Watch a video presentation of this article
Abbreviations
- CKD
chronic kidney disease
- CrCl
creatinine clearance
- ESRD
end‐stage renal disease
- GFR
glomerular filtration rate
- KALT
kidney after liver transplant
- KT
kidney‐pancreas transplant
- KTA
kidney transplant alone
- LAKT
liver after kidney transplant
- LT
liver transplantation
- MELD
Model for End‐Stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- RRT
renal replacement therapy
- SLK
simultaneous liver‐kidney transplantation
- SPKT
simultaneous pancreas‐kidney transplantation
- UNOS
United Network for Organ Sharing
Key Points
Patients with cirrhosis and kidney failure have a substantial survival benefit from transplant listing.
Patients with simultaneous liver‐kidney transplantation (SLK) have superior liver graft and patient survival compared with both liver‐only recipients who require post‐LT dialysis and kidney after liver transplant (KALT) recipients.
Highest risk patients have expected poor outcomes with SLK.
Kidney grafts for SLK have at least equivalent and perhaps superior long‐term survival when compared with counterparts transplanted as kidney only or simultaneous pancreas‐kidney transplantation (SPKT).
The effect of the new SLK allocation policy requires careful monitoring.
The dramatic increase in SLKs since the introduction of the Model for End‐Stage Liver Disease (MELD) allocation system in 2002 has generated considerable controversy in the fields of liver and kidney transplantation. Indeed, this phenomenon has prompted the transplant community to carefully examine SLK policy in numerous consensus conferences and committees. Because the MELD schema favors the contribution of kidney dysfunction over the other components (i.e., bilirubin and international normalized ratio), an ever‐rising proportion of SLKs was performed nationwide through the 2010s. In fact, in excess of 20% of the liver transplants at some high‐volume centers were SLKs. Concomitantly, concerns were being raised that high‐quality kidneys were being removed from the pool for kidney‐only recipients. Compounding this problem was a loophole that kept SLKs from affecting center‐specific outcomes, creating an unintended incentive to perform more SLKs. Thus, a new policy was developed to govern dual liver‐kidney allocation (Table 1). 1 Adopted nationwide by United Network for Organ Sharing/Organ Procurement and Transplantation Network (UNOS/OPTN), current policy dictates that stringent medical criteria are met and provides a safety net for patients with renal nonrecovery after LT alone. Although the results of this policy have not been fully realized, it is worthwhile to examine the rationale and criteria for SLK to determine whether the goal of a more equitable distribution is being met.
TABLE 1.
Updated OPTN Criteria for SLK
| If the candidate’s transplant nephrologist confirms a diagnosis of: | Then the transplant program must report to the OPTN contractor and document in the candidate’s medical record: |
| CKD with a measured or calculated GFR ≤60 mL/min for >90 consecutive days |
At least one of the following:
|
| Sustained acute kidney injury |
At least one of the following, or a combination of both of the following, for the last 6 weeks:
|
| If the candidate’s eligibility is not confirmed at least once every 7 days for the last 6 weeks, the candidate is not eligible to receive a liver and a kidney from the same donor. | |
| Metabolic disease |
A diagnosis of at least one of the following:
|
The Benefits of Listing
When patients with cirrhosis and end‐stage renal disease (ESRD) are evaluated for liver transplant, a clear dichotomy is seen based on listing status. To examine the relationship between access to transplant and survival among those patients with cirrhosis and concomitant renal failure, Allegretti et al. 2 reviewed a cohort of 472 patients, all of whom received dialysis. A dramatic survival difference was observed for patients with ESRD who were not listed compared with those listed for LT irrespective of ultimate transplant status. In this cohort, all of whom received renal replacement therapy (RRT), the probability of death (@75%) for those not listed for transplant was nearly equivalent to the likelihood of transplant for the listed patients 60 days from dialysis initiation irrespective of cause of kidney failure (hepatorenal syndrome versus acute tubular necrosis (ATN)). This stark difference in survival represents only the effects of LT listing, meaning that even before transplant the survival benefits of having access to a transplant program, undergoing evaluation, meeting LT criteria, and receiving the care associated with listing are substantial. In contrast, most patients with decompensated liver disease who experience concomitant ESRD are unlikely to receive the extensive care given to their listed counterparts. Indeed, many providers are reluctant to offer dialysis to patients with poor or failing liver function. Many of these patients subsequently decline rapidly or are transitioned to hospice level care.
The Benefits of SLK: More Than Just Survival
The benefits of combined liver‐kidney transplant are numerous. First, it has long been accepted that patients with chronic kidney disease (CKD) who require liver transplantation (LT) should receive consideration for both liver and kidney. Dialysis after transplant has been shown to predict both poor survival for patients and liver grafts. Recent studies continue to support this practice, with a UNOS review showing an approximately 35% increased risk for graft loss in liver‐only recipients with CKD who require dialysis after LT. 3 Second, in truly “simultaneous” LK, wherein both transplants are performed during the same operation, the recipient has a single exposure to the risks of anesthesia with concomitant savings in institutional resource utilization. Third, immunological benefits include exposure to one set of antigens and protection from both acute cellular and antibody‐mediated rejection for the kidney allograft. 4 In fact, recipients of LTA who subsequently require kidney transplant may have a harder time finding a suitable graft because of sensitization from both the liver graft and blood product administration at the initial transplant event. Thus, liver recipients with chronic kidney dysfunction clearly benefit from SLK, as do many patients with intermediate‐duration kidney failure. It is this latter group for whom the new listing criteria as outlined in Table 2 were developed with potential capture for KAL in the setting of kidney nonrecovery after LT.
TABLE 2.
OPTN Policy for Allocation of Kidneys to Prior Liver Recipients on the Kidney Waiting List
| If a kidney candidate received a liver transplant, but not a liver and kidney transplant from the same deceased donor, the candidate will be classified as a prior liver recipient. This classification gives priority to a kidney candidate if both of the following criteria are met: |
|
The concept of the safety net is an attractive one, posited on the fact that some SLKs were or are unnecessary, and that kidney recovery occurs in a larger percentage of patients than previously appreciated. However, review of this concept is warranted. Martin et al. 5 examined the early post‐MELD experience with SLK. They compared liver transplant alone (LTA) with SLK and KALT. On both univariate (Fig. 1A) and multivariate analysis, SLK was protective and offered a survival advantage when compared with KALT. Importantly, the authors showed that, on average, survival for KALT recipients was 10% worse than for patients with SLK. This was particularly pronounced with early (<3 months) and late (>12 months) KALT, suggesting that the contribution of kidney failure was underappreciated at the time of LT listing. No difference in survival was observed between the SLK and KALT groups when the latter received a kidney 6 to 12 months after the liver. These results are most instructive, because this is the group most likely to be eligible for the newly proposed safety net. Outcomes for liver graft survival similarly showed a clear benefit for SLK over KALT (Fig. 1B).
FIG 1.
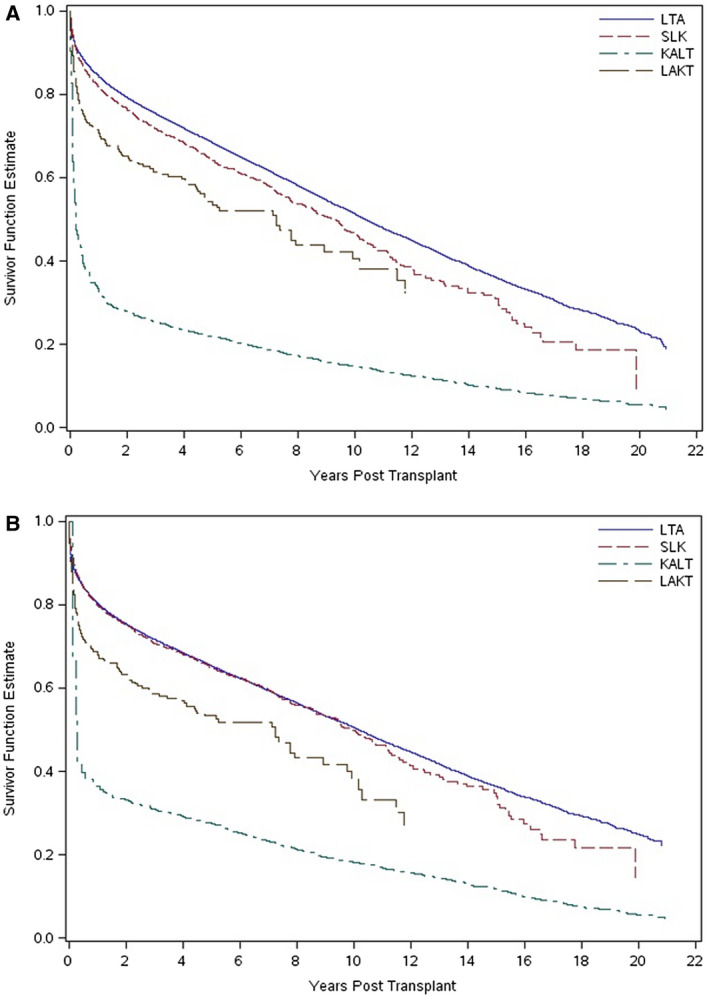
(A) Unadjusted Kaplan‐Meier curves for overall recipient survival with LTA, SLK, KALT, and LAKT. P < 0.001 for LTA versus SLK, KALT, and LAKT; P < 0.001 for SLK versus KALT; P = 0.003 for SLK versus LAKT; and P = 0.64 for KALT versus LAKT. (B) Unadjusted Kaplan‐Meier curves for overall graft survival with LTA, SLK, KALT, and LAKT. P = 0.27 for LTA versus SLK; P < 0.001 for LTA versus KALT and LAKT; P < 0.001 for SLK versus KALT and LAKT; and P < 0.001 for KALT versus LAKT. Reproduced with permission from Liver Transplantation. 5 Copyright 2012, American Association for the Study of Liver Diseases.
Avoiding Unnecessary SLK
The dual concepts of futile and unnecessary kidney transplants are two legitimate critiques of SLK. Prior to the strict allocation rules, multiple investigators have shown a proportion of SLK recipients end up with three functioning kidneys. 6 , 7 Combined with the concern for removing a high‐quality kidney from the pool of available organs for listed kidney recipients, any rationale for SLK certainly warrants examination. Some groups have in fact suggested that valuable graft years that might have otherwise benefited kidney transplant alone (KTA) recipients for a considerably longer duration are lost with SLK. 8 However, a well‐conducted review of UNOS data contradicts this notion. Comparing outcomes of high‐quality mate kidneys that went to SLK versus KTA or SPKT, Cannon et al. 9 showed that by 6.5 years from transplant, the kidney grafts in SLK had equivalent survival to the other groups (Fig. 2). By 10 years, the cumulative incidence rate of graft failure was actually higher in the KTA and SPKT groups compared with SLK (21% versus 11%; P < 0.01).
FIG 2.
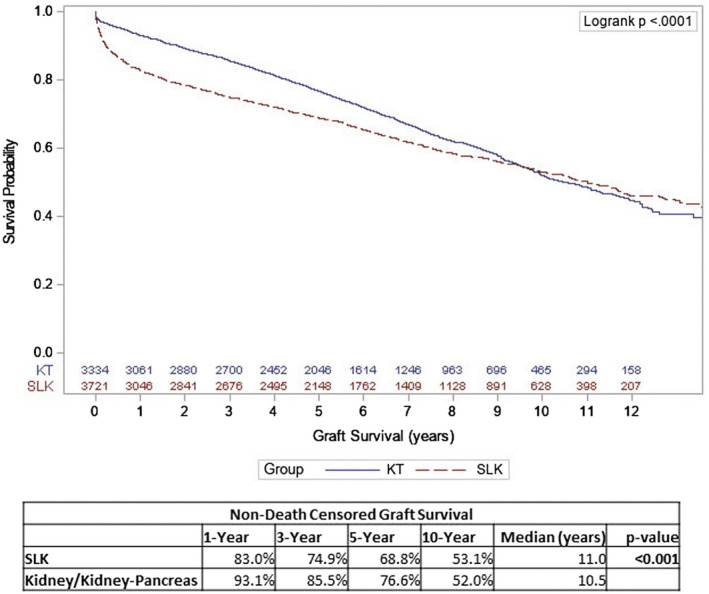
Kaplan‐Meier estimates of non‐death‐censored renal allograft survival for SLK and KTA or KT. Reproduced with permission from Journal of the American College of Surgeons. 9 Copyright 2019, American College of Surgeons.
Choosing the Match: Patient Selection and Organ Quality Matter
Determining not only which patients will benefit from SLK, but also which will actually tolerate a simultaneous dual‐organ transplant remains a clinical challenge. Lunsford et al. 10 examined a single large‐center experience spanning a decade with the aim of identifying futility in SLK. Patients with renal failure after SLK had profoundly inferior 1‐ and 3‐year survival rates compared with those with functioning kidney graft (18.2% and 13.5% versus 92.6% and 83.7%; P < 0.01). Predictors for renal graft failure included hyperlipidemia, longer duration of renal replacement, longer kidney cold time, and poorer kidney graft quality measured by the Kidney Donor Risk Index. Coupled with higher‐risk recipients, which included those with a higher MELD score at transplant and longer pretransplant hospitalization, these factors create a futile combination. In fact, a strong argument can be made that a substantial number of patients in this cohort should not have been considered for liver transplant to begin with. The authors are to be congratulated for pushing the limits and transplanting patients at the margins of futility, teaching us that careful consideration of these sickest patients with transplant only in the optimal setting (high‐quality grafts with short cold times) is essential.
Conclusions
Clearly, many patients do benefit from SLK, especially when compared with patients with persistent kidney dysfunction necessitating a KALT. Were there an overabundance of kidneys for transplant, this would not be a problem. But the current and continued organ shortage means that transplant practitioners must thoughtfully and justly allocate these precious resources. Can we find balance, where the “right” liver patients are helped without disadvantaging too many patients on the kidney wait list? The new allocation policy, with careful continued oversight, should both protect kidney‐only patients on the wait list and ensure a more equitable organ allocation. However, we must take care not to limit access to kidneys for liver recipients who would derive clear survival benefit from SLK. Careful follow‐up of the results for liver, dual‐organ, and kidney‐only patients is required with the newly adopted policy. In light of the clear benefit to patients with concomitant kidney and liver failure, SLK must remain the procedure of choice when these patients are indicated for transplantation.
Potential conflict of interest: Nothing to report.
References
- 1. Organ Procurement & Transplantation Network . Liver and kidney allocation policy. Available at: https://optn.transplant.hrsa.gov/governance/policies/. Published August 1, 2019. Accessed October 24, 2019.
- 2. Allegretti AS, Parada XV, Eneanya N D, et al. Prognosis of patients with cirrhosis and AKI who initiate RRT. Clin J Am Soc Nephrol 2018;13:16‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nagai S, Safwan M, Collins K, et al. Liver alone or simultaneous liver‐kidney transplant? Pretransplant chronic kidney disease and post‐transplant outcome ‐ a retrospective study. Transpl Int 2018;31:1028‐1040. [DOI] [PubMed] [Google Scholar]
- 4. Fong TL, Khemichian S, Shah T, et al. Combined liver‐kidney transplantation is preferable to liver transplant alone for cirrhotic patients with renal failure. Transplantation 2012;94:411‐416. [DOI] [PubMed] [Google Scholar]
- 5. Martin EF, Huang J, Xiang Q, et al. Recipient survival and graft survival are not diminished by simultaneous liver‐kidney transplantation: an analysis of the united network for organ sharing database. Liver Transpl 2012;18:914‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levitsky J, Baker T, Ahya SN, et al. Outcomes and native renal recovery following simultaneous liver‐kidney transplantation. Am J Transplant 2012;12:2949‐2957. [DOI] [PubMed] [Google Scholar]
- 7. Sharma P, Goodrich NP, Zhang M, et al. Short‐term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clin J Am Soc Nephrol 2013;8:1135‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choudhury RA, Reese PP, Goldberg D, et al. A paired kidney analysis of multiorgan transplantation: implications for allograft survival. Transplantation 2017;101:368‐376. [DOI] [PubMed] [Google Scholar]
- 9. Cannon RM, Davis EG, Jones CM. A tale of two kidneys: differences in graft survival for kidneys allocated to simultaneous liver kidney transplant compared with contralateral kidney from the same donor. J Am Coll Surg 2019;229:7‐17. [DOI] [PubMed] [Google Scholar]
- 10. Lunsford KE, Bodzin AS, Markovic D, et al. Avoiding futility in simultaneous liver‐kidney transplantation: analysis of 331 consecutive patients listed for dual organ replacement. Ann Surg 2017;265:1016‐1024. [DOI] [PubMed] [Google Scholar]


