Abstract
Although men of African ancestry have a high risk of prostate cancer (PCa), no genes or mutations have been identified that contribute to familial clustering of PCa in this population. We investigated whether the African ancestry–specific PCa risk variant at 8q24, rs72725854, is enriched in men with a PCa family history in 9052 cases, including 143 from high-risk families, and 8595 controls of African ancestry. We found the risk allele to be significantly associated with earlier age at diagnosis, more aggressive disease, and enriched in men with a PCa family history (32% of high-risk familial cases carried the variant vs 23% of cases without a family history and 12% of controls). For cases with two or more first-degree relatives with PCa who had at least one family member diagnosed at age <60 yr, the odds ratios for TA heterozygotes and TT homozygotes were 3.92 (95% confidence interval [CI] = 2.13–7.22) and 33.41 (95% CI = 10.86–102.84), respectively. Among men with a PCa family history, the absolute risk by age 60 yr reached 21% (95% CI = 17–25%) for TA heterozygotes and 38% (95% CI = 13–65%) for TT homozygotes. We estimate that in men of African ancestry, rs72725854 accounts for 32% of the total familial risk explained by all known PCa risk variants.
Keywords: 8q24, African ancestry, Familial prostate cancer, Family history, Genetic variant, Genetics, Health disparities, Prostate cancer
Patient summary:
We found that rs72725854, an African ancestry–specific risk variant, is more common in men with a family history of prostate cancer and in those diagnosed with prostate cancer at younger ages. Men of African ancestry may benefit from the knowledge of their carrier status for this genetic risk variant to guide decisions about prostate cancer screening.
Prostate cancer (PCa) is highly heritable, and having a first-degree relative with PCa is associated with a two- to three-fold increased risk [1]. With the exception of a rare HOXB13 European-specific mutation that accounts for ~5% of hereditary PCa [2,3] and variants in DNA repair pathway genes found in ~10% of inherited PCa cases [4], genes or mutations contributing to the familial clustering of PCa remain elusive. Despite the greater risk of PCa for men of African ancestry, no single mutation has been discovered that accounts for a large fraction of the aggregation of PCa within families of African ancestry.
We and others have shown that germline variation at 8q24 is the strongest PCa risk factor across racial and ethnic populations, with multiple independent risk variants discovered in the region (127.6–129.0 Mb) [5–7]. While most associations with 8q24 variants have been observed across racial/ethnic populations, less common, ancestry-specific variants have also been detected with odds ratios (ORs) >2.0. One such variant, rs72725854 (T risk allele frequency ~6%), is found only in men of African ancestry and is the strongest genome-wide signal for PCa (OR = 2.32; 95% confidence interval [CI] = 2.16–2.50; p = 1.1 × 10–109) discovered to date in this population [5]. Given the moderate effect size conveyed by this variant, we investigated whether the T allele is associated with PCa family history and age at diagnosis, characteristics that indicate a strong genetic influence of disease onset.
This study included 9052 PCa cases unselected for PCa family history (median age = 64 [interquartile range = 12] yr, 20% with a PCa family history), of which 2041 had high-risk disease and 692 lethal disease, and 8595 controls (median age = 64 [interquartile range = 13] yr, 9.0% with a PCa family history; Supplementary Tables 1 and 2), Among cases, 23.7% carried at least one copy of the T allele versus 11.6% of controls; the ORs were 2.29 (95% CI = 2.10–2.49) for TA heterozygotes and 5.04 (95% CI = 3.36–7.55) for TT homozygotes (Table 1 and Supplementary Fig. 1). The percentage of cases carrying the T allele was significantly greater for men with a PCa family history (27.4% in those with a first/second-degree relative with PCa vs 22.7% in those without, p = 0.002) and for men diagnosed at age <60 yr (28.2% vs 21.6% for those aged ≥60 yr at diagnosis, p = 0.002; Table 1). The mean age at diagnosis for TT homozygotes was 61.1 yr (standard deviation [SD] = 8.7) versus 62.7 yr (SD = 9.1) for TA heterozygotes and 64.3 yr (SD = 8.9) for AA homozygotes (p = 5.7E-14).
Table 1 –
Prostate cancer risk associated with the germline variant rs72725854 by family history a,b of prostate cancer and age at diagnosis
| Group | N | Risk allele frequency (%) | Carrier frequency (%) | Homozygote genotype frequency (%) | Heterozygote genotype | Homozygote variant genotype | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency (%) | OR (95% CI) | p value | Frequency (%) | OR (95% CI) | p value | |||||
| Controls (reference group) | 8595 | 6.0 | 11.6 | 88.3 | 11.3 | 0.35 | ||||
| Cases | 9052 | 12.6 | 23.7 | 76.3 | 22.3 | 2.29 (2.10–2.49) | 6.7E-81 | 1.4 | 5.04 (3.36–7.55) | 5.2E-15 |
| Cases with at least 1 first-degree relative with prostate cancer | 1795 | 14.8 | 27.4 | 72.6 | 25.1 | 2.67 (2.34–3.04) | 1.6E-48 | 2.3 | 8.66 (5.22–14.37) | 6.8E-17 |
| Cases with no family history of prostate cancer | 6125 | 11.9 | 22.7 | 77.4 | 21.5 | 2.17 (1.97–2.38) | 1.1E-59 | 1.2 | 4.31 (2.77–6.71) | 8.8E-11 |
| Cases with age at diagnosis <60 yr | 2923 | 15.1 | 28.2 | 71.8 | 26.2 | 2.63 (2.32–2.97) | 5.9E-52 | 2.0 | 6.84 (4.09–11.44) | 2.4E-13 |
| Cases with age at diagnosis ≥60 yr | 6129 | 11.4 | 21.6 | 78.4 | 20.4 | 2.10 (1.90–2.31) | 2.6E-49 | 1.2 | 4.22 (2.69–6.63) | 4.2E-10 |
| Cases with at least 1 first-degree relative with prostate cancer and case age at diagnosis <60 yr | 688 | 16.9 | 30.8 | 69.2 | 27.9 | 2.85 (2.33–3.49) | 1.4E-24 | 2.9 | 11.56 (6.00–22.27) | 2.5E-13 |
| Familial cases | 143 | 17.8 | 32.2 | 67.8 | 28.7 | 3.33 (2.29–4.85) | 3.3E-10 | 3.5 | 13.92 (5.15–37.63) | 2.1E-07 |
| Familial cases with ≥2 first-degree relatives with prostate cancer | 76 | 19.1 | 32.9 | 67.1 | 27.6 | 3.23 (1.92–5.41) | 8.8E-06 | 5.3 | 21.20 (7.04–63.83) | 5.6E-08 |
| Familial cases with ≥2 first-degree relatives with prostate cancer and age at diagnosis <60 yr (any relative) | 51 | 23.5 | 39.2 | 60.8 | 31.4 | 3.92 (2.13–7.22) | 1.2E-05 | 7.8 | 33.41 (10.86–102.84) | 9.5E-10 |
CI =confidence interval; OR = odds ratio.
Controls are limited to 8594 for comparisons with the 143 familial cases, as one control is a full sibling of one of the familial cases.
Family history in nonfamilial cases and controls includes first- or second-degree relatives with prostate cancer.
The percentage of cases carrying the T allele was highest among men with both a family history and an early diagnosis (30.8%; Table 1 and Supplementary Fig. 1). For men with a PCa family history and aged <60 yr at diagnosis versus controls, the ORs were 2.85 (95% CI = 2.33–3.49) for TA heterozygotes and 11.56 (95% CI = 6.00–22.27) for TT homozygotes.
The T allele was over-represented in advanced PCa cases (Supplementary Table 3), with the percentage of men carrying the T allele ranging from 26.0% for lethal PCa (metastatic disease, prostate-specific antigen [PSA] >100 ng/ml, or PCa death), 25.4% for high-risk disease (stage T3/T4, Gleason 8–10, or PSA = 20–100 ng/ml), 24.6% for intermediate-risk disease (Gleason 7, stage T1/T2, and PSA = 10–20 ng/ml), and 21.4% for low-risk disease (Gleason <7, stage T1/T2, and PSA < 10 ng/ml; heterogeneity p = 0.03). This pattern was consistent in cases with (35.1%, 28.8%, 27.4%, and 27.2%, respectively; heterogeneity p = 0.2) and without (24.8%, 24.2%, 24.1%, and 19.4%, respectively; heterogeneity p = 0.01) a PCa family history.
Given the enrichment of the T allele in cases with a PCa family history and early-onset disease, we examined a dose-response relationship between the risk allele and strength of family history using an independent sample of 143 high-risk families (Supplementary material and Supplementary Tables 1 and 2). Among affected probands, 32.2% carried the risk allele, with 3.5% being homozygotes. Comparing these familial cases with the 8595 controls, the ORs for TA heterozygotes and TT homozygotes were 3.33 (95% CI = 2.29–4.85) and 13.92 (95% CI = 5.15–37.63), respectively (Table 1). The percentage of cases carrying the T allele was greater for those with two or more first-degree relatives with PCa (n = 76, 32.9%) and for those who also had at least one family member aged <60 yr at diagnosis (n = 51, 39.2%). For this latter subset of cases, the ORs for TA heterozygotes and TT homozygotes were 3.92 (95% CI = 2.13–7.22) and 33.41 (95% CI = 10.86–102.84), respectively.
We estimated the absolute risk of PCa based on the log-additive effects of rs72725854 and family history (Supplementary material and Supplementary Table 4). Among men without a family history, the absolute risks for PCa by age 60 yr for TA heterozygotes and TT homozygotes were 9.0% (95% CI = 8.6–10%) and 16% (95% CI = 8.7–23%), respectively, versus 4.3% (95% CI = 4.1–4.5%) for nonrisk allele carriers (Fig. 1 and Supplementary Table 5). Among men with a PCa family history, the absolute risk by age 60 yr reached 21% (95% CI = 17–25%) for TA heterozygotes and 38% (95% CI = 13–65%) for TT homozygotes, versus 9.0% (95% CI = 8.2–9.8%) for nonrisk allele carriers. Lifetime absolute risk of PCa for heterozygotes and homozygotes with a family history reached 60% (95% CI = 53%−66%) and 79% (95% CI = 57–100%), respectively.
Fig. 1 –
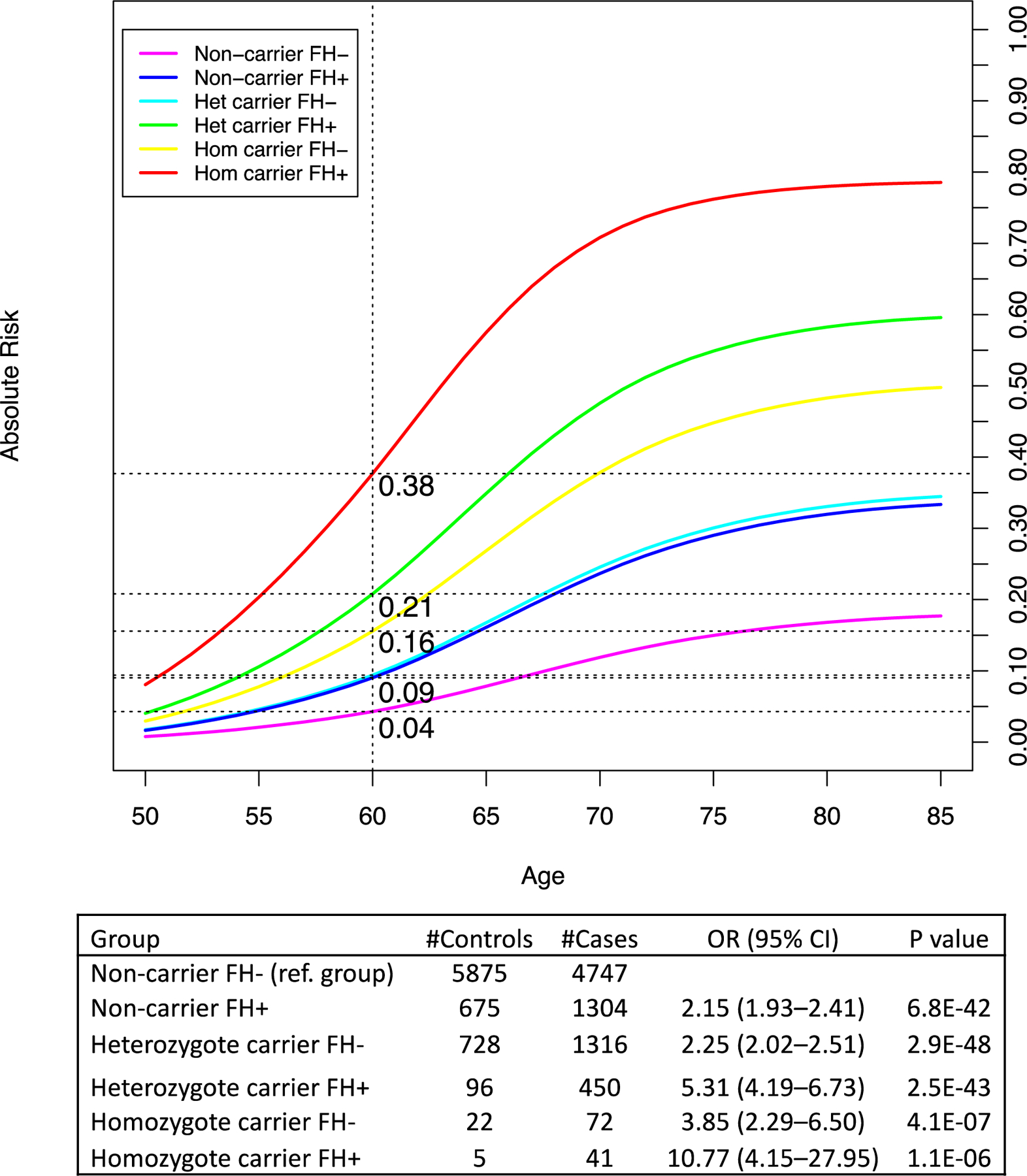
Absolute risk of prostate cancer by rs72725854 genotype and family history. Absolute risks are estimated using the odds ratios for each genotype/family history category, which are indicated in the table, combined with mortality and incidence rates for African-American men (see the Supplementary material).
CI = confidence interval; FH+ = participants with at least one first- or second-degree relative with prostate cancer; FH− = participants with no family history of prostate cancer; Hom = homozygote; Het = heterozygote; OR = odds ratio.
While the T allele is more common in men with a higher percentage of global African ancestry (Supplementary Fig. 2 and Supplementary material), its frequency varies widely between African populations. Based on genotype data from the current and previously published studies and from the 1000 Genomes Project, the T allele is found to range from 2.5% in Acholi men from North-Central Uganda to 15.0% in men from the Democratic Republic of the Congo (Supplementary Table 6), suggesting that in some populations, over 25% of men may be carriers of the risk allele (compared with 11.6% of unaffected men in our study, the majority of whom were African American).
We also evaluated four other 8q24 risk variants found to be associated with PCa in men of African ancestry [5] and found that these variants were not over-represented in men with a PCa family history (Supplementary Table 7). When combining the five 8q24 variants in a genetic risk score (GRS; Supplementary material), the PCa OR for the top 10% versus median 40–50% GRS was 2.33 (95% CI = 2.08–2.62). This was considerably diminished, after excluding rs72725854, to 1.54 (95% CI = 1.35–1.77; Supplementary Fig. 3). This association between PCa and a GRS excluding rs72725854 was similar between those with and without a PCa family history and those diagnosed at younger and older ages (Supplementary Table 8). Findings were similar for a GRS of the 180 known PCa risk variants, with ORs for the top 10% versus median 40–50% GRS of 2.84 (95% CI = 2.53–3.18) including and 2.28 (95% CI = 2.03–2.56) excluding rs72725854 (Supplementary Fig. 3 and Supplementary Table 8). These results suggest that rs72725854 is uniquely over-represented in men with PCa who have a family history and are diagnosed at a younger age.
We estimate that in men of African ancestry, the 180 known genetic risk variants collectively explain 29–38% of the familial relative risk of PCa (based on observed familial risk to first-degree relatives of PCa cases, ranging from 2.0 to 2.5, as described in the Supplementary material and Supplementary Table 9). The five African ancestry 8q24 variants explain 14–18% of familial risk of PCa (accounting for 49% of the total familial risk explained by the 180 variants; Supplementary Table 9), which is twice the amount of familial risk explained by 8q24 in men of European ancestry (~25%) [8]. The variant rs72725854 alone explains 9.2–12% of the familial relative risk (32% of the total familial risk explained by the 180 variants), a considerably larger contribution to familial risk than any other known risk variant (Supplementary Table 10), compared with the rare HOXB13 PCa risk variant found only in men of European ancestry, which is estimated to be ~5% of the total familial risk explained by all known PCa risk variants [8] and accounts for ~5% of all familial cases [3]. Our findings support the importance of 8q24 germline variation, while highlighting the much larger contribution of 8q24 risk variants to familial risk for men of African versus European ancestry.
We demonstrate that the germline variant rs72725854 is significantly enriched among PCa cases of African ancestry with a PCa family history. The T allele was observed in 32% of familial cases, with a 33-fold increase in the percentage of high-risk familial cases who were homozygous risk allele carriers (7.8%) compared with controls (0.35%). Although the biological and functional relevance of this risk variant has not been determined, it is located in a noncoding intergenic region near Prostate Cancer Associated Transcript 1 (PCAT1) and other PCa-associated long noncoding RNAs implicated in the promotion of PCa through regulation of the downstream MYC oncogene [9,10]. The lack of this variant in European ancestry populations emphasizes differences underlying the genetic architecture of PCa in men of African versus European descent.
Given the association of the germline variant rs72725854 with a high absolute PCa risk and an increased risk of aggressive and lethal disease, men of African ancestry may benefit from the knowledge of their carrier status for this genetic risk variant to guide decisions about PCa screening. Carriers of the rare HOXB13 mutation or BRCA2 mutations have been recommended to start PCa screening with a baseline PSA measurement at age 40 yr or 10 yr prior to the diagnosis of the youngest PCa patient in the family [11]. Men of African ancestry carrying the rs72725854 risk allele could benefit from similar screening recommendations. Further research is needed, particularly a prospective investigation of rs72725854 T allele carriers, to determine when and how to incorporate rs72725854 into PCa screening and whether combining rs72725854 genotype information with screening measures, such as PSA, the 4Kscore, the Stockholm-3 model, or the Prostate Health Index, could effectively identify PCa and aggressive disease earlier in men of African ancestry.
Supplementary Material
Acknowledgments:
A full list of acknowledgments is provided in the Supplementary material.
Funding/Support and role of the sponsor: This work was supported by the National Cancer Institute at the National Institutes of Health (grants U19 CA148537, U19 CA214253, R01 CA165862, and K99CA246063). Dr. Burcu F. Darst was supported in part by an award from the Achievement Rewards for College Scientists Foundation Los Angeles Founder Chapter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.
Additional members and affiliations are presented in the Supplementary material.
Financial disclosures: Christopher A. Haiman certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- [1].Stanford JL, Ostrander EA. Familial prostate cancer. Epidemiol Rev 2001;23:19–23. [DOI] [PubMed] [Google Scholar]
- [2].Ewing CM, Ray AM, Lange EM, et al. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med 2012;366:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xu J, Lange EM, Lu L, et al. HOXB13 is a susceptibility gene for prostate cancer: results from the International Consortium for Prostate Cancer Genetics (ICPCG). Hum Genet 2013;132:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Giri VN, Hegarty SE, Hyatt C, et al. Germline genetic testing for inherited prostate cancer in practice: implications for genetic testing, precision therapy, and cascade testing. Prostate 2019;79:333–9. [DOI] [PubMed] [Google Scholar]
- [5].Conti DV, Wang K, Sheng X, et al. Two novel susceptibility loci for prostate cancer in men of African ancestry. J Natl Cancer Inst. 2017;109:djx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Freedman ML, Haiman CA, Patterson N, et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci U S A 2006;103:14068–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Haiman CA, Patterson N, Freedman ML, et al. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat Genet 2007;39:638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Matejcic M, Saunders EJ, Dadaev T, et al. Germline variation at 8q24 and prostate cancer risk in men of European ancestry. Nat Commun 2018;9:4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Prensner JR, Chen W, Han S, et al. The long non-coding RNA PCAT-1 promotes prostate cancer cell proliferation through cMyc. Neoplasia 2014;16:900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim T, Cui R, Jeon YJ, et al. Long-range interaction and correlation between MYC enhancer and oncogenic long noncoding RNA CARLo-5. Proc Natl Acad Sci U S A 2014;111:4173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Giri VN, Knudsen KE, Kelly WK, et al. Role of genetic testing for inherited prostate cancer risk: Philadelphia Prostate Cancer Consensus Conference 2017. J Clin Oncol 2018;36:414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


