Abstract
The fidelity of chromosome segregation during mitosis is intimately linked to the function of kinetochores, which are large protein complexes assembled at sites of centromeric heterochromatin on mitotic chromosomes. These key “orchestrators” of mitosis physically connect chromosomes to spindle microtubules and transduce forces through these connections to congress chromosomes and silence the spindle assembly checkpoint. Kinetochore-microtubule attachments are highly regulated to ensure that incorrect attachments are not prematurely stabilized, but instead released and corrected. The kinase activity of the centromeric protein Aurora B is required for kinetochore-microtubule destabilization during mitosis, but how the kinase acts on outer kinetochore substrates to selectively destabilize immature and erroneous attachments remains debated. Here we review recent literature which sheds light on how Aurora B kinase is recruited to both centromeres and kinetochores and discuss possible mechanisms for how kinase interactions with substrates at distinct regions of mitotic chromosomes are regulated.
I. Introduction
Aurora B kinase regulates kinetochore-microtubule attachment stability in mitosis
Equal division of genetic material during mitosis requires that each sister chromatid of a mitotic chromosome stably attach to spindle microtubules emanating from each of the two opposite spindle poles. A complex network of proteins built on the centromere region of mitotic chromosomes, collectively called the kinetochore, mediates these attachments. Successful chromosome segregation also requires the precise regulation of kinetochore-microtubule attachment stability. In early mitosis, the mitotic spindle begins to form and establish its proper geometry at the same time that microtubules begin to dynamically probe for chromosomes, and as a result, erroneous kinetochore-microtubule attachments are likely to form (Figure 1) [1–6]. In order to prevent premature stabilization of kinetochore-microtubule attachments and to limit the accumulation of erroneous attachments, microtubule turnover at kinetochores is high during early mitosis; conversely, as mitosis progresses and chromosomes begin to bi-orient, kinetochore-microtubule turnover decreases and stably-bound microtubules accumulate at kinetochores [2, 6–10]. These stable attachments allow kinetochores to harness the forces generated from depolymerizing microtubule plus ends to power chromosome movements and to silence the spindle assembly checkpoint, which delays anaphase until all kinetochores are properly connected to microtubules [2, 6, 8, 11–13].
Figure 1. Kinetochore-microtubule attachments in mitosis.
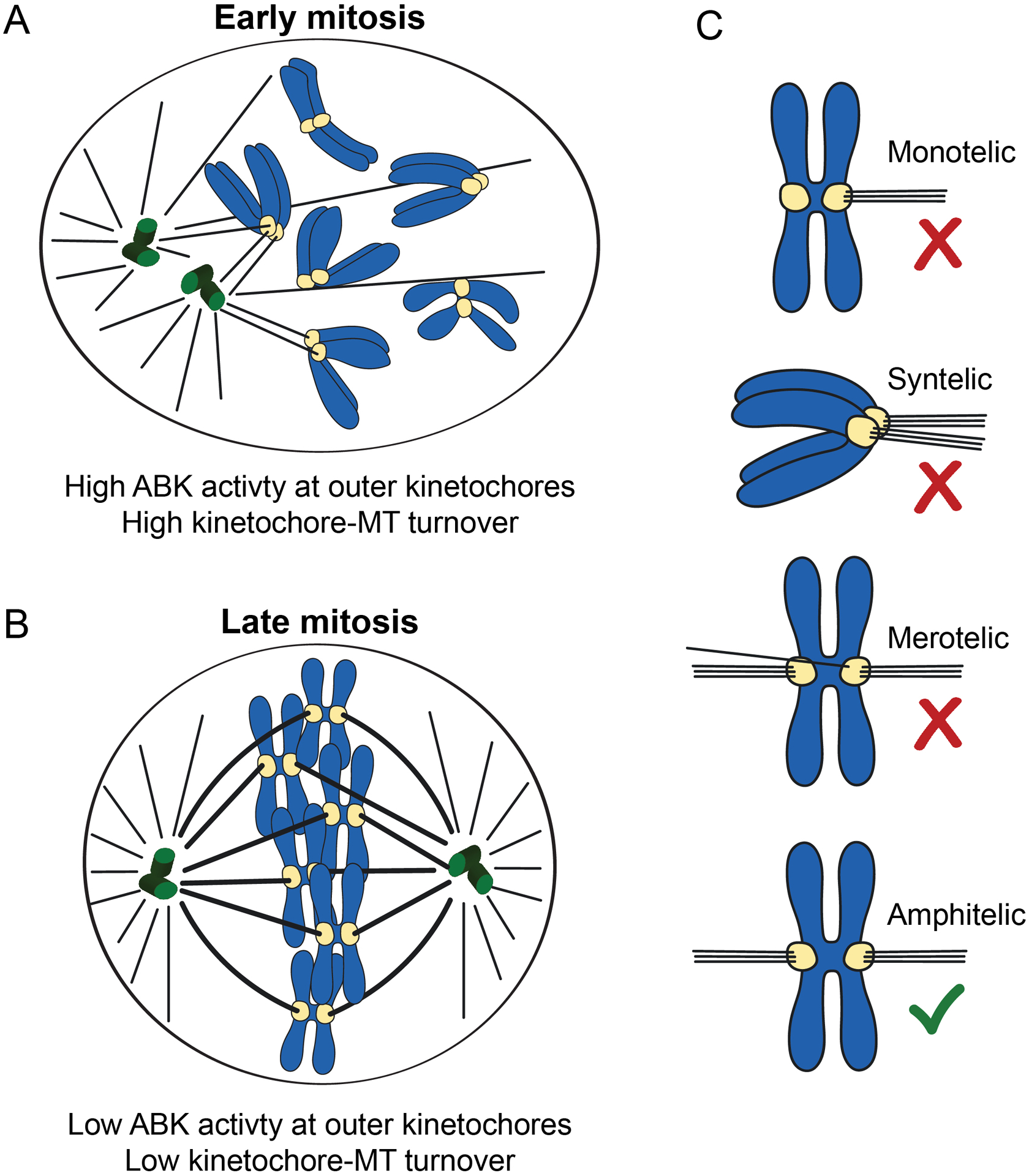
(A) In early mitosis, kinetochore-microtubule attachment errors are common, Aurora B kinase activity is high at outer kinetochores, and kinetochore-microtubule turnover is high to prevent premature stabilization of attachments. (B) In late mitosis, kinetochore-microtubule errors are infrequent, Aurora B kinase activity is low at outer kinetochores, and kinetochore-microtubule turnover is low to promote attachment stabilization. (C) Types of kinetochore-microtubule attachments are shown. Erroneous attachments include monotelic, syntelic, and merotelic attachments. Monotelic attachments occur when one kinetochore is attached to microtubules emanating from one spindle pole, and its sister kinetochore is unattached. Syntelic attachments occur when both sister kinetochores are attached to microtubules emanating from the same spindle pole. Merotelic attachments occur when one sister kinetochore is attached to microtubules emanating from one pole, and its sister is attached to microtubules emanating from both spindle poles. Correct, amphitelic attachments, in which one sister is attached to microtubules emanating from one spindle pole and its sister is attached to microtubules emanating from the opposite spindle pole, are also shown.
The Chromosomal Passenger Complex (CPC) is comprised of INCENP, Borealin, Survivin and Aurora B kinase, the enzymatic component of the complex which phosphorylates multiple substrates on mitotic chromosomes to ensure proper chromosome segregation [14–17]. One of the numerous functions of Aurora B kinase is to regulate kinetochore-microtubule attachment stability [14, 18–21]. For this purpose, Aurora B phosphorylates outer kinetochore-associated substrates, including Hec1 of the NDC80 complex, which directly links kinetochores to microtubules [8, 22, 23]. Phosphorylation of Hec1 decreases the affinity of NDC80 complexes for microtubules, and as a result, reduces kinetochore-microtubule attachment stability [24–27]. As mitosis progresses, Aurora B kinase-mediated phosphorylation of Hec1 and other outer kinetochore substrates decreases, resulting in increased stabilization of kinetochore-microtubule attachments, which in turn promotes chromosome congression and silencing of the spindle assembly checkpoint [12, 13, 24–27].
Current models for kinetochore-microtubule attachment regulation by Aurora B kinase
Although proper regulation of kinetochore-microtubule attachments requires that Aurora B phosphorylate substrates at outer kinetochores, the kinase itself, along with the rest of the CPC, resides prominently at inner centromeres during mitosis [28–32]. To explain how the centromere-localized kinase is capable of regulating the function of outer kinetochore proteins, researchers have proposed the “spatial positioning” model (Figure 2), which posits that activated Aurora B kinase emanates from the inner centromere as a diffusible gradient to phosphorylate its substrates [19, 33–38]. In this model, Aurora B is recruited to and activated at inner centromeres in early mitosis, prior to formation of kinetochore-microtubule attachments [31, 34–50]. Kinetochores in this case lack pulling forces from attached microtubules and are physically close to the inner centromere. As such, kinetochore substrates are situated within the reach of the kinase and are highly phosphorylated. As kinetochore-microtubule attachments are generated, microtubule-based pulling forces stretch kinetochores away from centromeres and outside the boundaries of the Aurora B gradient, which results in decreased phosphorylation of kinetochore substrates and subsequent stabilization of kinetochore-microtubule attachments [19, 33–38, 51]. In support of this model, Liu et al. [36] demonstrated that an ectopically targeted FRET sensor capable of detecting Aurora B kinase activity was phosphorylated when positioned at centromeres, but not at kinetochores, when kinetochores were properly bi-oriented. Additionally, ectopically targeting Aurora B to kinetochores using a Mis12-INCENP fusion protein destabilized kinetochore-microtubule attachments and delayed spindle assembly checkpoint silencing. This led the authors to conclude that stabilization of attachments in metaphase results from the spatial separation of outer kinetochore substrates from centromere-localized Aurora B kinase [36]. It is important to point out however, that other models describing Aurora B regulation of attachment stability (discussed below) predict that irreversibly targeting Aurora B to kinetochores in metaphase, when kinase activity at this region is known to be low, would lead to destabilization of attachments. Thus, the data presented by Liu et al., [36] do not necessarily rule out other mechanistic models for Aurora B regulation of kinetochore-microtubule attachment stability [36].
Figure 2. Spatial positioning model for Aurora B kinase-mediated regulation of kinetochore-microtubule attachment stability.
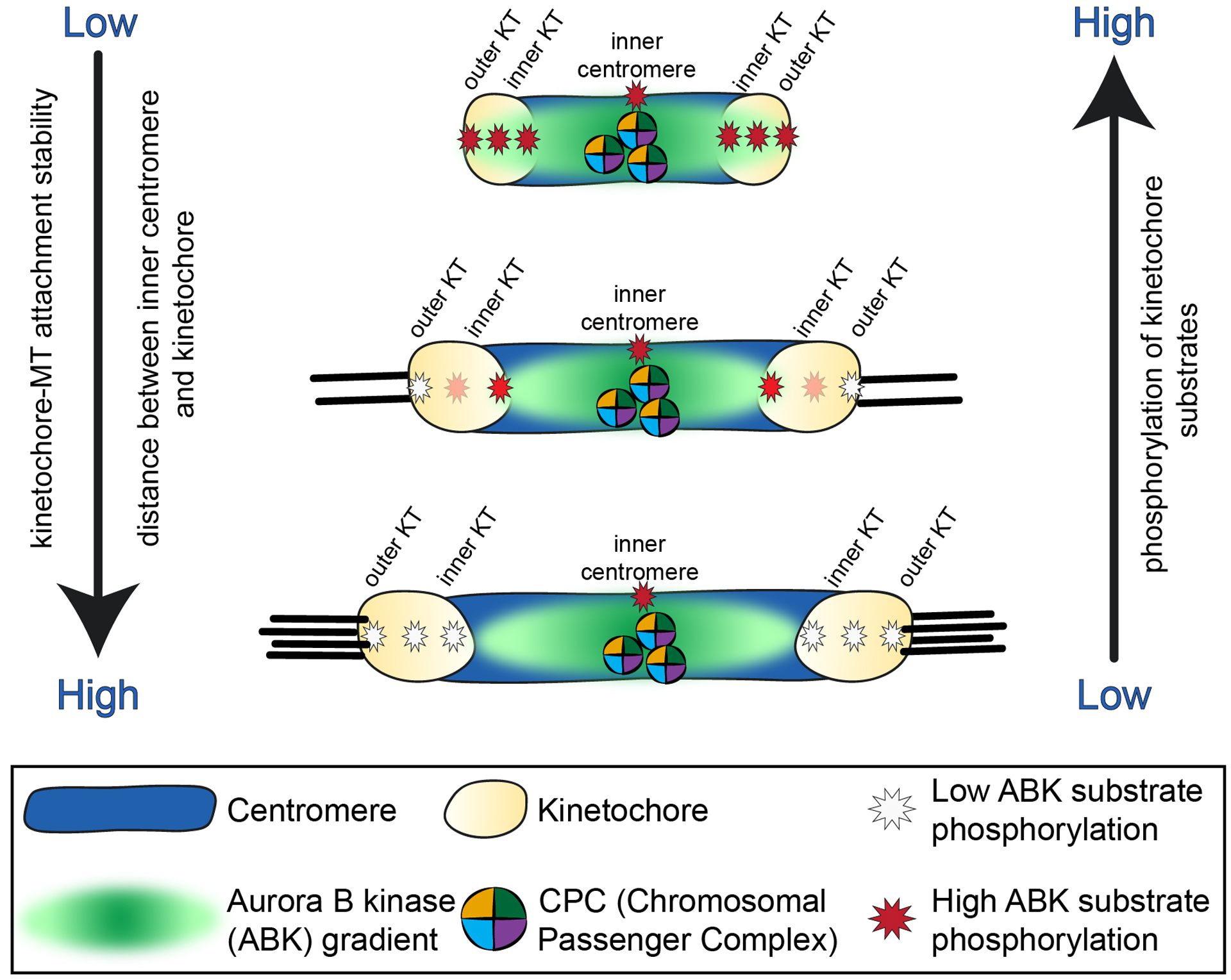
In this proposed mechanism Aurora B kinase is recruited to and activated at the inner centromere, and active kinase emanates as a diffusible gradient outward towards kinetochores. In early mitosis, when kinetochores lack stable microtubule-attachments, kinetochores are physically close to the inner centromere, and the kinase gradient reaches and phosphorylates kinetochore substrates, which promotes kinetochore-microtubule turnover. As stable attachments form and kinetochores experience pulling forces from the dynamics of attached microtubule plus ends, kinetochores are stretched away from the inner centromere region and out of the reach of the active kinase. Kinetochore substrate phosphorylation decreases, and attachments to microtubules are further stabilized.
The spatial positioning model described above is rooted in the idea that Aurora B kinase emanates from the inner centromere as a steep, diffusible gradient capable of differentially phosphorylating substrates within a short distance (~50–100 nm) [25, 26, 36, 37, 51–53]. The presence of such a fine-tuned gradient is debated, and the mechanisms for how the proposed gradient is established and maintained remain unknown [21, 38, 51, 52, 54, 55]. Similar to the spatial positioning model, the “dog leash” model accounts for differential activity of Aurora B kinase towards its substrates based on their distance from the centromere but does not rely on a diffusible gradient of the kinase. In this proposed mechanism, Aurora B’s “zone” of activity is restricted by its interaction with the CPC component INCENP, which contains a long single α-Helix (SAH) domain that may be capable of extending up to 80 nm to reach kinetochore substrates in early, but not late mitosis [21, 45, 47]. Consistent with this model, expression of a mutant version of chicken INCENP containing a shortened SAH domain in human cells resulted in decreased phosphorylation of outer kinetochore-, but not centromere-localized Aurora B substrates [45].
A third model to describe Aurora B regulation during mitosis suggests that spindle microtubules promote the activation of Aurora B kinase and facilitate phosphorylation of kinetochore substrates [31, 40–42, 44–50]. While the available data suggest a role for microtubules in activating Aurora B kinase, it seems unlikely that this is the major mechanism for regulating kinase activity at outer kinetochores in response to kinetochore-microtubule attachment, since the Hec1 tail domain and other kinetochore-associated Aurora B substrates remain highly phosphorylated when cells enter mitosis in the presence of the microtubule depolymerizing drug nocodazole [24, 37, 56].
A growing number of studies in both budding yeast and human cells have demonstrated that Aurora B kinase localizes not only to centromeres, but also to kinetochores, suggesting an alternative regulatory mechanism for controlling kinetochore-microtubule attachment stability, in which Aurora B kinase is recruited directly to kinetochores in early mitosis to phosphorylate its substrates and in turn, is evicted from kinetochores as stable attachments form (Figure 3) [21, 24, 43, 56–62]. In the sections below we discuss recent studies that shed light on the localization and functional properties of the CPC at both centromeres and kinetochores and how these new findings may lead to refinement of the current models for Aurora B kinase regulation of kinetochore-microtubule attachments during mitosis.
Figure 3. Direct recruitment model for Aurora B kinase-mediated regulation of kinetochore-microtubule attachment stability.
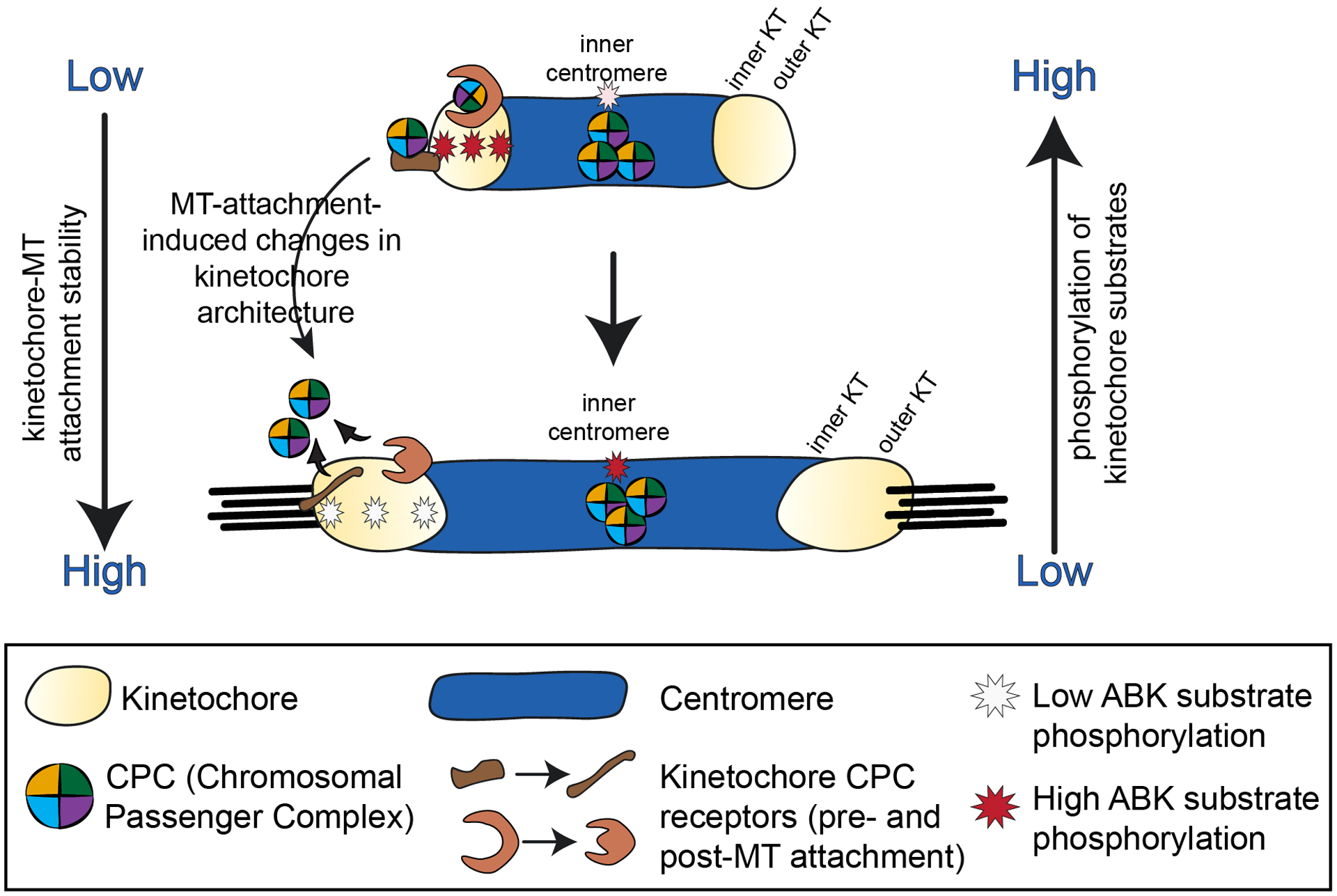
In this proposed mechanism, Aurora B kinase is directly recruited to both centromeres and kinetochores. In early mitosis, the kinase and its CPC cofactors are recruited to the inner centromere (via the phospho-histone marks pH2A and pH3, see Figure 4) and to kinetochores through specific kinetochore receptors that likely reside in both the inner and outer kinetochore. As mitosis progresses and tension-generating kinetochore-microtubule attachments form, changes in kinetochore architecture (and in specific CPC receptor protein structure) lead to loss of the kinetochore-associated Aurora B kinase/CPC binding sites and subsequent eviction of Aurora B and the CPC from kinetochores. Kinetochore substrate phosphorylation is reduced, and kinetochore-microtubule attachments are further stabilized. In this cartoon, while the CPC accumulates at inner centromeres in early mitosis, its activity increases in this region during later mitosis (see text for details). Activity on only one kinetochore of each pair is shown for clarity.
II. Aurora B localization and activity at centromeres
Aurora B and the CPC are recruited to centromeres via phosphorylated histones
Aurora B kinase and its CPC cofactors are recruited to the centromere region of mitotic chromosomes just prior to nuclear envelope breakdown [14, 40, 63], and this recruitment depends on phosphorylation of histones H3 and H2A (Figure 4) [64]. A significant body of work has demonstrated that Haspin kinase phosphorylates histone H3 at Thr3 (pH3-T3), which recruits the CPC component Survivin [64–67]. The BIR domain of Survivin directly interacts with pH3-T3 [28, 56, 66, 68, 69], while a separate helical domain of Survivin forms a three-helix bundle with Borealin and INCENP, which is connected to Aurora B through the C-terminal IN-Box of INCENP [32, 69]. It has also been demonstrated that Bub1 phosphorylates histone H2A at Thr120 which recruits the Shugoshin proteins Sgo1 and Sgo2 to centromeres, which in turn recruit the CPC [64, 70–73]. In metazoans, a number of studies have suggested that this linkage is mediated through Borealin [64, 70–73]. Antibodies to both pH3-T3 and pH2A-T120 localize to centromeres, and loss of either phospho-mark reduces centromeric CPC localization, which has led to a model in which Aurora B kinase and the CPC are recruited to regions of centromeric chromatin where the two marks overlap [64, 68, 72, 74]. This concentrated pool of centromere-localized Aurora B kinase is proposed to phosphorylate both centromere and kinetochore substrates to ensure proper chromosome congression and segregation [34, 36, 37, 64, 66, 71, 75, 76].
Figure 4. Recruitment pathways for centromere-localized Aurora B kinase.
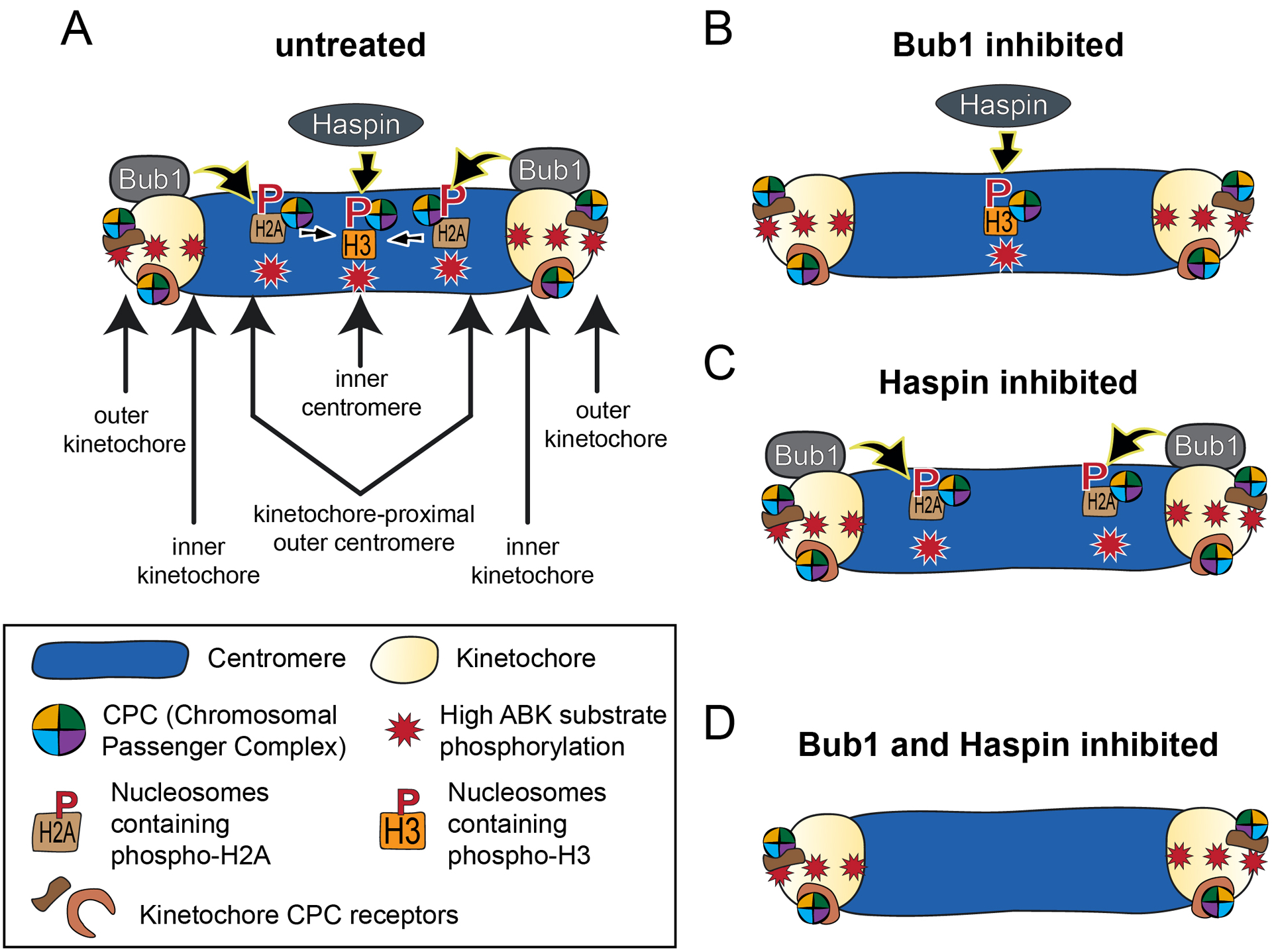
(A) Phosphorylated histone H3 recruits the CPC to inner centromeres through direct binding of Survivin. Phosphorylated histone H2A recruits the CPC to kinetochore-proximal outer centromere regions through Sgo1-dependent binding of the CPC, likely through direct interaction between Sgo1 and Borealin. Once recruited to the outer centromere, this population of the kinase is possibly translocated to the inner centromere (indicated by inward-directed arrows). (B) In Bub1 inhibited cells, phosphorylated histone H3 recruits the CPC to inner centromeres, and this activity is sufficient to support error-free chromosome segregation. (C) In Haspin inhibited cells, phosphorylated histone H2A recruits the CPC to kinetochore-proximal outer centromere regions. In this case, the kinase remains localized to these regions and is not relocated to inner centromeres. Similar to the scenario in (B), kinase recruitment to this region is sufficient to support error-free chromosome segregation. (D) In Bub1- and Haspin-inhibited cells, Aurora B fails to localize to centromeres, and chromosome segregation is impaired. In all cases (A-D), kinetochore-associated Aurora B kinase activity (in early mitosis) remains high, regardless of centromeric accumulation of the kinase. This model is based on data from references [56, 61, 62]
pH2A-T120 and pH3-T3 recruit the CPC to distinct locations within the centromere
In a series of recently published studies, three groups carried out experiments to directly test the above model for CPC localization; that is, to determine if CPC recruitment to inner centromeres requires the overlap of pH3-T3 and pH2A-T120 in human cells [56, 61, 62]. Using antibodies to both phospho-marks, the authors of all three studies reported that pH2A-T120 and pH3-T3 did not show significant overlap in cells; instead, immunostaining revealed that pH3-T3 localized distinctly as a single focus at the inner centromere, while pH2A-T120 localized as a pair of dots flanking the inner centromere [56, 61, 62]. This is consistent with previous data suggesting distinct localization patterns of the two histone marks [21, 58, 64, 66, 71, 77]. Line-scans and two-color localization experiments further revealed that pH2A is localized inside of the inner kinetochore protein CENP-C, on the order of ~100 nm, in both prometaphase and metaphase cells, which places this mark within the “kinetochore-proximal outer centromere” region, distinct from the pH3-T3-marked chromatin at the inner centromere [56, 61, 62]. Importantly, all three studies reported that each histone mark individually was sufficient to recruit Aurora B kinase and the CPC. Each group analyzed U2OS cells containing an ectopic Lac operator (LacO) array stably integrated in the short arm of chromosome one that were expressing fusions of either LacI-Haspin or LacI-Bub1 [78]. In cells expressing LacI-Bub1, the chromatin surrounding the LacO array was positive for pH2A-T120 but not pH3-T3, and the single phosphorylation mark (pH2A-T120) was sufficient for recruitment of Aurora B kinase in a manner dependent on Sgo1 [56, 61, 62]. Similarly, when Haspin was directed to the ectopic locus through expression of a LacI fusion protein, the local chromatin was positive for pH3-T3 but not pH2A-T120, and this single modification was also sufficient to recruit Aurora B kinase and its CPC partners [56, 61, 62]. Moreover, each histone mark was sufficient to recruit a population of the CPC to spatially distinct regions within the centromere region of mitotic chromosomes in human cells (Figure 4). While depletion of Haspin or inhibition of its kinase activity resulted in loss of both the pH3-T3 mark and accumulation of Aurora B at inner centromeres, a population of Aurora B remained localized to the kinetochore-proximal outer centromere, coincident with pH2A-T120 [56, 58, 61, 62]. Conversely, inhibition of Bub1 kinase activity resulted in loss of the pH2A-T120 mark, but Aurora B kinase and components of the CPC remained localized at the inner centromere coincident with pH3-T3 [56, 61, 62, 74]. Inhibition of both Bub1 and Haspin kinase activities; however, resulted in no detectable Aurora B and CPC components at centromeres [49, 56, 61, 62, 74].
In the studies described above, Aurora B kinase localized prominently to inner centromeres as a single focus, but was not clearly discernable at pH2A “marked” kinetochore-proximal outer centromere regions in control cells [56, 61, 62]. This population of the CPC became readily detectable however, when phosphorylation of H3-T3 was prevented through Haspin knockout or Haspin inhibition [56, 58, 61, 62], suggesting crosstalk between the two centromere-localized populations of the CPC. These results point to the possibility of a multifaceted loading process whereby a pool of Aurora B kinase is loaded directly to the inner centromere binding sites provided by pH3, and a second population of the complex is initially recruited to the kinetochore-proximal outer centromere by pH2A/Sgo1 and subsequently relocated to the inner centromere region. This mechanism is similar to what has been suggested for Sgo1, which first loads to kinetochores in early mitosis, which is required for its subsequent relocalization to the inner centromere, where it functions to protect cohesion and prevent premature sister chromatin separation [38, 73, 77, 79]. Furthermore, authors from the recent Liang et al. [56] study suggest that relocalization of Aurora B kinase from the pH2A-T120 binding sites to the inner centromere pH3-T3 binding sites in metaphase may be required to silence the spindle assembly checkpoint in response to kinetochore-microtubule attachment. The authors reported that experimentally-induced retention of Aurora B and the CPC at the kinetochore-proximal outer centromere in metaphase cells resulted in a small increase (by ~20%) in Aurora B kinase-mediated phosphorylation of the kinetochore scaffolding protein KNL1 which led to sustained checkpoint signaling [56]. In sum, three recent studies report the identification of discrete populations of Aurora B kinase within the centromere region that are recruited by distinct histone modifications. These studies suggest that the different populations of the CPC functionally interact and cooperatively contribute to the robust accumulation of Aurora B kinase at the inner centromere of mitotic chromosomes.
A role for centromere-localized Aurora B kinase in chromosome segregation
In light of the finding that each of the two histone marks recruits a distinct population of Aurora B and the CPC to centromeres, the authors of the studies described above [56, 61, 62] tested if either population is required for Aurora B kinase activity at kinetochores or for proper chromosome segregation. The three studies reported that inhibition of either pathway alone did not result in chromosome segregation errors or reduced phosphorylation of kinetochore Aurora B kinase substrates [56, 61, 62]. However, in cells inhibited for both Bub1 and Haspin kinase activities, chromosome segregation was compromised, although it was noted that the defects were less severe than those observed in cells inhibited for Aurora B kinase itself [56, 62]. Thus, the inner centromere and kinetochore-proximal outer centromere populations of Aurora B likely have redundant roles in ensuring proper chromosome segregation. Strikingly, in cells inhibited for both Bub1 and Haspin kinase activities, which resulted in a complete loss of centromere-localized CPC, Aurora B kinase localization remained high at kinetochores, and kinetochore substrate phosphorylation by Aurora B kinase was not largely reduced when compared to control cells (Figure 4) [56, 61, 62]. As such, the chromosome segregation defects resulting from loss of centromere-localized Aurora B could not be attributed to loss of phosphorylation of kinetochore substrates [56, 61, 62]. Importantly, these results provide evidence that centromere accumulation of the Aurora B kinase is not strictly coupled to Aurora B activity at kinetochores. This idea is consistent with a number of previous studies, the first of which demonstrated that centromere-localized Aurora B is not required for the regulation of kinetochore-microtubule attachments in chicken DT40 cells [80]. Here, cells depleted of endogenous Survivin and expressing a mutant version of Survivin defective for centromere localization completed mitosis normally with no detectable chromosome segregation defects [80]. In addition, kinetochore-microtubule attachment defects observed in HeLa cells depleted of the large kinetochore-associated scaffolding protein KNL1, which resulted in loss of kinetochore-associated Aurora B kinase activity, could not be rescued by ectopic targeting of the CPC to centromeres [57]. Finally, several studies have suggested that centromere accumulation of the CPC is uncoupled from kinetochore-associated Aurora kinase activity in budding yeast. Campbell and Desai [43] reported that budding yeast cells expressing INCENP/Sli15 mutants that fail to localize to centromeres exhibited normal chromosome bi-orientation and Aurora/Ipl1 kinase-mediated error correction. Recent studies have further demonstrated that the CPC is recruited directly to kinetochores in budding yeast, and this population is sufficient for Aurora kinase/Ipl1 activity at kinetochores and error-free chromosome segregation in the absence of centromere-localized CPC [59, 60].
An obvious question that emerges from the recent studies in human cells [56, 62] is what causes the observed chromosome segregation errors in the absence of centromere-localized Aurora B if the kinase is still able to phosphorylate kinetochore-associated substrates normally? A plausible explanation is that centromere-localized Aurora B phosphorylates centromere-localized substrates to promote proper chromosome segregation. Previous studies have demonstrated that Aurora B kinase regulates the activity and localization of MCAK, a centromere-localized kinesin-13 motor that promotes microtubule depolymerization, an activity that is implicated in correcting erroneous kinetochore-microtubule attachments [3, 9, 75, 76, 81–83]. Interestingly, the centromere localization of MCAK is perturbed in cells that are depleted of Haspin or inhibited for Bub1 kinase activity [62, 66, 67, 84, 85], consistent with the notion that alterations in MCAK activity and/or localization might contribute to the chromosome segregation errors observed in cells lacking centromere-localized Aurora B kinase. Further investigation into the role of MCAK and other centromere-localized Aurora B kinase substrates is needed to resolve this issue. It is interesting to note that while antibodies to CPC components localize prominently to the inner centromere in early mitosis, antibodies to phosphorylated, active Aurora B (pT232) and phosphorylated, active INCENP (pS893/pS894) show minimal inner centromere localization in early mitosis, but levels significantly increase as mitosis progresses [24, 61]. Consistent with this idea, Sgo1/2-PP2A antagonizes Aurora B activity at the centromere in early mitosis, which may explain this change in activity as mitosis progresses [86]. These results suggest that centromere substrates of Aurora B kinase that contribute to proper chromosome segregation may be phosphorylated and perhaps activated in late mitosis rather than in early mitosis.
Finally, it is important to point out that recent studies demonstrating that centromere-localized CPC is not explicitly required for phosphorylation of kinetochore substrates do not rule out the possibility that the centromere- and kinetochore-localized pools of the CPC may exhibit cross-talk and impact each others’ localization or activity. In fact, studies have demonstrated that while delocalization of CPC from centromeres did not result in decreased activity of Aurora B at kinetochores in human cells and in M-phase Xenopus egg extracts, the regulation of kinase activity in response to kinetochore-microtubule attachment was compromised [27, 65, 87]. Why this is the case is not clear, but in the future it will be important to resolve how the centromere pool of the CPC contributes to proper regulation of Aurora B kinase substrate phosphorylation at kinetochores in response to microtubule attachment.
III. Aurora B localization and activity at kinetochores
Aurora B kinase localizes to kinetochores
As previously mentioned, in addition to its centromere localization, Aurora B has also been detected at the kinetochore. Antibodies directed to active, phosphorylated Thr232, which resides in the T-loop of the kinase domain of Aurora B and is required for full activity of the kinase, recognize kinetochores in early mitosis, centromeres in late prometaphase and metaphase, and to the spindle midzone in anaphase [24, 54, 88–90]. A similar localization pattern was observed for phosphorylated Aurora B Ser331, a site whose phosphorylation is required for optimal Aurora B activity [91]. Furthermore, antibodies to phosphorylated, active INCENP (pS893/pS894) recognize both kinetochores and centromeres in early mitosis, and this localization shifts primarily to centromeres in late prometaphase and metaphase and the spindle midzone in anaphase [61]. Together, these studies raise the possibility that a population of Aurora B kinase, and likely the entire CPC, is recruited directly to kinetochores in early mitosis where it phosphorylates its kinetochore substrates to promote kinetochore-microtubule turnover. In such a model, as mitosis progresses and kinetochores accumulate bound microtubules, Aurora B is evicted from kinetochores, resulting in decreased kinetochore substrate phosphorylation, stabilization of attachments, and silencing of the spindle assembly checkpoint (Figure 3) [12, 13, 35, 61, 92–94].
How is Aurora B kinase recruited to the kinetochore to phosphorylate kinetochore substrates?
A major task that remains to be tackled is identifying the kinetochore binding sites for the CPC. In budding yeast, two research groups have made considerable headway on this front by demonstrating that inner kinetochore COMA (Ctf19/Okp1/Mcm21/Ame1) complex recruits the CPC through a direct interaction between INCENP/Sli15 and Ctf19 [59, 60]. Importantly, this kinetochore-associated population is sufficient to support Ipl1/Aurora kinase activity and normal chromosome segregation in the absence of centromere-localized CPC [59, 60]. In metazoan cells, however; the kinetochore binding sites for Aurora B and the CPC remain unknown. In a recent study discussed above [61], authors approximated the location of Aurora B kinase in early mitotic cells to ~22 nm outside of the inner kinetochore protein CENP-C, which places it ~8 nm inside of the N-terminus of the outer kinetochore protein Hec1. Many kinetochore proteins localize in this region of the kinetochore, making it difficult to predict specific binding sites. A previous study reported that Aurora B kinase localization to kinetochores is dependent on the kinetochore protein KNL1 [57], and Broad et al. [61] demonstrated that Aurora B localization is at least partially dependent on Bub1. Moreover, a recent study found that KNL1 undergoes significant conformational changes upon kinetochore-microtubule attachment [92]. Together, these findings make it tempting to speculate that KNL1 may directly or indirectly provide binding sites for CPC components in early mitosis. As mitosis progresses and kinetochore-microtubule attachments accumulate, KNL1 may undergo conformational changes that occlude these sites, leading to eviction of Aurora B kinase and its CPC cofactors (Figure 2). This speculative model remains to be tested.
Importantly, the available data suggest that Aurora B kinase may be recruited to multiple locations within the kinetochore to facilitate different functions. Based on the recent studies described above, we predict that Aurora B is recruited directly to outer kinetochores to phosphorylate outer kinetochore substrates involved in kinetochore-microtubule attachment regulation [21, 24, 57, 61, 89]. However, as mentioned above, in budding yeast, the CPC is recruited to the inner kinetochore COMA complex, whose homolog in humans is the CENP-O/P/Q/U complex, a component of the Constitutive Centromere Associated Network (CCAN) [59, 60]. Indeed, Aurora B kinase has substrates at the inner kinetochore that are important for mitotic progression. For example, the Mis12 complex component Dsn1 is phosphorylated by Aurora B in early mitosis to promote kinetochore assembly by facilitating an interaction between the inner-kinetochore protein CENP-C and the Mis12 complex [95–104]. A recent study from Bonner et al. [102] demonstrated that delocalization of the CPC from centromeres in M-phase Xenopus egg extracts resulted in decreased Dsn1 phosphorylation and consequently, failure to assemble the outer kinetochore. The authors of this study found that the SAH domain of INCENP was required, in a microtubule-independent manner, for Aurora B kinase-mediated phosphorylation of Dsn1 and kinetochore assembly. Ectopic targeting of INCENP lacking its central SAH domain to the inner kinetochore protein Nsl1 and subsequent recruitment of the CPC to this region rescued both Dsn1 phosphorylation and kinetochore assembly [102]. The authors concluded that the INCENP SAH domain is critical for localizing the CPC to the inner kinetochore, in close proximity to the Mis12 complex so that Aurora B is able to efficiently phosphorylate Dsn1 to promote outer kinetochore assembly [102]. These results also bring to light the idea that multiple kinetochore functions rely on kinetochore-associated Aurora B kinase activity, and the CPC may be recruited to different locations within the kinetochore to support these activities.
IV. Closing comments
A growing number of studies has demonstrated that centromere-localized Aurora B kinase is not explicitly required for Aurora B kinase activity at kinetochores. Based on these studies and data from numerous model systems, the current model for Aurora B kinase-mediated regulation of kinetochore-microtubule attachment stability (i.e. the “spatial positioning model”) should be re-evaluated. A growing number of studies have mapped Aurora B kinase to kinetochores, and ectopically targeting the CPC to kinetochores in multiple cell types rescues loss of the centromere-localized population. In budding yeast, at least one kinetochore binding site for the CPC has been identified, which suggests that Aurora/Ipl1 kinase localizes to kinetochores to specifically phosphorylate kinetochore substrates. This straightforward mechanism for CPC function at budding yeast kinetochores, in which the kinase is recruited to the region of mitotic chromosomes where its substrates are located, is likely utilized in metazoan cells as well. The next major challenge is to identify the binding site, or more likely, binding sites, for the CPC at metazoan kinetochores.
Acknowledgements
The authors thank members of the DeLuca lab for helpful discussions.
Funding
Work in the DeLuca lab related to the topic of the article is funded by grant R35GM130365 from the National Institutes of Health (NIH).
Footnotes
Declaration of Interests
The authors declare that there are no competing interests regarding this manuscript.
References
- 1.Musacchio A, Desai A. A Molecular View of Kinetochore Assembly and Function. Biology. 2017;6(4):5. doi: 10.3390/biology6010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cimini D, Wan X, Hirel CB, Salmon ED. Aurora Kinase Promotes Turnover of Kinetochore Microtubules to Reduce Chromosome Segregation Errors. Current Biology. 2006;16(17):1711–8. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Bakhoum SF, Genovese G, Compton DA. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr Biol. 2009;19(22):1937–42. Epub 2009/11/03. doi: 10.1016/j.cub.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santaguida S, Musacchio A. The life and miracles of kinetochores. The EMBO Journal. 2009;28(17):2511–31. doi: 10.1038/emboj.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicklas RB. How Cells Get the Right Chromosomes. Science. 1997;275(5300):632–7. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- 6.Zhai Y Kinetochore microtubule dynamics and the metaphase-anaphase transition. Journal of Cell Biology. 1995;131(3):721–34. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salmon ED, Cimini D, Cameron LA, Deluca JG. Merotelic kinetochores in mammalian tissue cells. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360(1455):553–68. doi: 10.1098/rstb.2004.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127(5):969–82. Epub 2006/11/30. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 9.Bakhoum SF, Thompson SL, Manning AL, Compton DA. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat Cell Biol. 2009;11(1):27–35. Epub 2008/12/09. doi: 10.1038/ncb1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godek KM, Kabeche L, Compton DA. Regulation of kinetochore–microtubule attachments through homeostatic control during mitosis. Nature Reviews: Molecular Cell Biology. 2014;16(1):57–64. doi: 10.1038/nrm3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etemad B, Kops GJ. Attachment issues: kinetochore transformations and spindle checkpoint silencing. Current Opinion Cell Biology. 2016;39:101–8. doi: 10.1016/j.ceb.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Tauchman EC, Boehm FJ, Deluca JG. Stable kinetochore–microtubule attachment is sufficient to silence the spindle assembly checkpoint in human cells. Nature Communications. 2015;6(1):10036. doi: 10.1038/ncomms10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etemad B, Kuijt TEF, Kops GJPL. Kinetochore–microtubule attachment is sufficient to satisfy the human spindle assembly checkpoint. Nature Communications. 2015;6(1):8987. doi: 10.1038/ncomms9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmena M, Wheelock M, Funabiki H, Earnshaw WC. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nature Reviews Molecular Cell Biology. 2012;13(12):789–803. doi: 10.1038/nrm3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol. 2001;153(4):865–80. Epub 2001/05/16. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews PD, Knatko E, Moore WJ, Swedlow JR. Mitotic mechanics: the auroras come into view. Curr Opin Cell Biol. 2003;15(6):672–83. Epub 2003/12/04. doi: 10.1016/j.ceb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Vader G, Medema RH, Lens SM. The chromosomal passenger complex: guiding Aurora-B through mitosis. J Cell Biol. 2006;173(6):833–7. Epub 2006/06/14. doi: 10.1083/jcb.200604032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biggins S, Severin FF, Bhalla N, Sassoon I, Hyman AA, Murray AW. The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes Dev. 1999;13(5):532–44. Epub 1999/03/11. doi: 10.1101/gad.13.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark MJR, Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora Kinase-INCENP) Complex Promotes Chromosome Bi-orientation by Altering Kinetochore-Spindle Pole Connections. Cell. 2002;108(3):317–29. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 20.Van Der Horst A, Lens SMA. Cell division: control of the chromosomal passenger complex in time and space. Chromosoma. 2014;123(1–2):25–42. doi: 10.1007/s00412-013-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krenn V, Musacchio A. The Aurora B Kinase in Chromosome Bi-Orientation and Spindle Checkpoint Signaling. Front Oncol. 2015;5:225 Epub 2015/11/04. doi: 10.3389/fonc.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The Conserved KMN Network Constitutes the Core Microtubule-Binding Site of the Kinetochore. Cell. 2006;127(5):983–97. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 23.DeLuca JG, Musacchio A. Structural organization of the kinetochore-microtubule interface. Curr Opin Cell Biol. 2012;24(1):48–56. Epub 2011/12/14. doi: 10.1016/j.ceb.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeLuca KF, Lens SM, DeLuca JG. Temporal changes in Hec1 phosphorylation control kinetochore-microtubule attachment stability during mitosis. J Cell Sci. 2011;124(Pt 4):622–34. Epub 2011/01/27. doi: 10.1242/jcs.072629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaytsev AV, Sundin LJ, DeLuca KF, Grishchuk EL, DeLuca JG. Accurate phosphoregulation of kinetochore-microtubule affinity requires unconstrained molecular interactions. J Cell Biol. 2014;206(1):45–59. Epub 2014/07/02. doi: 10.1083/jcb.201312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaytsev AV, Grishchuk EL. Basic mechanism for biorientation of mitotic chromosomes is provided by the kinetochore geometry and indiscriminate turnover of kinetochore microtubules. Mol Biol Cell. 2015;26(22):3985–98. Epub 2015/10/02. doi: 10.1091/mbc.E15-06-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoo TY, Choi JM, Conway W, Yu CH, Pappu RV, Needleman DJ. Measuring NDC80 binding reveals the molecular basis of tension-dependent kinetochore-microtubule attachments. Elife. 2018;7 Epub 2018/07/26. doi: 10.7554/eLife.36392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooke CA, Heck MM, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987;105(5):2053–67. Epub 1987/11/01. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, Nigg EA, Gerloff DL, Earnshaw WC. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol. 2004;166(2):179–91. Epub 2004/07/14. doi: 10.1083/jcb.200404001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uren AG, Wong L, Pakusch M, Fowler KJ, Burrows FJ, Vaux DL, Choo KH. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol. 2000;10(21):1319–28. Epub 2000/11/21. doi: 10.1016/s0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- 31.Wheatley SP, Kandels-Lewis SE, Adams RR, Ainsztein AM, Earnshaw WC. INCENP binds directly to tubulin and requires dynamic microtubules to target to the cleavage furrow. Exp Cell Res. 2001;262(2):122–7. Epub 2001/01/05. doi: 10.1006/excr.2000.5088. [DOI] [PubMed] [Google Scholar]
- 32.Adams RR, Wheatley SP, Gouldsworthy AM, Kandels-Lewis SE, Carmena M, Smythe C, Gerloff DL, Earnshaw WC. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr Biol. 2000;10(17):1075–8. Epub 2000/09/21. doi: 10.1016/s0960-9822(00)00673-4. [DOI] [PubMed] [Google Scholar]
- 33.Biggins S, Murray AW. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 2001;15(23):3118–29. Epub 2001/12/04. doi: 10.1101/gad.934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly AE, Sampath SC, Maniar TA, Woo EM, Chait BT, Funabiki H. Chromosomal Enrichment and Activation of the Aurora B Pathway Are Coupled to Spatially Regulate Spindle Assembly. Developmental Cell. 2007;12(1):31–43. doi: 10.1016/j.devcel.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchida KSK, Takagaki K, Kumada K, Hirayama Y, Noda T, Hirota T. Kinetochore stretching inactivates the spindle assembly checkpoint. The Journal of Cell Biology. 2009;184(3):383–90. doi: 10.1083/jcb.200811028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323(5919):1350–3. Epub 2009/01/20. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welburn JP, Vleugel M, Liu D, Yates JR 3rd, Lampson MA, Fukagawa T, Cheeseman IM. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell. 2010;38(3):383–92. Epub 2010/05/18. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang E, Ballister ER, Lampson MA. Aurora B dynamics at centromeres create a diffusion-based phosphorylation gradient. J Cell Biol. 2011;194(4):539–49. Epub 2011/08/17. doi: 10.1083/jcb.201103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ainsztein AM, Kandels-Lewis SE, Mackay AM, Earnshaw WC. INCENP Centromere and Spindle Targeting: Identification of Essential Conserved Motifs and Involvement of Heterochromatin Protein HP1 1998;143(7):1763–74. doi: 10.1083/jcb.143.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beardmore VA. Survivin dynamics increases at centromeres during G2/M phase transition and is regulated by microtubule-attachment and Aurora B kinase activity 2004;117(18):4033–42. doi: 10.1242/jcs.01242. [DOI] [PubMed] [Google Scholar]
- 41.Sandall S, Severin F, McLeod IX, Yates JR, Oegema K, Hyman A, Desai A. A Bir1-Sli15 Complex Connects Centromeres to Microtubules and Is Required to Sense Kinetochore Tension 2006;127(6):1179–91. doi: 10.1016/j.cell.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosasco-Nitcher SE, Lan W, Khorasanizadeh S, Stukenberg PT. Centromeric Aurora-B Activation Requires TD-60, Microtubules, and Substrate Priming Phosphorylation. Science. 2008;319(5862):469–72. doi: 10.1126/science.1148980. [DOI] [PubMed] [Google Scholar]
- 43.Campbell CS, Desai A. Tension sensing by Aurora B kinase is independent of survivin-based centromere localization. Nature. 2013;497(7447):118–21. doi: 10.1038/nature12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banerjee B, Kestner CA, Stukenberg PT. EB1 enables spindle microtubules to regulate centromeric recruitment of Aurora B. Journal of Cell Biology. 2014;204(6):947–63. doi: 10.1083/jcb.201307119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samejima K, Platani M, Wolny M, Ogawa H, Vargiu G, Knight PJ, Peckham M, Earnshaw WC. The Inner Centromere Protein (INCENP) Coil Is a Single alpha-Helix (SAH) Domain That Binds Directly to Microtubules and Is Important for Chromosome Passenger Complex (CPC) Localization and Function in Mitosis. J Biol Chem. 2015;290(35):21460–72. Epub 2015/07/16. doi: 10.1074/jbc.M115.645317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krupina K, Kleiss C, Metzger T, Fournane S, Schmucker S, Hofmann K, Fischer B, Paul N, Porter IM, Raffelsberger W, Poch O, Swedlow JR, Brino L, Sumara I. Ubiquitin Receptor Protein UBASH3B Drives Aurora B Recruitment to Mitotic Microtubules. Dev Cell. 2016;36(1):63–78. Epub 2016/01/15. doi: 10.1016/j.devcel.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheelock MS, Wynne DJ, Tseng BS, Funabiki H. Dual recognition of chromatin and microtubules by INCENP is important for mitotic progression. J Cell Biol. 2017;216(4):925–41. Epub 2017/03/21. doi: 10.1083/jcb.201609061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fink S, Turnbull K, Desai A, Campbell CS. An engineered minimal chromosomal passenger complex reveals a role for INCENP/Sli15 spindle association in chromosome biorientation. J Cell Biol. 2017;216(4):911–23. Epub 2017/03/21. doi: 10.1083/jcb.201609123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trivedi P, Zaytsev AV, Godzi M, Ataullakhanov FI, Grishchuk EL, Stukenberg PT. The binding of Borealin to microtubules underlies a tension independent kinetochore-microtubule error correction pathway. Nat Commun. 2019;10(1):682 Epub 2019/02/10. doi: 10.1038/s41467-019-08418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Funabiki H Correcting aberrant kinetochore microtubule attachments: a hidden regulation of Aurora B on microtubules. Curr Opin Cell Biol. 2019;58:34–41. Epub 2019/01/27. doi: 10.1016/j.ceb.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lampson MA, Cheeseman IM. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011;21(3):133–40. Epub 2010/11/26. doi: 10.1016/j.tcb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maresca TJ, Salmon ED. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol. 2009;184(3):373–81. Epub 2009/02/06. doi: 10.1083/jcb.200808130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lampson MA, Grishchuk EL. Mechanisms to Avoid and Correct Erroneous Kinetochore-Microtubule Attachments. Biology (Basel). 2017;6(1). Epub 2017/01/10. doi: 10.3390/biology6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuller BG, Lampson MA, Foley EA, Rosasco-Nitcher S, Le KV, Tobelmann P, Brautigan DL, Stukenberg PT, Kapoor TM. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature. 2008;453(7198):1132–6. Epub 2008/05/09. doi: 10.1038/nature06923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan L, Kapoor TM. Examining the dynamics of chromosomal passenger complex (CPC)-dependent phosphorylation during cell division. Proc Natl Acad Sci U S A. 2011;108(40):16675–80. Epub 2011/09/29. doi: 10.1073/pnas.1106748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang C, Zhang Z, Chen Q, Yan H, Zhang M, Zhou L, Xu J, Lu W, Wang F. Centromere-localized Aurora B kinase is required for the fidelity of chromosome segregation. J Cell Biol. 2020;219(2). Epub 2019/12/24. doi: 10.1083/jcb.201907092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caldas GV, DeLuca KF, DeLuca JG. KNL1 facilitates phosphorylation of outer kinetochore proteins by promoting Aurora B kinase activity. J Cell Biol. 2013;203(6):957–69. Epub 2013/12/18. doi: 10.1083/jcb.201306054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bekier ME, Mazur T, Rashid MS, Taylor WR. Borealin dimerization mediates optimal CPC checkpoint function by enhancing localization to centromeres and kinetochores. Nat Commun. 2015;6:6775 Epub 2015/04/10. doi: 10.1038/ncomms7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischbock-Halwachs J, Singh S, Potocnjak M, Hagemann G, Solis-Mezarino V, Woike S, Ghodgaonkar-Steger M, Weissmann F, Gallego LD, Rojas J, Andreani J, Kohler A, Herzog F. The COMA complex interacts with Cse4 and positions Sli15/Ipl1 at the budding yeast inner kinetochore. Elife. 2019;8 Epub 2019/05/22. doi: 10.7554/eLife.42879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia-Rodriguez LJ, Kasciukovic T, Denninger V, Tanaka TU. Aurora B-INCENP Localization at Centromeres/Inner Kinetochores Is Required for Chromosome Bi-orientation in Budding Yeast. Curr Biol. 2019;29(9):1536–44 e4. Epub 2019/04/23. doi: 10.1016/j.cub.2019.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broad AJ, DeLuca KF, DeLuca JG. Aurora B kinase is recruited to multiple discrete kinetochore and centromere regions in human cells. J Cell Biol. 2020;219(3). Epub 2020/02/07. doi: 10.1083/jcb.201905144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hadders MA, Hindriksen S, Truong MA, Mhaskar AN, Wopken JP, Vromans MJM, Lens SMA. Untangling the contribution of Haspin and Bub1 to Aurora B function during mitosis. J Cell Biol. 2020;219(3). Epub 2020/02/07. doi: 10.1083/jcb.201907087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bassett EA, Wood S, Salimian KJ, Ajith S, Foltz DR, Black BE. Epigenetic centromere specification directs aurora B accumulation but is insufficient to efficiently correct mitotic errors. J Cell Biol. 2010;190(2):177–85. Epub 2010/07/21. doi: 10.1083/jcb.201001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamagishi Y, Honda T, Tanno Y, Watanabe Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science. 2010;330(6001):239–43. Epub 2010/10/12. doi: 10.1126/science.1194498. [DOI] [PubMed] [Google Scholar]
- 65.Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science. 2010;330(6001):235–9. Epub 2010/08/14. doi: 10.1126/science.1189505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, Gorbsky GJ, Higgins JM. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science. 2010;330(6001):231–5. Epub 2010/08/14. doi: 10.1126/science.1189435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang F, Ulyanova NP, Daum JR, Patnaik D, Kateneva AV, Gorbsky GJ, Higgins JM. Haspin inhibitors reveal centromeric functions of Aurora B in chromosome segregation. J Cell Biol. 2012;199(2):251–68. Epub 2012/10/17. doi: 10.1083/jcb.201205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang F, Ulyanova NP, van der Waal MS, Patnaik D, Lens SM, Higgins JM. A positive feedback loop involving Haspin and Aurora B promotes CPC accumulation at centromeres in mitosis. Curr Biol. 2011;21(12):1061–9. Epub 2011/06/11. doi: 10.1016/j.cub.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131(2):271–85. Epub 2007/10/25. doi: 10.1016/j.cell.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 70.Kawashima SA, Tsukahara T, Langegger M, Hauf S, Kitajima TS, Watanabe Y. Shugoshin enables tension-generating attachment of kinetochores by loading Aurora to centromeres. Genes Dev. 2007;21(4):420–35. Epub 2007/02/27. doi: 10.1101/gad.1497307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science. 2010;327(5962):172–7. Epub 2009/12/08. doi: 10.1126/science.1180189. [DOI] [PubMed] [Google Scholar]
- 72.Tsukahara T, Tanno Y, Watanabe Y. Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature. 2010;467(7316):719–23. Epub 2010/08/27. doi: 10.1038/nature09390. [DOI] [PubMed] [Google Scholar]
- 73.Liu H, Qu Q, Warrington R, Rice A, Cheng N, Yu H. Mitotic Transcription Installs Sgo1 at Centromeres to Coordinate Chromosome Segregation. Mol Cell. 2015;59(3):426–36. Epub 2015/07/21. doi: 10.1016/j.molcel.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 74.Baron AP, von Schubert C, Cubizolles F, Siemeister G, Hitchcock M, Mengel A, Schroder J, Fernandez-Montalvan A, von Nussbaum F, Mumberg D, Nigg EA. Probing the catalytic functions of Bub1 kinase using the small molecule inhibitors BAY-320 and BAY-524. Elife. 2016;5 Epub 2016/02/18. doi: 10.7554/eLife.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6(2):253–68. Epub 2004/02/13. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- 76.Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol. 2004;14(4):273–86. Epub 2004/02/20. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 77.Hindriksen S, Lens SMA, Hadders MA. The Ins and Outs of Aurora B Inner Centromere Localization. Front Cell Dev Biol. 2017;5:112 Epub 2018/01/10. doi: 10.3389/fcell.2017.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janicki SM, Tsukamoto T, Salghetti SE, Tansey WP, Sachidanandam R, Prasanth KV, Ried T, Shav-Tal Y, Bertrand E, Singer RH, Spector DL. From silencing to gene expression: real-time analysis in single cells. Cell. 2004;116(5):683–98. Epub 2004/03/10. doi: 10.1016/s0092-8674(04)00171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu H, Jia L, Yu H. Phospho-H2A and Cohesin Specify Distinct Tension-Regulated Sgo1 Pools at Kinetochores and Inner Centromeres. Current Biology. 2013;23(19):1927–33. doi: 10.1016/j.cub.2013.07.078. [DOI] [PubMed] [Google Scholar]
- 80.Yue Z, Carvalho A, Xu Z, Yuan X, Cardinale S, Ribeiro S, Lai F, Ogawa H, Gudmundsdottir E, Gassmann R, Morrison CG, Ruchaud S, Earnshaw WC. Deconstructing Survivin: comprehensive genetic analysis of Survivin function by conditional knockout in a vertebrate cell line. J Cell Biol. 2008;183(2):279–96. Epub 2008/10/22. doi: 10.1083/jcb.200806118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knowlton AL, Lan W, Stukenberg PT. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol. 2006;16(17):1705–10. Epub 2006/09/05. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 82.Wordeman L, Wagenbach M, von Dassow G. MCAK facilitates chromosome movement by promoting kinetochore microtubule turnover. J Cell Biol. 2007;179(5):869–79. Epub 2007/11/28. doi: 10.1083/jcb.200707120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McHugh T, Zou J, Volkov VA, Bertin A, Talapatra SK, Rappsilber J, Dogterom M, Welburn JPI. The depolymerase activity of MCAK shows a graded response to Aurora B kinase phosphorylation through allosteric regulation. J Cell Sci. 2019;132(4). Epub 2018/12/24. doi: 10.1242/jcs.228353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tanno Y, Kitajima TS, Honda T, Ando Y, Ishiguro K, Watanabe Y. Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev. 2010;24(19):2169–79. Epub 2010/10/05. doi: 10.1101/gad.1945310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang H, Feng J, Famulski J, Rattner JB, Liu ST, Kao GD, Muschel R, Chan GK, Yen TJ. Tripin/hSgo2 recruits MCAK to the inner centromere to correct defective kinetochore attachments. J Cell Biol. 2007;177(3):413–24. Epub 2007/05/09. doi: 10.1083/jcb.200701122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hengeveld RCC, Vromans MJM, Vleugel M, Hadders MA, Lens SMA. Inner centromere localization of the CPC maintains centromere cohesion and allows mitotic checkpoint silencing. Nat Commun. 2017;8:15542 Epub 2017/06/01. doi: 10.1038/ncomms15542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haase J, Bonner MK, Halas H, Kelly AE. Distinct Roles of the Chromosomal Passenger Complex in the Detection of and Response to Errors in Kinetochore-Microtubule Attachment. Dev Cell. 2017;42(6):640–54 e5. Epub 2017/09/28. doi: 10.1016/j.devcel.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yasui Y, Urano T, Kawajiri A, Nagata K, Tatsuka M, Saya H, Furukawa K, Takahashi T, Izawa I, Inagaki M. Autophosphorylation of a newly identified site of Aurora-B is indispensable for cytokinesis. J Biol Chem. 2004;279(13):12997–3003. Epub 2004/01/15. doi: 10.1074/jbc.M311128200. [DOI] [PubMed] [Google Scholar]
- 89.Posch M, Khoudoli GA, Swift S, King EM, Deluca JG, Swedlow JR. Sds22 regulates aurora B activity and microtubule-kinetochore interactions at mitosis. J Cell Biol. 2010;191(1):61–74. Epub 2010/10/06. doi: 10.1083/jcb.200912046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shrestha RL, Conti D, Tamura N, Braun D, Ramalingam RA, Cieslinski K, Ries J, Draviam VM. Aurora-B kinase pathway controls the lateral to end-on conversion of kinetochore-microtubule attachments in human cells. Nat Commun. 2017;8(1):150 Epub 2017/07/29. doi: 10.1038/s41467-017-00209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petsalaki E, Akoumianaki T, Black EJ, Gillespie DA, Zachos G. Phosphorylation at serine 331 is required for Aurora B activation. J Cell Biol. 2011;195(3):449–66. Epub 2011/10/26. doi: 10.1083/jcb.201104023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roscioli E, Germanova TE, Smith CA, Embacher PA, Erent M, Thompson AI, Burroughs NJ, McAinsh AD. Ensemble-level organization of human kinetochores and evidence for distinct tension and attachment sensors. bioRxiv. 2019:685248. doi: 10.1101/685248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dudka D, Noatynska A, Smith CA, Liaudet N, McAinsh AD, Meraldi P. Complete microtubule-kinetochore occupancy favours the segregation of merotelic attachments. Nat Commun. 2018;9(1):2042 Epub 2018/05/26. doi: 10.1038/s41467-018-04427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suzuki A, Badger BL, Wan X, DeLuca JG, Salmon ED. The architecture of CCAN proteins creates a structural integrity to resist spindle forces and achieve proper Intrakinetochore stretch. Dev Cell. 2014;30(6):717–30. Epub 2014/10/01. doi: 10.1016/j.devcel.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dimitrova YN, Jenni S, Valverde R, Khin Y, Harrison SC. Structure of the MIND Complex Defines a Regulatory Focus for Yeast Kinetochore Assembly. Cell. 2016;167(4):1014–27 e12. Epub 2016/11/25. doi: 10.1016/j.cell.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petrovic A, Keller J, Liu Y, Overlack K, John J, Dimitrova YN, Jenni S, van Gerwen S, Stege P, Wohlgemuth S, Rombaut P, Herzog F, Harrison SC, Vetter IR, Musacchio A. Structure of the MIS12 Complex and Molecular Basis of Its Interaction with CENP-C at Human Kinetochores. Cell. 2016;167(4):1028–40 e15. Epub 2016/11/25. doi: 10.1016/j.cell.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Akiyoshi B, Nelson CR, Biggins S. The aurora B kinase promotes inner and outer kinetochore interactions in budding yeast. Genetics. 2013;194(3):785–9. Epub 2013/05/03. doi: 10.1534/genetics.113.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim S, Yu H. Multiple assembly mechanisms anchor the KMN spindle checkpoint platform at human mitotic kinetochores. J Cell Biol. 2015;208(2):181–96. Epub 2015/01/21. doi: 10.1083/jcb.201407074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rago F, Gascoigne KE, Cheeseman IM. Distinct organization and regulation of the outer kinetochore KMN network downstream of CENP-C and CENP-T. Curr Biol. 2015;25(5):671–7. Epub 2015/02/11. doi: 10.1016/j.cub.2015.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou X, Zheng F, Wang C, Wu M, Zhang X, Wang Q, Yao X, Fu C, Zhang X, Zang J. Phosphorylation of CENP-C by Aurora B facilitates kinetochore attachment error correction in mitosis. Proc Natl Acad Sci U S A. 2017;114(50):E10667–E76. Epub 2017/11/29. doi: 10.1073/pnas.1710506114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hara M, Ariyoshi M, Okumura EI, Hori T, Fukagawa T. Multiple phosphorylations control recruitment of the KMN network onto kinetochores. Nat Cell Biol. 2018;20(12):1378–88. Epub 2018/11/14. doi: 10.1038/s41556-018-0230-0. [DOI] [PubMed] [Google Scholar]
- 102.Bonner MK, Haase J, Swinderman J, Halas H, Miller Jenkins LM, Kelly AE. Enrichment of Aurora B kinase at the inner kinetochore controls outer kinetochore assembly. J Cell Biol. 2019;218(10):3237–57. Epub 2019/09/19. doi: 10.1083/jcb.201901004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang Y, Wu F, Ward T, Yan F, Wu Q, Wang Z, McGlothen T, Peng W, You T, Sun M, Cui T, Hu R, Dou Z, Zhu J, Xie W, Rao Z, Ding X, Yao X. Phosphorylation of HsMis13 by Aurora B kinase is essential for assembly of functional kinetochore. J Biol Chem. 2008;283(39):26726–36. Epub 2008/07/22. doi: 10.1074/jbc.M804207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Westermann S, Cheeseman IM, Anderson S, Yates JR 3rd, Drubin DG, Barnes G. Architecture of the budding yeast kinetochore reveals a conserved molecular core. J Cell Biol. 2003;163(2):215–22. Epub 2003/10/29. doi: 10.1083/jcb.200305100. [DOI] [PMC free article] [PubMed] [Google Scholar]


