Abstract
This prospective study aimed to analyze determinants that can influence bone mineral density evolution in childhood acute leukemia survivors. Patients included were selected from the long-term follow-up LEA cohort and had dual energy radiograph absorptiometry scan between 10 and 18 years and after the age of 18. All scans were centrally reviewed. Bone mineral density was measured at the lumbar spine, femoral neck, total hip, and whole body, and expressed as z-score. Eighty-nine patients (female 39, lymphoblastic leukemia 68, relapse 25, hematopoietic stem cell transplantation 44, and mean age 15.4 and 20.1 years at the first and second scans, respectively) were studied. The first and second scan z-scores were significantly correlated (P < 10−3). Mean femoral neck and total hip z-scores improved significantly between the first and second scans, whereas no significant evolution occurred at the lumbar spine and whole-body level. On the second evaluation, 14.6% of patients had z-score <−2 at the lumbar spine and 4.3% at the femoral neck level. Gender, type of leukemia, transplantation, relapse, cumulative corticosteroid doses, or growth hormone deficiency did not have any significant impact on z-score variation. Younger age at diagnosis (≤8.5 years) proved an unfavorable risk factor for z-score evolution at the lumbar spine (P = 0.041); the trend did not reach statistical significance for metabolic syndrome (P = 0.054). At the femoral neck, both were associated with unfavorable z-score evolution (P = 0.003 and 0.025, respectively). Patients treated at a younger age and those with metabolic syndrome seem to be at higher risk of bone mineral density decline and should benefit from specific interventions.
Introduction
Survival rates after childhood acute leukemia (AL) have reached more than 85% of patients with acute lymphoblastic leukemia (ALL) and 60% of patients with acute myeloid leukemia (AML)1 in recent years. Hence, the long-term side effects of the disease and its treatment have become major concerns. Bone morbidity has been largely reported at diagnosis and during treatment of AL,2,3 but among the long-term sequelae, conflicting results have been reported regarding the prevalence of low bone mineral density (BMD).4–8 In a previous study conducted in adults participating in the French long-term follow-up LEA (French acronym for “leukemia in children and adolescents”) cohort of childhood leukemia survivors, we found that those treated with chemotherapy only had a slight reduction in their lumbar BMD, whereas transplanted patients with gonadal deficiency had a reduced femoral BMD, which might increase their fracture risk later in life.9 However, evolution of BMD from the first long-term evaluation to that in adulthood and potential correlations between baseline and adult dual-energy radiograph absorptiometry (DXA) BMD values remain poorly documented.
In the long-term follow-up LEA program, BMD measurements are recommended at baseline when children reach the age of 10 and then when reaching adulthood. The aim of this prospective multicenter study, using LEA cohort data, was to describe the evolution of BMD in patients who underwent at least 2 DXA measurements, the first scan during childhood after completion of leukemia treatment, and the second scan during adulthood, and to analyze the determinants that can influence BMD recovery or decline.
Materials and methods
The study cohort
As detailed in previous reports,10 LEA is a French multicenter long-term follow-up program involving all childhood AL survivors treated since 1980 in the participating pediatric cancer centers. Patients in the LEA program are summoned to the follow-up clinic at predefined dates, starting 1 year after hematopoietic stem cell transplantation (HSCT) or after completion of chemotherapy. These visits repeat every 2 years until the age of 20 and at least 10 years of continuous complete remission, and every 4 years thereafter. The LEA study was approved by the French National Program for Clinical Research and by the French National Cancer Institute.
Patients in the LEA cohort were eligible for the present study if they (1) were older than 18 years at the last LEA evaluation; (2) had 2 consecutive DXA scans, the first one between the age of 10 and 18 years (scan 1) and the second after the age of 18 (scan 2); and (3) had been authorized to participate by the parents or legal guardian at first evaluation and had provided their own written informed consent after the age of 18.
Bone density measurement
Bone mineral density (g/cm2) was measured using DXA. Devices of 2 different manufacturers were used in this study: Hologic (Hologic Inc, Bedford, Massachusetts) and GE-Lunar (Madison, Wisconsin). For each patient, the 2 consecutive DXA scans were always performed in the same center on the same machine. Quality control of each DXA device was performed by measuring a local phantom every day when a patient was scanned and at least 3 times per week. These quality control tests were controlled each month by an independent office.
Because devices of different manufacturers give different absolute BMD values (due to the use of different technologies), patients’ results were expressed as z-scores. Z-score is calculated according to the following formula: (BMD of the patient – BMD of normal persons of the same age)/standard deviation (SD) of the BMD of normal persons of the same age). It is matched for age, ethnicity, sex, as well as for the technology used. Low BMD was defined as a z-score below −2. BMD of the lumbar spine was measured in all patients. BMD of the upper extremity of the femur (femoral neck and total hip) and of whole body was also measured in a subset of patients.
All DXA scan images were centrally reviewed by a rheumatologist to verify the quality of the scan: optimal positioning of the patient, absence of artifacts, and analysis of the scans according to the manufacturer’s recommendations were the main points that were checked to decide inclusion or exclusion of the examination.
Data processing
Patients’ characteristics such as disease type, treatment modalities, and long-term side effects were extracted from the LEA database.10 Metabolic syndrome (MS) was defined according to the National Cholesterol Education Program—Adult Treatment Panel III (NCEP-ATPIII) revised in 2005.11 In the LEA cohort, MS criteria are analyzed for patients older than 18 years. Hypogonadism was defined as low estradiol level with high follicle stimulating hormone (FSH) and high luteinizing hormone (LH) in women, low testosterone, or high FSH in men and/or hormone substitution for both genders.
Statistical methods
Categorical variables were expressed in number and percent. Quantitative variables were expressed as mean ± SD or median and range. Associations between z-score in the first and second scans were expressed with correlation coefficient and limits of agreement were graphically represented with Bland–Altman plots. Means of the z-scores at the 2 measurement times were compared with the paired student t test. Differences of the variation of z-scores between the 2 times of measurement and potential factors associated were analyzed with the linear general model for repeated measures. Statistical analysis was performed using SPSS 20.0 software (SPSS Inc., Chicago, Illinois) and Intercooled Stata 9.0 (Stata Corp., College Station, Texas)
Results
Patients’ characteristics
Among 4123 patients included in the LEA cohort as of November 2016, 113 adults fulfilled all eligibility criteria (ie, were identified in the LEA database as having had a bone density scan before the age of 18 years and a follow-up scan after the age of 18). Central review excluded 21 patients for whom we only had T-scores (not z-scores) or for whom we had scan results without images, and we were unable to perform Quality Control. Three further patients were excluded because the interval between the 2 scans was less than a year. As shown in the flowchart, 89 patients were studied (Figure 1). All of them had had 2 lumbar scans, of whom 69 had in addition 2 total hip and/or femoral neck scans, and 43 patients had 2 whole-body scans.
Figure 1.
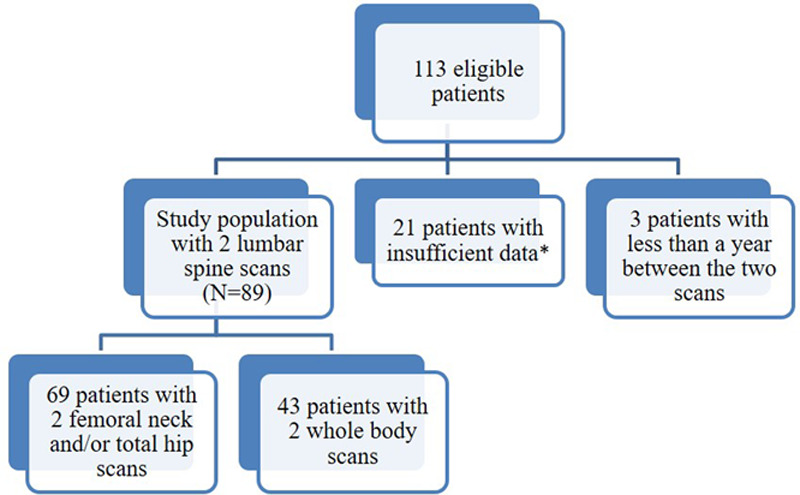
Flowchart. *Patients with insufficient data were those for whom we only had T-scores or scan results without images, and we were unable to perform quality control.
Comparing the study population of 89 patients with the group of 24 “eligible but not included” patients, we found no significant difference regarding the type of leukemia, the age at diagnosis, the follow-up duration from diagnosis to the last LEA evaluation, history of relapse, central nervous system radiation, or hematopoietic stem cell transplantation. Men were underrepresented in the “eligible but not included” group when compared to the study population (Table 1).
Table 1.
Comparison of Nonincluded and Included Patients.
| Eligible Nonincluded (N = 24)N (%) | Included (N = 89)N (%) | P | |
|---|---|---|---|
| Gender | |||
| Female | 17 (70.8) | 39 (43.8) | |
| Male | 7 (29.2) | 50 (56.2) | 0.019 |
| Type of leukemia | |||
| ALL | 19 (86.4) | 68 (76.4) | |
| AML | 3 (13.6) | 21 (23.6) | 0.31 |
| Age at diagnosis (Mean ± SD), y | 8.3 ± 4.7 | 8.5 ± 5.1 | 0.866 |
| CNS radiation | 6 (25) | 12 (13.6) | 0.179 |
| HSCT | 11 (45.8) | 44 (49.4) | 0.754 |
| History of relapse | 11 (45.8) | 25 (28.1) | 0.098 |
| Follow-up duration from diagnosis (Mean ± SD), y | 15.7 ± 6.3 | 13.8 ± 4.9 | 0.115 |
ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; CNS = central nervous system; HSCT = hematopoietic stem cell transplantation; SD = standard deviation.
Patients’ characteristics are presented in Table 2. Sixty-eight of them (76%) had ALL and 21 (24%) had AML. Mean age at the first and second scans was, respectively, 15.4 and 20.1 years old. Mean weight and height were, respectively, 50.9 kg/159.2 cm and 59.4 kg/165 cm.
Table 2.
Patients’ Characteristics (N = 89).
| N (%) | |
|---|---|
| Gender | |
| Female | 39 (43.8) |
| Male | 50 (56.2) |
| Type of leukemia | |
| ALL | 68 (76.4) |
| AML | 21 (23.6) |
| Age at diagnosis (Mean ± SD), y | 8.5 ± 5.1 |
| CNS radiation | 12 (13.5) |
| HSCT* | 44 (49.4) |
| History of relapse | 25 (28.1) |
| Follow-up duration from diagnosis (Mean ± SD), y | 13.8 ± 4.9 |
| Growth hormone deficiency | |
| No | 84 (94) |
| Yes without treatment | 0 (0) |
| Yes with treatment | 5 (6) |
| Hypogonadism | |
| No | 59 (66.3) |
| Yes without treatment | 4 (4.5) |
| Yes with treatment | 26 (29.2) |
| Metabolic syndrome (data for 77 patients) | 5 (6.5) |
| Corticosteroids | 66 (74) |
| Cumulative dose† (Mean ± SD) | 6118.2 ± 3197.9 |
| Interval from diagnosis to first scan (Mean ± SD), y | 7.0 ± 4.7 |
| Interval from diagnosis to second scan (Mean ± SD), y | 11.7 ± 5.2 |
| Interval between both scans | |
| Mean ± SD, y | 4.8 ± 2.6 |
| Median (minimum–maximum) | 3.8 (1.30–12.75) |
ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; CNS = central nervous system; HSCT = hematopoietic stem cell transplantation; SD = standard deviation.
*Of 44, 37 patients had total body irradiation.
†Dose of prednisone + dose of dexamethasone X 6.67 (in mg/m2).
MS was found in 5 patients (6.5%). These patients had 3 (n = 4) or 5 (n = 1) criteria according to MS definition, including increased waist circumference for 3 of them, increased blood pressure for 3 of them, decreased HDL-cholesterol in 4 cases, elevated fasting glucose in 4 cases, and increased triglycerides in 3 cases.
Correlation between first and second scans
As shown in Figure 2, the correlations between z-scores measured at the first and the second scans (paired for each patient) were highly significant. This was observed at all levels, lumbar spine, femoral neck, total hip, and whole body (P value <10−3 for each site) with acceptable agreement.
Figure 2.
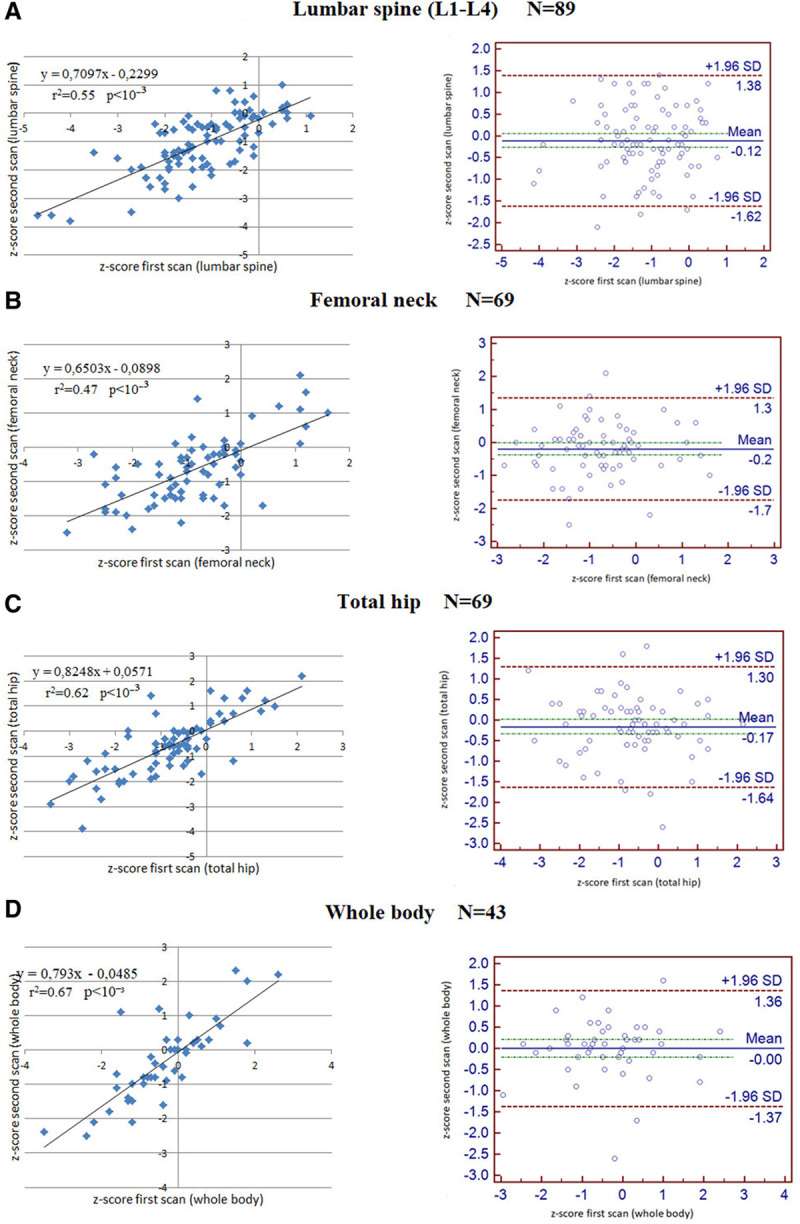
Correlation between z-scores of the first and the second scan and corresponding Bland Altman plots at lumbar spine (A), femoral neck (B), total hip (C), and whole body (D).
Overall change in BMD z-score from baseline to adult BMD
Overall, mean z-scores at the second evaluation improved significantly at the femoral neck (mean −0.85 ± 1.02 at the first scan, −0.64 ± 0.97 at the second scan; P = 0.033) and total hip (mean −0.78 ± 1.11 at the first scan, −0.59 ± 1.17 at the second scan; P = 0.036) level (Table 3). There was no significant evolution at lumbar spine and whole body.
Table 3.
Changes in Bone Mineral Density z-Scores Between First and Second Scans.
| z-Score at First Scan(Mean ± SD) | z-Score at Second Scan(Mean ± SD) | Difference z2-z1, Mean (95%CI) | P | |
|---|---|---|---|---|
| Lumbar spine (L1–L4) | −1.18 ± 1.10 | −1.07 ± 1.05 | 0.11 (−0.05 to 0.28) | 0.170 |
| Femoral neck | −0.85 ± 1.02 | −0.64 ± 0.97 | 0.21 (0.02–0.40) | 0.033 |
| Total hip | −0.78 ± 1.11 | −0.59 ± 1.17 | 0.19 (0.01–0.38) | 0.036 |
| Whole body | −0.36 ± 1.19 | −0.33 ± 1.16 | 0.03 (−0.19 to 0.25) | 0.815 |
CI = confidence interval; SD = standard deviation.
The distribution of the number of patients with low BMD (z-score < −2) at each site and stage is detailed in Table 4. The percentage of patients with a femoral neck z-score below −2 decreased from 14.5% to 4.3% (P = 0.04). We did not detect any significant difference at the other sites. Overall, 23 (24%) patients presented low BMD at 1 site or more at the first scan compared to 17 patients (19%) at follow-up evaluation.
Table 4.
Number and Proportion of Patients with Low Bone Mineral Density (z-Score < −2) at Each Site at the First and Second Scans.
| Number (%) of Patients With Low BMD | ||||
|---|---|---|---|---|
| Lumbar spine (L1–L4), N = 89 | Femoral Neck, N = 69 | Total Hip, N = 69 | Whole Body, N = 43 | |
| First scan | 14 (15.7%) | 10 (14.5%) | 10 (14.5%) | 3 (7%) |
| Second scan | 13 (14.6%) | 3 (4.3%) | 5 (7.2%) | 4 (9.3%) |
| P | 0.834 | 0.04 | 0.171 | 1 |
Determinants of BMD changes
Factors that could have influenced evolution of z-score between the 2 scans (z2-score over 18 years–z1-score under 18) are presented in Table 5 for the lumbar spine and in Table 6 for the femoral neck. At the lumbar spine site, the only statistically significant factor was the age at diagnosis of AL: z2–z1 score was 0.28 (improvement) for patients older than 8.5 years at diagnosis (the mean age of children at diagnosis), and −0.06 (decline) for those ≤8.5 years (P = 0.041). According to the presence of an MS, z2–z1 score was +0.16 (improvement) and −0.54 (decline) in patients without and with MS, respectively, but the difference did not reach statistical significance (P = 0.054). Both younger age at diagnosis and MS had a statistically significant unfavorable impact on femoral BMD (P = 0.003 and 0.025, respectively). Evolution of BMD according to the presence of MS or not is shown on Figure 3. Among patients with hypogonadism, those with substitutive treatment (n = 26) improved their lumbar z-scores (z2–z1 = +0.35), while those without treatment (n = 4) had a decline (z2–z1 = −0.20) but, may be due to the small number of patients, this trend is not statistically significant. We did not detect any significant risk factor for changes in total hip and whole-body analysis.
Table 5.
Factors Influencing Changes in z-Score at the Lumbar Spine L1–L4.
| z-Score L1–L4 (N = 89) | ||||||
|---|---|---|---|---|---|---|
| N | z1 (at Scan 1), Mean ± SD | z2 (at Scan 2), Mean ± SD | z2–z1 | b (95% CI) | P | |
| Gender | ||||||
| Female | 39 | −1.04 ± 0.91 | −0.79 ± 0.93 | 0.25 ± 0.62 | Ref | |
| Male | 50 | −1.30 ± 1.22 | −1.29 ± 1.10 | 0.01 ± 0.87 | −0.24 (−0.56 to 0.09) | 0.154 |
| Leukemia type | ||||||
| ALL | 68 | −1.30 ± 1.15 | −1.15 ± 1.07 | 0.15 ± 0.76 | Ref | |
| AML | 21 | −0.82 ± 0.81 | −0.82 ± 0.99 | −0.005 ± 0.84 | −0.15 (−0.54 to 0.23) | 0.426 |
| HSCT | ||||||
| No | 45 | −1.30 ± 1.30 | −1.12 ± 1.15 | 0.19 ± 0.72 | Ref | |
| Yes | 44 | −1.06 ± 0.84 | −1.02 ± 0.96 | 0.04 ± 0.83 | −0.15 (−0.47 to 0.18) | 0.374 |
| History of relapse | ||||||
| No | 64 | −1.15 ± 1.10 | −1.01 ± 1.02 | 0.15 ± 0.77 | Ref | |
| Yes | 25 | −1.26 ± 1.12 | −1.23 ± 1.14 | 0.03 ± 0.79 | −0.11 (−0.48 to 0.25) | 0.545 |
| Growth hormone deficiency | ||||||
| No | 84 | −1.16 ± 1.11 | −1.07 ± 1.07 | 0.10 ± 0.79 | Ref | |
| Yes | 5 | −1.55 ± 0.87 | −1.14 ± 0.80 | 0.41 ± 0.50 | 0.300 (−0.40 to 1.02) | 0.386 |
| Hypogonadism | ||||||
| No | 59 | −1.09 ± 1.15 | −1.05 ± 1.08 | 0.04 ± 0.71 | Ref | |
| Yes | 30 | −1.38 ± 0.96 | −1.11 ± 1.01 | 0.27 ± 0.89 | 0.23 (−0.12 to 0.58) | 0.188 |
| If yes substitution | ||||||
| No | 4 | −1.20 ± 0.62 | −1.40 ± 1.16 | −0.20 ± 0.60 | Ref | |
| Yes | 26 | −1.41 ± 1.01 | −1.06 ± 1.00 | 0.35 ± 0.92 | 0.55 (−0.43 to 1.53) | 0.264 |
| Metabolic syndrome | ||||||
| No | 72 | −1.14 ± 1.03 | −0.98 ± 1.02 | 0.16 ± 0.78 | Ref | |
| Yes | 5 | −0.66 ± 0.98 | −1.20 ± 0.97 | −0.54 ± 0.66 | −0.70 (−1.41 to 0.01) | 0.054 |
| Cumulative corticosteroid dose (as continuous variable) | 0.00001 (−0.00004 to 0.00007) | 0.691 | ||||
| Weight z-score difference between scan 1 and scan 2 (as continuous variable) | 0.07 (−0.016 to 0.16) | 0.11 | ||||
| Age at diagnosis (as continuous variable) | 0.03 (−0.003 to 0.06) | 0.074 | ||||
| Age at diagnosis | ||||||
| ≤8.5 y | 43 | −1.22 ± 0.97 | −1.28 ± 0.91 | −0.06 ± 0.75 | Ref | |
| >8.5 y | 46 | −1.15 ± 1.22 | −0.87 ± 1.15 | 0.28 ± 0.77 | 0.34 (0.01–0.66) | 0.041 |
| Total body irradiation | ||||||
| No | 52 | −1.24 ± 1.24 | −1.05 ± 1.12 | 0.19 ± 0.77 | Ref | |
| Yes | 37 | −1.11 ± 0.87 | −1.10 ± 0.96 | 0.01 ± 0.78 | −0.18 (−0.51 to 0.15) | 0.285 |
| CNS radiation | ||||||
| No | 76 | −1.14 ± 1.04 | −1.05 ± 1.02 | 0.09 ± 0.80 | Ref | |
| Yes | 13 | −1.40 ± 1.48 | −1.18 ± 1.35 | 0.23 ± 0.69 | 0.13 (−0.35 to 0.62) | 0.588 |
| Interval between scans (as continuous variable)* | 0.07 (−0.52 to 0.66) | 0.816 | ||||
ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; CNS = central nervous system; CI = confidence interval; HSCT = hematopoietic stem cell transplantation; SD = standard deviation.
*For this variable, a logarithmic transformation technique was implemented.
Table 6.
Factors Influencing Changes in z-Score at Femoral Neck.
| z-Score Femoral Neck (N = 69) | ||||||
|---|---|---|---|---|---|---|
| N | z1 (at Scan 1), Mean ± SD | z2 (at Scan 2), Mean ± SD | z2–z1 | b (95% CI) | P | |
| Gender | ||||||
| Female | 30 | −0.78 ± 01.09 | −0.61 ± 0.96 | 0.17 ± 0.78 | Ref | |
| Male | 39 | −0.91 ± 0.97 | −0.67 ± 1.00 | 0.24 ± 0.81 | 0.07 (−0.32 to 0.46) | 0.726 |
| Leukemia type | ||||||
| ALL | 54 | −0.92 ± 0.99 | −0.71 ± 0.99 | 0.21 ± 0.82 | Ref | |
| AML | 15 | −0.60 ± 1.13 | −0.41 ± 0.89 | 0.19 ± 0.73 | −0.03 (−0.49 to 0.44) | 0.905 |
| HSCT | ||||||
| No | 36 | −0.76 ± 1.16 | −0.44 ± 1.00 | 0.31 ± 0.74 | Ref | |
| Yes | 33 | −0.96 ± 0.85 | −0.86 ± 0.90 | 0.09 ± 0.85 | −0.22 (−0.60 to 0.16) | 0.254 |
| History of relapse | ||||||
| No | 51 | −0.81 ± 1.04 | −0.52 ± 0.95 | 0.29 ± 0.73 | Ref | |
| Yes | 18 | −0.97 ± 0.99 | −1.00 ± 0.96 | −0.03 ± 0.95 | −0.32 (−0.75 to 0.11) | 0.144 |
| Growth hormone deficiency | ||||||
| No | 67 | −0.80 ± 0.99 | −0.62 ± 0.97 | 0.19 ± 0.79 | NA | NA |
| Yes | 2 | Patient 1:−2.50 Patient 2:−2.50 |
Patient 1:−1.90 Patient 2:-1.10 |
Patient 1:0.60 Patient 2:1.40 |
||
| Hypogonadism | ||||||
| No | 50 | −0.80 ± 1.09 | −0.51 ± 1.03 | 0.29 ± 0.75 | Ref | |
| Yes | 19 | −0.99 ± 0.80 | −0.99 ± 0.71 | 0.005 ± 0.89 | −0.28 (−0.71 to 0.14) | 0.193 |
| If yes substitution | ||||||
| No | 4 | −0.78 ± 0.77 | −1.03 ± 1.14 | −0.25 ± 0.57 | Ref | |
| Yes | 15 | −1.05 ± 0.83 | −0.98 ± 0.61 | 0.07 ± 0.96 | 0.032 (−0.75 to 1.40) | 0.534 |
| Metabolic syndrome | ||||||
| No | 54 | −0.78 ± 1.01 | −0.51 ± 1.00 | 0.27 ± 0.65 | Ref | |
| Yes | 5 | −0.4 ± 0.95 | −0.84 ± 0.84 | −0.44 ± 0.73 | −0.71 (−1.33 to −0.09) | 0.025 |
| Cumulative corticosteroid dose (as continuous variable) | 0.00003 (−0.00003 to 0.00009) | 0.322 | ||||
| Weight z-score difference between scan 1 and scan 2 (as continuous variable) | 0.05 (-0.06-0.15) | 0.396 | ||||
| Age at diagnosis (as continuous variable) | 0.05 (0.01-0.09) | 0.013 | ||||
| Age at diagnosis | ||||||
| ≤8.5 y | 31 | −0.66 ± 1.04 | −0.76 ± 0.97 | −0.10 ± 0.61 | Ref | |
| >8.5 y | 38 | −1.01 ± 0.99 | −0.55 ± 0.98 | 0.46 ± 0.85 | 0.55 (0.19–0.92) | 0.003 |
| Total body irradiation | ||||||
| No | 41 | −0.75 ± 1.14 | −0.43 ± 1.01 | 0.31 ± 0.72 | Ref | |
| Yes | 28 | −1.01 ± 0.81 | −0.95 ± 0.83 | 0.05 ± 0.89 | −0.26 (−0.65 to 0.13) | 0.183 |
| CNS radiation | ||||||
| No | 60 | −0.85 ± 1.07 | −0.64 ± 0.99 | 0.21 ± 0.84 | Ref | |
| Yes | 9 | −0.89 ± 0.66 | −0.66 ± 0.90 | 0.23 ± 0.38 | 0.03 (−0.54 to 0.60) | 0.922 |
| Interval between scans (as continuous variable)* | −0.55 (−1.31 to 0.21) | 0.155 | ||||
ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; CNS = central nervous system; CI = confidence interval; HSCT = hematopoietic stem cell transplantation; NA = not applicable; SD = standard deviation.
*For this variable, a logarithmic transformation technique was implemented.
Figure 3.
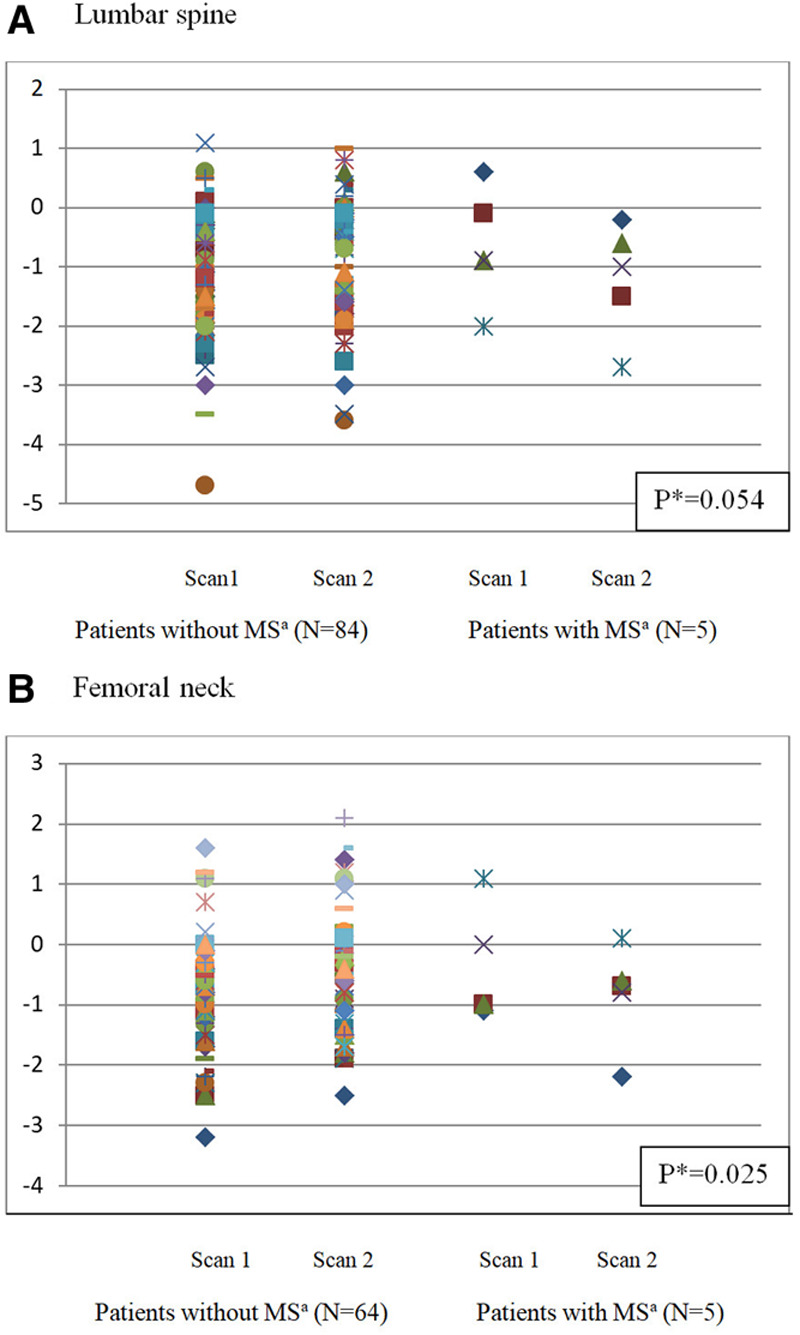
Comparison of z-score evolution between first and second scan depending on the existence of a metabolic syndrome at lumbar spine (A) and femoral neck (B). aMetabolic Syndrome. *P values correspond to the comparison of BMD evolutions between patients with or without metabolic syndrome.
Discussion
A low BMD is a well-known risk factor for fracture with advancing age. After the age of 50, 1 SD decrease in BMD value doubles the risk of fracture.12 In healthy young adults, a low but stable BMD is not a risk factor of imminent fracture, but detection of low BMD should lead to the implementation of nonpharmacological measures and to the prevention and/or treatment of any additional risk factors of BMD decline, as hypogonadism or tobacco smoking. Patients with added risk factors, including those requiring corticosteroid therapies, should benefit from a reinforced follow-up.
The main objective of this study, conducted in a population of AL survivors, was to describe the variation of BMD after the end of AL treatment, focusing on a period theoretically marked by a dramatic bone mass gain between adolescence and adulthood. Our results suggest that there is a significant improvement of BMD at the femoral neck and total hip but not at the lumbar spine level, from childhood to adulthood, with a mean interval between the 2 scans of 4.8 years.
Like many other pediatric oncology groups, the Children’s Oncology Group recommends a baseline BMD evaluation at entry into long-term follow-up13and considers DXA screening among “high-yield” tests for early detection of long-term complications in childhood cancer survivors.14 However, for each individual, the clinical relevance of this initial assessment to predict BMD in adulthood has not been studied, although a positive linear relationship has been described previously between BMD at the time of ALL diagnosis and during continuation therapy.2 In our cohort, we found at each site a strong correlation between the first and second BMD measurement, confirming the importance of baseline evaluation to allow adequate management in the case of low BMD. We therefore recommend BMD measurement when entering in the long-term follow-up period for all patients diagnosed with AL during childhood, because our study does not allow us to identify a group for which this first evaluation can be safely omitted.
Previous reports which evaluated longitudinally the variation of BMD have focused on ALL patients only.2–4,15–18 In survivors of childhood AML, data concerning BMD are available through small series of patients,19 or as part of a more global long-term late effects evaluation.20–22 In this prospective study, patients included were treated during childhood for either ALL or AML, with chemotherapy only or chemotherapy followed by HSCT. The constitution of the LEA cohort historically began with the inclusion of patients in pediatric cancer centers with high transplant activity, before expanding to other centers, which could explain the overrepresentation in our cohort of patients who underwent HSCT and/or with AML. As expected, the same occurs in our study population. However, as we did not find any impact of HSCT or type of leukemia on the evolution of BMD, it is unlikely that this recruitment bias influenced the results.
In our whole cohort of patients, we found that BMD trends to recover from childhood to adulthood, with significant improvement at the femoral neck and total hip sites. This is consistent with Gurney et al’s results.4 However, in this study from the St Jude Lifetime Cohort where lumbar trabecular volumetric BMD was determined from computed tomography, the proportion of patients under the age of 18 at the time of first assessment is not detailed. In a cross-sectional study, age and sex-standardized whole-body BMD z-scores also seemed to improve linearly as time elapses after the completion of therapy.7 As compared to controls, other previous studies that included children and young adults did not detect any impact of the disease and its treatment on BMD in survivors of childhood ALL.6,23 In contrast, during and after completion of therapy, a decline in BMD or a failure to improve z-scores were described in monocentric studies.3,16 In Kaste et al8 study, 21% of ALL survivors had low BMD, a proportion significantly greater than in the normal population, as also reported by other groups.5,24 In these studies, most patients had received cranial irradiation as part of their treatment. With a mean interval of 4 years from the end of treatment to evaluation, up to 37.9% of ALL survivors had low BMD or osteoporosis in a cross-sectional study conducted more recently in the Iranian population.25
Our results are more reassuring, with only 14.6% of patients with low lumbar BMD at adulthood, and 4.3% at the femoral neck. We identified factors that could negatively influence BMD evolution: a younger age at diagnosis for both lumbar spine and femoral neck BMD and the existence of an MS for femoral neck BMD. Prevention of BMD decline is important, as Van der Sluis et al26 suggested that the change in BMD rather than its absolute value could play a role in fracture risk. In previous studies, it was reported that a younger age at the time of treatment in male children with ALL24 or at the time of transplant9,19 was associated with a higher risk of low BMD. Among the factors that could account for this risk, growth impairment is one of the hypotheses. Despite the small number of patients with an MS in our cohort, we found that it has a significant negative impact on femoral BMD evolution, and a trend for an unfavorable evolution at the lumbar level, which warrants further comments. We previously found that, as compared to the general population,27 adults participating in the LEA cohort presented a higher risk of MS, regardless of antileukemic treatment. However, to our knowledge, the link between MS and BMD has not been studied in AL survivors. In other studies, published results are conflicting regarding the relationship between MS, BMD and fracture risk.28,29 A recent review focusing on the possible effects of MS on BMD in adolescents was in favor of a negative impact of MS on bone mass and some components of MS were negatively correlated with BMD.30 From a physiopathological standpoint, the authors underline that the components of MS can act on calcium homeostasis and/or bone resorption and osteoblasts differentiation. Wong et al29 also supported the hypothesis that MS and osteoporosis shared common underlying pathway. Specific nutritional and pharmacologic interventions against components of MS, as well as lifestyle modifications, could thus not only reduce the cardiovascular risk of patients but also improve their BMD.
With the advances in pharmacogenomics, other risk factors for bone morbidity and decreased BMD have been identified such as germline variation in the MTHFR or MTRR genes31 and single nucleotide polymorphism within CDH2 gene,32 a field that could be explored in the near future with the LEA cohort, because a collection of biological samples for genome-wide association studies is already in progress.
The first limitation of our work is its exploratory nature and the lack of primary hypothesis, raising the point of valid statistical inferences. Our significant results from univariate exploration of the determinants of the evolution of BMD are clearly exploratory results and corresponding hypothesis need to be tested in further confirmatory studies. Second, although tobacco smoking has been described as a potential risk factor for low BMD,33 it was not possible in this study to analyze the impact of smoking, because only qualitative declarative data were collected, as well as for the practice of sports activities. Another shortcoming of this study is that the patient’s family history of osteoporosis was not recorded, and no uniform recommendation was made in the LEA cohort for lifestyle intervention, calcium, or vitamin D supplementation and bisphosphonate therapy. In selected patients, bisphosphonate treatment could be considered, as suggested by Lee et al.34 Management of patients with low BMD or at risk of BMD decline should also continue to include assessment of calcium intake, vitamin D supplementation, and correction of hormone deficiency. In a longitudinal study, growth hormone therapy has been shown to provide benefits on BMD in young adults.35 Physical fitness should also be promoted, as it seems to be a major factor in developing and preserving BMD, that could also impinge on MS.36 To conclude, the strength of our study is to have measured BMD longitudinally, before and after peak bone mass achievement. Promoting bone health is a major issue for AL survivors, because during childhood they had a disease and received a treatment potentially deleterious for bone. A first BMD measurement is recommended at baseline few years after the end of leukemia treatment, and a follow-up BMD measurement should be made at the end of growth especially for patients who were young at the time of diagnosis, those with MS and/or with impaired BMD at the first evaluation. For these patients, additional risk factors for osteoporosis should be checked regularly (chronic inflammation, corticosteroid use, hypogonadism, hyperthyroidism, malabsorption, etc.). They could be offered once they have become adults a systematic consultation with a physician specialized in the management of osteoporosis. In case of fracture without evidence of trauma, this consultation will be mandatory, as pharmacological treatment will be indicated.
Acknowledgments
The authors would like to thank the patients and their families for participation, as well as the LEA study group, for data collection, and Murielle Jacob for her help in formatting.
Members of the LEA study group: Marseille Center: Anne-Lise Alloin, Pascal Auquier, Vincent Barlogis, Julie Berbis, Hervé Chambost, Catherine Curtillet, Sophie Esmiol, Claire Galambrun, Floriane Garnier, Gérard Michel, Delphine Orbicini, Claire Oudin, Isabelle Thuret, Camille Vercasson, Françoise Vion-Dury; Nancy Center: Pascal Chastagner, Laurence Clement, Audrey Contet, Fanny Fouyssac, Irène Lemelle, Ludovic Mansuy, Marjolaine Pernet, Aurore Perrot, Aurélie Phulpin, Cécile Pochon Cécile, Caroline Serrier; Nice Center: Anne Deville, Chokri Hathroubi, Marion Le Meignen-Diop, Fabrice Monpoux, Maryline Poirée, Christine Soler, Pierre-Simon Rohrlich; Clermont-Ferrand Center: Fanny Chambon, Eric Dore, Florentina Isfan, Justyna Kanold, Nadège Rouel; Grenoble Center: Dalila Adjaoud, Corinne Armari-Alla, Sophie Cacace, Anne Pagnier, Dominique Plantaz, Isabelle Schiff; Lyon Center: Yves Bertrand, Nathalie Garnier, Carine Halfon-Domenech, Julie-Yi Hu, Kamila Kebaïli, Marie Ouachee-Chardin, Cécile Renard; Paris Trousseau Center: Anne Auvrignon, Latéfa Bouayad Agha, Catherine Dollfus, Jean Donadieu, Judith Landman-Parker, Guy Leverger, Arnaud Petit, Marie-Dominique Tabone; St Etienne Center: Claire Berger, Audrey David, Françoise Odier, Jean-Louis Stephan, Sandrine Thouvenin; Rennes Center: Sophie Bayart, Jacinthe Bonneau, Céline Chappe, Virginie Gandemer, Anne-Marie Lamour, Phaktra Sok-Fedele, Sophie Taque, Fabienne Toutain; Montpellier Center: Stéphanie Haouy, Ornellia Mophawe, Laure Saumet, Anne Sirvent, Nicolas Sirvent; Paris St Louis – Robert Debré Center: André Baruchel, Benoît Brethon Thierry Leblanc, Marie-Noelle Moukoko, Anne-France Ray-Lunven, David Avran, Saba Azarnoush, Aurélie Cuinet, Jean-Hugues Dalle, Frédérique Duquesne, Raymonda Fahd, Brigitte Lescoeur, Karima Yakouben; Bordeaux Center: Nathalie Aladjidi, Sophie Ansoborlo, Céline De Bouyn-Icher, Stéphane Ducassou, Charlotte Jubert, Maria Merched, Anne Notz-Carrere, Cécile Verite-Goulard; Strasbourg Center: Sofia Ceron-Duran, Patrick Lutz, Catherine Paillard, Alexandra Spiegel; Angers Center: Elsa Berardi, Jean-François Brasme, Laure Colin, Emilie De Carli, Mylène Duplan, Isabelle Pellier, Stéphanie Proust-Houdemont.
Disclosures
The authors declare that they have no conflicts of interest with regard to the content of this report.
Sources of funding
The study was funded by the French National Clinical Research Program (PHRCN10.24.02), the French National Cancer Institute (InCA), the “Laurette Fugain” association, the French National Research Agency (ANR-10-COHO-09-03), the “Ligue contre le cancer” association, Cancéropôle PACA, the Regional Council PACA, the “111 des arts” association and the French Institute for Public Health Research (IRESP).
References
- 1.Lacour B, Goujon S, Guissou S, et al. Childhood cancer survival in France, 2000-2008. Eur J Cancer Prev.. 2014; 23:449–457 [DOI] [PubMed] [Google Scholar]
- 2.Rayar MS, Nayiager T, Webber CE, et al. Predictors of bony morbidity in children with acute lymphoblastic leukemia. Pediatr Blood Cancer.. 2012; 59:77–82 [DOI] [PubMed] [Google Scholar]
- 3.Maniadaki I, Stiakaki E, Germanakis I, et al. Evaluation of bone mineral density at different phases of therapy of childhood all. Pediatr Hematol Oncol.. 2006; 23:11–18 [DOI] [PubMed] [Google Scholar]
- 4.Gurney JG, Kaste SC, Liu W, et al. Bone mineral density among long-term survivors of childhood acute lymphoblastic leukemia: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer.. 2014; 61:1270–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoorweg-Nijman JJ, Kardos G, Roos JC, et al. Bone mineral density and markers of bone turnover in young adult survivors of childhood lymphoblastic leukaemia. Clin Endocrinol (Oxf).. 1999; 50:237–244 [DOI] [PubMed] [Google Scholar]
- 6.Jain S, Jain S, Kapoor G, Virmani A, Bajpai R. No impact of disease and its treatment on bone mineral density in survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer.. 2017; 64:e26271. [DOI] [PubMed] [Google Scholar]
- 7.Kadan-Lottick N, Marshall JA, Barón AE, et al. Normal bone mineral density after treatment for childhood acute lymphoblastic leukemia diagnosed between 1991 and 1998. J Pediatr.. 2001; 138:898–904 [DOI] [PubMed] [Google Scholar]
- 8.Kaste SC, Jones-Wallace D, Rose SR, et al. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: frequency of occurrence and risk factors for their development. Leukemia.. 2001; 15:728–734 [DOI] [PubMed] [Google Scholar]
- 9.Le Meignen M, Auquier P, Barlogis V, et al. Bone mineral density in adult survivors of childhood acute leukemia: impact of hematopoietic stem cell transplantation and other treatment modalities. Blood.. 2011; 118:1481–1489 [DOI] [PubMed] [Google Scholar]
- 10.Berbis J, Michel G, Baruchel A, et al. Cohort profile: the French childhood cancer survivor study for leukaemia (LEA cohort). Int J Epidemiol.. 2015; 44:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome. Circulation.. 2009; 120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 12.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res.. 2005; 20:1185–1194 [DOI] [PubMed] [Google Scholar]
- 13.Wasilewski-Masker K, Kaste SC, Hudson MM, et al. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics.. 2008; 121:e705–e713 [DOI] [PubMed] [Google Scholar]
- 14.Landier W, Armenian SH, Lee J, et al. Yield of screening for long-term complications using the children’s oncology group long-term follow-up guidelines. J Clin Oncol.. 2012; 30:4401–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Sluis IM, van den Heuvel-Eibrink MM, Hählen K, et al. Altered bone mineral density and body composition, and increased fracture risk in childhood acute lymphoblastic leukemia. J Pediatr.. 2002; 141:204–210 [DOI] [PubMed] [Google Scholar]
- 16.Vitanza NA, Hogan LE, Zhang G, et al. The progression of bone mineral density abnormalities after chemotherapy for childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol.. 2015; 37:356–361 [DOI] [PubMed] [Google Scholar]
- 17.Ward LM, Ma J, Lang B, et al. ; Bone morbidity and recovery in children with acute lymphoblastic leukemia: results of a six-year prospective cohort study. J Bone Miner Res.. 2018; 33:1435–1443 [DOI] [PubMed] [Google Scholar]
- 18.Swiatkiewicz V, Wysocki M, Odrowaz-Sypniewska G, et al. Bone mass and bone mineral metabolism at diagnosis and after intensive treatment in children with acute lymphoblastic leukemia. Med Pediatr Oncol.. 2003; 41:578–580 [DOI] [PubMed] [Google Scholar]
- 19.Bhatia S, Ramsay NK, Weisdorf D, et al. Bone mineral density in patients undergoing bone marrow transplantation for myeloid malignancies. Bone Marrow Transplant.. 1998; 22:87–90 [DOI] [PubMed] [Google Scholar]
- 20.Haddy TB, Mosher RB, Reaman GH. Late effects in long-term survivors after treatment for childhood acute leukemia. Clin Pediatr (Phila).. 2009; 48:601–608 [DOI] [PubMed] [Google Scholar]
- 21.Blijdorp K, van Waas M, van der Lely AJ, et al. Endocrine sequelae and metabolic syndrome in adult long-term survivors of childhood acute myeloid leukemia. Leuk Res.. 2013; 37:367–371 [DOI] [PubMed] [Google Scholar]
- 22.Perkins JL, Kunin-Batson AS, Youngren NM, et al. Long-term follow-up of children who underwent hematopoeitic cell transplant (HCT) for AML or ALL at less than 3 years of age. Pediatr Blood Cancer.. 2007; 49:958–963 [DOI] [PubMed] [Google Scholar]
- 23.Mandel K, Atkinson S, Barr RD, et al. Skeletal morbidity in childhood acute lymphoblastic leukemia. J Clin Oncol.. 2004; 22:1215–1221 [DOI] [PubMed] [Google Scholar]
- 24.Mäkitie O, Heikkinen R, Toiviainen-Salo S, et al. Long-term skeletal consequences of childhood acute lymphoblastic leukemia in adult males: a cohort study. Eur J Endocrinol.. 2013; 168:281–288 [DOI] [PubMed] [Google Scholar]
- 25.Rohani F, Arjmandi Rafsanjani Kh, Bahoush G, et al. Bone mineral density in survivors of childhood acute lymphoblastic leukemia Asian Pac J Cancer Prev.. 2017; 18:535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Sluis IM, de Muinck Keizer-Schrama SM, van den Heuvel-Eibrink MM. Bone mineral density in childhood acute lymphoblastic leukemia (ALL) during and after treatment. Pediatr Blood Cancer.. 2004; 43:182–183; discussion 184 [DOI] [PubMed] [Google Scholar]
- 27.Oudin C, Berbis J, Bertrand Y, et al. Prevalence and characteristics of metabolic syndrome in adults from the French childhood leukemia survivors’ cohort: a comparison with controls from the French population. Haematologica.. 2018; 103:645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esposito K, Chiodini P, Capuano A, et al. Fracture risk and bone mineral density in metabolic syndrome: a meta-analysis. J Clin Endocrinol Metab.. 2013; 98:3306–3314 [DOI] [PubMed] [Google Scholar]
- 29.Wong SK, Chin K-Y, Suhaimi FH, et al. The relationship between metabolic syndrome and osteoporosis: a review. Nutrients.. 2016; 8:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva VN, Fiorelli LN, da Silva CC, et al. Do metabolic syndrome and its components have an impact on bone mineral density in adolescents? Nutr Metab (Lond).. 2017; 14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.te Winkel ML, de Muinck Keizer-Schrama SM, de Jonge R, et al. Germline variation in the MTHFR and MTRR genes determines the nadir of bone density in pediatric acute lymphoblastic leukemia: a prospective study. Bone.. 2011; 48:571–577 [DOI] [PubMed] [Google Scholar]
- 32.Aaron M, Nadeau G, Ouimet-Grennan E, et al. Identification of a single-nucleotide polymorphism within CDH2 gene associated with bone morbidity in childhood acute lymphoblastic leukemia survivors. Pharmacogenomics.. 2019; 20:409–420 [DOI] [PubMed] [Google Scholar]
- 33.Thomas IH, Donohue JE, Ness KK, et al. Bone mineral density in young adult survivors of acute lymphoblastic leukemia. Cancer.. 2008; 113:3248–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JM, Kim JE, Bae SH, et al. Efficacy of pamidronate in children with low bone mineral density during and after chemotherapy for acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Res.. 2013; 48:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Heijkant S, Hoorweg-Nijman G, Huisman J, et al. Effects of growth hormone therapy on bone mass, metabolic balance, and well-being in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol.. 2011; 33:e231–e238 [DOI] [PubMed] [Google Scholar]
- 36.Jarfelt M, Fors H, Lannering B, et al. Bone mineral density and bone turnover in young adult survivors of childhood acute lymphoblastic leukaemia. Eur J Endocrinol.. 2006; 154:303–309 [DOI] [PubMed] [Google Scholar]


