Abstract
We discuss the evidence behind mask use, including evidence for homemade masks, social distancing, and the local coronavirus disease-2019 (COVID-19) epidemics in countries that initially employed more limited public health interventions. Given the absence of data for specific interventions in the rheumatic disease population, we reviewed the evidence available for the general population. The risk of poor outcomes with COVID-19 in patients with rheumatic diseases is a potential concern given the immunosuppression associated with these conditions and disease-modifying anti-rheumatic drug therapy, as well as advancing age and many of the comorbidities present in such patients. Infection prevention is key, for both individual patients and their community. Given the data collected from the general population, we recommend ongoing proper mask use, social distancing, and hand hygiene for patients with rheumatic diseases and encourage providers to counsel these patients in prevention strategies and attempt to dispel abundant misinformation.
Keywords: COVID-19, SARS-CoV-2, Mask, Social distancing, Public health, Herd immunity
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rapidly spread across the globe and was declared a pandemic by the World Health Organization on March 11, 2020. Public health interventions differed by region and country, but most employed mask use and social distancing with temporary closure of schools and businesses, limitations on large gatherings, and guidance to maintain distance from others to help limit respiratory viral transmission. Though some immunomodulatory pharmacological therapies such as remdesivir [1,2] and dexamethasone [3,4] have shown potential benefit to treat patients with coronavirus disease-2019 (COVID-19), they have not been shown to reduce transmission. In the absence of a therapy or vaccine to decrease the infectious period, further complicated by limited availability of widespread testing and surveillance, and transmission by asymptomatic cases, prevention remains our primary approach to combat this pandemic.
In response to the pandemic, the rheumatology community joined together to form the COVID-19 Global Rheumatology Alliance to address the knowledge gap of COVID-19 in rheumatic disease patients. However, data about the risk for infection and outcomes in this population remain limited [[5], [6], [7], [8]]. Currently, a very low percentage of patients hospitalized in the United States have underlying autoimmune disease, a percentage lower than that of the background population of the United States with such diseases. Whether this is due to the isolation and avoidance of infection by individuals who perceive themselves as potentially at higher risk or whether it is due in part to a protective nature of their immunosuppressive therapies is unclear [9]. There is much interest in using some biological and small molecular therapies employed in rheumatic diseases to quell the adverse immune response to SARS-CoV-2. Data regarding such therapies have been mixed. While negative results have generally been reported for IL-6-blocking therapies from randomized controll trials, encouraging results have been reported for baricitinib when used in combination with remdesivir to treat COVID-19 [6,10].
Regardless of any protective or negative effects of rheumatic disease therapies, many such patients are elderly or have comorbidities that put them at higher risk for severe outcomes with COVID-19. Accordingly, the prevention of infection is key. In this manuscript, we reviewed the evidence available in the general population for public health interventions meant to decrease virus transmission. We discuss the evidence behind mask use, including homemade masks, social distancing, and the local COVID-19 epidemics in countries that initially employed extremely limited public health interventions.
What is known regarding SARS-CoV-2 transmission?
COVID-19 is predominantly transmitted by droplets, though current evidence also indicates that viral particles of SARS-CoV-2 can be shed in aerosols [11,12]. The distance of 6 feet is the estimate of horizontal droplet transit, therefore the risk of exposure will be decreased if a greater distance is maintained [[13], [14], [15]]. There are situations where droplets can travel further than 6 feet, as seen with coughing and sneezing, which makes the use of masks just as important as social distancing to prevent transmission [16,17]. SARS-CoV-2 ribonucleic acid (RNA) from infected humans or animals has been detected as far as a 16.1 kilometer distance [[18], [19], [20]]. The environmental stability of SARS CoV-2 is poorly understood. Available evidence has shown the infectivity of SARS-CoV-2 persisting up to 16 hours after aerosolization at room temperature and with standard humidity [21,22]. Results from many studies that evaluated droplets and air samples in the environment have to be interpreted with caution, given most use viral RNA, which may not be infectious or distance from the source is unknown [[23], [24], [25], [26]]. Though the aerosolization of the virus is possible, data that evaluated the central air conditioning system on a cruise ship outbreak support that transmission is not primarily airborne [27].
Only limited data are available on the persistence of SARS-CoV-2 on commonly touched surface areas. However, the widespread distribution of viral RNA has been documented in clinical settings [25]. Studies have reported the infectivity of SARS-CoV-2 that persists up to 4 days at room temperature on plastic and metal and 2 days on glass, ceramic, and rubber surfaces [21,28]. Infectious SARS-CoV-2 particles have also been isolated from urine samples and stool, which suggest foodborne transmission may also be possible in the setting of diarrhea and gastroenteritis coupled with inadequate hand hygiene [[29], [30], [31], [32]]. Viral transmission is complex, with multiple dependent factors, including setting (indoor and outdoor), ventilation, wind, temperature, course of disease, viral shedding, hand hygiene, environmental contamination, and the duration of contact.
What is the value of social/physical isolation?
Social distancing is one tool that is frequently used by public health to help interrupt transmission during outbreaks and include temporary closure of schools, businesses, limitations on large gatherings, and guidance to remain 6 feet away from others to help limit respiratory viral transmission. The success of these measures depends largely on public cooperation and buy-in, which can be difficult to measure. Further complicating effect evaluation, decreased incidence in cases lags at least 2–3 weeks behind the implementation of social distancing measures due to the incubation time of SARS-CoV-2 [33]. The concept is based on reducing the basic reproduction number (R0, 2–6 for COVID-19) or the number of secondary infections each case is expected to cause during their illness in a susceptible population [34].
Data on the effects of social distancing in COVID-19 are largely derived from modeling studies with additional support from outbreaks and the increased cases observed following the reopening of schools and lifting social distancing measures [35,36]. Modeling studies using data from Ontario, Canada demonstrated the greatest effect of reducing epidemic peak, and hospital admissions were with restrictive physical distancing where individuals remain at home [37]. Their data suggest an attack rate of 56% with no interventions, which decreased to 2% with 13 months of social distancing [37]. Modeling of the pandemic in 89 countries by Lonergan and Chalmers demonstrated that lockdown and social distancing led to a reduction in R0. However, increasing contact by 20% could lead to peaks in most of the evaluated countries [38]. Additional models developed using data from Washington state, the location of the first COVID-19 case in the United States, demonstrated that a reduction of at least 25% of contacts for adults and 95% for those aged 60 years and older, could reduce the number of deaths and hospitalizations in the first 100 days of the epidemic by 78% [38]. A projected increase in cases was seen when social distancing interventions were lifted.
Ecological studies in Brazil, where mobile phone geolocation data and a distance algorithm were used to determine a social distancing index (SDI) for the population in different regions, provide an estimate of public adherence. The SDI is expressed as a percentage, where 100% indicates the entire population remains at home for a full day [39]. The authors demonstrated an impact in death rates based on the SDI [33,40]. Between March 16 and March 22, 2020, social distancing measures were implemented, including work from home and nonessential business closures. Prior to these interventions in Sao Paulo State, deaths increased at the rate of 5.2 cases per day and leveled off starting April 5, 2020, approximately two to three weeks after the implementation of social distancing measures [33]. An estimated SDI of over 52% is needed for a stable death rate, and an SDI of greater than 56%, to lead to a reduction in death rate. These indicate that over half the population would need to remain at home to stabilize COVID-19 transmission [33]. Additional data from some Scandinavian countries and Germany demonstrated decreased deaths and incident infections following the implementation of social distancing measures [41,42].
Both deaths and cases consistently stabilize or decline with social distancing measures in the published COVID-19 transmission modeling studies. These results collectively show that social distancing is necessary until an effective vaccine and/or therapies are widely available to reduce the number of deaths and avoid overwhelming the healthcare systems as seen in Italy, New York, Louisiana, and Texas [[43], [44], [45], [46]]. We encourage patients to avoid unnecessary public interactions, work from home if feasible, avoid close contact with people outside their household, and attempt to maintain a distance of 6 feet from others when out in public. If many are not wearing masks, particularly if coughing or sneezing, attempting to maintain greater than 6 feet distance is recommended. The impact of maintaining 6 feet distance between individuals is likely more relevant in indoor and poorly ventilated spaces as compared to outdoors, as multiple COVID-19 clusters are associated with indoor spaces [[47], [48], [49], [50], [51]]. When gathering with friends or family, we suggest outdoor gatherings where 6 feet can be maintained between households. Additionally, patients should avoid close contact such as hugs or handshakes, limit commonly touched surfaces or utensils, and encourage hand hygiene with hand sanitizer (≥60% alcohol) or hand washing. Most importantly, those who are sick, who have recently come in contact with an ill individual or someone with confirmed COVID-19 should self-isolate, even if they do not have symptoms [52].
What is the value of masks for rheumatic disease patients?
Masks are an essential tool, along with social distancing and hand hygiene, to help limit SARS-CoV-2 transmission. Cloth and surgical masks are used to decrease the spread of droplets during coughing, sneezing, and talking from the person wearing the mask; thus, protecting surrounding contacts and decreasing environmental contamination [53]. Decreased transmission with mask use has been seen in tuberculosis, influenza, and other respiratory viruses [[54], [55], [56], [57]]. Universal masking outside the home is recommended because of evidence of asymptomatic transmission of COVID-19 as well as delayed or limited testing availability in some areas, which make it impractical to recommend masks only for those with symptoms or confirmed disease [58,59]. In addition, patients should be educated about proper mask use – the mask should cover the nose and mouth, and hand hygiene should be performed before and after the mask is adjusted [53]. Masks should not be worn around the forehead, chin, or neck [60].
Multiple investigations of COVID-19 infection clusters support the use of universal masking outside the home. In China, a gentleman with COVID-19 rode two buses on an extended trip. He did not have a mask on the first bus; however, he purchased one prior to transfer and wore it on the second bus. The epidemiological investigation identified no secondary infections on the second bus as compared to 12.8% of the passengers subsequently infected on the first bus [61]. A public health investigation involving a hair salon in Missouri identified two stylists who worked from 5 to 8 days with symptoms before testing positive for COVID-19, exposing 139 clients [62]. Per city policy, the stylists and 98% of the clients were masked, and no secondary cases were linked to this public exposure with 48.2% who tested negative and 83.7% reporting no symptoms [62]. All household contacts of one of the two stylists subsequently developed symptoms and tested positive for COVID-19 [62]. In addition, multiple cases have been identified who were associated with airplane travel, where social distancing is not feasible, including two flights prior to recommendations for universal masking where genomic sequencing was not performed, and a more recent publication identifying identical viral strains between two passengers and two flight staff with incubation periods suggesting exposure to staff during the flight [[63], [64], [65]]. A contact investigation out of Australia identified 29 cases linked to a flight where mask use was infrequent with passengers from a known cruise ship outbreak, which resulted in multiple secondary cases identified by a distinct genetic cluster [66]. Secondary cases were clustered in the mid cabin but were dispersed over nine rows [66].
An ecological study looking at COVID-19 daily growth rate following universal mask mandates between April 8 and May 15, 2020 in the United States identified a significant decline, with the largest decrease of 2% three weeks following the mandate [67]. The authors estimate a lower estimate of 230,000 cases prevented with universal masking mandates [67]. More recently, a case-control study performed by the Center for Disease Control and Prevention (CDC) showed a statistically significant association between COVID-19 diagnosis and close contact with a known COVID-19 case, dining at a restaurant or going to a bar or coffee shop [68]. In those without a known COVID-19 contact, the odds ratio (OR) was greater for dining at a restaurant (OR = 2.8) or going to a bar or coffee shop (OR = 3.9), with 21% of those engaging in these activities reporting incomplete adherence to wearing masks or social distancing at the establishment [68]. There are limitations to this study; however, the data suggest activities where masking by many individuals may be inconsistent, such as places where people are eating or drinking, may be associated with COVID-19.
Is wearing masks context specific?
Masks should be worn outside the home or when in close contact with people outside one's household, particularly if unable to maintain appropriate social distancing [60]. The importance of universal mask use should be stressed in situations when people are indoors and may have close contact with people outside one's household [60,68]. If people become ill with fever, cough, or sneezing, recommendations are to remain home and to wear a mask around other household members to decrease transmission.
What is the effect of homemade masks?
Given the shortage of personal protective equipment and limited availability of medical masks, many have turned to homemade or cloth masks, and the CDC recommended use of cloth face masks when in public on April 3, 2020 [69]. A survey of 502 adults demonstrated rapid uptake of guidance with 61.9% who reported cloth mask use in public within a week, which increased to 76.4% approximately 1 month later [70]. At this point in the pandemic, there was limited evidence available to support the universal use of cloth masks, but more recent data continue to support this public health intervention.
A review by Lima et al. identified 9 studies evaluating the efficacy of cloth masks to filter particles from 0 μm to 1000 nm (SARS-CoV-2 is 50–200 nm) found 40%–97% efficacy of cloth masks, with variability resulting from number of layers, fabric type, and how frequently the mask was washed [71]. Heavier and denser fabrics such as felt, wool, quilt cotton, and denim provided greater protection than pillowcases, cotton, and linen. Cloth masks with double layers have been shown to work as well as surgical masks, with an additional study noting a minimal viral filtration efficiency of at least 50% [72,73]. Neupane et al. demonstrated a 20% decrease in effectiveness when double-layer cotton masks were washed more than four times [74]. More recently, Fischer and team utilized optical measurements to evaluate the transmitted droplet count during speech through 14 different masks. They identified that 2-layer cotton and cotton-polypropylene had similar droplet counts to surgical masks. In contrast, double-layered bandanas and fleece (neck gaiters) did not appear to decrease droplet transmission, with fleece actually increasing the transmitted droplet count. This suggests that fleece may fragment larger droplets as they pass through and lead to environmental contamination with smaller droplets [75]. Given these data, we recommend the use of double-layer masks made from a tightly woven fabric such as quilting cotton when in public and to avoid using bandanas or fleece fabric. If the mask becomes stretched after multiple washes and no longer fits properly, a new mask should be used. Instructions for mask construction are available on the CDC website (https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-to-make-cloth-face-covering.html) and can be provided to patients.
What personal protective equipment should be used in healthcare setting to reduce the risk of SARS-CoV-2 infection?
The proper use of personal protective equipment is critical to protect healthcare workers against COVID-19 infection. Respiratory protective devices such as N95 respirators are of particular importance. A meta-analysis that compares randomized controlled trials has shown low certainty evidence that medical masks provide similar protection against viral respiratory infection in healthcare workers during nonaerosol-generating care; this analysis recommends the preservation of N95 respirators for high-risk, aerosol-generating procedures [76]. Despite some conflicting evidence, the superiority of N95 for the prevention of viral infection, including coronaviruses over surgical masks has been demonstrated in a number of studies [77]. For viral infection like SARS-CoV-2, it is logical to use a respirator to offer more resistance to fluid penetration and to form a seal around the mouth and nose in contrast to surgical masks that provide barrier protection only against droplets, including large respiratory particles. For high-risk aerosol-generating procedures, additional personal protective equipment such as fluid-resistant gowns, face shields or goggles, double gloves, in addition to filtering face piece level 2–3 masks, or N95-99 respirator masks are recommended [78,79].
What do “experiments” like Sweden tell us about the value of these measures?
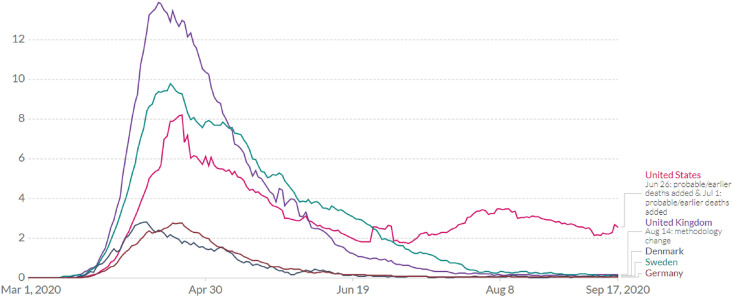
It is of value to consider infection control strategies taken by Sweden and neighbor countries and the intercountry differences in the course of the pandemic since March 2020. During the initial 3–4 months since COVID-19 was declared a pandemic in March 2020 [80], Sweden opted to take generally less stringent measures; large group gatherings (>50 persons) were banned and some distance learning was in place, but schools, bars, and restaurants were never closed and borders remained open [81,82]. This approach was largely based on the idea of allowing some infection to spread among the less vulnerable individuals to eventually reach a so-called “herd immunity,” which would provide indirect protection when the majority of the population is immune to the infection [34]. Like the United States, many European and Scandinavian countries such as Sweden, Netherlands, Germany, and United Kingdom initially took a “softer” approach. These countries focused on enforcing gradual measures, allowing some infection to spread in the population, while focusing on protecting the more vulnerable. However, after the Imperial College London released results in March 2020 from modeling studies predicting hospital overloads with COVID-19 patients, more stringent public health measures were implemented [83]. Unlike Sweden, neighboring countries such as Germany, Finland, and Denmark immediately closed their borders, bars, restaurants, and schools, and implemented population lockdowns between March and April of 2020 [41,84]. The difference in the daily number of confirmed COVID-19 deaths in Sweden and neighboring countries is striking (Fig. 1 ). Sweden had consistently higher death rates as compared to its neighboring countries, well beyond the initial few weeks of rapid surge in April 2020. The rapid surge was followed by gradual declines in Denmark, Finland, and United Kingdom, where rapid lockdown efforts seemed to have curtailed the initial infection surge. It is interesting to note that United Kingdom clearly surpassed Sweden in death rates earlier in the initial months, but like the other countries described above, the death rate rapidly declined following the implementation of strict social distancing measures.
Fig. 1.
Daily new confirmed COVID-19 death per million population in the rolling 7-day average. Source: European Center for Disease Control – Situation Update Worldwide – Last updated 17 September, 10:35 (London time).
It is important to note that other socioeconomic and geographical factors likely have had an impact on the course of the pandemic. Infection rates are high in the Stockholm area, where the high proportion of single households may have actually prevented viral spread between close contacts [42]. Sparse populations in the suburbs and the generally high level of wealth around the country, may also have played in favor for the pandemic response. However, failure to shield the most vulnerable individuals from COVID-19 may also have contributed to the high mortality rates; 40%–50% of COVID-19 cases in Sweden have been elderly nursing home residents [42,85]. Swedes are known to be open to voluntary cooperation with state requirements in crisis, rather than following strict legislative directives. While countrywide lockdowns were never ordered, Sweden relied on voluntary social distancing. Voluntary social distancing likely was followed at a much greater scale since Sweden's strategies based on herd immunity came to a greater media attention and many began to view Sweden's initial strategy as a failure.
It is only with more time that we will be able to fairly evaluate which strategies were more effective than others in pandemic response efforts. For now, without a widely available and efficacious antiviral treatment and while mass vaccination efforts are being made, prevention is the only and best tool we have for infection control and our patients with rheumatic diseases. It is of paramount importance for a majority of the population to follow preventive public health measures, either by mandate or voluntary, to minimize further infection and prevent adverse sequelae or deaths due to COVID-19.
Summary
Infection prevention for SARS-CoV-2 is key, for both individual patients and their community. Given the data collected from the general population, we recommend ongoing proper mask use when in contact with people outside one's household, social distancing, and hand hygiene for patients with rheumatic diseases. We encourage providers to counsel these patients in prevention strategies and attempt to dispel abundant misinformation.
Practice points.
-
•
The combination of social distancing, universal masking, and hand hygiene are essential public health tools to limit the transmission of SARS-CoV-2.
-
•
Masks should be worn outside the home or when in close contact with people who live outside one's household.
-
•
Homemade masks should be double-layer and constructed from a dense or tightly woven fabric such as quilt cotton; avoid using bandanas or fleece fabric.
Research agenda.
-
•
Given the limited data on outcomes and the risk of infection from COVID-19 in rheumatic disease patients, further research is needed in this area.
-
•
Further data are needed to evaluate the benefit of homemade masks and different fabrics to the individual wearing the mask.
-
•
Additional data are needed to identify which activities pose the greatest risk for infection, so we can better counsel our patients who may be at high risk for poor outcomes.
-
•
Research is needed to better understand the role or aerosol transmission, and how air filtration or ventilation systems may mitigate this risk.
-
•
More data are needed with regard to the risk of COVID-19 transmission outdoors.
Author's roles
Kevin Wintrhop are as follows; Consulting: Pfizer, AbbVie, Union Chimique Belge (UCB), Eli Lilly & Company, Galapagos, GlaxoSmithKline (GSK), Roche, Gilead, BMS, Regeneron, Sanofi, AstraZeneca, and Novartis.
Research: BMS and Pfizer.
Funding
None received for this review.
Declaration of competing interest
Cara Varley and Jennifer Ku have none.
References
- 1.Grein J., Ohmagari N., Shin D., et al. Compassionate use of remdesivir for patients with severe covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko W.C., Rolain J.M., Lee N.Y., et al. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020;55(4):105933. doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582(7813):469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 4.Abdolahi N., Kaheh E., Golsha R., et al. Letter to the editor: efficacy of different methods of combination regimen administrations including dexamethasone, intravenous immunoglobulin, and interferon-beta to treat critically ill COVID-19 patients: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):549. doi: 10.1186/s13063-020-04499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gianfrancesco M.A., Hyrich K.L., Gossec L., et al. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. 2020;2(5):e250–e253. doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McInnes I.B. COVID-19 and rheumatology: first steps towards a different future? Ann. Rheumat. Dis. 2020;79(5):551–552. doi: 10.1136/annrheumdis-2020-217494. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese C. COVID-19 and your rheumatology patients. Cleve Clin J Med. 2020 doi: 10.3949/ccjm.87a.ccc027. [DOI] [PubMed] [Google Scholar]
- 8.Gianfrancesco M., Hyrich K.L., Al-Adely S., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheumat. Dis. 2020;79(7):859. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prevention CfDCa . 2020. Coronavirus disease 2019 (COVID-19)-Associated hospitalization surveillance network (COVID-NET)https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html Available from: [Google Scholar]
- 10.Winthrop K.L., Mariette X. To immunosuppress: whom, when and how? That is the question with COVID-19. Ann. Rheumat. Dis. 2020;79(9):1129. doi: 10.1136/annrheumdis-2020-218694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan F., Ye T., Sun P., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Transboundary and Emerging Diseases; 2020. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papineni R.S., Rosenthal F.S. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10(2):105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 14.Dick E.C., Jennings L.C., Mink K.A., et al. Aerosol transmission of rhinovirus colds. J Infect Dis. 1987;156(3):442–448. doi: 10.1093/infdis/156.3.442. [DOI] [PubMed] [Google Scholar]
- 15.Duguid J.P. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. J Hyg (Lond). 1946;44(6):471–479. doi: 10.1017/s0022172400019288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dbouk T., Drikakis D. On coughing and airborne droplet transmission to humans. Phys Fluids (1994) 2020;32(5) doi: 10.1063/5.0011960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao B., Zhang Z., Li X. Numerical study of the transport of droplets or particles generated by respiratory system indoors. Build Environ. 2005;40(8):1032–1039. doi: 10.1016/j.buildenv.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alonso C., Goede D.P., Morrison R.B., et al. Evidence of infectivity of airborne porcine epidemic diarrhea virus and detection of airborne viral RNA at long distances from infected herds. Vet Res. 2014;45(1):73. doi: 10.1186/s13567-014-0073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao W.J., Wang M.L., Wei W., et al. Detection of SARS-CoV and RNA on aerosol samples from SARS-patients admitted to hospital. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2004;25(10):882–885. [PubMed] [Google Scholar]
- 20.Ge Z.Y., Yang L.M., Xia J.J., et al. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J Zhejiang Univ - Sci B. 2020;21(5):361–368. doi: 10.1631/jzus.B2010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fears A.C., Klimstra W.B., Duprex P., et al. Comparative dynamic aerosol efficiencies of three emergent coronaviruses and the unusual persistence of SARS-CoV-2 in aerosol suspensions. medRxiv. 2020 the preprint server for health sciences. [Google Scholar]
- 23.Liu Y., Ning Z., Chen Y., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 24.Chia P.Y., Coleman K.K., Tan Y.K., et al. Detection of air and surface contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in hospital rooms of infected patients. medRxiv. 2020 doi: 10.1101/2020.03.29.20046557. 2020.03.29.20046557. Now published in Nature Communications doi: 10.1038/s41467-020-16670-2. [DOI] [Google Scholar]
- 25.Ong S.W.X., Tan Y.K., Chia P.Y., et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santarpia J.L., Rivera D.N., Herrera V., et al. Aerosol and surface transmission potential of SARS-CoV-2. medRxiv. 2020 2020.03.23.20039446. [Google Scholar]
- 27.Xu P., Qian H., Miao T., et al. Transmission routes of covid-19 virus in the diamond princess cruise ship. medRxiv. 2020 2020.04.09.20059113. [Google Scholar]
- 28.Chin A.W.H., Chu J.T.S., Perera M.R.A., et al. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020;1(1):e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W., Du R.H., Li B., et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microb Infect. 2020;9(1):386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L., Li X., Chen H., et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343–348. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan J.F., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet (London, England) 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Y., Liu P., Shi X.L., et al. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020;69(6):1143–1144. doi: 10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- 33.Cruz CHB. Social distancing in Sao Paulo State: demonstrating the reduction in cases using time series analysis of deaths due to COVID-19. Rev Bras Epidemiol.23:e200056. [DOI] [PubMed]
- 34.Randolph H.E., Barreiro L.B. Herd immunity: understanding COVID-19. Immunity. 2020;52(5):737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorin J. 2020. Raging campus outbreaks send students home across the U.S. Bloomberg. [Google Scholar]
- 36.Lopez A.S., Hill M., Antezano J., et al. Transmission dynamics of COVID-19 outbreaks associated with child care facilities - Salt Lake city, Utah, April-July 2020. MMWR Morb. Mortality weekly Rep. 2020;69(37):1319–1323. doi: 10.15585/mmwr.mm6937e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuite A.R., Fisman D.N., Greer A.L. Mathematical modelling of COVID-19 transmission and mitigation strategies in the population of Ontario, Canada. Can Med Assoc J. 2020;192(19):E497–E505. doi: 10.1503/cmaj.200476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lonergan M., Chalmers J.D. Estimates of the ongoing need for social distancing and control measures post-"lockdown" from trajectories of COVID-19 cases and mortality. Eur Respir J. 2020;56(1):7. doi: 10.1183/13993003.01483-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moraes R.F.D. Determinants of physical distancing during the covid-19 epidemic in Brazil: effects from mandatory rules, numbers of cases and duration of rules. Ciência Saúde Coletiva. 2020;25:3393–3400. doi: 10.1590/1413-81232020259.21892020. [DOI] [PubMed] [Google Scholar]
- 40.Lins Filho P.C., Freitas J.L.D.M., Araujo M.M.S.D., et al. Predicting social distancing index during COVID-19 outbreak through online search engines trends. medRxiv. 2020 2020.05.28.20115816. [Google Scholar]
- 41.Orlowski E.J.W., Goldsmith D.J.A. Four months into the COVID-19 pandemic, Sweden's prized herd immunity is nowhere in sight. J R Soc Med. 2020;113(8):292–298. doi: 10.1177/0141076820945282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung F., Krieger V., Hufert F.T., Küpper J.H. Herd immunity or suppression strategy to combat COVID-19. Clin Hemorheol Microcirc. 2020;75:13–17. doi: 10.3233/CH-209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edgar Walters S.N. The Texas Tribune; 2020. Emma Platoff Texas hospitals are running out of drugs, beds, ventilators and even staff. [Google Scholar]
- 44.Brooks B. Reuters; 2020. Deaths, intubations swamp New Orleans doctors in coronavirus surge. [Google Scholar]
- 45.Glenza J.R., Ankita, Villarreal Alexandra. The Guardian; 2020. 'It's what was happening in Italy': the hospital at the center of New York's Covid-19 crisis. [Google Scholar]
- 46.Horowitz J. The New York Times; 2020. Italy's health care system groans under coronavirus — a warning to the World. [Google Scholar]
- 47.Nishiura H., Oshitani H., Kobayashi T., et al. medRxiv; 2020. Closed environments facilitate secondary transmission of coronavirus disease 2019 (COVID-19) [Google Scholar]
- 48.Shim E., Tariq A., Choi W., et al. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis. 2020;93:339–344. doi: 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leclerc Q.J., Fuller N.M., Knight L.E., et al. What settings have been linked to SARS-CoV-2 transmission clusters? Wellcome Open Res. 2020;5:83. doi: 10.12688/wellcomeopenres.15889.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bae S, Kim H, Jung TY, et al. Epidemiological characteristics of COVID-19 outbreak at fitness centers in Cheonan, Korea. J Kor Med Sci.35(31):e288. [DOI] [PMC free article] [PubMed]
- 51.James A, Eagle L, Phillips C, et al. High COVID-19 attack rate among attendees at events at a Church - Arkansas, March 2020. MMWR - Morbidity Mortality Weekly Rep.69(20):632-635. [DOI] [PubMed]
- 52.Prevention CfDCa . 2020. Coronavirus disease 2019 (COVID-19): personal and social activities.https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/personal-social-activities.html Available from: [Google Scholar]
- 53.Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res.25(1):32. [DOI] [PMC free article] [PubMed]
- 54.Dharmadhikari A.S., Mphahlele M., Stoltz A., et al. Surgical face masks worn by patients with multidrug-resistant tuberculosis: impact on infectivity of air on a hospital ward. Am J Respir Crit Care Med. 2012;185(10):1104–1109. doi: 10.1164/rccm.201107-1190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakayama DK. Surgical masks during the influenza pandemic of 1918-1920. Am Surg.86(6):557-559. [DOI] [PubMed]
- 56.Cowling B.J., Zhou Y., Ip D.K., et al. Face masks to prevent transmission of influenza virus: a systematic review. Epidemiol Infect. 2010;138(4):449–456. doi: 10.1017/S0950268809991658. [DOI] [PubMed] [Google Scholar]
- 57.MacIntyre C.R., Cauchemez S., Dwyer D.E., et al. Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis. 2009;15(2):233–241. doi: 10.3201/eid1502.081167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furukawa N., Brooks J., Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis J. 2020;26(7) doi: 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prevention CfDCa . 2020. Coronavirus disease 2019 (COVID-19): use of masks to help slow the spread of COVID-19. [Google Scholar]
- 61.Liu X, Zhang S. COVID-19: face masks and human-to-human transmission. Influenza Other Respir Virus.14(4):472-473. [DOI] [PMC free article] [PubMed]
- 62.Hendrix M.J., Walde C., Findley K., Trotman R. Absence of apparent transmission of SARS-CoV-2 from two stylists after exposure at a hair salon with a universal face covering policy - Springfield, Missouri. MMWR - Morbidity Mortality Weekly Rep. May 2020;69(28):930–932. doi: 10.15585/mmwr.mm6928e2. [DOI] [PubMed] [Google Scholar]
- 63.Qian G.Q., Yang N.B., Ding F., et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. Int J Med. 2020;113(7):474–481. doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi E.M., Chu D.K.W., Cheng P.K.C., et al. In-flight transmission of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(11) doi: 10.3201/eid2612.203910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khanh N.C., Thai P.Q., Quach H.L., et al. Transmission of severe acute respiratory syndrome coronavirus 2 during long flight. Emerg Infect Dis. 2020;26(11) doi: 10.3201/eid2612.203910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Speake H., Phillips A., Chong T., et al. Flight-associated transmission of severe acute respiratory syndrome coronavirus 2 corroborated by whole-genome sequencing. Emerg Infect Dis J. 2020;26(12) doi: 10.3201/eid2612.203910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esposito S., Principi N., Leung C.C., Migliori G.B. Universal use of face masks for success against COVID-19: evidence and implications for prevention policies. Eur Respir J. 2020;55(6):6. doi: 10.1183/13993003.01260-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fisher K.A., Tenforde M.W., Feldstein L.R., et al. Community and close contact exposures associated with COVID-19 among symptomatic adults >=18 Years in 11 outpatient health care facilities - United States, July 2020. MMWR Morb. Mortality weekly Rep. 2020;69(36):1258–1264. doi: 10.15585/mmwr.mm6936a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prevention CfDCa . US Department of Health and Human Services, CDC; Atlanta, GA: 2020. Coronavirus disease 2019 (COVID-19): recommendation for cloth face covers.https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html Available from: [Google Scholar]
- 70.Fisher K.A., Barile J.P., Guerin R.J., et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic - United States, April and may 2020. MMWR Morb. Mortality weekly Rep. 2020;69(28):933–937. doi: 10.15585/mmwr.mm6928e3. [DOI] [PubMed] [Google Scholar]
- 71.Lima MMS, Cavalcante FML, Macedo TS, et al.. Cloth face masks to prevent Covid-19 and other respiratory infections. Rev Latino-Am Enferm28:e3353. [DOI] [PMC free article] [PubMed]
- 72.Rodriguez-Palacios A., Cominelli F., Basson A.R., et al. Textile masks and surface covers-A spray simulation method and a "universal droplet reduction model" against respiratory pandemics. Front Med. 2020;7:260. doi: 10.3389/fmed.2020.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whiley H., Keerthirathne T.P., Nisar M.A., et al. Viral filtration efficiency of fabric masks compared with surgical and N95 masks. Pathogens. 2020;9(9) doi: 10.3390/pathogens9090762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neupane B.B., Mainali S., Sharma A., Giri B. Optical microscopic study of surface morphology and filtering efficiency of face masks. PeerJ. 2019;7 doi: 10.7717/peerj.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fischer E.P., Fischer M.C., Grass D., et al. Low-cost measurement of facemask efficacy for filtering expelled droplets during speech. Sci Adv. 2020 doi: 10.1126/sciadv.abd3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bartoszko J.J., Farooqi M.A.M., Alhazzani W., Loeb M. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses. 2020;14(4):365–373. doi: 10.1111/irv.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Umer F., Haji Z., Zafar K. Role of respirators in controlling the spread of novel coronavirus (COVID-19) amongst dental healthcare providers: a review. Int Endod J. 2020;53(8):1062–1067. doi: 10.1111/iej.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirschmann M.T., Hart A., Henckel J., et al. COVID-19 coronavirus: recommended personal protective equipment for the orthopaedic and trauma surgeon. Knee Surg Sports Traumatol Arthrosc. 2020;28(6):1690–1698. doi: 10.1007/s00167-020-06022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67(5):568–576. doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oranization W.H. 2020. Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update World health organization.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ [updated Sep 17 2020. Available from: [Google Scholar]
- 81.Sweden TPHAo More about COVID-19. 2020. https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/communicable-disease-control/covid-19-more-information/ [updated Sep 17 2020; cited 2020 Sep 17]. Available from:
- 82.Emergency Information from Swedish Authorities SCCA Restrictions and prohibitions. 2020. https://www.krisinformation.se/en/hazards-and-risks/disasters-and-incidents/2020/official-information-on-the-new-coronavirus/restriktioner-och-forbud [cited 2020 Sep 17]. Available from:
- 83.Ferguson DL N.M., Nedjati-Gilani G., Imai N., et al. Imperial College COVID-19 Response Team, 2020; 2020. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID- 19 mortality and healthcare demand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Departments and executive agencies EC. Coronavirus Response, Transport measures by country. 2020. https://ec.europa.eu/transport/coronavirus-response_en [updated Sep 17 2020. Available from:
- 85.Amér S., Molnar C., Tuutma M., et al. Almost two-thirds of the elderly with covid-19 surviving in nursing homes. Lakartidningen. 2020;117 [PubMed] [Google Scholar]