Abstract
Background
Characterizing the genetic diversity of malaria parasite populations in different endemic settings (from low to high) could be helpful in determining the effectiveness of malaria interventions. This study compared Plasmodium falciparum parasite population diversity from two sites with low (pre-elimination) and high transmission in Senegal and Nigeria, respectively.
Methods
Parasite genomic DNA was extracted from 187 dried blood spot collected from confirmed uncomplicated P. falciparum malaria infected patients in Senegal (94) and Nigeria (93). Allelic polymorphism at merozoite surface protein 1 (msp1) and merozoite surface protein- 2 (msp2) genes were assessed by nested PCR.
Results
The most frequent msp1 and msp2 allelic families are the K1 and IC3D7 allelotypes in both Senegal and Nigeria. Multiplicity of infection (MOI) of greater that 1 and thus complex infections was common in both study sites in Senegal (Thies:1.51/2.53; Kedougou:2.2/2.0 for msp1/2) than in Nigeria (Gbagada: 1.39/1.96; Oredo: 1.35/1.75]). The heterozygosity of msp1 gene was higher in P. falciparum isolates from Senegal (Thies: 0.62; Kedougou: 0.53) than isolates from Nigeria (Gbagada: 0.55; Oredo: 0.50). In Senegal, K1 alleles was associated with heavy than with moderate parasite density. Meanwhile, equal proportions of K1 were observed in both heavy and moderate infection types in Nigeria. The IC3D7 subtype allele of the msp2 family was the most frequent in heavily parasitaemic individuals from both countries than in the moderately infected participants.
Conclusion
The unexpectedly low genetic diversity of infections high endemic Nigerian setting compared to the low endemic settings in Senegal is suggestive of possible epidemic outbreak in Nigeria.
Keywords: msp1, msp2, Alleles, Diversity, Nigeria, Senegal, Plasmodium falciparum
Background
Malaria caused by Plasmodium falciparum continues to be a significant public health havoc in many endemic countries in tropical and sub-tropical parts of the world [1]. Though preventable, malaria accounted for 228 million cases and a mortality of 405,000, in 2018 alone [2]. During same period, the African region contributed a significant proportion of falciparum malaria cases (93%) and mortality (94%) [2]. Though, progress has been observed in malaria control, but in recent time there has been a plateau in advancement.
Malaria in Africa, majorly cause by P. falciparum continues to affect almost all age groups at risk of the disease in endemic areas. Senegal and Nigeria are both located in western part of Africa with different levels of interventions, heterogeneity in endemicity and transmission. In Senegal, malaria prevalence is generally low but, the entire population remain at risk and transmission increases gradually from the northern to the Southern part, corresponding to hyperendemicity from the south (annual incidence > 100/1000 inhabitants) to hypoendemicity in the North (annual incidence < 5/1000 inhabitants), respectively [3]. In 2017, malaria accounted for 395 706 cases, 284 deaths and by 33.45% among children under 5 in Senegal [4], despite intensified malaria control over the last 10 years. While malaria endemicity in Nigeria varies between the six geo-political zones of the country, with south west having a mix of meso- and hypo-endemic (1–50%) situation, the south south, south east, north east and north central uniformly hypo-endemic (20–39%), while the north western part being largely meso-endemic (40–49%). Annually, 40% of Nigeria gross domestic product is spent on malaria control [5], yet the country contribute about 25% of annual global incidence rates.
Hence, an effective malaria vaccine that can be readily available to at risk individuals in endemic areas remains imperative. Nevertheless, this initiative is being impeded by the enormous parasite diversity [6–8], which renders the vaccine almost ineffective in some populations. RTS, S, the only malaria vaccine gives a 30% protection even with multiple booster doses. This has mostly been attributed to the genetic diversity of the parasite, which also affects drug efficacy.
In moderate and high transmission areas such as in Africa, the probability of a person being infected with numerous parasite clones at the same time is very high [9–13]. These clones/strains are often a reflection of the transmission intensity or endemicity of an area and as such, impacts the immune system of persons residing in endemic regions. Ultimately, the interplay between parasite clones and the immune selective pressure has a profound impact on the success of any approved vaccine. Therefore, characterization of malaria parasite populations in different endemic settings (from low to high), becomes much more needed to help in the development of a second-generation vaccines and monitor current interventions. The asexual blood stage antigens merozoite surface protein 1 and 2 (MSP1 and MSP2) along with the glutamate-rich protein (GLURP) are highly diverse antigens being exploited for vaccine advancement [14, 15]. However, they have also been used in various studies in evaluating the different circulating clones of P. falciparum and/or determining the impact and progress of malaria intervention [16, 17].
The objective of this study was to compare repeat length polymorphism and genetic diversity of P. falciparum isolates from Nigeria and Senegal using the msp1 and msp2 genes. In addition, the multiplicity of infection and heterozygosity, both of which reflect the transmission intensity as affected by intervention were evaluated.
Methods
Ethical consideration
The study from Nigeria was approved by the Institutional Review Board (IRB/16/347), Nigerian Institute of Medical Research, Lagos and Lagos State Health Service Commission. Ethical approval for Senegal was obtained by by the National Ethics Committee for Health Research of Senegal. In addition, written and/or verbal consent where applicable were obtained from all recruited participants.
Study sites and samples collection
This study was carried out in two West African countries: Nigeria and Senegal.
In Nigeria, isolates were collected in two Local Government Areas of Lagos states namely Kosofe (06° 28′ N 003° 22′ E) and Ikorodu (06° 33′ N 003° 35′ E) from December 2016–March, 2017. Description of study area has been done in an earlier study [18]. Briefly, Lagos state shares a border with the Republic of Benin and is hypo-endemic in most part with a 1.9% prevalence rate in children age 6–59 months [19]. This is in part due to the expansion of insecticide-treated nets (ITNs) coverage, and active indoor residual spraying (IRS) in many of its LGAs [20].
In Senegal, samples were collected in two areas with different endemicity levels, Kedougou located in the south-east and Thiès in western Senegal in 2016. In Kedougou, malaria transmission is seasonal from July to December, with an entomological inoculation rate (EIR) of 20 to 100 infectious bites/person/year and an incidence > 25 malaria cases per 1000 habitants. While in Thies, the malaria situation is hypo-endemic, with an average 0–20 infectious mosquito bite annually and an incidence of 5–15 malaria cases per 1000 habitants. The malaria seasonal transmission in this area coincides with rainy season which generally last up to 4 months (September to December) [3, 21].
In both countries, a purposeful sampling was employed and only febrile patients (94 from Senegal and 93 from Nigeria) visiting health facilities in these areas during malaria transmission season were recruited. Blood samples were collected on filter-paper from patients who met the following inclusion criteria: residence 15-km radius of health facilities, presence of febrile condition (axillary temperature ≥ 37.5 °C) in the previous 48 h, age ranging from 6 months to 75 years and uncomplicated P. falciparum malaria with parasite density ≥ 1000 asexual forms per microlitre. Patients who presented signs or symptoms of severe malaria as defined by World Health Organization (WHO) [2] and pregnant women were not included.
“Pre-molecular” sample processing
Care Start® P.f (Access Bio Inc, USA) malaria RDT was used to initially detect P. falciparum following the manufacturer’s instruction, and samples found positive were processed for microscopy. Thin and thick blood films were prepared for each patient’s sample following a previously described protocol [20]. Positive Giemsa stained thick smear were counted against a minimum of 500 leucocytes and parasite density(PD) estimated using the 8000 white blood cells (WBCs)/ul following the formula below:
PD = (estimated parasite count × 8000)/number WBCs
PD was further classified into low (> 500 parasite/µl), moderate (500–< 1000 parasite/µl) and high PD (> 1000 parasite/µl) as per the World Health Organization classification system.
Molecular sample processing: parasite DNA extraction, species and allelic typing of P. falciparum msp1 and msp2 genes
Parasite genomic DNA was extracted from filter paper using QIAamp DNA Mini kit (QIAGEN, USA) according to the manufacturer’s instructions. Confirmation of P. falciparum isolates was done following the Snonou protocol [21] that targets the 18S rRNA of P. falciparum isolates. PCR amplification was carried out in a total volume of 25 µl with 1 µl of extracted DNA and 2 µl of nest 1 amplicon for the primary and nested PCR respectively using the Gotaq Green Master mix (Promega) as detailed in an earlier work [16]. Only confirmed P. falciparum (corresponding to 205 bp fragment size) were processed for the polymorphic loci genotyping of P. falciparum msp1 block 2 (K1, MAD20 and RO33) and msp2 central region (IC3D7 and FC27) following a previously described nested PCR protocol [22, 23] (Additional file 1: Table S1). All PCR reactions were carried out in a final volume of 20 μl containing: 1 μl of gDNA, 6 μl GoTaq Green Master Mix (Promega), 1 μl (0.5 μM) of each primer, and 11 μl nuclease free water. In both rounds of reaction, 1 μl of gDNA and PCR amplicon were used respectively as templates for nest 1 and 2 amplifications.
Cycling conditions for both PCR were as follows: initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 58 °C for 2 min (61 °C for 2 min for the nested reaction) and extension at 72 °C for 2 min; a final extension was carried out at 72 °C for 3 min. Positives (3D7 and Dd2) and negative (DNase free water) controls were systematically incorporated in each PCR run.
The nested PCR product were resolved in 2% agarose gels electrophoresis stained with ethidium bromide and visualized under UV trans-illumination (VersaDoc®, Bio-Rad, Hercules, USA). The sizes of PCR fragments were estimated using 100 bp molecular weight ladder (Maker). Presence of more than one genotype was taken as a polyclonal infection, while a single allele was considered as a monoclonal infection. If fragment sizes were within a 20 bp interval, alleles in each family were considered the same [24].
Statistical analysis
The online Biostatgv was used for statistical analysis. The Chi square test (χ2) was used to compare the frequencies of multiclonal isolates between sites and countries. The mean multiplicity of infection (MOI) was calculated as the quotient of the total number of genotypes for each marker and the number of PCR-positive samples. Thus, MOI was calculated by dividing the total number of alleles detected for msp1 and msp2 genes by the total number of samples [25]. Student’s t test was used to compare MOI between sites.
The expected heterozygosity (He, which is a measure of genetic diversity) was employed to assess population structure of parasites. Heterozygosity was calculated using the following formula He = [n/(n − 1)] [(1 − ΣPi2)], where n = sample size, Pi = allele frequency as described by [26]. A p value of ≤ 0.05 was considered suggestive of a statistically significant difference.
Results
A total of 187 malaria infected participants were recruited for the study with almost equal proportion from the two countries (94 from Senegal and 93 from Nigeria). The mean age was not different from each country and locality within the countries. Although, participants were randomly enrolled into the study, however, more males (108) partook in the study than females (79) (Table 1).
Table 1.
Characteristics of the study participants in four different endemic sites located in the two West African countries
| Variables | Senegal | Nigeria | ||
|---|---|---|---|---|
| Thies | Kedougou | Kosofe | Ikorodu | |
| Number (%) | 47 (50) | 47 (50) | 29 (31.2) | 64 (68.8) |
| Mean age (± SD) | 26.8 (15.2) | 20.4 (13.7) | 24 (14.4) | 27.8 (18.7) |
| PD (parasite/μl) mean (range) (Min–max) | 27,411.7 (900–164,700) | 35,348.9 (1350–207,000) | 37,673.9 (34–584,000) | 24,981 (149–905,600) |
| Male | 41 (87.2) | 23 (48.9) | 16 (55.2) | 28 (43.8) |
| Female | 6 (12.8) | 24 (51.1) | 13 (44.8) | 36 (56.2) |
PD parasite density
Genetic polymorphism of msp1 and msp2 genes in Senegal and Nigeria
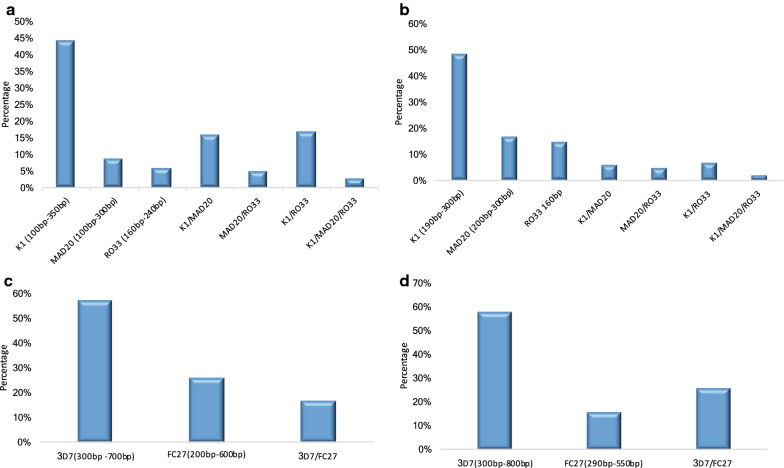
For msp1, gene diversity from the two countries were similar. K1 allelic family was predominant in Senegal and Nigeria with frequencies of 44% and 48%, respectively. RO33 allelic family was less represented in the both country with the frequency of 6% in Senegal and 15% in Nigeria. For polygenomic (complex) infections, K1/RO33 combination was the most frequent in both countries but higher in Senegal (17%), and Nigeria (7%). However, the K1/MAD20/RO33 trimorphic allelic infections were least frequent; Senegal (3%) and Nigeria (2%).
The fragment size of K1 allele type observed in Senegal (100-350 bp) was different from that seen in P. falciparum isolates from Nigeria (190-300 bp). Similar pattern was also observed with the MAD20 alleles, where sizes in Senegal ranged from 100-300 bp while those from Nigeria ranged from 200 to 300 bp. In addition, a different pattern was observed in RO33 where only one clone type was seen in Nigeria (160 bp) as against multiple clones in Senegal (160–240 bp) (Fig. 1a, b).
Fig. 1.
Prevalence of MSP 1 and 2 allele fragment sizes of P. falciparum; msp 1 in a Senegal, b Nigeria, and msp 2 in c Senegal, d Nigeria
For msp2 gene, there was no difference in the frequency of isolates with IC3D7 allele in Senegal (57%) and Nigeria (58%), while the FC27 frequency was more prevalent in Senegal (26%) than in Nigeria (16%). Similar pattern was noticed with the IC3D7/FC27 dimorphic allelic family in both countries. Allele sizes of the IC3D7 variant of msp2 ranged from 300 to 700 bp in Senegal and 300–800 bp in Nigerian P. falciparum isolates, while FC27 alleles varied in size from 200 to 600 bp in Senegal and 290–550 bp in Nigeria (Fig. 1c, d).
Multiplicity of P. falciparum infection and heterozygosity of msp1 and msp2
The presence of multiple clones in a single infection define by the MOI index was higher in both study sites in Senegal (Thies, 1.51/2.53; Kedougou, 2.2/2.0 for msp1/2) than the sites in Nigeria (Gbagada, 1.39/1.96; Oredo, 1.35/1.75). Consequently, the heterozygosity of msp1 gene was higher in P. falciparum isolates from Senegal (Thies, 0.62; Kedougou, 0.53) than isolates from Nigeria (Gbagada, 0.55; Oredo, 0.50). However, the heterozygosity of msp2 gene was not different for the two countries (Table 2).
Table 2.
Multiplicity of infection and heterozygosity of msp1 and msp2 of P. falciparum from Senegal and Nigeria
| Gene | Senegal | Nigeria | p-value | ||
|---|---|---|---|---|---|
| Thies | Kedougou | Gbagada | Oredo | ||
| msp1 | |||||
| MOI | 1.51 | 2.2 | 1.39 | 1.35 | 0.00* |
| He | 0.62 | 0.53 | 0.55 | 0.50 | 0.89 |
| msp2 | |||||
| MOI | 2.53 | 2.00 | 1.96 | 1.75 | 0.39 |
| He | 0.48 | 0.44 | 0.47 | 0.48 | 0.77 |
| MOI (both genes) | 2.65 | 2.68 | 1.64 | 1.57 | 0.72 |
MOI multiplicity of infection, He heterozygosity
* p-value less than 0.05 significant
Genetic polymorphism of msp1 and msp2 genes by parasite density
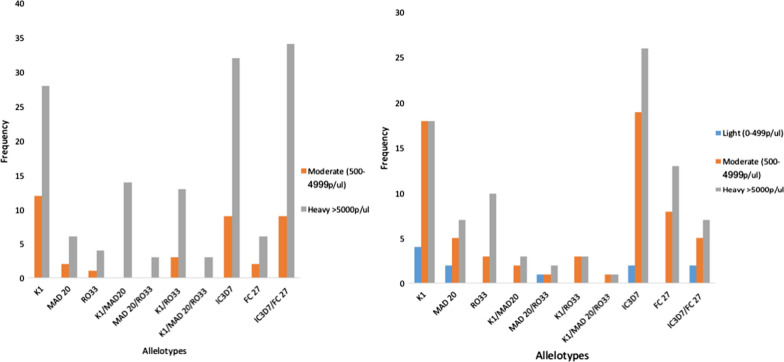
The distribution of various allele types in both msp1 and 2 in individuals infected with different parasite densities showed some level of stratification. For msp1 allele type, K1, MAD20, RO33 and their various allele combinations were not amplified in individuals with low parasitaemia infections (50–499 parasites/µl) from both countries. The K1 allele was more prevalent in individuals with higher parasitaemia infections (28) than those with moderate infections (18) from Senegal. However, in Nigeria, equal proportions of K1 were present in both moderate and high parasitaemic infections. Individuals with heavy infections from Senegal showed the highest proportion of K1/MAD20 (14) allele combination and same pattern was observed for the K1/RO33 mixture (13).
The IC3D7 subtype allele of the msp2 family showed high occurrence in high parasitaemic individuals from both countries (Senegal, 32; Nigeria, 26) than in the moderately infected participants. Similar pattern was noticed with the FC27 allele (Fig. 2a, b).
Fig. 2.
a Genetic diversity of P. falciparum msp1 and msp2 stratified by parasite density in infected individuals from Senegal. b Genetic diversity of P. falciparum msp1 and msp2 stratified by parasite density in infected individuals from Nigeria
Discussion
Control interventions targeting malaria parasite continues to face multiples hurdles from development of drug resistance in the parasite, insecticide resistance by the vectors, and unavailability of a reliable vaccine to confer the necessary protection against the parasite. Hence, there is need for continuous monitoring and evaluation of the effectiveness of control measures. Here, the diversity of P. falciparum msp 1 and msp 2 from malaria infected individual’s resident in two West African countries with significantly different levels of overall parasite endemicity; hypo-endemic area in Senegal and a meso-hyper endemic area of Nigeria was evaluated. Multiplicity of infection (MOI) index which is related to the number of clones per infection and usually associated with the level of malaria transmission [27–29] was also calculated. High MOI for both the msp1 and 2 genes were observed for both study locations in Senegal, but moderate MOI values were noted in Nigeria. Similar results for Senegal were reported by Niang et al. in 2017 [29]. This observation is unexpected as Senegal is generally categorized as a country under the malaria pre-elimination stage and as such, moderate to low MOI levels were expected. This could have several implications for the malaria control programme in Senegal: firstly, as control managers target a more focal control, parasite could be circulating and transmission going on in other not-targetted areas, subsequently, this high MOI observed and if neglected could lead to extensive parasite recombination and hence further diverse falciparum strains that could pose problems in employing the conventional control methods (use of artemisinin combination therapy). While Nigeria with more intense transmission across all regions should usually show high levels of MOI. However, the detection of low-moderate level of MOI in Nigeria shows that though transmission is high, but same clones of parasites are circulating the area. Hence, similar control strategy can be planned and implemented in the areas. Although, caution is needed when interpreting such results as samples were only collected from western Nigeria. Transmission of malaria in Kedougou is highest in Senegal and this region borders high transmission countries such as Mali and Guinea. On the other hand, the sites in Nigeria from the southern region are in proximity to the large Lagos metropolis where malaria prevalence is relatively low compared to the rest of the country. Low complexity infections have been reported in low prevalence urban dwellings across Africa.
Based on heterozygosity, which measures the level of genetic diversity at polymorphic loci, msp1 was more diverse in infections from Senegal than those from Nigeria. However, similar levels of diversity for msp2 was observed in both countries. This is in line with the differences in MOI probably due to differences in urbanization levels and recombination between genetically different clones. Indeed, there was a difference in the clonal structures of all allelic families (K1, MAD20, 3D7 and FC27) from both countries with Senegal showing the more sub-structured msp1 and msp2 populations. Nevertheless, similar trends has been observed in the Kingdom of Eswatini where a high genetic diversity was obtained in a low transmission area [30]. Circulation of different fragment sizes of K1 alleles in both countries is indicative of the presence of distinct falciparum clones occurring in both countries. Taken together, this result underscores the need for a more comprehensive evaluation of transmission using different epidemiological tools.
The high occurrence of msp1 K1 allele in high and low parasite infections from both countries has been previously observed by various studies [31], with this alleles associated more with these levels of parasite density. Whether the high parasite density is driving the selection of K1 or vice versa is yet to be determined. The strong presence of this allelic families was reported by many studies carried out in West Africa [28, 32], western Uganda [33] and Iran [34].
Similarly, high frequency of IC3D7 allele type of msp2 gene was also observed with both grades of parasite density, while no particular pattern was noticed with the FC27 allelotype. This finding is in agreement with the study of Hamid et al. in 2013 [35] where IC3D7 alleles was more predominant in moderate and heavily infected individuals.
Conclusion
Taken together, it can be concluded that evidence driving selection of these observed allelotypes in moderate and heavy P. falciparum individuals should be evaluated in a bidirectional manner and a more holistic approach should be employed in determining the true epidemiological situation of any malaria endemic country as this will help in a more targeted control measure.
Supplementary Information
Additional file 1: Table S1. Sequences of the primers used to amplify the msp 1 and msp 2 genes of P. falciparum isolates.
Acknowledgements
MAO is indebted to ECOWAS for tuition payment of her Ph.D. degree programme during which this study was conducted. We appreciate the study participants from both countries who consented for the study.
Abbreviations
- DNA
Deoxyribonucleic acid
- MOI
Multiplicity of infection
- MSP
Merozoite surface protein
- GLURP
Glutamate rich protein
- ITNs
Insecticide-treated nets
- IRS
Indoor residual spraying
- EIR
Entomological inoculation rate
- PCR
Polymerase chain reaction
Authors’ contribution
MAO and TN conceptualized study design, TN carried out laboratory assay, MAO and TN analysed experimental outcomes, MAO wrote the manuscript draft and received significant inputs from AA-N, KD. All authors read and approved the final manuscript.
Funding
This work did not receive funding from any body.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Ethics approval and consent to participate
The study was ethically approved by the Institutional Review Board of the Nigerian Institute of Medical Research, Lagos and the Senegalese National Ethics committee. Written informed consent and/or assent was obtained from the parents and guardian of children prior to recruitment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mary A. Oboh and Tolla Ndiaye contributed equally to this work
Contributor Information
Mary A. Oboh, Email: aigbi4god@gmail.com
Tolla Ndiaye, Email: ndiayetola@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-020-03563-4.
References
- 1.Blümel J, Burger R, Drosten C, Gröner A, Gürtler L, Heiden M, et al. Malaria. Transfus Med Hemother. 2009;36:48–60. doi: 10.1159/000197327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. World malaria report 2019. Geneva, World Health Organization. https://www.who.int/publications/i/item/world-malaria-report-2019.
- 3.Programme national de lutte contre le paludisme (PNLP). Bulletin épidémiologique annuel du paludisme au Senegal. Dakar. 2015. http://www.pnlp.sn/telechargements/Rapports/Bulletin-Epidemiologique-Annuel-2015-du-Paludisme-au-Senegal.pdf.
- 4.Programme national de lutte contre le paludisme (PNLP). Bulletin épidémiologique annuel du paludisme au Senegal. Dakar. 2017. https://fr.africacheck.org/wp-content/uploads/2018/04/Senegal-paludisme-bulletin-annuel-2017-PNLP.pdf.
- 5.Federal Ministry of Health. Nigeria Malaria Indicator Survey (MIS) 2015. Abuja, 2015.
- 6.Oboh MA, Idowu ET, Oyebola MK, Olukosi YA, Otubanjo O, Mafe M. Genetic diversity of Plasmodium falciparum among pregnant women in south-west Nigeria. Niger J Parasitol. 2017;38:104–110. doi: 10.4314/njpar.v38i1.19. [DOI] [Google Scholar]
- 7.Kolawole OM, Mokuolu OA, Olukosi YA, Oloyede TO. Population genomics diversity of Plasmodium falciparum in malaria patients attending Okelele Health Centre, Okelele, Ilorin, Kwara State, Nigeria. Afr Health Sci. 2016;16:704–711. doi: 10.4314/ahs.v16i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmedou-Salem MSO, Ndiaye M, Ouldabdallahi M, Lekweiry KM, Bogreau H, Konaté L, et al. Polymorphism of the merozoite surface protein-1 block 2 region in Plasmodium falciparum isolates from Mauritania. Malar J. 2014;13:26. doi: 10.1186/1475-2875-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aubouy A, Migot-nabias F, Deloron P. Polymorphism in two merozoite surface proteins of Plasmodium falciparum isolates from Gabon. Malar J. 2003;2:12. doi: 10.1186/1475-2875-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JT, Li J, Zha GC, Huang G, Huang ZX, Xie D, et al. Genetic diversity and allele frequencies of Plasmodium falciparum msp1 and msp2 in parasite isolates from Bioko Island, Equatorial. Malar J. 2018;17:458. doi: 10.1186/s12936-018-2611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apinjoh TO, Tata RB, Anchang-Kimbi JK, Chi HF, Fon EM, Mugri RN, et al. Plasmodium falciparum merozoite surface protein 1 block 2 gene polymorphism in field isolates along the slope of mount Cameroon: a cross—sectional study. BMC Infect Dis. 2015;15:309. doi: 10.1186/s12879-015-1066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyedeji SI, Awobode HO, Anumudu C, Kun J. Genetic diversity of Plasmodium falciparum isolates from naturally infected children in north-central Nigeria using the merozoite surface protein-2 as molecular marker. Asian Pac J Trop Med. 2013;6:589–594. doi: 10.1016/S1995-7645(13)60102-9. [DOI] [PubMed] [Google Scholar]
- 13.Mohammed H, Mindaye T, Belayneh M, Kassa M, Assefa A, Tadesse M, et al. Genetic diversity of Plasmodium falciparum isolates based on MSP-1 and MSP-2 genes from Kolla-Shele area, Arbaminch Zuria District, southwest Ethiopia. Malar J. 2015;14:73. doi: 10.1186/s12936-015-0604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes FJI, Richards JS. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev. 2016;40:343–372. doi: 10.1093/femsre/fuw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chauhan VS, Yazdani SS, Gaur D. Malaria vaccine development based on merozoite surface proteins of Plasmodium falciparum. Hum Vaccin. 2010;6:757–762. doi: 10.4161/hv.6.9.12468. [DOI] [PubMed] [Google Scholar]
- 16.Agyeman-budu A, Brown C, Adjei G, Adams M, Dosoo D, Dery D, et al. Trends in multiplicity of Plasmodium falciparum infections among asymptomatic residents in the middle belt of Ghana. Malar J. 2013;12:22. doi: 10.1186/1475-2875-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuesap J, Chaijaroenkul W, Ketprathum K, Tattiyapong P. Evolution of genetic polymorphisms of Plasmodium falciparum merozoite surface protein (PfMSP) in Thailand. Korean J Parasitol. 2014;52:105–109. doi: 10.3347/kjp.2014.52.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oboh MA, Badiane AS, Ntadom G, Ndiaye YD, Diongue K, Diallo MA, et al. Molecular identification of Plasmodium species responsible for malaria reveals Plasmodium vivax isolates in Duffy negative individuals from southwestern Nigeria. Malar J. 2018;17:439. doi: 10.1186/s12936-018-2588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odugbemi BA, Wright KO, Onajole AT, Kuyinu YA, Goodman OO, Odugbemi TO, et al. A malariometric survey of under—fives residing in indoor residual spraying—implementing and non-implementing communities of Lagos, Nigeria. Malar J. 2016;15:458. doi: 10.1186/s12936-016-1507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babamale O, Ugbomoiko U. Status of malaria infection in peri-urban community of north central region of Nigeria. J Bacteriol Parasitol. 2016;7:1–6. [Google Scholar]
- 21.Programme national de lutte contre le paludisme (PNLP). Cadre stratégique national de lutte contre le paludisme au Sénégal 2014–2018. http://www.pnlp.sn/wp-content/uploads/2016/08/PNLP_CADRE_STRATEGIQUE.pdf.
- 22.Snounou G, Zhu X, Spiripoon N, Jarra W, Thaithong S, Brown KN, et al. Biased distribution of msp1 and msp2 allelic variant in Plasmodium falciparum populations in Thailand. Trans R Soc Trop Med Hyg. 1999;93:369–374. doi: 10.1016/S0035-9203(99)90120-7. [DOI] [PubMed] [Google Scholar]
- 23.Papa Mze N, Ndiaye YD, Diedhiou CK, Rahamatou S, Dieye B, Daniels RF, et al. RDTs as a source of DNA to study Plasmodium falciparum drug resistance in isolates from Senegal and the Comoros Islands. Malar J. 2015;14:373. doi: 10.1186/s12936-015-0861-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammed H, Kassa M, Assefa A, Tadesse M, Kebede A. Genetic polymorphism of Merozoite Surface Protein-2 (MSP-2) in Plasmodium falciparum isolates from Pawe District, North West Ethiopia. PLoS One. 2017;12:e0177559. doi: 10.1371/journal.pone.0177559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayengue PI, Ndounga M, Davy MM, Tandou N, Ntoumi F. In vivo chloroquine resistance and prevalence of the pfcrt codon 76 mutation in Plasmodium falciparum isolates from the republic of Congo. Acta Trop. 2007;95:219–225. doi: 10.1016/j.actatropica.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978;89:583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kateera F, Nsobya SL, Tukwasibwe S, Mens PF, Hakizimana E, Grobusch MP, et al. Malaria case clinical profiles and Plasmodium falciparum parasite genetic diversity: a cross sectional survey at two sites of different malaria transmission intensities in Rwanda. Malar J. 2016;15:237. doi: 10.1186/s12936-016-1287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yavo W, Konaté A, Mawili-Mboumba DP, Kassi FK, Tshibola Mbuyi ML, Angora EK. Genetic polymorphism of msp1 and msp2 in Plasmodium falciparum isolates from Côte d’Ivoire versus Gabon. J Parasitol Res. 2016;2016:3074803. doi: 10.1155/2016/3074803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niang M, Thiam LG, Loucoubar C, Sow A, Sadio BD, Diallo M, et al. Spatio-temporal analysis of the genetic diversity and complexity of Plasmodium falciparum infections in Kédougou, Southeastern Senegal. Parasit Vectors. 2017;10:33. doi: 10.1186/s13071-017-1976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roh ME, Tessema SK, Murphy M, Nhlabathi N, Mkhonta N, Vilakati S, et al. High genetic diversity of Plasmodium falciparum in the low-transmission setting of the Kingdom of Eswatini. J Infect Dis. 2019;220:1346–1354. doi: 10.1093/infdis/jiz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghanchi NK, Hasan Z, Islam M, Beg MA. MAD 20 alleles of merozoite surface protein-1 (msp-1) are associated with severe Plasmodium falciparum malaria in Pakistan. J Microbiol Immunol Infect. 2015;48:213–218. doi: 10.1016/j.jmii.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Henry M, Diallo I, Bordes J, Ka S, Pradines B, Diatta B, et al. Urban malaria in Dakar, Senegal: chemosusceptibility and genetic diversity of Plasmodium falciparum isolates. Am J Trop Med Hyg. 2006;75:146–151. doi: 10.4269/ajtmh.2006.75.146. [DOI] [PubMed] [Google Scholar]
- 33.Peyerl-Hoffmann G, Jelinek T, Kilian A, Kabagambe G, Metzger WG, von Sonnenburg F. Genetic diversity of Plasmodium falciparum and its relationship to parasite density in an area with different malaria endemicities in West Ouganda. Trop Med Int Health. 2001;6:607–613. doi: 10.1046/j.1365-3156.2001.00761.x. [DOI] [PubMed] [Google Scholar]
- 34.Heidari A, Keshavarz H, B Rokni M, Jelinek T. Genetic diversity in merozoite surface protein msp1 and msp2 genes of Plasmodium falciparum in a major endemic region of Iran. Korean J Parasitol 2007;45:59–63. [DOI] [PMC free article] [PubMed]
- 35.Hamid MM, Mohammed SB, El Hassan IM. Genetic diversity of Plasmodium falciparum field isolates in Central Sudan inferred by PCR genotyping of merozoite surface protein 1 and 2. N Am J Med Sci. 2013;5:95–101. doi: 10.4103/1947-2714.107524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Sequences of the primers used to amplify the msp 1 and msp 2 genes of P. falciparum isolates.
Data Availability Statement
All data generated or analysed during this study are included in this published article.