Abstract
Background
The traditional general practitioner-based model (community-based rehabilitation [CBR]) for Chinese schizophrenia patients lacks sufficient content, usefulness, and theoretical basis for rehabilitation. Based on previous research, we postulate that Metacognitive Training (MCT) is effective in the community for schizophrenic patients.
Method
A randomized controlled, assessor-blinded trial was conducted. A total of 124 schizophrenia patients were recruited from Ningbo China and were randomly assigned to an intervention or a control group. A general practitioner (GP) training plan was carried out before intervention. Intervention and control groups received two CBR follow-ups once a month, while the intervention group, received an additional eight once-a-in-week session of MCT. The Positive and Negative Syndrome Scale (PANSS), and the Psychotic Symptom Rating Scales (PSYRATS) were the primary outcome instruments, while the Quality of Life Scale (SQLS) was the secondary outcome instrument.
Results
In the post-treatment between-groups assessment, the patients in the intervention group showed significantly more reductions on PSYRATS delusions, PSYRATS total, PANSS P6, PANSS core delusions, PANSS positive, PANSS negative, PANSS general and PANSS total, and a significant improvement in SQLS psychosocial aspect.
Conclusions
The study provides preliminary evidence for the usefulness of MCT as a complementary measure for community-based rehabilitation of schizophrenia patients.
Trial registration
ISRCTN, ISRCTN17333276. Registered 09 August 2020 - Retrospectively registered.
Keywords: Schizophrenia, Metacognition, General practitioner, Community rehabilitation
Background
Psychiatric disorders in the community: current challenges in China
Schizophrenia is a serious and highly disabling psychiatric disorder [1]. In China, the prevalence of psychotic disorders is 1.0% (0.8–1.1) [2], while the point prevalence of schizophrenia in urban areas is 0.68% (rural 0.35%) [3]. Over 90% of schizophrenia patients in China live with their families (in the community) rather than in a psychiatric institution [4]. There is therefore continuous demand for mental health and development (MHD) services at the community level [5].
Rehabilitation: from hospital-based to community-based
Efforts to improve rehabilitation services have intensified recently in China. For an extended period, the MHD system in China has been hospital-based. Psychiatric physicians conduct short-term rehabilitation in the hospital and follow the discharged patients via phone calls. However, due to various constraints, many of the resources in the hospitals are spent on inpatient treatments, and are not available to discharged patients. Notably, patients have little trust on community health centers and tend to seek health care at larger psychiatric hospitals [6]. However, distance prevents them from receiving hospital-based rehabilitation in several regions.
Hence, the burden of rehabilitation ultimately falls on family caregivers for patients with long-term illness [7]. Though the social burden will largely remain within the family, a supportive community-based rehabilitation (CBR) plan should be made available to help the affected families successfully care for mentally ill members [4]. In China, efforts by the government to shift the focus from hospital-based to community-based yielded plausible results [8], however, CBR services are underutilized [9].
CBR programs for mental health are weakly attached to the primary health care system in China as in other developing counties [5], and are mainly based on a three-tier system. At the municipal level, psychiatric hospital design rehabilitation solutions and pass the patient file to the district level, usually the District Center for Disease Control and Prevention (CDC). The District CDC is responsible for organizing and coordinating various resources. Patients are then assigned to a community health center (community rehabilitation), usually provided by the general practitioner (GP) at the community health center whose work generally includes follow-up and health education, such as recording the patient’s medication, symptoms, and side effects, helping patients complete their rehabilitation plan and giving lectures on mental illness. About 20 to 40 patients are assigned to a GP. Nonetheless, most patients do not have a strong sense of “being taken care of” and consider the follow-up “dispensable” and the health education “boring”. Moreover, the GPs need theoretical-based interventions for community rehabilitation.
Finding a theoretical-based intervention
There are two main goals in the CBR for mental health: supervision and rehabilitation. The latter aims to decrease psychiatric symptomatology [10]. Delusion is one of these symptoms considered to be significantly correlated with violent acts among psychiatric patients [11]. The challenge for the community is that patients with symptoms of delusion may sometimes cause engage in dangerous behaviors including assaulting others or even suicide [12] and, thus, have some severe consequences in the community.
It is believed that programs that combine active rehabilitation and medications achieve better outcomes as compared to medications alone [13]. Moritz and Woodward developed Metacognitive training (MCT) as an intervention for patients with schizophrenia [14]. Recent studies [15–19] have confirmed that MCT is exciting and can effectively change the patient’s delusional ideation [20]. The efficacy of MCT [21] in patients with schizophrenia spectrum disorder has been reported in several randomized controlled studies. Some of them showed the promising results in terms of immediate posttreatment effect [22–24] and long-term positive psychotic symptoms [16, 25–27]. Recent meta-analyses showed that the MCT can effectively improve the experience of delusions in schizophrenia patients with a small to moderate effect [28, 29]. The primary outcomes of MCT, such as a decline in positive symptoms [30], would meet the requirement of community rehabilitation for the chronic schizophrenic patients [31].
Overall, we aimed at determining whether implementation of MCT is effective under limited conditions such as trainer, recruitment, and location of courses, etc. at the community level.
About this study
The present study aimed to compare the outcomes of a combined intervention consisting of MCT and CBR with the control group receiving CBR only. The study goes further to confirm the superiority of MCT over CBR for the improvement of delusion.
Studies show that low quality of life generally associates with psychotic symptoms and comorbidities [32], cognitive impairment [33], social isolation, lack of access to environmental resources, and stigmatization of the illness [34].
Some studies have found that severe illness insight may lead to low quality of life [1, 35]. Schizophrenia patients with better insight can realistically evaluate their life and be aware of the enormous negative impact of their illness on their life conditions [36]. Given that illness insight is a target of the MCT [24], which means illness insight would be normally improved in the MCT course, we investigated whether MCT will decrease the quality of life. We also aimed to understand the changes of quality of life among schizophrenic patients pre- and post-intervention.
Finally, we expected to find the evidence of MCT feasibility on community rehabilitation, and for the government to tailor the community services by taking the MCT as a regular complement strategy to the CBR.
Method
Recruitment
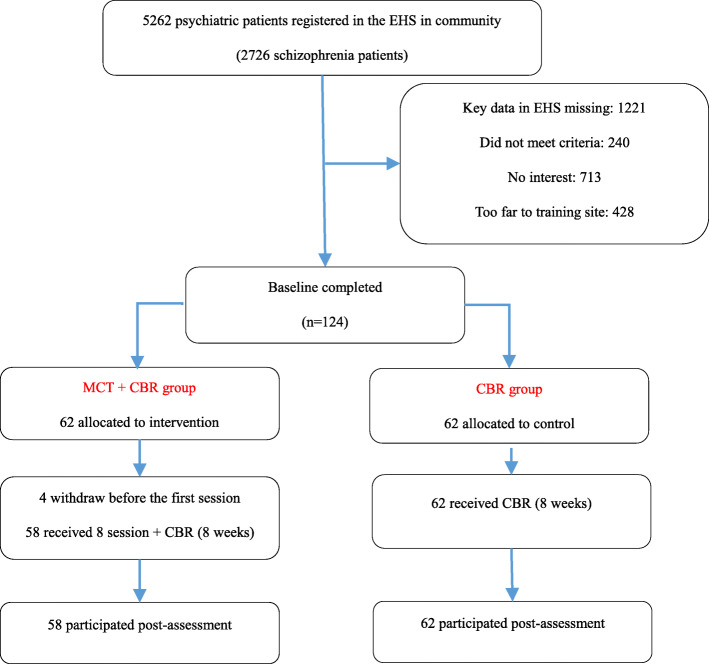
The trial was conducted at the Yinzhou District of Ningbo, China, with a population size of 1.294 million and a land area of 799.09 km2. There were 5262 registered psychiatric patients in the community in the Yinzhou Electronic Healthcare System (EHS) [37], of which 2726 had schizophrenia with a male-to-female sex ratio of 0.75:1 and an average age of 54.69 (12.14).
We first searched for all 2726 schizophrenic patients in the community who met the criteria in the Yinzhou EHS. A total of 1221 patients were excluded due to incomplete electronic records. The primary missing data in the EHS was the PANNS score. Recruitment was then conducted by a psychiatrist from Ningbo Mental Health Center who was assisted by three local GPs. A total of 713 patients had no interest in our project, 428 were too far from the training site and 240 did not meet the criteria. All EHS case data of 124 finally recruited patients were thoroughly reviewed before baseline investigation.
Design
We designed the study as a randomized controlled, assessor-blinded trial, considering some pragmatic aspects (such as flexibility in intervention delivery) to ensure generalizability of results in the community. The inclusion criteria were age 18–65, a diagnosis of schizophrenia in DSM-IV [38], and a total PANNS score between 50 and 120. Exclusion criteria included psychoactive substances and substance abuse over the last 6 months.
We established three intervention sites based on geographic location. After recruitment, patients were further divided into three groups based on their address. Patients in each group were then randomly assigned to the intervention or control group. Caregivers or patients in the intervention group were informed about the location and the schedule by independent community staff. To ensure safety, we requested a caregiver or a local community staff to accompany each patient during training. The caregiver or local staff stayed in a different room while the patient underwent training. The study was approved by the Ethics Committee of Health Commission of Ningbo (2016C05). All participants provided informed consent to participate in the study. The screening-to-inclusion ratio was 8.2% (see Fig. 1).
Fig. 1.
Flow chart
Intervention
The intervention group received CBR plus Metacognitive Training (MCT)21, Chinese ver.6.2 (see https://clinical-neuropsychology.de/metakognitives_training_psychose/). The MCT consists of 8 modules [14], which covers six cognitive and social biases (attribution biases, jumping to conclusions, belief inflexibility, overconfidence in errors, the theory of mind deficits, and depressive cognitive schemata). Each session lasted about 60 min, and a gift worth $5 was given to each patient in the intervention group after each session, the whole course lasted for 8 weeks. In our program, only four patients withdrew at the first lesson due to family reasons.
Each MCT group comprised of about ten patients, and each intervention site had two groups. Due to community constraints, we made two minor adjustments: (1) the MCT was administered once a week, although the manual guideline was twice a week. Secondly, the MCT was delivered by a trained GP at a local community health center, while the manual recommended psychologists, psychiatrists, psychiatric nurses, and occupational therapists in the institution. None of the GPs had ever attended formal training in CBT. We, therefore, designed a four-stage training plan. The first-stage was concept understanding and the underlying theory of MCT, the second-stage was MCT manual studying, the third-stage was trail lectures, and the fourth-stage was the seminar and feedback. The GP-training course lasted for 1 month. The training was conducted by an experienced psychiatrist.
Control group
The control group received a standard CBR for mental illness patients (see http://www.gov.cn/gongbao/content/2018/content_5338247.htm) for 8 weeks. In the first week, GPs together with patients and their respective families developed an individualized rehabilitation plan and a follow up plan conducted once during the project in the form of a phone call or home visits. The rehabilitation plan in CBR consisted of six aspects: medication training, relapse identification, physical management, life skills training, social skills training, and occupational rehabilitation training etc. Table 1 shows a comparison between MCT and CBR.
Table 1.
Comparison of interventions
| MCT | CBR | |
|---|---|---|
| Method | group activities | phone call, home visit, lessons |
| Frequency | once a week | once a quarter year |
| Duration | 60 min | around 30 min |
| intervention content | MCT courses | rehabilitation counseling |
Assessments
Assessments were conducted at baseline and at week 9. All ratings followed semi-structured interviews. To ensure correspondence, baseline interviews were administered by the same assessor for each patient while post-treatment assessment was performed by a different assessor. The two raters were project-independent psychiatrists from Ningbo Mental Health Center.
Psychopathological assessment
The primary target of symptom severity of delusion was assessed with the Positive and Negative Syndrome Scale (PANSS) [39], which is sensitive to the change of symptoms [40]. Since we primarily targeted delusions, to be in line with previous research [16], we computed a sub-score of delusion (sum of the following items: P1 delusions, P5 grandiosity, P6 suspiciousness/persecution), as one of the significant outcome parameters.
As items in PANSS are highly condensed, we adopted Psychotic Symptom Rating Scales (PSYRATS) [41], which consist of two subscales of hallucinations and delusions, to measure possible dissociations across different aspects of positive symptoms and the severity of the syndrome. The PSYRATS had yielded good inter-rater, re-test reliability and validity [42].
Quality of life assessment
To measure the quality of life of patients, the Schizophrenia Quality of Life Scale (SQLS) [7], which provides estimates for the aspects of psychosocial, motivation and energy, symptoms, and side effects, was administered as the secondary outcome. The internal consistency reliability and construct validity was satisfactory [7]. In the SQLS assessment, the higher the scale, the worse the quality of life.
Statistical analysis
We conducted per-protocol (pp) analyses. All statistical analyses were performed using SPSS 20.0. The patient’s baseline variables were compared between groups using independent t-tests or Chi-square tests. The repeated measure analysis of variance (ANOVA) was conducted to evaluate pre- and post-treatment effectiveness. The ANCOVA was applied to compare the effectiveness at post-treatment, with controlling for pre-treatment scores. Effect sizes were estimated using the partial eta squared (η2), the cut-off points of which were: small = 0.0099, medium = 0.0588, and large = 0.1379 [43]. All P-values are two-tailed, and P < 0.05 is considered statistically significant.
Multiple linear regression analyses (Stepwise) were performed to identify the factors that may independently contribute to quality of life. The scale change (post - pre) of three each aspect of SQLS was the dependent variable. We included group (intervention or control), sex, age, gender, age of onset, length of schizophrenia, years of formal education, marital status, length of illness, medication regimen, and improvement of several variables (PANSS core delusions, PANSS positive, PANSS negative, PANSS general, PSYRATS delusions, PSYRATS hallucinations, PSYRATS total) as dependent variables. To avoid co-linearity, we used tolerance to measure the strength of linear relationships (0.6 or above was acceptable). The 1-sample Kolmogorov-Smirnov test was performed to check the normality of the distributions for continuous variables.
Results
Baseline characteristics
Baseline characteristics for background and related psychopathological variables are shown in Table 2. There were no significant differences between the intervention and control groups. There were no significant differences in the percentage of patients who maintained the baseline medication plan between the two groups (86.20% vs. 93.55%, χ2 = 1.795, p = 0.181). Adherence was defined as the proportion of days the patients took their medication as prescribed in a month. A ratio of ≥90% was considered as regular. There was no significant difference between the two groups (79.31% vs. 67.74%, χ2 = 0.036, p = 0.849). Both groups had a chronic duration of schizophrenia (22.69 ± 12.05 vs. 29.55 ± 11.37, t = 0.217, p = 0.829), and a low level of education (6.32 ± 2.87 vs. 6.74 ± 2.46, t = 0.6.6, p = 0.547).
Table 2.
Baseline characteristics
| MCT + CBR(n = 58) | CBR(n = 62) | χ2 / t | p | |
|---|---|---|---|---|
| Age | 55.28 (9.51) | 52.90 (12.14) | 0.839 | 0.405 |
| Age of onset | 32.59 (12.05) | 29.55 (11.37) | 1.004 | 0.319 |
| Length of schizophrenia (years) | 22.69 (12.02) | 23.35 (12.70) | 0.217 | 0.829 |
| Years of formal education | 6.32 (2.87) | 6.74 (2.46) | 0.606 | 0.547 |
| Gender (male/female) | 24/34 | 24/38 | 0.089 | 0.765 |
| Marriage (Married/others) | 34/24 | 36/26 | 2.427 | 0.489 |
| Medication regimen (maintenance) | 86.20% | 93.55% | 1.795 | 0.181 |
| Taking adherence (regular) | 79.31% | 67.74% | 0.036 | 0.849 |
| Family history of Schizophrenia | 10.34% | 19.35% | 1.908 | 0.167 |
| Suffering from chronic diseases | 34.48% | 41.94% | 0.704 | 0.401 |
Outcomes
For PANSS, most of the scale assessments in the intervention group were significantly decreased at post-treatment as compared to pre-treatment, except for PANSS P5. However, in the control group, only PANSS P6, PANSS positive, PANSS negative, PANSS general, and PANSS total were significantly decreased at post-treatment.
After controlling for pre-treatment scores, a significant difference was found between the two groups in post-treatment period in terms of PANSS P6 (F (1,118) = 12.682, p = 0.001, η2partial = 0.182), PANSS core delusions (F (1,118) = 9.13, p = 0.004, η2partial = 0.138), PANSS positive syndrome (F (1,118) = 6.64, p = 0.013, η2partial = 0.104), PANSS negative (F (1,118) = 6.51, p = 0.013, η2partial = 0.102), PANSS general (F (1,118) = 12.039, p = 0.001, η2partial = 0.174), and PANSS total (F (1,118) = 11.46, p = 0.001, η2partial = 0.167).
For PSYRATS, significant effects were obtained for all three scales of the intervention group at post-treatment as compared to pre-treatment. In the control group, only PSYRATS total was significant at post-treatment compared with pre-treatment.
After controlling for pre-treatment scores, a significant difference was noted in the post-treatment period between the two groups in terms of PSYRATS delusions (F (1,118) = 4.43, p = 0.04, η2partial = 0.072), and PSYRATS total (F (1,118) = 4.32, p = 0.042, η2partial = 0.071).
In the intervention group, the three SQLS scales at post-treatment were different from those of pre-treatment. The control group was only significantly improved on SQLS symptoms and side-effects. While controlling for the pre-treatment scores, a significant difference was found in the two groups in the post-treatment in terms of the SQLS psychosocial (F (1,118) = 6.55, p = 0.013, η2partial = 0.103). See Table 3.
Table 3.
Group differences on measures of PSYRATS, PANSS, SQLS
| Variable | MCT + CBR(n = 58) | CBR(n = 62) | Post-treatment comparisons Between-group (ANCOVAs controlling for baseline scores) | ||||
|---|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | pre - post | Pre-treatment | Post-treatment | pre - post | ||
| PSYRATS delusions | 8.45 (5.35) | 6.61 (3.77) * | 1.84 | 8.00 (5.41) | 7.65 (5.08) | 0.35 | F (1,118) = 4.43, p = 0.04, η2partial = 0.072 |
| PSYRATS hallucinations | 14.03 (10.45) | 7.90 (9.17) *** | 6.13 | 13.10 (9.75) | 10.86 (9.93) | 2.24 | F (1,118) = 3.15, p = 0.081, η2partial = 0.052 |
| PSYRATS total | 22.48 (14.51) | 14.52 (11.97) ** | 7.96 | 21.10 (13.70) | 18.52 (13.28) * | 2.58 | F (1,118) = 4.32, p = 0.042, η2partial = 0.071 |
| PANSS P1 (delusions) | 2.74 (1.03) | 2.48 (0.89) ** | 0.26 | 2.66 (1.26) | 2.55 (1.06) | 0.11 | F (1,118) = 1.77, p = 0.189, η2partial = 0.030 |
| PANSS P5 (grandiosity) | 2.00 (1.10) | 1.74 (0.77) | 0.26 | 1.79 (1.05) | 1.86 (1.06) | −0.07 | F (1,118) = 2.34, p = 0.132, η2partial = 0.039 |
| PANSS P6 (suspiciousness) | 2.55 (1.63) | 1.58 (0.81) *** | 0.97 | 2.48 (1.50) | 2.24 (1.15) ** | 0.24 | F (1,118) = 12.682, p = 0.001, η2partial = 0.182 |
| PANSS core delusions | 7.29 (3.21) | 5.81 (1.80) *** | 1.48 | 6.93 (3.37) | 6.66 (2.94) | 0.27 | F (1,118) = 9.13, p = 0.004, η2partial = 0.138 |
| PANSS positive | 16.87 (6.23) | 13.61 (3.81) **** | 3.26 | 15.28 (5.89) | 14.62 (5.27) * | 0.66 | F (1,118) = 6.64, p = 0.013, η2partial = 0.104 |
| PANSS negative | 16.67 (4.45) | 14.10 (3.17) ** | 6.57 | 18.83 (5.57) | 17.24 (4.63) * | 1.59 | F (1,118) = 6.51, p = 0.013, η2partial = 0.102 |
| PANSS general | 38.16 (7.94) | 33.58 (5.34) **** | 4.58 | 39.59 (9.06) | 37.69 (6.84) ** | 1.9 | F (1,118) = 12.039, p = 0.001, η2partial = 0.174 |
| PANSS total score | 71.71 (2.72) | 61.29 (9.48) **** | 10.42 | 73.69 (17.53) | 69.55 (14.34) **** | 4.14 | F (1,118) = 11.46, p = 0.001, η2partial = 0.167 |
| SQLS psychosocial | 27.47 (12.32) | 24.3 (10.29) * | 3.17 | 26.63 (11.96) | 27.82 (10.55) | −1.19 | F (1,118) = 6.55, p = 0.013, η2partial = 0.103 |
| SQLS motivation and energy | 51.61 (14.31) | 47.93 (13.42) * | 3.68 | 59.85 (12.69) | 58.00 (12.06) | 1.85 | F (1,118) = 3.64, p = 0.062, η2partial = 0.06 |
| SQLS symptoms and side-effects | 22.28 (12.80) | 18.45 (10.56) * | 3.83 | 22.41 (15.98) | 20.37 (13.17) * | 2.04 | F (1,118) = 1.57, p = 0.216, η2partial = 0.027 |
Abbreviations: MCT Metacognitive Training, CBR Community-based rehabilitation, PANSS Positive and Negative Syndrome Scale, PSYRATS Psychotic Symptom Rating Scales, SQLS Quality of Life Scale
Significant difference from zero: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.005, ****p ≤ 0.001
The results of the multiple regression analyses conducted to identify the contributors to three aspects of SQLS are shown in Table 4. Improvement of SQLS psychosocial was independently associated with low PSYRATS hallucinations and low PANSS core delusions. Improvement of motivation and energy was independently associated with low PANSS core delusions. SQLS symptoms and side-effects were independently associated with low PSYRATS total.
Table 4.
Variables independently associated with the three aspect of SQLS in multiple linear regression analysis
| Dependent variable | Beta | T | P | 95%Confidence interval | |
|---|---|---|---|---|---|
| SQLS psychosocial | PSYRATS hallucinations | 0.305 | 3.089 | 0.003 | −0.107 to 0.503 |
| PANSS core delusions | 1.279 | 2.857 | 0.006 | 0.383 to 2.175 | |
| SQLS motivation and energy | PANSS core delusions | 0.938 | 2.051 | 0.045 | 0.022 to 1.854 |
| SQLS symptoms and side-effects | PSYRATS total | 0.317 | 4.347 | < 0.001 | 0.171 to 0.463 |
Discussion
This is the first trial conducted to examine the efficacy of MCT in community rehabilitation for patients with schizophrenia. The results confirmed our assumption that MCT can improve positive symptoms, particularly delusion symptoms [28].
By comparing several scores (PSYRATS total, PANSS positive, PANSS negative, PANSS general, and PANSS total) between pre-treatment and post-treatment, we found that the scores of both groups decreased to varying degrees, meaning, the overall psychiatric symptoms of both groups improved. It may be translated that the method taken by both groups was effective. While as in the intervention group, almost all the scales showed significant improved after the intervention, it indicated that the conventional CBR maybe effective to some extent but was not as comprehensive as CBR + MCT.
To further analyze the effect of MCT, we conducted ANCOVAs and controlled the baseline scores to compare post-treatment effects between the two groups. The PANSS core delusions showed significant improvement in intervention group when compared to the control group at post-treatment. Since the PANSS core delusions are the sum of P1, P5, and P6, we also computed the scores of each. Given that there was no significant difference in P1 and P5, the difference in PANSS core delusions may be mainly caused by P6 (suspiciousness/persecution). Some study indicated that the high risk of violence may sometimes be attributed to the delusions [44], among which patients are more likely to act on persecutory delusions [45]. Since our results show that MCT may improve the patients’ suspiciousness/persecution symptoms, we consider that MCT may decrease the risk of community violence.
The PANSS positive syndrome, PANSS negative, PANSS general, and the PANSS total scores were significantly different between the two groups at post-treatment. This showed that the MCT comprehensively improved the patient’s symptoms. A previous study [30] suggested that MCT can reduce positive symptoms in schizophrenia patients, and negative symptoms when combined with CBR at the community level. As for the reason why negative symptoms was improved, a study indicated that responses of patients to persecutory delusions may be associated with negative symptoms [45], herein, the MCT improved the persecutory delusions of the intervention group and thus affected the PANSS negative scale.
The PSYRATS scale enables a more detailed evaluation of delusions. As reported in a meta-analysis [28, 29], results of ANCOVAs confirmed that the intervention group showed significant improvements in the PSYRATS delusions and the PSYRATS total scale as compared to the control group.
The QOL of patients in the intervention group improved at post-treatment in all three dimensions: psychosocial, motivation and energy, and symptoms/side effects. The control group showed significant changes only in the symptom/side effect dimension at post-treatment. The improvement of symptoms/side effects domain maybe the result of the medication [9] and deinstitutionalization [46]. Since the percentage of patients who followed the baseline medication plan was not different between the two groups, we assumed that the improvement in symptoms/side effects was mainly because of the community intervention, either MCT or CRB.
Results of ANCOVAs analysis showed that the effect of the psychosocial dimension in SQLS of the intervention group was superior to that of the control group. In line with prior findings [24], MCT improved the quality of life, particularly its social aspects. The psychosocial dimension of SQLS mainly covers the patient’s emotional problems and attitudes towards the society and the future [7]. Thus, MCT may help patients to better control their emotions and build proper expectations for the future, especially their psychological well-being and social relationships [24]. Multiple regression analysis also showed that improvement of SQLS was independently associated with low positive psychotic symptoms. From this perspective, while MCT relieved positive symptoms, it may indirectly improve the patient’s quality of life. Previous study [47] reported that both positive and negative symptoms influence the quality of life of patients, our findings support this conclusion and again emphasize the importance of positive symptoms in determining the quality of life of schizophrenia patients in community.
In summary, our results show that MCT performed in the community, described as a hybrid of cognitive-behavioral therapy and psychoeducation [48], is effective. In China, CBR for patients with mental illness was mainly based on basic public health packages [6]. The services focus more on disease monitoring rather than community rehabilitation. Therefore, interventions that are relatively simple, exciting, and effective at using local community resources [49] are recommended.
Limitations
Community trails are usually characterized by limited resources [50]. However, flexibility in the program delivery might have influenced our findings. In our study, recruiters and patients were familiar with each other, making it easier to recruit patients and help patients adhere to the program. Good relationships may be a positive contributor to QOL in patients with prolonged illness [51], which would have a positive influence on the result. In addition, to increase patient participation in nearby MCT courses, we set up three intervention points, which may cause selection bias to some degree. Although we developed a reinforcement plan for the intervention group, some factors may affect the motivation for treatment, and may lead to higher compliance in the intervention group patient compared to the control group.
Second, the MCT courses used several Western characters and stories, which may affect understanding, and thus, influenced its effects. We shall conduct further localization research based on cultural characteristics, historical, and language characteristics.
Third, the study was limited by the shorter patient follow up. However, one of our major purposes was to find an effective intervention method for future implementation in the community. It should be noted that sustained effects for 6 months [28] and 6 months to 3 years were controversially reported [15, 52]. In the future, we may roll out regular MCT courses at the community level. The long-term effect and the effects of patients repeatedly attending these courses will be evaluated to find a more cost-benefit arrangement in the community. The developers of MCT are also exploring the possibility of online teaching [48]. Efforts should also be made to shorten the course (to reduce cost) [18].
Finally, future studies with a larger sample should take into consideration comorbid symptoms as moderator variables. Based on previous studies [53] it is expected that those with high on social anxiety and low on self-esteem will benefit most from the MCT intervention. We also suggest that the MCT app [48] should be translated into Chinese to augment its long-term effects especially in those with memory problems, for example due to comorbid neurological problems.
Conclusions
Our results show that MCT can be adopted in community rehabilitation for patients with schizophrenia.
Acknowledgements
We are much thankful to those who encouraged and supported this project.
Abbreviations
- CBR
Community-based rehabilitation
- MCT
Metacognitive Training
- GP
general practitioner
- PANSS
Positive and Negative Syndrome Scale
- PSYRATS
Psychotic Symptom Rating Scales
- SQLS
Quality of Life Scale
- MHD
mental health and development
- CDC
Center for Disease Control and Prevention
- EHS
Electronic Healthcare System
Authors’ contributions
QC contributed to the conception of the study, performed the data analyses and wrote the manuscript. YYS contributed to the GP training and helped with the manuscript writing. LFR contributed to the acquisition of data. JPW, YJC, MLZ contributed significantly to the intervention. GLB contributed to the design and manuscript. HYS contributed to the data analyses and language polishing. The authors read and approved the final version of the manuscript.
Funding
This project was supported by the Ningbo Medical Science and Technology Plan Project (2016C05). The funding was spent on sampling and community intervention.
Availability of data and materials
Data and materials of this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Ethical approval was granted by Yinzhou Center for Disease Control and Preventions’ Research Ethics Board, REC reference number 2016–11. Prior to participation, written informed consent was obtained from all participants and their legal guardians after a comprehensive explanation of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qi Chen, Email: 47229942@qq.com.
Yueyun Sang, Email: sangyueyun@sina.cn.
References
- 1.Karow A, Pajonk FG. Insight and quality of life in schizophrenia: recent findings and treatment implications. Curr Opin Psychiatry. 2006;19(6):637–641. doi: 10.1097/01.yco.0000245754.21621.c9. [DOI] [PubMed] [Google Scholar]
- 2.Phillips MR, Zhang J, Shi Q, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001–05: an epidemiological survey. Lancet. 2009;373(9680):2041–2053. doi: 10.1016/S0140-6736(09)60660-7. [DOI] [PubMed] [Google Scholar]
- 3.Chan KY, Zhao F, Meng S, et al. Prevalence of schizophrenia in China between 1990 and 2010. J Glob Health. 2015;5(1):10410. doi: 10.7189/jogh.05.010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang DF, Xu Y, Kleinman A, Kleinman J. Rehabilitation of Schizophrenia Patients in China. 2007. pp. 27–50. [Google Scholar]
- 5.Raja S, Boyce WF, Ramani S, Underhill C. Success indicators for integrating mental health interventions with community-based rehabilitation projects. Int J Rehabil Res. 2008;31(4):284–292. doi: 10.1097/MRR.0b013e3283013b0b. [DOI] [PubMed] [Google Scholar]
- 6.Yip WC, Hsiao WC, Chen W, Hu S, Ma J, Maynard A. Early appraisal of China's huge and complex health-care reforms. Lancet. 2012;379(9818):833–842. doi: 10.1016/S0140-6736(11)61880-1. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson G, Hesdon B, Wild D, Cookson R, Farina C, Sharma V, Fitzpatrick R, Jenkinson C. Self-report quality of life measure for people with schizophrenia: the SQLS. Br J Psychiatry. 2000;177:42–46. doi: 10.1192/bjp.177.1.42. [DOI] [PubMed] [Google Scholar]
- 8.Phillips MR. Can China's new mental health law substantially reduce the burden of illness attributable to mental disorders? Lancet. 2013;381(9882):1964–1966. doi: 10.1016/S0140-6736(13)61177-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang XQ, Petrini M, Morisky DE. Comparison of the Quality of Life, Perceived Stigma and Medication Adherence of Chinese with Schizophrenia: A Follow-Up Study. Arch Psychiatr Nurs. 2016;30(1):41–46. doi: 10.1016/j.apnu.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Xiong W, Phillips MR, Hu X, Wang R, Dai Q, Kleinman J, Kleinman A. Family-based intervention for schizophrenic patients in China. Brit J Psychiat. 1994;165(2):239–247. doi: 10.1192/bjp.165.2.239. [DOI] [PubMed] [Google Scholar]
- 11.Swanson JW, Borum R, Swartz MS, Monahan J. Psychotic symptoms and disorders and the risk of violent behaviour in the community. Crim Behav Ment Heal. 1996;6(4):309–329. doi: 10.1002/cbm.118. [DOI] [Google Scholar]
- 12.Freeman D, Garety P. Advances in understanding and treating persecutory delusions: a review. Soc Psych Psych Epid. 2014;49(8):1179–1189. doi: 10.1007/s00127-014-0928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falloon IR, Held T, Roncone R, Coverdale JH, Laidlaw TM. Optimal treatment strategies to enhance recovery from schizophrenia. Aust N Z J Psychiatry. 1998;32(1):43–49. doi: 10.3109/00048679809062704. [DOI] [PubMed] [Google Scholar]
- 14.Moritz S, Vitzthum F, Randjbar S, Veckenstedt R, Woodward TS. Detecting and defusing cognitive traps: metacognitive intervention in schizophrenia. Curr Opin Psychiatr. 2010;23(6):561–569. doi: 10.1097/YCO.0b013e32833d16a8. [DOI] [PubMed] [Google Scholar]
- 15.Moritz S, Veckenstedt R, Andreou C, et al. Sustained and “Sleeper” Effects of Group Metacognitive Training for Schizophrenia. Jama Psychiat. 2014;71(10):1103. doi: 10.1001/jamapsychiatry.2014.1038. [DOI] [PubMed] [Google Scholar]
- 16.Moritz S, Veckenstedt R, Bohn F, et al. Complementary group Metacognitive Training (MCT) reduces delusional ideation in schizophrenia. Schizophr Res. 2013;151(1):61–69. doi: 10.1016/j.schres.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Lam KCK, Ho CPS, Wa JC, Chan SMY, Yam KKN, Yeung OSF, Wong WCH, Balzan RP. Metacognitive training (MCT) for schizophrenia improves cognitive insight: a randomized controlled trial in a Chinese sample with schizophrenia spectrum disorders. Behav Res Ther. 2015;64:38–42. doi: 10.1016/j.brat.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Balzan RP, Delfabbro PH, Galletly CA, Woodward TS. Metacognitive training for patients with schizophrenia: preliminary evidence for a targeted, single-module programme. Australian New Zealand J Psychiatry. 2014;48(12):1126–1136. doi: 10.1177/0004867413508451. [DOI] [PubMed] [Google Scholar]
- 19.Vitzthum FB, Veckenstedt R, Moritz S. Individualized metacognitive therapy program for patients with psychosis (MCT+): introduction of a novel approach for psychotic symptoms. Behav Cogn Psychoth. 2014;42(1):105–110. doi: 10.1017/S1352465813000246. [DOI] [PubMed] [Google Scholar]
- 20.Moritz S, Woodward TS. Metacognitive training in schizophrenia: from basic research to knowledge translation and intervention. Curr Opin Psychiatr. 2007;20(6):619–625. doi: 10.1097/YCO.0b013e3282f0b8ed. [DOI] [PubMed] [Google Scholar]
- 21.Moritz S, Andreou C, Schneider BC, Wittekind CE, Menon M, Balzan RP, Woodward TS. Sowing the seeds of doubt: a narrative review on metacognitive training in schizophrenia. Clin Psychol Rev. 2014;34(4):358–366. doi: 10.1016/j.cpr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Briki M, Monnin J, Haffen E, et al. Metacognitive training for schizophrenia: a multicentre randomised controlled trial. Schizophr Res. 2014;157(1–3):99–106. doi: 10.1016/j.schres.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Gaweda L, Krezolek M, Olbrys J, Turska A, Kokoszka A. Decreasing self-reported cognitive biases and increasing clinical insight through meta-cognitive training in patients with chronic schizophrenia. J Behav Ther Exp Psychiatry. 2015;48:98–104. doi: 10.1016/j.jbtep.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Moritz S, Kerstan A, Veckenstedt R, Randjbar S, Vitzthum F, Schmidt C, Heise M, Woodward TS. Further evidence for the efficacy of a metacognitive group training in schizophrenia. Behav Res Ther. 2011;49(3):151–157. doi: 10.1016/j.brat.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Andreou C, Wittekind CE, Fieker M, Heitz U, Moritz S. Individualized metacognitive therapy for delusions: a randomized controlled rater-blind study. J Behav Ther Exp Psy. 2017;56:144. doi: 10.1016/j.jbtep.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Favrod J, Rexhaj S, Bardy S, Ferrari P, Hayoz C, Moritz S, Conus P, Bonsack C. Sustained antipsychotic effect of metacognitive training in psychosis: a randomized-controlled study. Eur Psychiat. 2014;29(5):275–281. doi: 10.1016/j.eurpsy.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Kuokkanen R, Lappalainen R, Repo-Tiihonen E, Tiihonen J. Metacognitive group training for forensic and dangerous non-forensic patients with schizophrenia: a randomised controlled feasibility trial. Criminal Behaviour Mental Health. 2014;24(5):345–357. doi: 10.1002/cbm.1905. [DOI] [PubMed] [Google Scholar]
- 28.Liu YC, Tang CC, Hung TT, Tsai PC, Lin MF. The Efficacy of Metacognitive Training for Delusions in Patients With Schizophrenia: A Meta-Analysis of Randomized Controlled Trials Informs Evidence-Based Practice. Worldviews on Evidence-Based Nursing. 2018;15(2):130–139. doi: 10.1111/wvn.12282. [DOI] [PubMed] [Google Scholar]
- 29.Eichner C, Berna F. Acceptance and efficacy of metacognitive training (MCT) on positive symptoms and delusions in patients with schizophrenia: a meta-analysis taking into account important moderators. Schizophrenia Bull. 2016;42(4):952–962. doi: 10.1093/schbul/sbv225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar D, Zia Ul Haq M, Dubey I, Dotivala KN, Veqar Siddiqui S, Prakash R, Abhishek P, Nizamie SH. Effect of meta-cognitive training in the reduction of positive symptoms in schizophrenia. Eur J Psychotherapy Counselling 2010;12(2):149–158.
- 31.Spaulding WD, Fleming SK, Reed D, Sullivan M, Storzbach D, Lam M. Cognitive Functioning in Schizophrenia: Implications for Psychiatric Rehabilitation. Schizophrenia Bull. 1999;25(2):275–289. doi: 10.1093/oxfordjournals.schbul.a033378. [DOI] [PubMed] [Google Scholar]
- 32.Awad AG, Voruganti LN. Quality of life and new antipsychotics in schizophrenia. Are patients better off? Int J Soc Psychiatry. 1999;45(4):268–275. doi: 10.1177/002076409904500405. [DOI] [PubMed] [Google Scholar]
- 33.Alptekin K, Akvardar Y, Kivircik AB, Dumlu K, Isik D, Pirincci F, Yahssin S, Kitis A. Is quality of life associated with cognitive impairment in schizophrenia? Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):239–244. doi: 10.1016/j.pnpbp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Sibitz I, Amering M, Unger A, et al. The impact of the social network, stigma and empowerment on the quality of life in patients with schizophrenia. Eur Psychiatry. 2011;26(1):28–33. doi: 10.1016/j.eurpsy.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Karow A, Pajonk FG, Reimer J, Hirdes F, Osterwald C, Naber D, Moritz S. The dilemma of insight into illness in schizophrenia: self- and expert-rated insight and quality of life. Eur Arch Psy Clin N. 2008;258(3):152–159. doi: 10.1007/s00406-007-0768-5. [DOI] [PubMed] [Google Scholar]
- 36.Xiang Y, Wang Y, Wang C, et al. Association of insight with sociodemographic and clinical factors, quality of life, and cognition in Chinese patients with schizophrenia. Compr Psychiat. 2012;53(2):140–144. doi: 10.1016/j.comppsych.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Lin H, Tang X, Shen P, Zhang D, Gao P. Using big data to improve cardiovascular care and outcomes in China: a protocol for the CHinese electronic health records research in Yinzhou (CHERRY) study. BMJ Open. 2018;8(2):e19698. doi: 10.1136/bmjopen-2017-019698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Association AP . Diagnostic and statistical manual of mental disorders (DSM-IV-TR) 1994. [Google Scholar]
- 39.Kay SR, Abraham F, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bull. 1987;2:2. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 40.Santor DA, Ascher-Svanum H, Lindenmayer JP, Obenchain RL. Item response analysis of the Positive and Negative Syndrome Scale. Bmc Psychiatry. 2007;7:66. doi: 10.1186/1471-244X-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.HADDOCK G, McCARRON J, TARRIER N, FARAGHER EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS) Psychol Med. 1999;29(4):879–889. doi: 10.1017/S0033291799008661. [DOI] [PubMed] [Google Scholar]
- 42.Drake R, Haddock G, Tarrier N, Bentall R, Lewis S. The psychotic symptom rating scales (PSYRATS): their usefulness and properties in first episode psychosis. Schizophr Res. 2007;89(1–3):119–122. doi: 10.1016/j.schres.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 43.Cohen J. Statistical power ANALYSIS for the Behavioral sciences. J Am Stat Assoc. 1988;2nd(334):499–500. [Google Scholar]
- 44.Walsh E, Gilvarry C, Samele C, et al. Predicting violence in schizophrenia: a prospective study. Schizophr Res. 2004;67(2–3):247–252. doi: 10.1016/S0920-9964(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 45.Freeman D, Garety PA, Kuipers E, Fowler D, Bebbington PE, Dunn G. Acting on persecutory delusions: the importance of safety seeking. Behav Res Ther. 2007;45(1):89–99. doi: 10.1016/j.brat.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Kunitoh N. From hospital to the community: the influence of deinstitutionalization on discharged long-stay psychiatric patients. Psychiat Clin Neuros. 2013;67(6):384–396. doi: 10.1111/pcn.12071. [DOI] [PubMed] [Google Scholar]
- 47.Browne S, Roe M, Lane A, Gervin M, Morris M, Kinsella A, Larkin C, O'Callaghan E. Quality of life in schizophrenia: its relationship to sociodemographic factors, symptomatology and tardive dyskinesia. Schizophr Res. 1996;18(2):238. doi: 10.1016/0920-9964(96)85728-8. [DOI] [PubMed] [Google Scholar]
- 48.Moritz S, Woodward TS, Balzan R. Is metacognitive training for psychosis effective? Expert Rev Neurother. 2016;16(2):105–107. doi: 10.1586/14737175.2016.1135737. [DOI] [PubMed] [Google Scholar]
- 49.Iemmi V, Kumar KS, Blanchet K, Gibson L, Kuper H. Community-based rehabilitation for people with physical and mental disabilities in low- and middle-income countries. Cochrane Db Syst Rev(Online). 2013(7).
- 50.Kelly L. RealWorld evaluation: Working under budget, time, data, and political constraints (2nd ed.). Vol 49: Routledge; 2018:117–118.
- 51.McCabe R, Roder-Wanner UU, Hoffmann K, Priebe S. Therapeutic relationships and quality of life: association of two subjective constructs in schizophrenia patients. Int J Soc Psychiatry. 1999;45(4):276–283. doi: 10.1177/002076409904500406. [DOI] [PubMed] [Google Scholar]
- 52.Andreou C, Wittekind CE, Fieker M, Heitz U, Veckenstedt R, Bohn F, Moritz S. Individualized metacognitive therapy for delusions: A randomized controlled rater-blind study. J Behav Ther Exp Psychiatry. 2017;56:144–151. doi: 10.1016/j.jbtep.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 53.Moritz S, Menon M, Andersen D, et al. Moderators of Symptomatic Outcome in Metacognitive Training for Psychosis (MCT). Who Benefits and Who Does Not? Cogn Ther Res. 2018;42:80–91.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials of this study are available from the corresponding author upon reasonable request.