Abstract
Background
Immunosuppression is regarded as the main cause of death induced by sepsis. Anti-programmed death-ligand 1 (PD-L1) therapy is promising in reversing sepsis-induced immunosuppression but no evidence is available on use of commercially available anti-PD-L1 medications for this indication. The present preclinical study was performed to investigate the therapeutic effect of an anti-PD-L1 nanobody (KN035) in sepsis.
Material/Methods
The level of expression of PD-L1 in PD-L1 humanized mice was confirmed with flow cytometry. Plasma concentrations of KN035 at different dosages at different time points were detected using an enzyme-linked immunosorbent assay. PD-L1 humanized mice were allocated into 4 groups: sham, cecal ligation and puncture (CLP), isotype (isotype+CLP), and PD-L1 (KN035+CLP). The 7-day survival rate was observed to investigate outcomes in CLP mice. Disease severity was assessed with histopathological scoring of mice lungs and livers. Immune status was assessed based on cell apoptosis in the spleen and bacterial clearance.
Results
PD-L1 levels were significantly elevated in peripheral lymphocytes, monocytes, and neutrophils after CLP surgery. Blood concentrations of KN035 showed that 2.5 mg/kg had potential to be an ideal dosage for KN035 therapy. Survival analysis demonstrated that KN035 was associated with significantly reduced mortality on Day 7 after surgery (P=0.0083). The histopathological tests showed that KN035 alleviated sepsis-induced injury in the lungs and liver. KN035 reduced the number of apoptotic cells in the spleen and almost eliminated bacterial colonies in the peritoneal lavage fluid from the CLP mice.
Conclusions
KN035, an anti-PD-L1 antibody, can improve the rate of survival in CLP mice and alleviate sepsis-induced apoptosis in the spleen.
MeSH Keywords: Antigens, CD274; Immunosuppression; Sepsis
Background
Sepsis, defined as infection-induced dysregulated host response and organ dysfunction, is the leading cause of death in Intensive Care Units (ICUs) [1]. Recently, some clinical trials have suggested that the sepsis survival rate has been decreasing, and the Surviving Sepsis Campaign Bundles have been widely applied in clinical settings [2–4]. However, because the incidence of sepsis is increasing, given the aging of the global population, mortality from the condition remains relatively high [5,6]. In China, the rate of mortality from sepsis is reported to be 66.7 per 100 000 population [7], which is higher than for most cancers, including brain tumors, gynecologic and kidney cancers, and lymphoma and leukemia [8].
In the 1990s, a pro-inflammatory response was considered as the main cause of death in patients with sepsis, thus, tumor necrosis factor (TNF)-α and interleukin (IL)-1 were used to treat the condition [9–11]. Unfortunately, several large-scale clinical trials of these approaches reported no positive results. Activated protein C was the next medication considered promising for improving outcomes in patients with sepsis [12], until 2 well-designed multicenter clinical trials, published in 2011, showed no mortality benefit [13,14]. Approval for use of Xigris in patients with severe sepsis then was withdrawn by the US Food and Drug Administration and the British Committee for Standards in Hematology [15]. These clinical trials remind us that we should reconsider the pathogenesis of sepsis.
Sepsis can induce profound immune disturbance, including both pro-inflammatory responses and immune tolerance reactions [16]. The function of major organs impaired by the hyperinflammation can be maintained with life support, such as mechanical ventilation, renal replacement therapy, and extracorporeal membrane oxygenation, but many patients with sepsis die from persistent nosocomial or secondary infections [17]. One autopsy study showed that profound immunosuppressive molecules were upregulated and immune cells were remarkably depleted in patients who died of sepsis [18]. Therefore, immune-stimulating therapy was considered as a new direction for treatment of the condition.
Programmed death-ligand 1 (PD-L1) is a co-inhibitory molecule that plays a role in immune tolerance in the antigen-presenting process. The process requires 2 signals, including the combination of MHC molecules and CD4/CD8, and the combination of co-stimulatory molecules. The co-stimulatory molecules are classified as positive and negative. The positive molecules include CD28, CD80, and CD86, and the negative ones are co-inhibitory molecules, including cytotoxic T-lymphocyte-associated protein 4, programmed cell death protein 1, PD-L1, and programmed death-ligand 2 [19]. Our previous study demonstrated that PD-L1 is an effective target for sepsis because the neutralizing antibody against PD-L1 can improve the survival of mice that have undergone cecal ligation and puncture (CLP) [19–21]. A subsequent study also showed that anti-PD-L1 peptide was beneficial for CLP mice [22]. Anti-PD-L1 antibody had been widely used to treat malignant tumors [23,24], but no information exists about the effect of these medications on sepsis. A study of the safety and pharmacology of BMS-936559 in severe sepsis was registered in ClinicalTrials.gov, but it was terminated because of a change in business objectives, according to the trial record on the website. KN035, a specific nanobody against PD-L1, was designed to block PD-L1 in humans, with high specificity. Several clinical trials of KN035 have been initiated in cancer, but whether it can be used to treat sepsis is unknown. The present study was performed to investigate the therapeutic role of KN035 in sepsis using a murine model of CLP.
Material and Methods
Mice and reagents
Male transgenic mice with PD-L1 humanization, aged 8 to 10 weeks and weighing 20 to 25 g, were purchased from the Shanghai Model Organisms Center, Inc. (Shanghai, China). Wild-type mice were obtained from the Animal Experimental Center of the Naval Medical University (Shanghai, China). Mice were acclimatized in our lab for at least 1 week before the experiments began. All experiments were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and performed with the approval of the Animal Care and Use Committee of our university. KN035 and the isotype antibody were produced by Alphamab Co., Ltd., Suzhou, Jiangsu, China. Other reagents were all commercially available from the respective companies.
Cecal ligation and puncture
The CLP model was performed as previously described [19,25]. In general, mice were anesthetized with sevoflurane (3%) (Hengrui, Lianyungang, Jiangsu, China), and subcutaneous butorphanol (4 mg/kg) was used for analgesia (Hengrui, Lianyungang, Jiangsu, China) [26]. The skin and peritoneal membrane were cut at the mid-line with a scissor, and the cecum was exposed with the protection of wet gauze. The cecum was then ligated by half with a 1-0 Prolene thread and punctured twice with a 22-gauge needle. Some cecal content was extruded slightly from the ligated cecum. Then, the intestine was returned to the peritoneal cavity and the abdominal wall was closed in 2 layers. The cecum was exposed without the ligation and puncture in the sham-operated mice. After surgery, the mice were resuscitated with 1 mL of subcutaneous saline. All mice were given free access to food and water after recovery from anesthesia. All mice were put to death by cervical vertebra dislocation after blood sampling by cardiac puncture or completion of the survival analysis.
KN035 administration
For pharmacokinetic analysis, wild-type C57BL/6 mice were divided into 3 groups: 1 mg/kg, 2.5 mg/kg, and 10 mg/kg (n=5 mice per group). The mice were injected intravenously (IV) via the tail vein with 1 mg/kg, 2.5 mg/kg, and 10 mg/kg of KN035, respectively. For pharmacodynamic experiments, PD-L1 humanized mice were allocated into sham, CLP, isotype+CLP, and KN035+CLP groups. At 3 h after CLP surgery, the mice in the CLP, isotype+CLP, and KN035+CLP groups were injected IV with 200 μL of saline, 2.5 mg/kg of isotype antibody, and 2.5 mg/kg of KN035, respectively. Survival analysis was performed in 10 mice per group within 7 days after surgery, and the other tests were performed in 6 mice per group at 24 h after surgery.
Flow cytometry
Flow cytometry was performed to detect the PD-L1 expression level in the PD-L1 humanized mice after CLP surgery. Blood samples were collected by heart puncture and anticoagulated in a BD Vacutainer (BD, Franklin Lakes, New Jersey, U.S.A.) 24 h after surgery. The white blood cells were stained with anti-CD3-APC, anti-CD11b-FITC, anti-Ly6C-APC, anti-Ly6G-APC, and anti-PD-L1-PE antibodies (purchased from eBioscience, San Jose, California, U.S.A.). Flow cytometry assays were performed with a FACSCalibur Flow Cytometer (BD Biosciences, Heidelberg, Germany) and the data were analyzed with FlowJo 7.6 software (Tree Star, Ashland, Oregon, U.S.A.). The level of PD-L1 expression was shown as the percentage of PD-L1-positive cells.
Pharmacokinetic assay
For the pharmacokinetic assay, an enzyme-linked immunosorbent assay (ELISA) plate (MaxiSorp NUNC-immuno plate, Thermo Scientific, Rockford, Illinois, U.S.A.) embedded with 3 μg/mL PD-L1-muFc was prepared. Plasma isolated from the mice injected with KN035 was incubated in the plates and anti-human immunoglobulin G (Fc-specific) peroxidase antibody produced in a goat was added as the secondary antibody. A standard curve was drawn using standard KN035 solution to calculate the concentration of plasma KN035.
Histopathological test
The lungs and livers were harvested 24 h after surgery and fixed in 4% paraformaldehyde. Paraffin sections were prepared and stained with hematoxylin-eosin (HE). The scoring criteria used were as previously described [25]. Generally, for lungs, 0, normal tissue; 1, minimal inflammatory change; 2, no obvious damage to the lung architecture; 3, thickening of the alveolar septae; 4, formation of nodules or areas of pneumonitis that distorted the normal architecture; and 5, total obliteration of the field. For livers: 0, none; 1, individual cell necrosis; 2, up to 30% lobular necrosis; 3, up to 60% lobular necrosis; 4, more than 60% lobular necrosis. The slides were examined by 2 pathologists who were unaware of the study groups.
TdT-mediated dUTP nick-end labeling assay
The TdT-mediated dUTP nick-end labeling (TUNEL) assay was performed to detect apoptosis in the spleen, which is a common feature of sepsis-induced immunosuppression. An In-Situ Cell Death Detection Kit (Roche, Basel, Switzerland) was used for the TUNEL assay. Each spleen sample was fixed in paraffin section and deparaffinized with xylene, and then hydrated with ethanol and pretreated with proteinase K. The endogenous peroxidase was blocked with 3% H2O2 and the sections were incubated in a terminal transferase reaction mixture. Tris-buffered saline solution was used as a negative control. Finally, the sections were incubated with streptavidin-HRP, colorized with diaminobenzidine, and counterstained with bis-benzamide. The level of apoptosis was expressed as the average number of TUNEL-positive cells per field.
Bacterial clearance in peritoneal lavage fluid
Peritoneal lavage fluid (PLF) was harvested by infusing 2 mL of phosphate-buffered saline into the peritoneal cavity 24 h after surgery and serially diluting it to 104- and 106-fold. A total of 100 μL of the diluted PLF was spread on tryptic soy agar blood agar plates and incubated at 37°C for 24 h. The colonies were counted and expressed as colony forming units (CFUs) per mL.
Statistical analyses
GraphPad Prism 6.0 software (San Diego, California, U.S.A.) was used for most of the statistical analyses. The exception was the comparison of blood concentrations of KN035, which was performed with the PROC NLMIXED method with Bonferoni correction using SAS 9.4 software (Cary, North Carolina, U.S.A.). Normal distribution of continuous data was shown as a mean±standard deviation (SD) and compared using t tests in 2 groups, whereas in 3 or more groups, a comparison was performed using analysis of variance. Histopathological scores were compared using Kruskal-Wallis tests followed by Dunn’s multiple comparison test for intergroup comparisons. Kaplan-Meier survival curves with Bonferroni correction were created for survival analysis. P<0.05 was considered statistically significant.
Results
PD-L1 expression was elevated in the main immune cells in CLP mice
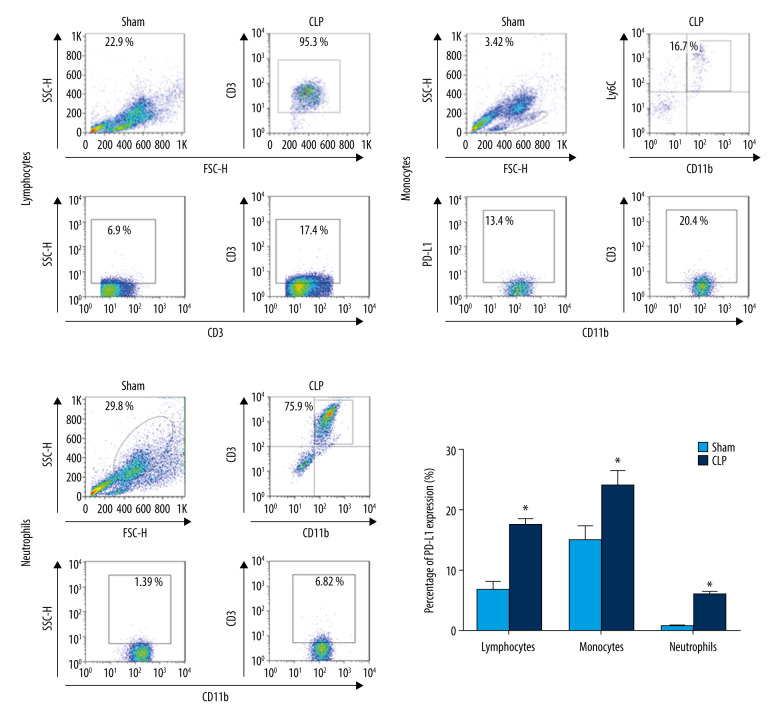
The experiment was designed based on the theory that PD-L1 was upregulated in immune cells during sepsis. Therefore, PD-L1 expression from CD3-positive T lymphocytes; CD11b-positive, Ly6C-positive monocytes; and CD11b-positive, Ly6G-positive neutrophils was assessed in the transgenic mice with humanized PD-L1. Similar to our previous studies with wild-type mice [19,21], PD-L1 was significantly upregulated significantly in lymphocytes, monocytes, and neutrophils 24 h after CLP surgery (Figure 1).
Figure 1.
Anti-programmed death-ligand 1 (PD-L1) expression is upregulated in peripheral lymphocytes, monocytes, and neutrophils from septic mice with PD-L1 humanization at 24 h after cecal ligation and puncture (CLP) surgery. Data shown as mean±standard deviation (SD) (n=6 per group). P<0.05 vs. sham group. CLP – cecal ligation and puncture.
Pharmacokinetics of KN035 in mice
To determine the dose of KN035 for treating sepsis, the plasma concentration was measured with ELISA at 1.5 h, 24 h, 48 h, 5 days, and 7 days, after IV injection of 1 mg/kg, 2.5 mg/kg, and 10 mg/kg of KN035. The peak concentration of KN035 occurred at 1.5 h at all 3 doses (Figure 2). The interindividual variability was greater at higher doses, with higher standard deviations. The plasma concentrations in mice were significantly different among the mice treated with the 3 different doses (P<0.001). Our previous studies showed that 10 μg/mL of anti-PD-L1 antibody was enough to reverse sepsis-induced dysfunction in T cells and monocytes in vitro [19]. The concentration of KN035 was close to 10 μg/mL within 5 days after injection into mice of the 2.5 mg/kg and 10 mg/kg dosages; therefore, a final dose of 2.5 mg/kg was chosen for the following experiments.
Figure 2.
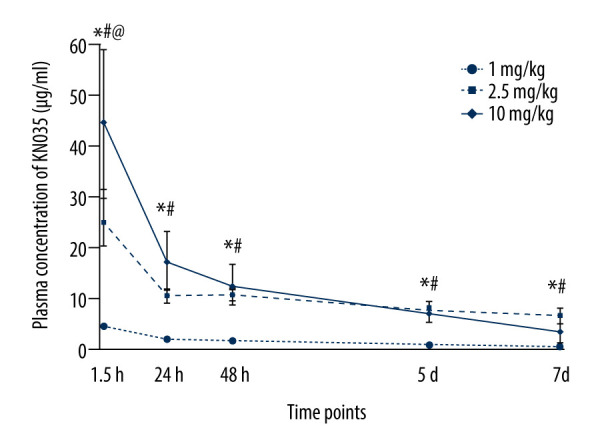
Blood concentration of KN035 after intravenous injection at 3 different dosage in mice. Data shown as mean±standard deviation (n=5 per group). * P<0.05 for 1 mg/kg vs. 2.5 mg/kg, # P<0.05 for 1 mg/kg vs. 10 mg/kg, @ P<0.05 for 2.5 mg/kg vs. 10 mg/kg.
KN035 improved the survival rate and alleviated organ injury in CLP mice
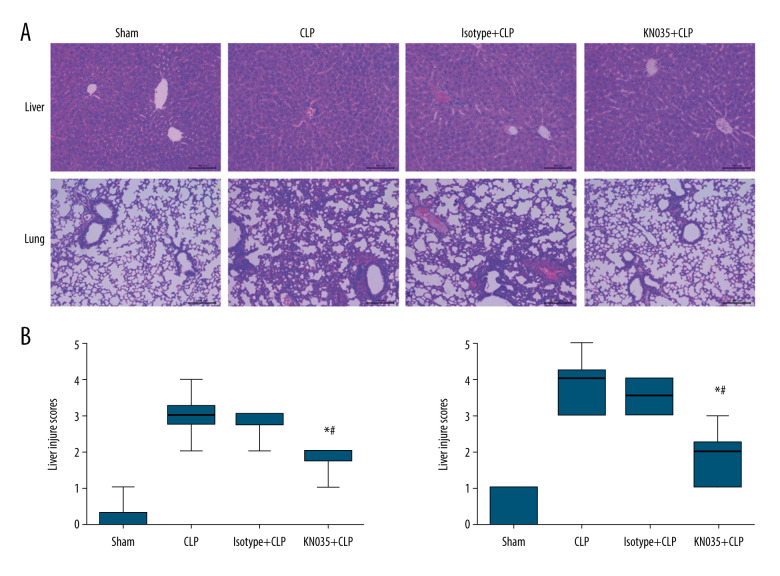
Seven-day survival is the criterion standard for determining the therapeutic effect of a drug against sepsis. Sepsis resulted in an 80% mortality rate in the CLP mice, and the 7-day survival rate was improved with the KN035 injection, but not the isotype antibody (P<0.0083). Interestingly, the comparison between the sham and KN035+CLP groups showed no significant difference (P=0.146) (Figure 3). Histopathological tests were performed to evaluate the effect of KN035 on sepsis-induced lung and liver injury. HE staining showed that the injury scores for lung and liver in the KN035+CLP group were both significantly lower than those in the CLP and isotype+CLP groups (P<0.05, KN035+CLP group vs. CLP group and isotype+CLP group), but remained a bit higher than in the sham group (Figure 4).
Figure 3.
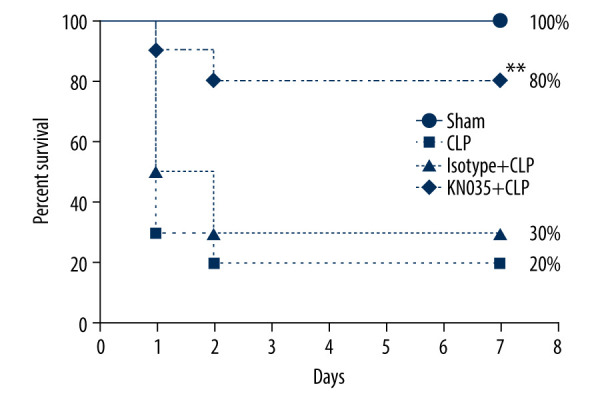
KN035 improved the survival rate in cecal ligation and puncture (CLP) mice. N=10 per group. * P=0.0083 vs. CLP group and isotype group. CLP – cecal ligation and puncture.
Figure 4.
(A, B) KN035 alleviated the sepsis-induced organ injury in the lungs and livers of cecal ligation and puncture (CLP) mice. Data shown as median, minimum, and maximum values (n=6 per group). * P<0.05 vs. CLP group and isotype group, # P<0.05 vs. sham group. CLP – cecal ligation and puncture.
KN035 reversed sepsis-induced apoptosis in the spleen and enhanced bacterial clearance
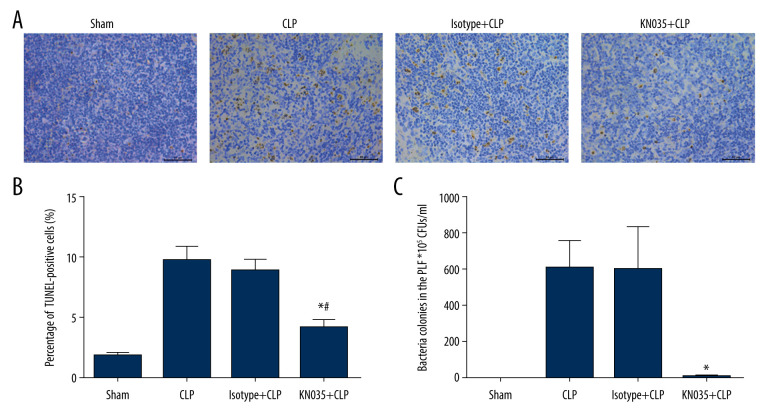
Sepsis-induced immunosuppression has been demonstrated in association with lymphocyte apoptosis in the thymus and spleen [27]. Thus, we assessed the extent of apoptosis in the spleen cells with a TUNEL assay (P<0.05) (Figure 5A, 5B). The percent of TUNEL-positive cells in the spleen was significantly lower in the KN035+CLP group than in the CLP and isotype+CLP groups.
Figure 5.
(A–C) KN035 reversed sepsis-induced apoptosis in the spleen (×400) and enhanced the bacterial clearance capacity. Data shown as mean±standard deviation (n=6 per group). * P<0.05 vs. Cecal ligation and puncture group and isotype group, # P<0.05 vs. sham group. CLP – cecal ligation and puncture; PLF – peritoneal lavage fluid.
Immunosuppression also can be validated by inability to clear bacteria in the abdominal cavity of CLP mice, which could be the cause of death when sepsis progresses. Bacterial cultural of PLF showed that KN035 almost eliminated bacterial reproduction, while the number of bacterial colonies remained rather high in the CLP and isotype+CLP groups (P<0.05), even after the PLF was diluted. The bacterial load in PLF in the KN035 group was comparable to that in the sham group (Figure 5C). The bacterial load in the blood was also assessed, but no bacterial growth was found in samples from any of the groups.
Discussion
The present study demonstrated that KN035, an anti-human PD-L1 nanobody, was capable of reversing apoptosis in immune cells and compromised bacterial clearance induced by sepsis, and thus could improve the survival rate and attenuate organ injury in a murine model of severe sepsis. The immune-stimulating effect was shown by the reduced apoptosis in the spleen and diminished bacteria in the PLF of CLP mice. The overall beneficial effect of KN035 on sepsis was confirmed by the improved 7-day survival rate and decreased injury score in the lungs and livers.
PD-L1 is an inhibitory co-stimulating factor involved in T cell responses. These responses require binding of MHC-II molecules and T cell receptors and a second signal transmitted by the binding of CD28 to CD80/CD86. The second signal may be inhibitory, so as to prevent overactivation of T cells, such as the PD-L1/PD-1 pathway. The inhibitory co-stimulating factors are involved in the immunological tolerance or immune escape in tumor pathogenesis. Therefore, the use of anti-PD-L1 antibody is becoming one of the most promising therapies against cancer [28,23]. Similar to tumor immune evasion, infection may also induce immunosuppression during sepsis, as shown by the increased apoptosis and dysfunction in immune cells. Our previous study showed that PD-L1 also was upregulated in lymphocytes, monocytes, and neutrophils in patients with sepsis and in CLP mice, and that anti-PD-L1 antibody could reverse sepsis-induced lymphocyte apoptosis and monocyte dysfunction in vitro and improve the survival of and bacterial clearance in CLP mice [20,21]. Similarly, our present study showed that PD-L1 was upregulated in lymphocytes, monocytes, and neutrophils in PD-L1-humanized mice subjected to CLP surgery. Therefore, PD-L1 could be an important participating factor and a potential therapeutic target for sepsis-induced immunosuppression. In our previous in vitro study, we demonstrated that 10 μg/mL of anti-PD-L1 antibody could reverse sepsis-induced dysfunction in lymphocytes and monocytes. Therefore, we assessed blood concentrations after IV injection of 3 different dosages of KN035 and determined that 2.5 mg/kg could achieve a blood concentration of 10 μg/mL.
Several anti-PD-L1 antibodies have been developed for tumor treatment, including atezolizumab [29], avelumab [30], and durvalumab [31]. In some patients with advanced cancer, these medications have shown great success in increasing survival time. In some patients with early-stage cancer, anti-PD-L1 also has been demonstrated to reduce rates of recurrence and mortality [32]. In addition, anti-PD-L1 antibody is useful for reversing PD-L1-mediated immune tolerance, which is also involved in immunosuppressive responses during sepsis.
KN035, which was derived from a single-domain antibody and developed to block the interaction between PD-L1 and PD1, has a lower molecular weight than normal monoclonal antibodies; this may result in more favorable physiochemical properties for it in promotion of anti-tumor activity. KN035 has an affinity for PD-L1 that is almost 1000-fold that of PD-1’s affinity for PD-L1 [33]. Thus, KN035 is quite promising in reversing PD-L1/PD-1-mediated immunosuppression. Its potential anti-tumor activity is being studied in several clinical trials in the United States, Japan, and China (NCT03101488, NCT03248843, NCT02827968). Its effect in sepsis-induced immunosuppression, however, has never been investigated. The present study showed that KN035 could improve survival of CLP mice and reverse sepsis-induced apoptosis of splenic cells and impaired bacterial clearance. These data provide important preclinical information to support future clinical trials. In accordance with its immune function, KN035 treatment resulted in higher rates of survival and less severe injury in the liver and lungs of CLP mice, demonstrating its ability to promote early elimination of bacteria and to prevent infection-related organ injury and mortality.
Performing clinical trials of treatments for sepsis remains challenging, as demonstrated by the fact that no positive results have been obtained with anti-TNF-α, anti-IL-1 receptor, granulocyte colony-stimulating factor, or granulocyte macrophage colony-stimulating factor (GM-CSF) [9–11,34]. Therefore, we believe that individualization of immunomodulatory therapy for sepsis is extremely important. Meisel et al. [34] used the monocyte HLA-DR level as a marker for immune status to assess treatment with GM-CSF. Although the sample size in their study was not large enough to identify a significant difference in mortality, they did find that GM-CSF was associated with lower Acute Physiology and Chronic Health Evaluation-II scores and shorter hospitalizations and intensive care unit stays in immunosuppressed patients who had HLA-DR levels <8000 monoclonal antibodies per cell. Therefore, immune-stimulatory therapy for sepsis should be carefully tailored and screening performed on patients to document immunocompromise. In addition, the criterion standard for diagnosing sepsis-induced immunosuppression should be established by several large-scale, multicenter, observational studies. We also may need to determine a cut-off value of PD-L1 level for distinguishing immunocompromise in patients with sepsis. Having that value could be extremely important for future clinical trials investigating the therapeutic effect of KN035 in immunosuppressed patients with sepsis.
Conclusions
KN035, an anti-PD-L1 nanobody, is protective in the pathogenesis of sepsis by reversing apoptosis of immune cells and dysfunction in bacterial clearance.
Acknowledgments
The authors sincerely thank Prof. Xiao-fei Ye of the Department of Health Statistics, Naval Medical University, Shanghai, China, for his kind and generous assistance with their statistical analysis.
Footnotes
Conflicts of interest
None.
Source of support: This work was supported by grants from the National Natural Science Foundation of China (81801955) and Pujiang Talent Program (16PJD002)
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevenson EK, Rubenstein AR, Radin GT, et al. Two decades of mortality trends among patients with severe sepsis: A comparative meta-analysis. Crit Care Med. 2014;42:625–31. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarzkopf D, Fleischmann-Struzek C, Ruddel H, et al. A risk-model for hospital mortality among patients with severe sepsis or septic shock based on German national administrative claims data. PLoS One. 2018;13:e0194371. doi: 10.1371/journal.pone.0194371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018;44:925–28. doi: 10.1007/s00134-018-5085-0. [DOI] [PubMed] [Google Scholar]
- 5.Alvaro-Meca A, Jimenez-Sousa MA, Micheloud D, et al. Epidemiological trends of sepsis in the twenty-first century (2000–2013): An analysis of incidence, mortality, and associated costs in Spain. Popul Health Metr. 2018;16:4. doi: 10.1186/s12963-018-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer N, Harhay MO, Small DS, et al. Temporal trends in incidence, sepsis-related mortality, and hospital-based acute care after sepsis. Crit Care Med. 2018;46:354–60. doi: 10.1097/CCM.0000000000002872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weng L, Zeng XY, Yin P, et al. Sepsis-related mortality in China: A descriptive analysis. Intensive Care Med. 2018;44:1071–80. doi: 10.1007/s00134-018-5203-z. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 9.Fisher CJ, Jr, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334:1697–702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 10.Abraham E, Wunderink R, Silverman H, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273:934–41. [PubMed] [Google Scholar]
- 11.Fisher CJ, Jr, Dhainaut JF, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271:1836–43. [PubMed] [Google Scholar]
- 12.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 13.Annane D, Timsit JF, Megarbane B, et al. Recombinant human activated protein C for adults with septic shock: A randomized controlled trial. Am J Respir Crit Care Med. 2013;187:1091–97. doi: 10.1164/rccm.201211-2020OC. [DOI] [PubMed] [Google Scholar]
- 14.Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 15.Mitka M. Drug for severe sepsis is withdrawn from market, fails to reduce mortality. JAMA. 2011;306:2439–40. doi: 10.1001/jama.2011.1755. [DOI] [PubMed] [Google Scholar]
- 16.Delano MJ, Ward PA. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol Rev. 2016;274:330–53. doi: 10.1111/imr.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutchins NA, Unsinger J, Hotchkiss RS, et al. The new normal: Immunomodulatory agents against sepsis immune suppression. Trends Mol Med. 2014;20:224–33. doi: 10.1016/j.molmed.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JF, Li JB, Zhao YJ, et al. Up-regulation of programmed cell death 1 ligand 1 on neutrophils may be involved in sepsis-induced immunosuppression: An animal study and a prospective case-control study. Anesthesiology. 2015;122:852–63. doi: 10.1097/ALN.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Li J, Lou J, et al. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care. 2011;15:R70. doi: 10.1186/cc10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Zhou Y, Lou J, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14:R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shindo Y, McDonough JS, Chang KC, et al. Anti-PD-L1 peptide improves survival in sepsis. J Surg Res. 2017;208:33–39. doi: 10.1016/j.jss.2016.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Jiang CC, Jin L, et al. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27:409–16. doi: 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- 24.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J, Wang J, Sheng Y, et al. Baicalin improves survival in a murine model of polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis. PLoS One. 2012;7:e35523. doi: 10.1371/journal.pone.0035523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsukamoto A, Iimuro M, Sato R, et al. Effect of midazolam and butorphanol premedication on inhalant isoflurane anesthesia in mice. Exp Anim. 2015;64:139–45. doi: 10.1538/expanim.14-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. 2018;14:121–37. doi: 10.1038/nrneph.2017.165. [DOI] [PubMed] [Google Scholar]
- 28.Yasunaga M. Antibody therapeutics and immunoregulation in cancer and autoimmune disease. Semin Cancer Biol. 2020;64:1–12. doi: 10.1016/j.semcancer.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–15. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–50. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Hu L. Immunomodulators targeting the PD-1/PD-L1 protein–protein interaction: From antibodies to small molecules. Med Res Rev. 2019;39:265–301. doi: 10.1002/med.21530. [DOI] [PubMed] [Google Scholar]
- 33.Zhang F, Wei H, Wang X, et al. Structural basis of a novel PD-L1 nanobody for immune checkpoint blockade. Cell Discov. 2017;3:17004. doi: 10.1038/celldisc.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meisel C, Schefold JC, Pschowski R, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: A double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–48. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]