Abstract
Anti-programmed cell death-1 (PD-1)/programmed death ligand-1 (PD-L1) antibodies are administered in varied human cancer types. The expression of PD-L1 within tumor cells has been identified as a predictive marker, although assessing its expression has benefitted only patients with non-small cell lung cancer (NSCLC) or head and neck cancer. Whereas, more than 75% of the patients with NSCLC showing partial response to PD-1 blockade therapy experienced long-term survival for more than 5-years Thus, identifying the responders to PD-1 blockade at early phase after its initiation is of clinical importance. The 2-deoxy-2-[fluorine-18] fluoro-D-glucose (18F-FDG) on positron emission tomography (PET) can evaluate any tumor shrinkage by assessing the metabolic tumor volume at an earlier phase than conventional modalities such as computed tomography (CT). While several reports describe the correlation of PD-L1 expression with 18F-FDG uptake rate in the tumor cells, it remains to be delineated whether this rate determined by the glucose metabolism and hypoxia is associated with the status of immune microenvironment, including the expression of PD-L1. Moreover, details of the relationship between expression of PD-L1 and 18F-FDG uptake is still unclear. Therefore, we reviewed the clinical significance of 18F-FDG uptake on PET as a predictor of the efficacy of PD-1 blockade therapy, by correlating with the expression of PD-L1, in patients with several neoplasms.
Keywords: 18F-FDG-PET, Immunotherapy, Immune checkpoint inhibitor, Human neoplasm, PD-L1
Introduction
Immune checkpoint inhibitors (ICIs) targeting programmed cell death-1 (PD-1) or programmed death ligand-1 (PD-L1) are administered to patients with varied tumor types. Although several novel ICIs are available, about 20% of the NSCLC patients who received anti-PD-1 antibody nivolumab showed low 5-year survival rate [1]. Moreover, the expression of PD-L1 in tumor cells has been described as predictor of response to therapy in patients with advanced NSCLC treated with anti-PD-1 pembrolizumab antibody [2]. However, apart from lung cancer or head and neck cancer [3, 4], the clinical significance of the PD-L1 expression as a predictive marker remains obscure. The tumor mutation burden (TMB) or peripheral blood mononuclear cells (PBMC) have been found useful for prediction of response to ICIs [5, 6]; however, except for PD-L1 expression by immunohistochemistry, biomarkers with better prognostic efficacy remain to be established.
Several recent studies have described the expression of PD-L1 within tumor cells to be closely associated with accumulation of 2-deoxy-2-[fluorine-18] fluoro-D-glucose (18F-FDG) in positron emission tomography (PET), replacing hypoxia inducible factor 1α (HIF-1α) as an alternative marker [7–15]. While the relationship between PD-L1 expression and the uptake of 18F-FDG in human neoplasms remains unclear, it is immunohistochemically supported by several studies [8–12]. The 18F-FDG-PET has been found useful for therapeutic monitoring after administration of systemic chemotherapy or molecular targeted therapy in patients with varied human cancer types, especially lung cancer [16–18]. However, only few reports describe the role of 18F-FDG-PET in therapeutic monitoring of anti-PD-1/PD-L1 antibodies. Our group has recently investigated the usefulness of 18F-FDG-PET in therapeutic evaluation at an early phase following initiation of nivolumab treatment in patients with previously treated NSCLC [19]. Our analysis suggested that 18F-FDG-PET potentially predicts precise therapeutic assessment at 1 month after nivolumab treatment, whereas, chest CT failed to differentiate a partial response from progressive disease at an early phase in the same duration. Furthermore, a prospective study is ongoing to confirm the results of our exploratory study [19].
Several reports from Western countries describe the relationship between uptake of 18F-FDG and ICIs or PD-L1 expression. Additionally, the correlation of 18F-FDG accumulation with PD-L1 expression within tumor cells reflects results similar to those obtained in previous investigation. Here, we have summarized the findings of previous reports to understand the potential of 18F-FDG-PET to predict the therapeutic potential of ICIs in human cancers.
Relationship between FDG accumulation and PD-L1 expression
The uptake of 18F-FDG by tumor cells is closely associated with glucose metabolism and hypoxia [20]. The expression of glucose transporter 1 (GLUT1) for glucose metabolism and HIF-1α in response to hypoxia play a crucial role in the accumulation of 18F-FDG, and correlate with tumor progression and spread. While the expression levels of PD-L1 are related to the therapeutic efficacy of treatment with anti-PD-1 antibody [2], studies have correlated 18F-FDG uptake with PD-L1 expression [8–10]. Moreover, it has been reported that the expression of PD-L1 is associated with GLUT1 and HIF-1α expressions in patients with pulmonary pleomorphic carcinoma and renal cell carcinoma (RCC) [11, 12]. In this section, we reviewed the relationship between 18F-FDG uptake and PD-L1 expression in cancer cells from the viewpoint of basic, pathological, and clinical evidence.
Basic research aspect
With respect to the mechanism of 18F-FDG uptake, HIF-1α is an essential factor linked to the upregulated expression of GLUT1. A recent study indicated that the increased expression of HIF-1α is associated with the enhanced expression of PD-L1, and contributes to the activation of T-cell function and mitogen-activated protein kinase (MAPK) and phosphoinositide-3-kinase (PI3K) signaling pathways [14]. Furthermore, HIF-1α directly binds to the hypoxia response element in the proximal promoter of PD-L1 and controls its expression under hypoxia [15]. These results recommend PD-L1 as an alternative target of HIF-1α, and the rate of glucose metabolism determined by HIF-1α may reflect the immune response based on the expression of PD-L1. Barsoum et al. reported that the up-regulation in expression of PD-L1 in tumor cells upon exposure to hypoxia, in vitro, increased the rate of apoptosis of cytotoxic T lymphocytes, which suggests that HIF-1α plays a crucial role in driving immune escape from cytotoxic T lymphocytes, and that the inhibition of PD-L1 expression in hypoxic tumor cells could be promising for cancer immunotherapy [21]. Moreover, Tomita et al. examined the effect of anti-PD-1 antibody on 18F-FDG uptake in an immune activated tumor system using the cyclic dinucleotide GMP-AMP (cGAMP)-injected B16F10 melanoma model [22]. Their study indicated that administration of a PD-1 inhibitor increased the rate of infiltration of immune cells into tumors and resulted in significantly lower levels of GLUT1high /hexokinase-IIhigh cells in CD45− cancer cells, suggesting that the change in glucose uptake activity is associated with a difference in levels of infiltration or activation of immune cells. They emphasized that the tumor immune microenvironment, upon treatment with PD-1 inhibitor, affects the glucose metabolism in tumor cells and influences the uptake of 18F-FDG via factors such as the immune cell infiltration, activation and composition [22]. Moreover, recent investigations described that the direct blockade of the PD-L1 in cancer cells suppresses glycolysis by inhibiting mTOR activity and expression of glycolysis enzymes [23]. Although supported by a few studies, the association between the expression of PD-L1 and 18F-FDG uptake mandates further investigation.
Clinicopathological aspect
Recent studies reported the positive correlation of PD-L1 expression with the uptake of 18F-FDG in patients with several human cancers, particularly lung cancer [7–10, 15, 24–30]. The relationship between PD-L1 expression and 18F-FDG uptake described in a review of literature is listed in Table 1. Of these 11 studies, seven were on lung cancer and four were on neoplasms originating in the colon and rectum, bladder, breast, and nasopharynx. We observed a significant correlation of 18F-FDG uptake with the expression of PD-L1 by immunohistochemistry, except in small cell lung cancer (SCLC) (Table 1). Patients with NSCLC also showed similar results, irrespective of the histological subtype or expression levels of PD-L1 [7–10, 25, 27]. Moreover, five studies performed immunohistochemical assessment of the correlation between 18F-FDG uptake and tumor infiltrative lymphocytes (TILs) [8, 9, 24, 27, 30]. While two studies indicated that the expression level of PD-L1 was not closely associated to the count of TILs, such as CD4, CD8, and Foxp3-regulatory T cells (Tregs) in NSCLC [8, 9], one study reported a statistically significant correlation between the maximum standardized uptake value (SUVmax) and expression of CD8 TILs, CD163 tumor-associated macrophages, and Tregs [27]. Kasahara et al. demonstrated that a high SUVmax on 18F-FDG-PET significantly correlated with low expression of CD4 and CD8 TILs in patients with SCLC, but not with that of Tregs and PD-L1 [24]. Hirakata et al. also examined the relationship between 18F-FDG uptake and levels of PD-L1/TILs in patients with breast cancer, and their results indicated a significant association between SUVmax and levels of CD8 TILs, and SUVmax and expression of PD-L1. Based on these evidences, the relationship between accumulation of 18F-FDG and PD-L1 expression in tumor cells appears meagre. However, the association between 18F-FDG uptake and TILs appeared to be different according to the histological grade and cancer type. Therefore, further investigation is warranted to elucidate the clinicopathological significance of 18F-FDG uptake in PET based on the number of TILs in several human neoplasms. The 18F-FDG uptake on PET based on the expression of PD-L1 has been represented in Fig. 1.
Table 1.
Relationship between PD-L1 expression and FDG uptake in review literatures
| Authors/References | Sample size | Cancer type | Histology | Correlation between FDG uptake and PD-L1 expression | Correlation of FDG uptake with TILs (statistical assessment) |
|
|---|---|---|---|---|---|---|
| p-value | PD-L1 clone | |||||
| Kasahara N / [8] | 167 | Lung cancer | SCC | 0.02 | E1L3N | Not significant |
| Kaira K / [9] | 315 | Lung cancer | AC | 0.01 | E1L3N/28–8 | Not significant |
| Takada K / [7] | 579 | Lung cancer | SCC/AC/other | < 0.001 | SP142 | NA |
| Zhang M / [10] | 84 | Lung cancer | SCC | 0.035 | 28–8 | NA |
| Kasahara N / [24] | 98 | Lung cancer | SCLC | 0.36 | E1L3N | Significant |
| Hu B / [25] | 362 | Lung cancer | SCC/AC | 0.001 | 28–8 | NA |
| Wang Y / [27] | 122 | Lung cancer | SCC/AC | 0.012 | NA | Significant |
| Jiang H / [26] | 65 | Colon cancer | AC | 0.000 | 28–8 | NA |
| Chen R / [15] | 63 | Bladder cancer | UC/SCC/SRC | 0.032 | NA | NA |
| Zhao L / [28] | 84 | NPC | SCC | < 0.001 | SP263 | NA |
| Hirakata T / [30] | 97 | Breast cancer | AC | < 0.001 | 28–8 | Significant |
Abbreviations: PD-L1 programmed death ligand-1, 18F-FDG 2-Deoxy-2-[18F] fluoro-D-glucose, TILs tumor infiltrative lymphocytes, SCC squamous cell carcinoma, AC adenocarcinoma, SCLC small cell lung cancer, UC urothelial cancer, SRC signet ring cell carcinoma, NPC nasopharyngeal carcinoma, NA not applicable
Fig. 1.
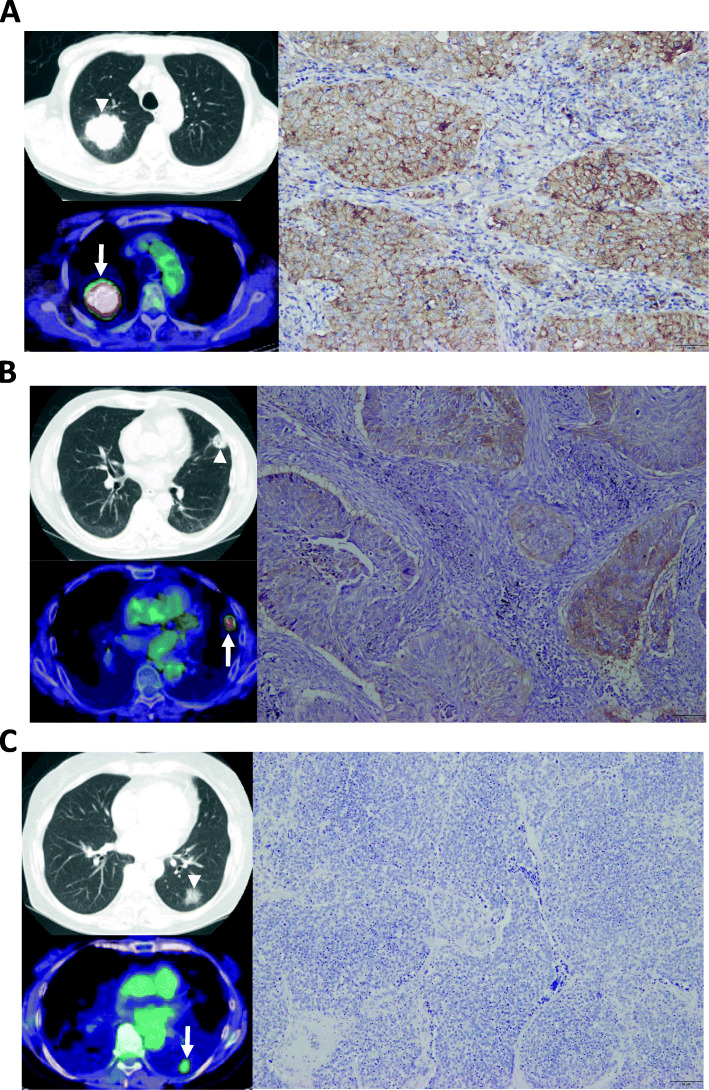
Different uptake of 18F-FDG on PET according to the expression level of PD-L1 within tumor cells. a 80-years old male with poorly differentiated adenocarcinoma (p-T2bN0M0); PET showed increased accumulation of 18F-FDG (white arrow) with SUVmax of 10.2 in the primary site corresponding to CT (white arrowhead). Immunohistochamical finding of this resected primary lesion exhibited high expression of PD-L1 with more than 50%. b 63-years old male with squamous cell carcinoma (p-T1bN0M0); moderate uptake of 18F-FDG (white arrow) with SUVmax of 5.6 was observed in the primary site corresponding to CT (white arrowhead). Moderate expression of PD-L1 with 1–49% was assessed in this resected primary site by immunohistochemistry. c 68-years female with well differentiated adenocarcinoma (p-T1bN0M0); PET revealed weak accumulation of 18F-FDG (white arrow) with SUVmax of 2.4 in the primary site corresponding to CT (white arrowhead). Immunohistochamical finding of this resected primary lesion displayed no expression of PD-L1 with less than 1%
The SUVmax is clinically utilized for evaluating the metabolic levels of 18F-FDG within the tumor cells. While SUVmax actually reflects the maximum extent of glucose metabolism within tumor cells, whether it can represent the complete metabolic tumor volume remains unclear. A recent meta-analysis found that metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are better predictive markers of 18F-FDG uptake than SUVmax [31]. In previous studies, it has been reported that the expression of PD-L1 was significantly correlated with the uptake of 18F-FDG by not only SUVmax but also TLG or MTV. However, it remains unclear whether the correlation of PD-L1 expression with SUVmax is stronger than that with TLG or MTV.
18F-FDG-PET in therapeutic monitoring of immunotherapy
The degree of effectiveness of 18F-FDG-PET to differentiate responders from non-responders at the early phase after administration of ICIs remains unclear. However, several reports are available regarding the usefulness of 18F-FDG-PET for the therapeutic monitoring of immunotherapy in patients with several human neoplasms, particularly NSCLC and malignant melanoma. In this section, we reviewed the clinical significance of 18F-FDG-PET for response evaluation before and after treatment with ICIs according to cancer types.
Malignant melanoma
The time-point for assessing the therapeutic response based on the uptake of 18F-FDG on PET before or after ICI administration remains to be validated. Seith et al. examined the potential of 18F-FDG on PET to identify complete responders to PD-1 therapy (nivolumab or pembrolizumab) at 2 weeks after its initiation in patients with metastatic melanoma [32]. They prospectively recruited 10 patients who underwent 18F-FDG-PET scan at three time points–before start of therapy, and two weeks and three months after initiation of therapy. Of these, three patients showed a partial metabolic response (PMR) at 2 weeks using 18F-FDG-PET and confirmed complete metabolic response (CMR) after 3 months. Four patients with progressive metabolic response (PMD) at 2 weeks exhibited the same response after 3 months. The results of their preliminary study suggested that 18F-FDG-PET could detect complete responders to anti-PD-1 therapy as early as 2 weeks after initiation of treatment for advanced melanoma. Further, Cho et al. prospectively investigated the prediction of response to ICIs (ipilimumab, BMS-936559, and nivolumab administered in 16, 3, and 1 patient, respectively) using 18F-FDG-PET before initiation of therapy, at days 21–28 and at 4 months [33] in 20 patients with advanced melanoma. Response evaluations performed at 21–28 days using response evaluation criteria in solid tumors (RECIST) by CT and PET evaluation criteria in solid tumors (PERCIST) by 18F-FDG-PET exhibited 75 and 70% accuracy in predicting best overall response at more than 4 months, respectively. Their study described that the combination of functional (18F-FDG-PET) and anatomical (CT) imaging at an early phase after treatment with ICIs improved the predictive potential of response to ICIs with 100% sensitivity, 93% specificity, and 95% accuracy. Additionally, several retrospective studies have elucidated the clinical predictive potential of 18F-FDG-PET [34, 35]. Annovazzi et al. observed that 18F-FDG-PET scan at 3–4 months after treatment with ICIs could accurately indicate response to treatment and predicted long-term outcome in 57 patients with metastatic melanoma (25 patients received ipilimumab and 32 patients received PD-1 inhibitors) [34]. Furthermore, a recent retrospective analysis of metastatic melanoma patients (n = 104) treated by PD-1 inhibitors demonstrated that most patients with a partial response (PR) based on the RECIST achieved CMR in 18F-FDG-PET at 1 year after initiation of treatment with a PD-1 inhibitor, and the majority of patients with CMR at 1 year exhibited continued response to treatment thereafter, suggesting the clinical utility of 18F-FDG-PET in predicting the long-term survival [35].
Non-small cell lung cancer
Our group prospectively investigated the therapeutic monitoring of 18F-FDG-PET as a predictive marker of early response after administration of nivolumab in 24 patients with previously treated NSCLC [19]. The results indicated significant efficacy of 18F-FDG uptake determined by TLG to predict the probability of a partial response (PR) (100% vs. 29%) and progressive disease (PD) (100% vs. 22.2%) at 1 month after treatment with nivolumab than that predicted by CT scans. Moreover, TLG and MTV were found to be better in appropriately assessing the metabolic activity than SUVmax by 18F-FDG uptake. A multi-institutional prospective study is currently ongoing to test the results of our preliminary study (jRCTs031180036). Further, Humbert et al. have reported the therapeutic significance of 18F-FDG-PET in the early assessment of anti-PD-1 immunotherapy (pembrolizumab and nivolumab) in patients (n = 50) with NSCLC [36], which was evaluated according to three points—at baseline, and after 7 weeks and 3 months of treatment. The analysis according to PERCIST, based on SUVmax, indicated a durable clinical benefit at 7 weeks after the treatment with 85.7% sensitivity, 62.1% specificity, and 72.0% accuracy. They also speculated that subsequent PET would be useful to identify more than 50% of the patients with an atypical response pattern among those with prior PD based on PERCIST. Next, a retrospective analysis of 28 NSCLC patients who received nivolumab evaluated the therapeutic assessment of 18F-FDG-PET before and after 2 months of treatment [37]. The sequential PET scan indicated CMR and PMR in 11 patients after 2 months of treatment, and 13 patients were identified with PMD, of whom 9 (69%) were confirmed as non-responders. In these three studies, the patients with PR on 18F-FDG-PET were found to be associated with favorable prognosis after immunotherapy [19, 36, 37], even though MTV and TLG were utilized in only one study [19]. Additionally, the potential of 18F-FDG-PET to predict the major pathological response (MPR) upon treatment with neoadjuvant anti-PD-1 antibody (sintilimab) was examined in 36 patients with resectable NSCLC [38]. The study indicated a significant correlation between the MPR and PET response based on PERCIST, and all the patients (100%) with PMR tumors were found to exhibit MPR, indicating the usefulness of 18F-FDG-PET in predicting the MPR to the neoadjuvant PD-1 blockade.
Next, several retrospective studies have described the prognostic significance of 18F-FDG uptake on PET before initiating treatment with PD-1 inhibitors [39–41]. Hashimoto et al. retrospectively investigated the prognostic significance of 18F-FDG uptake as a predictive marker before treatment with anti-PD-1 antibody in 85 patients with previously treated NSCLC [39]. The tumor metabolic activity assessed by TLG and MTV, but not SUVmax, was confirmed as a significant independent prognostic factor to predict the prognosis after treatment with PD-1 inhibitor based on the multivariate analysis [39]. Furthermore, in 109 patients with advanced NSCLC who underwent a baseline 18F-FDG-PET scan before the ICI monotherapy, MTV on PET was found to be closely associated with a worse outcome and absence of a durable clinical benefit, unlike SUVmax [40]. However, Takada et al. examined the clinical significance of pretreatment 18F-FDG-PET in recurrent patients (n = 89) treated with anti-PD-1 antibody, and the analysis indicated the average SUVmax of the responders to be significantly higher than that of the non-responders [41]. These results support the utility of SUVmax on PET as a potential predictor of therapeutic response to PD-1 inhibitors. Although several reports have suggested the predictive potential of 18F-FDG uptake, it remains unclear whether the assessment of 18F-FDG uptake based on MTV or TLG can better predict the outcome than SUVmax after initiation of treatment with ICIs. 18F-FDG-PET scan images depicting the patients’ response to the PD-1 inhibitor are shown in Figs. 2 and 3.
Fig. 2.
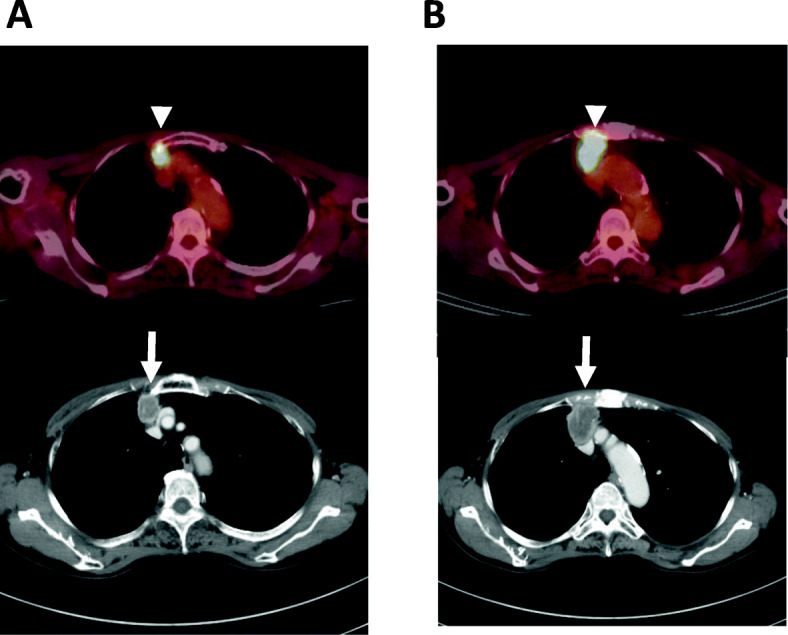
Therapeutic monitoring of 18F-FDG-PET in 71-years old male with advanced pulmonary adenocarcinoma who received pembrolizumab monotherapy as 1st line treatment. a 18F-FDG-PET/CT before pembrolizumab initiation shows primary site in the right upper lobe and hilum-mediastinal lymphadenopathy. Mediastinal lymphadenopathy before trachea revealed increased accumulation of 18F-FDG (black and white arrows) with SUVmax of 15, corresponding to that on CT scan (red arrow). b At 1 month after pmebrolizumab treatment, uptake of 18F-FDG on PET was obviously decreased with SUVmax of 3, but the morphological size of its lymphadenopathy was not changing (red arrow). c At 3 months after its treatment, there was no accumulation of 18F-FDG in primary site and lymphadenopathy (black and white arrows) with morphological shrinkage on CT scan (red arrow)
Fig. 3.
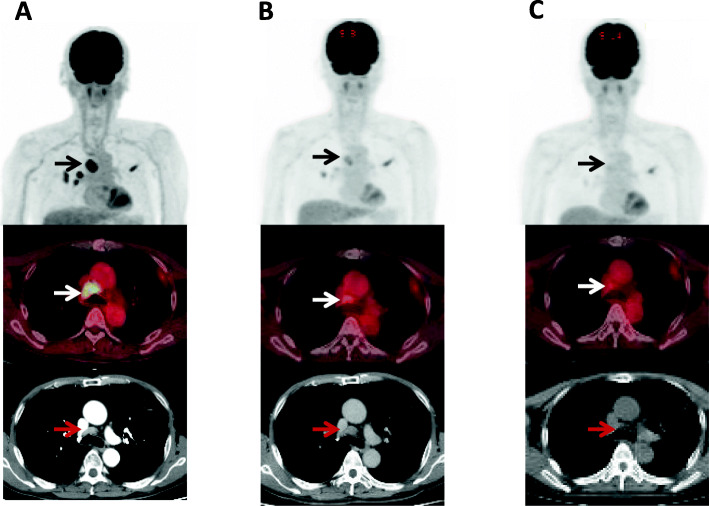
Imaging of 18F-FDG-PET in 74-years old female with advanced pulmonary adenocarcinoma who was treated with nivolumab monotherapy as 3rd line treatment. a PET imaging displayed the increased accumulation of 18F-FDG with SUVmax of 5.2 (white arrowhead) in the anterior mediastinal lymph node metastasis corresponding to CT (white arrow). b At 1 month after nivolumab initiation, the growth of the lymph node metastasis was observed on CT scan (white arrow) with increased uptake of 18F-FDG with SUVmax of 11.3 (white arrowhead)
Other malignancies
In nine patients with metastatic RCC who received nivolumab, 18F-FDG-PET was used to assess the early response at baseline and at 1 month after treatment [42]. The analysis confirmed that elevation in the uptake of 18F-FDG at 1 month was an independent predictor among therapy responders based on a multivariate logistic regression analysis. Furthermore, two studies reported that SUVmax and MTV on 18F-FDG uptake at 2 or 3 months after treatment with anti-PD-1 antibodies predict the outcome and response in patients with Hodgkin lymphoma [43, 44]. Moreover, Ferdinandus et al evaluated the PET response and survival of 27 patients with malignant mesothelioma who received 18F-FDG-PET at baseline and after at least 4 cycles pembrolizumab, and found that 18F-FDG PET metabolic volume response could predict outcome for such patients [45].
Discussion
Here, based on results of several studies, the expression of PD-L1 was found to be closely associated with the uptake of 18F-FDG on PET in different cancer types. As indicated in the basic research studies, PD-L1 was found to be a potential alternative marker of HIF-1. Moreover, the tumor glucose transporter and hypoxia were observed to be related to the expression of PD-L1 based on the immunohistochemical analysis. The aim of this review was to evaluate whether the levels of uptake of 18F-FDG could be useful for therapeutic monitoring of anti-PD-1/PD-L1 antibodies. However, the differences in the timing of 18F-FDG-PET after initiation of treatment with ICIs in each study make it difficult to predict their therapeutic efficacy. A morphological assessment using CT scan can predict the response of PD-1 inhibitor at approximately 9 weeks after its initiation. Although, since more than half of the patients who receive PD-1 inhibitor experience progressive disease at 9 weeks after its initiation, the responders should be distinguished from non-responders at early phase, such as 2 or 4 weeks after the treatment. Furthermore, two studies [19, 31] indicated that 18F-FDG-PET performed at 2 or 4 weeks after treatment with a PD-1 inhibitor permitted an early detection of responders and non-responders. Thus, these results should be validated using prospective studies to test whether the uptake level of 18F-FDG could accurately predict the efficacy at an early phase (2 or 4 weeks) after treatment with a PD-1 inhibitor.
In a recent analysis, Niemeijer et al. demonstrated that the expression of PD-1 and PD-L1 can be quantified using PET in patients with NSCLC [46]. They confirmed the safety and feasibility of in vivo molecular imaging of PD-1/PD-L1 using 18F-BMS-986192 and 89Zn-nivolumab; the tumor accumulation of 18F-BMS-986192 correlated with PD-L1 expression, while the uptake of 89Zn-nivolumab correlated with the expression of PD-1. The results of this study suggest that these PET tracers can be used to quantify the expression of PD-L1/PD-1 in studies with ICIs. Moreover, Bensch et al. reported the potential of 89Zn-atezolizumab PET imaging to assess the clinical response to PD-L1 blockade in first-in human cancer [47]. In their study, the tracer uptake of 89Zr-aterzolizumab was identified as a predictor of the response and outcome upon treatment with aterzolizumab. Thus, the PD-1/PD-L1 labeled PET tracer could serve as potential response predictor for PD-1 blockade therapy.
Conclusions
Although precise mechanism of how accumulation of 18F-FDG on PET correlates with expression of PD-L1 remains to be elucidated, the results of several independent studies are consistent irrespective of the cancer types. The uptake of 18F-FDG at an early phase after initiation of PD-1 blockade therapy could aid in therapeutic monitoring. However, the optimal timing of post-treatment 18F-FDG-PET is still obscure. Thus, further large-scale prospective studies are warranted to elucidate the novel possibility of therapeutic monitoring with 18F-FDG-PET after initiation of PD-1 inhibitor therapy.
Acknowledgements
Not applicable.
Abbreviations
- ICIs
Immune checkpoint inhibitors
- PD-1
Programmed cell death-1
- PD-L1
Programmed death ligand-1
- NSCLC
Non-small cell lung cancer
- TMB
Tumor mutation burden
- PBMC
Peripheral blood mononuclear cell
- 18F-FDG
2-deoxy-2-[fluorine-18] fluoro-D-glucose
- PET
Positron emission tomography
- CT
Computed tomography
- GLUT1
Glucose transporter 1
- HIF-1α
Hypoxia inducible factor-1α
- RCC
Renal cell carcinoma
- MAPK
Mitogen-activated protein kinase
- PI3K
Phosphoinositide-3-kinase
- cGAMP
Cyclic dinucleotide GMP-AMP
- TILs
Tumor infiltrative lymphocytes
- Tregs
Foxp3-regulatory T cells
- SUVmax
Maximum standardized uptake value
- MTV
Metabolic tumor volume
- TLG
Total lesion glycolysis
- PMR
Partial metabolic response
- CMR
Complete metabolic response
- PMD
Progressive metabolic response
- PERCIST
PET evaluation criteria in solid tumors
- RECIST
Response evaluation criteria in solid tumors
- PR
Partial response
- PD
Progressive disease
- MPR
Major pathological response
Authors’ contributions
The first author provided mainly technical input while the other two authors clinical input for the preparation of the manuscript. The authors read and approved the final manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Availability of data and materials
Not applicable for a review paper.
Ethics approval and consent to participate
Not applicable for a review paper.
Consent for publication
Not applicable.
Competing interests
KK and HK have received research grants and a speaker honorarium from Ono Pharmaceutical Company, Boehringer Ingelheim, Chugai Pharmaceutical, Taiho Pharmaceutical, Eli Lilly Japan and AstraZeneca.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gettinger S, Horn L, Jackman D, et al. Five-year follow-up of Nivolumab in previously treated advanced non-small-cell lung Cancer: results from the CA209-003 study. J Clin Oncol. 2018;36:1675–1684. doi: 10.1200/JCO.2017.77.0412. [DOI] [PubMed] [Google Scholar]
- 2.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 4.Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ready N, Hellmann MD, Awad MM, et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J Clin Oncol. 2019;37:992–1000. doi: 10.1200/JCO.18.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kagamu H, Kitano S, Yamaguchi O, et al. CD4+ T-cell Immunity in the Peripheral Blood Correlates with Response to Anti-PD-1 Therapy. Cancer Immunol Res. 2020;8:334–344. doi: 10.1158/2326-6066.CIR-19-0574. [DOI] [PubMed] [Google Scholar]
- 7.Takada K, Toyokawa G, Okamoto T, et al. Metabolic characteristics of programmed cell death-ligand 1-expressing lung cancer on 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Cancer Med. 2017;6:2552–2561. doi: 10.1002/cam4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasahara N, Kaira K, Bao P, et al. Correlation of tumor-related immunity with 18F-FDG-PET in pulmonary squamous-cell carcinoma. Lung Cancer. 2018;119:71–77. doi: 10.1016/j.lungcan.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Kaira K, Shimizu K, Kitahara S, et al. 2-Deoxy-2-[fluorine-18] fluoro-d-glucose uptake on positron emission tomography is associated with programmed death ligand-1 expression in patients with pulmonary adenocarcinoma. Eur J Cancer. 2018;101:181–190. doi: 10.1016/j.ejca.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Wang D, Sun Q, et al. Prognostic significance of PD-L1 expression and 18F-FDG PET/CT in surgical pulmonary squamous cell carcinoma. Oncotarget. 2017;8:51630–51640. doi: 10.18632/oncotarget.18257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang YL, Yang CY, Lin MW, et al. High co-expression of PD-L1 and HIF-1α correlates with tumour necrosis in pulmonary pleomorphic carcinoma. Eur J Cancer. 2016;60:125–135. doi: 10.1016/j.ejca.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Ruf M, Moch H, Schraml P. PD-L1 expression is regulated by hypoxia inducible factor in clear renal cell carcinoma. Int J Cancer. 2016;139:396–403. doi: 10.1002/ijc.30077. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Jiang CC, Jin L, et al. Regulation of PD-1:a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27:409–416. doi: 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- 14.Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R, Zhou X, Liu J, et al. Relationship between the expression of PD-1/PD-L1 and 18F-FDG uptake in bladder cancer. Eur J Nucl Med Mol Imaging. 2019;46:848–854. doi: 10.1007/s00259-018-4208-8. [DOI] [PubMed] [Google Scholar]
- 16.Kaira K, Higuchi T, Sunaga N, et al. Usefulness of 18F-α-Methyltyrosine PET for Therapeutic Monitoring of Patients with Advanced Lung Cancer. Anticancer Res. 2016;36:6481–6490. doi: 10.21873/anticanres.11247. [DOI] [PubMed] [Google Scholar]
- 17.Sunaga N, Oriuchi N, Kaira K, et al. Usefulness of FDG-PET for early prediction of the response to gefitinib in non-small cell lung cancer. Lung Cancer. 2008;59:203–210. doi: 10.1016/j.lungcan.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Lee DH, Kim SK, Lee HY, et al. Early prediction of response to first-line therapy using integrated 18F-FDG PET/CT for patients with advanced/metastatic non-small cell lung cancer. J Thorac Oncol. 2009;4:816–821. doi: 10.1097/JTO.0b013e3181a99fde. [DOI] [PubMed] [Google Scholar]
- 19.Kaira K, Higuchi T, Naruse I, et al. Metabolic activity by 18F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging. 2018;45:56–66. doi: 10.1007/s00259-017-3806-1. [DOI] [PubMed] [Google Scholar]
- 20.Kaira K, Endo M, Abe M, et al. Biologic correlation of 2-[18F]-fluoro-2-deoxy-D-glucose uptake on positron emission tomography in thymic epithelial tumors. J Clin Oncol. 2010;28:3746–3753. doi: 10.1200/JCO.2009.27.4662. [DOI] [PubMed] [Google Scholar]
- 21.Barsoum IB, Smallwood CA, Siemens DR, et al. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 22.Tomita M, Suzuki M, Kono Y, et al. Influence on [18F] FDG uptake by cancer cells after anti-PD-1 therapy in an enforced-immune activated mouse tumor. EJNMMI Res. 2020;10(1):24. doi: 10.1186/s13550-020-0608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasahara N, Kaira K, Yamaguchi K, et al. Fluorodeoxyglucose uptake is associated with low tumor-infiltrating lymphocyte levels in patients with small cell lung cancer. Lung Cancer. 2019;134:180–186. doi: 10.1016/j.lungcan.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Hu B, Chen W, Zhang Y, et al. 18F-FDG maximum standard uptake value predicts PD-L1 expression on tumor cells or tumor-infiltrating immune cells in non-small cell lung cancer. Ann Nucl Med. 2020;34:322–8. [DOI] [PubMed]
- 26.Jiang H, Zhang R, Jiang H, et al. Retrospective analysis of the prognostic value of PD-L1 expression and 18F-FDG PET/CT metabolic parameters in colorectal cancer. J Cancer. 2020;11:2864–2873. doi: 10.7150/jca.38689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Zhao N, Wu Z, et al. New insight on the correlation of metabolic status on 18F-FDG PET/CT with immune marker expression in patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2020;47:1127–1136. doi: 10.1007/s00259-019-04500-7. [DOI] [PubMed] [Google Scholar]
- 28.Zhao L, Zhuang Y, Fu K, et al. Usefulness of [18F]fluorodeoxyglucose PET/CT for evaluating the PD-L1 status in nasopharyngeal carcinoma. Eur J Nucl Med Mol Imaging. 2020;47:1065–1074. doi: 10.1007/s00259-019-04654-4. [DOI] [PubMed] [Google Scholar]
- 29.Togo M, Yokobori T, Shimizu K, et al. Diagnostic value of 18F-FDG-PET to predict the tumour immune status defined by tumoural PD-L1 and CD8+tumour-infiltrating lymphocytes in oral squamous cell carcinoma. Br J Cancer. 2020;122:1686–94. [DOI] [PMC free article] [PubMed]
- 30.Hirakata T, Fujii T, Kurozumi S, et al. FDG uptake reflects breast cancer immunological features: the PD-L1 expression and degree of TILs in primary breast cancer. Breast Cancer Res Treat. 2020;181:331–8. [DOI] [PubMed]
- 31.Im HJ, Pak K, Cheon GJ, et al. Prognostic value of volumetric parameters of (18) F-FDG PET in non-small-cell lung cancer: a meta-analysis. Eur J Nucl Med Mol Imaging. 2015;42:241–251. doi: 10.1007/s00259-014-2903-7. [DOI] [PubMed] [Google Scholar]
- 32.Seith F, Forschner A, Schmidt H, et al. 18F-FDG-PET detects complete response to PD-1 therapy in melanoma patients two weeks after therapy start. Eur J Nucl med Mol Imag. 2018;45:95–101. doi: 10.1007/s00259-017-3813-2. [DOI] [PubMed] [Google Scholar]
- 33.Cho SY, Lipson EJ, Im HJ, et al. Prediction of response to immune checkpoint inhibitor therapy using early-time-point 18F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med. 2017;58:1421–1428. doi: 10.2967/jnumed.116.188839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Annovazzi A, Vari S, Giannarelli D, et al. Comparison of 18F-FDG PET/CT criteria for the prediction of therapy response and clinical outcome in patients with metastatic melanoma treated with ipilimumab and PD-1 inhibitors. Clin Nucl Med. 2020;45:187–194. doi: 10.1097/RLU.0000000000002921. [DOI] [PubMed] [Google Scholar]
- 35.Tan AC, Emmett L, Lo S, et al. FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann Oncol. 2018;29:2115–2120. doi: 10.1093/annonc/mdy330. [DOI] [PubMed] [Google Scholar]
- 36.Humbert O, Cadour N, Paquet M, et al. 18F-FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: frequent and clinical significance of atypical evolutive patterns. Eur J Nucl Med Mol Imag. 2020;47:1158–1167. doi: 10.1007/s00259-019-04573-4. [DOI] [PubMed] [Google Scholar]
- 37.Goldfarb L, Duchemann B, Chouahnia K, et al. Monitoring anti-PD-1 based immunotherapy in non-small cell lung cancer with FDG-PET: introduction of iPERCIST. EJNMMI Res. 2019;9:8. doi: 10.1186/s13550-019-0473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao X, Li N, Wu N, et al. The efficiency of 18F-FDG PET-CT for predicting the major pathological response to the neoadjuvant PD-1 blockade in resectable non-small cell lung cancer. Eur J Nucl Med Mol Imag. 2020;47:1209–1219. doi: 10.1007/s00259-020-04711-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashimoto K, Kaira K, Yamaguchi O, et al. Potential of FDG-PET as prognostic significance after anti-PD-1 antibody against patients with previously treated non-small cell lung cancer. J Clin Med. 2020;9:725. doi: 10.3390/jcm9030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seban RD, Mezquita L, Berenbaum A, et al. Baseline metabolic tumor burden on FDG PET/CT scans predicts outcome in advanced NSCLC patients treated with immune checkpoint inhibitors. Eur J Nucl Med Mol Imag. 2020;47:1147–1157. doi: 10.1007/s00259-019-04615-x. [DOI] [PubMed] [Google Scholar]
- 41.Takada K, Toyokawa G, Yoneshima Y, et al. 18F-FDG uptake in PET/CT is a potential predictive biomarker of response to anti-PD-1 antibody therapy in non-small cell lung cancer. Sci Reports. 2019;9:13362. doi: 10.1038/s41598-019-50079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabei T, Nakaigawa N, Kaneta T, et al. Early assessment with 18F-2-fluoro-2-deoxyglucose positron emission tomography/computed tomography to predict short-term outcome in clear cell renal carcinoma treated with nivolumab. BMC Cancer. 2019;19:298. doi: 10.1186/s12885-019-5510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dercle L, Seban RD, Lazarovici J, et al. 18F-FDG PET and CT scans detect new imaging patterns of response and progression in patients with Hodgkin lymphoma treated by anti-programmed death 1 immune checkpoint inhibitor. J Nucl Med. 2018;59:14–24. doi: 10.2967/jnumed.117.193011. [DOI] [PubMed] [Google Scholar]
- 44.Chen A, Mokrane FZ, Schwartz LH, et al. Early 18F-FDG PET/CT response predicts survival in relapsed/refractory Hodgkin lymphoma treated with nivolumab. J Nucl Med. 2020;61:649–54. [DOI] [PubMed]
- 45.Ferdinandus J, Barbato F, Chodyla M, et al. Volumetric PET response assessment outperforms conventional criteria in patients receiving high-dose pembrolizumab for malignant mesothelioma. J Nucl Med. 2020;jnumed.120.245803. [DOI] [PubMed]
- 46.Niemeijer AN, Leung D, Huisman MC, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small cell lung cancer. Nat Commun. 2018;9:4664. doi: 10.1038/s41467-018-07131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bensch F, van der Veen EL, Lub-de Hooge MN, et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24:1852–1858. doi: 10.1038/s41591-018-0255-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable for a review paper.