Watch a video presentation of this article
Watch an interview with the author
Abbreviations
- ADH
alcohol dehydrogenase
- AH
alcoholic hepatitis
- ALD
alcohol‐associated liver disease
- ALDH
acetaldehyde dehydrogenase
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- LT
liver transplantation
- NIAAA
National Institute of Alcohol Abuse and Alcoholism
- PNPLA3
patatin‐like phospholipase domain‐containing protein 3
Alcohol‐associated liver disease (ALD) is one of the major causes of chronic liver diseases worldwide. ALD represents a spectrum of histopathological changes in patients with excessive alcohol use ranging from alcohol‐induced steatosis, alcoholic steatohepatitis, and cirrhosis.1 Alcoholic steatosis can occur in patients who consumed 120 to 150 g of alcohol per day for 2 to 3 weeks.2 Alcoholic hepatitis (AH) is a severe form of ALD with high morbidity and mortality.3 Approximately 8% to 20% of patients who drink alcohol excessively will progress to cirrhosis in their lifetime4 (Fig. 1). Approximately 27% of liver‐related deaths worldwide are attributed to alcohol consumption, with the highest in Europe and levels increasing globally.5 The overall mortality rates from ALD vary across countries in the Eastern and Western Hemispheres, and are closely correlated with the per capita alcohol consumption.6 Total alcohol per capita consumption is highest in the developed world, notably Eastern Europe, which has the highest per capita consumption.7 Although the per capita alcohol consumption is lower in several countries in Asia when compared with the West, there has been a rapid rise in per capita alcohol consumption in countries such as Indian and China with recent changes in the economies and increase in average incomes.1 For instance, the percentage of regular alcohol drinkers among the general population in different areas in China increased from 27.0% in 2000 to 66.2% in 2015, and the percentage of excessive drinkers increased from 0.21% in 1982 to 14.8% in 2000.8
Figure 1.
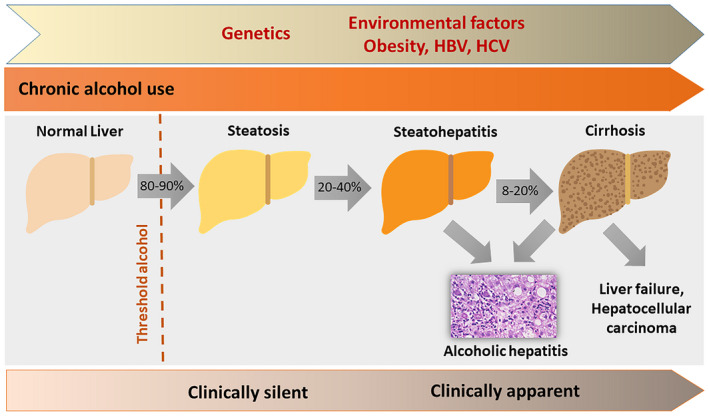
Schematic diagram on the histopathological spectrum of ALD.
Factors Associated With ALD
Several factors place patients at risk for ALD among excessive drinkers.1 Overweight and obesity were found to increase the risk for ALD based on studies in the United States, Europe, and Asia.1 Variants in genes encoding members of the alcohol dehydrogenase (ADH) family affect their ability to metabolize alcohol and determine the risk for alcohol dependence and ALD.1, 9 Patients with variants encoding for high levels of enzyme activity (ADH1B*2 and ADH1C*1 alleles) are believed to be at risk for ALD because of the accumulation of a toxic alcohol metabolite, acetaldehyde. A recent meta‐analysis confirmed this association in Asian populations but found no association in Western populations.10 The explanation in the difference between East and West populations is likely due to the low allele frequency for ADH1B*2 in Caucasians. Approximately 40% to 50% of Chinese people are homozygous or heterozygous for the ALDH2*2 allele with low ALDH2 activity.11 These individuals have high blood concentrations of acetaldehyde after alcohol consumption because of the inability to convert acetaldehyde to acetate, which makes them more susceptible to liver injury.1 The patatin‐like phospholipase domain‐containing protein 3 (PNPLA3) gene is located on the long arm of chromosome 22.12 Patients with PNPLA3 variant rs738409 have a 2.25‐fold increased risk for alcoholic cirrhosis13; the results were confirmed in a recent meta‐analysis, especially in the European cohort. A small study from India found that the rs738409 polymorphism increased the risk for ALD 2.1‐fold.14 However, further studies are needed to determine the association between PNPLA3 and ALD in Asian populations.
Clinical Presentation
The range of clinical features of ALD varies, from asymptomatic to end‐stage liver disease with portal hypertension (Fig. 1). Acute deterioration in patients with ALD, presenting as AH or acute‐on‐chronic liver failure manifesting as jaundice, ascites, and encephalopathy, is often the consequence of recent alcohol use. Many patients in Eastern and Western countries at the time of initial presentation of AH already have underlying alcoholic cirrhosis. Compared with Western populations, individuals with alcohol use disorders in Asia‐Pacific countries have increased rates of viral hepatitis infection, which might exacerbate the progression of underlying ALD.15 The mean age at which ALD is diagnosed in Asian countries is younger than those from the United States and several European countries.16, 17, 18, 19 The age differences between each geographic region are likely due to the data collection from the different health care settings and the study cohort being studied. Epidemiological data reported from each region have indicated a higher prevalence of ALD and its mortality in men.18, 19
Disease Burden
It is difficult to estimate the overall prevalence of alcoholic steatosis primarily because patients with alcoholic steatosis are mostly asymptomatic and thus remain undiagnosed. However, in several population‐based surveys in China, the reported prevalence rate is around 0.97% to 4.29%.8 The prevalence rate of alcoholic steatosis among U.S. adults was 4.7%, based on the 2001‐2016 National Health and Nutrition Examination Survey.16 The available data on the disease burden of AH from each geographic region are difficult to determine and compare because of the heterogeneity of the population being studied.1 The overall prevalence rate of AH was 7.4% from a large cohort of patients with excessive alcohol use who underwent liver biopsy.20 AH‐related hospitalization in the United States was 0.83% of total admissions in 2010 based on the analysis using National Inpatient Sample.19 The standardized incidence rate for AH hospitalization per 105 persons/year in South Korea was 14 in 2010, and the annual in‐hospital mortality rate ranged from 0.2% to 0.5%.21 The number of deaths caused by alcoholic cirrhosis among all ages and the age‐standardized death rate per 100,000 people in China was 41,792 and 5.23 in 1990 compared with 41,163 and 2.89 in 2013, respectively.8 Likewise, ALD was the leading cause of liver cirrhosis in Thailand, with the overall prevalence rate estimated at 40% of hospitalized patients and with increasing rates during 2009 to 2013.22 In a nationally representative cohort of privately insured persons in the United States, 36% of all patients with cirrhosis in the study cohort had alcoholic cirrhosis.23 Over the 7‐year study period, the estimated prevalence rate of alcoholic cirrhosis increased from 0.07% to 0.10%.23 Of importance, a recent study found a rapid increase in death rates among young people, aged 25 to 34 years old, with alcoholic cirrhosis in the United States.24 The evidence indicates that increases in participation of treatment for alcohol abuse and alcoholics anonymous programs have played an important role in reducing alcohol‐related morbidity and mortality in some countries.25
Management
Management of ALD depends on its clinical stage. Alcoholic steatosis is reversible on abstinence.1 For AH, the European and U.S. guidelines advocate corticosteroids therapy for patients with severe presentation as defined by Maddrey’s discriminant function ≥32.26, 27 In a recent meta‐analysis of individual data from controlled trials, therapy with corticosteroids modestly improves short‐term survival of patients with severe AH.28 Failure to improve after a week of corticosteroid therapy based on the Lille criteria was proposed as an indicator of treatment failure.29 Hence several combination therapies have been evaluated for enhancing the effectiveness of corticosteroids. Coadministration of intravenous N‐acetylcysteine with corticosteroids reduced 28‐day mortality with the lower incidence of infection and hepatorenal syndrome in European patients.30 The use of granulocyte colony‐stimulating factor to promote liver regeneration offered a favorable effect on the 90‐day survival of Indian patients with severe AH.31 The potential benefit of both treatments makes them appealing options, but it requires further validation. A large National Institute of Alcohol Abuse and Alcoholism (NIAAA)–sponsored clinical trial for patients with AH using interleukin‐1 receptor, granulocyte colony‐stimulating factor, and zinc sulfate is ongoing. Treatment approaches in China differ from Western countries with the use of traditional Chinese medicines and acupuncture.8
Alcoholic cirrhosis is one of the major indications for liver transplantation (LT) in the United States and Europe, and it has been increasing in some Asian countries.32, 33 Living donor LT is currently the predominant form of LT in Asia.32, 33 The principles of evaluating transplant candidates are similar to those of Western countries.34 Patient survival after LT in Eastern countries seems to be comparable with Western countries.32, 33, 35 Over the last decade, early LT has been increasingly offered in carefully selected patients with severe AH who are not responding to medical therapy. Similar to the European cohort, the U.S. and Asian experiences confirm the survival benefit of early LT for severe AH (Table 1).36, 37, 38, 39, 40 The incidence of alcohol use posttransplant was lower in Asian recipients with living donors, possibly due to stronger psychosocial support from their donors who are family members.
Table 1.
Early LT for Severe AH in Asia, Europe, and the United States
| The European Experience | The U.S. Experience | The Asian Experience | |||
|---|---|---|---|---|---|
| Author (year) | Mathurin et al. (2011)36 | Im et al. (2016)39 | Weeks et al. (2018)37 | Lee et al. (2018)40 | Choudhary et al. (2019)38 |
| N | 26 | 9 | 46 | 147 | 39 |
| Study design | Prospective | Retrospective | Retrospective | Retrospective | Retrospective |
| Comparison group | Historic, severe AH, no LT | Severe AH, no LT | Alcoholic cirrhosis with ≥6 months of abstinence, underwent LT | No comparison group | Alcoholic cirrhosis with mean of 4 months of abstinence, underwent LT |
| Median follow‐up | Not reported | 2.0 years | 1.3 years | 1.6 years | 2.3 years |
| Survival | 77% at 6 months | 89% at 6 months | 98% at 6 months | 94% at 1 year | 85% at 1 year |
| 71% at 1 year | 97% at 1 year | 84% at 3 years | |||
| Alcohol use post‐LT | 12% | 22% | 28% | 25% at 1 year | 13% |
| 34% at 3 years | |||||
| Median time to alcohol use post‐LT | 740 days | 132 days | 256 days | 160 days | Not reported |
Conclusion
The brief descriptions in this review underscore the unmet need for strategies to reduce the current global burdens from ALD. The expanding knowledge of the pathogenesis of ALD and the relative roles of environmental and genetic factors can be translated into clinical practice with the hope to identify new therapeutic agents for the treatment of ALD. Abstinence remains the cornerstone of treatment, with public awareness on the detrimental effects of alcohol use and the implementation of policies to restrict the availability of alcohol.
This study was partly supported by National Institutes of Health grants R01AA025208, R01DK107682, U01AA026917, UH2AA026903, and I01CX000361 (to S.L.).
Potential conflict of interest: S.L. consults for Durect Corporation. T.P. is on the speakers’ bureau and received grants from Gilead; is on the speakers’ bureau for Bristol‐Myers Squibb, Bayer, Abbott, Eisai, and MSD; and received grants from Roche, Janssen, FibroGen, and Vir.
References
- 1. Liangpunsakul S, Haber P, McCaughan GW. Alcoholic liver disease in Asia, Europe, and North America. Gastroenterology 2016;150:1786‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lieber CS, Jones DP, Decarli LM. Effects of prolonged ethanol intake: Production of fatty liver deposit adequate diets. J Clin Invest 1965;44:1009‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lourens S, Sunjaya DB, Singal A, et al. Acute alcoholic hepatitis: Natural history and predictors of mortality using a multicenter prospective study. Mayo Clin Proc Innov Qual Outcomes 2017;1:37‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mills SJ, Harrison SA. Comparison of the natural history of alcoholic and nonalcoholic fatty liver disease. Curr Gastroenterol Rep 2005;7:32‐36. [DOI] [PubMed] [Google Scholar]
- 5. Mokdad AH, Forouzanfar MH, Daoud F, et al. Global burden of diseases, injuries, and risk factors for young people's health during 1990‐2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2016;387:2383‐2401. [DOI] [PubMed] [Google Scholar]
- 6. Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013;59:160‐168. [DOI] [PubMed] [Google Scholar]
- 7. Global Information System on Alcohol and health (GISAH) . Total alcohol per capita (15+ years) consumption, in liters of pure alcohol, 2010. Available at: http://gamapserver.who.int/gho/interactive_charts/gisah/consumption_total/atlas.html. Published 2015.
- 8. Wang WJ, Xiao P, Xu HQ, et al. Growing burden of alcoholic liver disease in China: A review. World J Gastroenterol 2019;25:1445‐1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anstee QM, Seth D, Day CP. Genetic factors that affect risk of alcoholic and nonalcoholic fatty liver disease. Gastroenterology 2016;150:1728‐1744.e7. [DOI] [PubMed] [Google Scholar]
- 10. He L, Deng T, Luo HS. Genetic polymorphism in alcohol dehydrogenase 2 (ADH2) gene and alcoholic liver cirrhosis risk. Int J Clin Exp Med 2015;8:7786‐7793. [PMC free article] [PubMed] [Google Scholar]
- 11. Eng MY, Luczak SE, Wall TL. ALDH2, ADH1B, and ADH1C genotypes in Asians: A literature review. Alcohol Res Health 2007;30:22‐27. [PMC free article] [PubMed] [Google Scholar]
- 12. Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461‐1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tian C, Stokowski RP, Kershenobich D, et al. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet 2010;42:21‐23. [DOI] [PubMed] [Google Scholar]
- 14. Dutta AK. Genetic factors affecting susceptibility to alcoholic liver disease in an Indian population. Ann Hepatol 2013;12:901‐907. [PubMed] [Google Scholar]
- 15. Wong MCS, Huang JLW, George J, et al. The changing epidemiology of liver diseases in the Asia‐Pacific region. Nat Rev Gastroenterol Hepatol 2019;16:57‐73. [DOI] [PubMed] [Google Scholar]
- 16. Wong T, Dang K, Ladhani S, et al. Prevalence of alcoholic fatty liver disease among adults in the United States, 2001‐2016. JAMA 2019;321:1723‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol 2013;28(Suppl. 1):11‐17. [DOI] [PubMed] [Google Scholar]
- 18. Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol 2015;62:S38‐S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jinjuvadia R, Liangpunsakul S. Trends in alcoholic hepatitis‐related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol 2015;49:506‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naveau S, Giraud V, Borotto E, et al. Excess weight risk factor for alcoholic liver disease. Hepatology 1997;25:108‐111. [DOI] [PubMed] [Google Scholar]
- 21. Lee JY, Cho Y, Hong MH, et al. Incidence, inhospital mortality, and readmission among patients with alcoholic hepatitis in Korea: A nationwide study. J Gastroenterol Hepatol 2019;34:747‐754. [DOI] [PubMed] [Google Scholar]
- 22. Charatcharoenwitthaya P, Soonthornworasiri N, Karaketklang K, et al. Factors affecting mortality and resource use for hospitalized patients with cirrhosis: A population‐based study. Medicine (Baltimore) 2017;96:e7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodkin K, Heckman TG, Siegel K, et al. “Putting a face” on HIV infection/AIDS in older adults: A psychosocial context. J Acquir Immune Defic Syndr 2003;33:S171‐S184. [PubMed] [Google Scholar]
- 24. Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999‐2016: Observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smart RG, Mann RE. The impact of programs for high‐risk drinkers on population levels of alcohol problems. Addiction 2000;95:37‐51. [DOI] [PubMed] [Google Scholar]
- 26. Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol‐associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2020;71:306‐333. [DOI] [PubMed] [Google Scholar]
- 27. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: Management of alcohol‐related liver disease. J Hepatol 2018;69:154‐181. [DOI] [PubMed] [Google Scholar]
- 28. Louvet A, Thursz MR, Kim DJ, et al. Corticosteroids reduce risk of death within 28 days for patients with severe alcoholic hepatitis, compared with pentoxifylline or placebo‐a meta‐analysis of individual data from controlled trials. Gastroenterology 2018;155:458‐468.e8. [DOI] [PubMed] [Google Scholar]
- 29. Louvet A, Naveau S, Abdelnour M, et al. The Lille model: A new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007;45:1348‐1354. [DOI] [PubMed] [Google Scholar]
- 30. Nguyen‐Khac E, Thevenot T, Piquet MA, et al. Glucocorticoids plus N‐acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011;365:1781‐1789. [DOI] [PubMed] [Google Scholar]
- 31. Singh V, Sharma AK, Narasimhan RL, et al. Granulocyte colony‐stimulating factor in severe alcoholic hepatitis: A randomized pilot study. Am J Gastroenterol 2014;109:1417‐1423. [DOI] [PubMed] [Google Scholar]
- 32. Chen GH, Yang Y, Lu MQ, et al. Liver transplantation for end‐stage alcoholic liver disease: A single‐center experience from mainland China. Alcohol 2010;44:217‐221. [DOI] [PubMed] [Google Scholar]
- 33. Soyama A, Eguchi S, Egawa H. Liver transplantation in Japan. Liver Transpl 2016;22:1401‐1407. [DOI] [PubMed] [Google Scholar]
- 34. Shukla A, Vadeyar H, Rela M, et al. Liver transplantation: East versus West. J Clin Exp Hepatol 2013;3:243‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017;152:1090‐1099.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365:1790‐1800. [DOI] [PubMed] [Google Scholar]
- 37. Weeks SR, Sun Z, McCaul ME, et al. Liver transplantation for severe alcoholic hepatitis, updated lessons from the world's largest series. J Am Coll Surg 2018;226:549‐557. [DOI] [PubMed] [Google Scholar]
- 38. Choudhary NS, Saigal S, Gautam D, et al. Good outcome of living donor liver transplantation for severe alcoholic hepatitis not responding to medical management: A single center experience of 39 patients. Alcohol 2019;77:27‐30. [DOI] [PubMed] [Google Scholar]
- 39. Im GY, Kim‐Schluger L, Shenoy A, et al. Early liver transplantation for severe alcoholic hepatitis in the United States—A single‐center experience. Am J Transplant 2016;16:841‐849. [DOI] [PubMed] [Google Scholar]
- 40. Lee BP, Mehta N, Platt L, et al. Outcomes of early liver transplantation for patients with severe alcoholic hepatitis. Gastroenterology 2018;155:422‐430.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]


