Abstract
INTRODUCTION:
Fecal immunochemical testing (FIT) positivity is determined by a threshold decided by individual screening programs. Data are limited on correlation between FIT levels and pathology identified at colonoscopy. Our aim was to examine the correlation between FIT levels and pathology identified in a national colorectal cancer screening program.
METHODS:
FIT levels (n = 9,271) were analyzed and correlated with patient demographics and pathology identified, including adenomas, sessile serrated lesions, number/size of adenomas, and presence of dysplasia. Levels were divided into 2 categories: FIT levels were defined as “high” or “low” based on whether they were above or below the median (479 ngHb/mL). Multivariate analysis was performed.
RESULTS:
A total of 8,084 patients (87%) underwent colonoscopy. Those younger than 65 years (odds ratio [OR] 1.267, 95% confidence interval [CI] 1.107–1.45, P = 0.001), those with an adenoma >10 mm (OR 1.736, 95% CI 01.512–1.991, P < 0.001), and those with left-sided adenomas (OR 1.484, 95% CI 1.266–1.74, P < 0.001) had higher FIT levels. Cancers (OR 2.8, 95% CI 2.09–3.75, P < 0.001) and high-grade dysplasia (OR 1.356, 95% CI 1.08–1.7, P = 0.008) had higher FIT levels, but varied greatly. The number of adenomas was not significant.
DISCUSSION:
In this study, FIT levels were high for left-sided and large adenomas, suggesting that FIT has poor sensitivity for detection of diminutive and right-sided neoplasia. FIT levels had no association with gender and declined with age. Adenoma burden did not correlate with FIT levels; this is a novel finding. FIT levels vary greatly even in those with advanced neoplasia; therefore, FIT is unlikely to be useful as a risk stratification tool.
INTRODUCTION
Fecal immunochemical testing (FIT) (1) has been well established as a first-line screening tool for colorectal cancer (CRC) in at-risk patients. Higher FIT thresholds within screening programs show increased sensitivity and a decreased rate of normal colonoscopies/false positives.
As well as being sensitive for advanced pathology, FIT has been shown to increase participation in colon cancer screening by almost 10% compared with guaiac-based fecal occult blood test (2), due to only 1 sample being required and the lack of dietary restrictions in its use. A 2016 Scottish study (3) of patients' attitudes demonstrated that FIT was a more acceptable test to the patient than guaiac-based fecal occult blood test because it was easier to complete and “less disgusting.”
A prospective study by Chang et al. (4) analyzed results of more than 6,000 colonoscopies correlating with FIT between 2010 and 2014. Sensitivity for detection of sessile serrated polyps (SSPs) was 12.3%, 6.2%, and 6.2%, respectively at cutoff values of 10, 15, and 20 μg of Hb/g stool. Another study by the same group (4) showed that FIT levels in patients with a normal colonoscopy or small adenoma did not differ significantly from those with an SSP. Those with advanced/larger SSPs were less likely to have a positive FIT (odds ratio [OR] 0.44, 95% confidence interval [CI] 0.18–1.05), even if they had a concurrent conventional adenoma. There have been no subsequent publications to either validate or repudiate this finding. Most studies that have set out to establish the best threshold for FIT positivity have examined levels between 25 and 180 ngHb/mL (1,5,6). A 2014 post hoc analysis (7) of 2 blinded, multicenter prospective cross-sectional studies identified characteristics associated with a positive FIT. At a 100-ngHb/mL threshold, advanced neoplasia, pedunculated morphology, distal adenomas with high-grade dysplasia, and villous histology were all associated with a positive result.
In early 2014, the Irish BowelScreen program changed its FIT threshold from 100 to 225 ngHb/mL because there were a significant number of false positives seen with the lower threshold and the lower FIT generated a requirement for more colonoscopies than the national service could provide in a timely manner. The overall FIT positive rate for the first round was 5%. Although a lower threshold is ideal, health service costs and the burden on facilities and services are important for a screening program to be successful. A 2011 study showed that switching the FIT cutoff from >50 to >200 ngHb/mL decreased test positivity significantly (84%–37%), but did not have a significant effect on the rate of detection of CRC (8). The same study also found that the positive predictive value of FIT increases with age by over 20% (9).
The identification and removal of adenomatous polyps is an important aim of the BowelScreen program. The correlation between FIT level and adenoma location, number, size, and morphology is of interest. There are data to suggest that pedunculated lesions are more likely to be identified by FIT than flat lesions. This is understandable because these adenomas are more likely to have surface irritation or ulceration because of their morphology (10). FIT seems to be less sensitive for right-sided pathology than left (33% vs 20%) (10–12). We know from recent studies that sessile serrated adenomas are more common than previously thought, accounting for 10%–15% of adenomas identified, and that they are associated with synchronous advanced neoplasia (13). In a 2014 New England Journal of Medicine study that compared FIT with multitarget stool DNA testing, FIT had a poor sensitivity for SSPs—only 5% vs 42% (14). A study published in Gut last year analyzed more than 70,000 colonoscopies and found that there was a poor association between FIT level and SSPs (15), leading to the hypothesis that FIT may not be useful in identifying those patients who have small, flat, right-sided cancers. The aims of the study were to examine the correlation between FIT levels and pathology identified in a national screening program; to assess whether FIT may be useful as a tool to stratify risk and thereby facilitate planning and prioritising of patients, both within and outside a screening context; and to examine FIT levels specifically in relation to SSPs and to compare with normal colonoscopies and conventional adenomas.
METHODS
FIT levels from BowelScreen for 9,271 patients screened between November 6, 2012, and November 21, 2016, were available to us for analysis. FIT positivity threshold in the BowelScreen Program was increased from 100 to 225 ngHb/mL after 14 months. Ethical approval was granted by the Royal College of Physicians Ireland Ethics Committee. FIT levels, along with basic demographic data, were recorded for each patient and collated in a database with their endoscopic records, containing polyp endoscopic size, location, and whether or not it was resected and retrieved.
This was then correlated with the BowelScreen histology database. Data were recorded per patient and transformed into a single database. FIT levels were initially analyzed using continuous data; however, to infer statistical or clinical significance from the results, it was felt that a multivariate analysis through binary logistic regression would be the most useful statistical analysis. Subsequently, FIT was divided into 2 categories to do so, above the median of 479 ngHb/mL (high) and below the median (low). All patients included in the study had a positive FIT as defined by the screening program. To define a high or low FIT, we chose the median FIT level as we felt this had clinical relevance. The number of polyps was also divided into 4 categories: 0, 1, 2 or 3, and 4 or more. Polyp size was divided into 5 categories: <5, 5–9, 10–20, 20–30, and >30 mm. The histological sizing, where available, was used for the purposes of analysis. Dysplasia was classified as low or high grade, and neoplasias were categorized separately. SSPs in this study are defined by histological criteria—architectural disturbance of crypt bases/“boot-shape” crypts; at least 3 abnormal crypts; serrations and mature mucinous cells at the crypt bases; lacking the complexity of tubular adenomas, with our without evidence of dysplasia. It is worth noting that there are several different diagnostic criteria available, and a degree of interobserver variability is acknowledged (16–18).
SPSS v24 was used for all statistical analysis. Data were analyzed for descriptive characteristics. As the data were not normally distributed, median and interquartile ranges were used. Multivariate analysis was initially performed using linear regression. FIT scores were logged to do this. Sensitivity and specificity of FIT were confirmed with an ROC analysis.
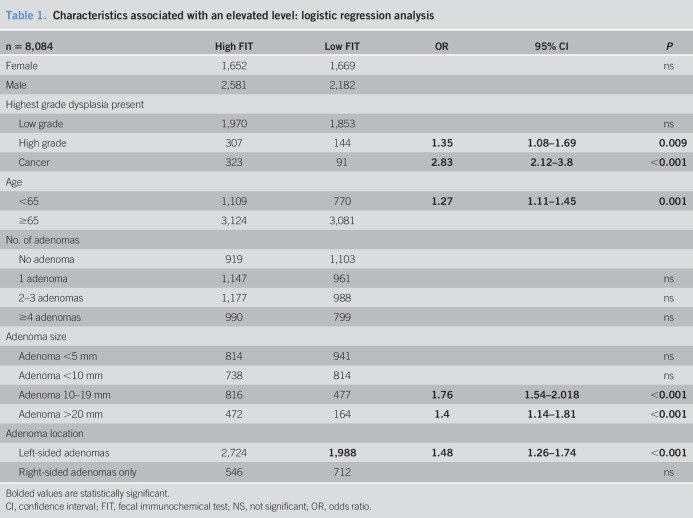
Initial analysis of these continuous data (Figure 1) demonstrated a large variance with a significant number of outliers. Although the results showed statistical significance, we chose to divide the FIT levels into “high” (>479 ngHb/mL) and “low” (<479 ngHb/mL), to perform a binary logistic regression for a multivariate analysis. This was to ensure that there was clinical relevance to our reported results.
Figure 1.
Characteristics associated with an elevated FIT level, using continuous data. FIT, fecal immunochemical test; SSL, sessile serrated lesion; TSA, traditional serrated adenoma.
RESULTS
A total of 196,440 patients returned a FIT test to BowelScreen between 2012 and 2016; 9,785 (5%) had a positive FIT, and 897 (9.2%) of these had a value of between 100 and 224 ngHb/mL. The remainder were >225 ngHb/mL. Median FIT level was 479 ngHb/mL. A total of 8084 attended for colonoscopy. Adenoma detection rate (inclusive of SSPs) was 54%. The total number of adenomas identified was 13,785, and the total number of cancers was 414 (5.1%). The mean number of adenomas identified per client was 2.3 (Table 1).
Table 1.
Characteristics associated with an elevated level: logistic regression analysis
| n = 8,084 | High FIT | Low FIT | OR | 95% CI | P |
| Female | 1,652 | 1,669 | ns | ||
| Male | 2,581 | 2,182 | |||
| Highest grade dysplasia present | |||||
| Low grade | 1,970 | 1,853 | ns | ||
| High grade | 307 | 144 | 1.35 | 1.08–1.69 | 0.009 |
| Cancer | 323 | 91 | 2.83 | 2.12–3.8 | <0.001 |
| Age | |||||
| <65 | 1,109 | 770 | 1.27 | 1.11–1.45 | 0.001 |
| ≥65 | 3,124 | 3,081 | |||
| No. of adenomas | |||||
| No adenoma | 919 | 1,103 | |||
| 1 adenoma | 1,147 | 961 | ns | ||
| 2–3 adenomas | 1,177 | 988 | ns | ||
| ≥4 adenomas | 990 | 799 | ns | ||
| Adenoma size | |||||
| Adenoma <5 mm | 814 | 941 | ns | ||
| Adenoma <10 mm | 738 | 814 | ns | ||
| Adenoma 10–19 mm | 816 | 477 | 1.76 | 1.54–2.018 | <0.001 |
| Adenoma >20 mm | 472 | 164 | 1.4 | 1.14–1.81 | <0.001 |
| Adenoma location | |||||
| Left-sided adenomas | 2,724 | 1,988 | 1.48 | 1.26–1.74 | <0.001 |
| Right-sided adenomas only | 546 | 712 | ns |
Bolded values are statistically significant.
CI, confidence interval; FIT, fecal immunochemical test; NS, not significant; OR, odds ratio.
FIT correlated with pathology detected: Cancers and high-grade dysplastic polyps were more likely to have higher FIT levels than low-grade dysplastic or hyperplastic polyps. FIT levels were more likely to be high if there was a polyp present than if the colonoscopy was normal, but the FIT level did not increase with increasing number of polyps detected.
On multivariate analysis, those younger than 65 years (OR 1.267, 95% CI 1.107–1.45, P = 0.001), those with a polyp over 10 mm (OR 1.736, 95% CI 01.512–1.991, P < 0.001), and those with left-sided polyps (OR 1.484, 95% CI 1.266–1.74, P < 0.001)) had higher FIT levels. Those with right-sided polyps had lower FIT levels, regardless of the presence of left-sided polyps (OR 0.867, 95% CI 0.75–0.99, P = 0.048.
Those with cancer (OR 2.71, 95% CI 2.1–3.5, P < 0.001) were significantly more likely to have a high FIT level; however, FIT scores in cancers showed a high degree of variation with one having a FIT level of 107 ngHb/mL, which is barely above the original FIT positivity threshold set at the start of the first round. High FITs were also seen in high-grade dysplasia (OR 1.44, 95% CI 1.16–1.78, P < 0.001) (Table 2).
Table 2.
FIT levels and pathology
| Pathology/variable | Median FIT level | IQR |
| Normal colonoscopy/no adenoma | 408 | 263–856 |
| Male | 496 | 291–1,299 |
| Female | 449 | 276–999 |
| <65 yr of age | 547 | 334–1,308 |
| ≥65 yr of age | 455 | 273–1,144 |
| Most advanced histology present | ||
| Hyperplastic | 439 | 274–971 |
| SSL | 411 | 268–846 |
| TSA | 518 | 294–1,601 |
| Adenoma | 489 | 291–1,175 |
| Cancer | 1,387 | 478–4,494 |
| Adenoma location | ||
| Left colon only | 546 | 312–1,450 |
| Right colon only | 390 | 263–926 |
| Both | 520 | 311–1,372 |
| Largest adenoma size | ||
| <5 mm | 421 | 265–953 |
| 5–9 mm | 429 | 276–898 |
| 10–19 mm | 620 | 339–1,544 |
| >20 mm | 1,157 | 442–3,374 |
| No. of adenomas | ||
| 1 adenoma | 505 | 286–1,266 |
| 2–3 adenomas | 497 | 297–1,258 |
| >4 adenomas | 513 | 302–1,274 |
| Most advanced dysplasia | ||
| Low | 468 | 286–1,102 |
| High | 815 | 385–1,909 |
| Cancer | 1,387 | 478–4,494 |
FIT, fecal immunochemical test; IQR, interquartile range; SSL, sessile serrated lesion; TSA, traditional serrated adenoma.
DISCUSSION
FIT-based CRC screening programs set a test positivity threshold, and patients are defined as either FIT positive or negative without consideration of individual FIT levels. Previous studies have demonstrated that FIT is more likely to be positive in those with left-sided and larger, pedunculated polyps as well as advanced polyps or cancers. It is not known whether FIT levels can be used to stratify the risk of advanced pathology among those with a positive test. Previous studies have shown that FIT levels correlate with the stage of dysplasia/neoplasia identified at colonoscopy (19). It is worth noting that although FIT is currently primarily a screening test for CRC, we are exploring its relationship—or lack thereof—to adenomas.
Although our study confirmed the previously reported correlation between advanced pathology and higher FIT levels, there were also advanced adenomas and cancers which had FIT levels just above the screening threshold. We identified a wide variation in FIT scores (107–129,532 ngHb/mL) in high-grade dysplasia and cancers. All the patients in this study were FIT positive, but the change in FIT threshold after approximately 1,900 positive results allows us to demonstrate that 10 cancers detected at the 100 threshold would have been missed by the new 225 threshold. This highlights the fact that some cancers (likely small, nonbleeding tumors) may cause a relatively low FIT elevation, a point that can often be difficult to explain when describing the aims of a screening program to the general public. The increase in the FIT positivity threshold after 2 years of the program meant there was a reduction in false-positive results, but this is likely balanced by a number of false negatives. It is important that patients understand that a negative FIT does not rule out pathology.
We know that SSPs are responsible for up to 30% of cancers in the colon, but remain a poorly understood entity from a molecular point of view. In general, detection levels of SSPs are relatively low (4% in this study), and so, large numbers are needed to adequately analyze factors associated with their detection. Those with right-sided polyps or SSPs alone were less likely to record a high FIT score, which demonstrates that pathology, and right-sided pathology in particular, may be missed by screening programs using FIT and/or sigmoidoscopy. FIT levels did not correlate with polyp burden, highlighting 1 large or advanced polyp, rather than multiple small polyps are more likely to be detected using FIT as a screening tool.
There are limitations to this study. It is retrospective in its design and susceptible to the inherent flaws of retrospective, observational studies. This study was limited to patients with a positive FIT, and as patients with a negative FIT did not undergo colonoscopy, we do not have information on pathology in those with a FIT level below the predetermined threshold. This means that absolute conclusions about all FIT levels and their correlation with pathology cannot be made. Our findings relating to FIT differences between age groups and genders are only indicative of FIT levels within a positive population. We had aimed to assess FIT's usefulness as a tool to stratify risk among those with a positive result, but given the lower sensitivity seen with advancing age, and lack of gender difference, we cannot draw the conclusion that it is a useful tool in this context. We also limited our scope to index FITs and colonoscopies for the purposes of this study. Although our data support previous studies showing FIT is not particularly sensitive for SSPs, it is difficult to draw any absolute conclusions relating to FIT and SSPs because of the small number of SSPs identified (14).
The change in FIT threshold in early 2014 was a factor beyond our control, as this was a retrospective study; however, we do not think it has affected the results of our study, which analyzes the relationship between FIT, patient factors, and the type and size of pathology identified. FIT positivity started at 8.6%, but fell to 4.1% after 2 years of the higher threshold, giving an overall positivity of 5%. The positive predictive value for detecting CRC for a positive FIT in the first 14 months (lower FIT threshold) was 4.75% (CI 3.91–5.75 as against 5.01% (CI 4.34–5.78) after adjustment (higher threshold) (20–22).This is also in keeping with findings from previous studies of FIT positivity in the general population of this age group (23) and resulted in a drop of false positives.
For the majority of our analysis, we chose to divide FIT levels into “high”—higher than the median—and “low”—below the median. When using continuous data for analysis, as represented in Figure 1, all results were indicated to be statistically significant because of the large population in this study. However, the variance was huge from patient to patient, and we felt that not all the statistically significant results were reflective of a clinical significance.
Despite these limitations, this study provides quantitative data on almost 10,000 positive FIT tests and 8,000 subsequent colonoscopies yielding more than 100,000 polyps. Data regarding endoscopic and pathological findings were prospectively entered by screening centers into a national database, and data were complete for all patients. Although it is a retrospective study, these numbers strengthen the data and are reflective of real-life clinical outcomes from a national screening program. As the population involved in CRC screening and follow-up surveillance continues to grow, it is important that screening programs continually assess FIT as a screening tool. This study provides us with new information about the quantitative value of FIT in a national screening population. Comparison of FIT levels in each patient from round to round of screening may be a useful predictor of pathology, but needs study. Those with SSPs or hyperplastic polyps alone had similar FIT levels to those with no polyps at all. This combined with the lower FIT levels associated with right-sided pathology is in keeping with previous studies suggesting FIT's poor sensitivity for SSPs (15). FIT levels overall were lower for older patients, suggesting that it may not be as sensitive a tool in the older cohort of screening patients. Although there is generally a significant rise in FIT with high-grade dysplasia, cancer, and large polyps, there was a high degree of variability in its levels. FIT was also not predictive of polyp burden, which is a novel finding. These findings suggest it cannot be used as a risk stratification tool for colorectal pathology in screening programs.
CONFLICTS OF INTEREST
Guarantor of the article: Susanne M. O'Reilly, MBBCh, BAO, MSc, MRCPI.
Specific author contributions: S.M.O.: study concept and design, acquisition, analysis and interpretation of data, and drafting of and critical revision of manuscript. S.M.: acquisition of data; material support. D.O.: study concept and design, supervision. T.M.: acquisition of data, material support, and study supervision. P.F.: study supervision. H.E.M.: analysis and interpretation of data, statistical analysis. G.C.: study concept and design, critical revision of manuscript for important intellectual content; obtained funding; study supervision.
Financial support: The first author completed this research during her MD, which was funded by Boston Scientific through a Newman Fellowship (University College Dublin).
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ FIT has largely replacedfecaloccult blood testing, because of its higher specificity. FIT positivity is determined by a threshold decided by individual screening programmed in each country. There are limited data on FIT values beyond the chosen cut-off for a “positive” result.
WHAT IS NEW HERE
✓ In this study of over 8,000 colorectal cancer screening patients, those older than 65 years of age, those with advanced adenomas (> 10 mm), and those with left-sided pathology had higher values.
✓ There was a wide variation in FIT values, even among those with high grade dysplasia and cancers.
✓ Adenoma burden does not affect the FIT value.
✓ Those with right-sided pathology or sessile serrated polyps alone had lower FIT values.
TRANSLATIONAL IMPACT
✓ FIT as a screening tool is not as sensitive for right-sided pathology or sessile serrated lesions so may miss these.
✓ Our findings overall suggest that FIT values cannot be used to stratify risk within a screening program.
Contributor Information
Sara MacNally, Email: s.mcnally@screeningservice.ie.
Diarmuid O'Donoghue, Email: dodonoghue0304@gmail.com.
Therese Mooney, Email: t.mooney@screeningservice.ie.
Patricia Fitzpatrick, Email: p.fitzpatrick@screeningservice.ie.
Hugh E. Mulcahy, Email: hemulc@hotmail.com.
Garret Cullen, Email: garretcullen@svhg.ie.
REFERENCES
- 1.Guittet L, Bouvier V, Mariotte N, et al. Comparison of a guaiac based and an immunochemical faecal occult blood test in screening for colorectal cancer in a general average risk population. Gut 2007;56(2):210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liles EG, Perrin N, Rosales AG, et al. Change to FIT increased CRC screening rates: Evaluation of a US screening outreach program. Am J Manag Care 2012;18(10):588–95. [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers JA, Callander AS, Grangeret R, et al. Attitudes towards the faecal occult blood test (FOBT) versus the faecal immunochemical test (FIT) for colorectal cancer screening: Perceived ease of completion and disgust. BMC Cancer 2016;16:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang LC, Shun CT, Hsu WF, et al. Fecal immunochemical test detects sessile serrated adenomas and polyps with a low level of sensitivity. Clin Gastroenterol Hepatol 2017;15(6):872–9.e1. [DOI] [PubMed] [Google Scholar]
- 5.van Rossum LG, van Rijn AF, Laheij RJ, et al. Cutoff value determines the performance of a semi-quantitative immunochemical faecal occult blood test in a colorectal cancer screening programme. Br J Cancer 2009;101(8):1274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raginel T, Puvinel J, Ferrand O, et al. A population-based comparison of immunochemical fecal occult blood tests for colorectal cancer screening. Gastroenterology 2013;144(5):918–25. [DOI] [PubMed] [Google Scholar]
- 7.Cubiella J, Castro I, Hernandez V, et al. Characteristics of adenomas detected by fecal immunochemical test in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev 2014;23(9):1884–92. [DOI] [PubMed] [Google Scholar]
- 8.Terhaar sive Droste JS, Oort FA, van der Hulst RW, et al. Higher fecal immunochemical test cutoff levels: Lower positivity rates but still acceptable detection rates for early-stage colorectal cancers. Cancer Epidemiol Biomarkers Prev 2011;20(2):272–80. [DOI] [PubMed] [Google Scholar]
- 9.Wieten E, Schreuders EH, Nieuwenburg SA, et al. Effects of increasing screening age and fecal hemoglobin cutoff concentrations in a colorectal cancer screening program. Clin Gastroenterol Hepatol 2016;14(12):1771–7. [DOI] [PubMed] [Google Scholar]
- 10.Haug U, Kuntz KM, Knudsen AB, et al. Sensitivity of immunochemical faecal occult blood testing for detecting left- vs right-sided colorectal neoplasia. Br J Cancer 2011;104(11):1779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner H, Niedermaier T, Chen H. Strong subsite-specific variation in detecting advanced adenomas by fecal immunochemical testing for hemoglobin. Int J Cancer 2017;140(9):2015–22. [DOI] [PubMed] [Google Scholar]
- 12.Widlak MM, Thomas CL, Thomas MG, et al. Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients. Aliment Pharmacol Ther 2017;45(2):354–63. [DOI] [PubMed] [Google Scholar]
- 13.IJspeert JE, de Wit K, van der Vlugt M, et al. Prevalence, distribution and risk of sessile serrated adenomas/polyps at a center with a high adenoma detection rate and experienced pathologists. Endoscopy 2016;48(8):740–6. [DOI] [PubMed] [Google Scholar]
- 14.Imperiale TF, Ransohoff DF, Itzkowitz SH. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014;371(2):187–8. [DOI] [PubMed] [Google Scholar]
- 15.Zorzi M, Senore C, Da Re F, et al. Detection rate and predictive factors of sessile serrated polyps in an organised colorectal cancer screening programme with immunochemical faecal occult blood test: The EQuIPE study (Evaluating Quality Indicators of the Performance of Endoscopy). Gut 2017;66(7):1233–40. [DOI] [PubMed] [Google Scholar]
- 16.Bateman AC, Shepherd NA. UK guidance for the pathological reporting of serrated lesions of the colorectum. J Clin Pathol 2015;68(8):585–91. [DOI] [PubMed] [Google Scholar]
- 17.Ensari A, Bilezikçi B, Carneiro F, et al. Serrated polyps of the colon: How reproducible is their classification? Virchows Arch 2012;461(5):495–504. [DOI] [PubMed] [Google Scholar]
- 18.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: Review and recommendations from an expert panel. Am J Gastroenterol 2012;107(9):1315–29; quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen LS, Yen AM, Chiu SY, et al. Baseline faecal occult blood concentration as a predictor of incident colorectal neoplasia: Longitudinal follow-up of a Taiwanese population-based colorectal cancer screening cohort. Lancet Oncol 2011;12(6):551–8. [DOI] [PubMed] [Google Scholar]
- 20.O'Donoghue D, Sheahan K, MacMathuna P, et al. A National Bowel Cancer Screening Programme using FIT: Achievements and Challenges. Cancer Prev Res (Phila) 2019;12(2):89–94. [DOI] [PubMed] [Google Scholar]
- 21.Ebell MH. FIT more acceptable with better detection rate than gFOBT for colorectal cancer screening. Am Fam Physician 2018;97(12):818. [PubMed] [Google Scholar]
- 22.Moss S, Mathews C, Day TJ, et al. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: Results from a pilot study within the national screening programme in England. Gut 2017;66(9):1631–44. [DOI] [PubMed] [Google Scholar]
- 23.Pellat A, Deyra J, Coriat R, et al. Results of the national organised colorectal cancer screening program with FIT in Paris. Sci Rep 2018;8(1):4162. [DOI] [PMC free article] [PubMed] [Google Scholar]