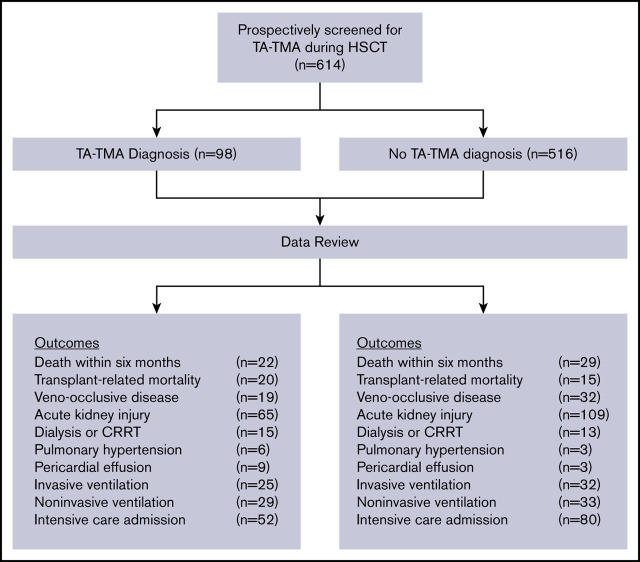
Key Points
In this multicenter study, we report a high incidence (16%) of TA-TMA after pediatric stem cell transplant.
Patients with TA-TMA have higher morbidity and mortality compared with patients without TA-TMA.
Abstract
Transplant-associated thrombotic microangiopathy (TA-TMA) is a severe complication of hematopoietic stem cell transplantation (HSCT). A single-center prospective screening study has shown that the incidence of TA-TMA is much higher than prior retrospective studies that did not systematically screen. These data have not been replicated in a multicenter study. Our objective was to determine the incidence and risk factors for TA-TMA and compare outcomes of pediatric HSCT patients with and without TA-TMA. Patients were prospectively screened for TA-TMA at participating centers using a simple to implement and inexpensive strategy from the start of the preparative regimen through day +100. TA-TMA was diagnosed if ≥4 of 7 laboratory/clinical markers diagnostic for TA-TMA were present concurrently or if tissue histology showed TA-TMA. A total of 614 patients (359 males; 58%) received prospective TA-TMA screening at 13 pediatric centers. TA-TMA was diagnosed in 98 patients (16%) at a median of 22 days (interquartile range, 14-44) posttransplant. Patients with TA-TMA had significantly increased bloodstream infections (38% [37/98] vs 21% [107/51], P ≤ .001), mean total hospitalization days (68; 95% confidence interval [CI], 63-74 vs 43; 95% CI, 41-45; P ≤ .001), and number of days spent in the intensive care unit (10.1; 95% CI, 6.4-14; vs 1.6; 95% CI, 1.1-2.2; P ≤ .001) in the first 100 days after HSCT compared with patients without TA-TMA. Overall survival was significantly higher in patients without TA-TMA (93%; 490/516) compared with patients with TA-TMA (78%; 76/98) (P ≤ .001). These data support the need for systematic screening for TA-TMA and demonstrate the feasibility and efficacy of an easy to implement strategy to do so.
Visual Abstract
Introduction
Hematopoietic stem cell transplantation (HSCT) is an effective treatment for many malignant conditions, marrow failure syndromes, and immune deficiencies in children, adolescents, and adults.1,2 Today, >50 000 HSCTs are carried out annually worldwide, and these numbers increase each year. Transplant strategies and supportive care have evolved over recent decades, resulting in improved overall survival (OS).3 Despite these advances, patients undergoing HSCT are at high risk for developing complications. Transplant-associated thrombotic microangiopathy (TA-TMA) is now recognized as a severe complication of HSCT.1,2 TA-TMA after HSCT can range from a mild self-limiting disease to a fulminant multiorgan disorder leading to death.3 Specifically, mortality as high as >80% has been reported 1 year after HSCT.4 The kidney is the organ most commonly affected by TA-TMA. However, other organs, such as the gastrointestinal tract and heart, can be affected as well.4,5 Patients diagnosed with TA-TMA can develop life-threatening pulmonary hypertension, pericardial effusions,6,7 bowel ischemia,4 and central nervous system hemorrhage.8 Patients with untreated severe TA-TMA can progress to multiple organ failure due to ongoing tissue injury, although patients with mild TA-TMA typically recover over time without specific treatment.
The lack of clarity regarding the incidence of TA-TMA is a consequence of the absence of any large prospective multicenter studies collecting data that might be considered generalizable to larger populations. In general, retrospective studies have reported low frequencies of TA-TMA because centers do not routinely screen for appropriate markers; thus, only the most severe cases are identified.9-13 Further, tissue diagnosis for TA-TMA is often not feasible in ill HSCT populations at high risk for bleeding.
Previous work in pediatrics, conducted mostly at a single center, suggests that simple and inexpensive markers applied to an entire HSCT population will be effective in correctly identifying TA-TMA cases, as well as in identifying those who need treatment.14,15 Recent prospective single-center TA-TMA studies in children and young adults have provided new insights into pathogenesis and genetic susceptibility to TA-TMA, raising awareness of this critical transplant complication and allowing for the identification of therapeutic options, including complement blockade.16,17 However, clinical experience is limited to few transplant centers.
In this study, we set out to establish whether a simple pragmatic approach to screening could be applied broadly at disparate transplant centers and would contribute to clinical care. We implemented prospective TA-TMA screening through a pragmatic research approach, using contemporary diagnostic criteria, in 13 pediatric transplant centers including small, medium, and large pediatric institutions as a combined Pediatric Transplant and Cellular Therapy Consortium and Pediatric Acute Lung Injury and Sepsis Investigators research effort. We then completed a multi-institutional data analysis to determine the incidence, timing, risk factors, and outcomes for TA-TMA after HSCT.
Methods
Prospective screening for TA-TMA
Prior to uniform implementation, members of the Pediatric Transplant and Cellular Therapy Consortium and Pediatric Acute Lung Injury and Sepsis Investigators network debated and agreed upon the screening and diagnosis algorithm that was modeled after evidence-based guidelines.8,9,12-14,18,19 Screening for TA-TMA was agreed upon by all providers at the participating centers and implemented into clinical practice in all pediatric patients undergoing HSCT between 2016 and 2019 (Figure 1A). To ensure reliability of TA-TMA screening, participating centers added TA-TMA screening to the transplant paper and electronic order sets. Additionally, local investigators verified adherence to the screening through regular audits. Data regarding clinical outcomes through the first 6 months post-HSCT were collected for all patients receiving TA-TMA screening. Prospective TA-TMA screening at the participating centers included daily complete blood count with differential, monitoring for schistocytes and transfusion requirements, renal panel and blood pressure measurement, twice-weekly lactate dehydrogenase (LDH), and weekly urine analysis (UA), with calculation of a random urine protein/creatinine ratio. Terminal complement activity measurement (blood sC5b-9) was requested by the treating physician only when TA-TMA was suspected. sC5b-9 testing was done internally or as an external send-out.
Figure 1.
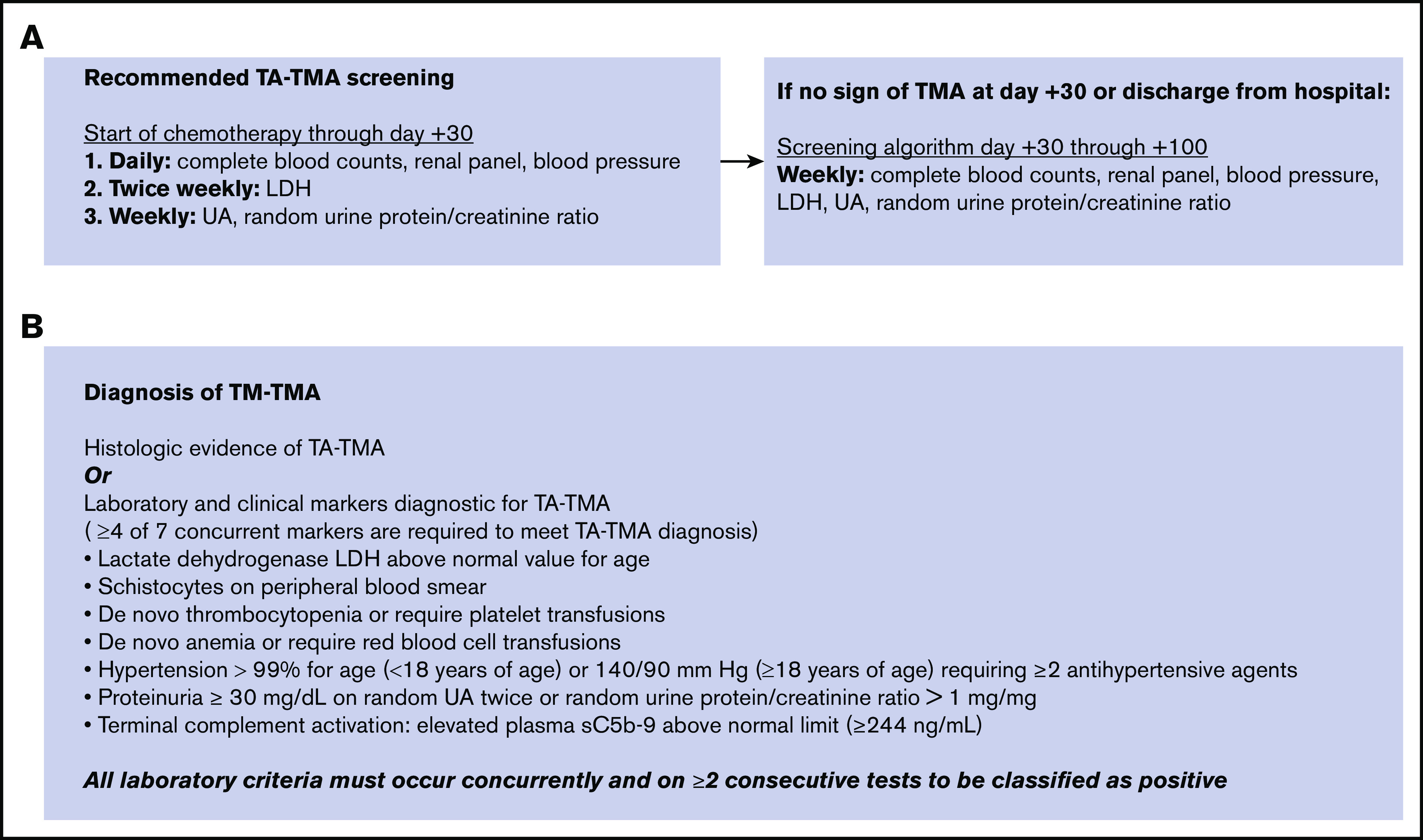
TA-TMA screening and diagnosis algorithms for patients at participating centers. (A) Participating institutions implemented standardized screening for TA-TMA. (B) Diagnostic criteria for TA-TMA.
Screening continued through the first 30 days posttransplant or until discharge from the hospital, whichever came later. Upon discharge, or at day +30 post-HSCT, LDH and UA were transitioned to weekly testing and continued through day +100 or resolution of TA-TMA, if later. Institutions used individual implementation strategies to ensure all patients were reliably screened.20,21
Diagnosis of TA-TMA
TA-TMA was diagnosed if ≥4 of 7 laboratory/clinical markers diagnostic for TA-TMA were concurrently present on 2 consecutive tests or if TA-TMA was identified by histology in a tissue biopsy. TA-TMA diagnostic markers were elevated LDH, schistocytes on the peripheral blood smear, de novo thrombocytopenia or anemia or transfusion dependence, hypertension defined as > 99% for age, proteinuria, and terminal complement activation (elevated plasma sC5b-9 above normal)9,13,14,17,22-24 (Figure 1B). Laboratory criteria for diagnosis of TA-TMA had to occur concurrently, as well as on ≥2 consecutive tests, to be classified as positive (Figure 1B). Additionally, centers were instructed to rule out thrombotic thrombocytopenic purpura by testing for ADAMTS13 activity before the diagnosis of TA-TMA was made, as previously described.10-12 Treatment and further workup after TA-TMA diagnosis was based on each institution’s standard of practice.
Clinical data review
The cohort consists of patients from 4 large transplant programs (>80 HSCTs per year), 4 medium-sized transplant programs (25-80 HSCTs per year), and 5 small institutions (<25 HSCTs per year). The study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center as the coordinating center, and the Institutional Review Board approved the study at each participating site prior to data review. Following Institutional Review Board approval of the uniform study protocol, each site completed a retrospective review of the consecutively screened patients from their center and entered deidentified data into a RedCap database prepared for this study. Data from all centers were aggregated to determine outcomes.
Patient-, disease-, and transplant-related variables
Patient data were described, including demographics, primary transplant diagnosis, therapy characteristics, transplant complications, and outcomes. The following variables were evaluated: sex, age at transplant, diagnosis, donor relationship, HLA match, stem cell graft source, graft-versus-host disease (GVHD) prophylaxis, and conditioning intensity.25 Currently accepted clinical criteria were used for the diagnosis of acute GVHD (aGVHD)26 and steroid-refractory GVHD (SR GVHD).27
Study outcomes and definitions
We assessed the cumulative incidence of TA-TMA in the first 100 days and compared overall survival (OS) and treatment-related mortality (TRM)28 at 6 months post-HSCT between patients who developed TA-TMA and those who did not. We determined the odds ratios (OR) of the following transplant-related complications in the first 100 days post-HSCT: respiratory failure requiring invasive and noninvasive ventilation; acute kidney injury (AKI), defined as doubling of creatinine from pretransplant baseline; veno-occlusive disease (VOD); pulmonary hemorrhage; pericardial effusion requiring surgical intervention (pericardiocentesis, drain, or pericardial window); pulmonary hypertension; and kidney failure requiring renal replacement therapy (RRT) in those with TA-TMA compared with those without. We also compared the number of hospitalization days, days spent in the intensive care unit, and the number of bloodstream infections in the first 100 days in patients with and without TA-TMA. Additionally, we compared the incidence of TRM, defined as death without progression or relapse of primary disease, in patients with TA-TMA compared with those without.28 Finally, in patients who developed TA-TMA, we compared OS in the first 6 months post-HSCT between patients with proteinuria and elevated sC5b-913 and those without, as well as those who received treatment and those who did not.
Statistical analysis
Patients were categorized into those who developed TA-TMA in the first 100 days post-HSCT and those who did not. We compared patient-, disease-, and transplant-related factors between groups using the χ2 test for categorical variables and the Wilcoxon 2-sample test for continuous variables. ORs with 95% confidence intervals (CIs) were determined between patients with and without TA-TMA. Multivariable logistic regression analysis with an examination of the proportional hazard assumption was used to evaluate potential risk factors for TA-TMA. If the proportional hazards assumption was violated, the variable was added as a time-dependent covariate. A stepwise selection procedure was used to identify the final model. The Kaplan-Meier method was used to estimate the probability of 6-month OS, which was compared by the log-rank test. The cumulative incidence function estimated the incidence of TA-TMA by 100 days. Statistical evaluation was performed using R (3.1.3), and P < .05 was considered significant.
Results
Six hundred and fourteen consecutive patients who underwent TA-TMA screening at 1 of the 13 participating centers were included in the analysis. The cost of TA-TMA screening was approximately $60 to $100 per week. Ninety-eight HSCT recipients (16%) were diagnosed with TA-TMA, a median of 22 days after HSCT (interquartile range [IQR], 14-44 days) (Figure 2A). Ninety patients (92%) were diagnosed with TA-TMA after meeting laboratory/clinical criteria alone (≥4 of 7 laboratory/clinical markers), and 8 patients (8%) were diagnosed by meeting laboratory/clinical criteria and having histologic findings of TA-TMA (kidney, n = 5; bowel, n = 2; lungs, n = 4; and skin, n = 1).
Figure 2.
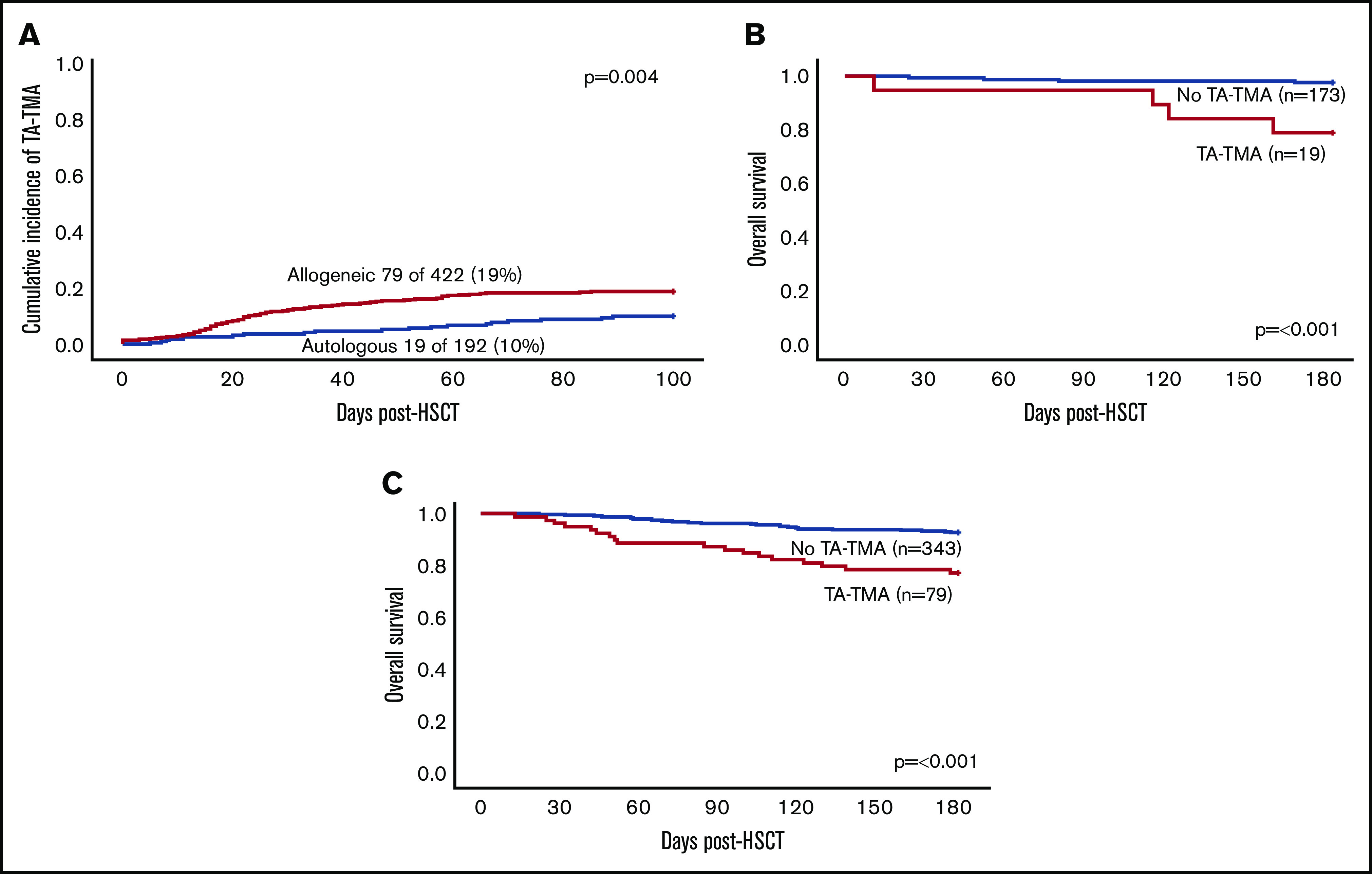
Cumulative incidence and 6-month survival in patients with and without TA-TMA. (A) Cumulative incidence of TA-TMA in all HSCT patients (N = 614). (B) Overall 6-month survival in allogeneic HSCT recipients with and without TA-TMA (n = 422). (C) Overall 6-month survival in autologous HSCT recipients with and without TMA (n = 192).
Nine of the 13 centers (70%) had ≥1 patient who was diagnosed with TA-TMA. The 4 centers that did not diagnose any patients with TA-TMA screened 6% (39/614) of the total patients in the cohort. Sixty-one patients underwent HSCT at a small center with 4 developing TA-TMA (7%), 142 underwent HSCT at a medium-sized center with 18 (13%) developing TA-TMA, and 411 underwent HSCT at large center with 76 (18%) developing TA-TMA. In univariate analyses, institution size was significantly associated with TA-TMA (P = .025); however, this was not statistically significant in multivariate analyses (Table 1).
Table 1.
Multivariate analysis of variables associated with TA-TMA
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| No TA-TMA (n = 516) | TA-TMA (n = 98) | P | OR (95% CI) | P | |
| Age, median (range), y | 6.7 (2.6-14.4) | 5.3 (1.9-12.7) | .11 | ||
| Sex | |||||
| Male | 311 | 64 | .37 | ||
| Female | 205 | 34 | |||
| Diagnosis | |||||
| Malignancy | 338 | 46 | <.001 | 1.0 | |
| Immune deficiency/dysregulation | 75 | 30 | 2.4 (1.3-4.5) | .006 | |
| Bone marrow failure | 54 | 12 | 1.4 (0.6-3.0) | .41 | |
| Nonmalignant hematology | 41 | 5 | 1.1 (0.3-2.9) | .92 | |
| Genetic/metabolic | 8 | 5 | 2.7 (0.7-9.2) | .11 | |
| Donor | |||||
| Autologous | 173 | 19 | .001 | 1.00 | |
| Related allogeneic | 142 | 20 | 1.1 (0.5-2.5) | .88 | |
| Unrelated allogeneic | 200 | 59 | 1.7 (0.8-3.7) | .19 | |
| Syngeneic | 1 | 0 | 0 | ||
| Graft source | |||||
| Bone marrow | 216 | 45 | .033 | 1.00 | |
| Peripheral blood stem cells | 266 | 40 | 1.1 (0.6-2.0) | .72 | |
| Cord blood unit | 34 | 13 | 2.0 (0.9-4.5) | .10 | |
| Preparative regimen | |||||
| Myeloablative | 398 | 69 | .33 | ||
| Nonmyeloablative | 19 | 3 | |||
| Reduced intensity | 96 | 26 | |||
| None | 3 | 0 | |||
| Institution size | |||||
| Small | 57 | 4 | .025 | 1.00 | |
| Medium | 124 | 18 | 1.9 (0.7-6.8) | .28 | |
| Large | 335 | 76 | 2.7 (1.0-9.3) | .07 | |
Univariate and multivariate analyses of variables associated with TA-TMA. Variables were included in the multivariate analysis if P < .1.
Allogeneic HSCT recipients
The incidence of TA-TMA was significantly higher in patients undergoing allogeneic HSCT (79/422; 19%) vs autologous HSCT (19/192; 10%; P = .004). Patients with an underlying immune deficiency had an increased risk for developing TA-TMA in multivariate analyses (OR, 2.4; 95% CI, 1.3-4.5; P = .006). Receiving a graft from an unrelated allogeneic donor was associated with TA-TMA (P = .001); however, this was not significant in multivariate analyses. Forty-two percent (13/31) of patients with primary hemophagocytic lymphohistiocytosis and 50% (4/8) of patients with Hurler syndrome were diagnosed with TA-TMA. Patient demographics and transplant characteristics are listed in Table 1. Univariate analysis of allogeneic HSCT recipients revealed a significant association between TA-TMA and use of alemtuzumab, cyclosporine, and methotrexate, as well as CD34 selection (supplemental Table 1). aGVHD (any grade) was diagnosed in 32% of allogeneic transplant recipients with TA-TMA and in 26% without TA-TMA (P = .33). Thirty-five allogeneic HSCT recipients were diagnosed with steroid-refractory aGVHD: 12 (15%) patients with TA-TMA and 23 (7%) patients without (P = .02).
Twenty-five patients were diagnosed with TA-TMA and aGVHD. In those patients, the median time from transplant to TA-TMA diagnosis was 24 days (IQR, 14-47), whereas the median time to aGVHD was 34 days (IQR, 25-45). Ten patients with aGVHD and TA-TMA were admitted to the intensive care unit; among those, 6 (60%) were diagnosed with TA-TMA prior to the aGVHD diagnosis. In the 12 patients with SR GVHD and TA-TMA, 7 patients (58%) were diagnosed with GVHD prior to the diagnosis of TA-TMA.
Autologous HSCT recipients
In the 192 subjects undergoing autologous HSCT, 72 patients had a primary diagnosis of neuroblastoma, and 120 patients had other solid tumors. Nineteen (10%) TA-TMA cases were diagnosed in the autologous HSCT cohort, and 13 (68%) occurred in patients with neuroblastoma, all of whom were scheduled to undergo tandem HSCT. Univariate analysis showed a significant association between TA-TMA and chemotherapy-preparative regimens (cyclophosphamide, carboplatin, thiotepa, and etoposide) of patients undergoing tandem autologous HSCT for neuroblastoma (supplemental Table 2).
Neuroblastoma and TA-TMA
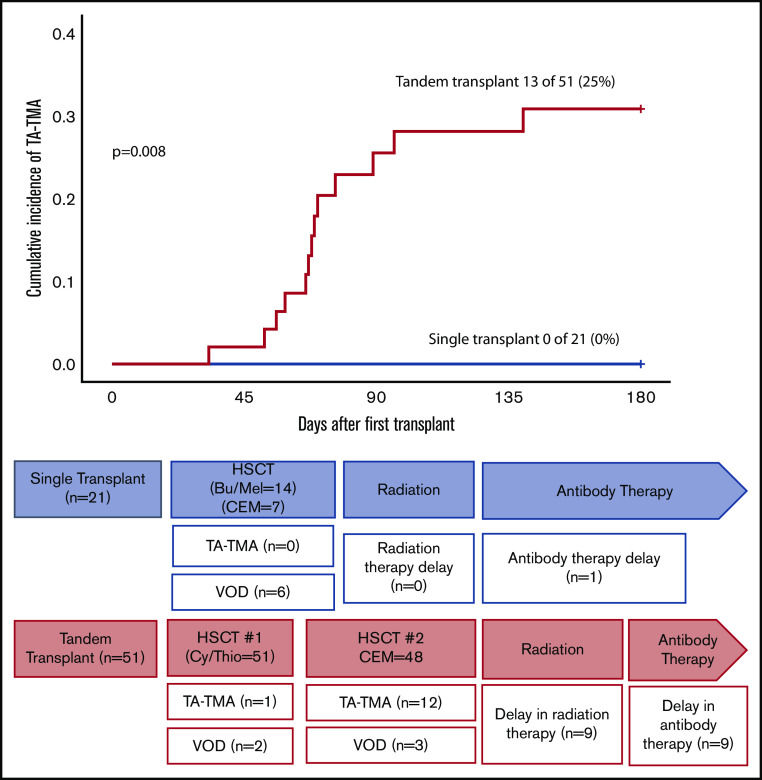
Seventy-two patients underwent autologous HSCT for neuroblastoma. Twenty-one patients (29%) were scheduled for a single transplant, and 51 (71%) were scheduled for a tandem transplant. TA-TMA did not occur in any patient in the single-transplant cohort, although 6 patients (29%) were diagnosed with VOD. No patient allocated to a single transplant had a delay in radiation; 1 patient had a delay in antibody therapy (unknown reason). Thirteen patients (25%) who were scheduled to undergo tandem transplant developed TA-TMA (1 after HSCT #1, 12 after HSCT #2), and 5 patients (10%) developed VOD. Three patients did not receive HSCT #2: 1 because of TA-TMA, 1 because of VOD, and 1 had disease relapse. Fifty-four percent of neuroblastoma patients with TA-TMA had a delay in starting posttransplant radiation therapy and 62% were delayed in receiving anti-GD2 antibody therapy compared with 10% and 7% of those without TA-TMA, respectively (Figure 3).
Figure 3.
Cumulative incidence of TA-TMA in neuroblastoma patients according to pretransplant allocation (n = 72). Seventy-two patients underwent autologous HSCT for neuroblastoma; 21 (29%) were scheduled for single transplant and 51 (71%) were scheduled for tandem transplant. No patient developed TA-TMA from the single transplant cohort; 6 (29%) were diagnosed with VOD. No patients allocated to a single transplant had a delay in radiation, and 1 patient had a delay in antibody therapy (unknown reason). Thirteen (25%) of the patients who were scheduled to undergo a tandem transplant developed TA-TMA (1 after HSCT #1, 12 after HSCT #2), and 5 (10%) developed VOD. Three patients did not receive HSCT #2: 1 because of TA-TMA, 1 developed VOD, and 1 had disease relapse. Nine patients (18%) had a delay in receiving radiation (5 with TA-TMA, 2 with TA-TMA + VOD, 2 with VOD), and 9 patients (18%) had a delay in antibody therapy (7 with TA-TMA, 1 with TA-TMA + VOD, 1 with VOD). Bu, busulfan; CEM, carboplatin, etoposide, melphalan; Cy, cyclophosphamide; Mel, melphalan; Thio, thiotepa.
Organ injury and transplant outcomes
In univariate analyses, all evaluated organ injury variables were significantly increased in patients with TA-TMA compared with patients without TA-TMA, including respiratory failure requiring intubation (26% vs 6%; P ≤ .001) or noninvasive ventilation (30% vs 6%; P ≤ .001), pericardial effusion requiring surgical interventions (9% vs <1%; P ≤ .001), pleural effusion requiring surgical intervention (4% vs <15%; P = .03), pulmonary hypertension (6% vs <1%; P = .001), renal failure requiring RRT (15% vs 3%; P < .001), and AKI (66% vs 21%; P < .001) (Table 2). Further, intensive care admission was significantly associated with TA-TMA (OR, 6.2; 95% CI, 1.4-6.3), pericardial effusion requiring surgical intervention (OR, 17.3; 95% CI, 4.5-66.9), pulmonary hypertension (OR, 11.2; 95% CI, 2.7-46.7), dialysis or continuous RRT (OR, 7.0; 95% CI, 3.2-15.5), death within the first 6 months post-HSCT (OR, 4.9; 95% CI, 2.6-9.0), and TRM (OR, 48.6; 95% CI, 4.1-17.7) (Figure 4).
Table 2.
Univariate outcomes of HSCT patients with and without TA-TMA
| TA-TMA (n = 98) | No TA-TMA (n = 516) | P | |
|---|---|---|---|
| Respiratory failure requiring intubation, n = 57 | 25 (26) | 32 (6) | <.001 |
| Respiratory failure requiring noninvasive ventilation, n = 62 | 29 (30) | 33 (6) | <.001 |
| PE requiring pericardiocentesis, drain, or window during the first 100 d, n = 12 | 9 (9) | 3 (<1) | <.001 |
| Pleural effusion requiring pleurocentesis and/or chest tube in the first 100 d, n = 8 | 4 (4) | 4 (<1) | .03 |
| Pulmonary hypertension diagnosed in first 100 d, n = 9 | 6 (6) | 3 (<1) | .001 |
| Required dialysis or continuous RRT in the first 100 d, n = 28 | 15 (15) | 13 (3) | <.001 |
| AKI in the first 100 d, n = 174 | 65 (66) | 109 (21) | <.001 |
With the exception of the P values, data are n (%).
PE, pericardial effusion.
Figure 4.

Outcomes of pediatric HSCT recipients with and without TA-TMA. CRRT, continuous RRT dialysis; ICU, intensive care unit.
Thirty-nine percent (38/98) of patients with TA-TMA and 21% (107/516) of those without TA-TMA had ≥1 bloodstream infection in the first 100 days posttransplant (P = .0002). Furthermore, 17% (n = 17) of patients with TA-TMA had ≥2 bloodstream infections vs 5% (n = 25) of those without (P = .0001). The mean number of days hospitalized in the first 100 days after HSCT was significantly higher in the TA-TMA group compared with those without TA-TMA (68 days; 95% CI, 63-74 vs 43 days; 95% CI, 41-45; P ≤ .001). Additionally, the mean number of days in the intensive care unit was significantly higher in patients with TA-TMA (10.1 days; 95% CI, 6.4-14 vs 1.6 days; 95% CI, 1.1-2.2; P ≤ .001).
OS in the first 6 months after transplant was significantly lower in allogeneic HSCT patients with TA-TMA compared with allogeneic HSCT patients without TA-TMA (77% vs 93%; P ≤ .001) (Figure 2B). TRM during the first 6 months was significantly higher in allogeneic HSCT recipients with TA-TMA compared with those without (20% vs 3%; P ≤ .0001). OS in autologous HSCT recipients during the first 6 months was also significantly lower in patients with TA-TMA than in those without (79% vs 98%; P = .001) (Figure 2C).
TA-TMA–targeted interventions
Fifty-eight of the 98 (59%) patients with TA-TMA received ≥1 targeted intervention based on treating physician discretion. Fifty (51%) were treated with the terminal complement blocker eculizumab, 6 (6%) were treated with therapeutic plasma exchange, 2 (2%) were treated with rituximab, and 3 (3%) received defibrotide. Clinicians adjusted the dose of calcineurin inhibitors in 12 allogeneic HSCT recipients with TA-TMA. Eculizumab was administered only to subjects who had high-risk TA-TMA features, TA-TMA–associated organ injury, or biopsy-proven TA-TMA. In patients treated with eculizumab, 96% had elevated sC5b-9 and/or proteinuria, which were previously identified as being high-risk for mortality in patients with TA-TMA.13 There was no statistical difference in the development of ≥1 bloodstream infection in the first 100 days post-HSCT in patients who were treated with eculizumab vs those who were not. In patients who were treated with eculizumab, 21 of 50 (42%) developed ≥1 bloodstream infection vs 17 of 48 patients (35%) who were not treated with eculizumab (P = .54).
Interestingly, 80% (n = 78) of patients with TA-TMA had proteinuria; 6-month mortality was 27% (21/78) in patients with proteinuria vs 5% (1/20) in patients without (P = .04) (Figure 5A). Patients with TA-TMA who received any treatment had a 6-month mortality of 29% (17/58) compared with 13% (5/40) in patients who did not receive any treatment (Figure 5B). Patients with TA-TMA who were treated with eculizumab had a 6-month mortality of 32% (16/50) compared with 13% (6/48) in patients with TA-TMA who were not treated with eculizumab (P = .03) (Figure 5C). It is important to highlight that there were no guidelines or recommendations for treatment of TA-TMA, and it is likely that the patients who received eculizumab had more severe disease compared with the patients who did not receive it. Patients with TA-TMA who were treated with eculizumab had an increased rate of intensive care admission (64% vs 42%; P = .04) and pulmonary hypertension (12% vs 0%; P = .03) compared with patients who were not treated with eculizumab (supplemental Table 3).
Figure 5.
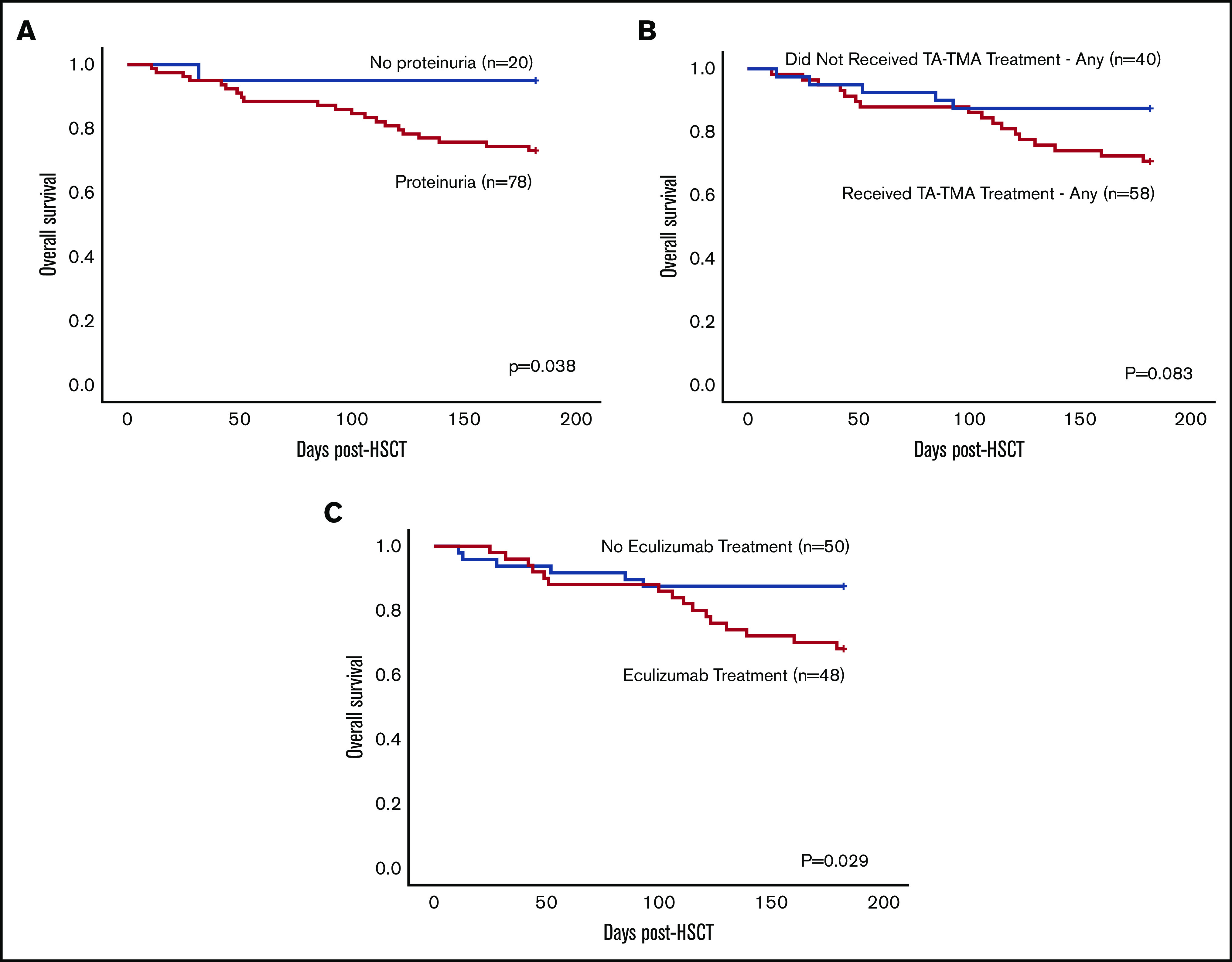
Comparison of diagnostic and treatment variables and 6-month OS in patients diagnosed with TA-TMA (n = 98). (A) Six-month OS in patients diagnosed with TA-TMA who had proteinuria vs those who did not. (B) Six-month OS in patients who received any treatment vs those who did not. (C) Six-month OS in patients treated with eculizumab vs those who were not.
Discussion
To our knowledge, this is the first multicenter analysis of incidence, risk factors, and outcomes of children with TA-TMA receiving prospective evidence-based screening for TA-TMA after HSCT. Our study cohort was composed of a large population of stem cell transplant recipients from small, medium, and large pediatric transplant centers. TA-TMA occurred in 19% of those undergoing allogeneic HSCT and in 10% of those undergoing autologous HSCT. These data confirm single-center data suggesting that TA-TMA is a frequent and important clinical problem after pediatric HSCT. We observed a higher incidence of TA-TMA in children with nonmalignant disorders, perhaps explaining, to some extent, differences between centers based on differences in clinical practice.13 The association between TA-TMA and immune and metabolic disorders is intriguing. Recent reports suggest coactivation of complement and interferon pathways as a potential culprit in the evolution of TMA in patients with immune dysregulation disorders like hemophagocytic lymphohistiocytosis, and it may explain the greater susceptibility to endothelial injury in such disease groups.29
TA-TMA after autologous HSCT was predominantly observed in patients with neuroblastoma undergoing myeloablative tandem HSCT29 using carboplatin/etoposide/melphalan. The use of carboplatin/etoposide/melphalan in neuroblastoma patients has been linked to high rates of TA-TMA in several single-institution retrospective studies.15,29-31 Our study data suggest that patients with neuroblastoma receiving this particular combination of chemotherapy are at high risk for developing TA-TMA; further investigations of endothelial injury mechanisms with these drugs are warranted. These data also support prospective TA-TMA screening and consideration for early interventions to reduce TA-TMA–associated organ injury because these patients require additional therapy with radiation and targeted antibody after HSCT that was commonly delayed in those with TA-TMA. Moreover, radiation therapy often encompasses kidneys, which commonly reactivates or exacerbates TA-TMA, leading to further morbidity.
Our study clearly demonstrates that patients diagnosed with TA-TMA had significantly lower OS and increased transplant-related complications requiring intensive care support, including respiratory failure and RRT, as well as increased health care resource utilization. This study plays a significant role in raising our awareness about TA-TMA’s impact on pediatric transplant recipients and will aid in planning studies in adults. TA-TMA had been considered an infrequent complication of HSCT until the last 5 years. In 2014, Jodele et al reported an 18% incidence of high-risk TA-TMA in a prospective cohort of pediatric stem cell transplant recipients.13 In this cohort, untreated high-risk TA-TMA was associated with significant organ injury and with poor 1-year posttransplant survival (<16.7%). Untreated TA-TMA has since been found to be associated with bacteremia,32 pulmonary hypertension,5,24,33,34 pericardial effusion,6,7,24 and bowel necrosis.4,35 The key role of complement activation in TA-TMA has been described and led to studies implementing complement blockade as a first-line therapy, with significantly improved clinical outcomes in patients with high-risk TA-TMA.8,17,19,23,36 Our current study confirms complement activation, as measured by elevated blood sC5b-9 and proteinuria, as a high-risk TA-TMA marker associated with increased TRM. These data will aid in the planning of future prospective studies based on TA-TMA risk stratifications. We believe that it is very important to identify high-risk patients early in the disease process, so that targeted intervention can be used in those who would benefit from such interventions and for the most economical use of medical resources.
Our study also documents a higher incidence of SR GVHD in patients with TA-TMA. Recent clinical and biomarker studies report endothelial injury in patients with SR GVHD, linking TA-TMA and GVHD with high mortality and suggesting that both complications should be treated in parallel to improve transplant outcomes.37,38 These data suggest that patients with GVHD and TA-TMA may need treatment of both processes to resolve symptomatology, possibly explaining the apparent lack of efficacy of steroids alone. Clinical trials of the efficacy of strategies to diagnose and treat both processes will be valuable.
The majority of the published clinical experience on TA-TMA in the United States has been reported as single-center studies, creating an urgent need to demonstrate whether findings are generalizable to all pediatric transplant populations. Moreover, it is important to determine whether simple and inexpensive screening measures can be incorporated into standard care at all institutions and are effective in identifying a population with a significantly increased risk for adverse outcomes.
The pragmatic research approach used in this study leverages innovative designs to inform “real-world” choices about clinical care.39 Califf and Sugarman defined pragmatic research as “research that is designed for the primary purpose of informing decision makers regarding the comparative balance of benefits, burdens and risks of a biomedical or behavioral health intervention at the individual or population level.”39 Pragmatic research and implementation work usually takes place in the clinical setting with an intention to avoid complex infrastructure and elevated costs to achieve generalizable results in an efficient manner.40 In our study, institutions adopted standardized evidence-based strategies to prospectively screen for TA-TMA in their practice/institution, and management was determined by the standard of care of their respective center. The intervention was easy to implement (addition of LDH and UAs, as well as close monitoring of cytopenias and hypertension) and had a low cost (∼$60-100 per week). Furthermore, the study was completed, with minimal expenses, by volunteer faculty at the respective centers using a uniform TA-TMA diagnostic algorithm and uniform remote deidentified data input. We believe that this approach has provided a platform for future collaborative pragmatic multi-institutional studies.
Multi-institutional studies necessarily include variability in clinical care practices at different centers, which is a strength and weakness of our study. We particularly wanted to ensure that approaches were suitable for large and small transplant centers, as well as more and less research-focused centers, but recognize that differences in standard care will occur. Our study did not include a standard treatment intervention; although we suspect that the increased mortality in those treated reflects management of more severe cases, we have insufficient data to verify this. An important future goal is to develop and validate a standardized treatment approach.
Despite limitations, this pragmatic multi-institutional study provides crucial data that TA-TMA affects outcomes in an important number of pediatric transplant recipients at all participating transplant centers, as well as that TA-TMA can be identified via prospective screening. Patients undergoing HSCT should have routine scheduled monitoring for TA-TMA, because these basic laboratory tests are inexpensive and available at all institutions.17 Specialized complement studies used for TA-TMA risk stratification are available as Clinical Laboratory Improvement Amendments (CLIA)-certified tests at reference laboratories. Our data clearly demonstrate that TA-TMA can lead to multiorgan failure; improved uniform treatment approaches are urgently needed. Prospective TA-TMA screening at all transplant centers is critical in proposing uniform TA-TMA care guidelines for patients with high-risk disease features and will likely improve long-term survival in children. Similar strategies should also be evaluated in adult HCST populations.
Supplementary Material
The full-text version of this article contains a data supplement.
Footnotes
Data sharing requests should be sent to Christopher E. Dandoy (e-mail: christopher.dandoy@cchmc.org).
Authorship
Contribution: C.E.D., S.J., J.J.A., R.C., and S.M.D. designed research, compiled centralized data, collected, analyzed, and interpreted the data, and wrote the manuscript; A.L. and C.E.D. performed statistical analyses; and S.R., P.B.A., A.K., C. Desmond, J. Huber, H.I., C.H., C.C.D., C. Duncan, M.S., L.L., M.C., J.K., B.D., R.P., K.M.M., S.K., N.L., M.V., K.M., G.W., A.N., P.K., D.B., N.G., E.A., J. Huo, and P.R. collected data at their individual centers, designed the research study, analyzed and interpreted the results, and wrote the manuscript.
Conflict-of-interest disclosure: C.C.D. has acted as a consultant for Alexion, Inc. and Omeros Corporation. C.E.D. has received travel support and honoraria for lectures at European Society for Blood and Marrow Transplantation (EBMT) and American Society of Transplantation and Cellular Therapy (ASTCT) meetings from Omeros. P.R. has served as a member of the speaker;s bureau for Sobi. R.P. has served on the advisory board for Orchard Therapeutics. S.J. has US patent applications under review, is a lead Principal Investigator for a National Institutes of Health–funded multi-institutional study with drug provided by Alexion Pharmaceuticals, and has received travel support and honoraria for lectures at EBMT and ASTCT meetings from Omeros. S.M.D. has acted as a consultant for Novartis and has research support from Alexion Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Christopher Dandoy, Division of Bone Marrow Transplant and Immune Deficiency, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, MLC 11027, Cincinnati, OH 45229; e-mail: christopher.dandoy@cchmc.org.
References
- 1.Barriga F, Ramírez P, Wietstruck A, Rojas N. Hematopoietic stem cell transplantation: clinical use and perspectives. Biol Res. 2012;45(3):307-316. [DOI] [PubMed] [Google Scholar]
- 2.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813-1826. [DOI] [PubMed] [Google Scholar]
- 3.Remberger M, Ackefors M, Berglund S, et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study. Biol Blood Marrow Transplant. 2011;17(11):1688-1697. [DOI] [PubMed] [Google Scholar]
- 4.El-Bietar J, Warren M, Dandoy C, et al. Histologic features of intestinal thrombotic microangiopathy in pediatric and young adult patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(11):1994-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dandoy CE, Jodele S, Paff Z, et al. Team-based approach to identify cardiac toxicity in critically ill hematopoietic stem cell transplant recipients. Pediatr Blood Cancer. 2017;64(10):e26513. [DOI] [PubMed] [Google Scholar]
- 6.Lerner D, Dandoy C, Hirsch R, Laskin B, Davies SM, Jodele S. Pericardial effusion in pediatric SCT recipients with thrombotic microangiopathy. Bone Marrow Transplant. 2014;49(6):862-863. [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer TM, Rotz SJ, Ryan TD, et al. Pericardial effusion requiring surgical intervention after stem cell transplantation: a case series. Bone Marrow Transplant. 2017;52(4):630-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jodele S, Laskin BL, Dandoy CE, et al. A new paradigm: diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29(3):191-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho BS, Yahng SA, Lee SE, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90(8):918-926. [DOI] [PubMed] [Google Scholar]
- 10.Willems E, Baron F, Seidel L, Frère P, Fillet G, Beguin Y. Comparison of thrombotic microangiopathy after allogeneic hematopoietic cell transplantation with high-dose or nonmyeloablative conditioning. Bone Marrow Transplant. 2010;45(4):689-693. [DOI] [PubMed] [Google Scholar]
- 11.Siami K, Kojouri K, Swisher KK, Selby GB, George JN, Laszik ZG. Thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation: an autopsy study. Transplantation. 2008;85(1):22-28. [DOI] [PubMed] [Google Scholar]
- 12.Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118(6):1452-1462. [DOI] [PubMed] [Google Scholar]
- 13.Jodele S, Davies SM, Lane A, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124(4):645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dvorak CC, Higham C, Shimano KA. Transplant-associated thrombotic microangiopathy in pediatric hematopoietic cell transplant recipients: a practical approach to diagnosis and management. Front Pediatr. 2019;7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jodele S, Dandoy CE, Myers K, et al. High-dose carboplatin/etoposide/melphalan increases risk of thrombotic microangiopathy and organ injury after autologous stem cell transplantation in patients with neuroblastoma. Bone Marrow Transplant. 2018;53(10):1311-1318. [DOI] [PubMed] [Google Scholar]
- 16.Jodele S, Fukuda T, Mizuno K, et al. Variable eculizumab clearance requires pharmacodynamic monitoring to optimize therapy for thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(2):307-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jodele S, Dandoy CE, Lane A, et al. Complement blockade for TA-TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood. 2020;135(13):1049-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571-575. [DOI] [PubMed] [Google Scholar]
- 19.Jodele S, Licht C, Goebel J, et al. Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Blood. 2013;122(12):2003-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pronovost PJ, Berenholtz SM, Goeschel CA, et al. Creating high reliability in health care organizations. Health Serv Res. 2006;41(4 Pt 2):1599-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langley G, Nolan K, Nolan T, Norman C, Provost L. The Improvement Guide: A Practical Approach To Enhancing Organizational Performance. San Francisco, CA: Jossey-Bass; 2009. [Google Scholar]
- 22.Luft T, Benner A, Jodele S, et al. EASIX in patients with acute graft-versus-host disease: a retrospective cohort analysis. Lancet Haematol. 2017;4(9):e414-e423. [DOI] [PubMed] [Google Scholar]
- 23.Jodele S, Dandoy CE, Myers KC, et al. New approaches in the diagnosis, pathophysiology, and treatment of pediatric hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transfus Apheresis Sci. 2016;54(2):181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dandoy CE, Davies SM, Hirsch R, et al. Abnormal echocardiography 7 days after stem cell transplantation may be an early indicator of thrombotic microangiopathy. Biol Blood Marrow Transplant. 2015;21(1):113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295-304. [DOI] [PubMed] [Google Scholar]
- 27.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109(10):4119-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mateos MK, O’Brien TA, Oswald C, et al. Transplant-related mortality following allogeneic hematopoeitic stem cell transplantation for pediatric acute lymphoblastic leukemia: 25-year retrospective review. Pediatr Blood Cancer. 2013;60(9):1520-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gloude NJ, Dandoy CE, Davies SM, et al. Thinking beyond HLH: clinical features of patients with concurrent presentation of hemophagocytic lymphohistiocytosis and thrombotic microangiopathy. J Clin Immunol. 2020;40(5):699-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tolbert VP, Dvorak CC, Golden C, et al. Risk factors for transplant-associated thrombotic microangiopathy after autologous hematopoietic cell transplant in high-risk neuroblastoma. Biol Blood Marrow Transplant. 2019;25(10):2031-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoettler M, Lehmann L, Li A, Ma C, Duncan C. Thrombotic microangiopathy following pediatric autologous hematopoietic cell transplantation: a report of significant end-organ dysfunction in eculizumab-treated survivors. Biol Blood Marrow Transplant. 2019;25(5):e163-e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dandoy CE, Haslam D, Lane A, et al. Healthcare burden, risk factors, and outcomes of mucosal barrier injury laboratory-confirmed bloodstream infections after stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(9):1671-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jodele S, Hirsch R, Laskin B, Davies S, Witte D, Chima R. Pulmonary arterial hypertension in pediatric patients with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2013;19(2):202-207. [DOI] [PubMed] [Google Scholar]
- 34.Dandoy CE, Hirsch R, Chima R, Davies SM, Jodele S. Pulmonary hypertension after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(11):1546-1556. [DOI] [PubMed] [Google Scholar]
- 35.Warren M, Jodele S, Dandoy C, et al. A complete histologic approach to gastrointestinal biopsy from hematopoietic stem cell transplant patients with evidence of transplant-associated gastrointestinal thrombotic microangiopathy. Arch Pathol Lab Med. 2017;141(11):1558-1566. [DOI] [PubMed] [Google Scholar]
- 36.Ricklin D, Cines DB. TMA: beware of complements. Blood. 2013;122(12):1997-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotz SJ, Dandoy CE, Davies SM. ST2 and endothelial injury as a link between GVHD and microangiopathy. N Engl J Med. 2017;376(12):1189-1190. [DOI] [PubMed] [Google Scholar]
- 38.Wall SA, Zhao Q, Yearsley M, et al. Complement-mediated thrombotic microangiopathy as a link between endothelial damage and steroid-refractory GVHD. Blood Adv. 2018;2(20):2619-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Califf RM, Sugarman J. Exploring the ethical and regulatory issues in pragmatic clinical trials. Clin Trials. 2015;12(5):436-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009;62(5):464-475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.