Abstract
This scientific commentary refers to ‘O-GlcNAcylation regulates dopamine neuron function, survival and degeneration in Parkinson disease’, by Lee et al. (doi:10.1093/brain/awaa320).
This scientific commentary refers to ‘O-GlcNAcylation regulates dopamine neuron function, survival and degeneration in Parkinson disease’, by Lee et al. (doi:10.1093/brain/awaa320).
Parkinson’s disease results from the progressive loss of midbrain dopamine-releasing neurons, which control voluntary movement, motivation and reward-related learning and memory. The degeneration of these specialized neurons, which is often accompanied by aggregation of α-synuclein and other proteins into Lewy bodies, leads to behavioural changes, such as shaking, stiffness, and difficulty with walking, balance and muscle coordination. But why are dopamine neurons so vulnerable to degeneration? In this issue of Brain, Lee and co-workers present the most comprehensive and convincing evidence to date that a post-translational modification called O-GlcNAcylation is essential for the survival and functioning of dopamine neurons, and that a reduction in this process contributes directly to progressive Parkinson’s disease (Lee et al., 2020).
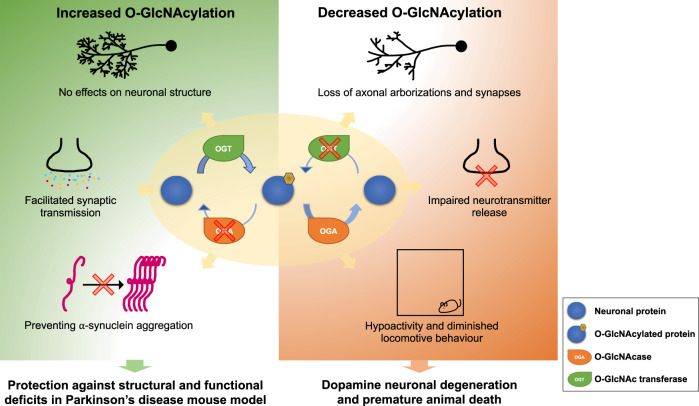
Figure 1.
Increased O-GlcNAcylation (OGN) in dopamine transporter (DAT)-expressing neurons protects against Parkinson’s disease pathology. Left: Protective effects of increased O-GlcNAcylation. Right: Pathological effects of reduced O-GlcNAcylation.
O-GlcNAcylation refers to the addition of a specific monosaccharide (N-acetylglucosamine, GlcNAc) to serine or threonine residues of nuclear and cytoplasmic proteins. The process serves as a nutrient sensor that regulates signalling, transcription, translation, and mitochondrial function in all cells (Hart, 2019). A dynamic interplay (often competitive) may be seen between O-GlcNAcylation and protein phosphorylation. After the pancreas, O-GlcNAcylation occurs most frequently in the brain, where as many as 40% of proteins are modified by this dynamic sugar, including nearly 2000 proteins in the synapse alone (Trinidad et al., 2012). O-GlcNAcylation has been directly linked to a range of neuronal functions, including AMPA receptor trafficking, synapse formation, neuronal excitability, and long-term potentiation and memory (Lagerlof and Hart, 2014).
In recent years, more than 100 publications have implicated reduced O-GlcNAcylation in the brain as a causative factor in late-onset Alzheimer’s disease and other tauopathies (Yuzwa and Vocadlo, 2014; Gong et al., 2016; Wani et al., 2017). The evidence for a direct role of O-GlcNAcylation in neurodegeneration associated with late-onset Alzheimer’s disease, in particular, is compelling (Yuzwa and Vocadlo, 2014; Gong et al., 2016; Wani et al., 2017): (i) in mice, fasting decreases O-GlcNAcylation and concomitantly increases phosphorylation of tau at several Alzheimer’s disease-associated sites; (ii) targeted brain deletion of O-GlcNAc transferase by Cre-Lox methods dramatically increases tau phosphorylation; (iii) paired helical filamentous tau (PHF-tau) undergoes ∼20% of the O-GlcNAcylation of normal tau; (iv) increasing O-GlcNAcylation by treating rodents with a very specific O-GlcNAcase inhibitor, Thiamet-G, reduces tau phosphorylation and improves learning and memory; (v) the five currently mapped O-GlcNAcylation sites on tau are phosphorylated when tau is hyperphosphorylated; (vi) O-GlcNAcylation increases the non-amyloidogenic secretase processing of amyloid precursor protein (APP) via O-GlcNAcylation of APP and nicastrin (subunit of γ-secretase that generates amyloid-β); (vii) the OGA gene, which encodes O-GlcNAcase—an enzyme that removes O-GlcNAc from target proteins—is located on chromosome 10q24.1, a locus associated with late-onset Alzheimer’s disease, and alternative splicing of OGA has been linked to Alzheimer’s disease; (viii) oral administration of Thiamet-G for 36 weeks to JNPL3 tau mice, which express the most common Alzheimer’s disease-causing APP mutation, markedly increased brain O-GlcNAcylation, and blocked cognitive decline as measured by a Morris water maze; (ix) synaptic loss is a hallmark of Alzheimer’s disease and O-GlcNAcylation helps regulate synaptic development and receptor cycling and trafficking to the plasma membrane (Lagerlof and Hart, 2014); and (x) a recent study in mice showed that hippocampal O-GlcNAc transferase expression and O-GlcNAcylation are decreased in aged mice, and that artificially decreasing O-GlcNAc transferase levels in young hippocampus impairs plasticity and cognition. Strikingly, increasing O-GlcNAc transferase in the hippocampus rescues these cognitive impairments, even in elderly mice (Wheatley et al., 2019).
However, with the exception of in vitro studies showing that O-GlcNAcylation of α-synuclein prevents its aggregation (Zhang et al., 2017; Levine et al., 2019), few studies have examined whether changes to OGN could also contribute to the degeneration of dopamine neurons in Parkinson’s disease. In their tour de force paper, Lee et al. therefore used targeted genetic manipulations of O-GlcNAcylation in murine dopamine neurons, along with pharmacological manipulations in mice, to increase or decrease O-GlcNAcylation. They combined these manipulations with biochemical and proteomic analyses of O-GlcNAcylation proteins in the midbrain dopamine area and striatum, and with analyses of dopamine neuronal function (Lee et al., 2020). The results revealed that O-GlcNAcylation is essential for the survival and maintenance of the midbrain dopamine system. Downregulation of O-GlcNAcylation in dopamine neurons caused severe motor defects, whereas upregulation of O-GlcNAcylation produced no adverse effects, but improved function at dopaminergic synapses.
Lee et al. (2020) explored the roles of O-GlcNAcylation in dopamine neurons and in the aetiology of Parkinson’s disease from many different angles. They performed targeted deletion of OGT in dopamine transporter (DAT)-expressing neurons and found that reduction of O-GlcNAcylation led to massive loss of axons, loss of axonal arborizations and loss of synapses in the dopamine neurons targeted, which was followed by degeneration and premature death. When O-GlcNAcylation was reduced in DAT neurons, the release of several neurotransmitters at synapses was also severely reduced. In contrast, targeted knockdown of OGA in DAT neurons increased O-GlcNAcylation and had no deleterious effects on neuronal structure or function; in fact it increased synaptic transmission. In other animal studies, knockout of OGT in DAT neurons led to reduced activity and reduced locomotor behaviour. To better understand the molecules involved in these effects, Lee et al. performed proteomic/glycomic analyses of the midbrain dopamine area and the striatum. They identified >900 O-GlcNAcylation proteins, including neurotransmitter transporters, synaptic adhesion molecules, post-synaptic density (PSD) proteins, GABA receptors and glutamate receptors. The authors then asked if increased O-GlcNAcylation could protect mice from Parkinson’s disease pathology induced by injection of an adeno-associated virus (AAV) bearing a mutant form of α-synuclein (A53T). They found that targeted knockdown of OGA not only increased O-GlcNAcylation, but also protected the mice from Parkinson’s disease pathology. They posited that upregulation of O-GlcNAcylation in vivo may make dopamine neurons less susceptible to pathological insults in Parkinson’s disease by blocking the aggregation of α-synuclein; a possibility supported by earlier in vitro studies (Levine et al., 2019). Behavioural analyses of the mice showed that increased O-GlcNAcylation in dopamine neurons normalized the behavioural abnormalities in this Parkinson’s disease model. Finally, using this same murine model, the authors showed that a specific O-GlcNAcase inhibitor, Thiamet-G, increased O-GlcNAcylation and effectively prevented and ameliorated Parkinson’s disease pathology.
The parallels between the findings of Lee et al. on the role of O-GlcNAcylation in Parkinson’s disease and the many similar studies on the role of O-GlcNAcylation in Alzheimer’s disease are striking. They suggest that reduced glucose utilization or uptake in parts of the ageing brain, leading to reduced O-GlcNAcylation of many brain proteins, contribute to fundamental mechanisms underlying neurodegeneration. This raises the exciting possibility that drugs that increase O-GlcNAcylation in the brain could be effective in preventing neurodegeneration and memory loss caused by both Alzheimer’s disease and Parkinson’s disease.
Glossary
O-GlcNAc transferase (OGT): Highly conserved enzyme that covalently attaches O-GlcNAc to thousands of proteins in all cells. OGT is highly abundant in neurons.
O-GlcNAcase (OGA): Highly conserved enzyme that selectively removes O-GlcNAc from serine and threonine residues on proteins. The combined action of OGT and OGA cause the cycling of O-GlcNAc, analogous to the cycling of phosphate residues on proteins.
O-GlcNAcylation (OGN): The cycling of N-acetylglucosamine on serine and threonine residues of proteins, which serves as a major nutrient sensor in all cells. The rate of cycling on and off proteins is site-dependent.
Paired helical filamentous tau (PHF-tau): Tau, which is an important microtubule bundling protein, forms insoluble fibres (PHF-tau) when it is hyperphosphorylated in Alzheimer’s disease and other tauopathies. In normal brain, tau is modified by O-GlcNAc, which helps prevent its phosphorylation and aggregation.
Thiamet-G (TMG): A highly specific inhibitor of OGA, the enzyme that removes O-GlcNAc. TMG analogues readily cross the blood–brain barrier and show promise for the treatment of neurodegenerative disease.
Funding
G.W.H. and C.-W.H. are supported by NIH R01GM116891 and by the Georgia Research Alliance.
Competing interests
The authors report no competing interests.
References
- Gong CX, Liu F, Iqbal K.. O–GlcNAcylation: a regulator of tau pathology and neurodegeneration. Alzheimers Dement 2016; 12: 1078–89. [DOI] [PubMed] [Google Scholar]
- Hart GW. Nutrient regulation of signaling & transcription. J Biol Chem 2019; 294: 2211–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerlof O, Hart GW.. O-GlcNAcylation of neuronal proteins: roles in neuronal functions and in neurodegeneration. Adv Neurobiol 2014; 9: 343–66. [DOI] [PubMed] [Google Scholar]
- Lee BE, Kim HY, Kim H-J, Jeong H, Kim B-G, Lee H-E, et al. O-GlcNAcylation regulates dopamine neuron function, survival, and degeneration in Parkinson disease. Brain 2020; 143: 3699–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine PM, Galesic A, Balana AT, Mahul-Mellier AL, Navarro MX, De Leon CA, et al. α-Synuclein O-GlcNAcylation alters aggregation and toxicity, revealing certain residues as potential inhibitors of Parkinson's disease. Proc Natl Acad Sci USA 2019; 116: 1511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinidad JC, Barkan DT, Gulledge BF, Thalhammer A, Sali A, Schoepfer R, et al. Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol Cell Proteomics 2012; 11: 215–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani WY, Chatham JC, Darley-Usmar V, McMahon LL, Zhang J.. O-GlcNAcylation and neurodegeneration. Brain Res Bull 2017; 133: 80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley EG, Albarran E, White CW 3rd, Bieri G, Sanchez-Diaz C, Pratt K, et al. Neuronal O-GlcNAcylation improves cognitive function in the aged mouse brain. Curr Biol 2019; 29: 3359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzwa SA, Vocadlo DJ.. O-GlcNAc and neurodegeneration: biochemical mechanisms and potential roles in Alzheimer's disease and beyond. Chem Soc Rev 2014; 43: 6839–58. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lei H, Chen Y, Ma YT, Jiang F, Tan J, et al. Enzymatic O-GlcNAcylation of α-synuclein reduces aggregation and increases SDS-resistant soluble oligomers. Neurosci Lett 2017; 655: 90–4. [DOI] [PubMed] [Google Scholar]