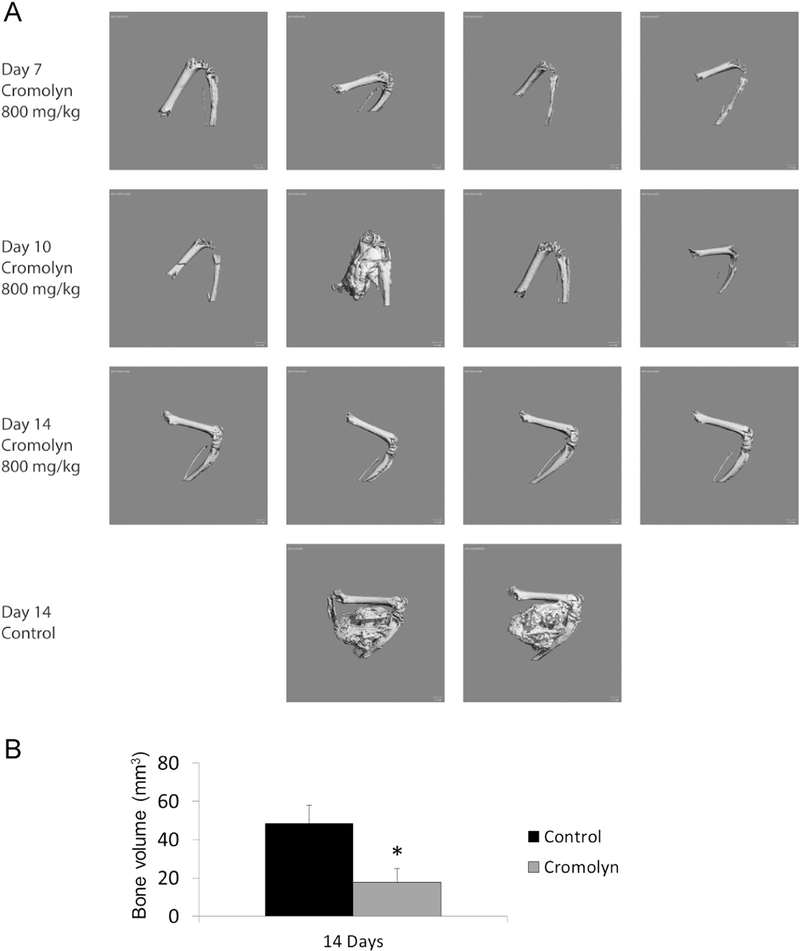
Fig. 1 shows that over a broad range of doses aprepitant does not inhibit heterotopic bone formation in the caACVR1 mouse model. In contrast, cromolyn dramatically reduces HO by day 14 after injury-induced lesion formation (Fig. 2).
Fig. 1.
Aprepitant does not reduceHOina caACVR1 mouse model of FOP. (A) Representative examples of HO formation by μCT. (B) Quantification of HO volume among the treatment doses of aprepitant.
Fig. 2.
Cromolyn effectively reduces HO in a caACVR1 mouse model of FOP. (A) Representative examples of HO formation by μCT. (B) Quantification of HO volume between the treatment groups. *, p < 0.05.
3.2. Mast cells are reduced in cromolyn- but not aprepitant-treated caACVR1 animals
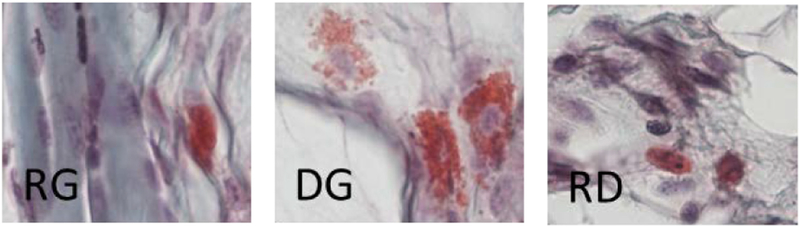
In pre-osseous lesions, mast cells can be detected by two days after injury induction (Fig. 3). The repertoire of mast cell subtypes, including activated DG cells, are present early and are identified by their characteristic appearances (see Material and methods — Histology section and Fig. 3).
Fig. 3.
Mast cell subtypes in early injury-induced lesions in a caACVR1 mouse model of FOP (day 2 after injury). Note the broken membrane and release of granules in activated (DG) mast cells. RG, resting granulated; DG, degranulating; RD, resting degranulated (RD).
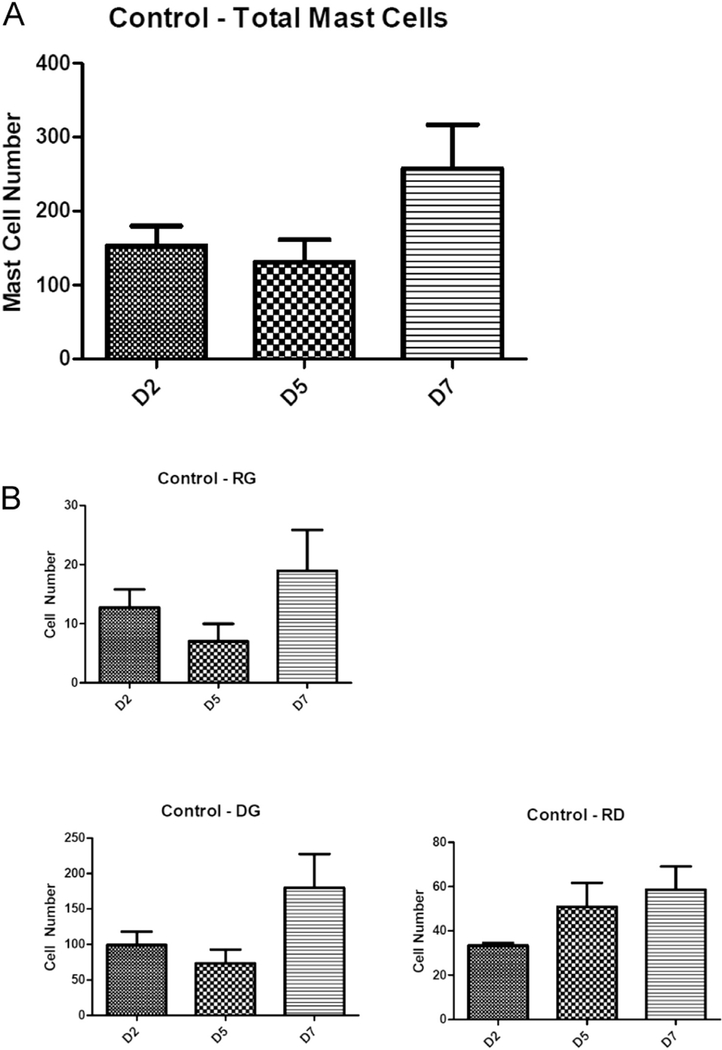
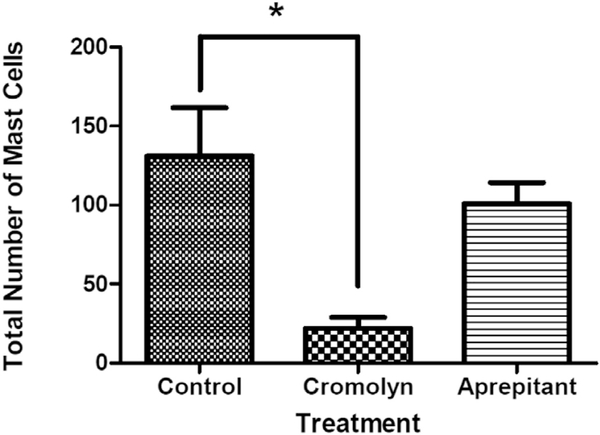
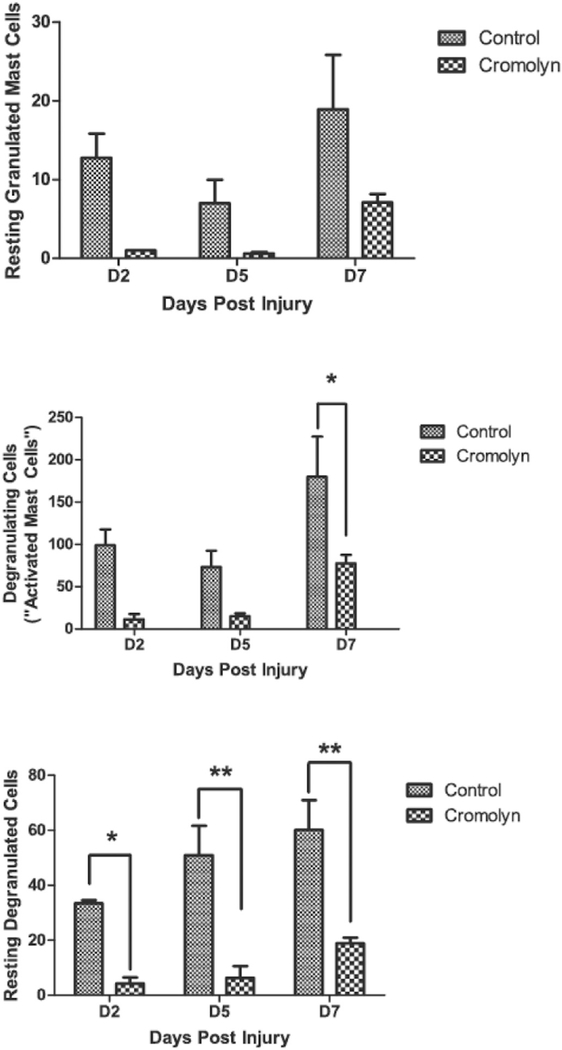
Fig. 4A demonstrates that in control lesional tissue sections, mast cells are present throughout the pre-osseous stages (up to day seven after injury). There is a statistically non-significant trend toward higher total mast cell number (Fig. 4A) and specific mast cell subtypes (Fig. 4B) by day seven. There was a statistically significant reduction in the total number of mast cells in the cromolyn-treated animal group compared to the control group, but not in the aprepitant-treated group (Fig. 5).
Fig. 4.
Quantification of total mast cells (A) and mast cell subtypes (B) in control caACVR1 mice. Data is shown for days two, five, and seven after muscle injury. D, day.
Fig. 5.
Total mast cell number isreduced in cromolyn- but not aprepitant-treated caACVR1 mice. Data shown is for day five after injury induction. *, p < 0.05.
Further analysis of the cromolyn-treated group revealed that reductions in specific mast cell subtypes are primarily responsible for the decrease in total mast cells compared to the control group (Fig. 6). Both DG and RD cells increased significantly post-injury (p b 0.05). Although all mast cell subtypes declined with cromolyn treatment over time, the most significant effects were with DG (p b 0.01) and RD cells (p b 0.0001). Post-hoc analysis demonstrated that DG cells on day seven after injury and RD cells at all time points after injury were significantly reduced by cromolyn treatment (Fig. 6).
Fig. 6.
Quantification of mast cell subtypes in cromolyn-treated caACVR1 mice. Data is shown for days two, five, and seven after muscle injury. *, p < 0.5; **, p < 0.01; D, day.
4. Discussion
The episodic connective tissue swelling and intramuscular edema seen with flare-ups in FOP are accompanied by highly angiogenic fibroproliferation seen histologically. Gannon et al. [7] showed that mast cell density at the periphery of FOP lesions is as much as 150-fold greater than in normal skeletal muscle or in uninvolved skeletal muscle from patients with FOP and up to 40-fold greater than in any other inflammatory myopathy. These findings demonstrate that mobilization and activation of inflammatory mast cells are pathological hallmarks of FOP lesions and serve as potential targets for pharmacologic intervention in this extremely disabling disease. Mast cell involvement has also been demonstrated in non-hereditary HO, strongly suggesting the cell type is critical to the pathology of HO [10,11].
Mast cells play a key role in the allergy response and anaphylaxis [2], and have been more recently recognized as mediators in tissue edema, wound repair, fibrosis, angiogenesis, pain pathophysiology, and tumor invasion [4,12–21]. Using Nse-BMP4/MOR (−/−) double mutant mice, in which μ-opioid receptor (MOR)-null mice over-express BMP4 in cells with an active Nse promoter, Kan et al. [22] showed attenuated mast cell activation and concomitant reduction in HO formation in response to injury. Opioid signaling may thus play a key role in mast cell activation and the downstream inflammatory responses associated with HO.
Following BMP induction, mast cells are recruited and activated by the potent neuro-inflammatory molecules Substance P (SP) and calcitonin gene related peptide (CGRP) [23]. Inhibition of the SP and CGRP signaling pathways via various genetic and pharmacological methods can reduce HO formation in animal models [24–26]. SP is also highly expressed in early stage lesional tissue from FOP patients [24]. SP acts on mast cells via binding to the NK-1R receptor, which induces mast cell degranulation and release of numerous pro-inflammatory factors [27].
Pharmacological inhibition of mast cells and SP is theoretically possible in two ways. The NK-1R receptor can be directly targeted by the NK-1R antagonist aprepitant, which has been successfully used to inhibit intracellular calcium ion flux, a readout of SP signaling [28]. However, at least in the caACVR1 FOP model, aprepitant was not effective in limiting injury-induced HO. Conversely, direct inhibition of mast cell degranulation and reduction of mast cell number by cromolyn was successful at significantly reducing HO. Our results that mast cells are present in early pre-osseous stages of lesion formation in the caACVR1 mouse model recapitulate the initial observation by Gannon et al. in FOP lesional tissue [7].
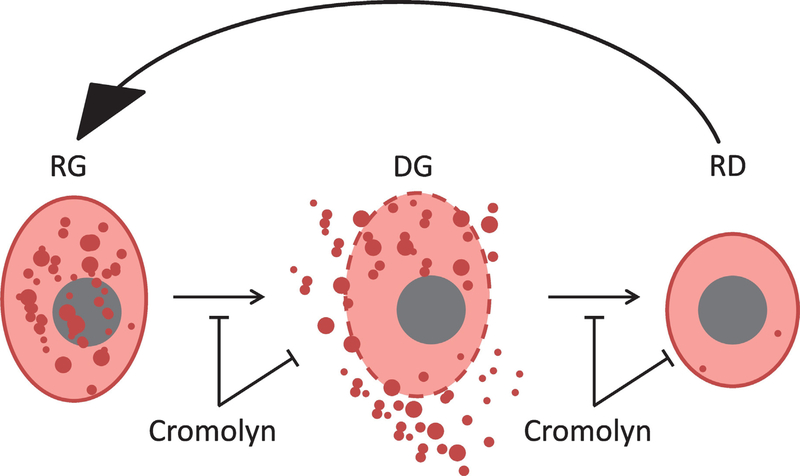
Cromolyn may disrupt the activation cycle of mast cells by multiple cellular mechanisms (Fig. 7). Cromolyn may antagonize both the activation of mast cells (formation of DG cells from RG cells) as well as the production of RD cells, preventing the regeneration of RG cells. Based on this latter mechanism, if cromolyn is given after an early FOP lesion has formed, it might potentiate the DG phase (by inhibiting the DG to RD cell transition) and potentially exacerbate a flare-up. This suggests that in its clinical use in FOP patients, as in the proof-of-concept FOP mouse model used here, cromolyn should be given prophylactically to prevent mast cell degranulation. It is also possible that cromolyn indirectly inhibits the DG to RD cell transition; that is, there are fewer RG mast cells because cromolyn effectively reduces the number of DG cells such that there are many fewer cells that can become RG cells. Cromolyn may also act by direct inhibition or removal of DG and RG cells from the regeneration cycle.
Fig. 7.
Schema showing cellular mechanisms of mast cell inhibition by cromolyn. Note that cromolyn antagonizes both the activation of mast cells (DG) from RG cells as well as the production of RD cells, preventing the regeneration of RG cells. Cromolyn may also act by directly inhibiting or removing DG and RG cells from the regeneration cycle. RG, resting granulated; DG, degranulating; RD, resting degranulated (RD).
Although its existing FDA indication for asthma may make cromolyn a potential option for FOP patients, its current routes of usual administration result in poor systemic distribution. Spray or aerosolized liquid forms for nasal or oral inhalation represent the best common routes for systemic distribution and cromolyn is currently used at the physician’s discretion as a Class II medication in current FOP treatment guidelines [29]. A liquid intravenous form of cromolyn has been reported [30,31]; however, it undergoes rapid elimination with possible hypertension and nausea as potential adverse effects [32]. An alternative option involves using the c-kit tyrosine kinase inhibitor imatinib to induce mast cell apoptosis, which has proven successful at reducing inflammation associated with rheumatoid arthritis [33] and severe refractory asthma [34], as well as reducing HO in an Achilles tendon injury model [35], and importantly, HO in a FOP mouse model [36].
5. Conclusions
Considering FOP as a type of localized mastocytosis may offer new therapeutic opportunities for treatment. Cromolyn is very safe and effective in abrogating HO in an injury-induced mouse model of FOP, but at doses in excess of those currently capable of being delivered in a safe and convenient manner. Improvement in the bioavailability of cromolyn, either by change in formulation or route of administration, may offer enhanced therapeutic benefits. Other mast cell inhibitors may offer similar and/or additional therapeutic benefits.
Acknowledgments
Funding
This work was supported by the Cali-Weldon Preclinical Drug Testing and Biomarker Development Program at the University of Pennsylvania, the Ian Cali Distinguished Clinician-Scientist Award, the Robert and Arlene Professorship in Geriatric Medicine at the Mayo Clinic, and the Radiant Hope Foundation (to RJP); the Center for Research in FOP and Related Disorders at the University of Pennsylvania Perelman School of Medicine; the Ian Cali Endowment for FOP Research; the Whitney Weldon Endowment for FOP Research and the Isaac and Rose Nassau Professorship of Orthopaedic Molecular Medicine (to FSK). This work wasalso supported by thePenn Center for Musculoskeletal Disorders (NIH P30-AR050950), and the National Institutes of Health (NIH R01-AR41916).
Footnotes
Conflict of interest
The authors declare that they have no competing interests.
References
- [1].Ito T, Smrz D, Jung MY, Bandara G, Desai A, Smrzova S, Kuehn HS, Beaven MA, Metcalfe DD, Gilfillan AM, Stem cell factor programs the mast cell activation phenotype, J. Immunol. 188 (11) (2012) 5428–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bischoff SC, Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data, Nat. Rev. Immunol. 7 (2) (2007) 93–104. [DOI] [PubMed] [Google Scholar]
- [3].Galli SJ, The mast cell-IgE paradox: from homeostasis to anaphylaxis, Am. J. Pathol. 186 (2) (2016) 212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Galli SJ, Grimbaldeston M, Tsai M, Immunomodulatory mast cells: negative, as well as positive, regulators of immunity, Nat. Rev. Immunol. 8 (6) (2008) 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dvorak AM,Galli SJ,Antigen-induced, IgE-mediated degranulation of cloned immature mast cells derived from normal mice, Am. J. Pathol. 126 (3) (1987) 535–545. [PMC free article] [PubMed] [Google Scholar]
- [6].Dvorak AM, Schleimer RP, Lichtenstein LM, Morphologic mast cell cycles, Cell. Immunol. 105 (1) (1987) 199–204. [DOI] [PubMed] [Google Scholar]
- [7].Gannon FH, Glaser D, Caron R, Thompson LD, Shore EM, Kaplan FS, Mast cell involvement in fibrodysplasia ossificans progressiva, Hum. Pathol 32 (8) (2001) 842–848. [DOI] [PubMed] [Google Scholar]
- [8].Fukuda T, Scott G, Komatsu Y, Araya R, Kawano M, Ray MK, Yamada M, Mishina Y, Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2, Genesis 44 (4) (2006) 159–167. [DOI] [PubMed] [Google Scholar]
- [9].Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD, BMP type I receptor inhibition reduces heterotopic [corrected] ossification, Nat. Med. 14 (12) (2008) 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Di Paolo N, Sacchi G, Lorenzoni P, Sansoni E, Gaggiotti E, Ossification of the peritoneal membrane, Perit. Dial. Int 24 (5) (2004) 471–477. [PubMed] [Google Scholar]
- [11].Mohler ER 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS, Bone formation and inflammation in cardiac valves, Circulation 103 (11) (2001) 1522–1528. [DOI] [PubMed] [Google Scholar]
- [12].Douaiher J, Succar J, Lancerotto L, Gurish MF, Orgill DP, Hamilton MJ, Krilis SA, Stevens RL, Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing, Adv. Immunol. 122 (2014) 211–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ehrlich HP, A snapshot of direct cell-cell communications in wound healing and scarring, Adv. Wound Care 2 (4) (2013) 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Farrugia BL, Whitelock JM, Jung M, McGrath B, O’Grady RL, McCarthy SJ, Lord MS, The localisation of inflammatory cells and expression of associated proteoglycans in response to implanted chitosan, Biomaterials 35 (5) (2014) 1462–1477. [DOI] [PubMed] [Google Scholar]
- [15].Frieri M, Patel R, Celestin J, Mast cell activation syndrome: a review, Curr. Allergy Asthma Rep 13 (1) (2013) 27–32. [DOI] [PubMed] [Google Scholar]
- [16].Gri G, Frossi B, D’Inca F, Danelli L, Betto E, Mion F, Sibilano R, Pucillo C, Mast cell: an emerging partner in immune interaction, Front. Immunol 3 (2012) 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Heron A, Dubayle D, A focus on mast cells and pain, J. Neuroimmunol. 264 (1–2) (2013) 1–7. [DOI] [PubMed] [Google Scholar]
- [18].Oldford SA, Marshall JS, Mast cells as targets for immunotherapy of solid tumors, Mol. Immunol. 63 (1) (2015) 113–124. [DOI] [PubMed] [Google Scholar]
- [19].Rodewald HR, Feyerabend TB, Widespread immunological functions of mast cells: fact or fiction? Immunity 37 (1) (2012) 13–24. [DOI] [PubMed] [Google Scholar]
- [20].Thevenot PT, Baker DW, Weng H, Sun MW, Tang L, The pivotal role of fibrocytes and mast cells in mediating fibrotic reactions to biomaterials, Biomaterials 32 (33) (2011) 8394–8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, Simone DA, Gupta K, Mast cell activation contributes to sickle cell pathobiology and pain in mice, Blood 122 (11) (2013) 1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kan L, Mutso AA, McGuire TL, Apkarian AV, Kessler JA, Opioid signaling in mast cells regulates injury responses associated with heterotopic ossification, Inflamm. Res. 63 (3) (2014) 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bucelli RC, Gonsiorek EA, Kim WY, Bruun D, Rabin RA, Higgins D, Lein PJ, Statins decrease expression of the proinflammatory neuropeptides calcitonin gene-related peptide and substance P in sensory neurons, J. Pharmacol. Exp. Ther. 324 (3) (2008) 1172–1180. [DOI] [PubMed] [Google Scholar]
- [24].Kan L, Lounev VY, Pignolo RJ, Duan L, Liu Y, Stock SR, McGuire TL, Lu B, Gerard NP, Shore EM, Kaplan FS, Kessler JA, Substance P signaling mediates BMP-dependent heterotopic ossification, J. Cell. Biochem. 112 (10) (2011) 2759–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Salisbury E, Rodenberg E, Sonnet C, Hipp J, Gannon FH, Vadakkan TJ, Dickinson ME, Olmsted-Davis EA, Davis AR, Sensory nerve induced inflammation contributes to heterotopic ossification, J. Cell. Biochem. 112 (10) (2011) 2748–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Salisbury E, Sonnet C, Heggeness M, Davis AR, Olmsted-Davis E, Heterotopic ossification has some nerve, Crit. Rev. Eukaryot. Gene Expr. 20 (4) (2010) 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F, The role of substance P in inflammatory disease, J. Cell. Physiol. 201 (2) (2004) 167–180. [DOI] [PubMed] [Google Scholar]
- [28].Manak MM, Moshkoff DA, Nguyen LT, Meshki J, Tebas P, Tuluc F, S Douglas, Anti-HIV-1 activity of the neurokinin-1 receptor antagonist aprepitant and synergistic interactions with other antiretrovirals, AIDS 24 (18) (2010) 2789–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kaplan FS, Shore EM, Pignolo RJ, I.C.C.o. FOP, The medical management of fibrodysplasia ossificans progressiva, Clin. Proc. Intl. Clin. Consort FOP 4 (2011) 1–100. [Google Scholar]
- [30].Neale MG, Brown K, Hodder RW, Auty RM, The pharmacokinetics of sodium cromoglycate in man after intravenous and inhalation administration, Br. J. Clin. Pharmacol. 22 (4) (1986) 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Podolsky ML, Expanded uses of cromolyn, Ann. Allergy 47 (1981) 126. [Google Scholar]
- [32].Walker SR, Evans ME, Richards AJ, Paterson JW, The fate of (14C)disodium cromoglycate in man, J. Pharm. Pharmacol. 24 (7) (1972) 525–531. [DOI] [PubMed] [Google Scholar]
- [33].Juurikivi A, Sandler C, Lindstedt KA, Kovanen PT, Juutilainen T, Leskinen MJ, Maki T, Eklund KK, Inhibition of c-kit tyrosine kinase by imatinib mesylate induces apoptosis in mast cells in rheumatoid synovia: a potential approach to the treatment of arthritis, Ann. Rheum. Dis. 64 (8) (2005) 1126–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cahill KN, Katz HR, Cui J, Lai J, Kazani S, Crosby-Thompson A, Garofalo D, Castro M, Jarjour N, DiMango E, Erzurum S, Trevor JL, Shenoy K, Chinchilli VM, Wechsler ME, Laidlaw TM, Boyce JA, Israel E, KIT inhibition by imatinib in patients with severe refractory asthma, N. Engl. J. Med. 376 (20) (2017) 1911–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Werner CM, Zimmermann SM, Wurgler-Hauri CC, Lane JM, Wanner GA, Simmen HP, Use of imatinib in the prevention of heterotopic ossification, HSS J 9 (2) (2013) 166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang H, Lindborg C, Lounev V, Kim JH, McCarrick-Walmsley R, Xu M, Mangiavini L, Groppe JC, Shore EM, Schipani E, Kaplan FS, Pignolo RJ, Cellular hypoxia promotes heterotopic ossification by amplifying BMP signaling, J. Bone Miner. Res. 31 (9) (2016) 1652–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]