Abstract
Multiplex experimentation that can assay multiple cellular signaling pathways in the same cells requires orthogonal genetically encoded reporters that report over large dynamic ranges. Luciferases are cost-effective, versatile candidates whose output signals can be sensitively detected in a multiplex fashion. Commonly used dual luciferase reporter assays detect one luciferase that is coupled to a single cellular pathway, and a second that is coupled to a control pathway for normalization purposes. We expanded this approach to multiplex hextuple luciferase assaying that can report on five cellular signaling pathways and one control, each of which is encoded by a unique luciferase. Light emission by the six luciferases can be distinguished by the use of two distinct substrates, each specific for three luciferases, followed by spectral decomposition of the light emitted by each of the three luciferase enzymes with bandpass filters. Here, we present detaileded protocols on how to perform multiplex hextuple luciferase assaying to monitor pathway fluxes through transcriptional response elements for five specific signaling pathways (i.e., c-Myc, NF-κβ, TGF-β, p53, and MAPK/JNK), using the constitutive CMV promoter as normalization control. Protocols are provided for preparing reporter vector plasmids for multiplex reporter assaying, performing cell culture and multiplex luciferase reporter vector plasmid transfection, executing multiplex luciferase assays, and analyzing and interpreting data obtained by a plate reader apppropriately equipped to detect the different luminescences. Protocols on how to tailor multiplex hextuple luciferase assaying to different cellular signaling pathways are explained in accompanying Current Protocols in Molecular Biology article (Sarrion-Perdigones et al., in press).
Basic Protocol 1:
Preparation of vectors for multiplex hextuple luciferase assaying
Basic Protocol 2:
Cell culture work for multiplex hextuple luciferase assays
Basic Protocol 3:
Transfection of luciferase reporter plasmids followed by drug and recombinant protein treatments
Basic Protocol 4:
Performing the multiplex hextuple luciferase assay
Keywords: Luciferase, assay, multiplex, hextuple, orthogonal, high-throughput, cellular signaling pathway, cell culture, transfection, pathway perturbation, plate reader
Introduction
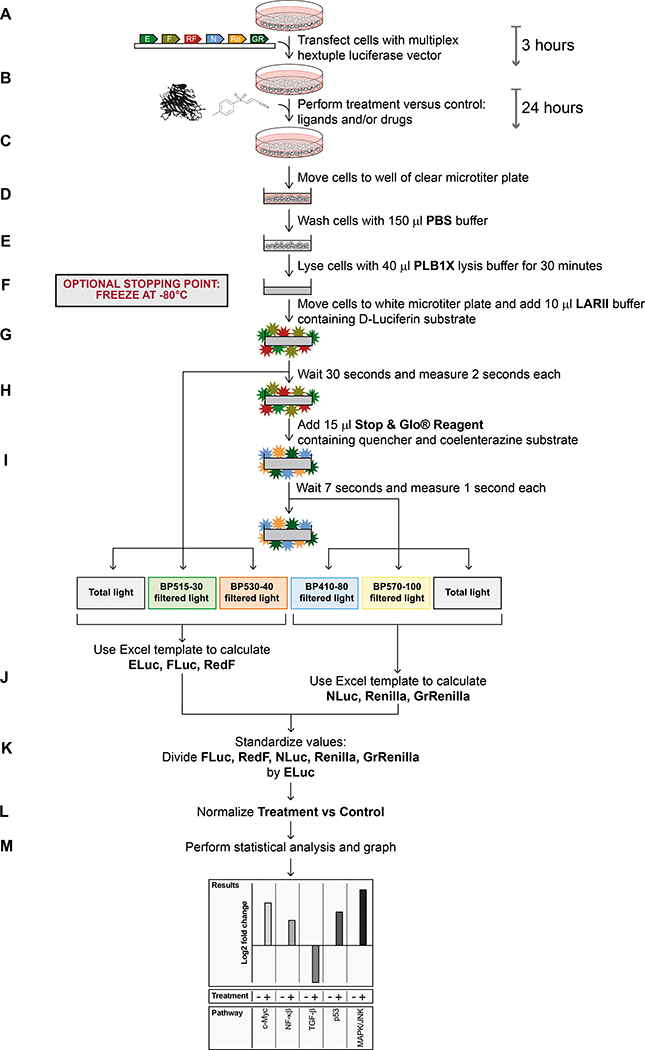
We recently developed a multiplex hextuple luciferase assay that allows the simultaneous measurement of signals emitted by six different luciferases in the same biological sample (Sarrion-Perdigones et al., 2019). This method utilizes reagents and protocols that are widely used in a commercial dual-luciferase assay, available from Promega, and then uses specific bandpass (BP) filters and mathematical equations to distinguish the light emitted by the six luciferases in a straightforward two-step protocol (Figure 1).
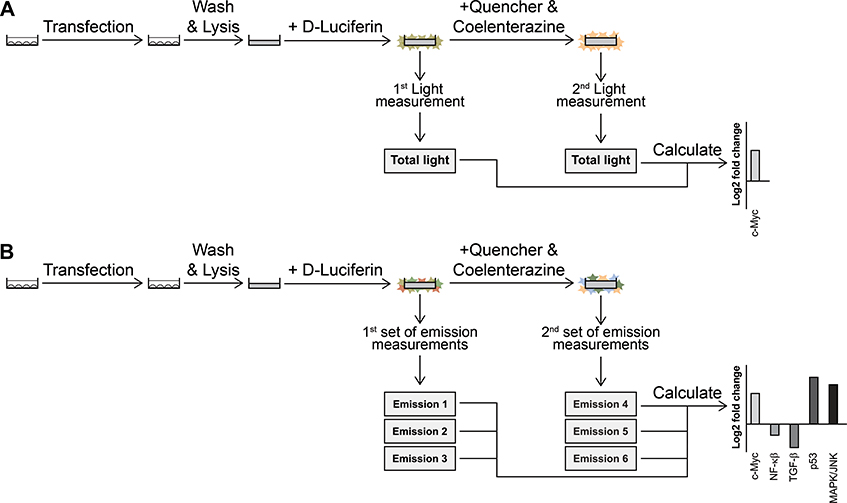
Figure 1. Workflow schematic of multiplex hextuple luciferase assaying.
(A) Transfect a test sample of cells with a multiplex luciferase reporter vector and incubate for 3 hours. The multiplex vector contains transcriptional reporter units for specific cellular pathways, each linked to one of six luciferases: ELuc (E), Flux (F), RedF (RF), NLuc (N), Renilla (Re), and GrRenilla (GR). (B) Treat cells with appropriate ligands and/or drugs, and incubate for an additional 24 hours. (C) Move transfected and treated cells to a well of a clear microtiter plate. (D) Wash cells with 150 μl PBS buffer. (E) Lyse cells for 30 minutes with 40 μl PLB1X lysis buffer. Optional: Store lysate in a −80°C freezer to continue the protocol at a later time point. When this step is included, thaw the sample to room temperature before proceeding to the next step. (F) Transfer an aliquot of lysate to a white microtiter plate and move plate to a plate reader equipped with appropriate bandpass filters, i.e., BP515–30 and BP530–40 for measuring D-Luciferin-consuming luciferase emissions, and BP410–80 and BP570–100 for measuring coelenterazine-luciferase emissions, and add 10 μl of D-Luciferin-containing LARII buffer. (G) Wait 30 seconds and record for 2 seconds each, total light, BP515–30-filtered light, and BP530–40-filtered light, emitted by the D-Luciferin-luciferases (ELuc, FLuc, and RedF). (H) Add 15 μl of quencher- and coelenterazine-containing Stop & Glo Reagent. (I) Wait 7 seconds and record for, 1 second each, BP410–80-filtered light, BP570–100-filtered light, and total light, emitted by the coelenterazine-luciferases (NLuc, Renilla, and GrRenilla). (J) Transfer the raw data given by the plate reader for the D-Luciferin and coelenterazine luciferases to the provided Excel template to calculate emission values for each luciferase. (K) Standardize the luciferase values by dividing values obtained for each of the experimental luciferases (i.e., for FLuc, RedF, NLuc, Renilla, and GrRenilla) by the value obtained for the control luciferase (ELuc). (L) Normalize the values by comparing treatment and control values. (M) Perform statistical analysis and graph accordingly.
In this assay, after the transfection of luciferase reporter plasmids followed by 24–48 hours of incubation, cells are washed and lysed with lysis buffer (Passive Lysis Buffer, PLB). Next, the cell lysate is incubated with D-Luciferin-containing buffer (Luciferase Assay Reagent II, LARII), and after 30 seconds, total light, BP515–30 filtered light (emitted light between 500 and 530 nm), and BP530–40 filtered light (emitted light between 510 and 550 nm) are measured serially during 2-second intervals. Next, the D-Luciferin luciferase quencher- and coelenterazine-containing buffer (Stop & Glo Reagent) is added, and after 7 seconds, BP510–80 filtered light (emitted light between 470 and 550 nm), BP570–100 filtered light (emitted light between 520 and 620 nm), and total light are measured serially during a 1-second interval, each. As a proof-of-concept, we engineered a luciferase assay tailored to probe pathway fluxes through c-Myc, NF-κβ, TGF-β, p53, and MAPK/JNK transcriptional response elements against a control constitutive CMV promoter, and measured changes occurring in these pathways for a number of breast cancer cell lines (Sarrion-Perdigones et al., 2019).
In this unit, we describe how to use the multiplex luciferase reporter vector to monitor the effects of ligands and chemical compound treatments on their target pathways as well as their collateral effects on the other four pathways, using the A549 lung cancer cell line as an example. We present protocols for each of four steps: preparation of the control reporter vectors needed to determine essential parameters needed to perform multiplex luciferase assaying, and the multiplex luciferase reporter used to determine changes in pathway activities for five different pathways (Basic Protocol 1), culturing (Basic Protocol 2) and transfection of the A549 cell line (Basic Protocol 3), and carrying out the multiplex luciferase assay followed by output data analysis (Basic Protocol 4). These protocols are applicable to other cell lines, as well as other cellular pathways needed to be probed.
Strategic planning
The multiplex luciferase reporter vectors generated for this protocol are built using a DNA assembly platform method based on the GoldenBraid 2.0 cloning system (Sarrion-Perdigones et al., 2011, 2013; see Current Protocols in Molecular Biology article by Vazquez-Vilar, et al., 2020). This system entails a one-step one-pot reaction, mixing multiple to-be-assembled DNA parts that are released and ligated together to generate defined assembly products in a destination plasmid. Using this assembly platform, multiple genetic elements can be combined into single transcriptional reporter units, that then can be further combined together to obtain multiple transcriptional reporter units in a single plasmid. The combination of all reporters onto a single vector reduces the higher variability between experiments for both reporter and control transcriptional units, compared to when all reporter units, each assembled in an individual plasmid, are cotransfected.
Previously, we designed the first hextuple multiplex luciferase assay to investigate transcriptional signaling through c-Myc, NF-κβ, TGF-β, p53, and MAPK/JNK response elements, against a constitutively control promoter (CMV promoter). We placed the luciferase ELuc under the control of the CMV promoter and the other five luciferases under control of DNA regulatory elements activated by one of the five specific cellular pathways. The control signal from the CMV promoter is used to normalize the readout from each pathway. We have deposited the multiplex hextuple luciferase vector and all required parts to build it in the public plasmid repository Addgene. In this protocol, we describe in detail the different steps performed during multiplex luciferase assaying, using the same multiplex hextuple luciferase vector, but applied to probe changes in five cellular signaling pathways in the A549 lung cancer cell line, demonstrating applicability of the same multiplex assay across different cellular model systems. However, it is important for researchers to note that the described multiplex approach can also be tailored to individual needs by incorporating other signaling pathways into the described assay, as explained in Current Protocols in Molecular Biology (Sarrion-Perdigones et al., in press).
Basic Protocol 1
Preparation of vectors for multiplex hextuple luciferase assays
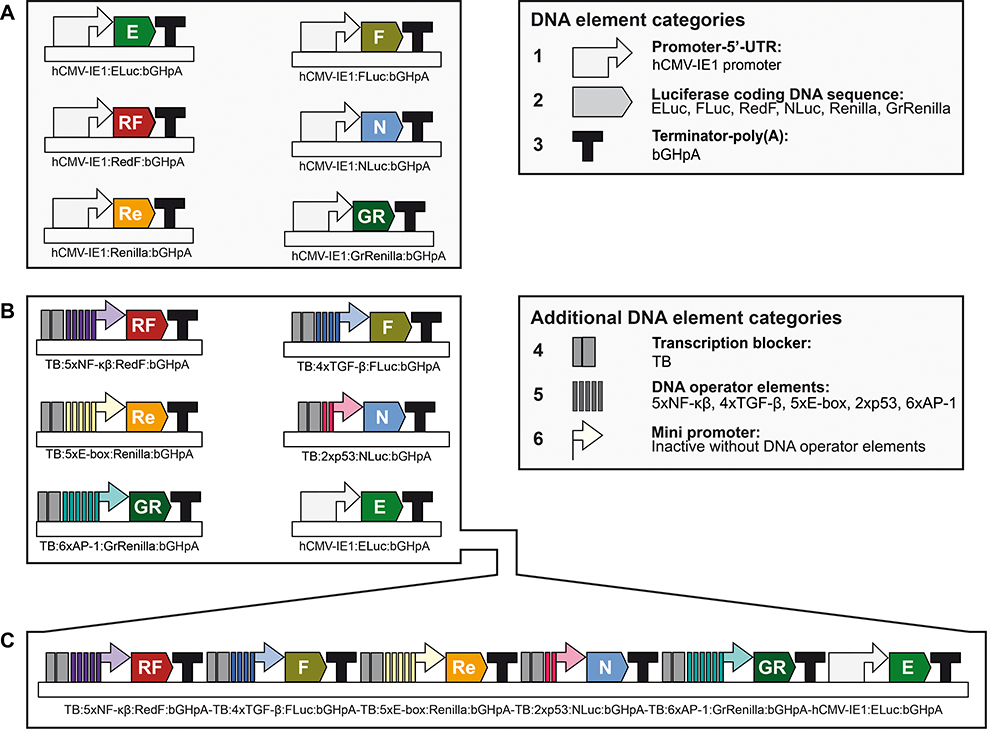
This protocol describes the preparation of the vectors needed to perform multiplex hextuple luciferase assaying, as described in Basic Protocol 4, and the verification of their integrity by restriction enzyme DNA fingerprinting. These vectors include the control constitutively expressed luciferase vectors needed to determine essential parameters needed to perform multiplex luciferase assaying (Figure 2A), and the multiplex luciferase reporter vector used to determine changes in pathway activities for five different pathways (Figure 2B and 2C). These vectors, listed in Table 1, can be obtained from Addgene.
Figure 2. Overview of vectors needed for multiplex luciferase assaying.
(A) Simplified schematic of the constitutively expressing luciferase reporter plasmids for all six luciferases (ELuc, FLuc, RedF, NLuc, Renilla, and GrRenilla), needed to determine the transmission coefficients (see Figure 7): hCMV-IE1:ELuc:bGHpA containing the control enhanced beetle luciferase reporter ELuc (E) (Addgene # 118062), hCMV-IE1:FLuc:bGHpA containing the firefly luciferase FLuc (F) (Addgene # 118063), hCMV-IE1:RedF:bGHpA containing the red firefly luciferase RedF (RF) (Addgene # 118064), hCMV-IE1:NLuc:bGHpA containing the nano luciferase NLuc (N) (Addgene # 118065), hCMV-IE1:Renilla:bGHpA containing the renilla luciferase Renilla (Re) (Addgene # 118066), and hCMV-IE1:GrRenilla:bGHpA containing the green renilla luciferase GrRenilla (GR)(Addgene # 118067) (see Table 1). Categories of DNA elements include: (1) full promoter and 5’UTR, the cytomegalovirus promoter, hCMV-IE1 promoter, used in all constitutively expressed transcriptional units; (2) luciferase-coding DNA sequence for Eluc, FLuc, RedF, NLuc, Renilla, or GrRenilla; and (3) terminator-poly(A) sequence, the terminator of the bovine growth hormone polyadenylation signal (bGHpA). (B) Simplified schematic of the six single pathway transcriptional reporter plasmids included in the multiplex hextuple luciferase vector (see C) to perform multiplex pathway analysis (see Figure 5) using multiplex luciferase assaying (see Figure 12): TB:5xNF-κβ:RedF:bGHpA reporting on NF-κβ pathway signaling using red firefly luciferase (RF), TB:4xTGF-β:FLuc:bGHpA reporting on TGF-β pathway signaling using firefly luciferase (F), TB:5xE-box:Renilla:bGHpA reporting on Myc pathway signaling using renilla luciferase (Re), TB:2xp53:NLuc:bGHpA reporting on p53 pathway signaling using nano luciferase (N), TB:6xAP-1:GrRenilla:bGHpA reporting on MAPK/JNK pathway signaling using green renilla luciferase (GR), and hCMV-IE1:ELuc:bGHpA (see A). Additional categories of DNA elements include: (4) transcription blocker (TB), consisting of a synthetic polyA terminator and the RNA polymerase II transcriptional pause signal from the human α2 globin gene, to prevent transcriptional interference between different pathway-responsive luciferase transcriptional units; (5) DNA operator elements, DNA pathway response elements whose activities are regulated by upstream cellular signaling through the NF-κβ (5xNF-κβ), TGF-β (4xTGF-β), Myc (5xE-box), p53 (2xp53), or MAPK/JNK pathway (6xAP-1); and (6) mini promoter, a synthetic minimal TATA-box promoter with low basal activity needed for transcription initiation driven by the different DNA pathway operator response elements. (C) Simplified schematic of the final multiplex hextuple luciferase vector consisting of the six transcriptional luciferase reporter units (see B) stitched together in a specified order to perform multiplex pathway analysis (see Figure 5) using multiplex luciferase assaying (see Figure 12): TB:5xNF-κβ:RedF:bGHpA-TB:4xTGF-β:FLuc:bGHpA-TB:5xE-box:Renilla:bGHpA-TB:2xp53:NLuc:bGHpA-TB:6xAP-1:GrRenilla:bGHpA-hCMV-IE1:ELuc:bGHpA (Addgene #118069) (see Table 1). For synthetic assembly details, see Current Protocols in Molecular Biology article (Sarrion-Perdigones et al., in press).
Table 1.
Summary of vectors described in this work.
| Type | Abbreviation | Description | Function | Vector | Resistance | Addgene |
|---|---|---|---|---|---|---|
| Single luciferase reporters | hCMV-IE1:ELuc | Constitutively expressed ELuc | Transcriptional Unit | pColE1_Alpha2 | Kanamycin | #118062 |
| hCMV-IE1:FLuc | Constitutively expressed FLuc | Transcriptional Unit | pColE1_Alpha2 | Kanamycin | #118063 | |
| hCMV-IE1:RedF | Constitutively expressed RedF | Transcriptional Unit | pColE1_Alpha2 | Kanamycin | #118064 | |
| hCMV-IE1:NLuc | Constitutively expressed NLuc | Transcriptional Unit | pColE1_Alpha2 | Kanamycin | #118065 | |
| hCMV-IE1:Renilla | Constitutively expressed Renilla | Transcriptional Unit | pColE1_Alpha2 | Kanamycin | #118066 | |
| hCMV-IE1:GrRenilla | Constitutively expressed GrRenilla | Transcriptional Unit | pColE1_Alpha2 | Kanamycin | #118067 | |
| Multiplex reporter | MLRV | Multi-luciferase reporter vector | Multigenic vector | pColE1_Alpha2 | Kanamycin | #118069 |
Materials: Reagents, solutions, and starting samples or test organisms/cells
Multiplex luciferase reporter vector (Addgene, plasmid ID 118069) (Table 1) as bacterial stab in agar.
Note 1: All plasmids used in these protocols have been deposited in Addgene for unrestricted accessibility by academic researchers. They are maintained in DH5α-T1R E.coli cells and have been tested in a variety of human cell lines. Upon arrival, streak out on bacterial plate within a few days.
Note 2: Genotype of DH5α-T1R (ThermoFisher Scientific) is F- φ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rk-, mk+) phoA supE44 thi-1 gyrA96 relA1 tonA (confers resistance to phage T1)
Constitutive luciferase vectors encoding ELuc, FLuc, RedF, NLuc, Renilla, and GrRenilla (Addgene, plasmid IDs 118062, 118063, 118064, 118065, 118066, and 118057, respectively) (Table 1) as bacterial stab in agar.
LB agar plates (VWR, cat. no. 25384–092) containing 30 μg mL−1 kanamycin.
LB liquid medium containing 30 μg mL−1 kanamycin
QIAprep Spin Miniprep Kit (QIAGEN, cat. no. 27106) or ChargeSwitch®-Pro Plasmid Miniprep Kit (ThermoFisher Scientific, cat. no. CS30250).
Restriction enzymes: KpnI-HF (New England Biolabs, cat. no. R3142S), BsmBI (New England Biolabs, cat. no. R0580S), MfeI-HF (New England Biolabs, cat. no. R3589S), NcoI-HF (New England Biolabs, cat. no. R3193S), and XhoI (New England Biolabs, cat. no. R0146S).
Restriction enzyme buffer: CutSmart® buffer (New England Biolabs, cat. no. B7204S) and NEBuffer™ 3.1 (New England Biolabs, cat. no. B7203S).
Reagents for agarose gel electrophoresis are described in Current Protocols in Molecular Biology (Voytas, 2001).
Materials: Hardware and instruments (e.g., glassware, disposables, microscopes, centrifuge)
1.7 mL microcentrifuge tubes (VWR, cat. no. 87003–294).
14 mL sterile bacterial culture tubes (VWR, cat. no. 60818–689).
50 mL sterile conical tubes (VWR, cat. no. 89401–562).
Table top centrifuge that can accommodate 14 mL and 50 mL tubes (Fisher Scientific, cat. no. 75230115).
Table top microcentrifuge that can hold 1.7 mL tubes (Fisher Scientific, cat. no. 75002435).
32°C and 37°C incubator-shakers (Amerex, cat. no. 747/747R).
DeNovix DS-11+ spectrophotometer (DeNovix, cat. no. DS-11).
55°C incubator (VWR, cat. no. 89409–216).
Equipment for agarose gel electrophoresis are described in Current Protocols in Molecular Biology (Voytas, 2001).
Prepare the control luciferase and the multiplex luciferase reporter vectors
-
1Obtain plasmids from Addgene (Table 1).
- Note: Alternatively, you can start the project with a custom plasmid built as described in Current Protocols in Molecular Biology (Sarrion-Perdigones et al., in press), but you will still need to prepare the six control plasmids.
-
2Use a sterile pipet tip or inoculating loop to scrape each bacterial stock from the Addgene bacterial stab, and streak it onto an LB agar plate containing 30 μg mL−1 kanamycin. Incubate plate at 32°C overnight.
- Upon arrival of the Addgene bacterial stabs, streak out on bacterial plate as soon as possible (within a few days).
- In our hands, plasmid yield isolated from commonly used bacteria (DH5α and DH10B) is often higher at 32°C, but standard growth at 37°C is also possible.
-
3
Pick a single colony from the multiplex luciferase reporter vector plate (#118069) to inoculate 15 mL of LB liquid medium containing 30 μg mL−1 kanamycin in a 50 mL conical tube. Pick a single colony from the control luciferase vectors (#118062, #118063, #118064, #118065, #118066, and #118057) to inoculate 5 mL of LB liquid medium containing 30 μg mL−1 kanamycin in a 14 mL culture tube. Grow overnight at 32°C for a maximum of 17 h.
-
4Isolate plasmids using the QIAprep Spin Miniprep Kit or the ChargeSwitch-Pro Plasmid Miniprep Kit. Divide 15 mL of multiplex luciferase reporter vector culture in three 5 mL aliquots and do three simultaneous preps.
- Note: To maximize DNA yield, elute in 50 μL of EB Buffer (QIAprep) or Elution Buffer (ChargeSwitch®-Pro), remove the elution tube and transfer the eluate back onto the same column. Re-insert the column in the tube and centrifuge at maximum speed for 30 seconds.
-
5
Quantify the DNA concentration of each miniprep using the DeNovix spectrophotometer.
Plasmid restriction enzyme digestion for verification
-
6Prepare restriction enzyme digestion mixtures as follows (for each of the six constitutive luciferase vectors):
- Quintuple BsmBI/KpnI/MfeI/NcoI/XhoI digestion
- 600 ng of constitutive luciferase vector
- 2.5 μL CutSmart® buffer
- 0.5 μL KpnI
- 0.5 μL MfeI-HF
- 0.5 μL NcoI-HF
- 0.5 μL XhoI
- 1 μL BsmBI
- Add water to a final volume of 25 μL.
- Uncut vector
- 600 ng of constitutive luciferase vector
- 2.5 μL CutSmart® buffer
- Add water to a final volume of 25 μL.
-
7Prepare restriction enzyme mixtures as follows for the multiplex luciferase reporter vector:
- XhoI Digestion
- 600 ng multiplex luciferase reporter vector
- 2.5 μL CutSmart® buffer
- 0.5 μL XhoI
- Add water to a final volume of 25 μL.
- BsmBI Digestion
- 600 ng multiplex luciferase reporter vector
- 2.5 μL NEBuffer™ 3.1
- 0.5 μL BsmBI
- Add water to a final volume of 25 μL.
- Uncut vector
- 600 ng multiplex luciferase reporter vector
- 2.5 μL CutSmart® buffer or NEBuffer™ 3.1
- Add water to a final volume of 25 μL.
-
8
Incubate all restriction enzyme digestions at 37°C (BsmBI/KpnI/MfeI/NcoI/XhoI and XhoI digestions, and Uncut Plasmid) and 55°C (BsmBI Digestion) for 1h.
-
9
Separate the digestion reactions using 1% agarose gel electrophoresis, according to Current Protocols in Molecular Biology (Voytas, 2001).
-
10
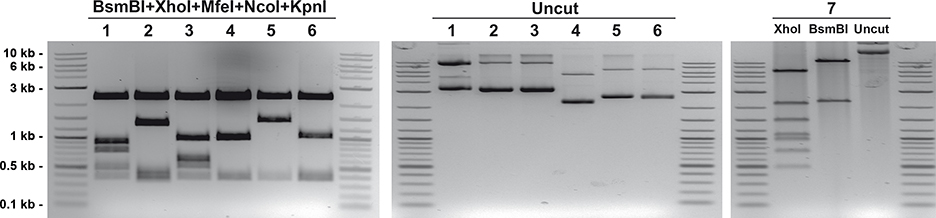
Image the agarose gel and compare it with Figure 3. After the quintuple BsmBI/KpnI/MfeI/NcoI/XhoI digestion, each constitutive control reporter vector will show a distinct set of bands. For the multiplex luciferase reporter vector, the XhoI digestion should have seven bands (6351, 2209, 1505, 1088, 979, 757, 494 bp), and the BsmBI digestion should have two bands (11056 and 2327 bp). For all plasmids, the uncut plasmid should show the supercoiled and circular DNA bands, proving the plasmid integrity.
Figure 3. Quality control of luciferase-encoding plasmid DNA preparations.
Purified plasmid DNA is analyzed by agarose gel electrophoresis two-ways: plasmid fingerprinting after restriction enzyme cutting (to confirm appropriate DNA banding patterns), and uncut (to confirm the absence of unwanted multimerizations). Plasmids are one of the six constitutive luciferases transcriptional units: ELuc (Plasmid 1), FLuc (Plasmid 2), RedF (Plasmid 3), NLuc (Plasmid 4), Renilla (Plasmid 5), and GrRenilla (Plasmid 6), and the multiplex luciferase reporter vector (Plasmid 7).
Basic Protocol 2
Cell culture work for multiplex hextuple luciferase assays
This protocol describes the culturing of the A549 lung cancer cell line, so that the cells can be transfected with luciferase reporter plasmids as described in Basic Protocol 3, followed by performing the multiplex hextuple luciferase assay as described in Basic Protocol 4.
Materials: Reagents, solutions, and starting samples or test organisms/cells
A549 lung cancer cell line (ATCC®, cat. no. CCL-185™).
DMEM/F-12, HEPES Culture media (ThermoFisher Scientific, cat. no. 11330032).
Fetal Bovine Serum (ThermoFisher Scientific, cat. no. 10437028).
Penicillin-Streptomycin 10,000 U/mL (ThermoFisher Scientific, cat. no. 15140148).
PBS, pH 7.2 (ThermoFisher Scientific, cat. no. 20012043).
TrypLE Express Enzyme (1X), phenol red (ThermoFisher Scientific, cat. no. 12605028).
Trypan Blue Solution, 0.4% (ThermoFisher Scientific, cat. no. 15250061).
Additional reagents and equipment for mammalian cell culture as described in Unit 1.1 (Phelan and May, 2017).
Materials: Hardware and instruments (e.g., glassware, disposables, microscopes, centrifuge)
T75 flask (VWR, cat. no. 10861–646).
Serological pipetes, 5 and 10 mL (VWR, cat. no. 53300–421 and 32314–006).
Clear and sterile 96 well tissue culture plate, flat bottom (VWR, cat. no. 10062–900).
Countess cell counting chamber slides (ThermoFisher Scientific, cat. no. C10228).
Countess automated cell counter (ThermoFisher Scientific, cat. no. AMQAX1000).
Cell culture
- Prepare DMEM/F-12+FBS culture media by adding:
- 500 mL DMEM/F-12, HEPES.
- 50 mL FBS, to a final concentration of 10%.
- 5 mL Penicillin-Streptomycin stock solution, to a final concentration of 100 U/mL.
- Thaw a frozen vial of A459 cells on ice and culture cells as described in Unit 1.1 (Phelan and May, 2017). Expand cells to a total volume of 12.5 mL using a T125 flask. Cells should be passaged twice a week in a 1:8 proportion.
- Note: Do not maintain your cultures for more than 30 passages. Cells should be passaged twice a week in a 1:8 proportion.
The day before the beginning of the multiplex luciferase experiment, wash the monolayer of cells with PBS for 2 minutes and then trypsinize the cells with 3 mL TrypL Express Enzyme for 5 minutes at 37°C. Stop the trypsinization with 5 mL of DMEM/F-12+FBS media.
Mix 10 μL of the cells with 10 μL of Trypan Blue Solution and apply to a Countess cell counting chamber slide. Count the number of cells using the Countess automated cell counter.
- Dilute the cells to 250,000 cells/mL, and dispense 100 μL of cells per well in a tissue culture treated 96 well plate, resulting in 25,000 cells/well. Incubate at 37°C overnight.
- Prepare 4 to 6 wells per experimental condition using the multiplex luciferase assay. Prepare also two wells for each constitutive luciferase control. We prepared 72 wells for the experiment as shown in Figure 4.
- The number of cells is optimized for the A459 cell line. When using other cell lines, the number of cells per well may need to be adjusted accordingly: difficult to transfect cells may need more cells to obtain decent enough luciferase emission signals.
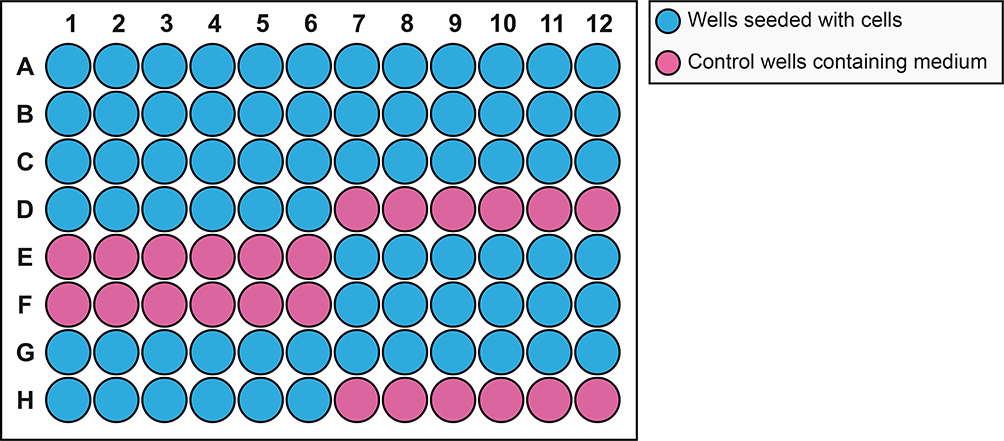
Figure 4. Plate schematic for seeding mammalian A549 cells to perform multiplex hextuple luciferase assaying.
Each well of a 96-well plate is seeded with 25,000 cells the day before plasmid transfection: blue wells indicate wells seeded with cells, while pink wells indicate wells where only culture medium is added (Control).
Basic Protocol 3
Transfection of luciferase reporter plasmids followed by drug and recombinant protein treatments
This protocol describes how to use the multiplex hextuple luciferase assay to analyze the effects of different treatments, for example pharmaceutical drugs or proteins on multiple cell signaling pathways. It uses the A549 lung cancer cell line as an example. The protocol describes how the multiplex luciferase reporter vector can be used to quantify responses on both targeted and non-targeted pathways.
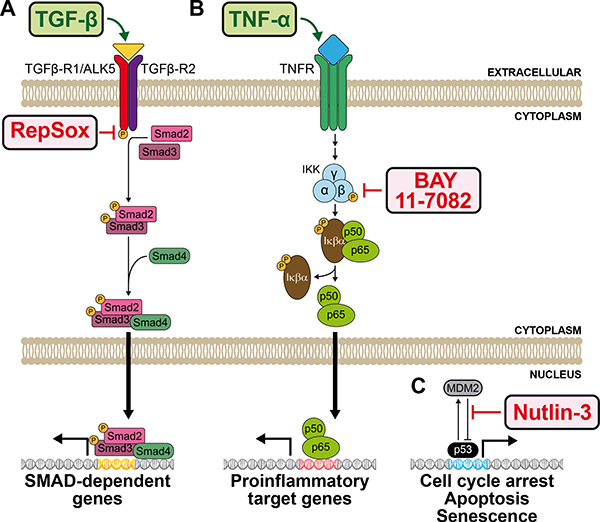
First, we describe how to probe the transforming growth factor beta (TGF-β) signaling pathway by treating A549 cells with recombinant human TGF-β. TGF-β binds to the TGF-β type II receptor (TGFBR2), which recruits and phosphorylates the TGF-β type I receptor (TGFBR1). The type I receptor then phosphorylates the signal transducing and transcriptional modulating proteins SMAD2 and SMAD3, which bind SMAD4. The SMAD complex then enters the nucleus where it activates transcription of SMAD dependent genes. Adding TGF-β should result in increased TGF-β pathway signaling. We also assessed the effect of the small molecule RepSox, a selective inhibitor of TGFBR1, anticipated to result in decreased TGF-β pathway signaling (Ichida et al., 2009), even when adding TGF-β (Figure 5A).
Figure 5. Pathway schematics of pharmaceutical and ligand interventions of three signaling pathways known to be active in mammalian A549 cells.
(A) Simplified schematic of the transforming growth factor beta (TGF-β) pathway. The ligand TGF-β (GeneCards ID: TGFB), binds its heterodimeric receptor (GeneCards ID: TGFBR1 and TGFBR2), resulting in the downstream activation of the signal transducing and transcriptional modulating SMAD cascade (GeneCards IDs: SMAD2, 3, and 4), subsequently followed by the transcriptional upregulation of SMAD-dependent genes. The TGF-β pathway-activating ligand is labelled in green, while the TGF-β pathway inhibiting pharmaceutical (RepSox) is labelled in red. (B) Simplified schematic of the tumor necrosis factor alpha (TNF-α) pathway. The ligand TNF-α (GeneCards ID: TNF) binds its trimeric receptor, consisting of tumor necrosis factor receptor superfamily members 1A and 1B proteins (GeneCards IDs: TNFRSF1A or TNFRSF1B), resulting in the downstream phosphorylation of the IκB kinase (IKK) complex, consisting of α/IKK-α (GeneCards ID: CHUK), β/IKK-β (GeneCards ID: IKBKB), and γ/IKK-γ (GeneCards ID: IKBKG). The IKK complex then activates the NF-κβ protein complex consisting of IκΒα/ nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (GeneCards ID: NFKBIA), p50/ Nuclear Factor NF-Kappa-B P50 Subunit (GeneCards ID: NFKB1), and p65/Nuclear Factor NF-Kappa-B P65 Subunit (GeneCards ID: RELA), through phosphorylation and release of IκΒα. The liberated p50/65 complex then transports to the nucleus followed by the transcriptional upregulation of proinflammatory target genes. The TNF-α pathway-activating ligand is labelled in green, while the TNF-α pathway inhibiting pharmaceutical (BAY11–7082) is labelled in red. (C) Simplified schematic of p53 pathway activation induced by Nutlin-3. Nutlin-3 selectively inhibits the interaction between the ubiquitin-protein ligase called mouse double minute 2 (MDM2) homolog (GeneCard ID: MDM2), and tumor protein p53 (GeneCard ID: TP53), resulting in p53 pathway activation. The p53 pathway inhibiting pharmaceutical (Nutlin-3) is labelled in red.
Second, we describe how to assay NF-κβ pathway signaling after the addition of the human cytokine tumor necrosis factor alpha (TNF-α). TNF-α binds its receptor, resulting in the downstream phosphorylation of the IκB kinase (IKK) complex. The phosphorylated IKK complex activates the NF-κβ protein complex consisting of IκΒα, p50, and p65, through phosphorylation and release of IκΒ. The liberated p50/65 complex then enters the nucleus where it induces transcriptional upregulation of proinflammatory target genes. Adding TNF-α should result in increased TNF-α pathway signaling. We also tested the effect of the small molecule Bay 11–7082, a known anti-inflammatory agent that inhibits TNF-α-induced IKK complex phosphorylation, anticipated to result in decreased TNF-α pathway signaling (Pierce et al., 1997), even when adding TNF-α (Figure 5B).
Finally, we describe how to investigate the p53 signaling pathway, by assaying the effect of Nutlin-3, a small molecule that selectively activates the p53 pathway through inhibition of the MDM2-mediated repression of p53 pathway signaling (Künkele et al., 2012). Treatment of A549 cells, that are wild-type for the p53 protein, results in increased p53 pathway signaling followed by cell cycle arrest, apoptosis, and senescence (Figure 5C).
Materials: Reagents, solutions, and starting samples or test organisms/cells
Multiplex luciferase reporter vector miniprep, quantified by spectrophotometry and verified by agarose gel DNA electrophoresis (see Basic Protocol 1).
96 well plate with 25,000 A549 cells/well, seeded the day before the experiment starts (see Basic Protocol 2).
Lipofectamine™ 3000 Transfection Reagent (ThermoFisher Scientific, cat. no. L3000015)
Note: Lipofectamine™ 3000 Transfection Reagent comes with P3000 reagent and Lipofectamine Reagent.
Opti-MEM I Reduced Serum Medium (ThermoFisher Scientific, cat. no. 31985070)
Dimethyl sulfoxide, DMSO (Millipore Sigma, cat.no. D8418)
A 20 mM nutlin-3 (VWR, cat.no. 89151–170) stock solution, diluted in DMSO. Store at −20°C.
A 10 mM BAY 11–7082 (Millipore Sigma, cat.no. 196870) stock solution, diluted in DMSO. Store at −20°C.
A 6 mM RepSox (VWR, cat.no. 10191–164) stock solution, diluted in DMSO. Store at −20°C.
A 5 μg/mL recombinant human TGF-β (VWR, cat.no. 10772–036) stock solution, reconstituted in 10mM Citric Acid, pH 3.0. Store at −80°C.
A 10 μg/mL recombinant rat TNF-α (VWR, cat.no. 10770–974) stock solution, reconstituted in miliQ water with 0.1% BSA. Store at −80°C.
Warm DMEM/F-12+FBS culture media at 37°C (see Basic Protocol 2).
Materials: Hardware and instruments
Multichannel repeater pipette (VWR, cat. no. 10827–926)
Note: The use of the multichannel repeater pipette is strongly preferred in order to reduce experimental variation between signals from wells of the same plate, and between different plates.
Transfection of the multiplex luciferase reporter vector
-
1
Dilute all vectors to be transfected to 150 ng/μL.
-
2
Prepare the following transfection reaction mixes:
| 1x | Each constitutive luciferase vector (3x) |
Multiplex luciferase reporter vector (66x) |
|
|---|---|---|---|
| Vector (150 ng/μL) | 1 μL | 3 μL | 66 μL |
| Opti-MEM | 15 μL | 45 μL | 990 μL |
| P3000 reagent | 0.3 μL | 0.9 μL | 19.8 μL |
| Lipofectamine reagent | 0.3 μL | 0.9 μL | 19.8 μL |
Multiply the amount of the transfection mix by the number of biological replicates and add at least 10%. For the experiments in this Unit, we needed to transfect 60 wells of the multiplex luciferase reporter vector, and 2 of each control vector, so we prepared a master mix consisting of 66x and 3x of the reaction mix for each vector, respectively.
-
3
Incubate the transfection reaction mixes for 30 minutes at room temperature.
-
4
Add 16.6 μL of the transfection mix per well as indicated in Figure 6, ideally using a multichannel repeater pipette to reduce experimental variation.
-
5
Incubate transfections for 3h in the cell incubator.
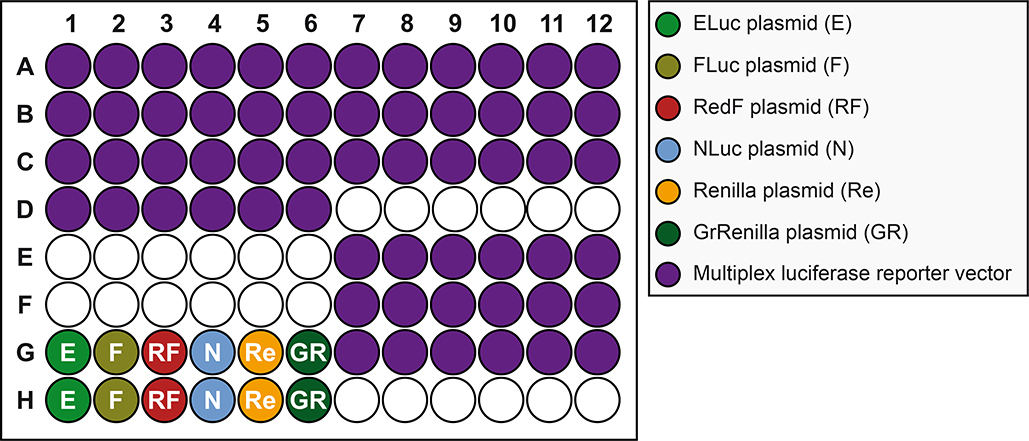
Figure 6. Plate schematic for transfection of mammalian A459 cells.
Wells seeded with cells (see Figure 4) are transfected with plasmid sample: bright green, dull green, red, blue, orange and dark green wells are transfected with constitutively expressing ELuc (E), FLuc (F), RedF (RF), NLuc (NL), Renilla (Re), or GrRenilla (GR) luciferase reporter vectors, respectively, used to determine the transmission coefficients (see Figure 8), while purple wells are transfected with the multiplex luciferase reporter vector containing five pathway transcriptional luciferase reporters (FLuc, RedF, NLuc, Renilla, and GrRenilla) and one control luciferase reporter (ELuc), used to measure pathway-specific manipulations (see Figure 12).
Treatments with drugs and recombinant proteins
-
6
Prepare a 10 μM Nutlin-3 in DMSO using the 20 mM Nutlin-3 stock solution.
-
7
Prepare the following dilutions in warm DMEM/F-12+FBS medium:
| DMEM/F-12+FBS | 5 μg/mL hTGF-β | 6 mM RepSox | DMSO | 10mM Citric Acid, pH 3.0 | |
|---|---|---|---|---|---|
| 5 ng/mL hTGF-β | 1 mL | 1 μL | - | 1 μL | - |
| 5 ng/mL hTGF-β + 6 μM RepSox | 1 mL | 1 μL | 1 μL | - | - |
| CONTROL | 1 mL | - | - | 1 μL | 1 μL |
| DMEM/F-12+FBS | 10 μg/mL TNF-α | 10 mM Bay11–7082 | DMSO | milliQ water with 0.1% BSA | |
|---|---|---|---|---|---|
| 10 ng/mL hTNF-α | 1 mL | 1 μL | - | 1 μL | - |
| 10 ng/mL hTNF-α+10 μM BAY11 | 1 mL | 1 μL | 1 μL | - | - |
| CONTROL | 1 mL | - | - | 1 μL | 1 μL |
| DMEM/F-12+FBS | 20 mM Nutlin-3 | 10 μM Nutlin-3 | DMSO | ||
|---|---|---|---|---|---|
| 20 μM Nutlin-3 | 1 mL | 1 μL | - | - | |
| 10 μM Nutlin-3 | 2 mL | 1 μL | - | - | |
| 2 μM Nutlin-3 | 0.8 mL | - | 0.2 mL | - | |
| CONTROL | 1 mL | - | - | 1 μL |
-
8Remove the medium from the wells using a multichannel pipette.
- Note: Do not use a vacuum aspirator. This will disturb the transfected cells, which become more fragile after the Lipofectamine 3000 transfection reaction.
-
9
Add 100 μL of each dilution to the 96 well plate as shown in Figure 7.
-
10
Incubate for 24h at 37°C in a cell culture incubator and proceed to Basic Protocol 4.
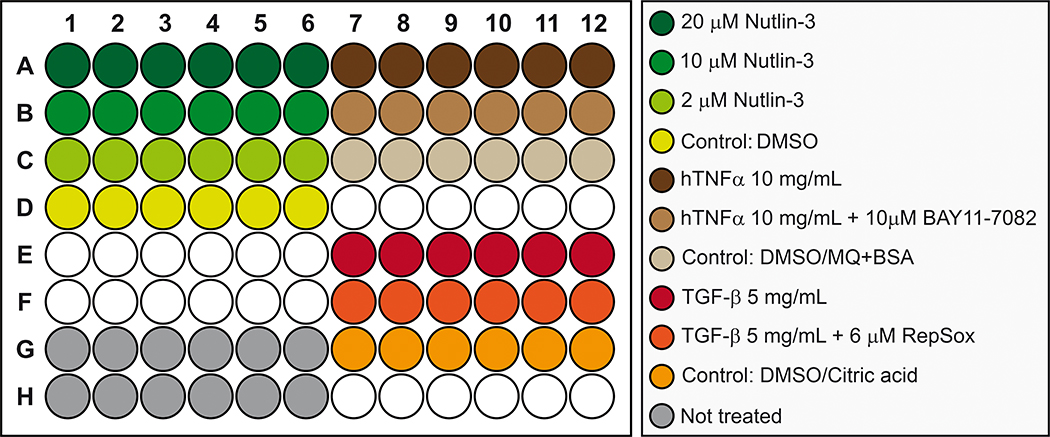
Figure 7. Plate schematic for drug treatments of mammalian A549 cells.
Transfected cells are treated with different concentrations of ligands and/or pharmaceuticals as indicated: 20 μM Nutlin-3, 10 μM Nutlin-3, 2 μM Nutlin-3, Nutlin-3 control (DMSO), 10 mg/ml hTNFα, 10 mg/ml hTNFα and 10 μM BAY11–7082, hTNFα/BAY11–7082 control (DMSO and MQ+BSA), 5 mg/ml TGF-β, 5 mg/ml TGF-β and 6 μM RepSox, TGF-β/RepSox control (DMSO and citric acid), and untreated control.
Basic Protocol 4
Performing the multiplex hextuple luciferase assay
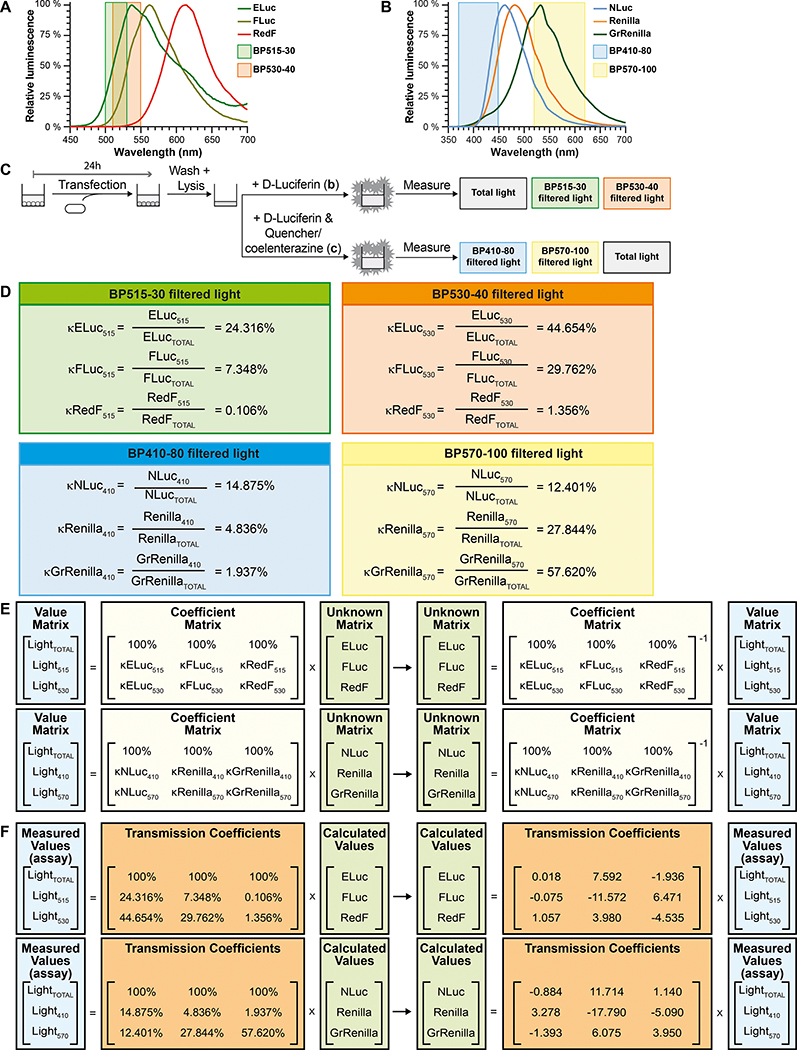
This protocol describes the use of a plate reader equipped with appropriate band pass emission filters capable of recording luciferase emission values for each of the luciferases, being total light emitted (unfiltered), or just a part of the total light emitted (filtered). First of all, the experimenter calibrates the luminometer using technical replicates of the cells transfected with the control constitutively expressed luciferases and determine how much emitted light from each luciferase is detected by the plate reader after light being unfiltered or filtered through bandpass filters (Figure 8A, 8B, and 8C). This calibration experiment results in transmission coefficients that are needed to calculate pathway signaling values from measured luciferase emission values obtained by multiplex luciferase assaying (Figure 8D). Then the experimenter carries out the multiplex luciferase assay using lysates of test samples. Next, the experimenter analyzes the data using the provided Excel template that automatically calculates the pathway signaling values from the measured values using the previously obtained transmission coefficients (Figure 8E and 8F). Finally, standardize the data between biological replicates with the ELuc constitutively expressed control luciferase, and perform statistical analysis.
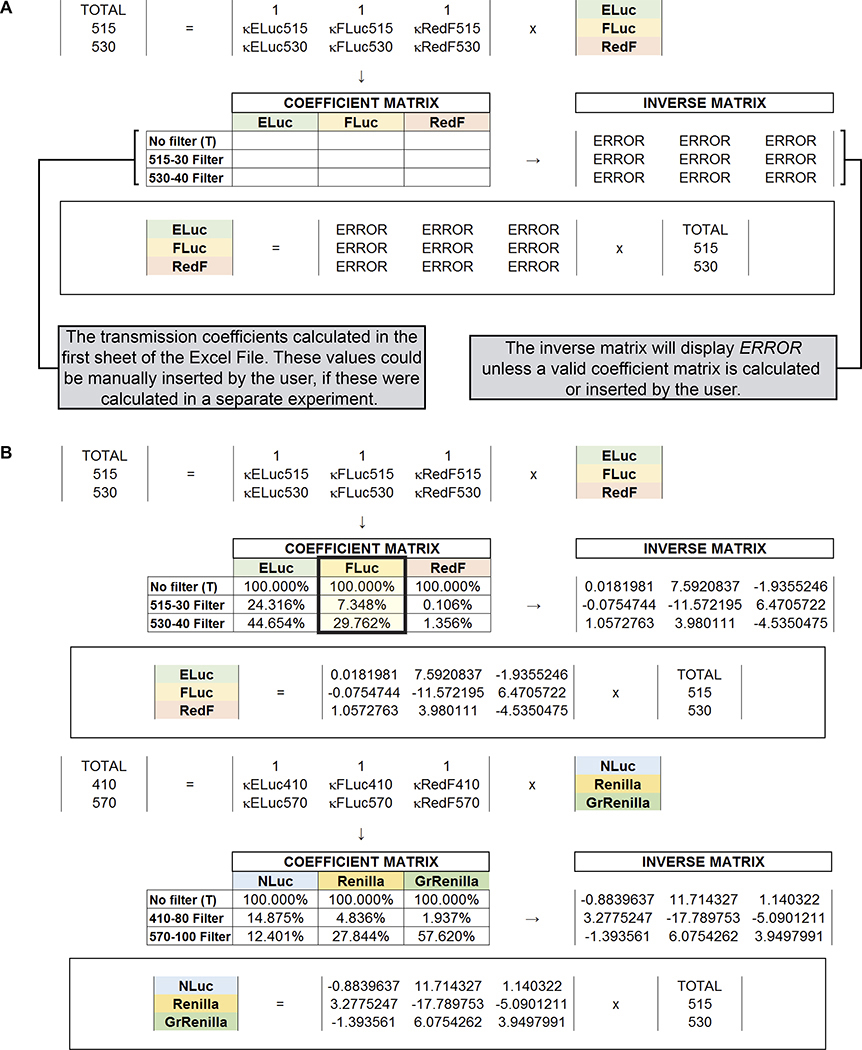
Figure 8. Schematic representation of the calculation of luciferase-specific emission contributions in a mixture of three luciferases using transmission coefficients and simultaneous equations.
(A) Emission spectra of the three D-Luciferin luciferases used in the multiplex hextuple luciferase assay demonstrate partial overlap between the three emission spectra. Hence, two bandpass emission filters, one measuring between 500 and 530 nm (BP515–30), and a second measuring between 510 and 550 nm (BP530–40), are used to capture select, and less partially overlapping portions of the three emission spectra that will be used for mathematical spectral unmixing of overlapping emission signals emitted by the three D-Luciferin luciferases during the assay (see C to F), and are indicated over the spectra. (B) Emission spectra of the three coelenterazine luciferases used in the multiplex hextuple luciferase assay demonstrate partial overlap between the three emission spectra. Hence, two additional bandpass emission filters, one measuring between 370 and 450 nm (BP410–80), and a second measuring between 520 and 620 nm (BP570–100), are used to capture select, and less partially overlapping portions of the emission spectrum that will be used for mathematical spectral unmixing of overlapping emission signals emitted by the three coelenterazine luciferases during the assay (see C to F), and are indicated over the spectra. (C) Simplified schematic of the experimental setup to determine transmission coefficients for each luciferase using total luminescence emission or filtered emission over the indicated bandpass emission filters. Cells are transfected with constitutively expressing ELuc, FLuc, or RedF luciferase, to determine the transmission coefficients for the D-Luciferin-responsive luciferases, or NLuc, Renilla, or GrRenilla luciferase to determine the transmission coefficients for the coelenterazine-responsive luciferases. (D) For the three D-Luciferin-responsive luciferases, κELuc515, κFLuc515, and κRedF515 represent the transmission coefficients over the BP515–30 bandpass emission filter (top left), while κELuc530, κFLuc530, and κRedF530 represent the transmission coefficients over the BP530–40 bandpass emission filter (top right). For the three coelenterazine-responsive luciferases, κNLuc410, κRenilla410, and κGrRenilla410 represent the transmission coefficients over the BP410–80 bandpass emission filter (bottom left), while κNLuc570, κRenilla570, and κGrRenilla570 represent the transmission coefficients over the BP570–100 bandpass emission filter (bottom right). (E) Simultaneous equations solving the D-Luciferin-responsive luciferase contributions have three unknowns corresponding to the amount of each D-Luciferin-responsive luciferase in a mix: ELuc, FLuc, and RedF. The value matrix includes the three measured values for the first step of the luciferase assay, while the coefficient matrix includes all the transmission coefficients for the luciferases in the equation system (see D). To solve the simultaneous equations for the D-Luciferin-responsive luciferases (unknown matrix representing the calculated values for each D-Luciferin luciferase), the inverse of the coefficient matrix is multiplied by the value matrix. LightTOTAL, Light515, and Light530 represent the total measured light values and the light filtered by the BP515–30 and BP530–40 bandpass emission filters for the D-Luciferin-responsive luciferases (ELuc, FLuc, and RedF). Similarly, simultaneous equations solving the coelenterazine-responsive luciferase contributions have three unknowns corresponding to the amount of the coelenterazine-responsive luciferase in a mix, namely NLuc, Renilla, and GrRenilla. The value matrix includes the three measured values for the second step of the luciferase assay, while the coefficient matrix includes all the transmission coefficients for the luciferases in the equation system (see D). To solve the simultaneous equations for the coelenterazine-responsive luciferases (the unknown matrix representing the calculated values for each coelenterazine luciferase), the inverse of the coefficient matrix is multiplied by the value matrix. LightTOTAL, Light410, and Light570 represent the total measured light values and the light filtered by the BP410–80 and BP570–100 bandpass emission filters for the coelenterazine-responsive luciferases (NLuc, Renilla, and GrRenilla). (F) To obtain calculated values for each D-Luciferin-responsive luciferase-linked reporter unit, a matrix inversion of the coefficient matrix (the matrix containing values for all transmission coefficients) obtained using the appropriate bandpass emission filters (see D), is multiplied by the value matrix (the matrix containing luminescence measurements obtained by the plate reader). Similarly to obtain calculated values for each coelenterazine-responsive luciferase-linked reporter unit, a matrix inversion of the coefficient matrix (the matrix containing values for all transmission coefficients) obtained using the appropriate bandpass emission filters (see D), is multiplied by the value matrix (the matrix containing luminescence measurements obtained by the plate reader).
Materials: Reagents, solutions, and starting samples or test organisms/cells
Dual-Luciferase® Reporter Assay System kit (Promega, cat. no. E1980). The kit contains:
5X Passive Lysis Buffer (5XPLB)
Lyophilized Luciferase Assay Substrate
Luciferase Assay Buffer II
50X Stop & Glo® Substrate
Stop & Glo® Buffer
1X PBS (see Basic Protocol 2).
Autoclaved milliQ water.
5 mL centrifuge tubes (VWR, cat. no. 10002–728)
CELLSTAR White 384-well plates (Greiner Bio-One, cat. no. #781073).
Materials: Hardware and instruments (e.g., glassware, disposables, microscopes, centrifuge)
CLARIOstar microplate reader (BMG LABTECH) equipped with appropriate emission bandpass (BP) filters:
BP410–80 emission filter, measuring light between 370 and 450 nm
BP515–30 emission filter, measuring light between 500 and 530 nm
BP530–40 emission filter, measuring light between 510 and 550 nm
BP570–100 emission filter, measuring light between 520 and 620 nm
Note: While our initial work developing and implementing the multiplex luciferase assay, as well as this work, is all performed using the CLARIOstar microplate reader and emission filters from BMG LABTECH, any microplate reader equipped with appropriate emission bandpass filters should be able to perform the needed measurements, after appropriate calibration.
Multichannel repeater pipette (VWR, cat. no. 10827–926).
Supplemental File, “Demo_Excel_Protected_2020_05_13_KV” includes sheets for the automatic calculation of transmission coefficients, simultaneous equations, unformatted measurements from a small group of samples, and unformatted measurements from a 96 well plate.
Substrate Preparation
-
1
Thaw the Luciferase Assay Substrate and the Stop & Glo® Buffer.
-
2Prepare the Luciferase Assay Reagent II (LAR II) buffer by resuspending the lyophilized Luciferase Assay Substrate with the Luciferase Assay Buffer II. Make 5 mL aliquots of LAR II buffer and store aliquots at −80°C.
- Note: Repeated freeze-thaw cycles will decrease assay performance. Also, aliquots are less stable at −20°C, so we recommend storing 2 or 3 at −20°C for short term storage.
-
3Make 5 mL aliquots of the Stop & Glo® Buffer, but don’t mix it with the Stop & Glo® substrate.
- Note: Stop & Glo® Buffer mixed with Stop & Glo® substrate (Stop & Glo® Reagent) should be prepared just before each use. Both reagents are stable at −20°C but once it is mixed with the substrate (Stop & Glo® Reagent), activity decreases in a few days.
Cell Lysis
-
4Prepare 3 mL of 1X Passive Lysis Buffer (1XPLB) by mixing 600 μL of the 5XPLB with 2.4 mL of MiliQ water.
- We recommend a minimum of 40 μL PLB1X per well in a 96 well plate. For our experiment, totaling 72 wells, we need at least 2.88 mL.
-
5Take the 96 well plate from the incubator, and remove the culture media with a multichannel pipette.
- Do not use a vacuum aspirator. This will disturb the cells.
-
6
Wash the wells once with 150 μL of PBS during 1 minute. Remove the PBS with a multichannel pipette.
-
7Add 40 μL of 1XPLB, and incubate at room temperature for 30 minutes. Lysates do not need to be cleared by centrifugation.
- After lysis, we recommend a 2h freeze at −80°C for improved lysis performance.
- Optional protocol stopping point. You can store the lysate at −80°C for up to a month for later luciferase measurements.
Calibrating the assay by calculating the transmission coefficients for each of the six luciferases
-
8Dilute 30 μL of the Stop & Glo® Substrate (50X) in 1470 μL of Stop & Glo® Buffer to generate 1.5 mL of Stop & Glo® Reagent.
- The minimum suggested reagent, for both LARII and Stop & Glo® Reagent, per injector in the CLARIOStar is 1.5 mL, regardless of the amount needed to ensure correct injector priming. For our experiment, totaling 72 wells, we need at least 0.72 mL (10 μL/well) and 1.08 mL (15 μL/well) of LARII and Stop & Glo® Reagent, respectively.
-
9Generate a multiplex luciferase protocol using the CLARIOstar microplate reader software with the following settings:
- Dispense 10 μL of LARII buffer
- Wait 30 seconds
- Measure the total light for 2 seconds
- Measure BP515–30 filtered light for 2 seconds
- Measure BP530–40 filtered light for 2 seconds
- Dispense 15 μL of the Stop & Glo® Reagent
- Wait 7 seconds
- Measure BP410–80 filtered light for 1 second
- Measure BP570–100 filtered light for 1 second
- Measure total light for 1 seconds
-
10
Load injector 1 with at least 1.5 mL LARII buffer and injector 2 with at least 1.5 mL Stop & Glo® Reagent.
-
11
Thaw the cell lysates if these had been frozen at −80°C.
-
12
Briefly spin down in centrifuge.
-
13
Transfer 5 μL of the cell lysates to a white 384-well plate for the constitutive luciferases, make four technical replicates, and insert the plate into the CLARIOStar luminometer.
-
14
Start the multiplex luciferase protocol using the CLARIOstar software, and export the recorded data.
-
15
Use the recorded values to calculate the transmission coefficients (κ) for each luciferase as shown schematically (Figure 8), as well as mathematically using the first sheet of the provided Excel file as described in Figure 9).
-
16
The coefficient matrices will be automatically calculated by the second sheet of the provided Excel template as described in Figure 10.
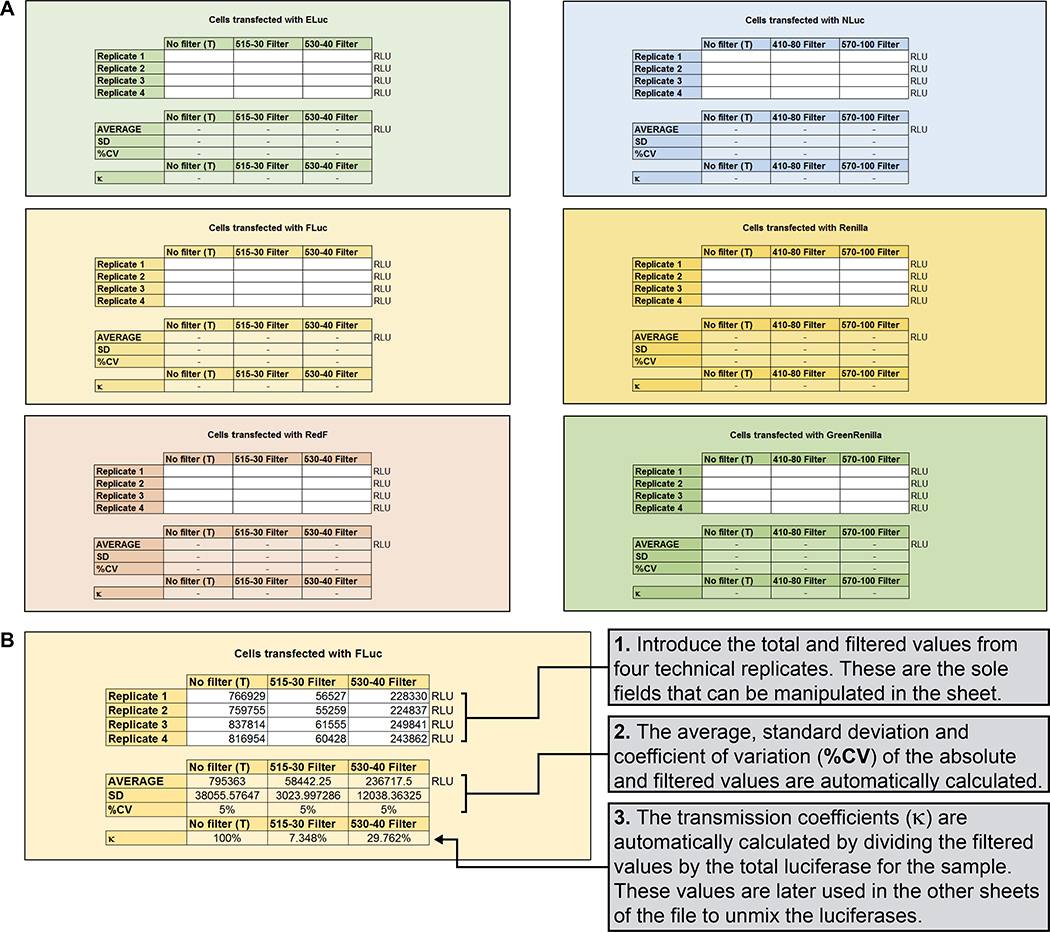
Figure 9. Automatic mathematical calculation of the transmission coefficients.
(A) Screenshot of the first worksheet of the provided Microsoft Excel template (see Supplemental File). (B) Real example to calculate the transmission coefficients for FLuc, with detailed instructions. Same instructions are followed to calculate transmission coefficients for the other five luciferases (ELuc, RedF, NLuc, Renilla, and GrRenilla).
Figure 10. Automatic mathematical calculation of the simultaneous equations.
(A) Screenshot of the second worksheet of the provided Microsoft Excel template (see Supplemental File), including simplified instructions to facilitate the generation of the simultaneous equations needed to spectrally unmix the obtained measurements into distinct values for the D-Luciferin luciferases. Distinct values for the coelenterazine luciferases are similarly obtained (not shown). (B) Real example of the automatic calculation of the simultaneous equations needed to spectrally unmix the luminescence for the D-Luciferin luciferases into distinct values. The transmission coefficients obtained for Fluc as previously illustrated (see Figure 9) are highlighted. Distinct values for the coelenterazine luciferases are similarly obtained.
Multiplex hextuple luciferase assaying
-
17
Thaw the lysates if these had been frozen at −80°C.
-
18
Briefly spin down in centrifuge.
-
19
Transfer the 40 μL of the lysates to a white 384-well plate, and insert the plate into the CLARIOStar microplate reader.
-
20
Start the multiplex luciferase protocol using the CLARIOstar software, and export the recorded data.
-
21
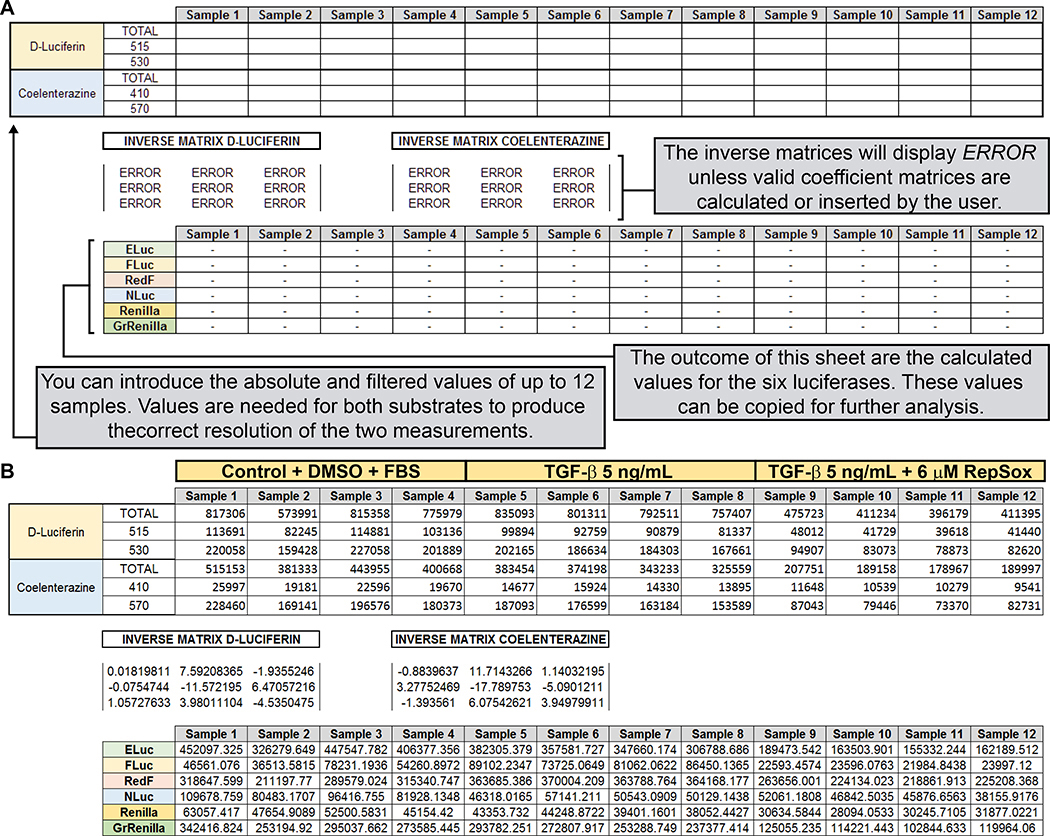
Use obtained values to calculate the individual contribution of each luciferase to the mix using either the third sheet of the provided Excel template (“Unformatted Measurements“) as described in Figure 11, or the fourth sheet of the provided Excel template (“96 well plate“ tab).
Figure 11. Calculation of spectrally unmixed values for the six luciferase values obtained for the experimental manipulations during the TGFβ/RepSox experiment.
(A) Screenshot of the third worksheet of the provided Microsoft Excel template (see Supplemental File), including simplified instructions on how to generate output values for the six luciferases from the measured values generated by the plate leader. This worksheet is prepared to process up to 12 samples. A fourth worksheet, able to process an entire 96-well plate, is provided separately (not shown) in the Microsoft Excel template. (B) Real example of the automatic calculation of the spectrally unmixed values for each luciferase, after the introduction of the luminescence values of 4 replicates for each experimental condition.
Normalization of the luciferase measurements by comparison with the control, calculation of statistics for the measurement, and analysis of the effects of the drugs and ligands
-
22Standardize all luciferase measurements in each sample by dividing the values of the FLuc, RedF, NLuc, Renilla and GrRenilla luciferases by the value obtained for ELuc.
-
23
Calculate the mean for each data set, and the standard error of the mean (SEM), as indicated here for the TGF-β treatment.
| CONTROL + DMSO + 10mM Citric Acid, pH 3.0 | +TGF-β 5 ng/mL | +TGF-β 5 ng/mL + 6 μM RepSox |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MEAN | SEM | N | MEAN | SEM | N | MEAN | SEM | N | |
| TGF-β | 0.1308 | 0.01600 | 4 | 0.2385 | 0.0158 | 4 | 0.1382 | 0.0065 | 4 |
| NF-κβ | 0.6938 | 0.0306 | 4 | 1.0549 | 0.0489 | 4 | 1.3900 | 0.0078 | 4 |
| P53 | 0.2266 | 0.0108 | 4 | 0.1474 | 0.0095 | 4 | 0.2730 | 0.013267 | 4 |
| c-MYC | 0.1285 | 0.0084 | 4 | 0.1186 | 0.0030 | 4 | 0.1812 | 0.0086 | 4 |
| MAPK/JNK | 0.7165 | 0.0293 | 4 | 0.7584 | 0.0102 | 4 | 0.6900 | 0.0187 | 4 |
-
24
For each pathway, normalize each treatment mean and SEM by dividing them by the value of the control, as indicated here for the TGF-β treatment.
| CONTROL + DMSO + 10mM Citric Acid, pH 3.0 | +TGF-β 5 ng/mL | +TGF-β 5 ng/mL + 6 μM RepSox |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MEAN | SEM | N | MEAN | SEM | N | MEAN | SEM | N | |
| N(TGF-β) | 1 | 0.1224 | 4 | 1.8237 | 0.1204 | 4 | 1.0570 | 0.0495 | 4 |
| N(NF-κβ) | 1 | 0.0441 | 4 | 1.5204 | 0.0704 | 4 | 2.0034 | 0.0113 | 4 |
| N(P53) | 1 | 0.0478 | 4 | 0.6506 | 0.0423 | 4 | 1.2046 | 0.0585 | 4 |
| N(c-MYC) | 1 | 0.0657 | 4 | 0.9232 | 0.0236 | 4 | 1.4101 | 0.0669 | 4 |
| N(MAPK/JNK) | 1 | 0.0410 | 4 | 1.0585 | 0.0142 | 4 | 0.9631 | 0.0262 | 4 |
-
25
Calculate the log2 fold-change, by doing the base 2 logarithm of the normalized values and propagate the errors, according to the formula:
| CONTROL + DMSO + 10mM Citric Acid, pH 3.0 | +TGF-β 5 ng/mL | +TGF-β 5 ng/mL + 6 μM RepSox |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| VALUE | ERROR | N | VALUE | ERROR | N | VALUE | ERROR | N | |
| Log2(TGF-β) | 0 | 0.1765 | 4 | 0.8669 | 0.0952 | 4 | 0.0800 | 0.0676 | 4 |
| Log2 (NF-κβ) | 0 | 0.0636 | 4 | 0.6045 | 0.0668 | 4 | 1.0025 | 0.0081 | 4 |
| Log2(P53) | 0 | 0.0690 | 4 | -0.6201 | 0.0937 | 4 | 0.2686 | 0.0700 | 4 |
| Log2(c-MYC) | 0 | 0.0948 | 4 | -0.1153 | 0.0369 | 4 | 0.4957 | 0.0685 | 4 |
| Log2(MAPK/JNK) | 0 | 0.0592 | 4 | 0.0820 | 0.0193 | 4 | -0.0542 | 0.0392 | 4 |
-
26
Determine the statistical significance of the log2 fold-change by the multiple t-test using the Holm-Sidak method with alpha = 0.05 (*P <0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, n.s. is non-significant), referred to the control values for each pathway.
| Control vs +TGF-β 5 ng/mL | Control vs +TGF-β 5 ng/mL + 6 μM RepSox |
|||||
|---|---|---|---|---|---|---|
| SIGNIFICANT | Adjusted P-value | Summary | SIGNIFICANT | Adjusted P-value | Summary | |
| TGF-β | YES | <0.0001 | **** | NO | 0.6025 | n.s. |
| NF-κβ | YES | <0.0001 | **** | YES | <0.0001 | **** |
| P53 | YES | <0.0001 | **** | YES | 0.0069 | ** |
| c-MYC | NO | 0.4136 | n.s. | YES | <0.0001 | **** |
| MAPK/JNK | NO | 0.5869 | n.s. | NO | 0.8037 | n.s. |
-
27
Represent the values in bar graphs, heat maps or boxes & whiskers, according to your preferences and preferred software. See Figure 12 for the results of the experiments performed in this work using the GraphPad Prism software for statistical analysis and graphing, followed by Creative Cloud Adobe Illustrator for illustration.
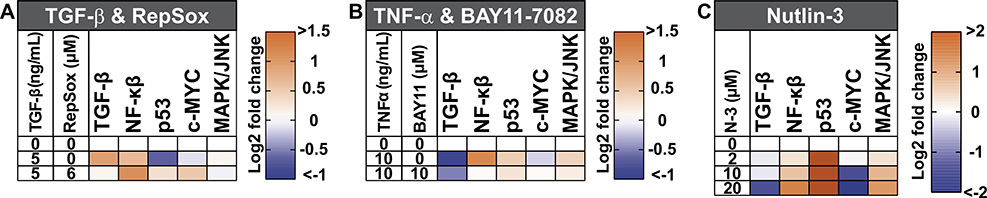
Figure 12. Multiplex luciferase analysis of effects of different ligand and drug treatments on signaling pathways in mammalian A549 lung cancer cells.
(A) The TGF-β pathway is one-fold activated after adding recombinant TGF-β ligand (5 ng/mL), but TGF-β also causes significant collateral activation of the NF-κβ and downregulation of the p53 pathways. Simultaneous addition of downstream RepSox inhibitor (6 μM) neutralizes the effects of recombinant TGF-β addition on TGF-β and p53 pathway signaling, while significantly activating the NF-κβ and c-MYC pathways. Changes in the other monitored pathways are non-significant. (B) The NF-κβ pathway is 1.5-fold activated after treatment with recombinant TNF-α ligand (10 ng/ml), but also has a strong downregulating effect in the TGF-β pathway. Simultaneous treatment with the downstream NF-κβ pathway inhibitor BAY11–7082 (10 μM) neutralizes the effect of recombinant TNF-α on the NF-κβ pathway and attenuates the downregulation of the TGF-β pathway. Changes in the other monitored pathways are non-significant. (C) Increasing concentrations of Nutlin-3 (N-3), from 2 to 20 μM, result in a strong activation of the p53 pathway, and incremental activation of the NF-κβ and MAPK/JNK pathways. At 10 μM, Nutlin-3 strongly downregulates the c-MYC pathway, and, at 20 μM, Nutlin-3 strongly downregulates the TGF-β and c-MYC pathways.
Reagents and Solutions
Dual-Luciferase® Reporter Assay System (Promega E1980)
The Dual-Luciferase® Reporter Assay System (Promega E1980) includes all essential reagents for the multiplex luciferase assay. After receiving the kit:
Store the 5X Passive Lysis Buffer (5XPLB) at −20°C and dilute 1:5 in MiliQ water before using.
Thaw the Luciferase Assay Buffer II and resuspend the lyophilized Luciferase Assay Substrate. The mix is known as Luciferase Assay Reagent II (LAR II). Prepare 5 mL aliquots, and store at −80°C for prolonged stability. Keep two working aliquots at −20°C.
Thaw the Stop & Glo® Buffer and prepare 5 mL aliquots. Store at −20°C.
Store the Stop & Glo® Substrate at −20°C. Dilute 1:50 in Stop & Glo® Buffer before using.
PBS, 10X
The protocol uses lab-made PBS to wash the cells before lysis with the 1XPLB. To prepare 1L of PBS 10X:
- Mix in 800 mL distilled water:
- 25.6 g Na2HPO4·7H2O
- 80 g NaCl
- 2 g KCl
- 2 g KH2PO4
Adjust the pH to 7.4 with HCl.
Add distilled water to a final volume of 1L.
Autoclave for 40 minutes at 121°C.
Commentary
Background information
Currently, most cell-based reporter assays rely on one measurement to probe a biomedical activity. The signal can be based on absorbance, fluorescence, or luminescence. However, biological systems are complex, and assays that measure a single quantity often yield only limited information. Even though additional single measurement assays can be performed to obtain more information, developing and carrying out such assays often requires lengthy assay development, and additional experimental time and expense to perform. Moreover, assays that are performed independently can’t be easily compared with one another, making controlled, comparative analysis across different experimental variables challenging (Garvey et al., 2016; Berg, 2017) For these reasons assays that provide multiple simultaneous measurements are useful (Taylor and Giuliano, 2005; Westwick and Lamerdin, 2011). Multiplexed cellular assays seek to address these limitations by measuring multiple readouts from a single experimental sample (Korn and Krausz, 2007; Michelini et al., 2008; Gustafsdottir et al., 2013). Because multiple measurements derive from the same sample, correlations between experimental variables and biological effects can be more accurately obtained and compared (Gerets et al., 2011).
Detection agents integrated in multiplex assays are required to detect orthogonally and preferentially do that over large dynamic ranges. Luciferases are cost-effective, versatile, genetically encoded candidates whose output signals can be sensitively detected. Luciferase reporters are used in biomedical research for a variety of applications, including analytic, immuno-, gene expression, drug screening, and many other assays (Kaskova et al., 2016). Advantages of luciferases over fluorescent proteins include higher sensitivity (they can report on little signal), wider dynamic detection range (they can detect over a broad range of signaling) (Branchini et al., 2018), and the absence of auto-luminescence in mammalian cells (Kaskova et al., 2016). Similar to fluorescent proteins, each luciferase has a unique emission spectrum (Nakatsu et al., 2006) that make possible the simultaneous quantification of signal from multiple different luciferases as of two as using detection filters are that distinguish between their emission spectra (Adams and Miller, 2014; Ohmiya, 2015).
The majority of today’s luciferase experiments relies on dual luciferase reporter assays (Schagat et al., 2007). Such assays consist of the sequential quantitative measurement of the activity of two luciferases in one cell lysate, eliminating pipetting errors that would result from measuring luciferase activities separately (Sherf et al., 1996). The first luciferase is used to monitor a cellular signaling event of interest, and the second is coupled to an internal control used for data normalization (Figure 13A).
Figure 13. Development of multiplex luciferase assays for the collateral analysis of cellular signaling events.
(A) Simplified schematic of the dual luciferase assay widely used for monitoring the activity of a single experimental cellular signaling event. (B) Simplified schematic of a multiplex hextuple luciferase assay that can be used for the collateral monitoring of five experimental cellular signaling events.
Recently, we demonstrated a multiplex luciferase assay multiplexing that monitor at least six pathways, while still using the same standard reagents (Sarrion-Perdigones et al., 2019). We employed six luciferases to monitor the activity of five experimental signaling pathways and one control pathway. Moreover, we assembled the multiplex reporter using a flexible synthetic assembly cloning pipeline that can be applied to any set of reporter genes. This approach (which we refer to as solotransfection) steps away from traditional cotransfection methods typically used for luciferase or any other reporter assays, in which we can ensure stoichiometric ratios of each transcriptional unit in each transfected cell, and results in lower experimental variation. In a previous paper, we further demonstrated, using a variety of lung and breast cancer cell lines, that luciferase multiplexing can be used to assay the effects of siRNA, ligands, and chemical compound treatments on a targeted pathway and on four other cellular pathways at the same time. These data demonstrated that multiplex luciferase assays have broad application potential to monitor multiple pathways simultaneously (Figure 13B).
Critical Parameters
It is essential to re-evaluate the transmission coefficients before performing experimental assays since changes in instrumentation, pH, temperature, or other variables can slightly affect the luciferase emission spectra and therefore the transmission coefficient values that are critical to calculate pathway signaling values from measured values (Feeney et al., 2016). The calibration of the assay is critical for the success of the multiplex luciferase protocol, especially if it is the first time the experiment is done using a new plate reader equipped with luminometer capabilities
The dynamic range of the assay spans from 106 to 104 relative luciferase units per second (RLU/s) for the D-Luciferin luciferases and from 107 to 105 RLU/s for the coelenterazine luciferases. Values higher or lower than the dynamic ranges will result in incorrect results.
Troubleshooting
Low miniprep yield of the multiplex luciferase reporter vector
To improve the bacterial lysis and higher DNA yield of significant vectors, double the amounts of:
Buffers P1, P2 and N3 (500 μL, 500 μL and 700 μL) if you use the QIAprep kit
Resuspension, Lysis, and Precipitation Buffers (500 μL each) if you use the ChargeSwitch®-Pro Kit.
Centrifuge for 10 minutes at maximum speed to pellet the debris as usual, and then transfer 800 μL of the supernatant to the column by pipetting. Centrifuge for 30 seconds, discard the flow-through and repeat the steps, applying the rest of the supernatant.
Luciferase values are higher than the dynamic range of the assay
Some cell lines (e.g., HEK293T/17) are easily transfected and express the luciferases very efficiently, so the readout may be higher than the dynamic range of the assay, 106 to 104 RLU/s for the D-Luciferin luciferases and from 107 to 105 RLU/s for the coelenterazine luciferases. We always recommend measuring one of the six biological replicates per experimental condition, to make sure that the values are within the ideal dynamic range. For values that are higher, dilute the rest of the lysates with 1X PLB 1:10, and measure again.
Luciferase values are lower than the dynamic range of the assay
Some cell lines (e.g., MCF7, ZR-75–1, MDA-MB-157) are poorly transfected and their lysates have little luciferase activity, so the readout may not go above the lower threshold of the dynamic range of the assay. To solve this problem, double the amount of cells and seed them in a 48 well plate and adjust the volumes of transfection reagents proportionally: twice the amounts of all reagents were used per sample to prepare the DNA-plasmid complexes. Perform lysis with 60 μL of 1XPLB. Alternatively, different transfection procedures need to be explored to obtain higher transfection efficiencies.
Statistical analysis
Data were analyzed using the provided Excel, followed by Prism 7 software (GraphPad) for statistical analysis and graphing. We analyzed our data by t-test using the Holm-Sidak method with alpha = 0.05 (*P <0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, n.s. is non-significant),
Understanding results
The purification of the vectors from the glycerol stocks should be straightforward as illustrated in Figure 3. Cell work, transfection and luciferase readings should not present any problems unless hard-to-transfect cell lines are chosen for the assay. High-enough efficiency transfection should result in luciferase emission readings that are statistically significant while determining the different transmission coefficients as illustrated in Figures 8 and 9. Those transmission coefficients will then be used to calculate pathway-associated luciferase contributions from the measured emission values through spectral unmixing using the simultaneous equations as illustrated in Figures 8 and 10. Adding known target pathway activators and inhibitors should result in expected increased or decreased pathway signaling activity, respectively, to which the other pathways can be directly compared, as illustrated in Figures 11 and 12. When novel observations are made during multiplex luciferase assaying, they need to be independently confirmed using the same assay and further validated through alternative experimental assays.
Time Considerations
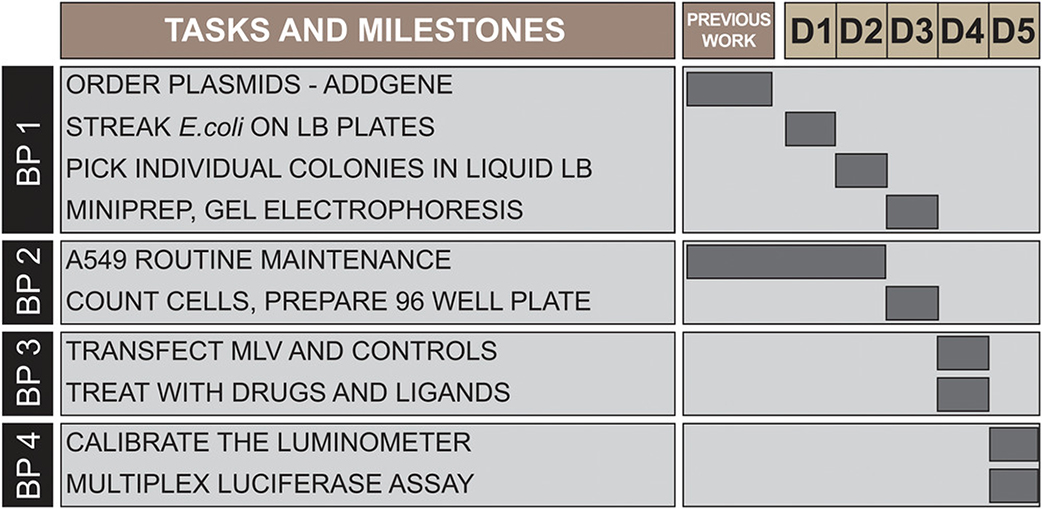
A549 cell maintenance will routinely start two weeks before the luciferase assay, to ensure that the cells are healthy. Once cells are ready, the complete procedure can be performed in a working week (see Figure 14). On day 1, streak E. coli on plates. On day 2, pick individual colonies into liquid LB medium. On day 3, isolate plasmids, cut with diagnostic restriction enzymes, and validate by gel electrophoresis. Also, count and plate the A549 cells as described here. On day 4, transfect the multiplex luciferase reporter vector and the control luciferase plasmids into the cells, and 3 hours later, treat the transfected cells with drugs and ligands. On day 5, wash and lyse the cells, and calibrate the luminometer with the constitutive CMV promoter control. Finally, perform the multiplex luciferase assay, and analyze the data.
Figure 14. Time considerations for the protocol.
Gantt chart illustrating tasks and milestones for the different protocols, as well as working days across two calendar weeks (D1 to D5).
Supplementary Material
Acknowledgments
This work was supported by start-up funds kindly provided by Baylor College of Medicine (D.W.Y. and K.J.T.V), the Albert and Margaret Alkek Foundation (K.J.T.V), the McNair Medical Institute at The Robert and Janice McNair Foundation (K.J.T.V), as well as a March of Dimes Foundation grant #1-FY14-315 (K.J.T.V), the Foundation For Angelman Syndrome Therapeutics grant FT2016-002 (K.J.T.V), the Cancer Prevention and Research Institute of Texas grants R1313 (K.J.T.V) and R1314 (D.W.Y.), and the National Institutes of Health grants 1R21GM110190 (K.J.T.V), 1R21OD022981 (K.J.T.V), R01GM109938 (K.J.T.V). The Dan L. Duncan Comprehensive Cancer Center is supported by Cancer Center Support Grant P30 CA125123 (National Institutes of Health and National Cancer Institute). Simple tandem repeat DNA fingerprinting of the A549 cells was performed by the MD Anderson Characterized Cell Line Core Facility, supported by Cancer Center Support Grant P30 CA016672 (National Institutes of Health and National Cancer Institute). Plasmids ID 118062, 118063, 118064, 118065, 118066, 118067, and 118069 are available through Addgene (https://www.addgene.org/).
This work is in memory of Alejandro Sarrion-Perdigones, a terrific scientist, colleague, mentor, and friend.
Footnotes
Conflicts of interest
The authors declare no conflicts of interests
Literature Cited
- Adams ST, and Miller SC (2014). Beyond D-luciferin: expanding the scope of bioluminescence imaging in vivo. Curr Opin Chem Biol 21, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg EL (2017). Phenotypic chemical biology for predicting safety and efficacy. Drug Discov Today Technol 23, 53–60. [DOI] [PubMed] [Google Scholar]
- Branchini BR, Southworth TL, Fontaine DM, Kohrt D, Florentine CM, and Grossel MJ (2018). A Firefly Luciferase Dual Color Bioluminescence Reporter Assay Using Two Substrates To Simultaneously Monitor Two Gene Expression Events. Sci Rep 8, 5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney KA, Putker M, Brancaccio M, and O’Neill JS (2016). In-depth Characterization of Firefly Luciferase as a Reporter of Circadian Gene Expression in Mammalian Cells. J. Biol. Rhythms 31, 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey CM, Spiller E, Lindsay D, Chiang C-T, Choi NC, Agus DB, Mallick P, Foo J, and Mumenthaler SM (2016). A high-content image-based method for quantitatively studying context-dependent cell population dynamics. Sci Rep 6, 29752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerets HHJ, Dhalluin S, and Atienzar FA (2011). Multiplexing cell viability assays. Methods Mol. Biol. 740, 91–101. [DOI] [PubMed] [Google Scholar]
- Gustafsdottir SM, Ljosa V, Sokolnicki KL, Anthony Wilson J, Walpita D, Kemp MM, Petri Seiler K, Carrel HA, Golub TR, Schreiber SL, et al. (2013). Multiplex cytological profiling assay to measure diverse cellular states. PLoS ONE 8, e80999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, Loh KM, Carter AC, Di Giorgio FP, Koszka K, et al. (2009). A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell 5, 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskova ZM, Tsarkova AS, and Yampolsky IV (2016). 1001 lights: luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chem. Soc. Rev 45, 6048–6077. [DOI] [PubMed] [Google Scholar]
- Korn K, and Krausz E (2007). Cell-based high-content screening of small-molecule libraries. Curr Opin Chem Biol 11, 503–510. [DOI] [PubMed] [Google Scholar]
- Künkele A, De Preter K, Heukamp L, Thor T, Pajtler KW, Hartmann W, Mittelbronn M, Grotzer MA, Deubzer HE, Speleman F, et al. (2012). Pharmacological activation of the p53 pathway by nutlin-3 exerts anti-tumoral effects in medulloblastomas. Neuro-Oncology 14, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini E, Cevenini L, Mezzanotte L, Ablamsky D, Southworth T, Branchini B, and Roda A (2008). Spectral-Resolved Gene Technology for Multiplexed Bioluminescence and High-Content Screening. Analytical Chemistry 80, 260–267. [DOI] [PubMed] [Google Scholar]
- Nakatsu T, Ichiyama S, Hiratake J, Saldanha A, Kobashi N, Sakata K, and Kato H (2006). Structural basis for the spectral difference in luciferase bioluminescence. Nature 440, 372–376. [DOI] [PubMed] [Google Scholar]
- Ohmiya Y (2015). Simultaneous multicolor luciferase reporter assays for monitoring of multiple genes expressions. Combinatorial Chemistry & High Throughput Screening 18, 937–945. [DOI] [PubMed] [Google Scholar]
- Phelan K, and May KM (2017). Mammalian Cell Tissue Culture Techniques. Curr Protoc Mol Biol 117, A.3F.1–A.3F.23. [DOI] [PubMed] [Google Scholar]
- Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, and Gerritsen ME (1997). Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272, 21096–21103. [DOI] [PubMed] [Google Scholar]
- Sarrion-Perdigones A, Falconi EE, Zandalinas SI, Juarez P, Fernandez-del-Carmen A, Granell A, and Orzaez D (2011). GoldenBraid: an iterative cloning system for standardized assembly of reusable genetic modules. PLoS.ONE. 6, e21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrion-Perdigones A, Vazquez-Vilar M, Palaci J, Castelijns B, Forment J, Ziarsolo P, Blanca J, Granell A, and Orzaez D (2013). GoldenBraid 2.0: a comprehensive DNA assembly framework for plant synthetic biology. Plant Physiol 162, 1618–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrion-Perdigones A, Chang L, Gonzalez Y, Gallego-Flores T, Young DW, and Venken KJT (2019). Examining multiple cellular pathways at once using multiplex hextuple luciferase assaying. Nat Commun 10, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagat T, Gaguio A, and Kopish K (2007). Normalizing genetic reporter assays: approaches and considerations for increasing consistency and statistical significance. Cell Notes 9–12. [Google Scholar]
- Sherf BA, Navarro SL, Hannah RR, and Wood KV (1996). Dual-LuciferaseTM Reporter Assay: An Advanced Co-Reporter Technology Integrating Firefly and Renilla Luciferase Assays. Promega Notes 57, 2–9. [Google Scholar]
- Taylor DL, and Giuliano KA (2005). Multiplexed high content screening assays create a systems cell biology approach to drug discovery. Drug Discov. Today Suppl, 13–18. [PubMed] [Google Scholar]
- Vazquez-Vilar M, Gandía M, García-Carpintero V, Marqués E, Sarrion-Perdigones A, Yenush L, Polaina J, Manzanares P, Marcos JF, & Orzaez D (2020). Multigene engineering by goldenbraid cloning: from plants to filamentous fungi and beyond. Current Protocols in Molecular Biology, 130, e116. doi: 10.1002/cpmb.116 [DOI] [PubMed] [Google Scholar]
- Voytas D (2001). Agarose gel electrophoresis. Curr Protoc Mol Biol Chapter 2, Unit2.5A. [DOI] [PubMed] [Google Scholar]
- Westwick JK, and Lamerdin JE (2011). Improving drug discovery with contextual assays and cellular systems analysis. Methods Mol. Biol 756, 61–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.