Abstract
The coronavirus disease 2019 (COVID-19) pandemic has caused untold disruption throughout the world. Understanding the mechanisms for transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is key to preventing further spread, but there is confusion over the meaning of ‘airborne’ whenever transmission is discussed. Scientific ambivalence originates from evidence published many years ago which has generated mythological beliefs that obscure current thinking. This article collates and explores some of the most commonly held dogmas on airborne transmission in order to stimulate revision of the science in the light of current evidence. Six ‘myths’ are presented, explained and ultimately refuted on the basis of recently published papers and expert opinion from previous work related to similar viruses. There is little doubt that SARS-CoV-2 is transmitted via a range of airborne particle sizes subject to all the usual ventilation parameters and human behaviour. Experts from specialties encompassing aerosol studies, ventilation, engineering, physics, virology and clinical medicine have joined together to produce this review to consolidate the evidence for airborne transmission mechanisms, and offer justification for modern strategies for prevention and control of COVID-19 in health care and the community.
Keywords: Virus, SARS-CoV-2, COVID-19, Air, Transmission, Aerosol
Introduction
As the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic rages on, so does the debate over what fraction of transmission occurs by aerosol exposure, as opposed to direct or indirect transmission by droplets and fomites [[1], [2], [3], [4], [5], [6], [7], [8], [9]].
This is an old debate that has been reignited by the appearance of yet another respiratory viral pandemic [[10], [11], [12], [13], [14]]. There is significant confusion over the definition and application of relevant terms, such as droplets, droplet nuclei, aerosols and particles (Table I ). Clearly, if there are differences amongst professionals in defining these terms, there will be problems in understanding the science. Ultimately, consensus will prove difficult, perhaps impossible, to achieve [15,16].
Table I.
Differences between clinicians, aerosol scientists and the general public in understanding of airborne terminology
| Term | Clinicians | Aerosol scientists | General public |
|---|---|---|---|
| Airborne | Long-distance transmission, such as measles; requires an N95/FFP2/FFP3 respirator (or equivalent) for infection control | Anything in the air | Anything in the air |
| Aerosol | Particle <5 μm that mediates airborne transmission; produced during aerosol-generating procedures and also requires an N95 respirator | Collection of solid or liquid particles of any size suspended in a gas | Hair spray and other personal/cleaning products |
| Droplet | Particle >5 μm that falls rapidly to the ground within a distance of 1–2 m from source; requires a surgical mask for infection control | Liquid particle | What comes out of an eyedropper |
| Droplet nuclei | Residue of a droplet that has evaporated to <5 μm; synonymous with ‘aerosol’ | A related term, ‘cloud condensation nuclei’, refers to small particles on to which water condenses to form cloud droplets | Never heard of |
| Particle | Virion | Tiny solid or liquid ‘blob’ in the air | Like soot or ash |
The way that evidence is being interpreted and applied differs between interested parties across the world. Baseline definitions of what constitutes sufficient evidence to support transmission by aerosols are many and varied. Without agreement, the debate will continue to drag on, confusing the issue, and placing more and more people at risk because the practical preventive interventions needed to control the virus are not adequately supported.
There is little, if any, direct evidence for the transmission of SARS-CoV-2 via any specific pathway. This statement applies to fomites and direct contact just as much as for large droplets and smaller airborne particles. It is notable that transmission through large droplets has never been demonstrated directly for any respiratory virus infection [7,17]. The proof required to elicit these routes of transmission should include genomic sequencing and matching of the target pathogen at source (e.g. on fomites or hands) with that causing subsequent disease in the recipient, along with sufficient evidence to exclude any other source of the pathogen strain before or during the study. However, genomic studies tracking a single virus are very difficult and expensive to perform, and they may fail [18].
To encourage both comprehension and consensus on airborne spread, this review presents a series of common ‘myths’ related to the science of viruses within aerosols. Use of the term ‘myth’ in this article implies a generally accepted statement about viral transmission that deserves fresh and unbiased consideration, especially in the light of the current pandemic. Each myth emanates from historical studies that merit evidence-based scrutiny to re-evaluate present-day opinion. By reviewing the science underpinning these myths, it is hoped that this article will facilitate understanding of why the common statements are outdated and why current evidence points in a different direction.
Myth 1: ‘aerosols are droplets with a diameter of 5 μm or less’
This myth originated from a historically incorrect definition, reported more recently by the World Health Organization as ‘… droplets <5 μm in diameter are referred to as droplet nuclei or aerosols’ [2].
Respiratory droplets, formed from respiratory secretions and saliva, are emitted through talking, coughing, sneezing and even breathing. Their diameters span a spectrum from <1 μm to >100 μm. The smaller droplets desiccate rapidly to 20–40% of their original diameter, leaving residues called ‘droplet nuclei’ which most clinicians believe to be synonymous with ‘aerosols’ [19].
Respiratory droplets with a wide range of diameters can remain suspended in the air and be considered airborne. The sizes of exhaled particles cover a continuum (Figure 1 ). One cannot definitively specify a cut-off for the diameter of airborne particles because the ability of a particle to remain suspended depends on many factors other than size, including the momentum with which they are expelled, and characteristics of the surrounding air flow (speed, turbulence, direction, temperature and relative humidity).
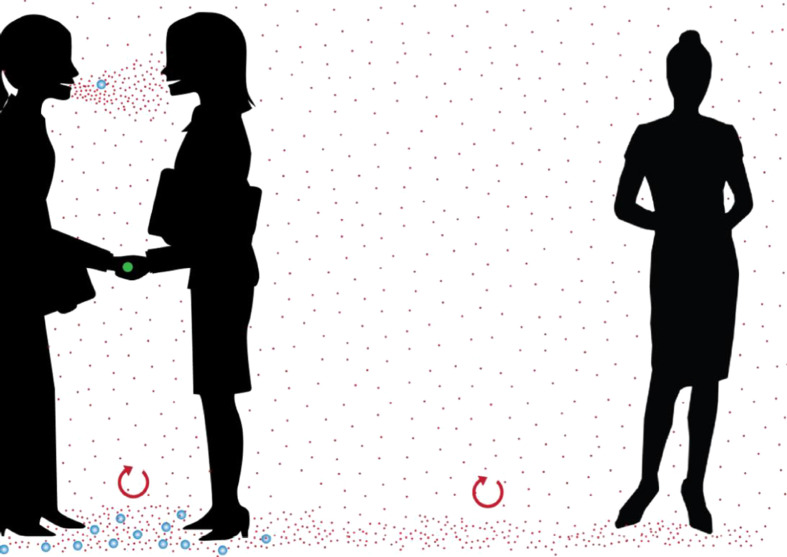
Figure 1.
Range of respiratory particles and potential spread over distance. Blue particles represent droplets, typically >100-μm diameter, that fall to the floor under gravity within 2 m of the source. Red particles represent aerosols, typically <100 μm, that stay suspended for longer, but eventually fall to the ground if the air is motionless for long enough (at least 30 min).
Depending upon airflow conditions, many particles that would previously have been classified as ‘large’ by this longstanding definition (diameter >5 μm) can travel much further than the ‘mythical’ 1–2 m distance within which such particles are claimed to fall to the ground. Taking this into account, even large particles can also behave like traditional ‘aerosols’. Both ‘aerosols’ and ‘droplets’ should be thought of as extremes of a size range for which their airborne pattern will vary depending on the local environmental conditions.
For the purpose of describing transmission, a more rational size threshold to distinguish droplets from aerosols, in terms of their physical behaviour and route of exposure, is 100 μm [20]. To clarify the terminology used in this review, therefore, droplets are particles that fall to the ground (or any surface including vertical surfaces) under the influence of gravity and/or the momentum of an infected person's exhaled air; and aerosols are particles that remain suspended due to size and/or environmental conditions. The term ‘particles’ will be used to refer to droplets/aerosols in general.
Myth 2: ‘all particles larger than 5 μm fall within 1–2 m of the source’
This is an oft-repeated but scientifically false statement. Exhaled particles of diameter 5–10 μm fall slowly to the ground under the influence of gravity in still indoor air. This takes 8–30 min from a height of 1.5 m. However, most rooms have typical ambient air currents of 0.1–0.2 m/s, which means that these particles are far too small to settle on the ground within 1–2 m of the source. A droplet must be larger than 50–100 μm to have a high probability of landing within 1–2 m of the emitting source indoors. Local turbulent air flows can extend this suspension time for even longer. It is known that droplets larger than 50–100 μm can be carried beyond 1–2 m in a jet of exhaled air, especially during sneezing or coughing [21,22].
Particles that are too small to settle rapidly under gravity can move upwards in a person's thermal plume. This is the upwardly moving column of warm air produced by a person's body heat [[23], [24], [25]]. Such particles can be influenced by other air flows generated by ventilation, people traffic, door movements and convective flows (e.g. air currents produced by warm electrical equipment and warm bodies) [26] before being inhaled. Transport by such flows is particularly important for particles of <5–10 μm, which can be carried over long (>2 m) distances.
In still air, particles of different sizes have different settling times that can be predicted accurately by physical laws (i.e. Stokes' law). Based on this, calculations show that even particles with a diameter of approximately 50 μm will take around 20 s to settle from a height of 1.5 m, and should be considered as aerosols [20]. The effect of turbulent air movements in busy hospital wards and clinics may result in particles of this size remaining airborne for even longer, and being capable of travelling >2 m from the source.
The time period that is clinically relevant for particles suspended in air depends on the ventilation. Hospital ventilation systems supply clean air which flush room air, and any particles it contains, out of the room. If a room has an uncontaminated air-exchange rate of six air changes per hour (ACH) from the combined effects of outdoor air, filtration and other air cleaners, the duration of interest is 10–30 min. If the room has an air-exchange rate of 12 ACH, the duration of interest is 5–15 min. Of course, some hospitals do not have mechanical ventilation systems, and, in the absence of open windows or doors, airborne particles could potentially take hours to settle on the ground [27]. The latter would constitute a risk for both staff and patients, especially if unprotected by distance from source or face masks.
Myth 3: ‘if it is short range, it cannot be airborne’
For the purpose of discussing this myth, the social distance proximity of 1–2 m is defined as the scale that differentiates between ‘short range’ and ‘long range’. It is commonly believed that long-range transmission is proof of airborne transmission, but the absence of detectable long-range transmission does not exclude airborne transmission. Specifically, airborne exposure and aerosol inhalation at short or close range (i.e. over conversational distance) may still be important, and even predominant, for the transmission of SARS-CoV-2, even if long-range transmission has not been demonstrated.
Delivery of the infectious agent by means of inhalation can occur over any distance, but it is more likely to occur at close range because aerosols are more concentrated nearer the source. A visual example of this can be seen by watching how smoke dissipates from a smoker over distance from the cigarette. A similar phenomenon can be experienced from smell. For example, if you are standing close enough to someone who has had garlic or alcohol for lunch, you may detect this when you inhale, but the odour fades as you move further away. However, if you do smell lunchtime odours in exhaled breath, you may also be inhaling any viruses present in that exhaled breath. Such encounters typically occur at a conversational distance (approximately 1 m or less). This has been confirmed by experiments and modelling studies of aerosol dynamics [17,[28], [29], [30], [31], [32], [33]].
It is known from influenza studies that exhaled breath and talking can carry viable viruses over conversational distances that can be inhaled by susceptible persons nearby [34,35]. These experiments demonstrate the presence of airborne viruses in different sized particles produced by infected persons over short conversational distances within 1 m.
Although, to date, there is no genotypic evidence that inhaled virus causes coronavirus disease (COVID-19) in humans, many outbreaks are difficult to explain other than inhalation of aerosolized SARS-CoV-2 [[36], [37], [38], [39], [40], [41]].
Aerosols are present at close range to an infectious emitter (<1 m) and, obviously, at much higher concentration than at longer range. At close range, one is exposed to the full spectrum of expired particles from ballistic ‘large droplets’ to tiny aerosols. Whether or not transmission occurs over longer ranges (beyond the social distancing range of 1–2 m) depends on several parameters. These include the quantity of airborne virions produced by the source; distribution of virions carried by different particle sizes; airflow patterns in the local environment; decay rate of virus infectivity; infectious dose needed to cause an infection in an individual; dilution of the inoculum at a distance; and timely removal by fresh air, ventilation or air cleaning.
The risk of longer range (>2 m) transmission may be smaller when compared with the risk of infection at close range (<1 m), but it could still occur and it could be significant. Unfortunately, longer-range transmission events for a pathogen can be very difficult to prove when that pathogen is already widespread in the community, with multiple sources able to emit the virus over various distances. A famous historical example is smallpox, for which long-range transmission could only be proven at the time of a single outbreak in Germany in the complete absence of ongoing community transmission [42].
Myth 4: ‘if the basic reproductive number, R0, is not as large as for measles, then it cannot be airborne’
The basic reproductive number (R 0) is generally defined as the average number of secondary cases arising from the presence of one single infected ‘index’ case in a population of uniformly distributed but otherwise totally susceptible individuals.
The key problem with this statement is that R 0 is not related directly to whether or not a disease is transmitted through aerosol inhalation. R 0 signifies how many people become infected after contact with one infected person, but the mechanism of transmission is irrelevant.
Various organisms can be disseminated by the airborne route but are not necessarily transmitted person-to-person. For example, hantaviruses, which cause hantavirus pulmonary syndrome, and Bacillus anthracis, which causes anthrax, have animal reservoirs and are acquired by inhalation, but they are not transmitted person-to-person. They have R 0=0 yet they are considered to be airborne diseases [43,44].
Furthermore, the value of R 0 is only as accurate as the ability to identify secondary cases. For viruses widely accepted to be airborne, such as measles and chickenpox, accurate identification of cases is relatively simple because these viruses cause distinctive skin pathology in >99% of infected cases. These can be diagnosed without laboratory testing, making identification and enumeration of secondary cases relatively easy. Estimates of R 0 are consequently much more accurate. As so many cases of COVID-19 are asymptomatic, R 0 is much more difficult to assess. A step further is the determination of R e, which is the ‘effective’ reproductive number. This is used when only a fraction of the exposed population may be susceptible to infections for which there is an effective vaccine (e.g. measles and chickenpox).
When patients present with an ‘influenza-like illness’, mild symptoms or none at all, the extent of any outbreak and, consequently, the number of secondary cases is much more difficult to ascertain. People will not necessarily know that they have been exposed, or be conscious of their ability to transmit the infection to others. They will not self-isolate and they will not be counted as potential secondary cases. This makes it impossible to contact trace and follow-up everyone involved in one specific exposure event, unless comprehensive details are recorded. Additionally, other contacts during their daily lives that could have led to the same infection from a different source cannot be excluded. Even in cases for which a single outbreak event can be associated with an infectious source, that same source may have already propagated other secondary cases that cannot be easily traced and counted. A substantial amount of pre-symptomatic transmission can occur with COVID-19, and similar to SARS-CoV-1, not all infected patients are equally contagious [45].
There is now good evidence that other respiratory viruses, such as influenza, SARS-CoV-1, Middle East respiratory syndrome coronavirus and respiratory syncytial virus, are transmitted through the air, so a similar application of this ‘myth-busting’ rationale can also be applied to the transmission of these viruses [[46], [47], [48], [49], [50]].
Myth 5a. ‘If it is airborne, surgical masks (or cloth face coverings) will not work’
This statement is false because it is essentially presented as an oversimplified binary scenario [i.e. masks work (completely) or do not work (at all) against viruses in respiratory particles].
Several laboratory studies have already shown that surgical and home-made masks are somewhat (but incompletely) effective in limiting exhaled particles and in protecting wearers from inhaling particles from others. Surgical masks can contain, and therefore reduce, the dissemination of viruses shed by an infected wearer by up to 3–4-fold (i.e. approximately 67–75%), and even 100% in the case of seasonal coronaviruses [34,51]. When an infectious person wears a mask or face covering, the size of the exhaled plume is also reduced, and this also helps to reduce the risk of exposure to those nearby.
Surgical masks also protect the wearer by reducing the exposure to incoming droplets and aerosols from infected individuals by an average of 6-fold (range 1.1- to 55-fold) [52,53]. The filtration capacity of surgical masks in the micron size range is often considerable, although it varies between brands [54]. It is known that the filtration capacity of N95/FFP2 respirators is better if they have been appropriately fit-tested to avoid leakage of aerosols around the side of the respirator into the breathing zone.
Even home-made cloth masks (made from tea towels or cotton t-shirts) can reduce the exposure from incoming particles by up to 2-4-fold (i.e. approximately 50–75%) [55,56]. This mainly depends on how the mask is made, what materials it is made from, the number of layers, and the characteristics of respiratory secretions to which it is exposed. Based on the evidence supporting a role for airborne transmission of COVID-19, the use of N95/FFP2/FFP3 respirators by front-line healthcare workers should be recommended. For those that cannot tolerate wearing these masks for long periods, the less restrictive surgical masks still offer some protection, but it needs to be acknowledged that these will not be quite as effective.
Myth 5b: ‘the virus is only 100 nm (0.1 μm) in size so filters and masks will not work’
This myth is related to Myth 5a. There are two levels of misunderstanding to be considered for this myth. Firstly, there is a lack of understanding of how high-efficiency particle air (HEPA) and other filters actually work. They do not act as simple ‘sieves’, but physically remove particles from the air stream using a combination of impaction and interception (where faster moving particles hit and stick to mask fibres via a direct collision or a glancing blow), diffusion (where slower moving particles touch and stick to mask fibres), and electrostatic forces (where oppositely charged particles and mask fibres adhere to each other). Together, these create a ‘dynamic collision trap’ as particles pass through the network of air channels between fibres at various speeds [57].
The minimum filtration efficiency typically occurs for particles of approximately 0.3 μm in diameter. Particles smaller than this ‘most penetrating particle size’ are captured with greater efficiency because their Brownian motion (allowing diffusion at an atomic level) causes them to collide with fibres in the filter at a high rate. Particles larger than this limiting diameter are removed efficiently through impaction and interception.
Secondly, viruses that are involved in transmission of infection are not generally ‘naked’. They are expelled from the human body in droplets containing water, salt, protein and other components of respiratory secretions. Salivary and mucous droplets are much larger than the virus [19], and it is the overall size that determines how the droplets and aerosols move and are captured by mask and filter fibres.
HEPA (or ‘arrestance’) filters can trap 99.97% or more of particles that are 0.3 μm (300 nm) in diameter. Exhaled salivary/mucous droplets start from approximately 0.5 μm in size and are removed entirely by HEPA filters. Indeed, HEPA filtration is not strictly needed in the ventilation systems of most commercial buildings other than health care, where specialist areas such as operating theatres, clean rooms, laboratories and isolation rooms benefit from single-pass capture of particles. Stand-alone ‘portable’ air cleaners that filter room air through built-in HEPA filters are an option for non-specialist areas such as offices and classrooms, although their performance may be limited by imperfect mixing, noise and draught effects [58].
Myth 6: ‘unless it grows in tissue culture, it is not infectious’
Viral culture is surprisingly difficult, which is one reason why virus isolation in cell culture is much less sensitive than detection by molecular methods. This is partly because it takes more than one virus to successfully initiate infection in a cell culture. For example, using influenza virus, Fabian et al. found that one TCID50 (i.e. the amount of virus required to infect 50% of in-vitro cell monolayers) represents approximately 300 genome copies; this is similar to previous estimates of 100–350 copies by Van Elden et al. but smaller than 650 copies reported by Wei et al. [[59], [60], [61]].
This difference in sensitivity is further compounded by currently available air-sampling techniques. Most studies use high-velocity ‘impingers’ which suck any airborne virus from the air into a bubbling liquid virus culture medium. However, these air-sampling devices generate high shear forces and vigorous mixing at the air–liquid interface, which may damage viral surface proteins and stop them growing in culture [62,63]. In contrast, natural human exhalation and inhalation flow velocities are much slower, which make them much less likely to cause shear stress damage to viruses [64,65]. Clearly, air-sampling technologies do not accurately replicate the mechanisms leading to human respiratory infection through inhalation.
As a consequence, failure to detect viable viruses in air samples does not necessarily prove the absence of live virus in samples where viral RNA was detected by molecular methods. Finding viral RNA in air samples should be interpreted as more likely to indicate the presence of live virus than not, as per the precautionary principle, which should always reinforce effective infection control [66].
For SARS-CoV-2, two different research groups have recently demonstrated the presence of infectious SARS-CoV-2 viruses in aerosol samples from patient rooms [67,68]. For the reasons stated above, these studies very likely underestimate the amount of viable airborne virus available for inhalation by others [69].
Conclusions
This review has attempted to clarify and dispel several common myths around the science underpinning airborne transmission of viruses. The myths presented are easily dismantled when consideration is given to the physical, epidemiological and virological principles of how respiratory aerosols are produced and disseminated; how secondary cases of infection can (or cannot) be readily identified; and how appropriate infection control measures can, and do, affect the risk of transmission. There is mounting evidence to support the presence and transmissibility of SARS-CoV-2 through inhalation of airborne viruses. Exposure to small airborne particles is equally, or even more, likely to lead to infection with SARS-CoV-2 as the more widely recognized transmission via larger respiratory droplets and/or direct contact with infected people or contaminated surfaces [70,71]. Some of the explanations and rationale for SARS-CoV-2 transmission can be applied to other respiratory viruses, but these should consider the numbers and types of studies available for those specific viruses [72,73].
What does this mean for infection control practitioners in health care, as well as the general population? Aside from the obvious benefits of personal protective equipment, the existing evidence is sufficiently strong to warrant engineering controls targeting airborne transmission as part of an overall strategy to limit the risk of infection indoors. These would include sufficient and effective ventilation, possibly enhanced by particle filtration and air disinfection; and the avoidance of systems that recirculate or mix air. Opening windows, subject to thermal comfort and security, provides more than a gesture towards reducing the risk of infection from lingering viral particles [70,71,73].
Measures to control overcrowding in both health care and confined indoor environments in the community, including public transport, are also relevant. There are a range of cost-effective measures aimed at diluting infectious airborne particles in homes and hospitals that are easily implemented, without major renovation or expenditure [70,72]. These will serve to protect everyone as the evidence required to further reduce the risk from COVID-19 is sought over the coming months and years. It is time to discard the myths and rewrite the science of viral transmission.
Conflict of interest statement
None declared.
Funding sources
None.
References
- 1.World Health Organization . WHO; Geneva: 2020. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations.https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations Available at: [last accessed December 2020] [Google Scholar]
- 2.World Health Organization . WHO; Geneva: 2020. Transmission of SARS-CoV-2: implications for infection prevention precautions.https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions Available at: [last accessed December 2020] [Google Scholar]
- 3.Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morawska L., Milton D.K. It is time to address airborne transmission of COVID-19. Clin Infect Dis. 2020;71:2311–2313. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conly J., Seto W.H., Pittet D., Holmes A., Chu M., Hunter P.R. WHO Infection Prevention and Control Research and Development Expert Group for COVID-19. Use of medical face masks versus particulate respirators as a component of personal protective equipment for health care workers in the context of the COVID-19 pandemic [published correction appears in Antimicrob Resist Infect Control 2020;9:151] Antimicrob Resist Infect Control. 2020;9:126. doi: 10.1186/s13756-020-00779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klompas M., Baker M.A., Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324:441–442. doi: 10.1001/jama.2020.12458. [DOI] [PubMed] [Google Scholar]
- 7.UK Scientific Advisory Group for Emergencies . SAGE; London: 2020. What is the evidence for the effectiveness of hand hygiene in preventing the transmission of respiratory viruses?https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/897598/S0574_NERVTAG-EMG_paper_-_hand_hygiene_010720_Redacted.pdf Available at: [last accessed December 2020] [Google Scholar]
- 8.Fennelly K.P. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med. 2020;8:914–924. doi: 10.1016/S2213-2600(20)30323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen J.G., Marr L.C. Recognizing and controlling airborne transmission of SARS-CoV-2 in indoor environments. Indoor Air. 2020;30:557–558. doi: 10.1111/ina.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interface. 2009;6(Suppl. 6):S783–S790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brankston G., Gitterman L., Hirji Z., Lemieux C., Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7:257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 13.Tang J.W., Li Y. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7:758–763. doi: 10.1016/S1473-3099(07)70268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seto W.H. Airborne transmission and precautions: facts and myths. J Hosp Infect. 2015;89:225–228. doi: 10.1016/j.jhin.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tellier R., Li Y., Cowling B.J., Tang J.W. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19:101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milton D.K. A Rosetta Stone for understanding infectious drops and aerosols. J Pediatric Infect Dis Soc. 2020;9:413–415. doi: 10.1093/jpids/piaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W., Zhang N., Wei J.J., Yen H.L., Li Y. Short-range airborne route dominates exposure of respiratory infection during close contact. Build Environ. 2020;176:106859. [Google Scholar]
- 18.Nguyen-Van-Tam J.S., Killingley B., Enstone J., Hewitt M., Pantelic J., Grantham M.L. Minimal transmission in an influenza A (H3N2) human challenge-transmission model within a controlled exposure environment. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marr L.C., Tang J.W., Van Mullekom J., Lakdawala S.S. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J R Soc Interface. 2019;16:20180298. doi: 10.1098/rsif.2018.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prather K.A., Marr L.C., Schooley R.T., McDiarmid M.A., Wilson M.E., Milton D.K. Airborne transmission of SARS-CoV-2. Science. 2020;370:303–304. doi: 10.1126/science.abf0521. [DOI] [PubMed] [Google Scholar]
- 21.Xie X., Li Y., Chwang A.T., Ho P.L., Seto W.H. How far droplets can move in indoor environments – revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 22.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323:1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 23.Licina D., Melikov A., Sekhar C., Tham K.W. Human convective boundary layer and its interaction with room ventilation flow. Indoor Air. 2015;25:21–35. doi: 10.1111/ina.12120. [DOI] [PubMed] [Google Scholar]
- 24.Licina D., Melikov A., Pantelic J., Sekhar C., Tham K.W. Human convection flow in spaces with and without ventilation: personal exposure to floor-released particles and cough-released droplets. Indoor Air. 2015;25:672–682. doi: 10.1111/ina.12177. [DOI] [PubMed] [Google Scholar]
- 25.Rim D., Novoselac A. Transport of particulate and gaseous pollutants in the vicinity of a human body. Build Environ. 2009;44:1840–1849. [Google Scholar]
- 26.Thatcher T.L., Lai A.C.K., Moreno-Jackson R., Sextro R.G., Nazaroff W.W. Effects of room furnishings and air speed on particle deposition rates indoors. Atmos Environ. 2002;36:1811–1819. [Google Scholar]
- 27.European Centre for Disease Control . ECDC; Stockholm: 2020. Heating, ventilation and air-conditioning systems in the context of COVID-19: first update.https://www.ecdc.europa.eu/sites/default/files/documents/Heating-ventilation-air-conditioning-systems-in-the-context-of-COVID-19-first-update.pdf Available at: [last accessed December 2020] [Google Scholar]
- 28.Bjørn E., Nielsen P.V. Dispersal of exhaled air and personal exposure in displacement ventilated rooms. Indoor Air. 2002;12:147–164. doi: 10.1034/j.1600-0668.2002.08126.x. [DOI] [PubMed] [Google Scholar]
- 29.Olmedo I., Nielsen P.V., Adana M.R.D., Jensen R.L., Grzelecki P. Distribution of exhaled contaminants and personal exposure in a room using three different air distribution strategies. Indoor Air. 2012;22:64–76. doi: 10.1111/j.1600-0668.2011.00736.x. [DOI] [PubMed] [Google Scholar]
- 30.Olmedo I., Nielsen P.V., Adana M.R.D., Jensen R.L. The risk of airborne cross-infection in a room with vertical low-velocity ventilation. Indoor Air. 2013;23:62–73. doi: 10.1111/j.1600-0668.2012.00794.x. [DOI] [PubMed] [Google Scholar]
- 31.Xu C., Nielsen P.V., Gong G., Liu L., Jensen R.L. Measuring the exhaled breath of a manikin and human subjects. Indoor Air. 2015;25:188–197. doi: 10.1111/ina.12129. [DOI] [PubMed] [Google Scholar]
- 32.Liu L., Li Y., Nielsen P.V., Wei J., Jensen R.L. Short-range airborne transmission of expiratory droplets between two people. Indoor Air. 2017;27:452–462. doi: 10.1111/ina.12314. [DOI] [PubMed] [Google Scholar]
- 33.Ai Z.T., Hashimoto K., Melikov A.K. Influence of pulmonary ventilation rate and breathing cycle period on the risk of cross-infection. Indoor Air. 2019;6:993–1004. doi: 10.1111/ina.12589. [DOI] [PubMed] [Google Scholar]
- 34.Milton D.K., Fabian M.P., Cowling B.J., Grantham M.L., McDevitt J.J. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan J., Grantham M., Pantelic J., Bueno de Mesquita P.J., Albert B., Liu F., EMIT Consortium Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc Natl Acad Sci USA. 2018;115:1081–1086. doi: 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J., Gu J., Li K., Xu C., Su W., Lai Z. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26:1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamner L., Dubbel P., Capron I., Ross A., Jordan A., Lee J. High SARS-CoV-2 attack rate following exposure at a choir practice – Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606–610. doi: 10.15585/mmwr.mm6919e6. [DOI] [PubMed] [Google Scholar]
- 38.Shen Y., Li C., Dong H., Wang Z., Martinez L., Sun Z. Community outbreak investigation of SARS-CoV-2 transmission among bus riders in Eastern China. JAMA Intern Med. 2020;180:1665–1671. doi: 10.1001/jamainternmed.2020.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller S.L., Nazaroff W.W., Jimenez J.L., Boerstra A., Buonanno G., Dancer S.J. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air. 2020 doi: 10.1111/ina.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Man P., Paltansing S., Ong D.S.Y., Vaessen N., van Nielen G., Koeleman J.G.M. Outbreak of COVID-19 in a nursing home associated with aerosol transmission as a result of inadequate ventilation. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1270. ciaa1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park S.Y., Kim Y.M., Yi S., Lee S., Na B.J., Kim C.B. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26:1666–1670. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gelfand H.M., Posch J. The recent outbreak of smallpox in Meschede, West Germany. Am J Epidemiol. 1971;93:234–237. doi: 10.1093/oxfordjournals.aje.a121251. [DOI] [PubMed] [Google Scholar]
- 43.US Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2012. Hantavirus pulmonary syndrome. Transmission.https://www.cdc.gov/hantavirus/hps/transmission.html#:∼:text=an%20infected%20rodent.,The%20hantaviruses%20that%20cause%20human%20illness%20in%20the%20United%20States,treated%20someone%20with%20the%20disease Available at: [last accessed December 2020] [Google Scholar]
- 44.US Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2015. Anthrax. How people are infected.https://www.cdc.gov/anthrax/basics/how-people-are-infected.html Available at: [last accessed December 2020] [Google Scholar]
- 45.Adam D.C., Wu P., Wong J.Y., Lau E.H.Y., Tsang T.K., Cauchemez S. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat Med. 2020;26:1714–1719. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- 46.Moser M.R., Bender T.R., Margolis H.S., Noble G.R., Kendal A.P., Ritter D.G. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110:1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- 47.Yu I.T., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 48.Booth T.F., Kournikakis B., Bastien N., Ho J., Kobasa D., Stadnyk L. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis. 2005;191:1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S.H., Chang S.Y., Sung M., Park J.H., Bin Kim H., Lee H. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS Isolation Wards. Clin Infect Dis. 2016;63:363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulkarni H., Smith C.M., Lee D.D.H., Hirst R.A., Easton A.J., O'Callaghan C. Evidence of respiratory syncytial virus spread by aerosol. Time to revisit infection control strategies? Am J Respir Crit Care Med. 2016;194:308–316. doi: 10.1164/rccm.201509-1833OC. [DOI] [PubMed] [Google Scholar]
- 51.Leung N.H.L., Chu D.K.W., Shiu E.Y.C., Chan K.H., McDevitt J.J., Hau B.J.P. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Health and Safety Executive UK . HSE; London: 2008. RR619 Evaluating the protection afforded by surgical masks against influenza bioaerosols.https://www.hse.gov.uk/research/rrhtm/rr619.htm Available at: [last accessed December 2020] [Google Scholar]
- 53.Makison Booth C., Clayton M., Crook B., Gawn J.M. Effectiveness of surgical masks against influenza bioaerosols. J Hosp Infect. 2013;84:22–26. doi: 10.1016/j.jhin.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Weber A., Willeke K., Marchioni R., Myojo T., McKay R., Donnelly J. Aerosol penetration and leakage characteristics of masks used in the health care industry. Am J Infect Control. 1993;21:167–173. doi: 10.1016/0196-6553(93)90027-2. [DOI] [PubMed] [Google Scholar]
- 55.van der Sande M., Teunis P., Sabel R. Professional and home-made face masks reduce exposure to respiratory infections among the general population. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davies A., Thompson K.A., Giri K., Kafatos G., Walker J., Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep. 2013;7:413–418. doi: 10.1017/dmp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tcharkhtchi A., Abbasnezhad N., Zarbini Seydani M., Zirak N., Farzaneh S., Shirinbayan M. An overview of filtration efficiency through the masks: mechanism of aerosol penetration. Bioact Mater. 2020;6:106–122. doi: 10.1016/j.bioactmat.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bluyssen P.M., Ortiz M., Zhang D. The effect of a mobile HEPA filter system on 'infectious' aerosols, sound and air velocity in the SenseLab. Build Environ. 2021;188:107475. doi: 10.1016/j.buildenv.2020.107475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fabian P., McDevitt J.J., DeHaan W.H., Fung R.O., Cowling B.J., Chan K.H. Influenza virus in human exhaled breath: an observational study. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Elden L.J., Nijhuis M., Schipper P., Schuurman R., van Loon A.M. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J Clin Microbiol. 2001;39:196–200. doi: 10.1128/JCM.39.1.196-200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei Z., McEvoy M., Razinkov V., Polozova A., Li E., Casas-Finet J. Biophysical characterization of influenza virus subpopulations using field flow fractionation and multiangle light scattering: correlation of particle counts, size distribution and infectivity. J Virol Methods. 2007;144:122–132. doi: 10.1016/j.jviromet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Verreault D., Moineau S., Duchaine C. Methods for sampling of airborne viruses. Microbiol Mol Biol Rev. 2008;72:413–444. doi: 10.1128/MMBR.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang J.W., Wilson P., Shetty N., Noakes C.J. Aerosol-transmitted infections – a new consideration for public health and infection control teams. Curr Treat Options Infect Dis. 2015;7:176–201. doi: 10.1007/s40506-015-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta J.K., Lin C.H., Chen Q. Characterizing exhaled airflow from breathing and talking. Indoor Air. 2010;20:31–39. doi: 10.1111/j.1600-0668.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 65.Tang J.W., Nicolle A.D., Klettner C.A., Pantelic J., Wang L., Suhaimi A.B. Airflow dynamics of human jets: sneezing and breathing – potential sources of infectious aerosols. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou J., Otter J.A., Price J.R., Cimpeanu C., Garcia D.M., Kinross J. Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa905. ciaa905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santarpia J.L., Rivera D.N., Herrera V.L., Morwitzer M.J., Creager H.M., Santarpia G.W. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care [published correction appears in Sci Rep 2020;10:13892] Sci Rep. 2020;10:12732. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lednicky J.A., Lauzardo M., Fan Z.H., Jutla A., Tilly T.B., Gangwar M. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown J.R., Tang J.W., Pankhurst L., Klein N., Gant V., Lai K.M. Influenza virus survival in aerosols and estimates of viable virus loss resulting from aerosolization and air-sampling. J Hosp Infect. 2015;91:278–281. doi: 10.1016/j.jhin.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 70.Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G. How can airborne transmission of COVID-19 indoors be minimised? Environ Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kampf G., Brüggemann Y., Kaba H.E.J., Steinmann J., Pfaender S., Scheithauer S. Potential sources, modes of transmission and effectiveness of prevention measures against SARS-CoV-2. J Hosp Infect. 2020;106:678–697. doi: 10.1016/j.jhin.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kutter J.S., Spronken M.I., Fraaij P.L., Fouchier R.A., Herfst S. Transmission routes of respiratory viruses among humans. Curr Opin Virol. 2018;28:142–151. doi: 10.1016/j.coviro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hobday R.A., Dancer S.J. Roles of sunlight and natural ventilation for controlling infection: historical and current perspectives. J Hosp Infect. 2013;84:271–282. doi: 10.1016/j.jhin.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]