Abstract
Gastrointestinal (GI) cancers are one of the most common malignancies worldwide, with high rates of morbidity and mortality. Myeloid-derived suppressor cells (MDSCs) are major components of the tumor microenvironment (TME). MDSCs facilitate the transformation of premalignant cells and play roles in tumor growth and metastasis. Moreover, in patients with GI malignancies, MDSCs can lead to the suppression of T cells and natural killer cells. Accordingly, a better understanding of the role and mechanism of action of MDSCs in the TME will aid in the development of novel immune-targeted therapies.
Keywords: Myeloid-derived suppressor cells, Gastrointestinal cancers, Immune checkpoint inhibitors, Tumor progression
Core Tip: In patients with cancer, the levels of myeloid-derived suppressor cells (MDSCs) are presumed to be of prognostic and predictive value. Recent studies have shown that MDSCs appear to be independent prognostic factors in gastrointestinal cancer. In addition, therapeutics that target MDSCs have been shown to enhance anti-tumor immune responses in animal models. Consequently, a better understanding of the role and mechanism of action of MDSCs in the tumor microenvironment may aid in the development of novel immune-targeted therapies.
INTRODUCTION
In 2018, 4.8 million new diagnoses of gastrointestinal (GI) cancer and 3.4 million related deaths were reported globally. The incidence of GI cancer is 26% worldwide, accounting for 35% of all cancer-associated mortalities[1]. Numerous stromal and immune cells and soluble markers are related to the immunosuppressive network in the tumor microenvironment (TME)[2]. This network is involved in tumor cell growth and the blockade of anti-tumor immune responses, which subsequently promote the progression and invasion of tumor cells[3]. Macrophages, monocytes, and dendritic cells (DCs) represent a subgroup of leukocytes called myeloid cells, which are generated from polymorphonuclear granulocytes[4]. Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells that arise from myeloid progenitor cells[5]. They play a key role in tumor-associated immune evasion, angiogenesis, and tumor metastasis[6,7].
In patients with cancer, the levels of MDSCs are thought to have prognostic and predictive significance[3]. Recent studies have examined the role of MDSCs in solid tumors, and discovered that they appear to be independent prognostic factors in GI cancer[8,9]. In a meta-analysis of 17 studies with 1115 patients with GI malignancies, patients with a higher number of MDSCs at tumor sites and peripheral blood had higher mortality rates (hazard ratio: 3.35, 95% confidence interval: 1.46-7.68; P = 0.0004), risk of relapse, and tumor progression. The authors concluded that MDSC levels have prognostic and predictive value in cancer patients[10]. Additionally, higher levels of MDSCs in patients with cancer are associated with advanced tumor stage and a poor clinical prognosis[11]. Shibata et al[12] evaluated 123 patients with advanced GI malignancy, including 62 with colorectal cancer (CRC), 43 with gastric cancer (GC), and 18 with esophageal malignancies, and found that overall survival (OS) was significantly shorter in stage IV GI cancer patients with high MDSC levels than in those with low MDSC levels (P < 0.05). Because MDSCs have a significant role in modulating cancer progression and metastasis by inhibiting the anti-tumor reactivity of T cells and natural killer (NK) cells, targeting MDSCs with immune checkpoint inhibitors (ICIs) can alleviate their pro-tumorigenic functions. Therefore, in this systematic review, we summarize the characteristics and proposed function of MDSCs in the TME and their relationship to prognosis in patients with GI cancers.
MATERIALS AND METHODS
PubMed/MEDLINE databases were explored with search strategies using search keywords “MDSCs,” “gastrointestinal cancers,” “prognosis,” “tumor progression,” and “mortality rate,” to categorize studies published between 2006 and 2020. A total of 128 articles were reviewed by the authors for relevance to MDSCs and GI cancers, including retrospective, cross-sectional, case reports, and cohort studies, of which 85 papers were selected that met our selection criteria.
MDSC MECHANISM OF ACTION
MDSCs are myeloid-derived heterogeneous cells with potent immune regulatory functions. They are derived from the myeloid lineage of immune cells that give rise to macrophages, granulocytes, and immature DCs[13]. Monocytic MDSCs (M-MDSCs) and polymorphonuclear MDSCs (PMN-MDSCs) are the two major myeloid subsets of MDSCs[14]. Phenotypically and morphologically, they are equivalent to monocytes and neutrophils, respectively (Table 1)[15].
Table 1.
Two main categories of myeloid-derived suppressor cells and their immunosuppressive functions[59]
|
Type of MDSC
|
Markers in humans
|
Immunosuppression mediator
|
Mechanism of immunosuppression
|
| PMN-MDSCs | CD11b+CD14−CD15+HLADR− or CD11b+CD14−CD66b+ or LOX-1+ | ARG1, ROS | Suppressing immune responses mainly in an antigen-specific manner; ROS production |
| M-MDSCs | CD11b+CD14+CD15−HLADRlow/− | NO, ARG1, and cytokines such as TGF-β and IL-10 | Suppressing T cell responses, both in antigen-specific and non-specific manners; production of NO and cytokines |
ARG1: Arginase 1; IL-10: Interleukin-10; M-MDSCs: Monocytic myeloid-derived suppressor cells; NO: Nitric oxide; PMN-MDSCs: Polymorphonuclear myeloid-derived suppressor cells; ROS: Reactive oxygen species; TGF-β: Transforming growth factor-beta; MDSC: Myeloid-derived suppressor cell.
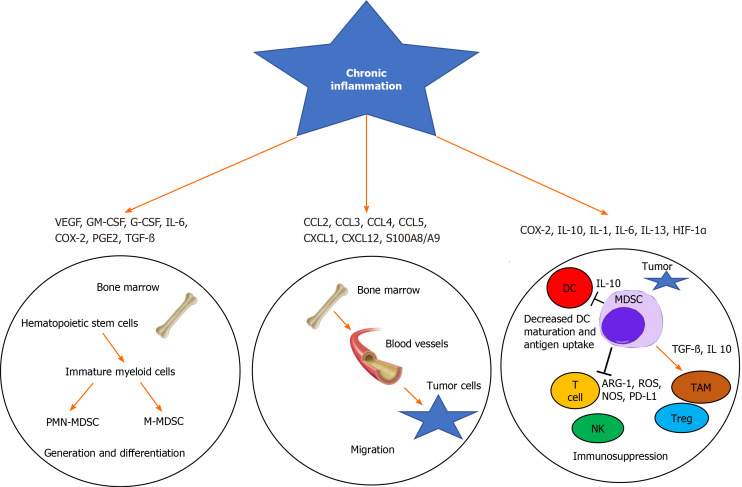
The process of myelopoiesis is driven by granulocyte-macrophage colony-stimulating factor (GM-CSF) in normal physiological conditions. Ultimately, GM-CSF and M-CSF induce the differentiation of granulocytes and macrophages from a common myeloid precursor that transforms into a common myeloblast[16]. Myeloid DCs eventually arise from monocytic as opposed to granulocytic lineages[17]. However, the hypersecretion of these mediator factors during chronic inflammation and cancer leads to the generation of MDSCs[18]. For example, inflammatory cytokines such as interleukin 6 (IL-6), IL-1β, and IL-3 and C-X-C chemokine receptor type 4 (CXCR4) and CXCL12 can lead to the induction and proliferation of MDSCs in peripheral blood and tumor sites in cancer patients[7,19]. The most important function of MDSCs is immunosuppression, mainly of target T cells (Figure 1)[14].
Figure 1.
Chronic inflammation activates myeloid-derived suppressor cell generation, migration, and immunosuppression in the tumor microenvironment. Several cytokines and stimulator factors secreted by stroma and tumor cells (e.g., VEGF, granulocyte-macrophage colony-stimulating factor, IL-1, IL-6, HIF-1α, TGF-β, COX-2) trigger myeloid-derived suppressor cell (MDSC) generation and migration. Cytokines (e.g., CCL2, CCL3, CCL4, CCL5, CXCL1) stimulate the migration of MDSCs into the tumor microenvironment. At the tumor site, MDSCs undergo activation (via TNF, IL-10, IL-1, IL-6, IFN-γ, COX-2, HIF-1α, etc.) and suppress the anti-tumor reactivity of T and natural killer cells. Cross-talk between MDSCs and dendritic cells (DCs) impairs DC function and promotes tumor progression[81]. GM-CSF: Granulocyte-macrophage colony-stimulating factor; PMN-MDSC: Polymorphonuclear myeloid-derived suppressor cells; M-MDSC: Monocytic myeloid-derived suppressor cells; MDSC: Myeloid-derived suppressor cell; DC: Dendritic cell; ARG-1: Arginase 1; NOS: Nitric oxide; PD-L1: Programmed death-ligand 1; NK: Natural killer; ROS: Reactive oxygen species.
Several in vitro and in vivo studies have documented the mechanisms underlying the immunosuppressive actions of MDSCs. Arginase 1 (ARG1), inducible nitric oxide (iNOS), reactive oxygen species, and reactive nitrogen species are important suppressive factors produced by MDSCs[20,21]. Cao et al[22] reported that PMN-MDSCs store ARG1 and secrete it to the TME. ARG1 and NOS activities lead to cellular depletion of L-arginine (referred to as L-arg), which is an essential substrate for T cell proliferation[22,23]. Similar to T cells, depletion of L-arg also impairs the function of NK cells[24].
In addition to immunosuppressive factors, MDSCs can overpower T cell functions by directly engaging with T cell inhibitory and apoptotic receptors. Activated MDSCs express high levels of Fas ligand (referred to as Fas L), programmed death-ligand 1 (PD-L1), and galectin-9. Subsequently, the interaction between these ligands with their receptors on T cells leads to T cell exhaustion via PD-L1/programmed cell death protein 1 (PD-1) or T cell apoptosis through the Fas L/Fas and galectin-9/T cell immunoglobulin and mucin domain-3 pathways[25]. Generally, M-MDSCs have more suppressive effects than PMN-MDSCs[26]. Moreover, MDSCs can stimulate and recruit regulatory T cells (Tregs) to the TME[27]. Tregs suppress anti-tumor immunity, and the interaction between MDSCs and Tregs create a strong blockade preventing cytotoxic immune cells from mounting an anti-tumor attack[28]. Elevated levels of Tregs are associated with poor survival in patients with hepatocellular carcinoma (HCC) and pancreatic cancer[29,30]. Regarding metastasis, MDSCs can promote angiogenesis by secreting IL-28 [interferon lambda (IFN-λ)] and matrix metalloproteinase (MMP)-9, promoting the invasion and migration of tumor cells[31].
MARKERS OF MDSCS IN PERIPHERAL BLOOD
MDSCs are categorized according to their phenotype, which includes several recognized surface markers, such as cluster of differentiation 33 (CD33), CD11b, or human leukocyte antigen-DR isotype (HLA-DR), as well as by the lack of expression of markers distinctive of mature lymphoid cells, such as CD3, CD19, and CD56[32]. Typically, flow cytometry is performed to isolate MDSCs from peripheral mononuclear blood cells (PBMCs)[33]. Fluorescent-labeled monoclonal antibodies are used to distinguish M-MDSCs and granulocytic MDSCs (G-MDSCs). M-MDSCs are identified as CD11b+CD14+CD33highHLA-DR-/low and CD66b, whereas G-MDSCs are recognized as CD11b+CD14-CD33lowHLA-DR-CD66b+[34]. MDSCs can be distinguished from other immune suppressor cells within the myeloid lineage, e.g., tumor-associated macrophages and macrophage type 2, by other specific surface markers, including CD163 and F4/80[35].
MDSCS IN GI MALIGNANCIES
Esophageal cancer
High levels of circulating MDSCs in esophageal cancer are associated with a poor prognosis[5]. Elevated MDSCs in the blood are correlated with elevated numbers of immunosuppressive cells, including Tregs[5]. Jiao et al[36] evaluated 31 esophageal cancer patients and 26 healthy controls (HCs), and found that MDSC numbers in the peripheral blood were increased 15-fold in esophageal cancer patients compared to HCs. The authors also showed that the plasma levels of ARG1 were 3-fold higher in cancer patients than in HCs. Xu et al[37] showed that 178 patients with esophageal cancer had a high level of G-MDSCs (> 82.5%), which were correlated with high morbidity due to the development of sepsis postoperatively after esophageal cancer surgery. The authors suggested that the level of G-MDSCs may be used to determine the incidence of sepsis in preoperative esophageal cancer patients postoperatively, and could improve the mortality of cancer-associated sepsis by targeting the level of MDSCs.
Additionally, a study by Chen et al[38] found that the levels of IL-6 and MDSCs predicted the prognosis and treatment response in mice with esophageal squamous cell carcinoma (SCC). The levels of MDSCs induced by IL-6 were linked to tumor growth and a poor prognosis. The authors concluded that targeted therapy against IL-6 with rapamycin or casein kinase 2 inhibitors might be a potential treatment modality for esophageal SCC[38,39].
GC
According to the Global Cancer Observatory (GLOBOCAN) 2018 database, GC is the fifth most common cancer and third most deadly cancer worldwide, with an estimated 783000 deaths in 2018[40]. Li et al[41] documented the levels of MDSCs in the peripheral blood of 21 GC patients who had not previously received treatment, and noted that the levels of MDSCs in these patients were about 4-fold higher than in the control groups. The authors concluded that cancer cell differentiation and lymph node metastasis are mostly related to the presence of M-MDSCs. They also showed that treatment with epirubicin and paclitaxel regimens can reduce the level of MDSCs in these patients, potentially leading to better outcomes for patients due to inhibition of cancer progression. Moreover, in 29 patients with GC and 18 HCs, MDSCs were increased in stage IV patients compared with HCs, and the 2-year survival rate of patients with higher levels of MDSCs was significantly poorer (median OS: 498 d vs 473 d; P = 0.048), but no significant difference was observed in survival among patients with stage I, II, and III GC[42]. Previously, we reported that schlafen (SLFN) 4-expressing myeloid cells recruited to the stomach during Helicobacter infection undergo a phenotypic shift to G-MDSCs under the influence of damage-associated molecular pattern (DAMP) signaling and the production of IFN-α[43,44]. SLFN4 is a myeloid cell differentiation factor that controls myelopoiesis[45]. These SLFN-expressing MDSCs secrete factors including microRNAs, which can be detected in the peripheral blood as a biomarker and promote epithelial cell growth. This sustained immune dysregulation creates a microenvironment capable of supporting GC development[46].
HCC
HCC is a leading cause of death in cirrhotic patients. Per the GLOBOCAN 2018 database, 841000 new cases of primary liver cancer and 782000 deaths due to HCC occurred that year[40]. In a prospective case-control study, Elwan et al[47] demonstrated a higher number of MDSCs in the peripheral blood of cirrhotic groups without HCC than in patients with cirrhosis and HCC compared to patients in control groups. They showed that mean MDSC counts in the peripheral blood of cirrhotics without HCC group and cirrhotics with HCC group were about 3.5-fold and 5-fold higher compared to the control groups, respectively. Although not statistically significant, the authors reported a low number of MDSCs in the ascitic fluid of patients with both cirrhosis and HCC. Additionally, they investigated the correlation of levels of IFN-γ and alpha-fetoprotein with MDSC level. Their data showed that alpha-fetoprotein was positively and INF-γ was negatively correlated with MDSC count in the HCC group[47]. A high frequency of MDSCs in the PBMCs of patients with HCC has been linked to more aggressive forms of HCC and poor clinical outcomes following local ablation, hepatectomy, or hepatic arterial infusion chemotherapy[48,49]. A cohort study by Bayik et al[50] showed an upsurge in circulating MDSC frequency in 114 patients with a secondary liver cancer, including CRC with liver metastases and neuroendocrine tumors, compared to individuals with benign lesions.
Data from animal models have shown that myeloid cells secrete MMPs, serine proteases, and cysteine cathepsins, which facilitate tumor cell invasion and metastasis by disrupting cell adhesions[51]. Tumor angiogenesis in HCC can be promoted by MDSCs by producing high levels of MMP-9 in HCC[52].
Pancreatic cancer
The incidence of pancreatic ductal adenocarcinoma has significantly increased worldwide over the last 30 years, with a 5-year survival time less than 8%[53]. Khaled et al[54] demonstrated that G-MDSCs, but not M-MDSCs, are much higher in circulation and in the tumor tissue of patients with pancreatic cancer compared to HCs or those with chronic pancreatitis. These results suggest that the high level of G-MDSCs in pancreatic cancer plays a key factor in tumor development and progression. A cohort study reported that the percentages of all subpopulations of MDSCs were higher in patients with intraductal papillary mucinous neoplasm (IPMN) than in HCs, and were even higher in those with pancreatic adenocarcinoma. Although there was a trend towards higher MDSC levels in pancreatic cancer vs IPMN, it was not statistically significant (P = 0.33)[55].
CRC
A recent study reported that CRC cells induce an increase in the number of MDSCs by producing inflammatory factors, such as transforming growth factor-beta, IL-10, and ARG1[56,57]. Consequently, T cell proliferation can be suppressed by tumor-derived MDSCs and promote tumor cell growth via oxidative metabolism. Previously, it was shown that the numbers of circulating Tregs and MDSCs are significantly reduced following tumor resection in patients with CRC. These data indicate that immunosuppression can be mitigated by reducing the number of MDSCs and Tregs in patients with CRC after reducing the tumor burden[57]. Tada et al[58] showed that patients with unresectable metastatic CRC with high M-MDSC, low CD4+, or low CD8+ effector memory T cell levels had significantly shorter progression-free survival.
MDSC-TARGETED THERAPY
Many studies have examined MDSCs as the core of targeted therapeutic strategies to improve tumor control in experimental animal models. These targeted therapies could be achieved by reducing MDSC numbers, hindering their trafficking and migration, or inhibiting their immunosuppressive function (Table 2)[59].
Table 2.
Potential therapeutic strategy for targeting myeloid-derived suppressor cells
|
Strategy
|
Agents
|
| Blocking TDFs from being produced or from reaching the bone marrow | Targeting the IL-6 receptor (tocilizumab)[83] |
| Key cytokines, such as IL-6 or S100A8/A9, could be directly targeted[82,84] | |
| Inhibiting generation of MDSCs from bone marrow progenitors or inducing apoptosis of circulating MDSCs[6] | Gemcitabine, 5-fluorouracil, sunitinib, and zolendronate[84] |
| Preventing trafficking of myeloid cells from the marrow to peripheral lymphoid organs or to the tumor microenvironment[6] | Drugs targeting chemokines CXCR2, CXCR4, and CSF1R[14] |
| Directly blocking MDSC suppression of T cells[85] | Phosphodiesterase type 5 inhibitors, e.g., sildenafil and tadalafil, or cyclooxygenase 2 inhibitors[63] |
| Drugs that would promote differentiation of MDSCs into proficient antigen-presenting cells that can stimulate tumor-specific T cells and/or into mature leukocytes[85] | All-trans retinoic acid, vitamin D3, and the DNA-methylating agent 5-azacytidine[85] |
MDSCs: Myeloid-derived suppressor cells; CSF1R: Colony-stimulating factor 1 receptor; CXCR2: C-X-C motif receptor 2; CXCR4: C-X-C motif receptor 4; IL-6: Interleukin-6; TDFs: Tumor-derived factors.
Because of variances in immunophenotype and mechanisms of suppression in the TME and diverse nature of human MDSCs, it is challenging to target human MDSCs[60]. Wang et al[61] treated pancreatic cancer patients with cytokine-induced killer (CIK) cell immunotherapy, CIK plus gemcitabine, and 5-fluorouracil (5-FU), and analyzed the levels of MDSCs in the peripheral blood pre- and post-treatment. The OS of metastatic pancreatic patients was increased with the combination of CIK and chemotherapy (gemcitabine and 5-FU) compared to patients treated with only CIK. Also, Jiang et al[62] reported that the quality of life and 2-year survival rate improved in patients with advanced GC following combining chemotherapy (5-FU and oxaliplatin) with CIK cell treatment compared to treatment with chemotherapy alone. Tadalafil, the Federal Drug Administration-approved phosphodiesterase-5 inhibitor, can suppress MDSCs through downregulation of ARG1 and iNOS activities in several preclinical models[63-65]. Rawat et al[66] showed in aflatoxin-induced HCC rats, that tadalafil reduced the level of glutamic oxaloacetic transaminase, an important enzyme that facilitates carbohydrate and protein metabolism in cancer cells. A previous study showed that treatment with tyrosine kinase inhibitors, such as sunitinib, reduced the number of MDSCs and Tregs in animals with intrahepatic colorectal metastases[67]. The authors also showed that the number of MDSCs was significantly reduced from 53.9% in phosphate-buffered saline-treated mice to 39% in sunitinib-treated mice. Sunitinib has established efficacy against advanced GI stromal tumors[68].
Treatment of esophageal SCC with 1α,25-dihydroxyvitamin D3 (calcitriol) has been shown to inhibit MDSC proliferation induced by IL-6 stimulation in C57 mice. Therefore, it has been proposed that this treatment may be a promising strategy for the prevention and treatment of esophageal SCC[69].
ICIs such as cytotoxic T-lymphocyte-associated protein 4 (ipilimumab and tremelimumab), PD-1 (pembrolizumab and nivolumab), and PD-L1 (atezolizumab, avelumab, and durvalumab) are promising treatment strategies that can be applied across numerous solid tumors[70]. Pembrolizumab is a therapeutic antibody that blocks PD-1, and has shown promising anti-tumor activity in advanced GC[71]. Although ICIs show great therapeutic benefits, substantial GI side effects such as colitis and GI bleeding can limit their use[72]. However, due to immunosuppression, which is regulated by MDSCs, some patients with cancer may develop resistance to ICIs[73]. Therefore, it is important to inhibit MDSC proliferation and migration to the TME by different strategies, such as anti-CXCR2 monoclonal antibody, to enhance PD-1 efficacy[74].
CORONAVIRUS-19 AND MDSCS
In this age of coronavirus-19 (COVID-19), we would be remiss not to address what is currently known about activation of the host’s immune response, in particular MDSCs, by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). Plasmacytoid DCs (pDCs) are the major source of tissue-derived type 1 IFNs in response to tissue antigens and activation of DAMPs. Typically, intracellular Toll-like receptors (TLRs) found on endosomes that mediate this pathway evolve to defend the cell against viral pathogens[75]. Thus, we queried whether coronavirus infections might be modulated by pDCs residing in the GI tract. Apparently, SARS-CoV-2 induces a massive anti-viral response by secretion of IFN-α from pDCs via TLR7[76]. The severity of COVID-19 infection might correlate with the activation of endosomal TLRs on pDCs in the GI tract and increased myeloid cell polarization to MDSCs. If this occurs, the cellular immune response to the virus could be rendered ineffective, suggesting that those with severe disease exhibit higher levels of MDSCs than those with mild disease. Indeed, the limited studies available of COVID-19 patients have shown that the MDSC population expands in those with severe disease[77,78]. Although T cell exhaustion from the cytokine storm that COVID-19 patients display is the leading cause for severe disease, massive production of immune suppressor cells also explains the lymphopenia occurring in many of these patients[79,80]. A better understanding of what controls MDSCs will facilitate not only therapeutic treatments but may ultimately help to predict who will respond to vaccination. Whether patients who recover from these infections are predisposed to chronic disorders, such as autoimmune diseases and cancer, will require long-term follow up of these patients over decades.
CONCLUSION
This review article provides a better understanding of the role and mechanism of action of MDSCs in GI malignancies. MDSCs are one of the most important elements in the TME. In patients with GI cancer, MDSCs can lead to immunosuppression, and they play an important role in premalignant cell transformation, tumor growth, and metastasis. A higher number of MDSCs at tumor sites and peripheral blood is correlated with higher mortality rates, risk of relapse, and tumor progression. Therefore, monitoring circulating MDSC levels might have prognostic and predictive value in patients with GI malignancies. The benefit of targeting treatment against MDSCs as a combination therapy has been shown. Consequently, a better comprehension of the role and mechanism of action of MDSCs in the TME may aid in the development of novel immune-targeted therapies. Further prospective studies are needed to understand the characterization and clinical value of MDSCs and more selective anti-MDSC therapies with improved therapeutic outcomes.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: August 25, 2020
First decision: November 16, 2020
Article in press: December 16, 2020
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gao F, Mortazavian AM S-Editor: Huang P L-Editor: A P-Editor: Li JH
Contributor Information
Maham Farshidpour, Inpatient Medicine, Banner University of Medical Center, Tucson, AZ 85724, United States. mfarshidpour@email.arizona.edu.
Monjur Ahmed, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Thomas Jefferson University, Philadelphia, PA 19107, United States.
Shilpa Junna, Division of Gastroenterology and Hepatology, Banner University of Medical Center, Tucson, AZ 85724, United States.
Juanita L Merchant, Division of Gastroenterology and Hepatology, Banner University of Medical Center, Tucson, AZ 85724, United States.
References
- 1.Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020; 159: 335-349. :e15. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffy A, Zhao F, Haile L, Gamrekelashvili J, Fioravanti S, Ma C, Kapanadze T, Compton K, Figg WD, Greten TF. Comparative analysis of monocytic and granulocytic myeloid-derived suppressor cell subsets in patients with gastrointestinal malignancies. Cancer Immunol Immunother. 2013;62:299–307. doi: 10.1007/s00262-012-1332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilcox RA. Cancer-associated myeloproliferation: old association, new therapeutic target. Mayo Clin Proc. 2010;85:656–663. doi: 10.4065/mcp.2010.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer Immunol Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol. 2013;190:794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- 9.Gao XH, Tian L, Wu J, Ma XL, Zhang CY, Zhou Y, Sun YF, Hu B, Qiu SJ, Zhou J, Fan J, Guo W, Yang XR. Circulating CD14+ HLA-DR-/low myeloid-derived suppressor cells predicted early recurrence of hepatocellular carcinoma after surgery. Hepatol Res. 2017;47:1061–1071. doi: 10.1111/hepr.12831. [DOI] [PubMed] [Google Scholar]
- 10.Hirbod-Mobarakeh A, Mirghorbani M, Hajiju F, Marvi M, Bashiri K, Rezaei N. Myeloid-derived suppressor cells in gastrointestinal cancers: A systematic review. J Gastroenterol Hepatol. 2016;31:1246–1256. doi: 10.1111/jgh.13284. [DOI] [PubMed] [Google Scholar]
- 11.Wang PF, Song SY, Wang TJ, Ji WJ, Li SW, Liu N, Yan CX. Prognostic role of pretreatment circulating MDSCs in patients with solid malignancies: A meta-analysis of 40 studies. Oncoimmunology. 2018;7:e1494113. doi: 10.1080/2162402X.2018.1494113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibata M, Gonda K, Nakajima T, Matsumoto Y, Nakamura I, Ohki S, Ohtake T, Kumamoto K, Shimura T, Takenoshita S, Abe N, Momma T. Pretreatment serum levels of circulating myeloid-derived suppressor cells (MDSC) as a prognostic indicator in patients with gastrointestinal cancer. In ASCO Annual Meeting 2014; Chicago. [Google Scholar]
- 13.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012;61:1155–1167. doi: 10.1007/s00262-012-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28:509–554. doi: 10.1016/j.dci.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chornoguz O, Grmai L, Sinha P, Artemenko KA, Zubarev RA, Ostrand-Rosenberg S. Proteomic pathway analysis reveals inflammation increases myeloid-derived suppressor cell resistance to apoptosis. Mol Cell Proteomics 2011; 10: M110. 2980:00. doi: 10.1074/mcp.M110.002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khaled YS, Ammori BJ, Elkord E. Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol Cell Biol. 2013;91:493–502. doi: 10.1038/icb.2013.29. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y, Feng Y, Zhang Y, Zhu X, Jin F. L-Arginine supplementation inhibits the growth of breast cancer by enhancing innate and adaptive immune responses mediated by suppression of MDSCs in vivo. BMC Cancer. 2016;16:343. doi: 10.1186/s12885-016-2376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goh CC, Roggerson KM, Lee HC, Golden-Mason L, Rosen HR, Hahn YS. Hepatitis C Virus-Induced Myeloid-Derived Suppressor Cells Suppress NK Cell IFN-γ Production by Altering Cellular Metabolism via Arginase-1. J Immunol. 2016;196:2283–2292. doi: 10.4049/jimmunol.1501881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinha P, Chornoguz O, Clements VK, Artemenko KA, Zubarev RA, Ostrand-Rosenberg S. Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood. 2011;117:5381–5390. doi: 10.1182/blood-2010-11-321752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 27.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 28.Goedegebuure P, Mitchem JB, Porembka MR, Tan MC, Belt BA, Wang-Gillam A, Gillanders WE, Hawkins WG, Linehan DC. Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr Cancer Drug Targets. 2011;11:734–751. doi: 10.2174/156800911796191024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu YX, Wang FS. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 30.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 31.Mucha J, Majchrzak K, Taciak B, Hellmén E, Król M. MDSCs mediate angiogenesis and predispose canine mammary tumor cells for metastasis via IL-28/IL-28RA (IFN-λ) signaling. PLoS One. 2014;9:e103249. doi: 10.1371/journal.pone.0103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–353. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 33.Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods. 2012;381:14–22. doi: 10.1016/j.jim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marini O, Spina C, Mimiola E, Cassaro A, Malerba G, Todeschini G, Perbellini O, Scupoli M, Carli G, Facchinelli D, Cassatella M, Scapini P, Tecchio C. Identification of granulocytic myeloid-derived suppressor cells (G-MDSCs) in the peripheral blood of Hodgkin and non-Hodgkin lymphoma patients. Oncotarget. 2016;7:27676–27688. doi: 10.18632/oncotarget.8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broz ML, Krummel MF. The emerging understanding of myeloid cells as partners and targets in tumor rejection. Cancer Immunol Res. 2015;3:313–319. doi: 10.1158/2326-6066.CIR-15-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiao ZJ, Gao JJ, Hua SH, Chen DY, Wang WH, Wang H, Wang XH, Xu HX. Correlation between circulating myeloid-derived suppressor cells and Th17 cells in esophageal cancer. World J Gastroenterol. 2012;18:5454–5461. doi: 10.3748/wjg.v18.i38.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Peng Y, Yang M, Guo N, Liu H, Gao H, Niu F, Wang R, Wang C, Yu K. Increased levels of myeloid-derived suppressor cells in esophageal cancer patients is associated with the complication of sepsis. Biomed Pharmacother. 2020;125:109864. doi: 10.1016/j.biopha.2020.109864. [DOI] [PubMed] [Google Scholar]
- 38.Chen MF, Kuan FC, Yen TC, Lu MS, Lin PY, Chung YH, Chen WC, Lee KD. IL-6-stimulated CD11b+ CD14+ HLA-DR- myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget. 2014;5:8716–8728. doi: 10.18632/oncotarget.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aparicio-Siegmund S, Sommer J, Monhasery N, Schwanbeck R, Keil E, Finkenstädt D, Pfeffer K, Rose-John S, Scheller J, Garbers C. Inhibition of protein kinase II (CK2) prevents induced signal transducer and activator of transcription (STAT) 1/3 and constitutive STAT3 activation. Oncotarget. 2014;5:2131–2148. doi: 10.18632/oncotarget.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 41.Li N, Han D, Sun J, Li Y, Zhang J, Zhang Y, Liu M, Peng R, Wang H, Zhang Z, Wang J, Liu Z, Ma J. Subtypes of MDSCs in mechanisms and prognosis of gastric cancer and are inhibited by epirubicin and paclitaxel. Discov Med. 2018;25:99–112. [PubMed] [Google Scholar]
- 42.Gonda K, Kanke Y, Yazawa T, Monma T, Nakamura SS, Ohki S, Shimura T, Ohto H, Takenoshita S. Circulating myeloid-derived suppressor cells (MDSC) and correlation to poor prognosis, Th2-polarization, inflammation, and nutritional damages in patients with gastric cancer. J Clin Oncol . 2013;31:3063–3063. [Google Scholar]
- 43.Ding L, Hayes MM, Photenhauer A, Eaton KA, Li Q, Ocadiz-Ruiz R, Merchant JL. Schlafen 4-expressing myeloid-derived suppressor cells are induced during murine gastric metaplasia. J Clin Invest. 2016;126:2867–2880. doi: 10.1172/JCI82529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merchant JL, Ding L. Hedgehog Signaling Links Chronic Inflammation to Gastric Cancer Precursor Lesions. Cell Mol Gastroenterol Hepatol. 2017;3:201–210. doi: 10.1016/j.jcmgh.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Companioni Nápoles O, Tsao AC, Sanz-Anquela JM, Sala N, Bonet C, Pardo ML, Ding L, Simo O, Saqui-Salces M, Blanco VP, Gonzalez CA, Merchant JL. SCHLAFEN 5 expression correlates with intestinal metaplasia that progresses to gastric cancer. J Gastroenterol. 2017;52:39–49. doi: 10.1007/s00535-016-1202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding L, Li Q, Chakrabarti J, Munoz A, Faure-Kumar E, Ocadiz-Ruiz R, Razumilava N, Zhang G, Hayes MH, Sontz RA, Mendoza ZE, Mahurkar S, Greenson JK, Perez-Perez G, Hanh NTH, Zavros Y, Samuelson LC, Iliopoulos D, Merchant JL. MiR130b from Schlafen4+ MDSCs stimulates epithelial proliferation and correlates with preneoplastic changes prior to gastric cancer. Gut. 2020;69:1750–1761. doi: 10.1136/gutjnl-2019-318817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elwan N, Salem ML, Kobtan A, El-Kalla F, Mansour L, Yousef M, Al-Sabbagh A, Zidan AA, Abd-Elsalam S. High numbers of myeloid derived suppressor cells in peripheral blood and ascitic fluid of cirrhotic and HCC patients. Immunol Invest. 2018;47:169–180. doi: 10.1080/08820139.2017.1407787. [DOI] [PubMed] [Google Scholar]
- 48.Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 2013;62:1421–1430. doi: 10.1007/s00262-013-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizukoshi E, Yamashita T, Arai K, Terashima T, Kitahara M, Nakagawa H, Iida N, Fushimi K, Kaneko S. Myeloid-derived suppressor cells correlate with patient outcomes in hepatic arterial infusion chemotherapy for hepatocellular carcinoma. Cancer Immunol Immunother. 2016;65:715–725. doi: 10.1007/s00262-016-1837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bayik D LA, Roversi GA, Serbinowski E, Acevedo-Moreno L, Lanigan C, Orujov M, Lo A, Alban TJ, Silver DJ, Brown JM, Allende DS, Aucejo FN, Lathia JD. Cholangiocarcinoma presents a distinct myeloid-derived suppressor cell signature compared to other hepatobiliary cancers. bioRxiv . 2019 [Google Scholar]
- 51.Aggarwal N, Sloane BF. Cathepsin B: multiple roles in cancer. Proteomics Clin Appl. 2014;8:427–437. doi: 10.1002/prca.201300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roderfeld M, Rath T, Lammert F, Dierkes C, Graf J, Roeb E. Innovative immunohistochemistry identifies MMP-9 expressing macrophages at the invasive front of murine HCC. World J Hepatol. 2010;2:175–179. doi: 10.4254/wjh.v2.i5.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, Weiss MJ, Zheng L, Wolfgang CL, He J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2018;267:936–945. doi: 10.1097/SLA.0000000000002234. [DOI] [PubMed] [Google Scholar]
- 54.Khaled YS, Ammori BJ, Elkord E. Increased levels of granulocytic myeloid-derived suppressor cells in peripheral blood and tumour tissue of pancreatic cancer patients. J Immunol Res. 2014;2014:879897. doi: 10.1155/2014/879897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma P, Beatty PL, McKolanis J, Brand R, Schoen RE, Finn OJ. Circulating Myeloid Derived Suppressor Cells (MDSC) That Accumulate in Premalignancy Share Phenotypic and Functional Characteristics With MDSC in Cancer. Front Immunol. 2019;10:1401. doi: 10.3389/fimmu.2019.01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, Fuchs JR, Eubank TD, Frankel WL, Bekaii-Saab T, Bloomston M, Lesinski GB. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73:3007–3018. doi: 10.1158/0008-5472.CAN-12-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.OuYang LY, Wu XJ, Ye SB, Zhang RX, Li ZL, Liao W, Pan ZZ, Zheng LM, Zhang XS, Wang Z, Li Q, Ma G, Li J. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J Transl Med. 2015;13:47. doi: 10.1186/s12967-015-0410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tada K, Kitano S, Shoji H, Nishimura T, Shimada Y, Nagashima K, Aoki K, Hiraoka N, Honma Y, Iwasa S, Okita N, Takashima A, Kato K, Yamada Y, Katayama N, Boku N, Heike Y, Hamaguchi T. Pretreatment Immune Status Correlates with Progression-Free Survival in Chemotherapy-Treated Metastatic Colorectal Cancer Patients. Cancer Immunol Res. 2016;4:592–599. doi: 10.1158/2326-6066.CIR-15-0298. [DOI] [PubMed] [Google Scholar]
- 59.Lu LC, Chang CJ, Hsu CH. Targeting myeloid-derived suppressor cells in the treatment of hepatocellular carcinoma: current state and future perspectives. J Hepatocell Carcinoma. 2019;6:71–84. doi: 10.2147/JHC.S159693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fultang L, Panetti S, Ng M, Collins P, Graef S, Rizkalla N, Booth S, Lenton R, Noyvert B, Shannon-Lowe C, Middleton G, Mussai F, De Santo C. MDSC targeting with Gemtuzumab ozogamicin restores T cell immunity and immunotherapy against cancers. EBioMedicine. 2019;47:235–246. doi: 10.1016/j.ebiom.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z, Liu Y, Zhang Y, Shang Y, Gao Q. MDSC-decreasing chemotherapy increases the efficacy of cytokine-induced killer cell immunotherapy in metastatic renal cell carcinoma and pancreatic cancer. Oncotarget. 2016;7:4760–4769. doi: 10.18632/oncotarget.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang J, Xu N, Wu C, Deng H, Lu M, Li M, Xu B, Wu J, Wang R, Xu J, Nilsson-Ehle P. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res. 2006;26:2237–2242. [PubMed] [Google Scholar]
- 63.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin S, Wang J, Wang L, Wen J, Guo Y, Qiao W, Zhou J, Xu G, Zhi F. Phosphodiesterase-5 inhibition suppresses colonic inflammation-induced tumorigenesis via blocking the recruitment of MDSC. Am J Cancer Res. 2017;7:41–52. [PMC free article] [PubMed] [Google Scholar]
- 65.Yu SJ, Ma C, Heinrich B, Brown ZJ, Sandhu M, Zhang Q, Fu Q, Agdashian D, Rosato U, Korangy F, Greten TF. Targeting the crosstalk between cytokine-induced killer cells and myeloid-derived suppressor cells in hepatocellular carcinoma. J Hepatol. 2019;70:449–457. doi: 10.1016/j.jhep.2018.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rawat D, Koiri RK. Tadalafil inhibits elevated glutamic oxaloacetic transaminase during alcohol aflatoxin induced hepatocellular carcinoma in rats. Int J Immunother Cancer Res. 2020;6:10–13. [Google Scholar]
- 67.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mulet-Margalef N, Garcia-Del-Muro X. Sunitinib in the treatment of gastrointestinal stromal tumor: patient selection and perspectives. Onco Targets Ther. 2016;9:7573–7582. doi: 10.2147/OTT.S101385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen PT, Hsieh CC, Wu CT, Yen TC, Lin PY, Chen WC, Chen MF. 1α,25-Dihydroxyvitamin D3 Inhibits Esophageal Squamous Cell Carcinoma Progression by Reducing IL6 Signaling. Mol Cancer Ther. 2015;14:1365–1375. doi: 10.1158/1535-7163.MCT-14-0952. [DOI] [PubMed] [Google Scholar]
- 70.Albertini MR. The age of enlightenment in melanoma immunotherapy. J Immunother Cancer. 2018;6:80. doi: 10.1186/s40425-018-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, McRee AJ, Lin CC, Pathiraja K, Lunceford J, Emancipator K, Juco J, Koshiji M, Bang YJ. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 72.Wang ZH, Shen L. Management of gastrointestinal adverse events induced by immune-checkpoint inhibitors. Chronic Dis Transl Med. 2018;4:1–7. doi: 10.1016/j.cdtm.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weber R, Fleming V, Hu X, Nagibin V, Groth C, Altevogt P, Utikal J, Umansky V. Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Front Immunol. 2018;9:1310. doi: 10.3389/fimmu.2018.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 76.Cervantes-Barragan L, Züst R, Weber F, Spiegel M, Lang KS, Akira S, Thiel V, Ludewig B. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agrati C, Sacchi A, Bordoni V, Cimini E, Notari S, Grassi G, Casetti R, Tartaglia E, Lalle E, D'Abramo A, Castilletti C, Marchioni L, Shi Y, Mariano A, Song JW, Zhang JY, Wang FS, Zhang C, Fimia GM, Capobianchi MR, Piacentini M, Antinori A, Nicastri E, Maeurer M, Zumla A, Ippolito G. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19) Cell Death Differ. 2020;27:3196–3207. doi: 10.1038/s41418-020-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bordoni V, Sacchi A, Cimini E, Notari S, Grassi G, Tartaglia E, Casetti R, Giancola ML, Bevilacqua N, Maeurer M, Zumla A, Locatelli F, De Benedetti F, Palmieri F, Marchioni L, Capobianchi MR, D'Offizi G, Petrosillo N, Antinori A, Nicastri E, Ippolito G, Agrati C. An Inflammatory Profile Correlates With Decreased Frequency of Cytotoxic Cells in Coronavirus Disease 2019. Clin Infect Dis. 2020;71:2272–2275. doi: 10.1093/cid/ciaa577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fathi N, Rezaei N. Lymphopenia in COVID-19: Therapeutic opportunities. Cell Biol Int. 2020;44:1792–1797. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Umansky V, Blattner C, Gebhardt C, Utikal J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines (Basel) 2016;4:36. doi: 10.3390/vaccines4040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Diaz-Montero CM, Finke J, Montero AJ. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol. 2014;41:174–184. doi: 10.1053/j.seminoncol.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tobin RP, Jordan KR, Kapoor P, Spongberg E, Davis D, Vorwald VM, Couts KL, Gao D, Smith DE, Borgers JSW, Robinson S, Amato C, Gonzalez R, Lewis KD, Robinson WA, Borges VF, McCarter MD. IL-6 and IL-8 Are Linked With Myeloid-Derived Suppressor Cell Accumulation and Correlate With Poor Clinical Outcomes in Melanoma Patients. Front Oncol. 2019;9:1223. doi: 10.3389/fonc.2019.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. 2013;3:49. doi: 10.3389/fonc.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Daurkin I, Eruslanov E, Vieweg J, Kusmartsev S. Generation of antigen-presenting cells from tumor-infiltrated CD11b myeloid cells with DNA demethylating agent 5-aza-2'-deoxycytidine. Cancer Immunol Immunother. 2010;59:697–706. doi: 10.1007/s00262-009-0786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]