Abstract
There is a lack of consensus on how we define heart failure with preserved ejection fraction (HFpEF), with wide variation in diagnostic criteria across society guidelines. This lack of uniformity in disease definition stems in part from an incomplete understanding of disease pathobiology, phenotypic heterogeneity, and natural history. We review current knowledge gaps and existing diagnostic tools and algorithms. We present a simple approach to implement these tools within the constraints of the current knowledge base, addressing separately (1) hospitalized individuals with rest congestion, where diagnosis is more straightforward, and (2) individuals with exercise intolerance, where diagnosis is more complex. Here, a potential role for advanced or provocative testing, including evaluation of hemodynamic responses to exercise is considered. More importantly, we propose focus areas for future studies to develop accurate and feasible diagnostic tools for HFpEF, including animal models that recapitulate human HFpEF, and human studies that both address fundamental understanding of HFpEF pathobiology as well as new diagnostic approaches and tools. In sum, there is an urgent need to more accurately define the syndrome of HFpEF, in order to inform diagnosis, patient selection for clinical trials, and ultimately future therapeutic approaches.
Keywords: Heart failure with preserved ejection fraction (HFpEF), Diagnosis, Exercise Testing
Introduction
Both the prevalence and incidence of heart failure with preserved ejection fraction (HFpEF) are rising relative to HF with reduced ejection fraction,1–3 yet there is continued lack of consensus on how we define HFpEF across various society guidelines and clinical trials. While most criteria rely on the presence of clinical symptoms and preserved ejection fraction (with variable cut-points), there is substantial variation regarding the use of biomarkers, abnormal cardiac structure and function ascertained by echocardiography, and previous hospitalizations to define HFpEF.3–5 In a recent study, the application of existing HFpEF criteria to 461 individuals with chronic dyspnea and preserved ejection fraction with extensive clinical, biochemical, and hemodynamic assessment resulted in anywhere between 12% to 90% of individuals being classified as having HFpEF using various society guideline criteria. Concomitantly, this diverse range in individuals labeled as having HFpEF had widely variable future cardiovascular events.6 While this prior study highlighted the lack of consensus around disease definition, it was suggested that the findings could be better understood within the context of the natural history of HFpEF.7
We examine existing pragmatic approaches to the diagnosis of HFpEF, recognizing that the lack of uniformity in disease definition stems in part from an incomplete understanding of disease pathobiology, and more importantly highlight current knowledge gaps with the goal of motivating future research. At the heart of the matter is the urgent need to more accurately define the syndrome of HFpEF, in order to inform patient selection, diagnosis and ultimately future therapeutic approaches.
Recognizing the Heterogeneity of Clinical Presentation in HFpEF
Unlike other diseases within cardiovascular medicine such as atrial fibrillation or hypertension where definitions are centered around a specific diagnostic test, HFpEF is a clinical syndrome for which we rely on a constellation of symptoms, signs, and other manifestations. As a clinical syndrome, the complexity of defining HFpEF arises in part from considerable heterogeneity in patients’ clinical presentation on multiple levels: (1) comorbidities i.e. co-existing conditions that modify clinical symptoms and signs; (2) organ system involvement, which may include both cardiac and non-cardiac manifestations; and (3) subset or stage of disease, where different phenotypes or stages of HFpEF may present with non-uniform clinical symptoms and signs.
Understanding HFpEF within the Context of Comorbidities
It is increasingly recognized that there is heterogeneity with respect to comorbid diseases upon HFpEF presentation (“HFpEF predisposition”, Figure 1). For example, while HFpEF was first recognized among elderly individuals with longstanding hypertension, more contemporary HFpEF samples include younger, predominantly obese individuals with cardiometabolic disease, characterized by lower natriuretic peptide levels and distinct exercise physiology.8,9 Recent data in Asian cohorts demonstrate that clustering of multimorbidities in both HFpEF and HFrEF relates differentially to patient quality of life and clinical outcomes.10 In this study, latent class analysis identified three HFpEF-predominant phenotypes, namely ‘Elderly/AF’ (older, high prevalence of atrial fibrillation); ‘Metabolic’ (obese, high prevalence of diabetes and hypertension) and ‘Lean diabetic’ (high prevalence of diabetes in absence of obesity).
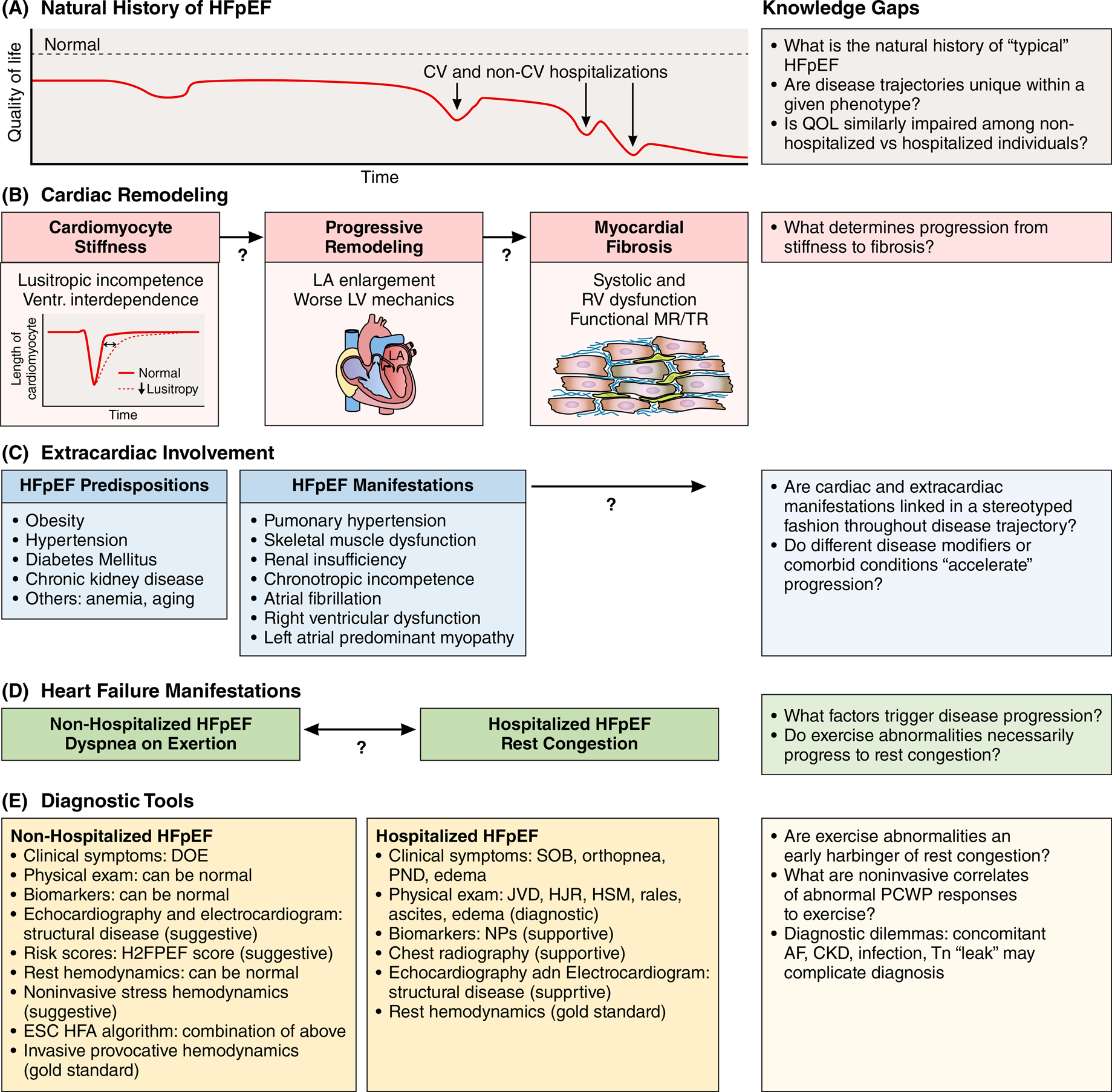
Figure 1. Contributors to diagnostic dilemma of HFpEF and summary of diagnostic tools.
We place HFpEF diagnosis within the context of the natural history of the disease (panel A). We recognize limited understanding with respect to factors driving progression of cardiac remodeling and extracardiac involvement (panels B and C). Broadly, we propose to categorize individuals with HFpEF into non-hospitalized individuals with exercise intolerance vs hospitalized individuals with rest congestion in order to examine relevant diagnostic tools and approaches (panel D and E). Central to this conceptual framework is the recognition of major current knowledge gaps (right-hand side of figure).
Understanding HFpEF within the Context of Organ System Involvement
Further heterogeneity exists across HFpEF phenotypic manifestations (“HFpEF manifestations”, Figure 1) with evidence of both cardiac and extra-cardiac organ system involvement, including contributions from right ventricular dysfunction, left atrial predominant myopathy, arterial stiffness, pulmonary hypertension, impaired peripheral oxygen extraction, skeletal muscle sarcopenia, and abnormal kidney function.11–15 While multiple physiologic abnormalities likely contribute to exercise intolerance in a given patient, significant variation in defects along the O2 pathway are also thought to underlie disease heterogeneity.16 In considering the many extracardiac manifestations and comorbidities among patients with HFpEF, it is not surprising that non-cardiovascular outcomes outweigh cardiovascular disease endpoints.17 Indeed, this diversity in clinical presentations has prompted proposals for HFpEF phenotype-guided approaches to treatment.18,19
Understanding HFpEF within the Context of Natural History
Even in the absence of heterogeneity in comorbidities or organ system involvement, disease severity or expression as manifested by symptoms and signs are inextricably linked to our ability to diagnose the clinical syndrome of HFpEF. For example, the sensitivity and specificity of detecting jugular venous distention to make a diagnosis of HFpEF will be greater in those with overt rest congestion and “more severe” disease compared with physical examination findings among patients “early” in the disease process. Thus, in addition to heterogeneity in comorbidities or organ system involvement, the natural history of the disease (or disease severity) must also be considered.
This concept is illustrated by our recent study where the definition of HFpEF was established based on the presence of elevated left ventricular (LV) filling pressures at rest or with exercise using invasive hemodynamic measurements during cardiopulmonary exercise testing (CPET).20 Among this physiologically-defined HFpEF sample, 91% of individuals met American College of Cardiology / American Heart Association (ACC/AHA) HFpEF diagnostic criteria, whereas only 17% met HFSA criteria. Notably, the application of HFSA criteria enriched for individuals with 4 times the event rate compared with the ACC/AHA sample, suggesting more advanced disease. These data indicate that ACC/AHA criteria are sensitive but non-specific, whereas HFSA criteria are highly insensitive for diagnosis but identify a high risk HFpEF phenotype. In addition, heterogeneity in outcomes among different HFpEF definitions may represent successive stages in disease progression.7 Notably, disease severity or stage may determine responsiveness to therapy, as illustrated in two large HFpEF trials where treatment benefit was observed among patient subgroups with lower natriuretic peptide levels in post-hoc analyses.21,22 Hence, it is essential that we understand HFpEF diagnosis within the context of its natural history or disease severity.
In contrast to HFrEF, where disease progression from risk factor to asymptomatic LV remodeling and clinically overt disease is relatively well understood and conceptualized within the framework of the ACC/AHA HF stages (A: risk factors, B: remodeling, C: clinical HF, D: end-stage HF),3 much remains to be uncovered about progression from HFpEF predisposing factors to overt disease.23 For example, pre-clinical diastolic dysfunction may precede HFpEF,24,25 yet many cardiac and extra-cardiac comorbidities also influence disease propensity and trajectory.26–28 Furthermore, once HFpEF is clinically recognized, non-cardiovascular comorbidities contribute significantly to outcomes including hospitalization and mortality.17,29 Lastly, whether different HFpEF subphenotypes are characterized by distinct disease trajectories remains unknown. For example, individuals with normal rest but abnormal exercise pulmonary capillary wedge pressure (PCWP, “early disease”) have lower exercise capacity,30 may develop exercise-induced pulmonary congestion,31 and are clearly at higher risk for future cardiovascular events including HF hospitalizations,6 yet progression to rest congestion may not be predestined.
In light of major knowledge gaps in disease heterogeneity and determinants of disease trajectory, we examine current approaches to HFpEF diagnosis. We acknowledge that disease definitions vary widely – for purposes of this paper, we will rely on the classic physiologic definition of HFpEF put forth by Dr. Eugene Braunwald as “the hearts inability to meet the metabolic demands of the body, or to do so at the expense of elevated filling pressures”, and we extend this to states of rest and exercise as has been embraced by a number of studies.32 It is clear that further research is needed in order to illuminate the use of specific diagnostic criteria that may in the future be tailored toward distinct HFpEF subphenotypes and/or disease stages.
A Practical Approach to HFpEF Diagnosis
Within the framework of the natural history of HFpEF, we can broadly divide patients into non-hospitalized HFpEF, where the main clinical manifestation is that of dyspnea on exertion and HF admission is rare, and hospitalized HFpEF marked by rest congestion (Figure 1D). Whether individuals with exercise intolerance and abnormal exercise reserve necessarily progress to rest congestion is unclear, though current data suggest greater future risk of HF hospitalizations even among the former group.6,33 In either case, a preserved LV ejection fraction ≥50% must also be confirmed to rule out HFrEF. We will outline currently available diagnostic approaches based on this dichotomy (Figure 2), acknowledging that individuals with HFpEF and exercise intolerance have a higher likelihood to progress to rest congestion:
Rest congestion (hospitalized HFpEF): The diagnosis of HFpEF among individuals hospitalized for rest congestion is more straightforward and can be made entirely based on a careful history and physical examination to elicit classic signs and symptoms of volume overload in the setting of preserved ejection fraction (Figure 1). Here, confirmatory or supportive evidence includes the use of natriuretic peptides, chest radiography, and echocardiography. Although these supportive objective tests are very helpful for ruling in the diagnosis in an overtly decompensated patient, there are notable exceptions. For example, up to 3 in 4 individuals with known HFpEF may not have LV hypertrophy on echocardiography.31,34–36 Similarly, 29% of obese individuals with HFpEF had normal natriuretic peptide levels despite the presence of clinically overt HF with elevated PCWP.37 Lastly, while routine invasive hemodynamic assessment via right heart catheterization is not recommended for the diagnosis of HFpEF,3 it provides definitive characterization of HFpEF when the history and physical examination or other testing prove ambiguous or at odds with one another. Diagnostic tools and specific cut-points and test characteristics are summarized in Table 1.
Exercise intolerance (non-hospitalized HFpEF): The diagnosis of HFpEF in patients without overt rest congestion is more complex. While exercise intolerance can severely limit quality of life, the etiology of breathlessness can be multifactorial, and the physical examination may be normal in the setting of normal LV filling pressures at rest.33,38 In the absence of rest congestion and with the high prevalence of obesity, it is not surprising that natriuretic peptide levels may not be elevated (mean N-terminal pro B-type natriuretic peptide [NT-proBNP] 104 pg/mL in the study by Borlaug et al).38 Supportive information may include the presence of other HFpEF predisposing factors or other manifestations such as atrial fibrillation, or the presence of structural heart disease on echocardiography.
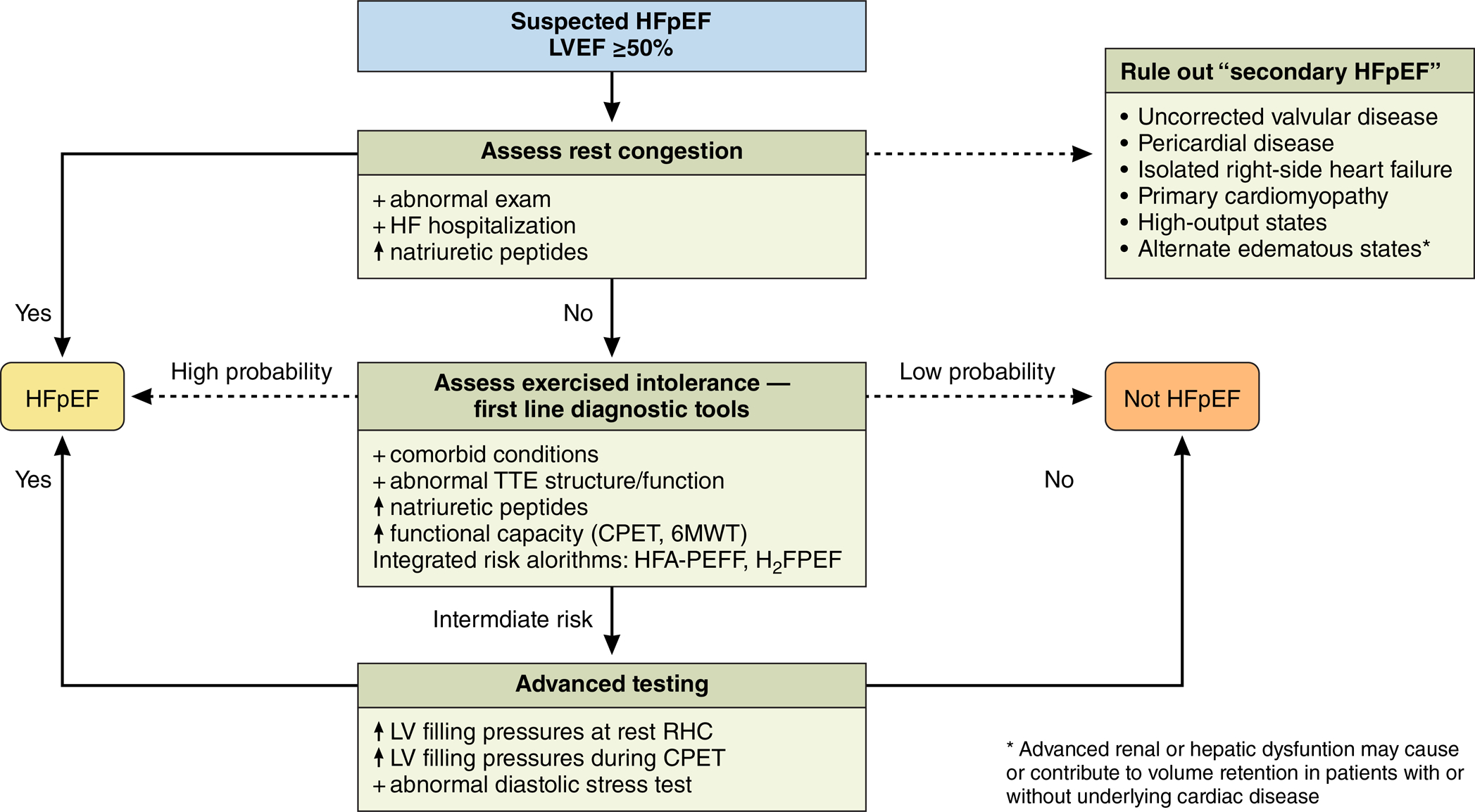
Figure 2. Practical approach to diagnostic tools.
In individuals suspected to have HFpEF, first steps include evaluating for rest congestion and consideration of potential secondary causes. If no diagnosis has been made, first-line diagnostic tools include echocardiography, natriuretic peptide levels, and objective assessment of functional capacity. Algorithms including HFA-PEFF and H2FPEF may be helpful in estimating probability of HFpEF. In individuals where diagnosis remains unclear, advanced testing can be considered including rest and exercise invasive hemodynamic measures, and diastolic stress testing in experienced centers.
Table 1.
Diagnostic Tools for HFpEF
| Non-hospitalized HFpEF | Hospitalized HFpEF | Criterion | Comments | Test Characteristics | |
|---|---|---|---|---|---|
| History | Dyspnea on exertion | Rest congestion | Orthopnea | ||
| Physical exam | May be normal | Diagnostic | JVP | ||
| Rales | |||||
| Peripheral edema | |||||
| Third heart sound | |||||
| Natriuretic peptides | Supportive | Supportive | Major ESC criterion: NT-proBNP>220 (660 in AF); minor ESC criterion: >125 (375 in AF)54 | Natriuretic peptides are 3–3.5 fold higher in AF compared with sinus rhythm68and may be falsely low in obese individuals with HF37 | High negative predictive value for overall HF5 although NPV less robust in HFpEF. Up to 20% with invasively-proven HFpEF have low NT-proBNP (<125)38,44,69; >125 cut-point: sensitivity 77% specificity 53%51 |
| Echocardiography | Supportive | Supportive | Diastolic function: Tissue Doppler of mitral septal and lateral e’ and E/e’ ratio | ESC criteria: septal e’<7 or lateral e’<10 (<75 yrs); septal e’<5 or lateral e’<7 (≥75 years); E/e’ ratio ≥15 (major), 9–14 (minor).54 | Septal e’<7: sensitivity 46%, specificity 76%; E/e’ ratio >9: sensitivity 78%, specificity 59%51. Systematic review: E/e’ correlated with invasive LV filling pressures with summary r 0.62 in 9 studies70 |
| TR jet velocity | ESC criteria: TR velocity > 2.8 m/s or PASP >35mmHg54 | PASP>35: sensitivity 46%, specificity 86%51 | |||
| Left atrial enlargement | ESC criteria advocate for separate cutpoints in sinus vs atrial fibrillation: Major - LAVI >34 (sinus), >40 (AF); minor - LAV 29–34 (sinus), 34–40 (AF)54 | LAVI >30 sensitivity 70%, specificity 71%51 | |||
| Left ventricular hypertrophy | ESC criteria emphasize concentric remodeling: Major - LVMI >=149 (men), >=122 (women) and relative wall thickness >0.42; minor - LVMI ≥115 (men), ≥95 (women) or relative wall thickness >0.42 or wall thickness ≥12mm54 | Can be normal. LVH sensitivity 26% specificity 88%51 | |||
| Invasive hemodynamics | May be normal | Confirmatory / diagnostic | Pulmonary capillary wedge pressure or left ventricular end-diastolic pressure | High filling pressure defined as LVEDP ≥16 or PCWP ≥15 mmHg | Diagnostic |
| Echocardiographic stress test | Suggestive | Exercise measures of diastolic function and TR velocity | Ideally semi-supine bicycle test with imaging during exercise, however universal protocols are lacking. Limited published data. TR velocity measurable in only about 50% of individuals with HFpEF54 | Addition of exercise average E/e’>14 or septal E/e’>15 to ESC algorithm improved sensitivity (90% from 60% for ESC alone) with similar specificity (71% from 75%) but higher false-positive rate to 29%44 | |
| Noninvasive CPET | Suggestive | Surrogate markers of cardiac functional limitation: peak VO2, VE/VCO2 slope | Mean peak VO2 in HFpEF is decreased relative to normals, however in intermediate range, shares overlap with non-cardiac dyspnea41,51 | Peak VO2 <14 discriminates HFpEF from non-cardiac dyspnea with sensitivity 91%, specificity 51%30 | |
| Invasive CPET (RHC) | Confirmatory / diagnostic | Exercise measures of pulmonary capillary wedge pressure | Universal protocols are lacking. Can use single cutpoint of peak PCWP ≥25 mmHg51,54versus change in PCWP indexed to change in flow (cardiac output)33 | Diagnostic |
The Role of Advanced or Provocative Testing
It is crucial to recognize that exercise intolerance is the defining symptom of HFpEF in this group of patients.39 As such, evaluation at rest may be normal, and exercise testing may be needed to unmask abnormal cardiovascular reserve in the absence of apparent volume overload.33,38,40 In a recent pooled analysis, one of the most significant impairments in exercise reserve among HFpEF patients was an exaggerated increase in PCWP with exercise.41 Noninvasive CPET can confirm limitations in overall exercise capacity, with peak oxygen consumption (VO2) being similarly prognostic among patients with HFpEF and HFrEF.42 Further, impaired peak VO2 is directly correlated with elevated LV filling pressures with exercise in HFpEF, although discrimination from non-cardiac causes of dyspnea using peak VO2 alone remains challenging.30 Thus, CPET or 6-minute walk testing can help to define limitations in functional capacity among individuals with suspected HFpEF, though they do not provide definitive diagnostic information or evaluation of multi-organ system abnormalities.
Noninvasive evaluation of LV diastolic performance
Beyond functional capacity, evaluation of LV diastolic performance during exercise can be assessed non-invasively using echocardiography with ascertainment of the mitral early inflow to mitral annular early diastolic velocity ratio (E/e’ ratio) and tricuspid regurgitation velocity at rest and with each stage of exercise.43,44 In this setting, exercise E/e’ ratio has been shown to correlate with invasively measured PCWP during exercise, improving sensitivity of HFpEF diagnosis when compared with invasive hemodynamic measures. It is notable that the association of E/e’ and invasively measured LV filling pressures is not consistently shown across studies.45,46 Further, widespread ascertainment of exercise diastolic function by echocardiography may be limited, with undetectable tricuspid regurgitation jet velocity in half of individuals, and ~20% without obtainable E/e’ ratio.44 In this context, diastolic stress testing with echocardiography may be considered in experienced echocardiography laboratories, though the gold standard for advanced evaluation remains exercise testing with invasive hemodynamic evaluation.
Invasive evaluation of LV diastolic performance
Invasive CPET allows for direct evaluation of LV hemodynamic exercise responses, recognizing that individuals with HFpEF may have normal rest PCWP, with “unmasking” of abnormal LV responses with exercise. It is important to note that reference values to define abnormal exercise PCWP remain uncertain, and both methods outlined below are used. A number of prior studies have used a single cut-point of peak exercise PCWP ≥25 mmHg, which has been linked to worse lung congestion.31,38 Other studies have indexed PCWP to cardiac output (CO) or work in order to account for variable “doses” of peak exercise achieved. This allows a relatively effort-independent assessment of LV hemodynamic responses and predicts clinical outcomes.6,47 This method involves serial hemodynamic measures of PCWP and CO throughout the duration of exercise to estimate ΔPCWP/ΔCO slope, with a steep ΔPCWP/ΔCO slope >2.0 mmHg/L/min indicative of HFpEF.33 This is analogous to recent consensus that increases in pulmonary artery pressure indexed to increases in CO are preferable to an absolute cut-point to define abnormal exercise pulmonary artery pressure responses.48,49 Among a sample of 461 individuals with dyspnea on exertion, we found that 129 (28%) had elevated rest PCWP, and 114 (25%) had normal rest but abnormal ΔPCWP/ΔCO slope with exercise, substantiating the importance of provocative testing to uncover abnormal exercise responses as outlined in other studies.20,38,41 Further, exercise provocation appears to be better than volume challenge or leg raise maneuvers in unmasking abnormal hemodynamic responses using provocative testing.38,50 It is important to acknowledge that advanced testing with hemodynamic assessment during exercise (either invasively or noninvasively) may not be widely available, and validation of more widely applicable noninvasive correlates for exercise diastolic function represents an important area for future study.
HFpEF Diagnostic Algorithms
Two recent diagnostic algorithms have emerged that incorporate multiple diagnostic tools to help guide HFpEF diagnosis. Both approaches leverage non-invasive data to identify low- or high-risk individuals, with additional testing recommended among patients with intermediate probability. For example, the H2FPEF score developed by Reddy et al incorporates six clinical and echocardiographic criteria to estimate a probability of HFpEF among patients with unexplained dyspnea (Table 1). When compared with HFpEF diagnosis based on invasive hemodynamic measure, the score had good discrimination (area under the receiver operator characteristic curve 0.84).51 While the score enables discrimination of HFpEF from non-cardiac causes of dyspnea, it is important to remember that the prevalence of HFpEF among the derivation and validation sample was high (64% and 61%). In this setting, the application of this score to broader samples with lower pre-test probability of HFpEF needs to be interpreted with caution. Studies examining the applicability of the H2FPEF score to external samples are emerging. For example, within the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial, the H2FPEF score was higher among participants from Americas vs. Russia/Georgia, identified patients at higher risk of adverse clinical events.52 Within the Alberta Heart Failure Etiology and Analysis Research Team sample, a H2FPEF score of > 2 had a sensitivity of 89–90% to detect clinically-adjudicated HFpEF and a H2FPEF score < 6 had a specificity of 82% to rule out HFpEF. However, it is important to acknowledge that HFpEF adjudication in this study was not validated against invasive hemodynamic assessment, limiting the conclusions that can be drawn.53
Second, the European Society of Cardiology recently proposed a stepwise algorithm to aid in HFpEF diagnosis that extended and updated previous approaches.54,55 This algorithm incorporates multiple diagnostic tools in a sequential approach to generate the HFA-PEFF score (HFA-PEFF; Heart Failure Association Pre-test assessment, Echocardiography & natriuretic peptide, Functional Testing, Final Etiology). This step-wise approach includes (1) pre-test assessment based on HFpEF predisposing and comorbid factors, (2) a score based on echocardiographic (structure and function) and natriuretic peptide levels separating patients into those with high scores (definite HFpEF), low (unlikely HFpEF), or intermediate scores (diagnostic uncertainty), with (3) further evaluation in those with diagnostic uncertainty, including functional testing with an exercise stress echocardiogram or invasive hemodynamic measurements at rest or with exercise. Initial validation of the HFA-PEFF score has been performed within the Maastricht cohort which included patients with suspected HFpEF (cases and non-cases), and the Northwestern (Chicago) HFpEF cohort (cases only).56 A high HFA-PEFF score (5–6 points) was shown to diagnose HFpEF with high specificity (93%), whereas a low HFA-PEFF score (0–1 points) ruled out HFpEF with a sensitivity of 99%; however a large proportion of 36% of patients fell in the intermediate category.
With respect to current validation efforts for both diagnostic approaches, it is notable that validation studies to date have largely relied on expert consensus drawing from various clinical, echocardiographic, and biomarker data for case definitions. In this context, future data with validation against invasive hemodynamic criteria as the “gold standard” are needed to complement current efforts. Further, how these multi-pronged approaches will aid in HFpEF diagnosis among broader at-risk samples remains to be seen and will be an important area of future research.
What is not considered HFpEF?
Many other conditions may lead to volume overload with preserved LV function, and potential “secondary HFpEF” causes are important to consider as this may lead to specific therapies (Figure 2). This includes underlying cardiac conditions (valvular heart disease, pericardial disease, pure right-ventricular failure, primary cardiomyopathies including amyloidosis), high-output states (anemia, thyroid disease), and fluid overload from kidney or liver disease. It is important to acknowledge that each of these secondary etiologies may warrant specific clinical management considerations. Therefore in this paper, we refer to HFpEF as “garden variety HFpEF”, whereby secondary conditions have been ruled out and the clinical presentation of HF in the setting of preserved EF has not been attributed directly to these other specific etiologies.
HFpEF Diagnosis: Major Knowledge Gaps and Unanswered Questions
HFpEF remains challenging to diagnose despite advances in cardiac biomarkers, noninvasive imaging modalities, and provocative testing. Fundamentally, it is important to recognize that part of the problem is that HFpEF is a clinical syndrome with a multitude of contributing risk factors, etiologies, and phenotypic manifestations.18 In order to address current knowledge gaps (outlined in Figure 1), we propose the following focus areas for future research:
Animal models that recapitulate human HFpEF: The search for preclinical models that resemble the complex human clinical phenotype of HFpEF has been challenging.57 While specific models focused on aging, obesity, or hypertension mimic certain aspects of disease, a recent ‘two-hit’ mouse model combining both metabolic and mechanical stress may most closely capture both systemic and cardiac manifestations arising on a background of multiple comorbidities in human disease.58 Future research should leverage such preclinical models to better understand disease pathogenesis, diverse disease manifestations, and natural history, in parallel with human studies, as fundamental understanding of disease biology will inform diagnostic approaches.
Human studies that address (1) careful phenotyping to untangle disease heterogeneity; (2) determinants of HFpEF disease progression and clinical trajectories; (3) the potential role of biomarkers (circulating, urinary, imaging) to distinguish HFpEF from non-cardiac causes of dyspnea or comorbidities including novel methods such as exercise-induced pulmonary B-lines; (4) the potential role of hemodynamic and/or activity monitors (invasive or non-invasive) in aiding the diagnosis of HFpEF or its pre-test probability; (5) prospective validation of any proposed diagnostic algorithm against “gold standard” invasive hemodynamic assessment in diverse populations, with particular attention to diagnostic accuracy in HFpEF patients with only exercise induced hemodynamic abnormalities.
Of the focus areas above, we would like to highlight that something as fundamental as the natural history of HFpEF remains largely understudied – future insights will inform approaches to diagnosis and therapies. Given heterogeneity in comorbid burden and disease manifestations, the determinants of disease trajectory may be unique for a given phenotype or underlying biological pathway with disease progression marked by distinct clinical manifestations. For example, progressive right ventricular dysfunction may occur in a subset of individuals with HFpEF and portends worse outcomes, whereas the development of LV systolic dysfunction remains rare over time.59,60 It also is unclear whether disease trajectories are invariably linear (progressing canonically from one predominant stage to the next) or non-linear. Do “early” manifestations of exercise intolerance lead to greater physical inactivity, which in turn may drive skeletal sarcopenia and impaired peripheral oxygen extraction as shown in many patients with HFpEF?12,61,62 Is there a subset of patients with a predisposition to pulmonary vascular dysfunction which may lead to predominant right ventricular failure later in the course?9,63 Do hypertension and chronic kidney disease perhaps lead to greater cardiac fibrosis and arterial stiffness as the predominant clinical feature in a subset of HFpEF?64,65 And is the ‘elderly with atrial fibrillation’ phenotype fundamentally different in clinical presentation and disease trajectory compared to the ‘young obese’ phenotype? How does inflammation relate to different phenotypes, and are there potential therapeutic implications?66,67
In sum, we highlight important knowledge gaps and challenges in HFpEF diagnosis and present an approach to implement available tools within the constraints of the current knowledge base. More importantly, we emphasize that further studies to develop accurate and feasible diagnostic tools for HFpEF are urgently needed. While invasive hemodynamic testing remains the gold standard, it is not yet accessible for all patients and a stepped invasive approach as outlined here may help identify indeterminate patients for referral. Further, academic institutions with capacity for advanced diagnostic testing including invasive evaluation of LV diastolic performance should prioritize research into development of novel diagnostic tools for HFpEF.
Funding:
Dr. Ho is supported by NIH grants R01-HL134893 and R01-HL140224.
Disclosures: Dr Ho has received research support from Bayer, Gilead Sciences, and EcoNugenics. Dr Lam is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, and Vifor Pharma; has served as consultant or on the Advisory Board/ Steering Committee/ Executive Committee for Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, Vifor Pharma, Novartis, Amgen, Merck, Janssen Research & Development LLC, Menarini, Boehringer Ingelheim, Novo Nordisk, Abbott Diagnostics, Corvia, Stealth BioTherapeutics, JanaCare, Biofourmis, Darma, Applied Therapeutics, MyoKardia, WebMD Global LLC, Radcliffe Group Ltd and Corpus.
Non-standard Abbreviations and Acronyms
- ACC/AHA
American College of Cardiology / American Heart Association
- CO
cardiac output
- CPET
cardiopulmonary exercise testing
- E/e’ ratio
mitral early inflow to mitral annular early diastolic velocity ratio
- HFA-PEFF
Heart Failure Association Pre-test assessment, Echocardiography & natriuretic peptide, Functional Testing, Final Etiology algorithm
- HFpEF
heart failure with preserved ejection fraction
- LV
left ventricular
- NT-proBNP
N-terminal pro B-type natriuretic peptide
- PCWP
pulmonary capillary wedge pressure
- TOPCAT
Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist
- VO2
oxygen consumption
REFERENCES
- 1.Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM, Vasan RS. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail. 2018;6:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–327. [DOI] [PubMed] [Google Scholar]
- 4.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WHW, Teerlink JR, Walsh MN. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–194. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, Van Der Meer P, Members AF. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 6.America HFSO, Ho JE, Zern EK, Wooster L, Bailey CS, Cunningham T, Eisman AS, Hardin KM, Zampierollo GA, Jarolim P, Pappagianopoulos PP, Malhotra R, Nayor M, Lewis GD. Differential Clinical Profiles, Exercise Responses, and Outcomes Associated With Existing HFpEF Definitions. Circulation. 2019;140:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senni M, Caravita S, Paulus WJ. Do Existing Definitions Identify Subgroup Phenotypes or Reflect the Natural History of Heart Failure With Preserved Ejection Fraction. Circulation. 2019;140:366–369. [DOI] [PubMed] [Google Scholar]
- 8.Tromp J, Shen L, Jhund PS, Anand IS, Carson PE, Desai AS, Granger CB, Komajda M, McKelvie RS, Pfeffer MA, Solomon SD, Køber L, Swedberg K, Zile MR, Pitt B, Lam CSP, McMurray JJV. Age-Related Characteristics and Outcomes of Patients With Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2019;74:601–612. [DOI] [PubMed] [Google Scholar]
- 9.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection FractionClinical Perspective. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tromp J, Tay WT, Ouwerkerk W, Teng TK, Yap J, MacDonald MR, Leineweber K, McMurray JJV, Zile MR, Anand IS, Richards AMR, Lam CSP, ASIAN-HF A. Multimorbidity in patients with heart failure from 11 Asian regions: A prospective cohort study using the ASIAN-HF registry. PLoS Med. 2018;15:e1002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obokata M, Kane GC, Reddy YNV, Melenovsky V, Olson TP, Jarolim P, Borlaug BA. The neurohormonal basis of pulmonary hypertension in heart failure with preserved ejection fraction. Eur Heart J. 2019;40:3707–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel RB, Alenezi F, Sun JL, Alhanti B, Vaduganathan M, Oh JK, Redfield MM, Butler J, Hernandez AF, Velazquez EJ, Shah SJ. Biomarker Profile of Left Atrial Myopathy in Heart Failure With Preserved Ejection Fraction: Insights From the RELAX Trial. J Card Fail. 2020;26:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD, Lewis GD. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Diagnosing and Ranking Its Causes Using Personalized O2 Pathway Analysis. Circulation. 2018;137:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal P, Loop M, Chen L, Brown TM, Durant RW, Safford MM, Levitan EB. Causes and Temporal Patterns of 30-Day Readmission Among Older Adults Hospitalized With Heart Failure With Preserved or Reduced Ejection Fraction. J Am Heart Assoc. 2018;7:e007785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senni M, Paulus WJ, Gavazzi A, Fraser AG, Díez J, Solomon SD, Smiseth OA, Guazzi M, Lam CSP, Maggioni AP, Tschöpe C, Metra M, Hummel SL, Edelmann F, Ambrosio G, Stewart Coats AJ, Filippatos GS, Gheorghiade M, Anker SD, Levy D, Pfeffer MA, Stough WG, Pieske BM. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J. 2014;35:2797–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho JE, Zern EK, Wooster L, Bailey CS, Cunnigham T, Eisman AS, Hardin KM, Zampierollo GA, Jarolim P, Pappagianopoulos PP, Malhotra R, Nayor M, Lewis GD. Differential Clinical Profiles, Exercise Responses and Outcomes Associated with Existing HFpEF Definitions. Circulation. 2019;140:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, McMurray JJ, Zile MR, Komajda M, Massie BM, Carson PE. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail. 2011;4:569–577. [DOI] [PubMed] [Google Scholar]
- 22.Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, Desai AS, O’Meara E, Fleg JL, Pfeffer MA, Pitt B, Solomon SD. Interaction Between Spironolactone and Natriuretic Peptides in Patients With Heart Failure and Preserved Ejection Fraction: From the TOPCAT Trial. JACC Heart Fail. 2017;5:241–252. [DOI] [PubMed] [Google Scholar]
- 23.Louridas GE, Lourida KG. Heart Failure in Patients with Preserved Ejection Fraction: Questions Concerning Clinical Progression. J Cardiovasc Dev Dis. 2016;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 25.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam CSP, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ, Vasan RS. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS, Ho JE. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart failure. 2018;6:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation. 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DS, Gona P, Albano I, Larson MG, Benjamin EJ, Levy D, Kannel WB, Vasan RS. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail. 2011;4:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic Correlates and Diagnostic Role of Cardiopulmonary Exercise Testing in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy YNV, Obokata M, Wiley B, Koepp KE, Jorgenson CC, Egbe A, Melenovsky V, Carter RE, Borlaug BA. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J. 2019;40:3721–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braunwald E, Grossman W. Clinical aspects of heart failure In: Braunwald E (ed). Heart Disease: A Textbook of Cardiovascular Medicine. 4th Ed. Philadelphia: Sunders; 1992. 444–463. [Google Scholar]
- 33.Eisman AS, Shah RV, Dhakal BP, Pappagianopoulos PP, Wooster L, Bailey C, Cunningham TF, Hardin KM, Baggish AL, Ho JE, Malhotra R, Lewis GD. Pulmonary Capillary Wedge Pressure Patterns During Exercise Predict Exercise Capacity and Incident Heart Failure. Circ Heart Fail. 2018;11:e004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE, I-PRESERVE I. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. [DOI] [PubMed] [Google Scholar]
- 36.Shah AM, Cikes M, Prasad N, Li G, Getchevski S, Claggett B, Rizkala A, Lukashevich I, O’Meara E, Ryan JJ, Shah SJ, Mullens W, Zile MR, Lam CSP, McMurray JJV, Solomon SD, PARAGON-HF I. Echocardiographic Features of Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction. J Am Coll Cardiol. 2019;74:2858–2873. [DOI] [PubMed] [Google Scholar]
- 37.Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, Shah SJ. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borlaug BA, Nishimura RA, Sorajja P, Lam CSP, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. [DOI] [PubMed] [Google Scholar]
- 40.Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;39:2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandey A, Khera R, Park B, Haykowsky M, Borlaug BA, Lewis GD, Kitzman DW, Butler J, Berry JD. Relative Impairments in Hemodynamic Exercise Reserve Parameters in Heart Failure With Preserved Ejection Fraction: A Study-Level Pooled Analysis. JACC Heart Fail. 2018;6:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nadruz W, West E, Sengeløv M, Santos M, Groarke JD, Forman DE, Claggett B, Skali H, Shah AM. Prognostic Value of Cardiopulmonary Exercise Testing in Heart Failure With Reduced, Midrange, and Preserved Ejection Fraction. J Am Heart Assoc. 2017;6:e006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad SB, Holland DJ, Atherton JJ. Diastolic stress echocardiography: from basic principles to clinical applications. Heart. 2018;104:1739–1748. [DOI] [PubMed] [Google Scholar]
- 44.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of Diastolic Stress Testing in the Evaluation for Heart Failure With Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation. 2017;135:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. [DOI] [PubMed] [Google Scholar]
- 46.Kim KH, Kane GC, Luong CL, Oh JK. Echocardiographic Diastolic Stress Testing: What Does It Add. Curr Cardiol Rep. 2019;21:109. [DOI] [PubMed] [Google Scholar]
- 47.Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle R-P, Pieske B, Neumann FJ. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3103–3112. [DOI] [PubMed] [Google Scholar]
- 48.Lewis GD, Bossone E, Naeije R, Grünig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128:1470–1479. [DOI] [PubMed] [Google Scholar]
- 49.Kovacs G, Hervé P, Barbera JA, Chaouat A, Chemla D, Condliffe R, Garcia G, Grünig E, Howard L, Humbert M, Lau E, Laveneziana P, Lewis GD, Naeije R, Peacock A, Rosenkranz S, Saggar R, Ulrich S, Vizza D, Vonk-Noordegraaf A, Olschewski H. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J. 2017;50:1700578. [DOI] [PubMed] [Google Scholar]
- 50.Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail. 2015;8:41–48. [DOI] [PubMed] [Google Scholar]
- 51.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation. 2018;138:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Segar MW, Patel KV, Berry JD, Grodin JL, Pandey A. Generalizability and Implications of the H2FPEF Score in a Cohort of Patients With Heart Failure With Preserved Ejection Fraction. Circulation. 2019;139:1851–1853. [DOI] [PubMed] [Google Scholar]
- 53.Sepehrvand N, Alemayehu W, Dyck GJB, Dyck JRB, Anderson T, Howlett J, Paterson I, McAlister FA, Ezekowitz JA. External Validation of the H2F-PEF Model in Diagnosing Patients With Heart Failure and Preserved Ejection Fraction. Circulation. 2019;139:2377–2379. [DOI] [PubMed] [Google Scholar]
- 54.Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 55.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. [DOI] [PubMed] [Google Scholar]
- 56.Aizpurua AB, Wijk SS, Rocca HB, Henkens M, Heymans S, Beussink-Nelson L, Shah SJ, van Empel VPM. Validation of the HFA-PEFF-score for the Diagnosis of Heart Failure with Preserved Ejection Fraction. Eur J Heart Fail. 2020;22:413–421. [DOI] [PubMed] [Google Scholar]
- 57.Valero-Muñoz M, Backman W, Sam F. Murine Models of Heart Failure with Preserved Ejection Fraction: a “Fishing Expedition”. JACC Basic Transl Sci. 2017;2:770–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV, Hill TM, Mammen PPA, Huang J, Lee DI, Hahn VS, Sharma K, Kass DA, Lavandero S, Gillette TG, Hill JA. Nitrosative stress drives heart failure with preserved ejection fraction. Nature. 2019;568:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lupón J, Gavidia-Bovadilla G, Ferrer E, de Antonio M, Perera-Lluna A, López-Ayerbe J, Domingo M, Núñez J, Zamora E, Moliner P, Santiago-Vacas E, Santesmases J, Bayés-Genis A. Heart Failure With Preserved Ejection Fraction Infrequently Evolves Toward a Reduced Phenotype in Long-Term Survivors. Circ Heart Fail. 2019;12:e005652. [DOI] [PubMed] [Google Scholar]
- 60.Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J. 2019;40:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melenovsky V, Hwang S-J, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reddy YNV, Andersen MJ, Obokata M, Koepp KE, Kane GC, Melenovsky V, Olson TP, Borlaug BA. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2017;70:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van de Wouw J, Broekhuizen M, Sorop O, Joles JA, Verhaar MC, Duncker DJ, Danser AHJ, Merkus D. Chronic Kidney Disease as a Risk Factor for Heart Failure With Preserved Ejection Fraction: A Focus on Microcirculatory Factors and Therapeutic Targets. Front Physiol. 2019;10:1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 67.Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti-Inflammatory Therapy With Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation. 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
- 68.van Doorn S, Geersing GJ, Kievit RF, van Mourik Y, Bertens LC, van Riet EES, Boonman-de Winter LJ, Moons KGM, Hoes AW, Rutten FH. Opportunistic screening for heart failure with natriuretic peptides in patients with atrial fibrillation: a meta-analysis of individual participant data of four screening studies. Heart. 2018;104:1236–1237. [DOI] [PubMed] [Google Scholar]
- 69.Meijers WC, Hoekstra T, Jaarsma T, van Veldhuisen DJ, de Boer RA. Patients with heart failure with preserved ejection fraction and low levels of natriuretic peptides. Neth Heart J. 2016;24:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nauta JF, Hummel YM, van der Meer P, Lam CSP, Voors AA, van Melle JP. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2018;20:1303–1311. [DOI] [PubMed] [Google Scholar]


