Abstract
Introduction
This paper describes the protocol for an ongoing project funded by the Royal Society, the Resilience After Individual Stress Exposure (RAISE) study; which aims to examine the factors and mechanisms that facilitate resilient functioning after childhood adversity (CA).
Methods and analysis
We aim to recruit up to 200 participants. We will use dimension reduction techniques (principal component analysis) on standard-normally transformed individual parameters of mental health, social functioning and CA to calculate a composite measure of adaptive (ie, ‘resilient’) psychosocial functioning. To examine the neuroimmune responses to stress and their relationship with the brain and social environment, we will use a well validated functional MRI task; the Montreal imaging stress task and venepuncture. We will run group or dimensional comparisons in multiple levels of biological and psychological outcomes, as well as mediation and moderation analyses to study how key biological systems (ie, the hypothalamic–pituitary–adrenal axis and the immune system) interrelate and interact with brain function and social influences in order to facilitate resilient functioning after CA. We hypothesise that resilient functioning will be facilitated by reduced morning cortisol and cytokine levels before and after the stressor and improved neural responses to such stress, as well as increased gray matter volume in the hippocampus and prefrontal cortex, enhanced inhibitory control and emotion regulation, and more friendship and family support.
Ethics and dissemination
This study has been reviewed and given favourable opinion by the National Research Ethics Service, NRES Committee East of England-Cambridge Central and external reviewers from the Royal Society (RGF\R1\180064 and RGF\EA\180029). The results of the RAISE study will be disseminated through (1) publications in scientific peer reviewed journals, (2) presentations on relevant scientific conferences and meetings, (3) publications and presentations for the general public and (4) through social media.
Keywords: child & adolescent psychiatry, immunology, magnetic resonance imaging
Strengths and limitations of this study.
The Resilience After Individual Stress Exposure study will provide a comprehensive evaluation of the neurobiological mechanisms that contribute to adolescent resilience.
We will use standardised and validated instruments of psychological functioning, childhood adversity, cognitive tasks, venepuncture and neuroimaging.
The exclusion of psychiatric patients will restraint the data to the resilience side of the spectrum.
Child adversity will be assessed from self-reports that are subjected to reporting biases.
A longer recruitment period may be required due to COVID-19.
Introduction
Childhood adversity (CA) is the leading preventable risk factor for mental illness and substance abuse.1–8 This kind of experiences, which can happen within the family environment (eg, in the form of childhood maltreatment and/or intrafamily adversity) or outside the household (eg, trauma and bullying), can have a detrimental impact on a wide range of functions. For example, CA has been associated with physical (eg, failure to thrive, poor adult health and high mortality), cognitive (eg, impaired inhibitory control and emotion regulation) and personal and interpersonal problems (eg, negative self-cognitions, suicidal behaviours, increased peer rejection, social withdrawal, sexual maladjustment, aggression and criminality).8–16
Importantly, adolescents with CA are at increased risk for and are more sensitive to psychosocial stress. In response to acute stress, the body reacts by releasing pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumour necrosis factor alpha (TNF-α).17 These cytokines play a key role in stress reactivity and stress recovery.18 Specifically, proinflammatory cytokines stimulate the hypothalamic–pituitary–adrenal (HPA) axis to release glucocorticoid hormones such as cortisol. Glucocorticoids, in turn, suppress the further release of cytokines from the immune system.19 Thus, cortisol is an important anti-inflammatory compound in the body that is crucial for stress recovery. Proinflammatory cytokines and chemokines can cross the blood–brain barrier and negatively impact the function of brain regions involved in threat, reward and executive functioning.20 21 Indeed, acute stress has been associated with increased levels of proinflammatory cytokines in the amygdala and decreased proinflammatory cytokines in the medial prefrontal cortex (MPFC)22; regions associated with executive functions and emotion regulation.23–28 Therefore, it is plausible that the alteration of these processes, or the inability to properly value and manage emotions, can lead to anxiety and/or depression in situations of negative affect.29–31
However, although CA is associated with considerably lowered odds of adequate mental and physical health functioning later in life, a significant proportion of individuals with a history of CA function ‘better than expected,’ or, in other words, are ‘functioning resiliently’.32 These individuals may have benefited from protective ‘resilience factors’33 34 which exist across social, cognitive, neuronal, physiological and genetic levels. For example, good mental health after CA has been associated with increased hippocampal volume and greater connectivity between the central executive network and limbic regions as well as a greater ability to regulate emotions,35 higher self-esteem36 37 and social support.32 38
Objectives and hypothesis
Resilient functioning has been associated with various components, ranging from genes and cellular mechanisms to higher-order biological systems and the social environment (see reviews in refs. 39–41; figure 1). However, it is yet unknown whether and how neuro-immune responses to psychosocial stress differ in resilient vs. vulnerable adolescents with a history of CA. In this study, we aim to test the factors and mechanisms that facilitate resilient functioning, the interactions between those factors, and how they explain resilient responses to future stress. Specifically, we will address resilience by investigating how key biological systems (ie, the HPA axis and the immune system) interrelate and interact with brain structure, brain function and social influences to facilitate resilient functioning after CA.
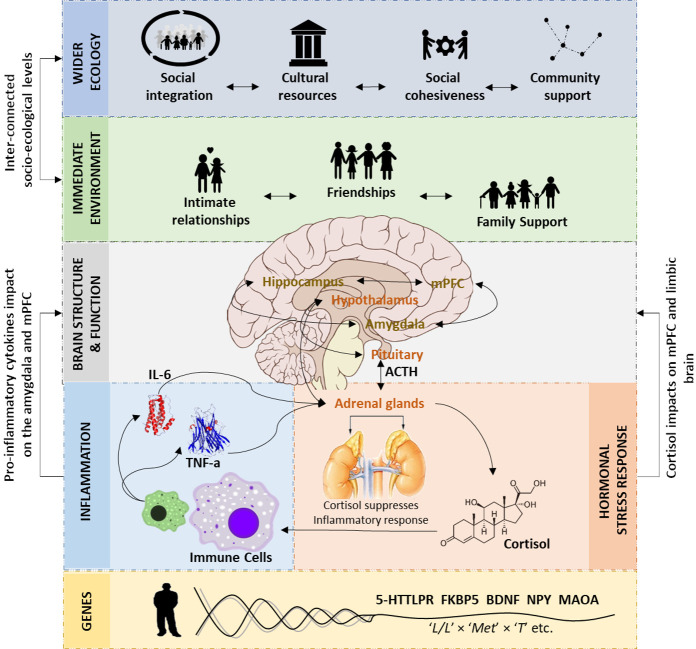
Figure 1.
The complex neurobiology of resilience after childhood maltreatment (CM).39 Resilient functioning in those individuals who have experienced CM may be facilitated by larger prefrontal cortex (PFC) and hippocampal volume and connectivity, the ability to adequately regulate emotions and dampen stress responsivity, cortisol and proinflammatory baseline and responses, polygenic resilience effects, social support from the immediate environment, and the wider ecology. For readability, the location of the hippocampus is not correct. 5-HTTLPR, serotonin-transporter-linked polymorphic region; ACTH, adrenocorticotropic hormone; BDNF, brain-derived neurotrophic factor; FKBP5, FK binding protein 5; IL-6, interleukin 6; MAOA, monoamine oxidase A; mPFC, medial PFC; NPY, neuropeptide-Y; TNFα, tumour necrosis factor-α.
All included participants will complete an online assessment to assess psychological functioning and early life experiences, an in-unit assessment day to assess neuroimmune and cognitive responses to stress and an online follow-up assessment to assess psychological functioning after stress exposure. Resilient functioning will be quantified as the degree to which an individual functions better or worse than expected given their self-reported CA experiences (32 39; figure 2). Specifically, to examine the neuroimmune responses to stress and their relationship with brain structure and function and the social environment, we will use a well-validated functional MRI (fMRI) task, the Montreal imaging stress task (MIST) and venepuncture. Since stress increases circulating inflammatory protein levels in the blood, and high levels of inflammation predict later mental health disorders, we will examine whether resilience is related to lower levels of inflammation in response to psychosocial stress, and whether this is explained by improved brain responses to stress.
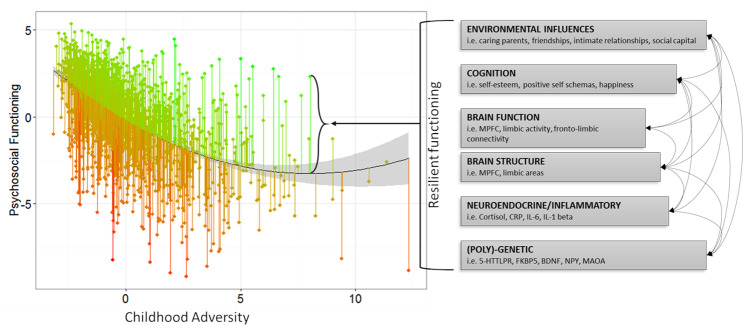
Figure 2.
Risk to Resilient functioning in the NSPN sample of n=1980 adolescents.32 Green and red lines indicate functioning that is better or ‘resilient’ (green) or worse ‘vulnerable’ (red) than expected. Psychosocial functioning reflects a factor score (mean=0, SD=1) derived from multiple measures of psychiatric symptomatology, personality traits and mental well-being. CA reflects a factor score (mean=0, SD=1) from two measures which assess early life family experiences. 5-HTTLPR, serotonin-transporter-linked polymorphic region; BDNF, brain-derived neurotrophic factor; CA, childhood adversity; FKBP5, FK binding protein 5; IL-6, interleukin 6; MAOA, monoamine oxidase A; MPFC, medial prefrontal cortex; NPY, neuropeptide-Y; NSPN, Neuroscience in Psychiatry Network.
We hypothesise that resilient functioning will be facilitated by
Reduced morning cortisol and cytokine levels before and after the stressor (ie, a latent factor constructed by serum high-sensitivity C reactive protein (hs-CRP), TNF-α, IL-6 and IL-1 factor beta (IL-1β) levels, as these have been shown to be increased in those with CA and mental illness.42–46
Reduced stress-related brain responses (ie, amygdala, insula) and increased modulatory responses in the MPFC and anterior cingulate cortex (ACC)47–49 during the MIST.
Increased functional connectivity between the dorsal MPFC (DMPFC) and emotion processing regions (eg, insula, amygdala and hippocampus) during the MIST.50
Increased grey matter volume in the hippocampus and prefrontal cortex.35 51–57
Enhanced cognitive and emotional executive performance in behavioural tasks of inhibitory control and emotion regulation.29
Higher self-esteem and more friendship and family support which will aid lower anxiety and perceived stress after the MIST.36 58
Finally, we expect that the neurocognitive mechanisms that facilitate resilient functioning to stress at in-unit assessment will be related to improved cognitive and emotional functioning at follow-up (lower rumination, lower interpersonal stress, improved mood) in line with the neuroimmune network hypothesis21 (figure 3).
Figure 3.
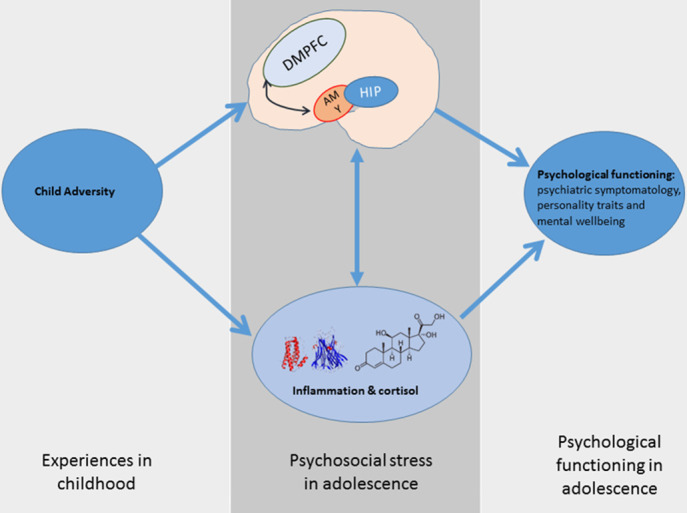
The neuroimmune network hypothesis of child maltreatment. Inflammatory protein and brain responses to psychosocial stress mediate the relationship between CA and psychological functioning. CA, childhood adversity; DMPFC, dorsal medial prefrontal cortex.
Methods
Recruitment and eligibility
Recent research suggests that the link between CA and immune markers in the blood are small for hsCRP immune biomarkers in adults with depression (hsCRP r=0.1559). However, there are no studies investigating immune biomarkers in response to stress in adolescents. Therefore, it is difficult to determine the necessary sample size for our study. For instance, sample sizes for an intended power of 80% are r=0.15: n=345; r=0.2: n=193; r=0.3: n=84. Furthermore, it is well established that sample correlations show fluctuations and are unstable in smaller samples. Simulation studies show that for a stable (ie, replicable and generalisable) correlation, any sample would need to approach N=250 individuals.60 For this reason, to increase power to find small effects, we will recruit N=200 participants. With this sample size, our study is appropriately powered to detect correlations from r>0.19.
Participants will be recruited from the general population and from previous studies conducted in the Department of Psychiatry of the University of Cambridge. First, we will contact participants from the Neuroscience in Psychiatry Network (NSPN) study who agreed to be contacted again. NSPN is a multicentre accelerated longitudinal community cohort study (N=2389) focusing on normative adolescent to young adult development (between the ages of 14 and 24). Overall, this sample can be described as healthy, reporting low levels of psychopathological symptoms, behaviours and/or personality traits, and average mental well-being scores. From this cohort, we will contact those who agreed to be contacted for future research of whom family adversity scores were in the highest 25% of the entire NSPN cohort (≥75%=597 eligible individuals) as assessed by van Harmelen et al.32 Family adversity scores in NSPN were calculated using a principal component analysis (PCA) on standard-normally transformed sum scores for the Measure of Parenting Style (MOPS) and the Alabama Parenting Questionnaire (APQ). Eligible participants will be contacted via email first. This email will include the participant information sheet (PIS) of the study. A reminder email will be sent after a month. Finally, we will contact those participants who cannot be reached by email via phone call. To recruit participants from the general population we will distribute flyers and advertisements in colleges, Addenbrooke’s hospital and online. Individuals expressing an interest in the study could either email or telephone a member of the research team and leave their contact details. A member of the Resilience After Individual Stress Exposure (RAISE) study research team will then phone interested individuals. During the telephone call a member of the research team will discuss the content of the PIS and assess the inclusion and exclusion criteria in order to ensure they are eligible and fully aware of the nature of the study. Eligible participants will be emailed the PIS of the study. Potential participants will be given the opportunity to raise any queries regarding any aspect of the study including confidentiality, anonymity, storage and use of data, as well as the right to withdraw.
The inclusion criteria will be: aged 16–26 years old; able and willing to give informed consent; able to speak, write and understand English; body mass index (BMI) between 18.5 and 29.9 kg/m2; have experienced adverse life experiences and/or CA within the family environment including childhood maltreatment (eg, emotional, sexual and/or physical abuse, emotional and/or physical neglect) and intrafamily adversity (eg, marital distress/conflict, parental mental health problems and/or parental alcohol dependence, violence and/or aggressive behaviour) before the age of 16; and willing to abstain from strenuous exercise for 72 hours prior to the in-unit assessment.
The exclusion criteria will be: alcohol or substance use disorder within the past 6 months; current disorders likely to compromise the interpretation of the data (including, but not limited to, psychiatric disorders, immunological disorders, cardiovascular disorders, endocrine and autoimmune disorders, malignancies or infections, or any other condition to be determined by the principal investigator or delegate); current medication likely to compromise the interpretation of immunological data (including, but not limited to, corticosteroids or any other substance to be determined by the principal investigator or delegate); and contraindications to MRI (eg, pacemaker or other implantable device or pregnancy).
Testing protocol and procedure
All included participants will be asked to complete three phases:
Phase I: an online assessment to assess psychological functioning and early life experiences; phase II: an in-unit assessment to assess neuroimmune and cognitive responses to stress, and phase III: an online follow-up assessment to assess psychological functioning after stress exposure. Please see figure 4 and sections below for a description of the measures included in each phase.
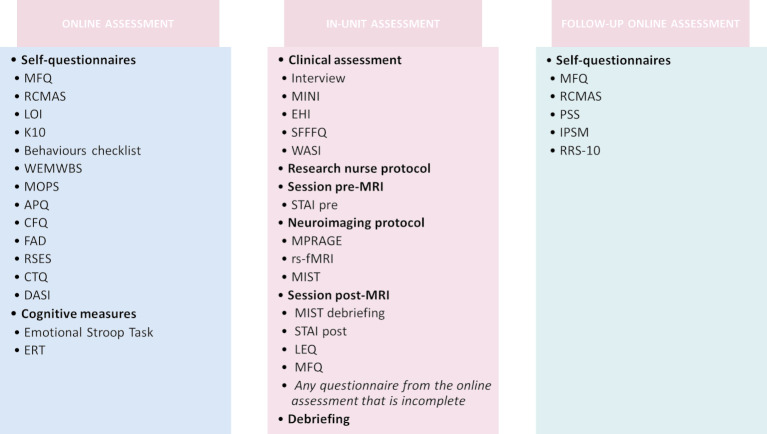
Figure 4.
Summary of the study protocol. APQ, Alabama Parenting Questionnaire; CBC, Child Behaviours Checklist; CFQ, Cambridge Friendship Questionnaire; CTQ, Childhood Trauma Questionnaire; DASI, Drugs Alcohol and Self-Injury; EHI, Edinburgh Handedness Inventory; ERT, Emotion Regulation Task; FAD, Family Assessment Device; IPSM, Interpersonal Sensitivity Measure; K10, Kessler Psychological Distress Scale; LEQ, Life-Events Questionnaire; LOI, Leyton Obsessional Inventory; MFQ, Mood and Feelings Questionnaire; MINI, Mini-International Neuropsychiatric Interview; MIST, Montreal Imaging Stress Task; MOPS, Measureof Parenting Style; MPRAGE, T1-Weighted rapid three-dimensional radient-echo; PSS, Perceived Stress Scale; RCMAS, Revised Children's Manifest Anxiety Scale; RRS-10, Ruminative Response Scale; RSES, Rosenberg Self-Esteem Scale; rs-fMRI, restingstate functional MRI; SFFFQ, Short-Form Frequency Food Questionnaire; STAI, State-Trait Anxiety Inventory; WASI, Wechsler Abbreviated Scale of Intelligence; WEMWBS, Warwick-Edinburgh Mental Well-Being Scale.
Phase I: online assessment
The online assessment will include signing an informed consent form (ICF), the completion of a set of self-report questionnaires and two cognitive tasks online. The self-report questionnaires included in this assessment will be the Mood and Feelings Questionnaire (MFQ61), Revised Children’s Manifest Anxiety Scale (RCMAS62), Leyton Obsessional Inventory (LOI63), the 10-item version of the Kessler Psychological Distress Scale (K1064), the Child Behaviours Checklist (CBC65), the Warwick-Edinburgh Mental Well-being Scale (WEMWBS66), the MOPS67, the APQ68, the Cambridge Friendship Questionnaire, Family Assessment Device (FAD69 70), Rosenberg Self-Esteem Scale71, Childhood Trauma Questionnaire (CTQ72) and the Drugs, Alcohol and Self-Injury Inventory.73. These questionnaires will be used to calculate a composite measure of resilient functioning as described below. The cognitive tasks will be the Emotional Stroop task74 and Emotional Regulation task.75 More information about these measures is provided in the online supplemental material.
bmjopen-2020-040394supp001.pdf (664.6KB, pdf)
Phase II: in-unit assessment
Following completion of the online assessment, participants will be contacted to schedule an appointment at Addenbrooke’s Hospital (Cambridge, UK), after which they will receive a letter with the appointment details. This letter will include clothing and make-up regulations for brain scanning, time and location of the facilities where the evaluations will take place, as well as the research team’s contact details. It will also reiterate that participation is voluntary and that participants can withdraw at any given time during the study. The second assessment will have a duration of 5 hours and will include (1) the completion of a second ICF, (2) a clinical evaluation, (3) a research nurse protocol, (4) an MRI session and (5) the completion of a second set of self-report questionnaires. Please see the sections below for a description of the instruments included in each assessment.
Clinical evaluation
Participants will be asked to attend the clinical research facility at Addenbrooke’s Hospital at 9:00 hours, where they will be given a detailed overview of the study, asked to sign the ICF, and receive a low-fat breakfast adapted to their nutritional needs. The breakfast provided will exclude antioxidants such as glutathione and vitamins E and C to reduce the effects of these variables on the inflammatory markers addressed.76 Subsequently, the clinical evaluation will take place. This session will include the evaluation of psychiatric disorders, handedness, physical activity, sleep and eating patterns, medications (eg, oral contraception) and intellectual functioning. Specifically, we will use the following measures: The Mini-International Neuropsychiatric Interview77, The Edinburgh handedness inventory,78 The Short-form Frequency Food questionnaire79 and The Wechsler Abbreviated Scale of IntelligenceWASI80. The measures used will be supplemented with an interview. See the online supplemental material for a description of the instruments used in this evaluation.
Physiological evaluation protocol
The clinical assessment will be followed by a physiological evaluation protocol. A research nurse will assess physical variables such as body temperature, height, weight, waist circumference, blood pressure (systolic and diastolic) and will implant a cannula to take blood samples during the assessment day.
Venepuncture
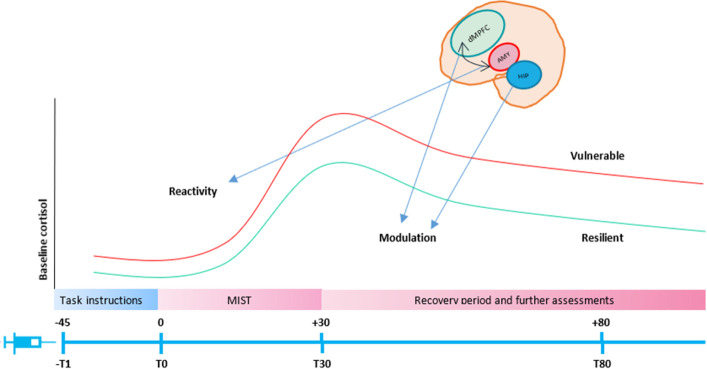
The implantation of the cannula will be conducted according to the standard Cambridge Clinical Research Facility protocol with risk protocols in place. These procedures include a brief interview about adverse experiences with blood assessment (such as fainting), as well as their preference for one arm or the other and if they want to lie down, or sit upright during the implantation of the cannula. Up to 30 mL of venous blood will be collected per participant for the measurement of cortisol, blood cytokines and immunophenotyping (ie, basic immune cell counts and cell phenotyping). Blood will be extracted using an intravenous catheter inserted in the antecubital vein of the arm of the participants. A 30 min following catheter insertion, the participants will undergo MRI scanning and will perform the psychological stress task (ie, MIST). A 1.2 mL K2 Ethylenediamine Tetraacetic Acid (EDTA) tubes will be taken for the analysis of immunophenotyping, 2.6 mL serum white tap tubes for the analysis of cytokines and 4.6 mL serum brown tap tubes for the analysis of cortisol. Bloods will be acquired at four time points: (−T1) 45 min before the start of the task (baseline line), (T0) right before the start of the task, (T30) right after the end of the task (peak cortisol) (T80) 80 min after the start of the task (delayed immune reactions).81 Please see figure 5 for a representation of the venepuncture protocol.
Figure 5.
Venepuncture protocol. Bloods will be acquired at four time points: (−T1) 45 min before the start of the task, (T0) Just before the start of the task (T30) right after the start of the task, (T80) 80 min after the start of the task. dMPFC, dorsal medial prefrontal cortex; MIST, Montreal Imaging Stress Task
Neuroimaging protocol
Before the scan, participants will complete an MRI screening form, the State-Trait Anxiety Inventory,82 and practice the MIST. Each participant will be in the MRI scanner for about 50 min. The MRI scanning session will comprise the following MRI sequences:
T1-weighted three-dimensional magnetisation-prepared rapid gradient-echo (MPRAGE) (6 mins). High spatial resolution T1-weighted structural scans will be used to aid normalisation and visualisation of each of the other MRI modalities (as described below) and analyse brain structure (ie, cortical thickness, grey matter volume).
Resting state functional MRI (fMRI) (7 mins). A resting state fMRI will be used to investigate effects of inflammation on brain functional connectivity.
MIST (30 mins). We will use a modified version of the MIST83. This task comprises a series of computerised mental arithmetic tasks with an induced failure component. The protocol consists of a training session conducted outside the imaging unit, and a test session during which the functional images are acquired. Please see the online supplemental material for a description of the paradigm.
After the MRI session, participants will be debriefed. We will tell them that the task was designed to be impossible to accomplish and that it did not truly assess their ability to perform mental arithmetic. Then, we will ask them to complete the STAI-State, MFQ, Life Events Questionnaire,84 and any questionnaire from the online assessment that is incomplete. After completion of the post-MRI session, the participants will have a standard meal and be given time to relax. In addition, we will have a protocol for debriefing, an information letter with relevant types of support available, and a distress protocol in case the participant reports severe distress.
Phase III: follow-up online assessment
The follow-up and final assessment will be completed online within a month from the in-unit assessment and will include the third and last online ICF, the MFQ, RCMAS and LEQ, as well as the following measures described in the online supplemental material: Perceived Stress Scale85, Interpersonal Sensitivity Measure86 and the 10-item Ruminative Response Scale.87
Please see the online supplemental material for a relation of the main risk and ethical issues associated with this protocol.
Patient and public involvement
A group of three adolescents participated in a Lived Experience Advisory group to assess the protocol and materials included in the study (eg, PIS, consent forms, questionnaires, etc). We have made the following changes as a result of their feedback: (1) we have included a risk protocol in case a participant feel distressed during the completion of the questionnaires, (2) we have increased the payment for the completion of the study to account for the time burden of the questionnaires and the distress associated with the cannulation and (3) we have modified the PIS in accordance with their suggestions.
Analyses
Clinical, questionnaire and immunological data will be descriptively summarised. The significance threshold will be set at p<0.05 and Family-Wise Error (FWE) corrections will be applied to correct for multiple comparisons.
Preprocessing
Quantification of resilient functioning
Using the data collected during the first online assessment, we will calculate gender and age-related degree of resilient functioning based on the model described in van Harmelen et al 32; see also Ioannidis et al 39 for a description of the benefits and pitfalls of this method. Specifically, we will conduct two PCAs; one for psychosocial functioning using standard-normally transformed individual total scores on the MFQ, RCMAS, LOI, CBC, K10 and WEMWBS; and another one for CA, including standard-normally transformed sum scores for the MOPS, APQ, FAD and CTQ. From both analyses, we will extract individual scores for the first component to reflect individual current psychosocial functioning and recalled CA experience scores. Next, we will regress the psychosocial functioning component score against the CA score, testing for possible linear, quadratic or cubic relationships. From this model, we will extract the residual scores as a measure of individual degree of resilient functioning: the extent to which an individual has better, or worse, psychosocial functioning than the average score expected given their CA experiences. For parsimony, we will refer to this as degree of ‘resilient functioning’ with higher scores reflecting better (conditional) psychosocial functioning outcomes. These individual resilient functioning scores will be used in the analyses described below.
Imaging preprocessing
Task-evoked and resting state fMRI data will be preprocessed using Statistical Parametric Mapping 12 (SPM12) (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and the CONN toolbox (https://web.conn-toolbox.org/) implemented in MatLab R2019b (Mathworks, Natick, Massachusetts, USA). Images will be corrected for movement artefacts, coregistered with the T1-weighted images, normalised to a standard EPI template in the Montreal Neurological Institute space and spatially smoothed with an 8 mm FWHM Gaussian kernel. Quality control will be performed after each preprocessing step. Structural MRI will be analysed using the FreeSurfer image analysis suite, which is widely documented and freely available online (http://surfer.nmr.mgh.harvard.edu/).
Cortisol and immune markers preprocessing
Blood samples will be processed at the Core Biochemical Assay Laboratory (blood cytokines), Pathology (cortisol) and Immunology laboratory (immunophenotyping) at Addenbrookes Hospital (Cambridge, UK). A 1 mL of blood will be diluted 1:1 with phosphate buffered saline and stimulated with lipopolysaccharide (LPS) (LPS challenge) to analyse the production of IL-6 and other cytokines difficult to detect.88 89 LPS-stimulated IL-6 and IL-1β production in peripheral leukocytes will be processed following a protocol originally developed by DeRijk et al. 90 All cytokines, including serum TNF-α and hs-CRP will be measured using the MSD platform.
Phenotyping
A 50–100 uL of blood will be used for the enumeration of the major populations of immune cells (monocytes, granulocytes, NK cells, NKT cells, CD4+ and CD8+T cells and B cells). We will use multicolour flow cytometry to count and analyse the size, shape and properties of individual cells within heterogeneous populations. We will use multivariate methods (partial least squares (PLS) and PCA) to reduce dimensionality and define populations of differentially coexpressed cell counts and the unbiased gating algorithm Spanning-tree Progression Analysis of Density-normalised Events (SPADE). SPADE generates an immune cell hierarchy by clustering phenotypically similar cells into groups which can be enumerated.
Statistical analyses
Age, gender, socioeconomic status and education level will be included as covariates in all analyses. Additionally, for the imaging analysis, we will include the total volume of grey matter (for the analyses of grey matter), a high-pass filter uses to remove low-frequency drifts in the data (for the analysis of fMRI data), and the signal fluctuations in white matter and cerebrospinal fluid and the subject-specific six realignment parameters and their first order derivatives (for the analyses of functional connectivity). Finally, phase of menstrual cycle, BMI, and tobacco smoking will be used in the analyses involving immune/cortisol markers.
Hypothesis 1: Individuals with higher resilient functioning will display lower baseline cortisol and blood cytokines, faster habituation and less cortisol volatility.
We will use a PCA to derive a factor score for endocrine or inflammatory markers at the different time points. We expect there will be two components: one relating to baseline cortisol (or index of immune biomarkers), and one relating to cortisol change (or index of immune responsivity; see91 for a similar approach). We will then use area under curve (AUC) analyses to examine whether degree of resilient functioning is more strongly associated with general immune status (ie, the baseline cortisol measure) or cortisol responsivity (ie, area under the curve with respect to ground (AUCG) and area under the curve with respect to increase (AUCI) (see92 for specifics). We will validate this result using a simple multivariate regression to examine whether the two components of immune functioning independently predict resilient functioning outcomes.
Hypotheses 2–4: Higher resilient functioning is associated with more balanced and integrated neural systems:
For the event-related fMRI analysis, we will use both whole-brain and regions of interest (ROIs) approaches. To examine the effect of stress, we will examine brain responses to the contrast ‘experimental +control condition’ vs ‘rest condition’. Then, in a second level analysis, we will conduct a multiple regression analysis with resilience scores as regressor of interest. The ROIs use will be the MPFC, ACC, amygdala, insula and hippocampus. Finally, we will run a psychophysiological interaction analysis to test hypothesis 3 (ie, increased functional connectivity between the DMPFC and emotion processing regions during the MIST).
For the analysis of resting state fMRI, we will use whole-brain and ROIs approaches. Our first approach will be a data-driven analysis. We will use intrinsic connectivity contrast (ICC93). The use of ICC does not require a priori selection of a seed region but instead objectively defines how well each voxel is connected to the rest of the brain. Following the calculation of a resting state ICC map for each participant, we will test associations between ICC maps and resilient functioning. Specifically, we will correlate resilient functioning with the voxel level ICC using a multiple regression analyses in SPM. Finally, we will calculate functional connectivity maps and network involvement of the regions found to be associated with resilient functioning.
The significance thresholds will be set at p<0.05 after FWE correction for multiple comparisons across the whole-brain (pFWE <0.05) or the voxels of the different ROIs (ie, using small volume correction (SVC) procedures (pFWE-SVC <0.05).
Hypotheses 5 and 6: Individuals with higher resilient functioning will display better cognitive control and greater levels of social support.
We will use Structural Equation Modelling to examine the relations and interrelations described in hypotheses 5 and 6. Specifically, we hypothesise that, using a multiple indicators multiple causes model, we will observe that individual differences in outcome (cognitive functioning) will be explained by partially independent and complementary neural systems (ie, all paths shown will be significant when estimated simultaneously).
Hypothesis 7: Neurocognitive mechanisms that facilitate resilient functioning to stress are related to improved cognitive and emotional functioning at follow-up.
Using mediation modelling (eg, figure 2), we will examine whether the mechanisms that facilitate resilient functioning to stress are related to improved cognitive and emotional functioning (ie, rumination, interpersonal stress, mood, etc) at follow-up.
Immunophenotyping analyses
We will assess immunophenotyping data to determine whether or not resilient adolescents can be distinguished in terms of immunophenotype levels. The proportion of each immune cell type will be used as the predictor variables in a PLS analysis, with resilient functioning as the response variable. Using this method, we hope to identify immune patterns that are associated with resilient functioning.
Current status
This study has been reviewed and given favourable opinion by the National Research Ethics Service, NRES Committee East of England-Cambridge Central and external reviewers from the Royal Society (RGF\R1\180 064 and RGF\EA\180029). We are currently at year 3 of the project. As of March 2020, 102 participants have been recruited for the study. From those, 62 have completed phases I, II and II of the study. Only ten participants have withdrawn following informed consent due to various reasons (eg, scheduling conflicts, presence of mental health disorders or found images presented in the Emotional Regulation Task overly distressing). Additionally, of the adolescents screened, the most common reasons for ineligibility are MRI incompatibility (eg, dental braces), current psychotropic medication and BMI outside 18–30. Due to the uncertainty caused by the COVID-19 outbreak, we cannot anticipate when we will be able to finish the recruitment of our participants. However, we are approaching community organisations and agencies across Cambridge, including agencies working with victims of trauma to aid in the recruitment of participants with CA, and therefore, we anticipate the finalisation of the recruitment in 3 months from the time we start recruiting again.
Discussion
The RAISE study aims to examine how resilient adolescents react to psychosocial stress in order to better understand the neurobiological mechanisms that contribute to adolescent resilience. We will examine how key biological systems (ie, the HPA axis and the immune system) interrelate and interact with brain function and social influences in order to facilitate resilient functioning after CA. We hypothesise that resilient functioning would be facilitated by reduced baseline cortisol and cytokine levels before and after the stressor (ie, a latent factor constructed by serum hs-CRP and TNF-α and IL-6 and IL-1β levels) as these have been shown to be increased in those with CA, mental illness, reduced stress related brain responses (ie, amygdala, insula), and increased modulatory responses in the MPFC and ACC during the MIST. Moreover, we expect to find that the neurocognitive mechanisms that facilitate resilient functioning to stress during the in-unit assessment will be related to improved cognitive and emotional functioning at follow-up (lower rumination, lower interpersonal stress, improved mood). The findings from this study will help us to determine what sets resilient individuals apart on a neurobiological level. Such knowledge will be helpful to inform intervention strategies for individuals with a history of CA to prevent the development of mental health disorders, and ultimately, increase resilience in individuals who have experienced adversity in early life. Although we will take a dimensional approach, examining individual variation in degree of resilient functioning and including individuals with past histories of psychiatric disorders and subthreshold mental health disorders, the exclusion of patients with current psychiatric disorders will limit the interpretability of the data and our findings. Future studies should include participants with current mental health disorders to ensure the representation of both dimensions of the spectrum.
Supplementary Material
Acknowledgments
The authors would like to thank the adolescents and young adults who participated in this project, as well as the research nurses at the Cambridge Clinical Research Facility (Addenbrooke’s Hospital) for their contributions towards recruitment of study participants. They also are grateful to current and former members of the Risk and Resilience group who have contributed to this project.
Footnotes
Twitter: @drannelaura
Collaborators: RAISE Consortium list of authors: Alicia J. Smith (Department of Psychiatry, University of Cambridge, UK, Medical Research Council Cognition and Brain Sciences Unit, University of Cambridge, UK), Camilla Nord (Medical Research Council Cognition and Brain Sciences Unit, University of Cambridge, UK), Eugenia M. Davidson (Department of Psychiatry, University of Cambridge, UK), Nadia González-García (Hospital Infantil de México Federico Gómez. Laboratorio de Neurociencias, Ciudad de México, México), Oksana Berhe (Department of Psychiatry and Psychotherapy, Central Institute of Mental Health, Medical Faculty Mannheim, University of Heidelberg, Mannheim, Germany), Paul Fletcher(Department of Psychiatry, University of Cambridge, UK, Cambridgeshire and Peterborough NHS Foundation Trust, Fulbourn, UK), Paul Wilkinson(Department of Psychiatry, University of Cambridge, UK, Cambridgeshire and Peterborough NHS Foundation Trust, Fulbourn, UK), Rosalie Shrofer (Department of Psychiatry, University of Cambridge, UK), Sofia Orellana (Department of Psychiatry, University of Cambridge, UK), Sophie L. Bellow(Department of Psychiatry, University of Cambridge, UK), Suzanne Schweizer (Medical Research Council Cognition and Brain Sciences Unit, University of Cambridge, UK, Institute of Cognitive Neuroscience, University College London, London, UK).
Contributors: LM-L led the recruitment and testing of participants and drafted the manuscript. SNS, KI, MK, KS, ADA and LT were instrumental to the design of the study and reviewed the manuscript. The RAISE consortium members were or are instrumental to the setup and/or assessment of the RAISE study. A-LvH conceptualised and designed the study, drafted the original grant proposal, obtained financial support, oversaw all study procedures, and reviewed and revised this manuscript. All authors approved the final manuscript and agree to be accountable for all aspects of the work presented.
Funding: The RAISE study is funded by two grants from the Royal Society to A-LvH (RGF\EA\180029 & RGF\R1\180064). This work was further supported by a Royal Society Dorothy Hodgkin fellowship for A-LvH (DH150176) and a Wolfe Health fellowship for LM-L.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
RAISE Consortium:
Alicia J Smith, Camilla Nord, Eugenia M Davidson, Nadia González-García, Oksana Berhe, Paul Fletcher, Paul Wilkinson, Rosalie Shrofer, Sofia Orellana, Sophie L Bellow, and Suzanne Schweizer
References
- 1. Agnew-Blais J, Danese A. Childhood maltreatment and unfavourable clinical outcomes in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry 2016;3:342–9. 10.1016/S2215-0366(15)00544-1 [DOI] [PubMed] [Google Scholar]
- 2. Hazel NA, Hammen C, Brennan PA. Early childhood adversity and adolescent depression: the mediating role of continued stress. Psychol Med 2008;38:581–9. 10.1017/S0033291708002857 [DOI] [PubMed] [Google Scholar]
- 3. Hawker DSJ, Boulton MJ. Twenty years’ research on peer victimization and psychosocial maladjustment: A meta-analytic review of cross-sectional studies. J Child Psychol Psychiatry Allied Discip 2000. [PubMed] [Google Scholar]
- 4. Iffland B, Sansen LM, Catani C, et al. Emotional but not physical maltreatment is independently related to psychopathology in subjects with various degrees of social anxiety: a web-based Internet survey. BMC Psychiatry 2012;12 10.1186/1471-244X-12-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mullen PE, Martin JL, Anderson JC, et al. The long-term impact of the physical, emotional, and sexual abuse of children: a community study. Child Abuse Negl 1996;20:7–21. 10.1016/0145-2134(95)00112-3 [DOI] [PubMed] [Google Scholar]
- 6. Spinhoven P, Elzinga BM, Hovens JGFM. The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. J Affect Disord 2010;126:103–12. 10.1016/j.jad.2010.02.132 [DOI] [PubMed] [Google Scholar]
- 7. Vachon DD, Krueger RF, Rogosch FA, et al. Assessment of the harmful psychiatric and behavioral effects of different forms of child maltreatment. JAMA Psychiatry 2015;72:1135. 10.1001/jamapsychiatry.2015.1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wright Margaret O'Dougherty, Crawford E, Del Castillo D. Childhood emotional maltreatment and later psychological distress among college students: the mediating role of maladaptive schemas. Child Abuse Negl 2009;33:59–68. 10.1016/j.chiabu.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 9. Danese A, Pariante CM, Caspi A. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Egeland B. Taking stock: childhood emotional maltreatment and developmental psychopathology. Child Abuse Negl 2009;33:22–6. 10.1016/j.chiabu.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 11. Majer M, Nater UM, Lin J-MS, et al. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurol 2010;10:61. 10.1186/1471-2377-10-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry 2012. [DOI] [PubMed] [Google Scholar]
- 13. Savitz JB, van der Merwe L, Stein DJ, et al. Neuropsychological task performance in bipolar spectrum illness: genetics, alcohol abuse, medication and childhood trauma. Bipolar Disord 2008;10:479–94. 10.1111/j.1399-5618.2008.00591.x [DOI] [PubMed] [Google Scholar]
- 14. Shaffer A, Yates TM, Egeland BR. The relation of emotional maltreatment to early adolescent competence: developmental processes in a prospective study. Child Abuse Negl 2009;33:36–44. 10.1016/j.chiabu.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 15. Trickett PK, Mennen FE, Kim K. Emotional abuse in a sample of multiply maltreated, urban young adolescents: issues of definition and identification. Child Abus Negl 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yates TM, Wekerle C. The long-term consequences of childhood emotional maltreatment on development: (mal)adaptation in adolescence and young adulthood. Child Abuse Negl 2009;33:19–21. 10.1016/j.chiabu.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 17. Pongratz G, Straub RH. The sympathetic nervous response in inflammation. Arthritis Res Ther 2014;16 10.1186/s13075-014-0504-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Danese A, van Harmelen A-L. The hidden wounds of childhood trauma. Eur J Psychotraumatol 2017;8:1375840. 10.1080/20008198.2017.1375840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci 2012;1261:55–63. 10.1111/j.1749-6632.2012.06633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Danese A, Baldwin JR. Hidden wounds? inflammatory links between childhood trauma and psychopathology. Annu Rev Psychol 2017;68:517–44. 10.1146/annurev-psych-010416-044208 [DOI] [PubMed] [Google Scholar]
- 21. Nusslock R, Miller GE. Early-Life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry 2016;80:23–32. 10.1016/j.biopsych.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vecchiarelli HA, Gandhi CP, Gray JM. Divergent responses of inflammatory mediators within the amygdala and medial prefrontal cortex to acute psychological stress. Brain Behav Immun 2016;51:70–91. 10.1016/j.bbi.2015.07.026 [DOI] [PubMed] [Google Scholar]
- 23. Davidson RJ, Lewis DA, Alloy LB, et al. Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry 2002;52:478–502. 10.1016/S0006-3223(02)01458-0 [DOI] [PubMed] [Google Scholar]
- 24. Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci 2015;16:693–700. 10.1038/nrn4044 [DOI] [PubMed] [Google Scholar]
- 25. Johnstone T, van Reekum CM, Urry HL, et al. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci 2007;27:8877–84. 10.1523/JNEUROSCI.2063-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci 2009;29:11614–8. 10.1523/JNEUROSCI.2335-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci 2005;9:242–9. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 28. Wager TD, Davidson ML, Hughes BL. Neural mechanisms of emotion regulation: evidence for two independent prefrontal-subcortical pathways. NIH Public Access 2009. [Google Scholar]
- 29. Davidovich S, Collishaw S, Thapar AK. Do better executive functions buffer the effect of current parental depression on adolescent depressive symptoms? J Affect Disord 2016;199:54–64. 10.1016/j.jad.2016.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Lissnyder E, Koster EHW, Derakshan N. The association between depressive symptoms and executive control impairments in response to emotional and non-emotional information. Cogn Emot 2010. [Google Scholar]
- 31. Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology 2012;37:117–36. 10.1038/npp.2011.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Harmelen A-L, Kievit RA, Ioannidis K. Adolescent friendships predict later resilient functioning across psychosocial domains in a healthy community cohort. Psychol Med 2017;47:2312–22. 10.1017/S0033291717000836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalisch R, Baker DG, Basten U, et al. The resilience framework as a strategy to combat stress-related disorders. Nat Hum Behav 2017;1:784–90. 10.1038/s41562-017-0200-8 [DOI] [PubMed] [Google Scholar]
- 34. Rutter M. Resilience in the face of adversity. protective factors and resistance to psychiatric disorder. Br J Psychiatry 1985;147:598–611. 10.1192/bjp.147.6.598 [DOI] [PubMed] [Google Scholar]
- 35. Moreno-López L, Ioannidis K, Askelund AD. The resilient emotional brain: a scoping review of the medial prefrontal cortex and limbic structure and function in resilient adults with a history of childhood maltreatment. Biol Psychiatry Cogn Neurosci Neuroimaging 2020;5:392–402. 10.1016/j.bpsc.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 36. Fritz J, de Graaff AM, Caisley H. A systematic review of amenable resilience factors that moderate and/or mediate the relationship between childhood adversity and mental health in young people. Front Psychiatry 2018;9:230. 10.3389/fpsyt.2018.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Askelund AD, Schweizer S, Goodyer IM. Positive memory specificity is associated with reduced vulnerability to depression. Nat Hum Behav 2019. [DOI] [PubMed] [Google Scholar]
- 38. van Harmelen A-L, Gibson JL, St Clair MC, et al. Friendships and family support reduce subsequent depressive symptoms in at-risk adolescents. PLoS One 2016;11:e0153715 10.1371/journal.pone.0153715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ioannidis K, Askelund AD, Kievit RA, et al. The complex neurobiology of resilient functioning after childhood maltreatment. BMC Med 2020;18 10.1186/s12916-020-1490-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Charney DS. Psychobiological mechanism of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry 2004. [DOI] [PubMed] [Google Scholar]
- 41. Russo SJ, Murrough JW, Han MH. Neurobiology of resilience. Nat Neurosci 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chamberlain SR, Cavanagh J, de Boer P, et al. Treatment-Resistant depression and peripheral C-reactive protein. Br J Psychiatry 2019;214:11–19. 10.1192/bjp.2018.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baumeister D, Akhtar R, Ciufolini S. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry 2016;21:642–9. 10.1038/mp.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carpenter LL, Gawuga CE, Tyrka AR, et al. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology 2010;35:2617–23. 10.1038/npp.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Danese A, Caspi A, Williams B, et al. Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry 2011;16:244–6. 10.1038/mp.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hartwell KJ, Moran-Santa Maria MM, Twal WO, et al. Association of elevated cytokines with childhood adversity in a sample of healthy adults. J Psychiatr Res 2013;47:604–10. 10.1016/j.jpsychires.2013.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moor BG, Güroğlu B, Op de Macks ZA, et al. Social exclusion and punishment of excluders: neural correlates and developmental trajectories. Neuroimage 2012;59:708–17. 10.1016/j.neuroimage.2011.07.028 [DOI] [PubMed] [Google Scholar]
- 48. van Harmelen A-L, Hauber K, Gunther Moor B, et al. Childhood emotional maltreatment severity is associated with dorsal medial prefrontal cortex responsivity to social exclusion in young adults. PLoS One 2014;9:e85107. 10.1371/journal.pone.0085107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van Harmelen AL, Van Tol MJ, Dalgleish T. Hypoactive medial prefrontal cortex functioning in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gee DG, Gabard-Durnam LJ, Flannery J. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A 2013;110:15638–43. 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mehta MA, Golembo NI, Nosarti C, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian adoptees study pilot. J Child Psychol Psychiatry 2009;50:943–51. 10.1111/j.1469-7610.2009.02084.x [DOI] [PubMed] [Google Scholar]
- 52. Morey RA, Haswell CC, Hooper SR, et al. Amygdala, hippocampus, and ventral medial prefrontal cortex volumes differ in Maltreated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacology 2016;41:791–801. 10.1038/npp.2015.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thomaes K, Dorrepaal E, Draijer N. Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. J Clin Psychiatry 2010;71:1636–44. 10.4088/JCP.08m04754blu [DOI] [PubMed] [Google Scholar]
- 54. Tottenham N, Hare TA, Quinn BT. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci 2010;13:46–61. 10.1111/j.1467-7687.2009.00852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Rooij SJH, Kennis M, Sjouwerman R. Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychol Med 2015;45:2737–46. 10.1017/S0033291715000707 [DOI] [PubMed] [Google Scholar]
- 56. Vythilingam M, Heim C, Newport J, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 2002;159:2072–80. 10.1176/appi.ajp.159.12.2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Whittle S, Dennison M, Vijayakumar N, et al. Childhood maltreatment and psychopathology affect brain development during adolescence. J Am Acad Child Adolesc Psychiatry 2013;52:940–52. 10.1016/j.jaac.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 58. Gee DG, Gabard-Durnam L, Telzer EH. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci 2014;25:2067–78. 10.1177/0956797614550878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rasmussen LJH, Moffitt TE, Eugen-Olsen J, et al. Cumulative childhood risk is associated with a new measure of chronic inflammation in adulthood. J Child Psychol Psychiatry 2019;60:199–208. 10.1111/jcpp.12928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schönbrodt FD, Perugini M. At what sample size do correlations stabilize? J Res Pers 2013. [Google Scholar]
- 61. Angold A, Costello J, Van Kämmen W. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents: factor composition and structure across development. Int J Methods Psychiatr Res 1996. [Google Scholar]
- 62. Reynolds CR, Richmond BO. What I think and feel: a revised measure of children’s manifest anxiety. J Abnorm Child Psychol 1997;25:15–20. 10.1023/A:1025751206600 [DOI] [PubMed] [Google Scholar]
- 63. Bamber D, Tamplin A, Park RJ, et al. Development of a short leyton obsessional inventory for children and adolescents. J Am Acad Child Adolesc Psychiatry 2002;41:1246–52. 10.1097/00004583-200210000-00015 [DOI] [PubMed] [Google Scholar]
- 64. Kessler RC, Andrews G, Colpe LJ. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med 2002. [DOI] [PubMed] [Google Scholar]
- 65. Achenbach T. Manual for child behavior Checklist/ 4–18 and 1991 profile. University. Burlington, 1991. [Google Scholar]
- 66. Tennant R, Hiller L, Fishwick R, et al. The Warwick-Dinburgh mental well-being scale (WEMWBS): development and UK validation. Health Qual Life Outcomes 2007;5:63. 10.1186/1477-7525-5-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Parker G, Roussos J, Hadzi-Pavlovic D. The development of a refined measure of dysfunctional parenting and assessment of its relevance in patients with affective disorders. Psychol Med 1997;27:1193–203. 10.1017/S003329179700545X [DOI] [PubMed] [Google Scholar]
- 68. Elgar FJ, Waschbusch DA, Dadds MR. Development and validation of a short form of the Alabama parenting questionnaire. J Child Fam Stud 2007. [Google Scholar]
- 69. Epstein NB, Baldwin LM, Bishop DS. The McMaster family assessment device. J Marital Fam Ther 1983. [Google Scholar]
- 70. Miller IW, Epstein NB, Bishop DS. The McMaster family assessment device: reliability and validity. J Marital Fam Ther 1985. [Google Scholar]
- 71. Rosenberg M. Society and the adolescent self-image, 2015. [Google Scholar]
- 72. Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 1994;151:1132–6. 10.1176/ajp.151.8.1132 [DOI] [PubMed] [Google Scholar]
- 73. Cassels M, van Harmelen A-L, Neufeld S, et al. Poor family functioning mediates the link between childhood adversity and adolescent nonsuicidal self-injury. J Child Psychol Psychiatry 2018;59:881–7. 10.1111/jcpp.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Preston SD, Stansfield RB. I know how you feel: task-irrelevant facial expressions are spontaneously processed at a semantic level. Cogn Affect Behav Neurosci 2008;8:54–64. 10.3758/CABN.8.1.54 [DOI] [PubMed] [Google Scholar]
- 75. Kanske P, Heissler J, Schönfelder S, et al. How to regulate emotion? neural networks for reappraisal and distraction. Cereb Cortex 2011;21:1379–88. 10.1093/cercor/bhq216 [DOI] [PubMed] [Google Scholar]
- 76. Zhou X, Fragala MS, McElhaney JE, et al. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care 2010;13:541–7. 10.1097/MCO.0b013e32833cf3bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:22–33. [PubMed] [Google Scholar]
- 78. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- 79. Cleghorn CL, Harrison RA, Ransley JK, et al. Can a dietary quality score derived from a short-form FFQ assess dietary quality in UK adult population surveys? Public Health Nutr 2016;19:2915–23. 10.1017/S1368980016001099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dumont R, Willis JO, Veizel K. Wechsler abbreviated scale of intelligence In: Encyclopedia of special education, 2014. [Google Scholar]
- 81. Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun 2007;21:901–12. 10.1016/j.bbi.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 82. Spielberger CD. State-trait anxiety inventory In: The Corsini encyclopedia of psychology, 2010. [Google Scholar]
- 83. Dedovic K, Renwick R, Mahani NK. The Montreal imaging stress task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci 2005;30:319–25. [PMC free article] [PubMed] [Google Scholar]
- 84. Goodyer IM, Herbert J, Tamplin A, et al. Short-Term outcome of major depression: II. life events, family dysfunction, and friendship difficulties as predictors of persistent disorder. J Am Acad Child Adolesc Psychiatry 1997;36:474–80. 10.1097/00004583-199704000-00009 [DOI] [PubMed] [Google Scholar]
- 85. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 86. Harb GC, Heimberg RG, Fresco DM, et al. The psychometric properties of the interpersonal sensitivity measure in social anxiety disorder. Behav Res Ther 2002;40:961–79. 10.1016/S0005-7967(01)00125-5 [DOI] [PubMed] [Google Scholar]
- 87. Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: a psychometric analysis. Cognit Ther Res 2003;27:247–59. 10.1023/A:1023910315561 [DOI] [Google Scholar]
- 88. Bellingrath S, Rohleder N, Kudielka BM. Effort-reward-imbalance in healthy teachers is associated with higher LPS-stimulated production and lower glucocorticoid sensitivity of interleukin-6 in vitro. Biol Psychol 2013;92:403–9. 10.1016/j.biopsycho.2012.12.003 [DOI] [PubMed] [Google Scholar]
- 89. Decker M-L, Grobusch MP, Ritz N. Influence of age and other factors on cytokine expression profiles in healthy Children-A systematic review. Front Pediatr 2017;5:255. 10.3389/fped.2017.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. DeRijk RH, Petrides J, Deuster P. Changes in corticosteroid sensitivity of peripheral blood lymphocytes after strenuous exercise in humans. J Clin Endocrinol Metab 1996;81:228–35. 10.1210/jcem.81.1.8550757 [DOI] [PubMed] [Google Scholar]
- 91. Khoury JE, Gonzalez A, Levitan RD, et al. Summary cortisol reactivity indicators: interrelations and meaning. Neurobiol Stress 2015;2:34–43. 10.1016/j.ynstr.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fekedulegn DB, Andrew ME, Burchfiel CM. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med 2007;69:651–9. 10.1097/PSY.0b013e31814c405c [DOI] [PubMed] [Google Scholar]
- 93. Martuzzi R, Ramani R, Qiu M, et al. A whole-brain voxel based measure of intrinsic connectivity contrast reveals local changes in tissue connectivity with anesthetic without a priori assumptions on thresholds or regions of interest. Neuroimage 2011;58:1044–50. 10.1016/j.neuroimage.2011.06.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-040394supp001.pdf (664.6KB, pdf)