Abstract
The aggressive lymphoma, extranodal natural killer/T-cell lymphoma-nasal type, is strongly associated with Epstein-Barr virus (EBV) and is most common in Asia and in South and Central America. By contrast, incidence is low in the United States and Europe, where extranodal natural killer/T-cell lymphoma represents only 0.2%-0.4% of all newly diagnosed non-Hodgkin lymphomas. At diagnosis, it is important to test for EBV DNA in plasma by polymerase chain reaction and to carry out positron emission tomography/computer tomography and magnetic resonance imaging of the nasopharynx. In stage I/II disease, radiotherapy is the most important treatment modality, but in high-risk stage I/II disease (stage II, age > 60 y, elevated lactate dehydrogenase, Eastern Cooperative Oncology Group performance score ≥2, primary tumor invasion), it should be combined with chemotherapy. The most optimal responses are reached with nonmultidrug resistance-based therapy (eg, asparaginase- or platinum-based therapy). Therapeutic approaches consist of either platinum-based concurrent chemoradiotherapy or sequential chemoradiotherapy. The minimum dose of radiotherapy should be 50-56 Gy. Treatment of stage III/IV disease consists of 3 cycles of chemotherapy followed by autologous hematopoietic cell transplantation. Allogeneic hematopoietic cell transplantation should only be considered in case of relapsed disease or after difficulty reaching complete remission. During treatment and follow-up, plasma EBV levels should be monitored as a marker of tumor load.
Introduction
Extranodal natural killer/T-cell lymphoma (ENKTL) is an aggressive lymphoma that is closely associated with Epstein-Barr virus (EBV). Incidence in the United States and Europe is low, with only 0.2%-0.4% of newly diagnosed non-Hodgkin lymphomas of the ENKTL type.1,2 ENKTL is more common in Asia and in Central and South America, where ENKTL represents 5%-15% of all newly diagnosed lymphomas.3 Around 85% of ENKTLs originate from natural killer (NK) cells and 15% from T-cells.4 The most common site of occurrence is the nasopharynx, but other common locations include the sinuses, tonsils, Waldeyer ring, and oropharynx. About 70%-90% of patients have stage I or II lymphoma at presentation,5 and the majority present with nasal obstruction, epistaxis, or tumor growth through anatomic structures such as the palatum or orbita. Common additional symptoms include fever, weight loss, and malaise. In stage III/IV disease, the most frequent sites of occurrence include the skin, salivary glands, lymph nodes, testis, and the gastrointestinal tract, accompanied by gastrointestinal bleeding as a common presenting symptom. In up to 11% of cases, the presenting symptom is hemophagocytic syndrome.6,7
Diagnosis
Diagnosis of ENKTL by tissue biopsy is not always straightforward. In nasal mucosa, in particular, where extensive ulceration is often present, it can be difficult to differentiate between lymphoma and other conditions such as granulomatous polyangiitis, infection, or cocaine-induced destruction. ENKTL shows a diffuse and invasive growth pattern, often accompanied by an angiocentric and angiodestructive pattern that can result in necrosis and significant inflammation (Figure 1). Among individual cases, the cytological spectrum of tumor cells is broad, ranging from small and barely atypical to large and anaplastic. Nuclear contour can be irregular, the cytoplasm is often pale, and mitotic figures are frequently numerous.8 Epstein-Barr virus-encoded small RNAs in situ hybridization is always positive, and in most cases, the NK cell marker CD56 and cytotoxic markers granzyme B and TIA-1 are expressed and detectable by immunohistochemistry. Of the traditional T-cell markers, CD2 is positive and CD3 shows only cytoplasmic positivity, whereas CD4, CD8, CD5, and CD7 are usually negative.8 T-cell receptor clonality analysis by polymerase chain reaction (PCR) usually shows no rearrangement products, consistent with the NK cell origin of ENKTL in 85% of cases. In daily practice, Epstein-Barr virus-encoded small RNA and CD56 staining are very helpful in distinguishing ENKTL from other inflammatory and ulcerating processes. In view of the newly available therapeutic options, it is worth mentioning that 95% of ENKTLs are positive for CD38, about 30% for CD30 and up to 70% for programmed death-ligand 1 (PD-L1) (CD274), with positivity even reaching almost 100% in nodal cases.9,10
Figure 1.

Histology of an NK/T-cell lymphoma showing *extensive lymphocytic infiltration, **necrosis, and ***angiocentric growth. NK = natural killer.
ENKTL is also fluorodeoxyglucose (FDG)-avid in almost 100% of cases, although maximum standardized uptake values are lower compared to diffuse large B-cell lymphoma.11,12 Therefore, positron emission tomography (PET)/computer tomography (CT) should be performed at diagnosis in order to stage the disease. A magnetic resonance imaging of the nasopharynx is especially recommended in cases with nasal localization in order to determine the radiation fields in stage I/II disease. Random biopsies of the nasopharynx are also recommended if nasal localization is not found on imaging of stage I/II disease, due to the possible need for radiation therapy if there is nasopharynx involvement.3
Plasma EBV load is a reliable marker for tumor load at diagnosis and can be used at follow-up for early detection of recurrent disease.13-15 PCR analysis of EBV plasma DNA is therefore advised at diagnosis as a baseline measurement.
ENKTL is often accompanied by hemophagocytosis (HLH). If there is a clinical suggestion of HLH, further diagnostics for HLH should be performed. Finally, staging of the disease includes a bone marrow biopsy.
Prognostic models
Using the Ann Arbor staging system, most ENKTL patients are categorized as early stage, which does not correlate with the poor survival. Recently the Asia lymphoma study group published a new and improved correlating staging system.16 With stage I defined as limited nasal disease without tumor invasion in the surrounding structures, stage II nonnasal localization or nasal localization with tumor invasion, stage III nasal disease with regional lymph node involvement, and stage IV disease with nonregional lymph node involvement or lymph node involvement on both sides of the diaphragm. As mentioned by the authors, the suggested staging system needs to be validated, preferably in a prospective study.
In the past, the International Prognostic Index and NK/T-cell lymphoma prognostic index were used for risk stratification. Following the introduction of nonanthracycline containing treatment regimens, these scoring systems were no longer adequate, and in 2016 the prognostic index of natural killer lymphoma (PINK) was introduced (Table 1).17 This new scoring system attributes 1 point for every risk factor, and a modification of the PINK score is the prognostic index of natural killer lymphoma-EBV score that includes all factors from the PINK score together with EBV plasma load.17 Since 2017, the PINK score has been included in the National Comprehensive Cancer Network (NCCN) guideline for peripheral T-cell lymphoma.18
Table 1.
Prognostic Score Algorithm for NK/T-Cell Lymphoma.
| PINK | PINK-E |
|---|---|
| Age > 60 y | Age > 60 yr |
| Stage III/IV disease | Stage III/IV disease |
| Nonnasal primary localization | Nonnasal primary localization |
| Distant lymph node involvement* | Distant lymph node involvement* |
| Detectable plasma EBV DNA | |
| Low: 0 | Low: 0-1 |
| Intermediate: 1 | Intermediate: 2 |
| High: 2-4 | High: 3-5 |
| 3-y OS | 3-y OS |
| Low: 81% | Low: 81% |
| Intermediate: 62% | Intermediate: 55% |
| High: 25% | High: 28% |
Every item scores 1 point.
EBV = Epstein-Barr virus; ENKTL = extranodal natural killer/T-cell lymphoma; NK = natural killer; OS = overall survival; PINK = prognostic index of natural killer lymphoma; PINK-E = prognostic index of natural killer lymphoma-EBV.
*Axillary, infraclavicular, mediastinal in case of primary nasal ENKTL.
Treatment
Due to differences in treatment modalities for stage I/II and stage III/IV disease, adequate staging at diagnosis is crucial. Although almost all data are obtained from phase I/II studies or retrospective studies, it has been established that the most important treatment modality in stage I/II disease is radiotherapy, which often is combined with chemotherapy. Stage III/IV disease treatment consists of chemotherapy and hemopoietic stem cell transplantation (Figure 2).
Figure 2.
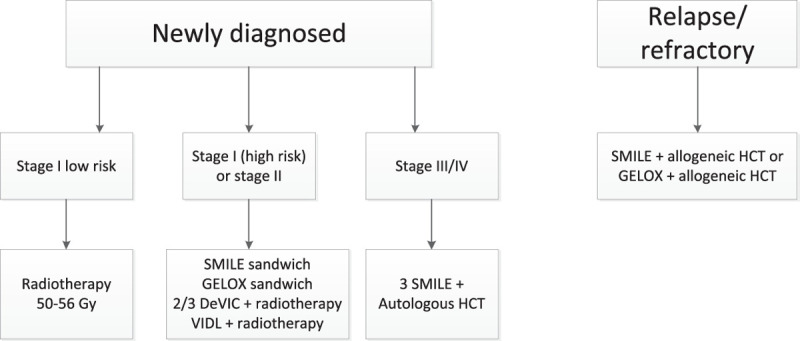
Proposed treatment algorithm. DeVIC = dexamethasone, etoposide, ifosfamide, and carboplatin; GELOX = gemcitabine, oxaliplatin, and L-asparaginase; HCT = hematopoietic cell transplantation; SMILE = dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide; VIDL = etoposide, ifosfamide, dexamethasone, and L-asparaginase.
Owing to the complexity of diagnostics and treatment, it is important that a patient is treated by a multidisciplinary team in a center with expertise in NK/T-cell lymphoma treatment.19
Treatment of a stage I/II disease
In stage I/II disease, the choice of therapy depends on the risk of disease. A large retrospective study by Yang et al,5 which included 1273 patients with stage I/II disease, showed that combined chemotherapy/radiotherapy in standard-risk disease has no additional value compared to radiotherapy alone, with a 5-year overall survival (OS) of 87% for the group with radiotherapy alone versus 88% for the group with combined chemoradiotherapy. By contrast, in high-risk disease, the 5-year OS for combined chemoradiotherapy was 72%, which compares favorably with the 60% 5-year OS for radiotherapy alone. Yang et al5 defined disease as high risk when 1 or more of the following factors were present:
Ann Arbor stage II
Age > 60 years
Elevated lactate dehydrogenase
Eastern Cooperative Oncology Group performance score ≥2
Primary tumor invasion into surrounding anatomic structures
These outcome data and risk stratifications were confirmed in a retrospective study by Liu et al20 that also included patients treated with nonmultidrug resistance (MDR)-based treatment. These authors found no difference between treatment with radiotherapy or chemoradiotherapy in the low risk (none of above factors present) and intermediate risk (1 factor present) categories, although there was a clear trend in favor of treatment with radiotherapy combined with a non-MDR–based chemotherapy regime. However, patients in whom ≥ 2 factors were present derived clear survival benefit when treated with radiotherapy combined with a non-MDR–based chemotherapy regime.20
In a retrospective study including 642 patients with stage I-II disease, Vargo et al21 showed that patients treated with chemotherapy alone had an inferior OS (32% 5-y OS in the chemotherapy alone group compared to 53% in the radiotherapy alone group and 58% in the combined chemoradiotherapy group). These investigators also found that OS was significantly lower with radiation doses of less than 50 Gy (5-y OS < 50 Gy 38% versus ≥ 50 Gy 53%). Another retrospective study has also shown that the risk of a locoregional relapse increases with doses < 50-52 Gy.22
Most existing clinical data are based on the use of outdated 3D conformal radiation therapy, but radiotherapy techniques have improved over the last decades. Newer techniques such as intensity-modulated radiotherapy (IMRT) improve target coverage23 and make it possible to reduce doses to organs at risk (OARs).24 Compared to IMRT, volumetric-modulated arc therapy reduces treatment time but delivers higher mean doses to OARs.24 Further reduction of dose to OARs, especially the integral dose to the body that is relevant to the induction of secondary cancers, can be achieved using proton therapy.25
The International Lymphoma Radiation Oncology Group (ILROG) guideline states that the clinical target volume includes the entire involved cavity and adjacent structures. This is because ENKTL is locally destructive and may have infiltrated in adjacent tissues without macroscopic changes. Magnetic resonance imaging and PET differentiation between tumor and inflammatory tissue/retained mucus is also frequently challenging.26 The guideline recommends that the delivered radiotherapy dose should be at least 50 Gy preferably using an IMRT technique, with eventually 5-10 Gy boost in case of residual disease.18
Choice of chemotherapy
In the past chemotherapeutic treatments consisted of cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP)-like regimens and achieved 5-year OS rates of less than 50%. In the mid-1990s, it was demonstrated that ENKTL expresses the MDR1/ABCB1 gene and its product P-glycoprotein. This indicated that anthracycline-containing regimens should be avoided and non-MDR–dependent therapies should be the first choice of therapy, examples of which are asparaginase- or platinum-based therapies.3,19
Asparagine is a nonessential amino acid that can be synthesized from aspartic acid in healthy cells (Figure 3) and cellular synthesis of asparagine is accomplished by the enzymatic action of asparagine synthetase. Asparaginase is an enzyme that breaks down asparagine, reducing cellular levels of the amino acid. Insufficient levels of cellular asparagine lead to reduced DNA, RNA, and protein synthesis, inhibition of cell growth, and ultimately to the activation of apoptotic cell death.27 As glutamine can be used as an amino group donor in asparagine synthesis, it has been suggested that both asparaginase and glutamine should be depleted for optimal therapeutic effect.28 Importantly, NK cells lack the asparagine synthase activity found in most normal cells, and asparaginase has been shown to induce apoptosis in NK/T-cell lymphoma cell lines in vitro.29 It was subsequently shown that asparaginase is effective in patients relapsing after CHOP-based therapy.30,31
Figure 3.
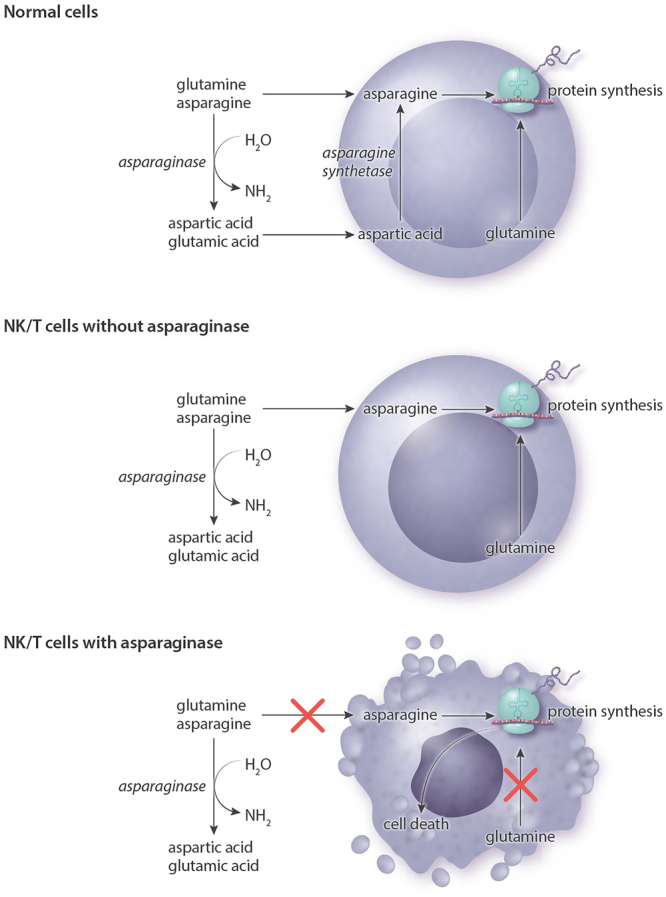
Mechanism of action of asparaginase. The lack of asparagine synthetase in NK/T-cells means that they are dependent on exogenous asparagine and glutamine. By degrading asparagine and glutamine to aspartic acid and glutamic acid, asparaginase inhibits essential intracellular protein synthesis and triggers cell death. NK = natural killer.
The 2 main treatment strategies used for high-risk profile stage I/II disease, either concurrent chemoradiotherapy (CCRT) or a sandwich approach, showed no differences in outcome in a large retrospective study.32
A commonly used CCRT schedule combines radiotherapy (50 Gy) and 3 cycles of dexamethasone, etoposide, ifosfamide, and carboplatin (DeVIC). A phase 2 study showed a complete remission (CR) rate of 77%, a 5-year OS of 70%, and progression-free survival (PFS) of 63%.33 These outcomes were later confirmed in a large retrospective study in Japan.34
A schedule used in Korea combined 40-44 Gy radiotherapy with cisplatin once a week, followed by 2 cycles of VIDL (etoposide, ifosfamide, dexamethasone, and L-asparaginase). Eighty-seven percent of the patients reached CR and the 5-year OS and PFS were 73% and 60%, respectively.35 Two other CCRT treatment protocols used in Korea are VIPD (etoposide, ifosfamide, cisplatin, and dexamethasone) and MIDLE (methotrexate, ifosfamide, dexamethasone, L-asparaginase, and etoposide). In the VIPD study, 80% of patients reached CR and the 3-year PFS and OS were 85% and 86%, respectively.36 The study using the MIDLE regimen reported that 82% of patients reached CR at 3-year follow-up, with an OS and PFS were 82% and 74%, respectively.37
The most commonly used asparaginase-containing sequential regimen is sandwich therapy with 2 cycles of SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide) followed by 50 Gy radiotherapy and again 2 cycles of SMILE. Eighty-two percent of patients reached CR and long-term response was 90%.38 A small series of non-Asian patients whereby L-asparaginase was replaced with pegylated (PEG)-asparaginase (modified SMILE) showed a 2-year OS of 100% and a PFS of 83%.39 The most common side effects were neutropenia and severe infections. Five patients died in the first study due to severe infection, after adding granulocyte-colony stimulating factor (G-CSF) support, no more deaths were seen due to infection.31 SMILE should only be prescribed to fit patients and when accompanied by G-CSF support.
Gemcitabine-based therapies have been combined with asparaginase in some studies. A study by Wang et al40 combined GELOX (gemcitabine, oxaliplatin, and L-asparaginase) treatment with 56 Gy radiotherapy and reported a CR rate of 74% and a 2-year OS and PFS of 86%. This study also found less hematological toxicity than studies in which courses of SMILE were used (see also Table 2).
Table 2.
Treatment of Stage I/II Disease.
| Treatment | Stage | No. Patients (n) | Response (%) | PFS (%) | OS (%) | Grade 3/4 Toxicity (%) | References |
|---|---|---|---|---|---|---|---|
| Radiotherapy 50-56 Gy | I low risk | 298 | NA | 5-y 79 | 5-y 89 | NA | Yang et al5 |
| 2/3 DeVIC + 50 Gy radiotherapy | I/II | 33 | CR 77 | 5-y 63 | 5-y 70 | Neutropenia 93 | Yamaguchi et al33 |
| Infection/febrile neutropenia 15 | |||||||
| TRM 0 | |||||||
| 2/3 DeVIC + 50 Gy radiotherapy | I/II | 150 | NA | 5-y 61 | 5-y 72 | Neutropenia NA | Yamaguchi et al34 |
| Infection/febrile neutropenia 22 | |||||||
| TRM 0 | |||||||
| Cisplatin + 45 Gy radiotherapy + 2 VIDL | I/II | 30 | CR 87 | 5-y 60 | 5-y 73 | Neutropenia 80 | Kim et al35 |
| Infection/febrile neutropenia 17 | |||||||
| TRM 0 | |||||||
| Cisplatin + 45 Gy radiotherapy + 2 MIDLE | I/II | 28 | CR 82 | 3-y 74 | 3-y 82 | Neutropenia 91 | Yoon et al37 |
| Infection/febrile neutropenia 44 | |||||||
| TRM 4 | |||||||
| Cisplatin + 45 Gy radiotherapy + 3 VIDP | I/II | 30 | CR 80 | 3-y 80 | 3-y 86 | Neutropenia 47 | Kim et al36 |
| Infection/febrile neutropenia 15 | |||||||
| TRM 7 | |||||||
| SMILE + > 40 Gy radiotherapy | I/II | 17 | CR 69 | NA | NA | Neutropenia 67 | Kwong et al38 |
| Infection/febrile neutropenia 31 | |||||||
| TRM 6 | |||||||
| mSMILE + 45 Gy radiotherapy | I/II | 11 | NA | 2-y 83 | 2-y 100 | NA | Qi et al39 |
| GELOX + 56 Gy radiotherapy | I/II | 27 | CR 74 | 2-y 86 | 2-y 86 | Neutropenia 33 | Wang et al40 |
| Infection NA | |||||||
| TRM 0 |
CR = complete remission; DeVIC = dexamethasone, etoposide, ifosfamide, and carboplatin; GELOX = gemcitabine, oxaliplatin, and L-asparaginase; MIDLE = methotrexate, ifosfamide, dexamethasone, L-asparaginase, and etoposide; mSMILE = dexamethasone, methotrexate, ifosfamide, PEG-asparginase; NA = not available; OS = overall survival; PFS = progression-free survival; SMILE = dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide; TRM = treatment-related mortality; VIDL = etoposide, ifosfamide, dexamethasone, and L-asparaginase; VIDP = etoposide, ifosfamide, dexamethasone, L-asparaginase.
Treatment of stage III/IV disease
In a phase II study in stage IV disease, the response after 2 cycles of SMILE was 80%, whereby 40% reached a CR,31 the OS after 1 year was 55% and the PFS 53%. L-asparaginase could be replaced with PEG-asparaginase 1500-2500 IE/m2 on day 839and the cycle shortened to 21 days with PEG-asparaginase. If an allergic reaction to L-asparaginase or PEG-asparaginase occurs, an alternative is Erwinia asparaginase 25 000 IE/m2 3 times a week (6 doses in total) as a replacement for 1 dose of PEG-asparaginase or 7 doses of L-asparaginase.41
In a retrospective study of 25 patients with stage III/IV or relapsed disease, treatment with P-GEMOX (PEG-asparaginase, gemcitabine, oxaliplatin) was promising, with responses of 80%, including 51% with CR. However, as 2-year PFS was only 39% responses seem to be short-lived.42 Another combination in use is GDP (gemcitabine, dexamethasone, and cisplatin) (see also Table 3).43 To our knowledge, only 1 study has directly compared a gemcitabine/platinum-based regimen to a SMILE regimen.44 In this study, DDGP (PEG-asparaginase, gemcitabine, cisplatin, and dexamethasone) was randomized against SMILE. After 3 years, PFS was 57% for the DDGP group and 42% for the SMILE group, which was accompanied by a 5-year OS of 74% for the DDGP group and 52% for the SMILE group.45 Although the outcome of the DDGP-treated arm appears promising, there are several caveats associated with this study. First, reported outcomes for the DDGP group are much better than for other previously reported gemcitabine-based regimens, even though differences in medication are minimal. Second, a considerable number of patients in the SMILE group did not complete treatment, with only 42% completing 6 cycles and 19% not even completing 1 cycle. Third, more patients died due to toxicity in the SMILE group than expected, probably due to lack of G-CSF support in the SMILE group. And finally, this study compared L-asparaginase with PEG-asparaginase. We know from studies of acute lymphoblastic leukemia that the latter causes fewer allergic reactions and is probably more effective due to less antibody-mediated neutralization. Thus, due to the early dropout in the SMILE group, intention-to-treat analysis was probably not the best approach when comparing the 2 groups, and a per-protocol analysis of the patients that completed at least 1 cycle would have been a better comparison in terms of effectiveness. In conclusion, DDGP is probably less toxic than SMILE, but SMILE is probably more effective than reported here and the side effects are acceptable when good support is provided. Nonetheless, gemcitabine/platinum-based therapies may possibly represent more effective treatments in less fit patients.
Table 3.
Treatment of Stage III/IV/Relapsed Disease.
| Treatment | Stage | No. Patients (n) | Response (%) | PFS (%) | OS (%) | Grade 3/4 Toxicity (%) | References |
|---|---|---|---|---|---|---|---|
| SMILE | III/IV, refractory, relapsed | 38 | ORR 79 | 1-y 53 | 1-y 55 | Neutropenia 100 | Yamaguchi et al31 |
| CR 45 | Infection/febrile neutropenia 61 | ||||||
| TRM 0 | |||||||
| SMILE | III/IV, refractory, relapsed | 47 | ORR III/IV 81 | 4-y R/R 68,2 | 5-y R/R 52,3 | Neutropenia 67 | Kwong et al38 |
| ORR R/R 75 | St III/IV NA | St III/IV NA | Infection/febrile neutropenia 31 | ||||
| TRM 6 | |||||||
| DDGP | III/IV, refractory, relapsed | 80 | ORR 90 | 3-y 57 | 5-y 74 | Neutropenia 71 | Li et al44 |
| Infection/febrile neutropenia NA | |||||||
| TRM 0 | |||||||
| P-GEMOX | III/IV, refractory, relapsed | 35 | ORR 94 | 2-y 39 | 2-y 65 | Neutropenia 40 | Wang et al42 |
| CR 26 | Infection/febrile neutropenia NA | ||||||
| TRM 0 | |||||||
| GDP | III/IV, refractory, relapsed | 41 | ORR 83 | 1-y 55 | 1-y 73 | Neutropenia 34 | Wang et al43 |
| CR 42 | Infection/febrile neutropenia NA | ||||||
| TRM 0 |
CR = complete remission; DDGP = PEG-asparaginase, gemcitabine, cisplatin, and dexamethasone; GDP = gemcitabine, dexamethasone, and cisplatin; NA = not available; ORR = overall response rate; OS = overall survival; PEG = pegylated; PFS = progression-free survival; P-GEMOX = PEG-asparaginase, gemcitabine, and oxaliplatin; SMILE = dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide; TRM = treatment-related mortality.
Radiotherapy has no standard role in the treatment of stage III/IV disease, but some small series have reported positive trends when radiotherapy was used following chemotherapy in cases with a bulky mass.46 Patients with residual disease have reportedly converted to CR (6/8 patients converted from partial remission [PR] to CR) and the 2-year OS for the group undergoing radiotherapy was 81.5% compared to 40.2% with no radiotherapy.46 It should be noted that 67% of the patients in this 2015 study were treated with a nonasparaginase-based therapy. Nevertheless, further studies to establish the role of radiotherapy in stage III/IV disease are warranted.
Refractory or relapsed disease
Patients previously treated with SMILE or refractory to SMILE can be treated with gemcitabine and platinum-based regimens. Examples include P-GEMOX, possibly followed by consolidation with allogeneic hematopoietic cell transplantation (HCT). Rechallenge with an asparaginase-based therapy is possible if 6 months have passed since last treatment with SMILE, as a retrospective study by Lim et al47 showed a 50% response rate, whereas patients retreated within 6 months were nonresponders.
Autologous hematopoietic cell transplantation
In the prospective studies by Yamaguchi et al,31 Kwong et al,38 and Wang et al,42 a proportion of patients received either autologous HCT (Wang et al42 20%, Yamaguchi et al31 11%, and Kwong et al38 16%) or allogeneic HCT (Yamaguchi et al31 45% and Kwong et al38 9%) as a form of consolidation therapy. A prospective Korean study using a SMILE regimen and autologous HCT described transplants in 11 of 27 patients; the 2-year OS was 63% and the 2-year PFS was 55%.48 Of the 3 patients who reached CR but were not consolidated with an autologous HCT, 2 died due to relapse. The study by Kwong et al38 mentioned that of those who did not receive a consolidation, 41% stayed in CR. Two recent retrospective studies from the European Society for Blood and Marrow Transplantation and American Society for Blood and Marrow Transplantation showed that consolidation with an autologous HCT has no place in the first line in the case of stage I/II disease associated with good survival (>70%) following sandwich chemoradiotherapy.49,50 In stage III/IV disease, an autologous HCT may have added value and is recommended in both the European Society for Medical Oncology and NCCN guidelines.18,51
The number of cycles before transplantation is not established. In the Kwong et al38 and Yamaguchi et al31 studies, 2-3 cycles were administered before transplantation. In the study by Kim et al,48 3 cycles were administered before transplantation.
Allogeneic hematopoietic cell transplantation
In patients who underwent an earlier autologous HCT and relapsed or in patients who did not achieve CR after first line of therapy, an allogeneic HCT could be considered. There is limited (retrospective) data available on the role of allogeneic HCT. The largest series is found in a retrospective study based on data available at the Center for International Blood and Marrow Transplant Research (CIBMTR), gathered between 2000 and 2014.52 This study included 82 patients, with 30% receiving an allogeneic HCT in the first line and 60% after >1 line of therapy (10% unknown). Fifty-nine percent received reduced intensity conditioning and 38% myeloablative conditioning. The 3-year PFS and OS were 28% for the reduced intensity conditioning group and 34% for the myeloablative conditioning group, respectively. No further relapses were seen after 24 months, and there was no significant difference in PFS and OS between upfront allogeneic HCT versus transplantation at relapse. It should be noted that just 38% of the patients received asparaginase-based therapy before allogeneic HCT.52 A retrospective Asian study of allogeneic HCT in 18 patients with mostly stage III/IV or relapsed disease showed a 5-year OS of 51%, and no further relapses at 20 months.53
New therapeutic strategies
A new and promising therapy therapeutic option are the immune checkpoint inhibitors. ENKTL cells show upregulation of PD-L1, and 1 mechanism of action is via EBV triggering of latent membrane protein 1 (LMP1), which then upregulates PD-L1 expression through activation of the mitogen-activated protein kinases pathway/nuclear factor kappa B pathway (Figure 4).10 This suggests that programmed cell death protein 1/PD-L1 inhibitors are a rational choice. In 5 small retrospective series, responses of up to 100% and CR rates of up to 71% were found, despite extensive earlier treatment with L-asparaginase and platinum-based therapy.54-57 The 2 largest series, reported by Kwong et al54 and Couronné et al,57 included 7 and 13 patients, respectively. In the Kwong et al54 study, 5 patients reached CR (2 after previous allogeneic HCT) and 2 achieved PR. In the Couronné et al57 series, only 3 of 13 patients reached CR and 2 PR. Another study, in this case from China (Orient-4), used an anti-programmed cell death protein 1 antibody, sintilimab, and reported CR or PR in 68% of the patients.58 Several studies of sintilimab are ongoing in China (NCT04004572, NCT04127227, NCT03936452), and phase II studies are currently being conducted in the United States using pembrolizumab in patients with NK/T-cell lymphoma, including both stage I/II and III/IV disease (NCT03728972, NCT03586024).
Figure 4.
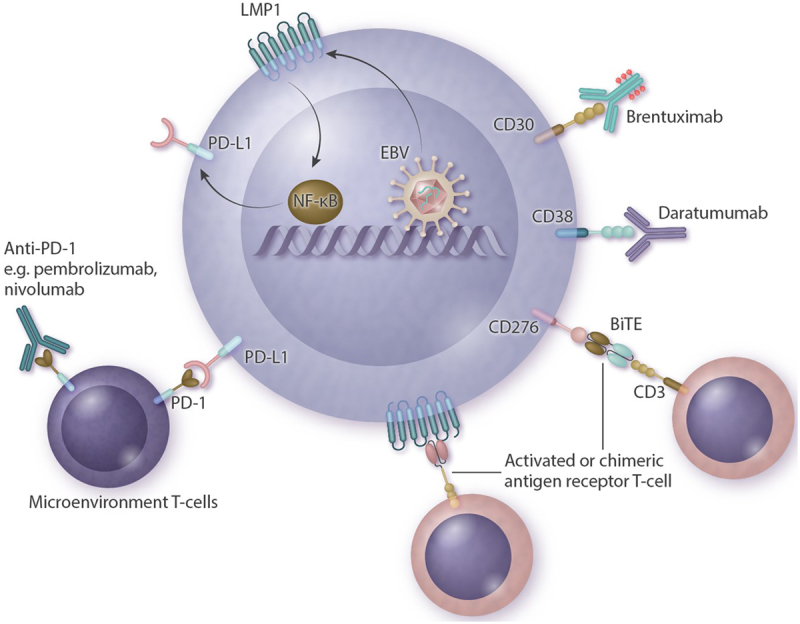
Summary of possible new therapeutic strategies with mechanisms of action. Antibody drugs target proteins on the cellular membrane and include brentuximab-vedotin (directed against CD30) and daratumumab (directed against CD38). Anti-PD-1 antibodies (pembrolizumab, nivolumab) target microenvironmental T-cells that become inactivated when bound to PD-L1 expressed on a lymphoma. LMP1 is a transmembrane protein produced by EBV that activates the NF-kB pathway and leads to proliferation and lymphomagenesis. This, in turn, upregulates PD-L1. Other possible targets are CAR-T or BiTE directed against LMP1 or CD276 (B7-H3). BiTE = bispecific antigen engager; CAR-T = chimeric antigen receptor T-cells; EBV = Epstein-Barr virus; LMP1 = latent membrane protein 1; NF-kB = nuclear factor kappa B; PD-1 = programmed cell death protein 1; PD-L1 = programmed death-ligand 1.
Other potential targets include CD38 and CD30. Two case reports have described successful treatment with daratumumab (an antibody directed against CD38),59,60 although a phase II study with daratumumab monotherapy showed barely any response. Of the 32 patients included in the latter study, the responses included 0 reaching CR, 8 PR, 5 stable disease, 14 progressive disease, and 5 patients were not evaluable.61 Median response duration was 55 days. In addition, 2 case reports have described treatment with brentuximab-vedotin (antibody-drug conjugate directed against CD30), 1 as a monotherapy and 1 in combination with bendamustine, but both with reportedly good responses.62,63
Studies are currently underway using chimeric antigen receptor T-cells directed against LMP1.64 In an earlier study with autologous cytotoxic T-lymphocytes directed against LMP1, 3 out of 11 patients achieved long-term remission.65 Promising results were also seen in vitro and in mice with chimeric antigen receptor T-cells and bispecific T-cell engager directed against B7-H3 (CD276).66
As Xiong et al67 demonstrated in their recent publication, mutations in ENKTL are very heterogeneous with different subtypes and with different responses to therapy. More personalized treatment approached will probably be developed in the future. A possible target might be the Janus kinases/signal transducer and activator of transcription protein pathway, especially as Janus kinases 3/signal transducer and activator of transcription protein 3 and STAT5B seem to be upregulated in ENKTL.68-70
Follow-up
After intensive treatment, strict follow-up is important and the focus should lie on toxicity following chemotherapy and radiotherapy, together with early identification of recurrent disease. Standard follow-up should include plasma EBV-PCR, as any sign of an increase in EBV means that relapse is inventible, even when a patient has been in remission for years.3 Serial measurement of EBV load is therefore strongly recommended. Toxicity following chemotherapy is highly dependent on the chemotherapy schedule used. One very specific radiotherapy-related toxicity is the development of obstruction of the nasopharynx and nasal secretion, which might be due to fibrosis and inflammation but could also be a sign of recurrent disease. To further differentiate between fibrosis and recurrence or residual disease, an FDG PET-CT should be performed. Within the first months after treatment, a positive FDG PET-CT is not uncommon due to posttreatment inflammation. In case of FDG-PET positive lesions, a biopsy should be taken. In case of nasal obstruction, a nasal lavage or cleavage of fibrosis is often needed.
Disclosures
The authors declare no competing interest.
References
- 1.van Doesum JA, Nijland M, Brink M, Huls GA, van Meerten T. A West-European cohort of patients with NK/T-cell lymphoma, a population-based analysis. HemaSphere. 2020; 4S1EP1238 [Google Scholar]
- 2.Karkera AC, Marshall Parson B, Borgert A, Go RS. NK/T cell lymphoma in the U.S.: a population-based study using the national cancer database from 1998-2012. J Clin Oncol. 2016; 3415_supple19038 [Google Scholar]
- 3.Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood. 2013; 121:4997–5005 [DOI] [PubMed] [Google Scholar]
- 4.de Mel S, Soon GS, Mok Y, et al. The genomics and molecular biology of natural killer/T-cell lymphoma: opportunities for translation. Int J Mol Sci. 2018; 19:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Zhu Y, Cao JZ, et al. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood. 2015; 126:1424–1432; quiz 1517 [DOI] [PubMed] [Google Scholar]
- 6.Cheung MM, Chan JK, Lau WH, et al. Primary non-Hodgkin’s lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol. 1998; 16:70–77 [DOI] [PubMed] [Google Scholar]
- 7.Jia J, Song Y, Lin N, et al. Clinical features and survival of extranodal natural killer/T cell lymphoma with and without hemophagocytic syndrome. Ann Hematol. 2016; 95:2023–2031 [DOI] [PubMed] [Google Scholar]
- 8.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127:2375–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Wang H, Li PF, et al. CD38 expression predicts poor prognosis and might be a potential therapy target in extranodal NK/T cell lymphoma, nasal type. Ann Hematol. 2015; 94:1381–1388 [DOI] [PubMed] [Google Scholar]
- 10.Hu B, Oki Y. Novel immunotherapy options for extranodal NK/T-cell lymphoma. Front Oncol. 2018; 8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khong PL, Pang CB, Liang R, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008; 87:613–621 [DOI] [PubMed] [Google Scholar]
- 12.Chan WK, Au WY, Wong CY, et al. Metabolic activity measured by F-18 FDG PET in natural killer-cell lymphoma compared to aggressive B- and T-cell lymphomas. Clin Nucl Med. 2010; 35:571–575 [DOI] [PubMed] [Google Scholar]
- 13.Kwong YL, Anderson BO, Advani R, et al. ; Asian Oncology Summit. Management of T-cell and natural-killer-cell neoplasms in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009; 10:1093–1101 [DOI] [PubMed] [Google Scholar]
- 14.Au WY, Pang A, Choy C, et al. Quantification of circulating Epstein-Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood. 2004; 104:243–249 [DOI] [PubMed] [Google Scholar]
- 15.Suzuki R, Yamaguchi M, Izutsu K, et al. ; NK-cell Tumor Study Group. Prospective measurement of Epstein-Barr virus-DNA in plasma and peripheral blood mononuclear cells of extranodal NK/T-cell lymphoma, nasal type. Blood. 2011; 118:6018–6022 [DOI] [PubMed] [Google Scholar]
- 16.Hong H, Li Y, Lim ST, et al. A proposal for a new staging system for extranodal natural killer T-cell lymphoma: a multicenter study from China and Asia Lymphoma Study Group. Leukemia. 2020; 34:2243–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016; 17:389–400 [DOI] [PubMed] [Google Scholar]
- 18.Horwitz SM, Ansell SM, Ai WZ, et al. NCCN guidelines insights: T-cell lymphomas, version 2.2018. J Natl Compr Canc Netw. 2018; 16:123–135 [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood. 2018; 131:2528–2540 [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Wu T, Zhu SY, et al. Risk-dependent conditional survival and failure hazard after radiotherapy for early-stage extranodal natural killer/T-cell lymphoma. JAMA Netw Open. 2019; 2:e190194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vargo JA, Patel A, Glaser SM, et al. The impact of the omission or inadequate dosing of radiotherapy in extranodal natural killer T-cell lymphoma, nasal type, in the United States. Cancer. 2017; 123:3176–3185 [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Cao JZ, Lan SM, et al. Association of improved locoregional control with prolonged survival in early-stage extranodal nasal-type natural killer/T-cell lymphoma. JAMA Oncol. 2017; 3:83–91 [DOI] [PubMed] [Google Scholar]
- 23.Tomita N, Kodaira T, Tachibana H, et al. A comparison of radiation treatment plans using IMRT with helical tomotherapy and 3D conformal radiotherapy for nasal natural killer/T-cell lymphoma. Br J Radiol. 2009; 82:756–763 [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Huang E, Wang Y, et al. Dosimetric comparison of helical tomotherapy, VMAT, fixed-field IMRT and 3D-conformal radiotherapy for stage I-II nasal natural killer T-cell lymphoma. Radiat Oncol. 2017; 12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng YD, Cutter DJ, Plastaras JP, et al. Evidence-based review on the use of proton therapy in lymphoma from the Particle Therapy Cooperative Group (PTCOG) lymphoma subcommittee. Int J Radiat Oncol Biol Phys. 2017; 99:825–842 [DOI] [PubMed] [Google Scholar]
- 26.Yahalom J, Illidge T, Specht L, et al. ; International Lymphoma Radiation Oncology Group. Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015; 92:11–31 [DOI] [PubMed] [Google Scholar]
- 27.Müller HJ, Boos J. Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998; 28:97–113 [DOI] [PubMed] [Google Scholar]
- 28.Panosyan EH, Grigoryan RS, Avramis IA, et al. Deamination of glutamine is a prerequisite for optimal asparagine deamination by asparaginases in vivo (CCG-1961). Anticancer Res. 2004; 24:1121–1125 [PubMed] [Google Scholar]
- 29.Ando M, Sugimoto K, Kitoh T, et al. Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol. 2005; 130:860–868 [DOI] [PubMed] [Google Scholar]
- 30.Jaccard A, Petit B, Girault S, et al. L-asparaginase-based treatment of 15 western patients with extranodal NK/T-cell lymphoma and leukemia and a review of the literature. Ann Oncol. 2009; 20:110–116 [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011; 29:4410–4416 [DOI] [PubMed] [Google Scholar]
- 32.Kwong YL, Kim SJ, Tse E, et al. Sequential chemotherapy/radiotherapy was comparable with concurrent chemoradiotherapy for stage I/II NK/T-cell lymphoma. Ann Oncol. 2018; 29:256–263 [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi M, Tobinai K, Oguchi M, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol. 2009; 27:5594–5600 [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi M, Suzuki R, Oguchi M, et al. Treatments and outcomes of patients with extranodal natural killer/T-cell lymphoma diagnosed between 2000 and 2013: a cooperative study in Japan. J Clin Oncol. 2017; 35:32–39 [DOI] [PubMed] [Google Scholar]
- 35.Kim SJ, Yang DH, Kim JS, et al. Concurrent chemoradiotherapy followed by L-asparaginase-containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08-01 phase II study. Ann Hematol. 2014; 93:1895–1901 [DOI] [PubMed] [Google Scholar]
- 36.Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009; 27:6027–6032 [DOI] [PubMed] [Google Scholar]
- 37.Yoon DH, Kim SJ, Jeong SH, et al. Phase II trial of concurrent chemoradiotherapy with L-asparaginase and MIDLE chemotherapy for newly diagnosed stage I/II extranodal NK/T-cell lymphoma, nasal type (CISL-1008). Oncotarget. 2016; 7:85584–85591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012; 120:2973–2980 [DOI] [PubMed] [Google Scholar]
- 39.Qi S, Yahalom J, Hsu M, et al. Encouraging experience in the treatment of nasal type extra-nodal NK/T-cell lymphoma in a non-Asian population. Leuk Lymphoma. 2016; 57:2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Wang ZH, Chen XQ, et al. First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer. 2013; 119:348–355 [DOI] [PubMed] [Google Scholar]
- 41.Salzer WL, Asselin B, Supko JG, et al. Erwinia asparaginase achieves therapeutic activity after pegaspargase allergy: a report from the Children’s Oncology Group. Blood. 2013; 122:507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang JH, Wang L, Liu CC, et al. Efficacy of combined gemcitabine, oxaliplatin and pegaspargase (P-gemox regimen) in patients with newly diagnosed advanced-stage or relapsed/refractory extranodal NK/T-cell lymphoma. Oncotarget. 2016; 7:29092–29101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang JJ, Dong M, He XH, et al. GDP (gemcitabine, dexamethasone, and cisplatin) is highly effective and well-tolerated for newly diagnosed stage IV and relapsed/refractory extranodal natural killer/T-cell lymphoma, nasal type. Medicine (Baltimore). 2016; 95:e2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Cui Y, Sun Z, et al. DDGP versus SMILE in newly diagnosed advanced natural killer/T-cell lymphoma: a randomized controlled, multicenter, open-label study in China. Clin Cancer Res. 2016; 22:5223–5228 [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Zhang L, Liu X, et al. Efficacy and survival in newly diagnosed advanced extranodal natural killer/T-cell lymphoma: a randomized, controlled, multicenter and open-labled study with Ddgp regimen versus SMILE regimen. Blood. 2019; 134suppl_1463–463 [Google Scholar]
- 46.Bi XW, Jiang WQ, Zhang WW, et al. Treatment outcome of patients with advanced stage natural killer/T-cell lymphoma: elucidating the effects of asparaginase and postchemotherapeutic radiotherapy. Ann Hematol. 2015; 94:1175–1184 [DOI] [PubMed] [Google Scholar]
- 47.Lim SH, Hong JY, Lim ST, et al. Beyond first-line non-anthracycline-based chemotherapy for extranodal NK/T-cell lymphoma: clinical outcome and current perspectives on salvage therapy for patients after first relapse and progression of disease. Ann Oncol. 2017; 28:2199–2205 [DOI] [PubMed] [Google Scholar]
- 48.Kim SJ, Park S, Kang ES, et al. Induction treatment with SMILE and consolidation with autologous stem cell transplantation for newly diagnosed stage IV extranodal natural killer/T-cell lymphoma patients. Ann Hematol. 2015; 94:71–78 [DOI] [PubMed] [Google Scholar]
- 49.Fox CP, Boumendil A, Schmitz N, et al. High-dose therapy and autologous stem cell transplantation for extra-nodal NK/T lymphoma in patients from the Western hemisphere: a study from the European Society for Blood and Marrow Transplantation. Leuk Lymphoma. 2015; 56:3295–3300 [DOI] [PubMed] [Google Scholar]
- 50.Yhim HY, Kim JS, Mun YC, et al. ; Consortium for Improving Survival of Lymphoma Study. Clinical outcomes and prognostic factors of up-front autologous stem cell transplantation in patients with extranodal natural killer/T cell lymphoma. Biol Blood Marrow Transplant. 2015; 21:1597–1604 [DOI] [PubMed] [Google Scholar]
- 51.d’Amore F, Gaulard P, Trumper L, et al. ; ESMO Guidelines Committee. Peripheral T-cell lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015; 26suppl 5v108–115 [DOI] [PubMed] [Google Scholar]
- 52.Kanate AS, DiGilio A, Ahn KW, et al. Allogeneic haematopoietic cell transplantation for extranodal natural killer/T-cell lymphoma, nasal type: a CIBMTR analysis. Br J Haematol. 2018; 182:916–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tse E, Chan TS, Koh LP, et al. Allogeneic haematopoietic SCT for natural killer/T-cell lymphoma: a multicentre analysis from the Asia Lymphoma Study Group. Bone Marrow Transplant. 2014; 49:902–906 [DOI] [PubMed] [Google Scholar]
- 54.Kwong YL, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017; 129:2437–2442 [DOI] [PubMed] [Google Scholar]
- 55.Li X, Cheng Y, Zhang M, et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 2018; 11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan TSY, Li J, Loong F, et al. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: efficacy and safety. Ann Hematol. 2018; 97:193–196 [DOI] [PubMed] [Google Scholar]
- 57.Couronné L, Chaubard S, Bruneau J, et al. PD-1 blockade in a French series of 13 relapsed/refractory NK/T-cell lymphoma patients. Hematol Oncol. 2019; 37:272–273 [Google Scholar]
- 58.Tao R, Fan L, Song Y, et al. Sintilimab for relapsed/refractory (R/R) extranodal NK/T cell lymphoma (ENKTL): a multicenter, single-arm, phase 2 trail (ORIENT-4). J Clin Oncol. 2019; 37:389–390 [Google Scholar]
- 59.Hari P, Raj RV, Olteanu H. Targeting CD38 in refractory extranodal natural killer cell-T-cell lymphoma. N Engl J Med. 2016; 375:1501–1502 [DOI] [PubMed] [Google Scholar]
- 60.Aeppli S, Driessen C, Graf L, et al. Systemic treatment of a patient with relapsed and refractory extranodal NK/T-cell lymphoma (ENKL) and meningeosis leukemica with daratumumab. Hematol Oncol. 2018; 36:713–714 [DOI] [PubMed] [Google Scholar]
- 61.Huang H-q, Kim W-S, Yao M, et al. Daratumumab monotherapy for patients with relapsed or refractory (R/R) natural killer/T-cell lymphoma (NKTCL), nasal type: updated results from an open-label, single-arm, multicenter phase 2 study. Blood. 2019; 134suppl_1156831698420 [Google Scholar]
- 62.Kim HK, Moon SM, Moon JH, et al. Complete remission in CD30-positive refractory extranodal NK/T-cell lymphoma with brentuximab vedotin. Blood Res. 2015; 50:254–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poon LM, Kwong YL. Complete remission of refractory disseminated NK/T cell lymphoma with brentuximab vedotin and bendamustine. Ann Hematol. 2016; 95:847–849 [DOI] [PubMed] [Google Scholar]
- 64.Prockop S, Reshef R, Tsai DE, et al. Long-term outcomes of subjects with Epstein-Barr virus-driven post-transplant lymphoproliferative disorder (EBV+PTLD) following solid organ (SOT) or allogeneic hematopoietic cell transplants (HCT) treated with tabelecleucel on an expanded access program. Blood. 2019; 134suppl_14071 [Google Scholar]
- 65.Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014; 32:798–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng M, Yu L, Hu J, et al. Efficacy of B7-H3-redirected BiTE and CAR-T immunotherapies against extranodal nasal natural killer/T cell lymphoma. Transl Oncol. 2020; 13:100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiong J, Cui BW, Wang N, et al. Genomic and transcriptomic characterization of natural killer T cell lymphoma. Cancer Cell. 2020; 37:403–419.e6 [DOI] [PubMed] [Google Scholar]
- 68.Koo GC, Tan SY, Tang T, et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2012; 2:591–597 [DOI] [PubMed] [Google Scholar]
- 69.Küçük C, Jiang B, Hu X, et al. Activating mutatio~ns of STAT5B and STAT3 in lymphomas derived from γδ-T or NK cells. Nat Commun. 2015; 6:6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee S, Park HY, Kang SY, et al. Genetic alterations of JAK/STAT cascade and histone modification in extranodal NK/T-cell lymphoma nasal type. Oncotarget. 2015; 6:17764–17776 [DOI] [PMC free article] [PubMed] [Google Scholar]


