Watch a video presentation of this article
Watch an interview with the author
Abbreviations
- CI
confidence interval
- DAA
direct‐acting antiviral agent
- DCV
daclatasvir
- ELISA
enzyme‐linked immunosorbent assay
- HCV
hepatitis C virus
- IDU
injection drug use
- LDV
ledipasvir
- LMIC
low‐ and middle‐income country
- MMPHCRF
Mukh‐Mantri Punjab Hepatitis C Relief Fund
- NAT
nucleic acid test
- NSP
needle and syringe program
- OST
opioid substitution therapy
- PCP
primary care provider
- PLHCV
person living with hepatitis C virus
- PWID
person who injects drugs
- QALY
quality‐adjusted life‐year
- SOF
sofosbuvir
- SVR
sustained virological response
- UMIC
upper‐ and middle‐income country
- VEL
velpatasvir
- WHO
World Health Organization
Defining the Public Health Problem of Hepatitis C
Hepatitis C virus (HCV) infection remains a matter of global concern despite concerted efforts by the World Health Organization (WHO), national bodies, and civil society groups. Despite ready availability of cost‐effective and efficacious generic direct‐acting antiviral agents (DAAs), the majority of the estimated 71 million persons living with HCV (PLHCVs) remain untreated. Viral hepatitis has become the seventh leading cause of death worldwide. 1 More than 90% of the deaths and disability caused by viral hepatitis can be attributed to HCV and hepatitis B virus infections. 2 The WHO target of HCV elimination by 2030 requires that 90% of PLHCVs need to be diagnosed, and 80% of these individuals need to be treated in conjunction with means to reduce the incidence of HCV in high‐risk groups, such as persons who inject drugs (PWIDs), dialysis patients, and recipients of unsafe blood transfusion. In 2015, it was estimated that China has 10 million PLHCVs, followed by Pakistan (7.2 million), India (6.2 million), and Egypt (5.6 million), and these account for 40% of global PLHCVs. The main source of transmission is unsafe medical practices and in the pre‐HCV screening birth cohort was blood transfusion. 3 The main source of HCV incidence is in PWIDs, leading to two distinct groups of PLHCVs: (1) an older cohort who were infected by unsafe medical practices and have comorbidities, such as obesity, alcohol use, and diabetes, are at higher risk for cirrhosis; and (2) a younger group with recent infection caused by injection drug use (IDU). 4
Implementing the HCV Elimination by 2030 Manifesto
There are six main platforms for the rollout of the Global HCV Elimination Coalition. First, the meteoric success of DAAs ensures cure rates of >95% with even short courses of 8 to 12 weeks and have shifted the focus of HCV elimination programs from “control” to “cure.” The second platform is allocation of resources in terms of finances, logistics, and manpower. Not only a reduced cost of drugs, but the availability of comprehensive, affordable, validated point‐of‐care tests and nucleic acid tests (NATs) for HCV will make the laboratory diagnosis and management of HCV simpler. 5 Third, education and training of medical health personnel to provide safe injections and prevention of cross‐infection, and patient education services that counsel those with the disease, as well as cater to prevention of HCV incidence, are essential services in such a setting. Fourth, integration of services in the available health care systems to serve socially marginalized groups, such as PWIDs, prison populations, and poor households, who may not be catered to by routine health care, is important. Fifth, inclusion of a primary health care system as the frontline for treating HCV in a decentralized fashion is key to success. 6 The Project Extension for Community Health Outcomes is another such model for treatment of HCV. 7 This uses a tele‐mentoring system for primary care providers (PCPs) and nurse practitioners by specialists in a hub‐and‐spoke fashion. This method of collaborative care enables PCPs to provide specialized health care for HCV under guidance from specialists who provide didactic teaching with case‐based learning. 6 The major health care programs started in HCV across different WHO regions are shown in Fig. 1. The WHO requires every country to look at its existing health care system and to develop a cascade of care (Figs. 2 and 3) to ensure capacity building, performance amplification at each step, and surveillance mechanisms to ensure case capture and treatment. Lastly, other than state‐run public health programs, several initiatives from civil society, such as Buyer’s Club (FixHepC, South‐East Asia Buyer’s Club, Eastern Europe Buyer’s Club, Hepatitis C Treatment Without Borders, etc.), screening programs, and health awareness campaigns pave the way for inclusive health care. 8 , 9
FIG 1.
Global programs for HCV elimination. Reproduced with permission from Global Hepatitis Report 2017. Geneva: World Health Organization; 2017. Licence: CC BY‐NC‐SA 3.0 IGO.
FIG 2.

(A) Global prevalence estimates of HCV as per WHO data (2015). (B) Global incidence estimates of HCV as per WHO data (2015). Reprinted with permission from World Health Organization (WHO), work conducted by the Centre for Disease Analysis, Global Hepatitis Report 2017.
Cost‐Effectiveness Analysis For HCV Elimination
Several studies have shown the use of DAAs to be cost‐effective because they effectively curtail the progression of HCV and reduce risk for cirrhosis, decompensation, and HCC, thus making them cost saving in public health terms in the long run. 10 In the United States, an estimated 3.5 million PLHCVs were in need of treatment in 2015. 2 The initial list price for sofosbuvir (SOF) was US $1000 per pill when first introduced, making the cost of the initial regimen US $94,500. 5 An 8‐week course of glecaprevir‐pibrentasvir costs US $26,400 in the United States. 4 Treating HCV even at originator drug price is favorable due to significant reduction in cost of managing complications of severe stages. Such treatment is cost‐effective, that is, has incremental cost‐effectiveness ratio per quality‐adjusted life‐year (QALY) of less than annual per capita national income. However, despite cost‐effectiveness, the uptake of HCV treatment is lower than expected because of lack of access to care.
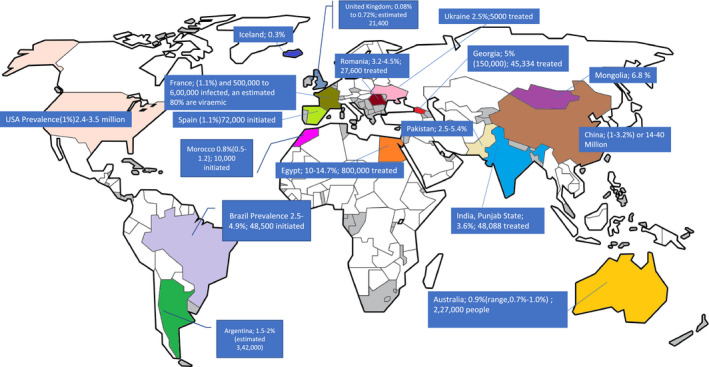
Iyenger et al. systematically compared the price and affordability of SOF ± ledipasvir (LDV) across 26 member countries of the Organisation for Economic Cooperation and Development and reported that 90% of the analyses would conclude DAAs to be cost‐effective for genotypes 2 to 5 at the discounted price of US $40,000, irrespective of the presence of cirrhosis, prior treatment history, or the region of the study. Furthermore, the listed originator price of DAAs (US $539‐$94,500) was substantially lower than the threshold price (US $144,400‐$225,000) at which they would be deemed cost‐effective. However, the threshold price at which DAAs would be deemed cost saving was in the range of US $17,300 to $25,400. In several low‐ and middle‐income countries (LMICs), DAAs are available at highly discounted prices. Therefore, treatment of HCV infection with DAAs at the prices prevalent in LMIC is not only cost‐effective, but also cost saving. Several countries, including Argentina, Brazil, Egypt, Georgia, Indonesia, Morocco, Nigeria, Pakistan, Thailand, and Ukraine, are getting drugs for HCV elimination thanks to the WHO and partners’ aid for treatment access and favorable licensing agreements (Table 1). In India, the market price of generic SOF+velpatasvir (VEL) ranges from US $ 714 to $857 for a 12‐week course, which is almost four times the price at which the Punjab state government is procuring the same combination and is providing it free to patients.
TABLE 1.
Cost of DAAs in Various Countries With Available Data for Drug Prices in the Public and Private Sectors
| Country | Registered DAAs | Price, Public Sector, per 28‐Day Supply | Price, Private Sector, per 28‐Day Supply | National Guidelines |
|---|---|---|---|---|
| Indonesia (LMIC) | SOF/LDV/VEL/DCV | US $280 | HCV guidelines in 2014; more than 47,035 treated under a private sector program | |
| Pakistan (LMIC) | SOF/LDV/VEL/DCV | Public Health Programme initiated | ||
| SOF (generic) | US $56 | |||
| SOF/LDV | US $15‐$42 | US $420 | ||
| Egypt (LMIC) | DCV | US $7‐$167 | US $300 | Active Public Health program since 2015 |
| Simeprevir | US $250 | |||
| SOF | US $51 | US $76 | ||
| Brazil (UMIC) | SOF | US $850 | US $2292 | Estimated incidence rate of 11.9 cases per 100,000 people, or approximately 0.71% of Brazil’s total population; program since 2015 |
| Argentina (UMIC) | SOF | US $501 | US $2086 | 1.5%‐2.5% of adults are infected; ongoing program |
This implies that the cost of treatment is offset by the savings in future health care costs, thus leading to an overall monetary savings while at the same time increasing QALYs. 11 Other than cost of the drugs itself, another factor contributing to overall expenditure is the cost of screening tests and the confirmatory nucleic acid tests (NATs) for diagnosing viremia and sustained virological response (SVR12). Table 2 shows the costs of testing for HCV under the Punjab HCV treatment program (Mukh‐Mantri Punjab Hepatitis C Relief Fund [MMPHCRF]) and the market price in the state of Punjab in India. With the advent of the National Viral Hepatitis Control Programme, these costs have been reduced further by use of standard pan‐genotypic regimens, that is, SOF/daclatasvir (DCV) for persons without cirrhosis, SOF/VEL for compensated cirrhosis, and SOF/VEL/weight‐based ribavirin for decompensated cirrhosis, which obviates the need for a genotype test. Another measure under the program was to perform the NATs at the time of diagnosis and at the time of SVR12, reducing testing costs.
TABLE 2.
Comparative Prices for Laboratory Diagnosis of HCV Under the National Viral Hepatitis Control Program in India
| Cost of Diagnostic Tests | Base Value | Lower Limit | Upper Limit |
|---|---|---|---|
| Program rates (price, public sector; in US $) | |||
| ELISA | 0.77 | 0.62 | 1.15 |
| HCV‐RNA | 13.54 | 10.83 | 20.31 |
| Routine tests | 7.69 | 6.15 | 11.54 |
| Genotyping | 13.77 | 11.02 | 20.65 |
| Market prices (price, private sector; in US $) | |||
| ELISA | 1.54 | 1.08 | 2.00 |
| HCV‐RNA | 76.92 | 53.85 | 100.00 |
| Routine tests | 10.77 | 7.54 | 14.00 |
| Genotyping | 84.62 | 59.23 | 110.00 |
National Strategies For HCV Elimination
The HCV elimination program will require collaboration with insurance agencies to ensure wider access to screening and linkage to care. 12 In the United Kingdom, there were an estimated 2,14,000 PLHCVs in 2016, and DAA treatment led to a decline of almost 10% in the annual number of HCV‐related deaths between 2014 and 2016. The National Health Service sponsors the treatment, and the price of a combination of SOF and VEL in 2016 ranged from approximately US $10,500 to US $17,000 for a 28‐day supply. 13 The Australian Programme has a unique volume purchase licensing agreement that allows PLHCVs to be treated under the Pharmaceutical Benefits Scheme (which reimburses patients’ treatment expenses), treating an estimated 62,000 people for AUS $1 billion. 14
The Mongolian strategy has incorporated HCV treatment in the National Health Insurance System, which covers 98% of the population. All PLHCVs are reimbursed $265 regardless of using public or private health care providers. Yehia et al. 12 performed a meta‐analysis that suggests that the programs have a steep slope to cover. The use of generic drugs available in countries with licensing agreements has reduced the cost of treatment to a fraction (Table 1). 13 , 14 , 15 , 16 , 17 , 18
Of all of the PLHCVs, only a small fraction go on to have their disease diagnosed and viremic status conformed by NATs, and fewer still have the disease staged and initiated on treatment. Until DAAs were available, only 9% of the total PLHCV burden was cured, which is a far cry from the 80% required to achieve HCV elimination by 2030. 12
Another model to rationalize costs of DAAs is the subscription‐based flat‐fee model similar to Netflix, a video‐streaming service that provides unlimited content for a flat fee. This model engages a drug corporation in a subscription‐based arrangement to pay for HCV treatment for the state’s residents. Australia instituted the “Netflix model” in 2015, agreeing to spend about AUS $1 billion to treat an estimated 104,000 people with the disease over 5 years, saving about AUS $6.5 billion more than if it had tried to treat the same number of people under the traditional pricing method. 19 , 20
Targeting New HCV Infections
Role of Opioid Substitution and Needle and Syringe Programs in PWIDs
In the HCV elimination 2030 strategy, prevention of new infections is of equal importance to screening, diagnosing, and treating the existing HCV pool. One of the primary areas of concern is terminating transmission in PWIDs. Coupling HCV treatment with needle and syringe programs (NSPs), counseling, and deaddiction services or opioid substitution therapy (OST) under one roof will reduce risk for HCV reinfection (Table 3). Recently, the SIMPLIFY trial reported that currently injecting PWIDs achieved SVR12 of 94%, irrespective of IDU before or during therapy. 21 In the ANCHOR study, those receiving OST were more adherent to follow‐up and change in high‐risk behavior. 22 These studies suggest that active drug use during HCV treatment did not impact treatment outcomes.
TABLE 3.
PWIDs Prevalence and NSP/OST Coverage Across WHO Regions
| WHO Region | Size of the Population Injecting Drugs | Proportion of Countries With NSPs | Needle and Syringe Substitution | |||
|---|---|---|---|---|---|---|
| Number (millions) | Prevalence (%) in the Population 15‐64 Years | Proportion of Countries Reporting Data on PWIDs | % of Countries With Data | Median Number of Syringes/PWIDs/Year | ||
| African region | 0.52 | 0.1 | 30 | 11 | 2 | 6 |
| Region of the Americas | 2.75 | 0.42 | 34 | 26 | 9 | 22 |
| Eastern Mediterranean region | 0.92 | 0.23 | 43 | 38 | 14 | 25 |
| European | 3.97 | 0.66 | 92 | 91 | 58 | 59 |
| South‐East Asia region | 0.56 | 0.04 | 82 | 55 | 55 | 29 |
| Western Pacific region | 3.03 | 0.23 | 33 | 33 | 26 | 57 |
| World | 11.75 | 0.25 | 53 | 36 | 26 | 27 |
Reprinted with permission from Global Hepatitis Report 2017, World Health Organization.
A systematic review concluded that OST (relative risk [RR] = 0.50, 95% confidence interval [CI] = 0.40‐0.63) reduces risk for new HCV infection and is strengthened in combination with NSP. There is weaker evidence for the impact of NSP (RR = 0.79, 95% CI = 0.39‐1.61), although stronger evidence that high coverage is associated with reduced risk in Europe. 23
A target of 90% reduction in new HCV is needed for preventing new infections. Secondary measures include reuse prevention syringes, blood banking regulation, better biomedical waste disposal, and education of dialysis and medical care personnel for improved medical care safety.
Elimination of HCV by 2030: Treatment Trends
Chen et al. 24 performed a modeling study on the HCV burden, treatment capacity, and clinical landscape of HCV treatment in five European countries: France, Germany, Italy, Spain, and the United Kingdom. First, the number of PLHCVs with SVR12 will exceed the number diagnosed but have not yet received DAAs. Second, the patients already diagnosed with HCV will have received treatment by the end of 2020, which implies that aggressive screening strategies are required to treat the bulk of the iceberg, that is, the undiagnosed burden of PLHCVs. Hence rather than birth cohort or high‐risk screening strategy, the trend will shift toward universal screening and treatment. Third, the capacity‐building measures for a nonrestrictive treatment of PLHCV will be required. Initially, these five states treated only F4 patients, and then gradually included F3, then F2, and finally F0‐F1. For example, the number of patients receiving treatment would remain steady in France and Italy until at least 2020, whereas the number of patients receiving treatment will decline substantially in Germany, Spain, and the United Kingdom by 2020, unless the diagnosis rate is increased. Therefore, a low treatment rate limits HCV coverage in France and Italy; whereas in the United Kingdom, Germany, and Spain, a restrictive diagnosis rate due to current screening practice is the bottleneck. 24
Screening Strategies
Screening strategies suggest appreciable prevention benefits could be achieved from WHO’s treat‐all strategy, although greater benefits per treatment can be achieved through targeting PWIDs. Under the current process, treatment coverage achieved will be between 65% and 74%, a far cry from the target of 90%. Hence the restrictive strategy should be phased out. Higher impact will be achieved in countries with high population growth. Analyses have been performed in several countries with a high prevalence of HCV, including the United States, Japan, and Egypt. Kim et al. reported that one‐time screening for HCV in South Korea is likely to be highly cost‐effective in people aged 40 to 69 years at current levels of treatment uptake due to a higher cost of managing cirrhosis. 25 Similarly, Japan has adopted national screening for both the general population and the high‐risk groups. 26
Another issue faced by public health programs is the cumulative treatment failure cases, which are about 8% of all cases. 6 These PLHCVs, already linked to care, require longer duration and expensive and multidrug rescue regimens. This is a necessary cost because successful retreatment will go a long way in reducing decompensation, HCC, and liver‐related mortality.
Conclusion
Global elimination of HCV requires a multipronged approach as mandated by the WHO. Every state needs to formulate a locally relevant health care policy targeting not only the high‐risk population but also the general population to curb transmission of HCV. Adopting the dual approach of depleting the reservoir of HCV and decreasing the incidence of new infection would help curtail the disease and decrease liver‐related mortality attributable to HCV.
FIG 3.
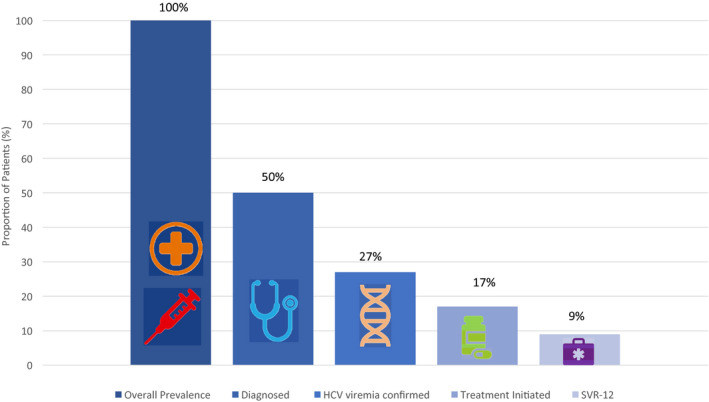
HCV cascade of care. Of the 3.5 million estimated PLHCVs in the United States, only 20% have been screened by serological tests and then only 27% have NATs for confirmation; still fewer with disease staging, treatment initiation, and adherence; and finally, only 9% of the total pool achieves cure.
Potential conflict of interest: Nothing to report.
References
- 1. World Health Organization . Global Health Sector Strategy on Viral Hepatitis 2016‐2021: towards ending viral hepatitis. WHO/HIV/2016.06. Available at: http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/. Accessed June 15, 2019.
- 2. World Health Organization . Global Hepatitis Report. Geneva: World Health Organization; 2017. [Google Scholar]
- 3. Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016;388:1081‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Progress report on access to hepatitis C treatment. Focus on overcoming barriers in low‐ and middle‐income countries. WHO/CDS/HIV/18.4. Available at: http://apps.who.int/iris/bitstream/10665/260445/1/WHO-CDS-HIV-18.4-eng.pdf?ua=1. Published March 2018. Accessed June 21, 2019.
- 5. Chugh Y, Dhiman RK, Premkumar M, et al. Real‐world cost‐effectiveness of pan‐genotypic Sofosbuvir‐Velpatasvir combination versus genotype dependent directly acting anti‐viral drugs for treatment of hepatitis C patients in the universal coverage scheme of Punjab state in India. PLoS One 2019;14:e0221769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhiman RK, Grover GS, Premkumar M, et al.; MMPHCRF Investigators . Decentralized care with generic direct‐acting antivirals in the management of chronic hepatitis C in a public health care setting. J Hepatol 2019;71:1076‐1085. [DOI] [PubMed] [Google Scholar]
- 7. Arora S, Thornton K, Murata G, et al. Outcomes of hepatitis C treatment by primary care providers. N Engl J Med 2011;364:2199‐2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dhiman RK, Grover GS, Premkumar M, et al. Direct‐acting antiviral therapy is safe and effective in pediatric chronic hepatitis C: the public health perspective. J Pediatr Gastroenterol Nutr 2019;68:74‐80. [DOI] [PubMed] [Google Scholar]
- 9. Chhatwal J, Chen Q, Bethea ED, et al. The impact of direct‐acting antivirals on the hepatitis C care cascade: identifying progress and gaps towards hepatitis C elimination in the United States. Aliment Pharmacol Ther 2019;50:66‐74. [DOI] [PubMed] [Google Scholar]
- 10. Premkumar M, Grover GS, Dhiman RK. Chronic hepatitis C: do generics work as well as branded drugs? J Clin Exp Hepatol 2017;7:253‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iyengar S, Tay‐Teo K, Vogler S, et al. Prices, costs, and affordability of new medicines for hepatitis C in 30 countries: an economic analysis. PLoS Med 2016;13:e1002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yehia BR, Schranz AJ, Umscheid CA, et al. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta‐analysis. PLoS One 2014;9:e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Public Health England . Hepatitis C in the UK, 2017 report: working to eliminate hepatitis C as a major public health threat. London: Public Health England; 2017. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/632465/HCV_in_the_uk_report_2017.pdf. Published July 2017. Accessed September 4, 2019. [Google Scholar]
- 14. The Kirby Institute, UNSW Sydney . Monitoring hepatitis C treatment uptake in Australia Issue 7, July 2017. Available at: https://kirby.unsw.edu.au/report/monitoring-hepatitis-c-treatment-uptake-australia-issue-7-july-2017. Published July 2017. Accessed September 4, 2019. [Google Scholar]
- 15. Tsertsvadze T, Gamkrelidze A, Chkhartishvili N, et al. Progress towards achieving hepatitis c elimination in the country of Georgia, April 2015 – March 2018. J Hepatol 2019;70(Suppl. 258):e141‐e382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elsharkawy A, Fouad R, El Akel W, et al. Sofosbuvir‐based treatment regimens: real life results of 14 409 chronic HCV genotype 4 patients in Egypt. Aliment Pharmacol Ther 2017;45:681‐687. [DOI] [PubMed] [Google Scholar]
- 17. Wasitthankasem R, Posuwan N, Vichaiwattana P, et al. Decreasing hepatitis C virus infection in Thailand in the past decade: evidence from the 2014 national survey. PLoS One 2016;11:e0149362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDonald SA, Mohamed R, Dahlui M, et al. Bridging the data gaps in the epidemiology of hepatitis C virus infection in Malaysia using multi‐parameter evidence synthesis. BMC Infect Dis 2014;14:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haridy J, Wigg A, Muller K, et al. Real‐world outcomes of unrestricted direct‐acting antiviral treatment for hepatitis HCV in Australia: the South Australian statewide experience. J Viral Hepat 2018;25:1287‐1297. [DOI] [PubMed] [Google Scholar]
- 20. Trusheim MR, Cassidy WM, Bach PB. Alternative state‐level financing for hepatitis C treatment—the “netflix model”. JAMA 2018;320:1977‐1978. [DOI] [PubMed] [Google Scholar]
- 21. Grebely J, Dalgard O, Conway B, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open‐label, single‐arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol 2018;3:153‐161. [DOI] [PubMed] [Google Scholar]
- 22. Dore GJ, Altice F, Litwin AH, et al.; C‐EDGE CO‐STAR Study Group . Elbasvir‐grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med 2016;165:625‐634. [DOI] [PubMed] [Google Scholar]
- 23. Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta‐analysis. Addiction 2018;113:545‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Q, Ayer T, Bethea E, et al. Changes in hepatitis C burden and treatment trends in Europe during the era of direct‐acting antivirals: a modelling study. BMJ Open 2019;9:e026726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim DY, Han K-H, Jun B, et al. Estimating the Cost‐Effectiveness of One‐Time Screening and Treatment for Hepatitis C in Korea. PLoS ONE 2019;12:e026726 10.1371/journal.pone.0167770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tanaka J, Akita T, Ko K, et al. Epidemiological Research Group on Viral Hepatitis and its Long‐term Course, Ministry of Health, Labour and Welfare of Japan. Countermeasures against viral hepatitis B and C in Japan: An epidemiological point of view. Hepatol Res 2019;49:990‐1002. 10.1111/hepr.13417 [DOI] [PMC free article] [PubMed] [Google Scholar]


