Abstract
BACKGROUND
Sepsis is a severe clinical syndrome related to the host response to infection. The severity of infections is due to an activation cascade that will lead to an auto amplifying cytokine production: The cytokine storm. Hemoadsorption by CytoSorb® therapy is a new technology that helps to address the cytokine storm and to regain control over various inflammatory conditions.
AIM
To evaluate prospectively CytoSorb® therapy used as an adjunctive therapy along with standard of care in septic patients admitted to intensive care unit (ICU).
METHODS
This was a prospective, real time, investigator initiated, observational multicenter study conducted in patients admitted to the ICU with sepsis and septic shock. The improvement of mean arterial pressure and reduction of vasopressor needs were evaluated as primary outcome. The change in laboratory parameters, sepsis scores [acute physiology and chronic health evaluation (APACHE II) and sequential organ failure assessment (SOFA)] and vital parameters were considered as secondary outcome. The outcomes were also evaluated in the survivor and non-survivor group. Descriptive statistics were used; a P value < 0.05 was considered to be statistically significant.
RESULTS
Overall, 45 patients aged ≥ 18 and ≤ 80 years were included; the majority were men (n = 31; 69.0%), with mean age 47.16 ± 14.11 years. Post CytoSorb® therapy, 26 patients survived and 3 patients were lost to follow-up. In the survivor group, the percentage dose reduction in vasopressor was norepinephrine (51.4%), epinephrine (69.4%) and vasopressin (13.9%). A reduction in interleukin-6 levels (52.3%) was observed in the survivor group. Platelet count improved to 30.1% (P = 0.2938), and total lung capacity count significantly reduced by 33% (P < 0.0001). Serum creatinine and serum lactate were reduced by 33.3% (P = 0.0190) and 39.4% (P = 0.0120), respectively. The mean APACHE II score was 25.46 ± 2.91 and SOFA scores was 12.90 ± 4.02 before initiation of CytoSorb® therapy, and they were reduced significantly post therapy (APACHE II 20.1 ± 2.47; P < 0.0001 and SOFA 9.04 ± 3.00; P = 0.0003) in the survivor group. The predicted mortality in our patient population before CytoSorb® therapy was 56.5%, and it was reduced to 48.8% (actual mortality) after CytoSorb® therapy. We reported 75% survival rate in patients given treatment in < 24 h of ICU admission and 68% survival rates in patients given treatment within 24-48 h of ICU admission. In the survivor group, the average number of days spent in the ICU was 4.44 ± 1.66 d; while in the non-survivor group, the average number of days spent in ICU was 8.5 ± 15.9 d. CytoSorb® therapy was safe and well tolerated with no adverse events reported.
CONCLUSION
CytoSorb® might be an effective adjuvant therapy in stabilizing sepsis and septic shock patients. However, it is advisable to start the therapy at an early stage (preferably within 24 h after onset of septic shock).
Keywords: Acute physiology and chronic health evaluation score, Hemadsorption, Sepsis, Sequential organ failure assessment score, Vasopressor
Core Tip: This prospective, real time, observational multicenter study was conducted in 45 patients with sepsis and septic shock. Post therapy, 26 patients survived and dose reduction in norepinephrine, epinephrine and vasopressin was 51.4%, 69.4% and 13.9%, respectively. Interleukin-6 level reduction was 52.3%, and platelet count improved significantly to 30.1%. Mean acute physiology and chronic health evaluation and sequential organ failure assessment scores were reduced significantly. Predicted mortality before CytoSorb® therapy was 56.5%, and mortality reduced to 48.8% after CytoSorb® therapy. The survival rate in patients given treatment in < 24 h of intensive care unit admission was 75% and 68% when given within 24-48 h of intensive care unit admission. CytoSorb® therapy was safe and well tolerated with no adverse events reported.
INTRODUCTION
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection with a high mortality rate, ranging from 30%-50% or more[1]. Extracorporeal cytokine hemoadsorption attenuates the overwhelming inflammatory response in sepsis and helps in immunomodulation[1,2]. Septic shock is defined as sepsis with hyperlactataemia and concurrent hypotension[3,4]. In intensive care units (ICUs), sepsis is a leading cause of death and the 11th leading cause of death overall. In India, more than one million estimated new cases of sepsis are treated in ICUs each year, accounting for one out of every four patients in the ICUs. A recent study conducted by the Indian Society of Critical Care across 17 states of India in 4209 patients (the Indian intensive care case mix and practice patterns study) reported mortality as high as 46% in patients with septic shock and 42.2% overall in septic patients, compared with 17.8% mortality for ICU patients who did not develop sepsis[5].
The management of patients with septic shock includes early resuscitation with fluid and vasopressor therapy, support by mechanical ventilation, renal replacement therapy and appropriate antibiotic initiation[6]. An initial goal to treat patients with septic shock and sepsis is to maintain mean arterial pressure (MAP) and cardiac output. Patients who fail to respond to adequate fluid resuscitation are prescribed vasopressors [norepinephrine (NE), dopamine, epinephrine (E), vasopressin (V), phenylephrine] and inotropes (dobutamine, milrinone) in order to maintain hemodynamic parameters[7]. These agents help to maintain adequate blood pressure and organ perfusion. However, they can have substantial adverse effects like profound vasoconstriction, causing hypoperfusion and arrhythmic events[8]. Thus, their optimized use is crucial. CytoSorb® is an International Science Organization 10993 biocompatible device that is approved in the United States under International Science Organization 13485 certification. It is also approved as an extracorporeal cytokine adsorber in the European Union and marketed in 29 countries across the globe for all the indications that are associated with high cytokine levels[9-11]. It is a CE-approved hemoadsorption device designed to remove excess levels of inflammatory mediators like cytokines and other mid-molecular weight molecules through size selective removal and surface adsorption[12,13]. Unlike metabolic approaches to anti-inflammation, CytoSorb® is able to capture directly and reduce mid-molecular weight inflammatory mediators (approximately 10-60 kDa) in blood, including both pro- and anti-inflammatory cytokines, chemokines and bacterial exotoxins[14]. It is reported to work most effectively when treatment is initiated within 24 h of diagnosed sepsis[15]. As a result of adsorption of inflammatory metabolites like cytokines it is inferred that hemodynamic and metabolic stabilization will follow[16].
In addition to standard treatment, including renal replacement and cardiac support, recent studies showed promising results with the use of extracorporeal cytokine hemoadsorption therapy[17-20]. CytoSorb® therapy along with standard of care is also utilized in the treatment of allergic reactions, burn injuries, and liver and pulmonary failure. Other potential indications for use of CytoSorb® therapy are trauma, hemophagocytic lymphohistiocytosis, pancreatitis and rhabdomyolysis[21,22].
To date, scarce scientific evidence including few case series and randomized controlled trials are available on the use of CytoSorb® therapy[10,15]. An international registry of 22 countries on use of Cytosorb® therapy in 198 patients noted that sepsis was the most common indication for use of Cytosorb® therapy (n = 135) and reported improved interleukin (IL)-6 levels and improved actual mortality (AM, 65%) vs predicted mortality (PM, 78%) for these patients[10].
CytoSorb® has been used in India for several years. Therefore, the purpose of this prospective study was to collect data and evaluate the clinical outcomes with CytoSorb® therapy in patients with sepsis and septic shock.
MATERIALS AND METHODS
Study design
This was a prospective, real time, investigator initiated, observational, multicenter study conducted for 8 mo (including enrollment and completion) across four different tertiary care ICUs in India. The study protocol was approved by the local scientific and ethical committee. The study was conducted in compliance with the current International Council for Harmonization, Good clinical practice (ICH GCP), Schedule Y and Indian Council of Medical Research guidelines. A written informed consent was obtained from all the patients/relatives before initiating the therapy. The patients/ caretakers received information about the usage, advantages and disadvantages of treatment.
Inclusion/exclusion criteria
We enrolled patients admitted in the ICU with sepsis and septic shock who were initially managed for at least 6 h as recommended by the surviving sepsis guidelines[2]. Of these, we included those patients who had evidence of at least one new onset organ dysfunction during the course of sepsis.
Patients were excluded if: Diagnosed with septic shock for > 48 h; Had symptoms of uncontrolled hemorrhage in the last 24 h; Had more than three failed organs on presentation; Had received chemotherapy or radiation treatment within last 60 d; Diagnosed with chronic kidney disease stage 5 or end stage hepatic liver failure; Had a history of immunosuppressive disorders or admitted with acute coronary syndrome or life-threatening cardiac arrhythmia.
Study procedure
Before initiating the CytoSorb® therapy, the baseline patient data, including relevant demographic details, vital signs, clinical diagnosis, progression of clinical condition and laboratory parameters, were recorded in the case record form (CRF). To monitor the effects of CytoSorb therapy, all relevant parameters were recorded before and after the CytoSorb® treatment.
Primary outcomes
The following outcomes were considered as primary end points:
Change in vasopressor requirement: As per vasopressor or inotropic requirement, the MAP was targeted > 65 mmHg. Dose and number of drugs (i.e. NE, E and V) and change in MAP before and after CytoSorb® therapy were recorded.
Cytokine assay: Serum samples for multi cytokine assay (i.e. IL-1, IL-6) were collected pre-(baseline) and post-(after the last treatment) CytoSorb® therapy and analyzed in Syngene Lab (Bangalore, India). The post CytoSorb® samples were collected before disconnecting the device. Change in pre and post cytokine values were recorded in CRF.
Percentage reduction in vasopressor dose/cytokine level was calculated as (difference in average pre and average post vasopressor dose or cytokine level/average pre vasopressor dose or pre cytokine level dose) × 100. To monitor the vasopressor-MAP relationship, the MAP/NE ratio was used.
Secondary outcomes
Evaluation of laboratory parameters: We recorded the complete blood count and biochemistry test results both at baseline, during and at the completion of CytoSorb® therapy in the CRF. Change in laboratory parameter values for pre and post CytoSorb® therapy were evaluated.
Organ function
Acute physiology and chronic health evaluation (APACHE II) and Sequential organ failure assessment (SOFA) scores were recorded at baseline and post therapy. Vital parameters were recorded at baseline and on each day of CytoSorb® treatment. MAP, X–ray findings, ventilator requirement and oxygenation parameters (fraction of inspiration O2, alveolar oxygen partial pressure, partial pressure of carbon dioxide) were also documented in CRF. At the end of treatment, change in pre and post therapy values were calculated. APACHE-II calculator was used as a severity score and mortality estimation tool[23].
Survival outcomes
Survival outcomes were determined on the basis of length of patients’ stay in ICU (total number of days spent by the patient in ICU before, during and post CytoSorb® therapy) and mechanical ventilation/dialysis requirement (frequency at which the patients required mechanical ventilation and dialysis before and after the treatment).
Length of treatment
The duration of CytoSorb® treatment in hours and number of CytoSorb® devices used were decided as per the patient’s condition and clinical outcomes. We used a minimum of two devices for each patient. Each day one CytoSorb® device was used for 8-12 h in hemodialysis machine or for maximum of 24 h in continuous renal replacement therapy machines.
Safety evaluation
Any event that was not expected due to the course of disease and concurrent medications was recorded and evaluated.
Statistical analysis
A sample size calculation was not performed due to the exploratory character of the study. Data were primarily recorded in Microsoft Excel 2016. Data are summarized according to data distribution (normal or not-normal), and the appropriate parametric or non-parametric statistical tests were used to evaluate the difference in clinical outcomes and the change in clinical and laboratory parameters before and after CytoSorb® therapy. The level of significance was defined as P < 0.05.
RESULTS
Study population
A total of 45 patients aged ≥ 18 and ≤ 80 years were included in the study. Majority of the patients were men (n = 31; 69.0%) with mean age 47.16 ± 14.11 years. The mean age of women patients was 48.14 ± 19.04 years. Prior to CytoSorb® therapy, the percentage of patients who required mechanical ventilation and dialysis were 78% and 49%, respectively. The rest of the demographics are summarized in Table 1. Twenty-six patients (57.8%) survived the full course of CytoSorb® therapy, and 3 patients were lost to follow up.
Table 1.
Baseline characteristics of all the patients before initiating the therapy
|
Baseline characteristics
|
Findings, mean ± SD
|
| Age, yr | 47.46 ± 15.56 |
| Heart rate, beats/min | 117 ± 22.05 |
| MAP, mmHg | 69.15 ± 9.19 |
| GCS | 9.04 ± 3.06 |
| APACHE-II | 25.46 ± 5.06 |
| SOFA | 12.90 ± 4.37 |
| Leucocytes, µL | 15311.44 ± 7140.54 |
| Platelets, cells/mm3 | 139153.48 ± 89467.72 |
| S. Creatinine, mg/dL | 2.74 ± 1.72 |
| S. Lactate, mmoL/L | 4.61 ± 2.87 |
| PaCO2 | 43.37 ± 18.22 |
| PaO2 | 94.02 ± 49.09 |
| FiO2 | 48.78 ± 43.28 |
| PaO2/FiO2 | 118.6 ± 58.01 |
APACHE II: Acute physiology and chronic health evaluation; FiO2: Fraction of inspiration O2; GCS: Glasgow coma scale; MAP: Mean arterial pressure; SD: Standard deviation; SOFA: Sequential organ failure assessment; PaCO2: Partial pressure of carbon dioxide; PaO2: Alveolar oxygen partial pressure.
Evaluation of primary outcomes
Vasopressor requirement: Table 2 shows the change in vasopressor drugs in the survivor group from the start after the termination of CytoSorb® therapy. Overall, before CytoSorb® therapy, 21 patients required NE, 4 patients were on E and 9 were on V. In general, there was a tendency of reduced need for vasopressors post CytoSorb® therapy, but it did not reach statistical significance. Amongst the patients in the non-survivor group, the use of vasopressor drugs increased or remained unchanged (data not shown).
Table 2.
Percentage decrease in patients and vasopressor doses (survivors)
|
Vasopressor drug, µg/kg/min
|
Pre CytoSorb®, therapy patient number (n), dose (median)
|
Post CytoSorb® Therapy, patient number (n), dose (median)
|
% Decrease in dose
|
P
value (dose)
|
| Norepinephrine | 21; 1 | 18; 0.45 | 43.3 | 0.160 |
| Epinephrine | 4; 0.055 | 1; 0.055 | 64.4 | - |
| Vasopressin | 9; 1.5 | 7; 1 | 15.4 | 0.816 |
Change in laboratory parameters in survivors (Table 3): Total lymphocyte count reduced significantly at the end of the therapy. Serum creatinine and lactate levels also reduced significantly. There was no other significant change in any of the investigated parameters (Table 3).
Table 3.
Change in laboratory parameters for survivors
|
Parameters
|
Pre CytoSorb
®
therapy
|
Post CytoSorb
®
therapy
|
P
value
|
| Hb, g/dL | 10.01 ± 2.20 | 9.28 ± 1.53 | 0.1830 |
| HCT, % | 29.74 ± 8.4 | 25.75 ± 7.67 | 0.0909 |
| Leucocytes, µL | 16724 ± 5425 | 11215 ± 3317 | 0.00011 |
| Platelets, cells/mm3 | 139256 ± 88029 | 181203 ± 181381 | 0.2938 |
| S. Creatinine, mg/dL | 3.13 ± 1.92 | 2.08 ± 1.02 | 0.01901 |
| S. Lactate, mmol/L | 4.75 ± 2.77 | 2.88 ± 2.39 | 0.01201 |
| SGOT, U/L | 488.44 ± 1570.42 | 369.95 ± 1134.74 | 0.7661 |
| SGPT, U/L | 192.72 ± 298.99 | 145.90 ± 236.97 | 0.5503 |
| BUN, mg/dL | 76.21 ± 61.88 | 62.39 ± 52.28 | 0.4076 |
| Bilirubin, mg/dL | 9.91 ± 36.77 | 8.35 ± 31.36 | 0.8730 |
| Sodium, mmol/L | 134.38 ± 25.69 | 134.32 ± 6.20 | 0.9908 |
| Potassium, mmol/L | 3.98 ± 0.95 | 3.73 ± 1.05 | 0.3723 |
| Albumin, g/L | 2.65 ± 0.93 | 2.71 ± 0.95 | 0.8261 |
| Arterial pH | 7.35 ± 0.100 | 7.36 ± 0.105 | 0.7291 |
| Bicarbonate | 24.89 ± 10.71 | 24.75 ± 9.21 | 0.9599 |
Significant value P < 0.05, all values are defined as mean ± SD.
BUN: Blood urea nitrogen; Hb: Hemoglobin; HCT: Hematocrit; SD: Standard deviation; SGOT: Serum glutamic oxaloacetic transaminase; SGPT: Serum glutamic-pyruvic transaminase.
There was some reduction in the inflammatory marker levels for both IL-1 and IL-6, but it did not reach statistical significance (Table 4).
Table 4.
Cytokine assay results for survivors
|
Cytokine
|
Pre CytoSorb
®
therapy, mean ± SD
|
Post CytoSorb
®
therapy, mean ± SD
|
Percentage change
|
P
value
|
| IL1, pg/mL | 10.74 ± 9.70 | 9.54 ± 9.66 | 11.11 | 0.5580 |
| IL6, pg/mL | 889.15 ± 1307.43 | 423.69 ± 1105.55 | 52.34 | 0.0792 |
IL: Interleukin.
Change in vital parameters in survivors (Table 5): After CytoSorb® therapy, there was a 15.8% significant increase in MAP. Among non-survivors (n = 19) there was also a significant increase in MAP (from 69.56 ± 7.84 to 72.13 ± 13.2 mmHg, P = 0.036). Post CytoSorb® therapy, both heart rate and the Glasgow coma score improved significantly in the survivor group. The rest of the data for survivors are shown in Table 5.
Table 5.
Change in vital parameters in survivors
|
Parameters
|
Survivor group
|
P
value
|
|
|
|
Pre CytoSorb
®
therapy, mean ± SD
|
Post CytoSorb
®
therapy, mean ± SD
|
|
| Heart rate, beats/min | 118.57 ± 19.8 | 103.07 ± 19.38 | 0.00651 |
| MAP, mmHg | 68.61 ± 9.62 | 79.42 ± 9.05 | 0.00011 |
| GCS | 9.86 ± 2.34 | 12.20 ± 1.47 | 0.00011 |
| PaCO2 | 43.32 ± 18.63 | 38.57 ± 11.66 | 0.2757 |
| PaO2/FiO2 | 162.09 ± 82.99 | 161.20 ± 66.58 | 0.9704 |
Significant P value < 0.05.
All values are defined as mean ± SD. FiO2: Fraction of inspiration O2; GCS: Glasgow coma scale; MAP: Mean arterial pressure; PaCO2: Partial pressure of carbon dioxide; PaO2: Alveolar oxygen partial pressure; SD: Standard deviation.
Evaluation of secondary outcomes
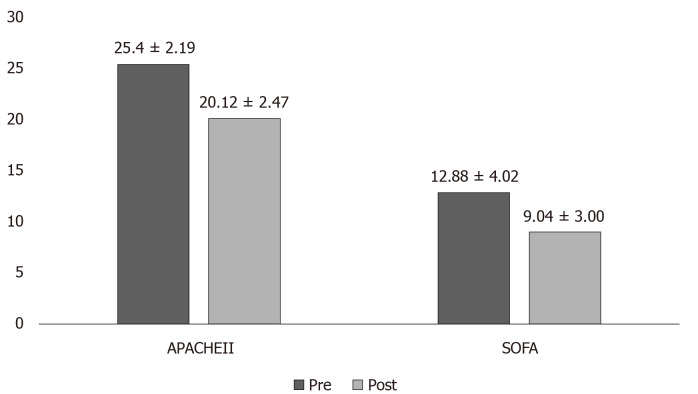
Assessment of sepsis scores: Both APACHE II and SOFA scores were significantly reduced by the end of the treatment among survivors (Figure 1). Overall, there was a 20.7% reduction in APACHE II and 29.8% reduction in SOFA scores. In the non-survivor group, APACHE II scores increased from 26.5 ± 5.2 to 27.93 ± 5.2, and SOFA scores also increased from 13.56 ± 4.53 to 15.38 ± 4.29.
Figure 1.
Sepsis scores in survivor group (pre and post Cytosorb® therapy). Significant P values obtained for both acute physiology and chronic health evaluation (P < 0.0001) and sequential organ failure assessment scores (P = 0.0003). APACHE II: acute physiology and chronic health evaluation; SOFA: Sequential organ failure assessment scores.
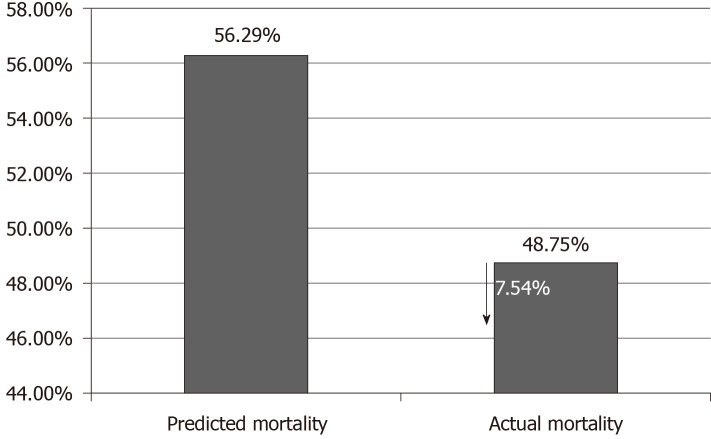
Predicted mortality: The PM before CytoSorb® therapy was 56.5% in the overall population; the actual mortality after CytoSorb® therapy was 48.8% (Figure 2).
Figure 2.
Predicted mortality vs actual mortality based on acute physiology and chronic health evaluation.
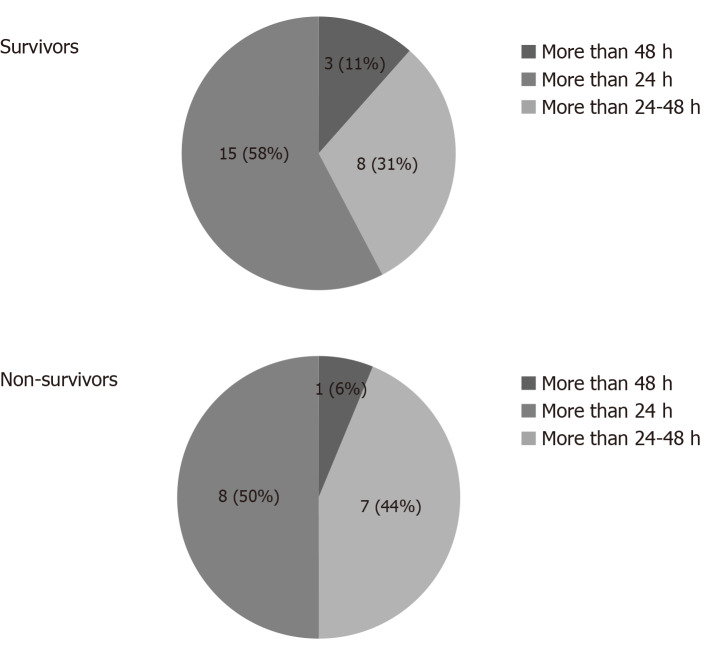
Initiation of CytoSorb® therapy: In most patients, treatment was commenced between 24-48 h after ICU admission (Figure 3). Only 3 patients among survivors and 1 in non-survivors received therapy within < 24 h, and in 16 cases treatment was started > 48 h after ICU.
Figure 3.
Time of initiation of CytoSorb® therapy in survivors and non-survivors.
Overall, 50% (n = 8) of patients survived after 72 h of therapy. In the survivor group, the average number of days spent in ICU was 4.44 ± 1.66 d; while in the non-survivor group, it was 8.5 ± 15.9 d.
Evaluation of safety parameters: We could not observe any CytoSorb® related side effects or adverse events. There was no significant change in platelet or albumin levels (Table 3). Only 1 single patient showed clot formation in the device when used without heparin due to the clinical condition of the patient, which led to the stoppage of the therapy when used for the second time. One patient was diagnosed with ventricular tachycardia and needed injection of amiodarone.
DISCUSSION
Various adjuvant therapies are included in current treatment modalities for controlling cytokine storm; immunoglobulin therapy, endotoxin-binding polymyxin B hemoperfusion, dialysis and plasma filtration, etc. The mortality rate still remains high with these techniques[24-26]. Direct hemoperfusion using a polymyxin B endotoxin-adsorbing column was studied in clinical trials (ABDOMIX Study). The study could not confirm its clinical efficacy due to the nephrotoxic effects of the technique and associated high risks of cartridge clotting resulting in acute blood loss in patients admitted in ICU[27]. Similarly, anti-IL-1RA, anti-IL-1β, anti-tumor necrosis factor-α and anti-lipopolysaccharide showed disappointing results in both preclinical and clinical trials, despite their ability to reduce significantly serum cytokine concentrations[28,29]. A recent Cochrane review reported low-quality evidence for high-volume hemofiltration in the treatment of critically ill patients with sepsis and suggested that more multicenter randomized controlled trials are required before these therapies can be recommended for routine use[12].
Extracorporeal cytokine adsorption is a recent adjuvant alternative introduced into clinical practice less than a decade ago. Its aim was to reduce cytokine storm by the bulk removal of mediators of inflammation. Later, this treatment was reported as safe and well-tolerated in more than 300 human treatments in very sick patients with the worst forms of sepsis and lung injury, and to date, the treatment has emerged as the safest in nearly 1500 human treatments overall[30]. Though published data suggest that using CytoSorb® in conjunction with standard care including mechanical ventilation and dialysis may decrease the level of pro-inflammatory cytokines and improve hemodynamics in sepsis and septic shock, high-quality data from clinical trials are not available yet[16].
The present study evaluated some aspects of the clinical outcomes with CytoSorb® device treatment along with current standard of care in management of sepsis and septic shock. We observed improved clinical outcomes of patients with septic shock in terms of reduced mortality as compared to predicted, improved hemodynamics as indicated by MAP, and reduced use of vasopressors and their doses.
We studied patients requiring increasing vasopressor dose to maintain MAP > 65 mmHg. In our study, MAP increased significantly during CytoSorb® therapy in both survivors and non-survivors. This improvement in MAP was accompanied by a non-significant reduction in vasopressor dose as also indicated by the increase in the MAP/NE ratio. These results are in accord with our previous study conducted in 10 ICU patients where an overall reduction in all the vasopressor drugs after CytoSorb® therapy was reported[31]. Of the nine patients who were given vasopressin, five were weaned off V, two had a reduced dose and two were on the same dose as before.
Our results were consistent with the results reported by a prospective single center study with 20 patients; wherein the CytoSorb® treatment included NE dose that was significantly reduced after 6 h (−0.4 µg/kg/min; P = 0.03) and 12 h (−0.6 µg/kg/min; P = 0.001). Shock reversal was achieved in 13 (65%) patients; 28 d survival was 45%. The study reported shock reversal in two-thirds of these patients after using CytoSorb® adsorption therapy[10]. The findings of our study are supported by some more recent case reports demonstrating that CytoSorb® might be an effective adjuvant therapy, decreasing vasopressor requirements and stabilizing hemodynamics of septic shock patients[15,31-35].
Cytokines play an important role in the pathophysiology of sepsis and other clinical conditions with systemic inflammation. An elevated circulating levels of pro-inflammatory cytokines causes dysregulation in immune response and results in multi organ failure causing prolonged ICU stay and high mortality in ICU patients[36,37]. Specifically, elevated serum IL-1 and IL-6 appear to correlate with sepsis severity and end-organ damage[38]. We performed a cytokine assay, and our results showed improved levels of IL-6 in survivor group.
In addition to all above parameters, our results showed significant reduction in laboratory parameters like total lung capacity count improved (P < 0.00001) and overall improvement in HR (P = 0.0065), Glasgow coma score (P < 0.0001) and other biomarkers like serum creatinine (P = 0.0190) and lactate (P = 0.0120). An insignificant improvement was seen in other parameters also (i.e. respiratory parameters, liver and kidney profile). There was significant reduction in the SOFA scores (P = 0.0003) in the survivor group. The current findings are well consistent with other published studies[33,39,40].
We also investigated the time of initiation of CytoSorb® (in less than 24 h or 48 h of admission in ICU). Although there was a tendency that survivors received therapy earlier compared to non-survivors, the numbers are very small to make firm conclusions regarding timing and outcome. Nevertheless, the tendency of this pattern provide some further support to those studies, which also reported that starting therapy within 24 h after the onset of septic shock is the most beneficial[16,24].
PM was 54% in survivor group and 60% in non-survivor group using acute APACHE II calculator[23]. However, the AM was 42%. Our findings are similar to those of Kogelmann et al[15] who used CytoSorb® as an adjunctive therapy in 26 critically ill patients with septic shock and in need of renal replacement therapy. They reported that AM was lower in the overall patient population than PM. The actual 28 d, ICU and hospital mortality was 61.54%, 73.08% and 80.77%, respectively. However, mortality as predicted by APACHE II score in the overall patient population was 89.9%. Another previously published study reported that hemoadsorption with CytoSorb® results in a decreased observed vs expected 28 d mortality in patients with septic shock, and the mean PM (based on SOFA) was 75% (95%CI: 71%-79%) while AM was found to be 48% (mean difference-27%, 95%CI: 38%-15%, P < 0.001)[11].
Regarding safety, our results provide further data that the therapy is safe, as we could not find any device related AE or laboratory deterioration. In fact, platelet count remained unchanged or rather slightly increased, rather than decreased. This is contrast with some other reports, where thrombocytopenia had been observed[41].
This study has certain limitations. The sample size is relatively small, several circumstances were not standardized and there was no control group. Detailed hemodynamic evaluation and conventionally used inflammatory markers, such as C-reactive protein or procalcitonin, were not measured. However, a very recent retrospective, propensity score matched study reported very positive results on around 100 patients without measuring inflammatory markers[11].
CONCLUSION
Overall, the current study showed improvement in hemodynamic stability and organ function and reduction in IL-6 levels. Our results provide further support to the notion that outcomes are better if cytokine adsorption (CytoSorb®) is initiated early after the onset of septic shock. We can also conclude that we could not find any treatment related AE. Further, studies should be performed to help us to identify the appropriate patient population and timing of therapy and also to test the positive results of retrospective and observational studies, just like the current results, in the setting of randomized clinical trials.
ARTICLE HIGHLIGHTS
Research background
Sepsis is one of the oldest and most elusive syndromes in medicine, and yet it remains the most significant unmet medical need. In India, more than one million estimated new cases of sepsis are treated in intensive care units (ICUs) each year. CytoSorb® is an International Science Organization 10993 biocompatible device that is approved in the United States under International Science Organization 13485 certification. It is also approved as an extracorporeal cytokine adsorber in the European Union and marketed in 29 countries. In this study, clinical outcomes of patients with septic shock were assessed in terms of reduced mortality as compared to predicted, improved hemodynamics as indicated by mean arterial pressure (MAP) and reduced use of vasopressors and their doses.
Research motivation
Sepsis and septic shock is the leading cause of death among hospitalized patients. CytoSorb® therapy showed promising results in hyperinflammatory condition of critically ill septic patients. This study was conducted to evaluate clinical outcomes in these patients. This study will help clinicians to evaluate the use of CytoSorb® therapy for the patients considering clinical outcomes like MAP and use of vasopressors drugs.
Research objectives
The objective of the study was to evaluate CytoSorb® use as an adjunctive therapy along with the standard of care. The study showed improvement in hemodynamic stability and organ function and reduction in interleukin-6 levels.
Research methods
This was a prospective, real time, investigator initiated, observational multicenter study conducted in the patients admitted to the ICU with sepsis and septic shock. The improvement of MAP and reduction of vasopressor needs were evaluated as primary outcome. The change in laboratory parameters, sepsis scores [acute physiology and chronic health evaluation (APACHE II) and sequential organ failure assessment (SOFA)] and vital parameters were considered as secondary outcome. The outcomes were also evaluated in the survivor and non-survivor group. Descriptive statistics were used; a P value < 0.05 was considered to be statistically significant.
Research results
A total of 45 patients aged ≥ 18 and ≤ 80 years were included; a majority were men (n = 31; 69.0%) with mean age; 47.16 ± 14.11 years. Post CytoSorb® therapy, 26 patients survived and 3 patients were lost to follow-up. In the survivor group, the percentage dose reduction in vasopressor was NE (51.4%), E (69.4%) and V (13.9%). A reduction in interleukin-6 levels (52.3%) was observed in the survivor group. Platelet count improved to 30.1% (P = 0.2938), total lung capacity count significantly reduced by 33% (P < 0.0001). Serum creatinine and serum lactate were reduced by 33.3% (P = 0.0190) and 39.4% (P = 0.0120), respectively. The mean APACHE II score was 25.46 ± 2.91, and SOFA scores was 12.90 ± 4.02 before initiation of CytoSorb® therapy and reduced significantly post therapy (APACHE II 20.1 ± 2.47; P < 0.0001 and SOFA 9.04 ± 3.00; P = 0.0003) in the survivor group. The predicted mortality in our patient population before CytoSorb® therapy was 56.5%, and it reduced to 48.8% (actual mortality) after CytoSorb® therapy. We reported 75% survival rate in patients given treatment in < 24 h of ICU admission and 68% survival rates in patients given treatment within 24-48 h of ICU admission. In the survivor group, the average number of days spent by patients in ICU was 4.44 ± 1.66 d; while in the non-survivor group, the average number of days spent by patients in ICU was 8.5 ± 15.9 d. CytoSorb® therapy was safe and well tolerated with no adverse events reported.
Research conclusions
Early initiation of CytoSorb® therapy significantly improves clinical outcomes.
Research perspectives
In the future, adding a standard of control group and conducting a study that is powered to compare the time of initiation of CytoSorb® therapy will be necessary.
ACKNOWLEDGEMENTS
The authors acknowledge Mr. Pradeep Yanamala and Dr Vikram Shetty for end-to-end coordination.
Footnotes
Institutional review board statement: This study was reviewed and approved by an institutional ethics committee.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: June 2, 2020
First decision: July 4, 2020
Article in press: November 28, 2020
Specialty type: Critical care medicine
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wan TT S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li JH
Contributor Information
Rajib Paul, Department of Internal Medicine and Critical Care, Apollo Health City, Hyderabad 500033, India. rajib.paulcriticalcare@gmail.com / drrajibpaul@gmail.com.
Prachee Sathe, Department of Critical Care Medicine, Ruby Hall Clinic, Pune 411001, India.
Senthil Kumar, Department of Critical Care Medicine, Apollo Hospital, Chennai 600006, India.
Shiva Prasad, Department of Anesthesiology and Critical Care, Narayana Institute of Cardiac Sciences, Bangaluru 560099, India.
Ma Aleem, Department of Internal Medicine and Critical Care, Apollo Health City, Hyderabad 500033, India.
Prashant Sakhalvalkar, Department of Critical Care Medicine, Ruby Hall Clinic, Pune 411001, India.
Data sharing statement
There is no additional data available.
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 3.van der Linde G, Grootendorst A. First case of toxic shock treated with haemoadsorption by CytoSorb® in the Netherlands. Neth J Crit Care. 2016;24:27–9. [Google Scholar]
- 4.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Divatia JV, Amin PR, Ramakrishnan N, Kapadia FN, Todi S, Sahu S, Govil D, Chawla R, Kulkarni AP, Samavedam S, Jani CK, Rungta N, Samaddar DP, Mehta S, Venkataraman R, Hegde A, Bande BD, Dhanuka S, Singh V, Tewari R, Zirpe K, Sathe P INDICAPS Study Investigators. Intensive Care in India: The Indian Intensive Care Case Mix and Practice Patterns Study. Indian J Crit Care Med. 2016;20:216–225. doi: 10.4103/0972-5229.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annane D, Aegerter P, Jars-Guincestre MC, Guidet B CUB-Réa Network. Current epidemiology of septic shock: the CUB-Réa Network. Am J Respir Crit Care Med. 2003;168:165–172. doi: 10.1164/rccm.2201087. [DOI] [PubMed] [Google Scholar]
- 7.Pollard S, Edwin SB, Alaniz C. Vasopressor and Inotropic Management of Patients with Septic Shock. P T. 2015;40:438–450. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Mao Z, Zeng X, Kang H, Liu H, Pan L, Hou PC. Vasopressors in septic shock: a systematic review and network meta-analysis. Ther Clin Risk Manag. 2015;11:1047–1059. doi: 10.2147/TCRM.S80060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shroff G. Establishment and characterization of a neuronal cell line derived from a 2-cell stage human embryo: clinically tested cell-based therapy for neurological disorders. Int J Recent Sci Res. 2015;6:3730–8. [Google Scholar]
- 10.Friesecke S, Träger K, Schittek GA, Molnar Z, Bach F, Kogelmann K, Bogdanski R, Weyland A, Nierhaus A, Nestler F, Olboeter D, Tomescu D, Jacob D, Haake H, Grigoryev E, Nitsch M, Baumann A, Quintel M, Schott M, Kielstein JT, Meier-Hellmann A, Born F, Schumacher U, Singer M, Kellum J, Brunkhorst FM. International registry on the use of the CytoSorb® adsorber in ICU patients : Study protocol and preliminary results. Med Klin Intensivmed Notfmed. 2019;114:699–707. doi: 10.1007/s00063-017-0342-5. [DOI] [PubMed] [Google Scholar]
- 11.Brouwer WP, Duran S, Kuijper M, Ince C. Hemoadsorption with CytoSorb shows a decreased observed vs expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study. Crit Care. 2019;23:317. doi: 10.1186/s13054-019-2588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borthwick EM, Hill CJ, Rabindranath KS, Maxwell AP, McAuley DF, Blackwood B. High-volume haemofiltration for sepsis in adults. Cochrane Database Syst Rev. 2017;1:CD008075. doi: 10.1002/14651858.CD008075.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei CL, Miura T, Robson P, Lim SK, Xu XQ, Lee MY, Gupta S, Stanton L, Luo Y, Schmitt J, Thies S, Wang W, Khrebtukova I, Zhou D, Liu ET, Ruan YJ, Rao M, Lim B. Transcriptome profiling of human and murine ESCs identifies divergent paths required to maintain the stem cell state. Stem Cells. 2005;23:166–185. doi: 10.1634/stemcells.2004-0162. [DOI] [PubMed] [Google Scholar]
- 14.Wiegele M, Krenn CG. Cytosorb™ in a patient with Legionella pneumonia-associated rhabdomyolysis: a case report. ASAIO J. 2015;61:e14–e16. doi: 10.1097/MAT.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 15.Kogelmann K, Jarczak D, Scheller M, Drüner M. Hemoadsorption by CytoSorb in septic patients: a case series. Crit Care. 2017;21:74. doi: 10.1186/s13054-017-1662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonavia A, Groff A, Karamchandani K, Singbartl K. Clinical Utility of Extracorporeal Cytokine Hemoadsorption Therapy: A Literature Review. Blood Purif. 2018;46:337–349. doi: 10.1159/000492379. [DOI] [PubMed] [Google Scholar]
- 17.Basu R, Pathak S, Goyal J, Chaudhry R, Goel RB, Barwal A. Use of a novel hemoadsorption device for cytokine removal as adjuvant therapy in a patient with septic shock with multi-organ dysfunction: A case study. Indian J Crit Care Med. 2014;18:822–824. doi: 10.4103/0972-5229.146321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minkov GA, Halacheva KS, Yovtchev YP, Gulubova MV. Pathophysiological mechanisms of acute pancreatitis define inflammatory markers of clinical prognosis. Pancreas. 2015;44:713–717. doi: 10.1097/MPA.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 19.Hassan K, Kannmacher J, Wohlmuth P, Budde U, Schmoeckel M, Geidel S. Cytosorb Adsorption During Emergency Cardiac Operations in Patients at High Risk of Bleeding. Ann Thorac Surg. 2019;108:45–51. doi: 10.1016/j.athoracsur.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 20.Tomescu D, Popescu M, David C, Dima S. Clinical effects of hemoadsorption with CytoSorb® in patients with severe acute pancreatitis: A case series. Int J Artif Organs. 2019;42:190–193. doi: 10.1177/0391398818823762. [DOI] [PubMed] [Google Scholar]
- 21.Shroff G. A novel approach of human embryonic stem cells therapy in treatment of Friedreich's ataxia. IJCRI. 2015;6:261–6. [Google Scholar]
- 22.Cytosorbents Corporation. CytoSorb Literature Database, 2019. Last accessed on 5th August 2019. Available from: https://Literature.cytosorb.com/
- 23.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 24.Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, Malcangi V, Petrini F, Volta G, Bobbio Pallavicini FM, Rottoli F, Giunta F, Ronco C. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445–2452. doi: 10.1001/jama.2009.856. [DOI] [PubMed] [Google Scholar]
- 25.Kreymann KG, de Heer G, Nierhaus A, Kluge S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med. 2007;35:2677–2685. [PubMed] [Google Scholar]
- 26.Bellomo R, Baldwin I, Ronco C. Extracorporeal blood purification therapy for sepsis and systemic inflammation: its biological rationale. Contrib Nephrol. :2001: 367–374. doi: 10.1159/000060105. [DOI] [PubMed] [Google Scholar]
- 27.Payen DM, Guilhot J, Launey Y, Lukaszewicz AC, Kaaki M, Veber B, Pottecher J, Joannes-Boyau O, Martin-Lefevre L, Jabaudon M, Mimoz O, Coudroy R, Ferrandière M, Kipnis E, Vela C, Chevallier S, Mallat J, Robert R ABDOMIX Group. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. 2015;41:975–984. doi: 10.1007/s00134-015-3751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Opal SM, Fisher CJ Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, Shelly MP, Pribble JP, LaBrecque JF, Lookabaugh J, Donovan H, Dubin H, Baughman R, Norman J, DeMaria E, Matzel K, Abraham E, Seneff M. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25:1115–1124. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Bernard GR, Francois B, Mira JP, Vincent JL, Dellinger RP, Russell JA, Larosa SP, Laterre PF, Levy MM, Dankner W, Schmitt N, Lindemann J, Wittebole X. Evaluating the efficacy and safety of two doses of the polyclonal anti-tumor necrosis factor-α fragment antibody AZD9773 in adult patients with severe sepsis and/or septic shock: randomized, double-blind, placebo-controlled phase IIb study*. Crit Care Med. 2014;42:504–511. doi: 10.1097/CCM.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 30.Reshma Tewari DRBG. Role of Cytosorb® in Optimization of Vasopressors and Reduction of Sepsis scores: A Case Series: Poster presented at Second International Cytosorb User Meeting, 2015. Last accessed on 8 May 2019. Available from: https://cytosorb-therapy.com/wp-content/uploads/2015/10/Tewari_Poster_2015_10_02_CytoSorb.pdf .
- 31.Mitzner SR, Gloger M, Henschel J, Koball S. Improvement of hemodynamic and inflammatory parameters by combined hemoadsorption and hemodiafiltration in septic shock: a case report. Blood Purif. 2013;35:314–315. doi: 10.1159/000351206. [DOI] [PubMed] [Google Scholar]
- 32.Hetz H, Berger R, Recknagel P, Steltzer H. Septic shock secondary to β-hemolytic streptococcus-induced necrotizing fasciitis treated with a novel cytokine adsorption therapy. Int J Artif Organs. 2014;37:422–426. doi: 10.5301/ijao.5000315. [DOI] [PubMed] [Google Scholar]
- 33.Träger K, Fritzler D, Fischer G, Schröder J, Skrabal C, Liebold A, Reinelt H. Treatment of post-cardiopulmonary bypass SIRS by hemoadsorption: a case series. Int J Artif Organs. 2016;39:141–146. doi: 10.5301/ijao.5000492. [DOI] [PubMed] [Google Scholar]
- 34.Hinz B, Jauch O, Noky T, Friesecke S, Abel P, Kaiser R. CytoSorb, a novel therapeutic approach for patients with septic shock: a case report. Int J Artif Organs. 2015;38:461–464. doi: 10.5301/ijao.5000429. [DOI] [PubMed] [Google Scholar]
- 35.Nemeth E, Kovacs E, Racz K, Soltesz A, Szigeti S, Kiss N, Csikos G, Koritsanszky KB, Berzsenyi V, Trembickij G, Fabry S, Prohaszka Z, Merkely B, Gal J. Impact of intraoperative cytokine adsorption on outcome of patients undergoing orthotopic heart transplantation-an observational study. Clin Transplant. 2018;32:e13211. doi: 10.1111/ctr.13211. [DOI] [PubMed] [Google Scholar]
- 36.Gouel-Chéron A, Allaouchiche B, Guignant C, Davin F, Floccard B, Monneret G AzuRea Group. Early interleukin-6 and slope of monocyte human leukocyte antigen-DR: a powerful association to predict the development of sepsis after major trauma. PLoS One. 2012;7:e33095. doi: 10.1371/journal.pone.0033095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mera S, Tatulescu D, Cismaru C, Bondor C, Slavcovici A, Zanc V, Carstina D, Oltean M. Multiplex cytokine profiling in patients with sepsis. APMIS. 2011;119:155–163. doi: 10.1111/j.1600-0463.2010.02705.x. [DOI] [PubMed] [Google Scholar]
- 38.Singbartl K, Miller L, Ruiz-Velasco V, Kellum JA. Reversal of Acute Kidney Injury-Induced Neutrophil Dysfunction: A Critical Role for Resistin. Crit Care Med. 2016;44:e492–e501. doi: 10.1097/CCM.0000000000001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawchar F, László I, Öveges N, Trásy D, Ondrik Z, Molnar Z. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J Crit Care. 2019;49:172–178. doi: 10.1016/j.jcrc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Träger K, Skrabal C, Fischer G, Datzmann T, Schroeder J, Fritzler D, Hartmann J, Liebold A, Reinelt H. Hemoadsorption treatment of patients with acute infective endocarditis during surgery with cardiopulmonary bypass - a case series. Int J Artif Organs. 2017;40:240–249. doi: 10.5301/ijao.5000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barsam SJ, Psaila B, Forestier M, Page LK, Sloane PA, Geyer JT, Villarica GO, Ruisi MM, Gernsheimer TB, Beer JH, Bussel JB. Platelet production and platelet destruction: assessing mechanisms of treatment effect in immune thrombocytopenia. Blood. 2011;117:5723–5732. doi: 10.1182/blood-2010-11-321398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no additional data available.