Abstract
BACKGROUND
Transanal total mesorectal excision (taTME) is a new technique with many potential technical advantages. Laparoscopy-assisted taTME is a combination of transabdominal taTME and transluminal endoscopic surgery taTME. Laparoscopy-assisted taTME is a combination of techniques such as minimally invasive surgery, intersphincter-assisted resection, natural orifice extraction, ta minimally invasive surgery, and ultralow-level preservation of the anus.
AIM
To verify the feasibility and safety of an innovative technique of taTME for treatment of cancer located in the lower rectum.
METHODS
From January 2016 to March 2018, we attempted to perform laparoscopy-assisted taTME surgery in 24 patients with lower rectal cancer.
RESULTS
The new technique of laparoscopy-assisted taTME was successfully performed in all 24 patients. Mean operating time was 310.0 min and mean intraoperative blood loss was 69.1 mL. The mean time to passing of first flatus was 3.1 d, and mean postoperative hospital stay was 9.2 d. Two patients were given postoperative analgesics due to anal pain. Twenty-three patients were able to walk in first 2 d, and five patients had postoperative complications.
CONCLUSION
Laparoscopy-assisted taTME is suitable for selected patients with lower rectal cancer, and this technique is worthy of further recommendation.
Keywords: Laparoscopy-assisted, Total mesorectal excision, Technique, Lower rectal cancer, Trans-abdominal, Trans-anus
Core Tip: We report our initial experience with transanal total mesorectal excision for distal rectal cancer, with a 100% success rate of intraoperative preservation of the anal sphincter. The patients in this study had a narrow pelvis, mild obesity, and distal rectal lesions, making the operation extremely difficult. Most of the patients had undergone neoadjuvant chemoradiation. We believe that this procedure is feasible for selected patients with lower rectal cancer, and is worthy of further recommendation.
INTRODUCTION
The introduction of total mesorectal excision (TME) in combination with advanced neoadjuvant and adjuvant oncological treatment regimens has improved overall survival and local recurrence in rectal cancer patients[1]. Although minimal invasive procedures are being used more frequently and generally add to this positive development, dissection in the pelvic cavity remains a technical challenge[2,3]. Concerns about adequate distal resection margin, bulky tumors and inadequate view of the operating field in the lower pelvis may negatively affect the surgical as well as oncological quality of the procedure, which reduces the advantages of laparoscopy-assisted approaches[4]. Laparoscopy-assisted transanal (ta) TME has been developed as a means of reducing the above disadvantages. This approach is reasonable in lower rectal cancer patients with a strong desire for preserving anus, narrow pelvic, or obesity, or male patients[5-8]. Between January 2016 and March 2018, 24 patients with lower rectal cancer were treated by laparoscopy-assisted taTME.
MATERIALS AND METHODS
Patients
From January 2016 to March 2018, we attempted to perform laparoscopy-assisted taTME for 24 patients with lower rectal cancer (Table 1). Currently, there is no international standard surgical indication for taTME. The suggested indications are: Low rectal cancer, distance 0-8 cm from anal verge (defined by magnetic resonance imaging [MRI]), histological biopsy showing adenocarcinoma stage I-III (by MRI and abdominal computed tomography). All patients underwent a standard clinical examination including rigid proctoscopy, MRI of the rectum, and thoracoabdominal computed tomography. Distant metastasis was excluded by imaging examination. All operations were performed by a stationary surgical team. The patients were selected to have a detailed understanding of the taTME surgical procedure and risks, have a strong desire to retain the anus and choose the surgical method, and take risks.
Table 1.
Patient characteristics
|
Cases
|
Age in yr
|
Gender
|
BMI, kg/m2
|
Tumor height from anorectal junction in cm
|
Clinical stage
|
CRT
|
Pathological stage
|
Tumor size in cm
|
Technique
|
Operation time in min
|
Blood loss in mL
|
POHS
|
Time to first flatus in d
|
Complications
|
| 1 | 64 | F | 21.94 | 5.0 | T3N2b | Yes | ypT0N0 | 7.0 | taTME | 198 | 100 | 9 | 2 | N |
| 2 | 48 | M | 30.78 | 5.0 | T3N2b | Yes | ypT3N1b | 1.5 | taTME | 270 | 100 | 12 | 3 | N |
| 3 | 64 | M | 30.06 | 5.0 | T3N2b | Yes | ypT3N0 | 1.5 | taTME (Hartmann) | 364 | 100 | 9 | 2 | N |
| 4 | 67 | M | 25.24 | 6.0 | T3N1 | Yes | ypT2N1a | 2.0 | taTME | 270 | 100 | 11 | 2 | Fever |
| 5 | 57 | M | 27.11 | 5.0 | T3N2 | Yes | ypT2N0 | 2.0 | taTME | 392 | 100 | 9 | 3 | N |
| 6 | 66 | M | 20.44 | 3.0 | T3N+ | Yes | ypT2N0 | 1.0 | taTME | 262 | 50 | 9 | 2 | N |
| 7 | 45 | M | 26.67 | 4.0 | T3N0 | Yes | ypT3N0 | 1.2 | taTME | 317 | 200 | 8 | 2 | N |
| 8 | 38 | M | 25.82 | 3.0 | T3bN2a | Yes | ypT2N0 | 2.0 | taTME | 266 | 200 | 9 | 2 | N |
| 9 | 47 | M | 24.39 | 5.0 | T3N+ | Yes | ypT2N1 | 2.5 | taTME | 375 | 100 | 14 | 10 | Obstruction |
| 10 | 61 | M | 26.20 | 4.0 | T3N2b | Yes | ypT3N1a | 1.3 | taTME | 293 | 100 | 9 | 3 | N |
| 11 | 62 | M | 29.41 | 6.0 | T4aN+ | Yes | ypT3N2b | 2.0 | taTME | 337 | 100 | 10 | 2 | N |
| 12 | 72 | M | 36.37 | 3.0 | T4N+ | Yes | ypT0N1a | 0.5 | taTME | 405 | 200 | 12 | 2 | Fever |
| 13 | 65 | M | 24.0 | 7.0 | T3N+ | Yes | ypT3N1a | 2.0 | taTME | 290 | 100 | 7 | 2 | N |
| 14 | 51 | M | 25.76 | 2.0 | T3N2b | Yes | ypT1N1b | 2.0 | taTME | 364 | 200 | 9 | 2 | N |
| 15 | 55 | M | 23.66 | 8.0 | T3N+ | Yes | ypT3N0 | 4.0 | taTME | 400 | 100 | 10 | 2 | N |
| 16 | 30 | M | 26.17 | 3.0 | T3N0 | Yes | ypT0N0 | 0.0 | taTME | 283 | 100 | 10 | 2 | N |
| 17 | 61 | M | 26.99 | 5.0 | T2N2 | Yes | ypT2N0 | 1.5 | taTME | 245 | 50 | 7 | 2 | Fever |
| 18 | 49 | F | 26.34 | 2.0 | T3N+ | Yes | ypT2N0 | 1.1 | taTME | 226 | 100 | 7 | 2 | N |
| 19 | 58 | M | 20.96 | 2.0 | T3N+ | Yes | ypT3N0 | 1.0 | taTME | 342 | 200 | 10 | 2 | N |
| 20 | 60 | M | 26.90 | 5.0 | T3N1 | No | ypT3N0 | 5 | taTME | 271 | 100 | 7 | 2 | N |
| 21 | 71 | M | 25.3 | 5.0 | T3N1 | Yes | ypT2N0 | 0.5 | taTME | 310 | 100 | 8 | 2 | Anastomotic fracture |
| 22 | 69 | M | 23.9 | 3.0 | T3N2 | Yes | ypTIN0 | 1.5 | taTME | 317 | 50 | 8 | 2 | N |
| 23 | 39 | F | 24.2 | 4.0 | Neuroendocrine tumor G2 | No | Neuroendocrine tumor G2 | 1.0 | taTME | 235 | 50 | 12 | 2 | N |
| 24 | 59 | M | 23.39 | 4.0 | T3N+ | Yes | ypT2N0 | 1.0 | taTME | 266 | 50 | 8 | 2 | N |
BMI: Body mass index; CRT:chemoradiotherapy; POHS: Post-operative hospital stay; F: Female; M: Male; taTME: Transanal total mesorectal excision; N: None.
The training phase included cadaver dissection, technical and practical courses, and participation as an assistant in a human taTME procedure.
All patients received standard mechanical bowel preparation and followed a standard postoperative enhanced recovery program for colorectal resection.
TaTME technique
After induction of general anesthesia, proctoscopy was performed to ensure adequate preoperative bowel preparation and to perform washout of the rectum with Povidone-iodine (PVP-I).
The procedure started with the laparoscopic transabdominal part of the dissection according to TME principles and high ligation of the inferior mesenteric artery. To ease the transanal extraction of the specimen, the splenic flexure was mobilized entirely to the midline in some patients. The mobilization continued caudally/posteriorly to the sacral promontory and anteriorly to the peritoneal reflection of the rectum. and anteriorly to the level of vagina/seminal vesicles (Figure 1).
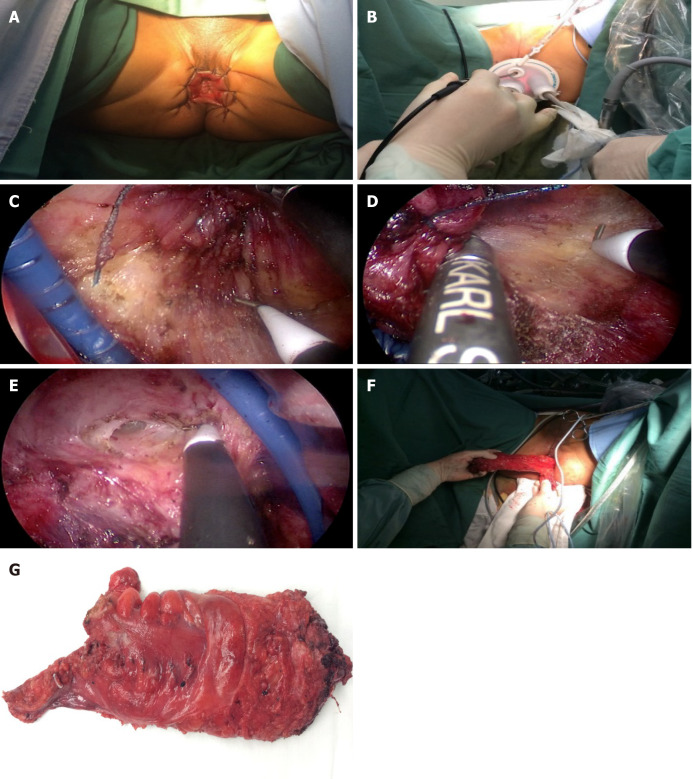
Figure 1.
Transanal total mesorectal excision surgical procedure presentation. A: Anal exposure (anal and perianal skin suture); B: Place the operation platform (Star port); C: Left lateral dissection; D: Right lateral dissection; E: Anterior dissection; F: Specimen extraction.
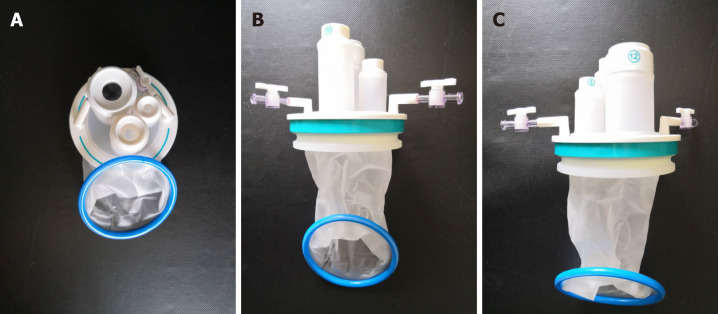
We commenced by inserting an Applied Medical STARPORT Path Trans Anal Access Platform and closed the rectum with a purse string suture under direct visualization of the tumor (Figure 2). This provided optimal conditions for identifying the appropriate distal margin at least 1 cm below the tumor.
Figure 2.
Photos of the transanal STARPORT used in transanal total mesorectal excision surgery. The transanal STARPORT consists of an air-tight cover and an anal dilator. A: Positive view; B: Back view; C: Side view.
A full thickness incision of the entire rectum wall provided access to the anatomical planes. Mobilization of the mesorectum started posteriorly and was then continued anteriorly. Finally, the dissection progressed laterally, to avoid the specimen blocking the operating field during the posterior dissection and to ensure the correct lateral plane to avoid nerve injury (taTME video).
When communication to the peritoneal cavity was achieved and the ta and transabdominal dissection planes met, one surgeon returned to the abdominal group and assisted with the final mobilization of the rectum. Depending on the tumor size, the specimen was extracted either via a Pfannenstiel incision or transanally, using the Wound Protector.
The remaining part of the colonic mesentery was divided extracorporeally above the division of the inferior mesenteric artery, testing the marginal artery for sufficient blood flow by division and subsequent ligation. A purse string was made and the anvil of the circular stapler was inserted and fastened. The end of the colon could now be repositioned into the abdomen and the anal distal stamp was pre-closed with a purse string suture, stitching the anvil before connecting with the central rode of the stapler. The anastomosis was performed as end-to-end or side-to-end using a 28-33 mm circular stapling device depending on the colonic caliber.
Diverting loop ileostomy was usually done,as well as full-thicken strengthen of the anastomosis. Anastomotic leaks were graded according to the classification system proposed by the International Study Group of Rectal Cancer and complications by the Clavien-Dindo classification. The quality of the specimens was assessed by the surgeon and pathology department.
Follow-up
We followed up patients every 3 mo with physical examination and laboratory tests, including tumor markers (carcinoembryonic antigen and carbohydrate antigen 19-9). Abdominal computed tomography was performed every 6 mo after the operation, and endoscopy was performed 1 year postoperatively. To evaluate the ano(neo)rectal function after taTME, the low anterior resection syndrome (LARS) questionnaire[9] was used. The LARS questionnaire of taTME group was sent 6 mo after ileostomy closure. The LARS score was categorized into no LARS (0-20 points), minor LARS (21-29 points), and major LARS (30-42 points) (Table 2)[9]. The follow-up time was 3-13 mo, and the last follow-up date was March 30, 2018.
Table 2.
Low anterior resection syndrome questionnaire and scoring[16]
|
LARS Score: Scoring instructions
|
| Add the scores from each 5 answers to one final score. |
| Do you ever have occasions when you cannot control your flatus (wind)? |
| □ No, never |
| □ Yes, less than once per week |
| □ Yes, at least once per week |
| Do you ever have any accidental leakage of liquid stool? |
| □ No, never |
| □ Yes, less than once per week |
| □ Yes, at least once per week |
| How often do you open your bowels? |
| □ More than 7 times per day (24 h) |
| □ 4-7 times per day (24 h) |
| □ 1-3 times per day (24 h) |
| □ Less than once per day (24 h) |
| Do you ever have to open your bowels again within 1 hour of the last bowel opening? |
| □ No, never |
| □ Yes, less than once per week |
| □ Yes, at least once per week |
| Do you ever have such a strong urge to open your bowels that you have to rush to the toilet? |
| □ No, never |
| □ Yes, less than once per week |
| □ Yes, at least once per week |
| Total Score: |
| Interpretation: |
| 0-20: No LARS |
| 21-29: Minor LARS |
| 30-42: Major LARS |
LARS: Low anterior resection syndrome.
RESULTS
We successfully completed laparoscopy-assisted taTME in 24 patients with lower rectal cancer. Among them, one underwent additional lateral lymph node dissection and one Hartmann surgery. In 24 patients, mean operation time was 310.0 min, mean intraoperative blood loss was 69.1 mL, and mean time to passing of first flatus was 3.1 d. The mean postoperative hospital stay was 9.2 d. Postoperative pain associated with taTME surgery was scored by the patient according to the subjective simulated pain scale (numeric rating scale) ranging from 0 to 10, with 0 representing no pain at all and 10 the worst pain imaginable. Pain was also assessed by a rehabilitation physician at 24 h and 72 h after surgery. Patients suffering from 4-7 score pain were given analgesics. Twenty patients experienced slight anal pain after operation, and only 4 patients received analgesics. Twenty-three patients were able to walk within 2 d. Five patients had postoperative complications. One patient had anastomotic fracture, followed by Hartmann operation (the patient developed an anastomotic rupture followed by a pelvic infection. Despite the prophylactic ileostomy, the patient developed proximal colonic retraction, which was followed by Hartmann operation). One patient had intestinal obstruction, which was confirmed by abdominal X-ray on the postoperative day 8, and he was cured by indwelling gastric tube methods. Three patients developed fever within 7 d after taTME operation, and were diagnosed as pelvic infection. After antibiotic treatment, pelvic infection was cured. No patient had cancer recurrence during the follow-up period. Two of the 24 patients had fecal incontinence after the operation; the other patients could control their defecation easily.
DISCUSSION
Our primary aim for the taTME technique was to gain experience with this novel surgical approach, especially in patients with lower and midrectal cancer, as we believe the technique will potentially improve the surgical and oncologic outcome in selected cases (obesity, male patients, narrow pelvis, neoadjuvant therapy). TaTME is a new technique that seems to provide technical advantages. Laparoscopy-assisted taTME is also known as trans-abdominal taTME or hybrid-natural orifice transluminal endoscopic surgery (NOTES) taTME. Laparoscopy-assisted taTME is a combination of techniques, such as minimally invasive surgery, intersphincteric resection (ISR), natural orifice specimen extraction (NOSE), minimally invasive surgery, and ultralow-level preservation of the anus[6].
The quality of our specimens seems to be equivalent to the series by Nagtegaal et al[10]. All the specimens were intact and achieved good quality. Shortterm results were satisfactory with a low morbidity and consequently a median length of stay of 9.2 d and no mortality.
The results of our initial cases presented in this paper were obtained during our learning curve and as we continue to learn by each procedure performed, we do not expect to have reached the top of the curve yet. Our initial experience confirmed our impression during the preparation phase that the technique presents many challenges both operatively and in the use of the necessary equipment. Having tried out the procedure in more recent cases with an instrument that provides continuous low flow carbon dioxide gas inflation, we found that it eases the procedure remarkably.
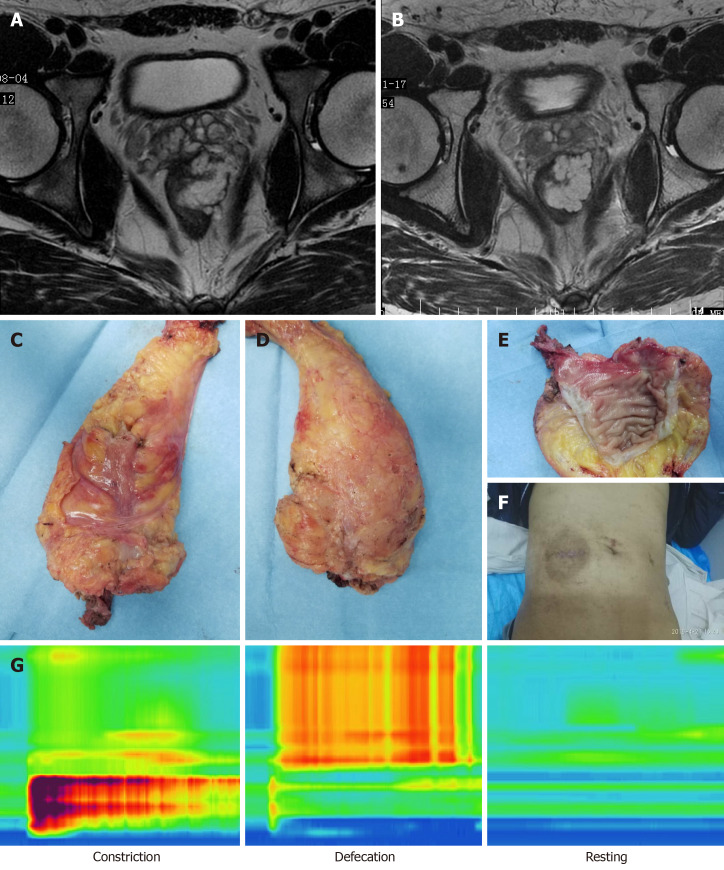
We presented the case of a young patient with lower bulky rectal cancer who highlighted the possibility of abdominoperineal resection (Figure 3). A 30-year-old stout man was diagnosed in October 2017 with stage IIIA lower rectal bulky mucinous adenocarcinoma and treated with preoperative chemoradiotherapy and subsequently with four cycles of capecitabine and oxaliplatin. After neoadjuvant therapy, the tumor size remained unchanged with mucus components. It was difficult to preserve the anus using the routine surgical technique. We successfully completed taTME operation for this patient, and found that the tumor was 3 cm from the anus and the anus was retained. Postoperative evaluation of the anal pressure function and urinary and sexual function was normal. Postoperative pathology indicated PCR of the tumor. Recently, the patient had reversal of an ileostomy operation. The advantages of taTME for patients with lower rectal cancer are significant, especially those with obesity, male, narrow pelvis and neoadjuvant therapy. TaTME is helpful for preservation of the anal sphincter.
Figure 3.
One case of a 30-year-old male patient. A: Magnetic resonance image before neoadjuvant therapy; B: Magnetic resonance image after neoadjuvant therapy; C: Front view of specimen; D: Back view of specimen; E: Specimen; F: Abdominal appearance; G: Anal pressure measurement and pressure variation diagram (constriction pressure [121.0 ± 11.6 mmHg], defecation pressure, resting pressure [41.5 ± 8.6 mmHg]).
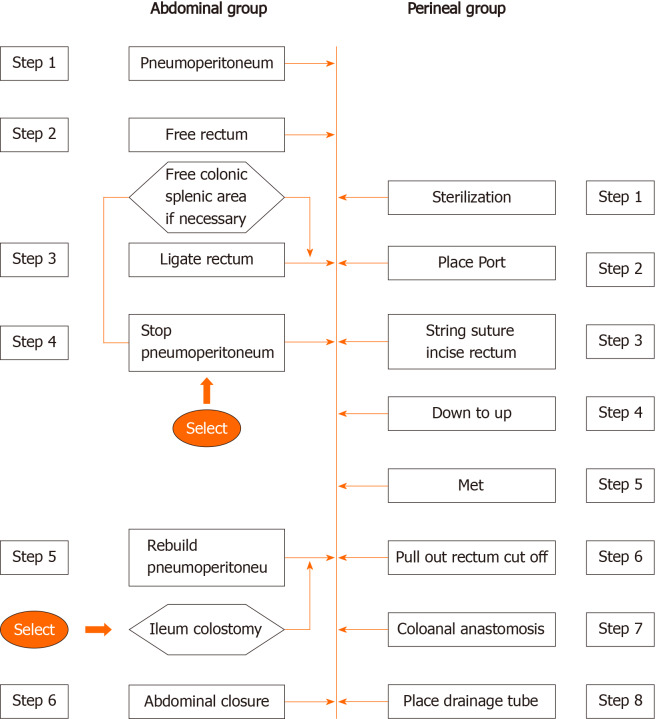
TaTME can be relatively easy to complete free and low rectum and mesorectum excision to guarantee the quality of TME-resected specimens, and may have advantages of NOTES, NOSE without abdominal incision, ISR, and single-port laparoscopic minimally invasive technique[11,12]. However, the technological requirements of taTME are high, the learning curve is long, and the operation is difficult. It has been reported that taTME has an effect on postoperative short-term voiding function and anal function[13,14]. Whether there are long-term effects needs further observation. Notably, the longest duration of taTME surgery in this study was 402 min. The main reason is that the operation was performed by one group of doctorsbetween transabdominal and transanal parts, and the operation platform was constantly changed. However, theoretically taTME can be performed by the transabdominal group and the ta group at the same time, so it is possible to reduce the operation time. After we adjusted the procedure (Figure 4), the overall operation time was reduced. In the abdominal operation group pelvic autonomic nerve preservation was performed, and the nerves were better protected than using the pure NOTES taTME. According to the 24 patients with operation time, average operation time was 310 min. After optimizing the operation process and adjusting the surgical approach, the average operation time was shortened to 240 min, and the learning curve was stable.
Figure 4.
The laparoscopy-assisted transanal total mesorectal excision was divided into the transabdominal and transanal parts. This operation can make full use of the advantages of transabdominal and transanal surgery. Laparoscopic surgery can complete laparoscopic exploration, vascular ligation and lymph node dissection, middle and upper mesentery dissociation, while transanal surgery can complete the lower mesentery migration and specimen removal, and then complete abdominal and transanal anastomosis reconstruction.
Concerns about this technique focus on bacterial contamination and tumor cell contamination[15,16]. However, several studies can help to remove these doubts[12,16]. For example, it has been confirmed that intracorporeal bowel opening for anastomosis completion does not increase the risk of infection during colorectal surgery. Tumor cell contamination can be prevented by suturing predetermined margins and closing the intestinal cavity to isolate the tumor. From our follow-up data, we confirmed that surgical procedures on the anus had no influence on bacterial and tumor cell contamination.
As a new technique applied in clinic, safety is the most important. Even at the beginning of the learning curve, when the risk of complications is high, safety is particularly important. The same is true for taTME. In this study, the safety of taTME was verified by the amount of blood loss, postoperative complications, postoperative mortality and postoperative hospital stay. In the taTME group, the average blood loss of patients undergoing surgical treatment was less than 100 mL, which was not significantly different from the traditional laparoscopic surgery group and the open surgery group. In the first 20 taTME surgeries performed by Atallah et al[15], the average patient lost 153 mL of blood. In 720 samples enrolled study, 61.2% of patients had intraoperative blood loss less than 100 mL, and 1% had intraoperative blood loss greater than 1 L[11]. From the perspective of complications, there were 5 complications in this group. The most serious patient had anastomotic rupture and therefore received sigmoidostomy (Hartmann operation) for treatment; 1 patient had postoperative intestinal obstruction which was improved by conservative treatment and 3 patients had fever and other infection symptoms which were controlled by antibiotic treatment. Burke et al[14] studied 50 patients in their early taTME study and found that 12% needed a second operation within 30 d of surgery. The main causes of secondary operation were ileostomy dysfunction, pelvic effusion and anastomotic leakage[15]. In this study, no postoperative death occurred in the taTME group. Penna et al[11] conducted a study with 720 samples, showing that the postoperative mortality rate was 0.5%, suggesting that taTME is a safe surgical method.
At present, taTME is mainly suitable for malignant tumors requiring accurate anatomy and resection of the middle and lower rectum and mesangial. The indications of taTME for the treatment of malignant rectal tumors should be limited to low and medium rectal cancers, especially low rectal cancers. TaTME may be more advantageous for rectal cancer patients with “difficult pelvis,” such as male, prostatic hypertrophy, obesity, tumor diameter of > 4 cm, rectal mesangial hypertrophy, lower anterior rectal wall tumor, pelvic stenosis, and unclear tissue plane caused by neoadjuvant radiotherapy.
CONCLUSION
Laparoscopy-assisted taTME is suitable for selected patients with lower rectal cancer. Bulky tumor, obesity, male, narrow pelvis, neoadjuvant therapy, and the lower position of the tumor may be the indications to perform this technique. The short-term outcomes of this technique are adjudged to be satisfactory. Laparoscopy-assisted taTME is safe and feasible for patients with lower rectal cancer, and this technique is worthy of further recommendation. Of course, our data are preliminary and the clinical outcome of taTME technique must be confirmed through more cases.
ARTICLE HIGHLIGHTS
Research background
Transanal total mesorectal excision (taTME) is a new technique that might have many technical advantages. Laparoscopy-assisted taTME is also known as transabdominal taTME or hybrid-natural orifice transluminal endoscopic surgery taTME. Laparoscopy-assisted taTME is a combination of techniques, such as minimally invasive surgery, intersphincter-assisted resection, natural orifice extraction, ta minimally invasive surgery, and ultralow-level preservation of the anus.
Research motivation
Laparoscopy-assisted taTME surgery was reported by literature with relatively small amount of cases. However, there has been little published data on laparoscopy-assisted taTME surgery on the Chinese population. The safety and feasibility of laparoscopy-assisted taTME is still lack of report.
Research objectives
This study was designed to investigate the utility of laparoscopy-assisted taTME technique with both favorable and unfavorable factors.
Research methods
Laparoscopy-assisted taTME surgery was done by a standard laparoscopic platform (STARPORT Port). Patients’ characteristics, surgery duration, pathological diagnosis and postoperative complications (Clavien-Dindo classification) were collected.
Research results
Laparoscopy-assisted taTME could be safe and feasible technique to rectal tumor. Laparoscopic surgeons would be proficient for laparoscopy-assisted taTME with approximately 20 cases. Laparoscopy-assisted taTME may provide an alternative to traditional surgical methods for accurate anal retention. This study demonstrated the first piece of evidence of peri-operative data and short-term outcome in patients treated with laparoscopy-assisted taTME in Chinese tertiary hospital.
Research conclusions
Laparoscopy-assisted taTME is suitable for selected patients with lower rectal cancer, and this technique is worthy of further recommendation.
Research perspectives
At present, taTME is mainly suitable for malignant tumors requiring accurate anatomy and resection of the middle and lower rectum and mesangial. The indications of taTME for the treatment of malignant rectal tumors should be limited to low and medium rectal cancers, especially low rectal cancers. TaTME may be more advantageous for rectal cancer patients with “difficult pelvis,” such as male, prostatic hypertrophy, obesity, tumor diameter of > 4 cm, rectal mesangial hypertrophy, lower anterior rectal tumor, anterior rectal wall tumor, narrow pelvic, and unclear tissue, and unclear tissue plane caused by neoadjuvant radiotherapy. In addition, taTME can be performed in combination with sphincter resection (ISR) for ultra-low rectal cancer patients. TaTME surgery may have indications for the treatment of colorectal benign diseases: Large benign tumors of the middle and lower rectum that cannot be removed locally, inflammatory bowel disease requiring rectal excision, familial adenomatous polyposis, and radioactive proctitis.
ACKNOWLEDGEMENTS
We greatly appreciate the following staff members who contributed to this work: Professor Zhang XY, Dong QS and Li ZW at Beijing Cancer Hospital.
Footnotes
Institutional review board statement: This study and the protocol were reviewed and approved by the Ethics Committee at Beijing Cancer Hospital (approval no. 2017-p2-181-01).
Clinical trial registration statement: This registration policy applies to prospective, randomized, controlled trials only.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare no conflict of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: August 17, 2020
First decision: September 16, 2020
Article in press: December 16, 2020
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Ammendola M, Burke D S-Editor: Chen XF L-Editor: Filipodia P-Editor: Li JH
Contributor Information
Ying-Jie Li, Gastrointestinal Cancer Center Unit III, Beijing Cancer Hospital and Beijing Institute for Cancer Research, Beijing 100142, China; Department of Gastrointestinal Surgery, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Beijing Cancer Hospital and Beijing Institute for Cancer Research, Beijing 100142, China.
Lin Wang, Gastrointestinal Cancer Center Unit III, Beijing Cancer Hospital and Beijing Institute for Cancer Research, Beijing 100142, China.
Ting-Ting Sun, Gastrointestinal Cancer Center Unit III, Beijing Cancer Hospital and Beijing Institute for Cancer Research, Beijing 100142, China.
Ai-Wen Wu, Gastrointestinal Cancer Center Unit III, Beijing Cancer Hospital and Beijing Institute for Cancer Research, Beijing 100142, China; Department of Gastrointestinal Surgery, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Beijing Cancer Hospital and Beijing Institute for Cancer Research, Beijing 100142, China. drwuaw@sina.com.
Data sharing statement
No additional data are available.
References
- 1.van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 2.van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 3.Battersby NJ, How P, Moran B, Stelzner S, West NP, Branagan G, Strassburg J, Quirke P, Tekkis P, Pedersen BG, Gudgeon M, Heald B, Brown G MERCURY II Study Group. Prospective Validation of a Low Rectal Cancer Magnetic Resonance Imaging Staging System and Development of a Local Recurrence Risk Stratification Model: The MERCURY II Study. Ann Surg. 2016;263:751–760. doi: 10.1097/SLA.0000000000001193. [DOI] [PubMed] [Google Scholar]
- 4.Hompes R, Arnold S, Warusavitarne J. Towards the safe introduction of transanal total mesorectal excision: the role of a clinical registry. Colorectal Dis. 2014;16:498–501. doi: 10.1111/codi.12661. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Hevia M, Delgado S, Castells A, Tasende M, Momblan D, Díaz del Gobbo G, DeLacy B, Balust J, Lacy AM. Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg. 2015;261:221–227. doi: 10.1097/SLA.0000000000000865. [DOI] [PubMed] [Google Scholar]
- 6.Atallah S. Transanal total mesorectal excision: full steam ahead. Tech Coloproctol. 2015;19:57–61. doi: 10.1007/s10151-014-1254-5. [DOI] [PubMed] [Google Scholar]
- 7.de Lacy AM, Rattner DW, Adelsdorfer C, Tasende MM, Fernández M, Delgado S, Sylla P, Martínez-Palli G. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: "down-to-up" total mesorectal excision (TME)--short-term outcomes in the first 20 cases. Surg Endosc. 2013;27:3165–3172. doi: 10.1007/s00464-013-2872-0. [DOI] [PubMed] [Google Scholar]
- 8.Hompes R, Guy R, Jones O, Lindsey I, Mortensen N, Cunningham C. Transanal total mesorectal excision with a side-to-end stapled anastomosis - a video vignette. Colorectal Dis. 2014;16:567. doi: 10.1111/codi.12660. [DOI] [PubMed] [Google Scholar]
- 9.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 10.Nagtegaal ID, van de Velde CJ, van der Worp E, Kapiteijn E, Quirke P, van Krieken JH Cooperative Clinical Investigators of the Dutch Colorectal Cancer Group. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20:1729–1734. doi: 10.1200/JCO.2002.07.010. [DOI] [PubMed] [Google Scholar]
- 11. Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP; TaTME Registry Collaborative. Transanal Total Mesorectal Excision: International Registry Results of the First 720 Cases. Ann Surg. 2017;266:111–117. doi: 10.1097/SLA.0000000000001948. [DOI] [PubMed] [Google Scholar]
- 12. Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP; International TaTME Registry Collaborative. Incidence and Risk Factors for Anastomotic Failure in 1594 Patients Treated by Transanal Total Mesorectal Excision: Results From the International TaTME Registry. Ann Surg. 2019;269:700–711. doi: 10.1097/SLA.0000000000002653. [DOI] [PubMed] [Google Scholar]
- 13. Marks JH, Myers EA, Zeger EL, Denittis AS, Gummadi M, Marks GJ. Long-term outcomes by a transanal approach to total mesorectal excision for rectal cancer. Surg Endosc. 2017;31:5248–5257. doi: 10.1007/s00464-017-5597-7. [DOI] [PubMed] [Google Scholar]
- 14. Burke JP, Martin-Perez B, Khan A, Nassif G, de Beche-Adams T, Larach SW, Albert MR, Atallah S. Transanal total mesorectal excision for rectal cancer: early outcomes in 50 consecutive patients. Colorectal Dis. 2016;18:570–577. doi: 10.1111/codi.13263. [DOI] [PubMed] [Google Scholar]
- 15. Atallah S, Martin-Perez B, Albert M, deBeche-Adams T, Nassif G, Hunter L, Larach S. Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): results and experience with the first 20 patients undergoing curative-intent rectal cancer surgery at a single institution. Tech Coloproctol. 2014;18:473–480. doi: 10.1007/s10151-013-1095-7. [DOI] [PubMed] [Google Scholar]
- 16. Araujo SE, Crawshaw B, Mendes CR, Delaney CP. Transanal total mesorectal excision: a systematic review of the experimental and clinical evidence. Tech Coloproctol. 2015;19:69–82. doi: 10.1007/s10151-014-1233-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.