See Krone and Vyazovskiy (doi:10.1093/brain/awaa396) for a scientific commentary on this article.
Using TMS and EEG in awake patients with focal cortical injuries, Sarasso et al. reveal the presence of sleep-like dynamics in perilesional areas, coexisting with typical wakefulness cortical reactivity in control areas. Reversing perilesional sleep-like dynamics could represent a novel strategy for rehabilitation.
Keywords: stroke, TMS/EEG, sleep
Abstract
The functional consequences of focal brain injury are thought to be contingent on neuronal alterations extending beyond the area of structural damage. This phenomenon, also known as diaschisis, has clinical and metabolic correlates but lacks a clear electrophysiological counterpart, except for the long-standing evidence of a relative EEG slowing over the injured hemisphere. Here, we aim at testing whether this EEG slowing is linked to the pathological intrusion of sleep-like cortical dynamics within an awake brain. We used a combination of transcranial magnetic stimulation and electroencephalography (TMS/EEG) to study cortical reactivity in a cohort of 30 conscious awake patients with chronic focal and multifocal brain injuries of ischaemic, haemorrhagic and traumatic aetiology. We found that different patterns of cortical reactivity typically associated with different brain states (coma, sleep, wakefulness) can coexist within the same brain. Specifically, we detected the occurrence of prominent sleep-like TMS-evoked slow waves and off-periods—reflecting transient suppressions of neuronal activity—in the area surrounding focal cortical injuries. These perilesional sleep-like responses were associated with a local disruption of signal complexity whereas complex responses typical of the awake brain were present when stimulating the contralesional hemisphere. These results shed light on the electrophysiological properties of the tissue surrounding focal brain injuries in humans. Perilesional sleep-like off-periods can disrupt network activity but are potentially reversible, thus representing a principled read-out for the neurophysiological assessment of stroke patients, as well as an interesting target for rehabilitation.
See Krone and Vyazovskiy (doi:10.1093/brain/awaa396) for a scientific commentary on this article.
Introduction
The functional consequences of a focal brain lesion are due to direct structural damage as well as to alteration in the dynamics of neighbouring and connected areas (von Monakow, 1914). Detecting the electrophysiological changes occurring in these areas and understanding their neuronal underpinnings has been so far elusive.
A lateralized slowing of the wake EEG over the zone surrounding focal lesions is a classic notion derived from early recordings in acute stroke patients (Nuwer et al., 1987). However, since the use of electrophysiology in stroke research was superseded by structural and metabolic imaging techniques, our understanding of stroke-related EEG slow rhythms in humans proceeded at a rather low pace. By contrast, over the past 20 years the cellular/network mechanisms of physiological slow waves have been clearly identified in in vivo, in vitro as well as in silico models (Steriade et al., 1993; Sanchez-Vives and McCormick, 2000; Compte et al., 2003).
Compelling evidence ascertained that sleep EEG slow waves reflect the occurrence of brief interruptions of neuronal firing (off-periods) associated with a slow oscillation of the membrane potential (Steriade et al., 1993). Off-periods are due to the tendency of cortical neurons to fall into a silent hyperpolarized state after an initial activation [a phenomenon known as cortical bistability (Steriade et al., 1993; Timofeev et al., 2001)] and represent the most dramatic change in the functional regime of cortical circuits that can be readily detected as suppressions of high frequency power associated with slow waves (Mukovski et al., 2007) as shown in scalp (Piantoni et al., 2013) and intracranial recordings (Cash et al., 2009; Lewis et al., 2012). The mechanisms for such cellular behaviour rely on both neuronal as well as network properties, such as enhanced adaptation mechanisms (Compte et al., 2003) and shifts in the excitation/inhibition balance (Timofeev et al., 2001). Although similar alterations may also occur as a consequence of brain injury (Nita et al., 2007; Clarkson et al., 2010), the lateralized slow waves occurring after focal brain injury have never been explicitly connected to the electrophysiology of physiological sleep slow waves.
Such a putative connection is particularly interesting when considering that slow waves and neuronal off-periods can occur locally not only during sleep (Nir et al., 2011), but also during wakefulness with important motor/cognitive behavioural consequences (Vyazovskiy et al., 2011; Nir et al., 2017).
In this work we test the hypothesis that the electrophysiological alteration affecting structurally intact perilesional areas reflects a tendency of cortical circuits to fall into a silent off-period, i.e. a pathological form of local sleep patterns in the awake injured brain.
By virtue of their activity-dependent nature, cortical bistability and the associated off-periods can be better revealed above and beyond spontaneous activity by recording the cortical response to direct perturbations (Pigorini et al., 2015; Usami et al., 2015; Rosanova et al., 2018). Here, by applying transcranial magnetic stimulation (TMS) perturbations in a cohort of 30 conscious awake brain-injured patients we reveal the presence of local sleep-like off-periods in the perilesional area surrounding focal cortical lesions. These local off-periods were found even when not manifest in the spontaneous EEG, were associated with a disruption of local signal complexity and were not present when stimulating a control contralateral site.
By connecting the notion of local sleep to the pathophysiology of focal brain injury and stroke, perilesional off-periods may represent a valid read-out of the state of discrete cortical circuits following brain injury as well as a potential target for the development of novel therapeutic interventions and physical rehabilitation aimed at fostering functional recovery.
Materials and methods
Patients
Three groups of conscious awake brain-injured patients (total n = 30) of various aetiologies and disease severity were included in the study (Supplementary Table 1). Specifically, we included (i) a group of 10 patients affected by unilateral cortico-subcortical lesions due to an ischaemic occlusion of the middle cerebral artery [MCA ischaemia group; four females; mean age ± standard error of the mean (SEM): 68 ± 4 years old]; (ii) a group of 10 patients affected by severe ischaemic, haemorrhagic and traumatic multifocal cortico-subcortical lesions (severe multifocal lesions group; four females; mean age ± SEM: 53 ± 6.3 years old); and (iii) a group of 10 patients affected by unilateral lacunar ischaemic or typical haemorrhagic purely subcortical lesions (subcortical lesions group; six females; mean age ± SEM: 72 ± 2.6 years old). All patients were in a subacute to chronic stage (>1 month; mean duration in months ± SEM: 12.9 ± 3.9; 7.6 ± 2.5; 19.5 ± 8.2, respectively). Individual lesion volumes were quantified for the MCA ischaemia and subcortical lesions group (mean volume ± SEM: 107.8 ± 16.7 and 2.6 ± 0.8 cm3, respectively; Wilcoxon rank sum test Z = 3.7, P = 0.0001). The identified lesion was slice-by-slice segmented with a manual contouring of the area of interest, by an experienced radiologist (M.Q.) on the fluid attenuated inversion recovery (FLAIR) images (repetition time = 11 000 ms, echo time = 140 ms, no gap between slices, coronal plane) using the OsiriX Lite DICOM viewer (v.11.0.3; Pixmeo SARL) open-source software. To calculate the entire lesion volume every segmented area was multiplied to the slice thickness volume (i.e. 5 mm). Because of the inclusion of TBI patients (4/10) and the associated presence of diffuse axonal injury, we were not able to compute lesion volumes for the severe multifocal lesions group.
Clinical evaluation of the MCA ischaemia and subcortical lesion groups, included the NIH Stroke Scale [NIHSS (Brott et al., 1989); average (min/max) NIHSS score: 8 (4/17) and 5.5 (2/10), respectively; Wilcoxon rank sum test Z = 2.1, P = 0.03). The Coma Recovery Scale-Revised [CRS-R (Giacino et al., 2004)] was applied for the severe multifocal lesions group which included patients in a minimally conscious state (MCS; n = 6) as well as patients who emerged from the MCS and recovered functional communication (emerged MCS; n = 4).
The local ethical committees of IRCCS Fondazione Don Carlo Gnocchi Onlus, Comitato Etico Interaziendale Milano Area A in Milan, Italy, and the Faculty of Medicine of the University of Liege in Liege, Belgium approved the experimental protocols. Written informed consent was obtained from all the patients or their legal surrogates (as in the case of MCS patients).
Experimental procedures
Each patient underwent a single experimental session including two TMS/EEG measurements with TMS targeted to intact cortical portions of both the affected (perilesional stimulation site) and the unaffected (contralesional stimulation site) hemispheres. During the experiment, patients were awake with their eyes open. If signs of drowsiness appeared, recordings were momentarily interrupted and patients were stimulated using the CRS-R arousal facilitation protocols (Giacino et al., 2004). Anatomical lesions were identified on T1-weighted MRI or CT scans acquired within 1 week prior to the experimental sessions and used to operationalize the selection of TMS targets (Rosanova et al., 2018). Specifically, in the case of the MCA ischaemia group, the perilesional stimulation site corresponded to the intact portion of the same Brodmann Area (BA) affected by the lesion (either BA4, BA6 or BA7) within 2 cm of the lesion boundary, while the contralesional stimulation site corresponded to the homologue contralateral cortical area. The same criteria were applied for the severe multifocal lesions group. For this group, in case the contralesional stimulation site was also directly affected by a lesion or inaccessible to TMS due to the presence of intracerebral drainage/shunt, we chose another BA over either the same (Patients 16 and 18) or the contralateral (Patients 11 and 19) hemisphere. Finally, for the subcortical lesion group, perilesional and contralesional stimulation sites consisted in the same frontal target (either BA4 or BA6) over the affected or the unaffected hemisphere, respectively.
Of note, for all patients, when stimulating BA4 we explicitly avoided inducing muscle twitches (hand/arm, face, shoulder/trunk and foot/leg) in order to avoid TMS-evoked EEG potentials (TEPs) contamination by the sensory re-entry of proprioceptive feedback (Fecchio et al., 2017). A detailed description regarding the individual patient lesions as well as stimulation sites are shown in Supplementary Table 1.
TMS pulses were delivered with a focal bipulse figure-of-eight coil (mean/outer winding diameter ∼50/70 mm, biphasic pulse shape, pulse length ∼280 µs, focal area of the stimulation 0.68 cm2) driven by a Mobile Stimulator Unit (eXimia TMS Stimulator, Nexstim Ltd.). For all TMS/EEG measurements, we aimed at the convexity of the targeted cortical gyrus with the induced current perpendicular to its main axis. The estimated electric field was of ∼120 V/m corresponding to a percentage of the maximal stimulator output intensity comparable between contralesional and perilesional stimulation sites (mean ± SEM: 65.3 ± 1.97 versus 67.1 ± 2.61 for the MCA ischaemia group; 62.6 ± 3.7 versus 67.8 ± 2.58 for the severe multifocal lesions group; 59.2 ± 1.73 versus 60.9 ± 2.24 for the subcortical lesion group; for all comparisons P > 0.05, paired t-test) as well as across groups [mixed-model ANOVA categorical predictor: Group; within-subject factor: Site Group × Site interaction effect: F(2,27) = 0.3, P = 0.73933]. In each TMS/EEG measurement, at least 200 pulses were delivered with an interstimulus interval randomly jittering between 2000 and 2300 ms. The precision and reproducibility of the TMS pulses with respect to the selected targets was guaranteed by means of a Navigated Brain Stimulation (NBS) system (Nexstim Ltd.). EEG data were recorded using a TMS-compatible 60-channel amplifier (Nexstim Ltd.). Raw recordings were online referenced to an additional forehead electrode, filtered between 0.1 Hz and 350 Hz and sampled at 1450 Hz. Two additional sensors were applied to record the electrooculogram (EOG). During all TMS/EEG recordings a masking sound was played via earphones and a thin layer of foam was placed between the coil and the scalp for abolishing the sensory (auditory and somatosensory) inputs associated with TMS coil discharge.
TMS/EEG data recording and analysis
TMS/EEG data were band-pass filtered (1–45 Hz, zero-phase shift Butterworth, 3rd order), down-sampled to 725 Hz and segmented in a time window of ± 600 ms around the stimulus. Bad channels (≤10% of channels per recording) were interpolated using spherical splines. A comparable number of good trials between contralesional and perilesional stimulation sites (mean ± SEM: 184 ± 16 versus 184 ± 14 for the MCA ischaemia group; 150 ± 12 versus 137 ± 13 for the severe multifocal lesion group; 184 ± 20 versus 196 ± 23 for the subcortical lesion group; for all comparisons P > 0.05, paired t-test) were re-referenced to the average reference and baseline corrected. Finally, after reducing the number of independent components to the number of good, non-interpolated channels by performing singular value decomposition (SVD), independent component analysis (ICA) was applied (Rosanova et al., 2018).
To detect the presence of local sleep-like cortical reactivity we followed the methodological rationale of Pigorini et al. (2015) and Rosanova et al. (2018). Specifically, for each group of patients, we aimed at quantifying (i) the presence of TMS-evoked slow waves over the perilesional area; and (ii) the correspondent occurrence of a cortical off-period. Operationally, these variables can be quantified respectively as (i) the amplitude of low-frequency EEG components (<4 Hz); and (ii) the modulation of post-stimulus high-frequency EEG power (>20 Hz) (Mukovski et al., 2007; Cash et al., 2009; Piantoni et al., 2013). We refer the reader to Pigorini et al. (2015) and Rosanova et al. (2018) for a detailed methodological description. In brief, single trials were low-pass filtered (4 Hz, second-order Chebyshev filter), re-referenced to the mathematically-linked mastoids, averaged and rectified. For each channel, the slow wave amplitude was computed as the cumulated amplitude of the rectified signal within the 8–350-ms time window. On the other hand, we applied the event-related spectral perturbation (ERSP) procedure (Grandchamp and Delorme, 2011). For each channel, high-frequency EEG power was obtained as the integral of the significant high-frequency (>20 Hz) power averaged between 150 and 350 ms. For each TMS/EEG measurement, slow wave amplitude and high-frequency EEG power were averaged over the four channels closest to the stimulation site (Casarotto et al., 2013; Rosanova et al., 2018) for group analysis (Fig. 2B, D and F).
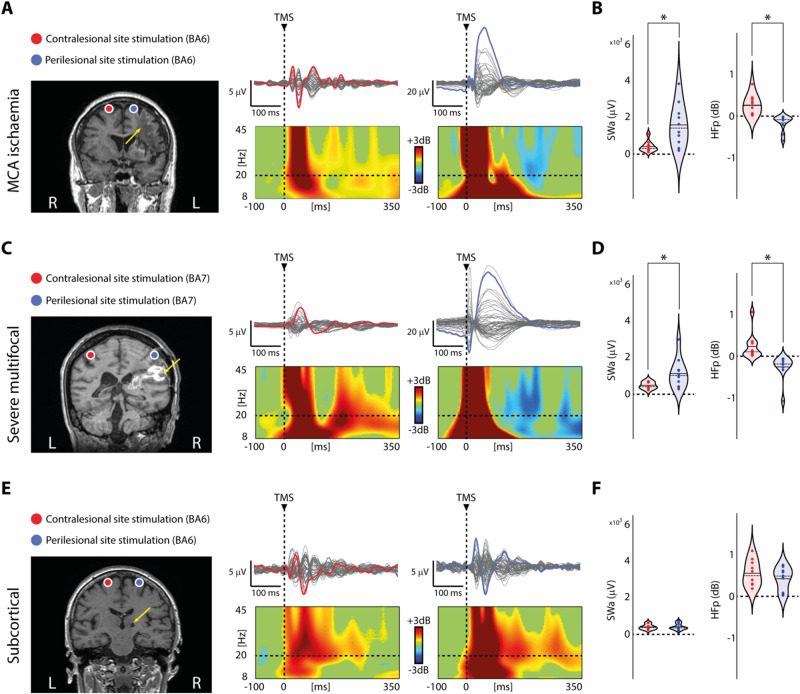
Figure 2.
Perilesional cortical off-periods are present in all patients with cortico-subcortical lesions irrespective of the aetiology. (A, C and E) Results from three representative patients, one for each group (Patients 4, 12 and 23 from Supplementary Table 1, respectively) are shown for both contralesional (red) and perilesional (blue) stimulation sites. For each panel, MRIs and cortical targets as estimated by the NBS system are shown (left). The yellow arrows highlight lesion location. Butterfly plots of the TEPs recorded at all 60 electrodes (traces) are depicted (right). A dashed vertical line marks the occurrence of TMS. ERSP is presented for the EEG electrode (coloured trace) with the largest early response, selected among the four channels closest to TMS. In the ERSP plot, significance for bootstrap statistics is set at α = 0.05 (absence of any significant difference form baseline spectrum is coloured in green). Statistically significant increases of power compared to baseline are coloured in yellow/red, while blue represents significant power decreases. The dashed horizontal line indicates the 20 Hz frequency bin. (B, D and F) Violin plots and individual values of slow wave amplitude (SWa, left) and high-frequency power (HFp, right) calculated for the contralesional (red) and perilesional (blue) stimulation sites for the three groups. Violin plots display the median (dashed line) and the mean (solid line) of the kernel density. *P < 0.05; Wilcoxon signed-rank test.
For each patient, we then estimated the maximum global spatiotemporal dynamics of TEPs by quantifying the temporal complexity of the principal components of the EEG response to TMS (PCIst) as described in Comolatti et al. (2019). Briefly, principal component analysis of the response is performed in order to obtain the spatial modes of the signal and a method derived from recurrence quantification analysis (RQA) is then applied to quantify the number of ‘state transitions’ present in each component. PCIst reflects the overall capacity of thalamocortical circuits to engage in complex patterns of causal interactions, typically present in conscious awake individuals. In addition, we applied a generalization of the original method and, for each stimulation session, we calculated PCIst restricted to the four channels closest to the stimulation site to assess the impact of perilesional sleep-like off-periods on local signal complexity. For both the global and local signal complexity estimates, PCIst was computed using the source-code available at github.com/renzocom/PCIst using the same parameters for component selection (max_var = 99%; min_snr = 1.1) and state transition quantification (k = 1.2) as in Comolatti et al. (2019).
Resting-state EEG data recording and analysis
Preceding TMS/EEG measurements, a wake spontaneous EEG recording (up to 10 min with eyes open) was performed with the same apparatus for the MCA ischaemia patients (n = 10) to assess the presence of the lateralized slowing typical of unilateral brain injuries characterized by cortical infarction (Macdonell et al., 1988).
Spontaneous EEG data were offline filtered (0.5–40 Hz, zero-phase shift Butterworth, 3rd order). Continuous data were then split into contiguous 2-s segments. Visually inspected artefactual segments were excluded from the analysis (max/min retained EEG segments: 284/69). Bad channels were rejected (≤10% of channels per recording) and interpolated using spherical splines. Signals were re-referenced to the average reference, SVD and ICA were applied and power spectral density (PSD) estimates were computed across segments for each channel using the Welch’s method (2-s Hanning windows, 50% overlap). For each patient, PSD was averaged across the same channels used for TMS/EEG analysis (four contralesional and four perilesional) and further averaged across bins pertaining to the classical EEG frequency ranges (Fig. 3A). In parallel to the PSD analysis, filtered continuous EEG data were re-referenced according to a longitudinal bipolar montage based on the 10-20 system and visually inspected by a clinical neurophysiologist blind to the anatomical site of the lesion to assess the presence of EEG anomalies (Table 1 and Fig. 3B).
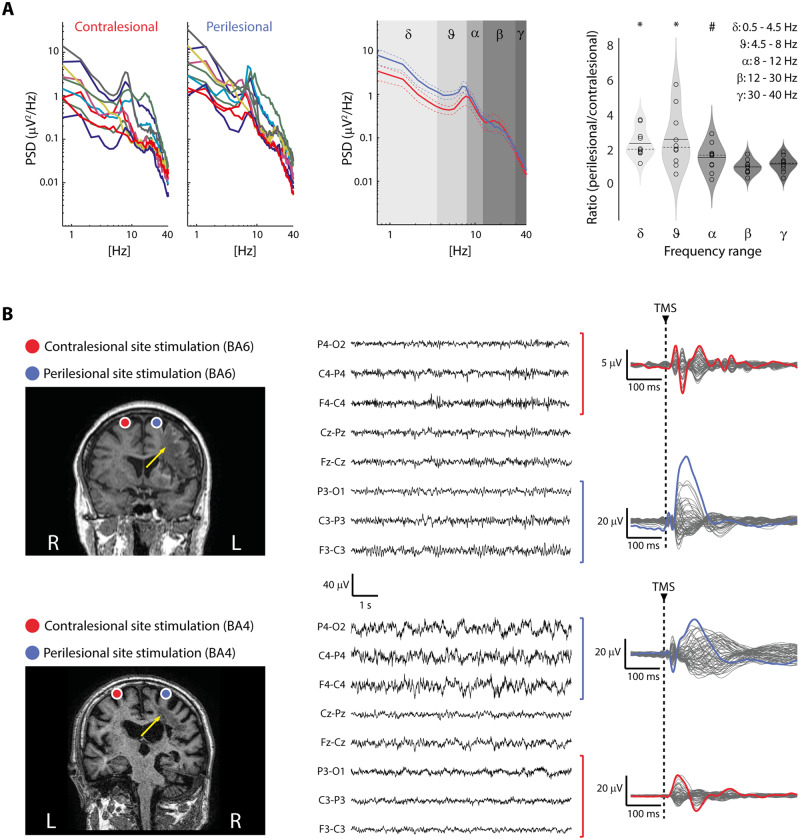
Figure 3.
Resting-state EEG recordings in the MCA ischaemia group. (A) For each patient, colour-coded individual PSD calculated over the same four channels used for TMS/EEG analysis is shown (left). Average PSD (±SEM) across patients (contralesional, red; perilesional, blue) is also shown with shaded grey boxes identifying classical frequency ranges (middle). Violin plots and individual values of the ratio between perilesional and contralesional site PSD averaged across bins divided into classical EEG frequency ranges: delta (0.5–4.5Hz), theta (4.5–8 Hz), alpha (8–12 Hz), beta (12–30 Hz) and gamma (30–40 Hz) (right). Group analysis showed a significant increase of delta and theta EEG activity over the perilesional site (*P-values <0.008 for both delta and theta; Bonferroni corrected α = 0.01), confirming the typical EEG pattern found in unilateral brain injuries characterized by cortical infarction (Macdonell et al., 1988). Alpha frequency was also found increased over the perilesional site (#P = 0.02, uncorrected). Violin plots display the median (dashed line) and the mean (solid line) of the kernel density. (B) MRIs and cortical targets as estimated by the NBS system are shown for two representative patients (Patients 4 and 5 from Supplementary Table 1, left). The yellow arrows highlight lesion location. The visual inspection of EEG (here displayed with a reduced longitudinal bipolar montage focused on the regions explored by TMS, middle) confirmed the PSD findings and highlighted heterogeneous EEG patterns (Table 1) characterized by the lateralized presence of either theta rhythms (top) or slow waves (bottom). Notably, in both cases, applying TMS (dashed vertical line) resulted in a clear-cut evoked EEG slow wave over the perilesional site (blue traces on the right), thus showing the added value of TMS in revealing the presence of perilesional slow waves (and of the associated off-periods, not shown here, but see Figs 1 and 2) irrespective of the presence of slow waves spontaneously occurring in the background EEG.
Table 1.
Spontaneous EEG clinical assessment of MCA ischaemia patients
| Patient number | Lateralized anomalies | Focal anomalies |
Bilateral anomalies |
|||
|---|---|---|---|---|---|---|
| Location | Frequency band | Incidence | Location | Side prevalence | ||
| 1 | Marked | Right F-C-T | Delta-theta | Subcontinuous | − | − |
| 2 | None | − | − | − | − | − |
| 3 | Mild | Right T | Sharp waves | Intermittent | − | − |
| 4 | Mild | Left F-C | Theta | Intermittent | − | − |
| 5 | Marked | Right C-P-T | Delta | Continuous | − | − |
| 6 | Mild | Right P | Delta | Subcontinuous | Bifrontal | Left |
| 7 | Marked | Right T-P | Delta-theta | Subcontinuous | − | − |
| 8 | None | − | − | − | − | − |
| 9 | Mild | Left F-T | Delta | Sporadic | − | − |
| 10 | Mild | Right P-O | Theta | Subcontinuous | − | − |
Continuous EEG data were re-referenced according to a longitudinal bipolar montage based on the 10-20 system including the following EEG derivations: ‘Fp1_F5’, ‘F7_T3’, ‘T3_T5’, ‘T5_O1’ ‘Fp1_F3’, ‘F3_C3’, ‘C3_P3’, ‘P3_O1’, ‘Fz_Cz’, ‘Cz_Pz’, ‘Fp2_F6’, ‘F6_T4’, ‘T4_T6’, ‘T6_O2’, ‘Fp2_F4’, ‘F4_C4’, ‘C4_P4’, and ‘P4_O2’. These data were then visually inspected by a clinical neurophysiologist blind to the anatomical site of the lesion to assess the presence of EEG anomalies. C = central; F = frontal; O = occipital; P = parietal; T = temporal.
Statistical analyses
For slow wave amplitude, high-frequency EEG power, local signal complexity, and resting-state PSD, within-group comparisons between stimulation sites (perilesional, contralesional) were performed by means of the non-parametric Wilcoxon signed-rank test (α = 0.05; n = 10). When testing between-group differences, either mixed-model ANOVAs were performed with GROUP as categorical predictor and SITE as within-subject factor (α = 0.05; n = 30) with post hoc two-tailed t-tests (α = 0.05, Bonferroni correction), or Wilcoxon rank-sum test (α = 0.05; n = 20) were used.
Data availability
All data needed to evaluate the paper's conclusions are present in the paper. Additional data related to this paper may be requested from the corresponding author.
Results
TMS reveals perilesional sleep-like cortical off-periods
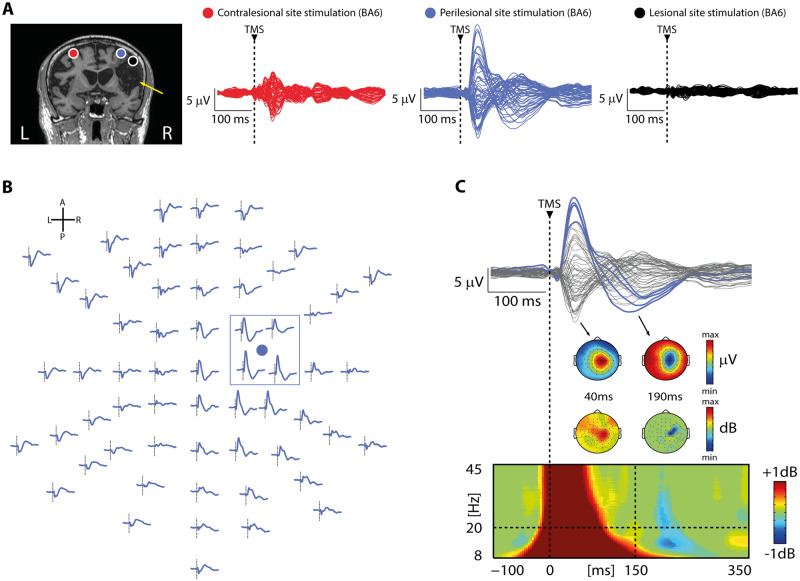
In the MCA ischaemia group, TEPs obtained from the stimulation of the contralesional site were composed by low-amplitude, fast-frequency recurrent waves similar to those previously observed in healthy awake individuals (Rosanova et al., 2009, 2018) (Figs 1A and 2A, red trace). When TMS was applied using the same stimulation parameters over the perilesional site, TEPs were characterized by a local slow EEG potential (Figs 1A–C and 2A, blue traces) associated with an initial broad-band activation followed by a significant suppression of high frequency EEG power starting roughly at 150 ms after TMS (mean ± SEM: 167 ± 14 ms) over the four channels closest to the stimulation site (Fig. 1B and C). Notably, this local pattern of activation, matching the criteria for an off-period, was found for all 10 patients only over the perilesional stimulation site and never over the contralateral unaffected stimulation site. Consistently, slow wave amplitude obtained over the perilesional site was significantly higher compared to the contralesional homologue site (Wilcoxon signed-rank test Z = 2.4, P = 0.01; Fig. 2B). Also, high-frequency EEG power was significantly different between the two stimulated sites (Wilcoxon signed-rank test Z = 2.8, P = 0.005), and suppressed for all patients only over the perilesional stimulation site (range: −0.01/−0.6 dB, Fig. 2B). In the same group of patients, spontaneous EEG showed the typical lateralized slowing (encompassing delta, theta and alpha frequency bands) over the affected hemisphere (Fig. 3A). Notably though, perilesional off-periods were invariably found in all subjects irrespective of the presence of lateralized slow waves spontaneously occurring in their background EEG (Fig. 3B and Table 1).
Figure 1.
TMS reveals local, sleep-like slow waves associated with cortical off-periods over the affected hemisphere. (A) Results from one representative patient (Patient 10 from Supplementary Table 1) are shown for both the contralesional (red) and perilesional (blue) stimulation sites as well as for an additional TMS/EEG measurement performed in this patient directly targeting the cortical lesion (black). Left: MRI and cortical targets (BA6) as estimated by the NBS system are shown. The yellow arrow highlights lesion location. Right: Butterfly plots of the TEPs recorded at all 60 electrodes for the three stimulation sites. Note the absence of EEG reactivity to TMS of the lesion. (B) TEPs of the perilesional stimulation site for all EEG sensors is displayed (based on the channel layout; anterior is up, posterior is down, left is left, right is right). For each channel, a dashed vertical line marks the occurrence of TMS. The blue circle represents the position of the coil focus over the scalp. The blue box highlights the four channels closest to TMS. Note the occurrence of a local slow wave over the right hemisphere. (C) Butterfly plot of the TEPs recorded at all 60 electrodes for the perilesional stimulation site (top, same as in A). The four EEG electrode closest to TMS (indicated by the blue box in B) are displayed in colour. The instantaneous voltage topography of the positive and negative deflections of the slow wave is depicted below with the corresponding time points. ERSP averaged across the four EEG electrodes closest to TMS (bottom). Statistically significant increases of power compared to baseline are coloured in red, while blue represents significant power decreases. The dashed horizontal line indicates the 20 Hz frequency bin while the dashed vertical line at 150 ms indicates the beginning of the time window used for high-frequency (>20 Hz) power quantification (see ‘Materials and methods’ section). The instantaneous voltage topography of the averaged power between 20 Hz and 45 Hz is depicted above for the same time points. Note the occurrence of a local significant suppression of high-frequency power limited to the perilesional stimulation site concurrent with the negative deflection of the slow wave. For A–C, a dashed vertical line at time 0 marks the occurrence of TMS.
Similar results were obtained in the severe multifocal lesions group (Fig. 2C). Also in this case, perilesional stimulation was characterized by a slow wave associated with an initial broad-band activation followed by a significant suppression of high frequency EEG power beginning at 158 ± 16 ms (mean ± SEM) over the four channels closest to the stimulation site. Consistently both slow wave amplitude and high-frequency EEG power (Fig. 2D) obtained over the perilesional site were significantly different compared to the contralesional site (Wilcoxon signed-rank test Z = 2.3, P = 0.01 and Z = 2.8, P = 0.005, respectively). Importantly, also in this group, the high-frequency EEG power suppression was present for all 10 patients only over the perilesional stimulation site (range: −0.06/−1.09 dB, Fig. 2D).
Overall, results in these two groups of patients confirmed that, irrespective of the aetiology, TMS in the presence of cortico-subcortical lesions featured a sleep-like slow wave associated with the presence of a cortical off-period confined to the perilesional stimulated site. Further confirming the sleep-like nature of the observed findings, Supplementary Fig. 1 shows the results obtained for a representative patient (Patient 14, see Supplementary Table 1) from the severe multifocal lesions group. In this patient, in addition to the two TMS/EEG measurements performed during wakefulness (Supplementary Fig. 1B), the same cortical targets were also stimulated while the patient was asleep for the entire duration of the recording (Supplementary Fig. 1C). Results show a striking similarity (both in terms of overall slow wave amplitude and significant high-frequency EEG power suppression) between the TEPs obtained over the perilesional stimulation site during wakefulness and those obtained over both stimulation sites during sleep.
At odds with the above, TEPs obtained in the subcortical lesions group were never associated with the presence of a clear-cut slow wave or high frequency EEG power suppression over neither the perilesional nor the contralesional stimulation site (Wilcoxon signed-rank test Z = 0.7, P = 0.44 for slow wave amplitude and Z = 0.8, P = 0.38 for high-frequency EEG power; Fig. 2E and F). Interestingly, in this group of patients both contralesional and perilesional slow wave amplitude and high-frequency EEG power values were similar to those obtained over the contralesional stimulation site in both MCA ischaemia and severe multifocal lesions groups (Fig. 2B, D and F). To test this, we performed separate mixed-model ANOVAs for slow wave amplitude and high-frequency EEG power (categorical predictor: Group; within-subject factor: Site). Values obtained in the subcortical lesions group over both perilesional and contralesional sites were not statistically different from those obtained over the contralesional site of both MCA ischaemia and severe multifocal lesions, as revealed by post hoc comparisons (all P-values >0.2, Bonferroni corrected). Further, in one patient affected by multiple unilateral lacunar periventricular ischaemic white matter lesions (Patient 24; Supplementary Table 1) we performed a more extensive mapping including two additional stimulations targets (BA7 of both hemispheres; Fig. 4) confirming the absence of TMS-evoked slow waves and off-periods. Though slow waves and off-periods were not detectable, in this group of patients we observed an overall slowing of TMS-evoked oscillations (i.e. natural frequency: 15.8 ± 2.2 Hz and 15.3 ± 2.2 Hz for the contralesional and perilesional stimulation sites, respectively) compared to that previously observed for healthy controls over the same stimulation sites [average: 26.5 Hz for BA6 and 21.8 Hz for BA4; overall range: 19–29 Hz (Ferrarelli et al., 2012)].
Figure 4.
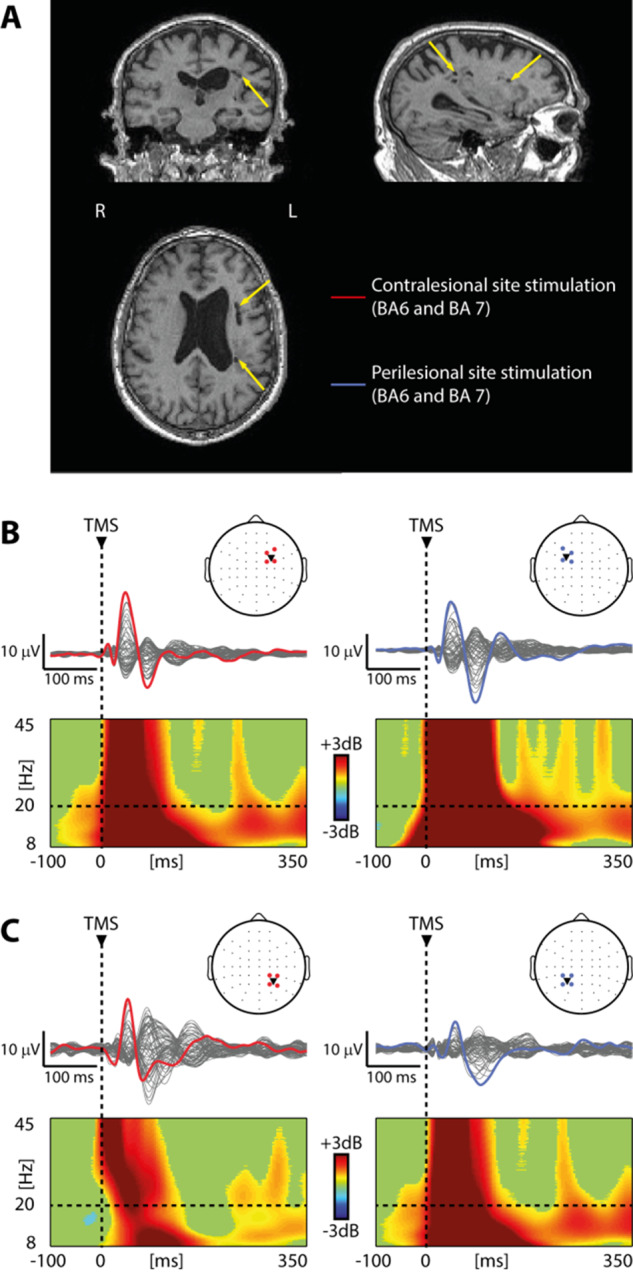
The absence of TMS-evoked slow waves and off-periods irrespective of the stimulated hemisphere and cortical area in the group of patients affected by unilateral lacunar ischaemic or haemorrhagic subcortical lesions. Results from one representative patient (Patient 24 from Supplementary Table 1) are shown for both the contralesional (red) and perilesional (blue) stimulation sites. (A) The MRI of the patient is shown. The yellow arrows highlight lesion location. (B and C) Butterfly plots of TEPs recorded at all 60 electrodes (traces) after TMS of both BA6 (B) and both BA7 (C) are depicted. A dashed vertical line marks the occurrence of TMS. ERSP is presented for the EEG electrode (coloured trace) with the largest early response, selected among the four channels closest to TMS (all presented in colour in the channel layout next to each butterfly plot). The dashed horizontal line indicates the 20 Hz frequency bin. Note the absence of TMS-evoked slow waves and off-periods across all stimulated sites.
The presence of perilesional sleep-like cortical off-periods affects local signal complexity
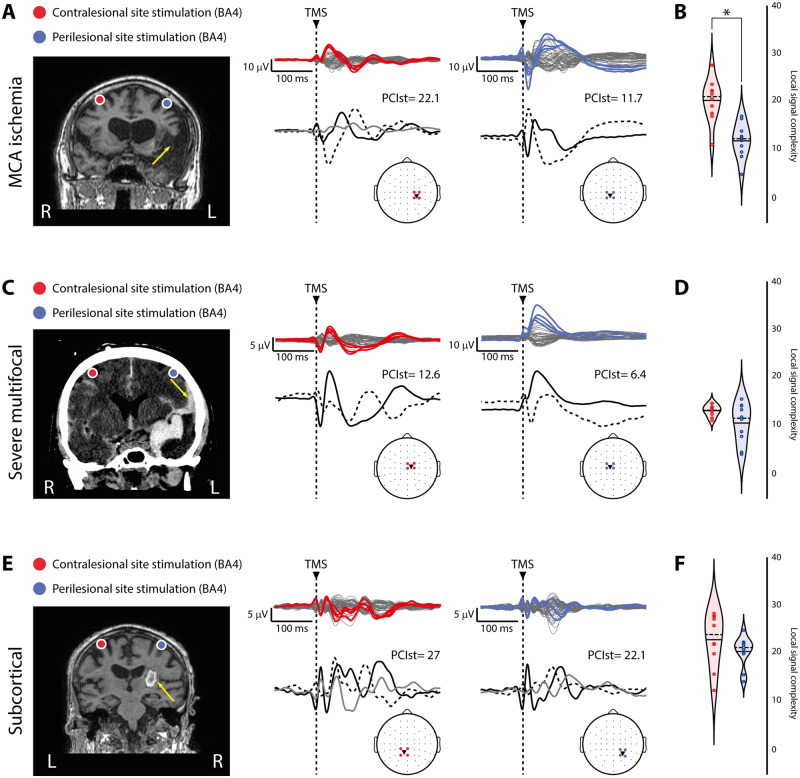
For each patient and stimulated site, we then quantified the temporal complexity of the principal components of the signals calculated over the four channels closest to TMS (Fig. 5A, C and E) and found a significant reduction of local signal complexity over the perilesional compared to the contralesional stimulation site for the MCA ischaemia group (Wilcoxon signed-rank test Z = 2.4, P = 0.01; Fig. 5B). A similar, albeit not statistically significant result was found for the severe multifocal lesions group where differences were only showing a statistical trend (Wilcoxon signed-rank test Z = 1.5, P = 0.1; Fig. 5D). The same analysis applied on the subcortical lesions group showed no differences (Wilcoxon signed-rank test Z = 1.2, P = 0.2; Fig. 5F). Altogether, these findings suggest that, when present, local sleep-like cortical off-periods affect local signal complexity.
Figure 5.
The presence of local, sleep-like cortical off-periods affects local signal complexity. (A, C and E) For each panel, brain imaging (MRI for A and E, CT scan for C) and cortical targets as estimated by the NBS system are shown for three representative patients, one for each group of patients (Patients 1, 13 and 22 from Supplementary Table 1, respectively) are shown for both the contralesional (red) and perilesional (blue) stimulation sites. The yellow arrows highlight lesion location. Butterfly plots of the TMS-evoked EEG potentials recorded at all 60 electrodes (traces) are depicted (top). For each stimulated site, the four EEG electrode closest to TMS are displayed in colour and topographically projected on the template channel layout (bottom). The correspondent local signal complexity values (calculated by applying PCIst restricted to the four channels, see ‘Materials and methods’ section) and the time course of the principal components based on singular value decomposition are depicted (bottom). A dashed vertical line marks the occurrence of TMS. (B, D and F) Violin plots and individual values of local signal complexity calculated for the contralesional (red) and perilesional (blue) stimulation sites for the three groups. Violin plots display the median (dashed line) and the mean (solid line) of the kernel density. *P < 0.05; Wilcoxon signed-rank test.
Of note, the somehow smaller difference observed in the group of patients affected by severe multifocal lesions may be explained by the presence of brain lesions often involving both hemispheres (Supplementary Table 1), potentially affecting also the local signal complexity of the contralesional stimulation site. This was confirmed by marginal effect observed in the mixed-model ANOVA [categorical predictor: Group; within-subject factor: Site; Group × Site interaction effect: F(2,27) = 3.3, P = 0.04985]. In particular, for the severe multifocal lesions group local signal complexity was found similar to that observed for the MCA ischaemia group (P > 0.05, Bonferroni corrected; Fig. 5B and D) and significantly lower than that observed in the subcortical lesions group (P = 0.000001, Bonferroni corrected; Fig. 5D and F) over the perilesional stimulation site, while it was found reduced compared to the other two groups (all P-values <0.0002, Bonferroni corrected; Fig. 5B, D and F) over the contralesional stimulation site.
We finally assessed the impact of focal brain injuries on the global spatiotemporal dynamics of TEPs calculated on all 60 channels. We observed that, for all three groups, maximum PCIst values were above the empirical threshold for consciousness (mean ± SEM: 41.1 ± 2.4, 28.5 ± 3.1 and 42.5 ± 2, for the MCA ischaemia, the severe multifocal and the subcortical lesion groups, respectively. PCIst threshold value: 23.02) obtained from a benchmark population (382 TMS/hd-EEG sessions performed on 108 healthy subjects) (Comolatti et al., 2019). However, a one-way ANOVA [Group effect F(2,27) = 9.3, P = 0.00082] showed a significantly lower maximum PCIst value for the severe multifocal lesions group compared to the other two groups (all P-values < 0.004, Bonferroni corrected), confirming the role of multifocal brain lesions in affecting overall signal complexity.
Discussion
This study provides a novel insight about the nature of the electrophysiological consequences of brain injury. Reviving the old notion of EEG slowing in perilesional areas, here we draw an explicit connection between the electrophysiology of local sleep, thoroughly described in the sleep literature, and the pathophysiology of focal brain injury and stroke. The present findings demonstrate that different patterns of cortical reactivity to a direct perturbation typically associated with different global brain states (coma, sleep, wakefulness) can coexist locally within the same brain. As shown in Fig.1, TEPs were (i) absent, as in the case of severe post-anoxic patients, when directly stimulating the lesion; (ii) simple, as those previously observed in healthy sleeping subjects, for TMS of the perilesional areas; and (iii) complex, similar to those found in neurotypical awake subjects, when stimulating the contralesional hemisphere (Figs 1 and 2). The dramatic changes in EEG reactivity to TMS applied across adjacent scalp regions, suggest that TEPs can provide genuine information regarding the state of the underlying local cortical circuits beyond potential confounding factors due to sensory co-stimulation (Belardinelli et al., 2019; Conde et al., 2019; Siebner et al., 2019). More generally, the present work reinforces and generalizes the idea that TMS/EEG represents an interesting venue for the identification of relevant neurophysiological markers of stroke (Sato et al., 2015; Mensen et al., 2019) expanding its potential beyond the motor domain (Borich et al., 2016; Gray et al., 2017; Palmer et al., 2019; Tscherpel et al., 2020).
The hallmark of physiological sleep is the occurrence of slow waves and off-periods, often referred to in the sleep literature as cortical bistability (Steriade et al., 1993; Timofeev et al., 2001). Off-periods are caused by the enhancement of adaptation (or activity-dependent) K+ currents, brought about by decreased levels of neuromodulation from brainstem activating systems (McCormick and Williamson, 1989) and/or by increased inhibition (Funk et al., 2017; Zucca et al., 2017). Because of these mechanisms, cortical neurons tend to plunge into a silent, hyperpolarized state, lasting a few hundred milliseconds, after an initial activation (Timofeev et al., 2001). The occurrence of synchronous membrane hyperpolarization and synaptic silence in cortical neurons is reflected in extracellular spontaneous slow waves associated with transient suppressions of high-frequency (>20 Hz) activity detectable both by local field potential (Mukovski et al., 2007) and EEG (Piantoni et al., 2013) recordings. However, because of its activity-dependent nature, cortical bistability and the associated off-periods can be better revealed using a perturbational approach, whereby the impulse-response properties of cortical neurons are challenged by means of direct activations (Pigorini et al., 2015; Usami et al., 2015). Consistently, TMS-evoked EEG events similar to those reported here were previously found in sleeping subjects (Rosanova et al., 2018), but never in awake healthy subjects (Rosanova et al., 2009, 2018).
A key finding of the present work is the demonstration of a pathological form of local cortical bistability similar to that ubiquitously observed during sleep (Cash et al., 2009; Vyazovskiy et al., 2011; Menicucci et al., 2013; Pigorini et al., 2015; Rosanova et al., 2018) but occurring (i) during wakefulness; and (ii) in circumscribed intact portions of the cortex adjacent to focal brain injuries.
Interestingly, in the past few years, science has produced compelling evidence supporting the idea that sleep is under local regulation (Krueger et al., 2019). Experiments in isolated cortical slabs (Kristiansen and Courtois, 1949), as well as in slice preparations (Steriade et al., 1993) and cell cultures (Hinard et al., 2012), confirmed that sleep—in the form of the occurrence of slow waves and off-periods—is essentially an intrinsic property of cortical cells ensembles. In parallel, a growing body of evidence, expanded the notion of local sleep partially redefining the classical definition of wake and sleep as separate, discrete states (Sarasso et al., 2014). Along these lines, studies in both rodents and humans demonstrated the occurrence of local sleep-like graphoelements intruding behavioural wakefulness over circumscribed cortical regions (Vyazovskiy et al., 2011; Nir et al., 2017).
Particularly relevant in the present context is that sleep features may be promoted locally by cytokines such as interleukin-1 (IL-1) and tumour necrosis factor α (TNFα) (Imeri and Opp, 2009). Notably, local cortical unilateral microinjections of both these cytokines in rats result in an acute lateralized increase in the number of EEG slow waves during subsequent sleep (Yoshida et al., 2004; Yasuda et al., 2005). Similarly, the local immune response taking place over perilesional areas may be responsible for the occurrence of the lateralized EEG slow waves typically observed in the acute post-stroke phase (Butz et al., 2004).
In parallel, the reduction of the ATP/ADP ratio following ischaemia, activates K-ATP sensitive channels causing membrane hyperpolarization and suppression of activity in local neuronal populations surrounding the lesion (Sun and Feng, 2013). This phenomenon, although initially neuroprotective, may have chronic functional consequences depending on the length of the hypoxic challenge, which may explain the sleep-like reactivity profile observed here in chronic patients.
Besides the local effects of inflammatory and metabolic changes, pathological alterations of the excitation/inhibition balance similar to those occurring during physiological sleep (Timofeev et al., 2001) may play a major role after brain injury. Specifically, in a mouse model of stroke, tonic neuronal inhibition was found increased in the peri-infarct zone (Clarkson et al., 2010). Notably, the pharmacological dampening of tonic inhibition produced an early and sustained recovery of motor function. On the other hand, lesions may induce local cortical bistability by engendering a state of cortico-cortical disfacilitation, that is by reducing recurrent excitation (Timofeev et al., 2001) from the lost tissue. Since cortical neurons receive most of their inputs from nearby cells, they tend to become hyperpolarized when their neighbours drastically reduce their spiking activity [e.g. during sleep (Vyazovskiy and Harris, 2013)]. Cortical lesions of various aetiologies (including traumatic) represent an extreme case of such, resulting in an intracortical fibre-mediated hyperpolarization and depression of function in intact cortical areas neighbouring the injured site, i.e. diaschisis associativa (von Monakow, 1914).
Cortical bistability has been also experimentally induced in the animal model by means of cortical deafferentation. As an example, severing the white matter with a cortical undercut results in slow waves and in a continuous alternation between on and off periods in the partially deafferented gyrus, even when the animal, and the rest of the brain, is awake (Nita et al., 2007). Deafferentation may thus induce cortical bistability due to the interruption of a critical amount of fibres from the ascending activating systems and may occur in patients following both large vascular (e.g. MCA ischaemia) and traumatic (diffuse axonal injury) white matter lesions. Deafferentation and EEG slowing may also occur in ischaemic conditions due to presynaptic transmission failure (Hofmeijer and van Putten, 2012; van Putten and Hofmeijer, 2016).
Irrespective of the specific mechanism involved, direct cortical perturbations with TMS could best reveal the presence of local off-periods regardless of the background EEG pattern (Fig. 3) and, most importantly, of the prevalence of spontaneously lateralized slow waves (found, with variable incidence, in 5 of 10 MCA ischaemia patients, see Supplementary Table 1). This evidence clearly highlights the potential of TMS/EEG as an informative investigational tool complementing spontaneous recordings (Butz et al., 2004) as TMS may effectively reveal covert forms of bistability possibly by massively recruiting local inhibitory circuits if the excitation/inhibition balance is biased towards the latter, or by triggering activity-dependent K+ currents if K+ channels are de-inactivated.
In this context, the presence of lesions involving both white and grey matter in both MCA ischaemia and severe multifocal patients seems to be particularly suited in producing EEG slow waves and off-periods in response to a direct cortical perturbation. Although the precise assessment of the effects of lesion size and location on the observed findings is beyond the scope of the present work, the inclusion of a large sample of patients affected by cortico-subcortical lesion with different volumes and locations (Tscherpel et al., 2020) represents an interesting venue for future studies. At the same time, performing multiple stimulations while systematically increasing the distance with respect to the lesion boundaries will also allow assessing the precise spatial extent of perilesional areas.
On the other hand, small lacunar ischaemic and haemorrhagic subcortical lesions, such as those characterizing our group of patients affected by unilateral subcortical lesions, may not result in clear-cut slow wave responses although the TMS-evoked oscillatory activity was found systematically slower than in typical wakefulness (Fig.4). This finding is in line with previous TMS/EEG work in patients with unilateral subcortical lesions where TEPs were characterized by a sequence of positive and negative polarity deflections with similar morphology compared to healthy controls albeit with a reduced amplitude of fast frequency oscillatory components (Pellicciari et al., 2018).
Finally, the presence of off-periods in areas that are not directly affected by the lesion may have profound behavioural consequences. In an extreme case, the ubiquitous presence of pathological off-periods in response to TMS across cortical islands spared by anatomical lesions in awake vegetative state/unresponsive wakefulness syndrome patients was shown to prevent the build-up of global complexity, resulting in behavioural unresponsiveness (Casarotto et al., 2016; Rosanova et al., 2018). On the other hand of the spectrum, even in the absence of lesions, local sleep slow waves and off-periods in the awake brain of sleep-deprived rodents (Vyazovskiy et al., 2011) and humans (Nir et al., 2017) are still associated with selective motor/cognitive impairments depending on the specific cortical regions involved. Here, the occurrence of local off-periods associated with the disruption of local signal complexity (Fig. 5) over perilesional areas suggests their potential involvement in the behavioural consequence of brain injury. Establishing a systematic link between the presence of local perilesional off-periods and their behavioural consequences would ultimately require longitudinal measurements paralleled by appropriate domain-specific clinical assessments.
Supplementary Material
Acknowledgements
The authors thank Dr Angela Comanducci, Dr Andrea Pigorini, Dr Ezequiel Mikulan, and Dr Simone Russo for their help and comments on the manuscript draft.
Funding
This work was supported by the European Union’s Horizon 2020 Framework Program for Research and Innovation under the Specific Grant Agreement No. 785907 (Human Brain Project SGA2) (to M.M., M.R., and S.L.) and No. 945539 (Human Brain Project SGA3) (to M.M., M.R., and S.L.), by Fondazione Regionale per la Ricerca Biomedica (Regione Lombardia), Project ERAPERMED2019-101, GA 779282 (to M.R.) and by the EU grant H2020, FETOPEN 2014-2015-RIA no. 686764 “Luminous” (to M.M. and S.L.). This work has been also supported by the grant “Sinergia” CRSII3_160803/1 of the Swiss National Science Foundation (to M.M.), by the James S. McDonnell Foundation Scholar Award 2013 (to M.M.), by the Tiny Blue Dot Foundation (to M.M.), by the Belgian National Funds for Scientific Research (F.R.S-FNRS; to S.L. and O.G.), by the Fondazione Europea di Ricerca Biomedica (to S.L. and O.G.), by the BIAL Foundation (to S.L. and O.G.), by AstraZeneca (to S.L. and O.G.) and by the Foundation Roi Baudouin (to S.L. and O.G.).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
Glossary
- ERSP=
event-related spectral perturbation
- MCA=
middle cerebral artery
- PSD=
power spectral density
- TEP=
TMS-evoked EEG potential
- TMS=
transcranial magnetic stimulation
References
- Belardinelli P, Biabani M, Blumberger DM, Bortoletto M, Casarotto S, David O, et al. Reproducibility in TMS-EEG studies: a call for data sharing, standard procedures and effective experimental control. Brain Stimul 2019; 12: 787–90. [DOI] [PubMed] [Google Scholar]
- Borich MR, Wheaton LA, Brodie SM, Lakhani B, Boyd LA.. Evaluating interhemispheric cortical responses to transcranial magnetic stimulation in chronic stroke: A TMS-EEG investigation. Neurosci Lett 2016; 618: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20: 864–70. [DOI] [PubMed] [Google Scholar]
- Butz M, Gross J, Timmermann L, Moll M, Freund H-J, Witte OW, et al. Perilesional pathological oscillatory activity in the magnetoencephalogram of patients with cortical brain lesions. Neurosci Lett 2004; 355: 93–6. [DOI] [PubMed] [Google Scholar]
- Casarotto S, Canali P, Rosanova M, Pigorini A, Fecchio M, Mariotti M, et al. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topogr 2013; 26: 326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotto S, Comanducci A, Rosanova M, Sarasso S, Fecchio M, Napolitani M, et al. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann Neurol 2016; 80: 718–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash SS, Halgren E, Dehghani N, Rossetti AO, Thesen T, Wang C, et al. The human K-complex represents an isolated cortical down-state. Science 2009; 324: 1084–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST.. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 2010; 468: 305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comolatti R, Pigorini A, Casarotto S, Fecchio M, Faria G, Sarasso S, et al. A fast and general method to empirically estimate the complexity of brain responses to transcranial and intracranial stimulations. Brain Stimul 2019; 12: 1280–9. [DOI] [PubMed] [Google Scholar]
- Compte A, Sanchez-Vives MV, McCormick DA, Wang X-J.. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol 2003; 89: 2707–25. [DOI] [PubMed] [Google Scholar]
- Conde V, Tomasevic L, Akopian I, Stanek K, Saturnino GB, Thielscher A, et al. The non-transcranial TMS-evoked potential is an inherent source of ambiguity in TMS-EEG studies. Neuroimage 2019; 185: 300–12. [DOI] [PubMed] [Google Scholar]
- Fecchio M, Pigorini A, Comanducci A, Sarasso S, Casarotto S, Premoli I, et al. The spectral features of EEG responses to transcranial magnetic stimulation of the primary motor cortex depend on the amplitude of the motor evoked potentials. PLoS One 2017; 12: e0184910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Sarasso S, Guller Y, Riedner BA, Peterson MJ, Bellesi M, et al. Reduced natural oscillatory frequency of frontal thalamocortical circuits in schizophrenia. Arch Gen Psychiatry 2012; 69: 766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CM, Peelman K, Bellesi M, Marshall W, Cirelli C, Tononi G.. Role of somatostatin-positive cortical interneurons in the generation of sleep slow waves. J Neurosci 2017; 37: 9132–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K, Whyte J.. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004; 85: 2020–9. [DOI] [PubMed] [Google Scholar]
- Grandchamp R, Delorme A.. Single-trial normalization for event-related spectral decomposition reduces sensitivity to noisy trials. Front Psychol 2011; 2: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WA, Palmer JA, Wolf SL, Borich MR.. Abnormal EEG responses to TMS during the cortical silent period are associated with hand function in chronic stroke. Neurorehabil Neural Repair 2017; 31: 666–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinard V, Mikhail C, Pradervand S, Curie T, Houtkooper RH, Auwerx J, et al. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J Neurosci 2012; 32: 12506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeijer J, van Putten MJAM.. Ischemic cerebral damage: an appraisal of synaptic failure. Stroke 2012; 43: 607–15. [DOI] [PubMed] [Google Scholar]
- Imeri L, Opp MR.. How (and why) the immune system makes us sleep. Nat Rev Neurosci 2009; 10: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen K, Courtois G.. Rhythmic electrical activity from isolated cerebral cortex. Electroencephalogr Clin Neurophysiol 1949; 1: 265–72. [PubMed] [Google Scholar]
- Krueger JM, Nguyen JT, Dykstra-Aiello CJ, Taishi P.. Local sleep. Sleep Med Rev 2019; 43: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LD, Weiner VS, Mukamel EA, Donoghue JA, Eskandar EN, Madsen JR, et al. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci USA 2012; 109: E3377–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonell RA, Donnan GA, Bladin PF, Berkovic SF, Wriedt CH.. The electroencephalogram and acute ischemic stroke. Distinguishing cortical from lacunar infarction. Arch Neurol 1988; 45: 520–4. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Williamson A.. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci USA 1989; 86: 8098–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menicucci D, Piarulli A, Allegrini P, Laurino M, Mastorci F, Sebastiani L, et al. Fragments of wake-like activity frame down-states of sleep slow oscillations in humans: new vistas for studying homeostatic processes during sleep. Int J Psychophysiol 2013; 89: 151–7. [DOI] [PubMed] [Google Scholar]
- Mensen A, Pigorini A, Facchin L, Schöne C, D’Ambrosio S, Jendoubi J, et al. Sleep as a model to understand neuroplasticity and recovery after stroke: observational, perturbational and interventional approaches. J Neurosci Methods 2019; 313: 37–43. [DOI] [PubMed] [Google Scholar]
- Mukovski M, Chauvette S, Timofeev I, Volgushev M.. Detection of active and silent states in neocortical neurons from the field potential signal during slow-wave sleep. Cereb Cortex 2007; 17: 400–14. [DOI] [PubMed] [Google Scholar]
- Nir Y, Andrillon T, Marmelshtein A, Suthana N, Cirelli C, Tononi G, et al. Selective neuronal lapses precede human cognitive lapses following sleep deprivation. Nat Med 2017; 23: 1474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, et al. Regional slow waves and spindles in human sleep. Neuron 2011; 70: 153–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nita DA, Cissé Y, Timofeev I, Steriade M.. Waking-sleep modulation of paroxysmal activities induced by partial cortical deafferentation. Cereb Cortex 2007; 17: 272–83. [DOI] [PubMed] [Google Scholar]
- Nuwer MR, Jordan SE, Ahn SS.. Evaluation of stroke using EEG frequency analysis and topographic mapping. Neurology 1987; 37: 1153–9. [DOI] [PubMed] [Google Scholar]
- Palmer JA, Wheaton LA, Gray WA, Saltão da Silva MA, Wolf SL, Borich MR.. Role of interhemispheric cortical interactions in poststroke motor function. Neurorehabil Neural Repair 2019; 33: 762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicciari MC, Bonnì S, Ponzo V, Cinnera AM, Mancini M, Casula EP, et al. Dynamic reorganization of TMS-evoked activity in subcortical stroke patients. Neuroimage 2018; 175: 365–78. [DOI] [PubMed] [Google Scholar]
- Piantoni G, Astill RG, Raymann RJEM, Vis JC, Coppens JE, Van Someren EJW.. Modulation of γ and spindle-range power by slow oscillations in scalp sleep EEG of children. Int J Psychophysiol 2013; 89: 252–8. [DOI] [PubMed] [Google Scholar]
- Pigorini A, Sarasso S, Proserpio P, Szymanski C, Arnulfo G, Casarotto S, et al. Bistability breaks-off deterministic responses to intracortical stimulation during non-REM sleep. Neuroimage 2015; 112: 105–13. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M.. Natural frequencies of human corticothalamic circuits. J Neurosci 2009; 29: 7679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Fecchio M, Casarotto S, Sarasso S, Casali AG, Pigorini A, et al. Sleep-like cortical OFF-periods disrupt causality and complexity in the brain of unresponsive wakefulness syndrome patients. Nat Commun 2018; 9: 4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA.. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 2000; 3: 1027–34. [DOI] [PubMed] [Google Scholar]
- Sarasso S, Pigorini A, Proserpio P, Gibbs SA, Massimini M, Nobili L.. Fluid boundaries between wake and sleep: experimental evidence from Stereo-EEG recordings. Arch Ital Biol 2014; 152: 169–77. [DOI] [PubMed] [Google Scholar]
- Sato S, Bergmann TO, Borich MR.. Opportunities for concurrent transcranial magnetic stimulation and electroencephalography to characterize cortical activity in stroke. Front Hum Neurosci 2015; 9: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Conde V, Tomasevic L, Thielscher A, Bergmann TO.. Distilling the essence of TMS-evoked EEG potentials (TEPs): A call for securing mechanistic specificity and experimental rigor. Brain Stimul 2019; 12: 1051–4. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F.. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 1993; 13: 3252–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Feng Z.. Neuroprotective role of ATP-sensitive potassium channels in cerebral ischemia. Acta Pharmacol Sin 2013; 34: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M.. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. Proc Natl Acad Sci USA 2001; 98: 1924–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscherpel C, Dern S, Hensel L, Ziemann U, Fink GR, Grefkes C.. Brain responsivity provides an individual readout for motor recovery after stroke. Brain 2020; 143: 1873–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami K, Matsumoto R, Kobayashi K, Hitomi T, Shimotake A, Kikuchi T, et al. Sleep modulates cortical connectivity and excitability in humans: direct evidence from neural activity induced by single-pulse electrical stimulation. Hum Brain Mapp 2015; 36: 4714–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Putten MJAM, Hofmeijer J.. EEG monitoring in cerebral ischemia: basic concepts and clinical applications. J Clin Neurophysiol 2016; 33: 203–10. [DOI] [PubMed] [Google Scholar]
- von Monakow C. Die lokalisation im grosshirn und der abbau der funktion durch kortikale herde. Jama 1914; LXIII: 797. [Google Scholar]
- Vyazovskiy VV, Harris KD.. Sleep and the single neuron: the role of global slow oscillations in individual cell rest. Nat Rev Neurosci 2013; 14: 443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G.. Local sleep in awake rats. Nature 2011; 472: 443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Yoshida H, Garcia-Garcia F, Kay D, Krueger JM.. Interleukin-1beta has a role in cerebral cortical state-dependent electroencephalographic slow-wave activity. Sleep 2005; 28: 177–84. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Peterfi Z, García-García F, Kirkpatrick R, Yasuda T, Krueger JM.. State-specific asymmetries in EEG slow wave activity induced by local application of TNFalpha. Brain Res 2004; 1009: 129–36. [DOI] [PubMed] [Google Scholar]
- Zucca S, D’Urso G, Pasquale V, Vecchia D, Pica G, Bovetti S, et al. An inhibitory gate for state transition in cortex. Elife 2017; 6: e26177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the paper's conclusions are present in the paper. Additional data related to this paper may be requested from the corresponding author.