Abstract
Exposure to polycyclic aromatic hydrocarbons (PAHs) is a risk factor for human health. Workers are a vulnerable group due to their high exposure and therefore require special attention to mitigation measurements; however, some groups of workers are especially vulnerable, precarious workers. The objective of this research was to evaluate mixtures of hydroxylated PAHs (OH-PAHs) in precarious workers in Mexico. The following activities were evaluated: (i) brickmakers (TER), stonemasons (ESC), indigenous workers (TOC) and mercury miners (CAM). Ten OH-PAHS were analyzed: 1-hydroxynaphtalene and 2-hydroxynaphtalene; 2-,3- and 9-hydroxyfluorene; 1-,2-,3- and 4-hydroxyphenanthrene; and 1-hydroxypyrene in urine by GC-MS, chemical fingerprints of the sites were established by multivariate analysis. One hundred forty-nine precarious workers participated in the study. The populations presented total OH-PAHs concentrations of 9.20 (6.65–97.57), 14.8 (9.32–18.85), 15.7 (6.92–195.0), and 101.2 (8.02–134.4) μg/L for CAM, ESC, TER, and TOC, respectively (median (IQR)). The results of the multivariate analysis indicate that the indigenous population presented a different fingerprint compared to the three scenarios. The chemical fingerprints among the brickmakers and mercury mining population were similar. The results of the concentrations were similar and in some metabolites higher than workers in occupations classified as carcinogenic by the IARC; therefore, the control of exposure in these occupations acquires great importance and surveillance through biological monitoring of OH-PAHs should be applied to better estimate exposure in these working populations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-021-12413-y.
Keywords: Polycyclic aromatic hydrocarbons, Chemical fingerprints, Precarious work
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a group of permanent organic pollutants that include two or more benzene rings fused together. They are a ubiquitous group of several hundred chemically related compounds, environmentally permanent with diverse structures and broad toxicity (Kim et al. 2013). These compounds are mainly generated during incomplete combustion of organic material; they occur by natural generation (e.g., forest fires, volcanic eruptions); however, the predominant sources are anthropogenic activities involving the burning of fossil fuels, industrial processes, automobile emissions, and cooking with biomass, among others (Kim et al. 2013).
PAHs containing up to four rings are called “light” and those containing more than four rings are called “heavy”. Heavy PAHs are more stable and toxic than light ones, specific PAHs concentration ratios are widely used for qualitative source determination and are commonly used to determine concentrations in air, soils, and sediments (Dat and Chang 2017). Besides, they distinguish between pollution from oil products, oil combustion, and the combustion of biomass or coal. The compounds involved in each relation have the same molecular weight, so they are assumed to present similar physicochemical properties. The Agency for Toxic Substances and Disease Registry (ATSDR) of the USA states that the produced health effects by each one of the PAHs are not the same; the following 16 PAHs are considered in a cluster for complete monitoring: acenaphthene, acenaphthylene, anthracene, benzo[a]anthracene, benzo[a]pyrene, benzo[e]pyrene, benzo[b]fluoranthene, benzo[g,h,i]perylene, benzo[j]fluoranthene, benzo[k]fluoranthene, chrysene, dibenzo[a,h]anthracene, fluoranthene, fluorine, indeno[1,2,3-c,d]pyrene, phenanthrene, and pyrene (ASTDR 1995). These 16 PAHs are suspected of being more toxic than the others and exhibit harmful effects that are representative of PAHs; there is also a greater possibility of greater exposure to these compounds because they are produced in higher concentrations.
On the other hand, the health effects of individual PAHs are not the same. In fact, the International Agency for Research on Cancer (IARC) classifies some PAHs as known, possibly or probably carcinogenic to humans (group 1, 2A, or 2B). These include benzo [a] pyrene (group 1), naphthalene, chrysene, benz [a] anthracene, benzo [k] fluoranthene, and benzo [b] fluoranthene (group 2B) (IARC 2010).
The exposure pathways to PAHs can be by inhalation, ingestion, and dermal exposure, through multiple routes and sources (Alomirah et al. 2011, Bhargava et al. 2004, Liu et al. 2007). Within the main exposure, scenarios are the occupational environments, where it has been reported greater exposure to high concentrations of PAHs; in addition, epidemiological evidence has shown that in exposed workers, especially in coke ovens and aluminum foundries, have shown high incidences of lung cancer and very suggestive excesses of bladder cancer (Kogevinas 2020, Petit et al. 2019, Shankar et al. 2019, Vimercati et al. 2020). IARC has reported two mixtures containing PAHs (coal tar soot, coal tar pitch) and occupational exposures in four PAH-related industries (coal tar distillation, coal gasification, coke production, aluminum production) as group 1 carcinogens (Armstrong et al. 2004, IARC 2010, Rota et al. 2014).
The biomonitoring is a powerful tool for population studies, the determination of hydroxylated metabolites in urine is performed to measure exposure to PAHs in humans, and these compounds have relatively short half-life elimination times that range from 5 to 35 h and are considered useful for estimating recent exposures to PAHs (Grova et al. 2017, Guo et al. 2013, Woudneh et al. 2016).
There are several biomonitoring studies of PAHs exposure that include coke oven workers, aluminum smelter workers, foundry workers, and road workers (Campo et al. 2020, Iamiceli et al. 2020, Persoons et al. 2020, Zhang et al. 2020). It is evident that workers are a vulnerable group, requiring special attention to mitigation measurements; however, some groups of workers are especially vulnerable; those so-called precarious works, characterized by abuse of their labor rights (low salaries, without access to social security, health services, lack of training, null protection, among many other factors) (Julià et al. 2017, Kachi et al. 2014, Medina-Gómez and López-Arellano 2019) and which also present health risks due to high exposure to pollutants. The above, in scenarios with high degrees of marginalization, where they generally perform their work activities within the household, exposing the worker and in consequence their families (Flores-Ramirez et al. 2018, Pérez-Herrera et al. 2019). Therefore, the objective of this research was to evaluate the mixtures of hydroxylated PAHs in precarious workers of four activities with high toxicity in Mexico.
Materials and methods
Population and study design
The present study was conducted in four work scenarios under precarious conditions: (i) Artisanal mercury mine workers (CAM) are located in the community of Camargo, Peñamiller, in the state of Querétaro, Mexico (21° 06′ 45″ N, 99° 43′ 55″ W). It has a total population of 852 inhabitants; in this area, mercury has been extracted since pre-colonial times; the extraction process is still rudimentary. After the cinnabar (mercury sulfide) material is obtained from the mines, it is crushed, sieved, and incinerated in a clay oven with wood; the mercury is collected once the vapors generated in the oven are cooled (Fig. 1a). (ii) Stonemasons (ESC) are located in the community of Escalerillas; this area is the main quarrying region in the state of San Luis Potosi, Mexico (22° 06′ 40″ N, 101° 04′ 36″ W); with a total population of 6226 inhabitants, the locality presents a high degree of marginalization. The main economic activity of the region is the work of artisan quarry and its exposure to PAHs is by the use of fuels for its cutters and high vehicle traffic (Fig. 1b). (iii) Brickmakers (TER) are located in the brick zone “Las terceras” in San Luis Potosi, Mexico (22° 12′ 04″ N, 100° 51′ 26″ W); the municipality has a population of 824,229 inhabitants; in this scenario, there are more than 120 brick kilns, which use wood tires, waste, used oil, and plastics as fuel. (Flores-Ramirez et al. 2018) (Fig. 1c). And (iv) indigenous workers (TOC) are the community of the Tocoy area that is located in the municipality of San Antonio in the state of San Luis Potosi, Mexico. This community of the Huasteca Potosina is characterized by a high degree of marginalization, is a recognized area of the Tenek ethnic group and has a population of approximately 1061 people. (Díaz de León-Martínez et al. 2019). The main economic activities in the area are agriculture (maize, sugarcane, beans), domestic work, and the main source of exposure to PAHs is through the use of biomass for cooking, the burning of garbage, and the practice of slash-and-burn to prepare the land for harvesting (Fig. 1d).
Fig. 1.
Different occupational exposure scenarios in the study. a Miners (CAM), b brickmakers (TER), c stonemasons (ESC), and d indigenous people (TOC)
The protocol was approved by the Research Ethics Committee of the Faculty of Medicine of the Autonomous University of San Luis Potosí and the Bioethics Commission of the State of San Luis Potosi (CEI-2018-002). A cross-sectional sampling was conducted in November and December 2019 and January 2020. An open invitation was extended to the population considering the following inclusion criteria: (i) older than 30 years, (ii) residence of over three years at the site of study, (iii) signature of informed consent, and (iv) to be active workers in the study communities. Women who reported menarche at the time of the sample collection were excluded from the study. Anthropometric data were collected from the study population (height and weight, BMI); also, a questionnaire was applied in which socioeconomic status, risk activities, exposure to pollutants, and general health status were assessed.
Sample collection
The study participants were required to collect the first morning’s urine under 8-h fasting conditions and at the end of the working week, the sample was collected in sterile 50 mL polypropylene cups and then transported at 4 °C, after which aliquots were made for the determination of the following proteins and metabolites: (a) for the determination of monohydroxy-polycyclic aromatic hydrocarbons (OH-PAH) and (b) for general urine analysis. All samples were frozen at − 80 °C until further analysis.
Determination of OH-PAHs in urine
OH-PAHs determination in urine was performed with some modifications of the methodology established by the Center for Disease Control and Prevention (CDC) for the determination of Monohydroxy-Polycyclic Aromatic Hydrocarbons by gas chromatography-mass spectrometry-electronic impact ionization (GC-MS-EI) (CDC 2013). Ten hydroxylated metabolites were analyzed, 1-hydroxynaphtalene (1-OH-NAP) and 2-hydroxynaphtalene (2-OH-NAP); 2-,3- and 9-hydroxyfluorene (2-OH-FLU, 3-OH-FLU, 9-OH-FLU); 1-,2-,3- and 4-hydroxyphenanthrene (1-OH-PHE, 2-OH-PHE, 3-OH-PHE, 4-OH-PHE); and 1-hydroxypyrene (1-OH-PYR). The analytical standards were obtained from LCG standards (Dr. Ehrenstrofer reference materials). In brief, enzymatic hydrolysis of 2 mL of previously filtered urine was first performed, 20 μL of the enzyme β-glucuronidase/arylsulfatase (Merck Millipore, Massachusetts, USA) and 2 mL of acetate buffer (1 M, pH 5.5) were added, and the samples were afterwards incubated for 17 h at 37 °C under constant agitation. Following incubation, liquid-liquid extraction was performed with a solution of pentane and toluene (80:20 v:v), which was then evaporated under a stream of nitrogen at 45 °C to a volume of 10 μL; then, 10 μL of N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) derivatizing agent (Merck Millipore, Massachusetts, USA) and 2.5 μL of internal standard 13C6 1-OH-PYR (Cambridge Isotope Laboratories) at 25 ng/mL were added, and the solution was calibrated to 100 μL with toluene. Subsequently, the solution was subjected to a derivatization process at 60 °C for 30 min. The samples and calibration curves were analyzed by gas chromatography (GC) (Agilent 6890) coupled to a mass spectrometry detector (MS) (Agilent 5975) in electron impact ionization mode (EI). The injection port was operated in splitless mode, its temperature was of 270 °C; helium was used as carrier gas at a pressure of 36 psi with a constant flow of 0.9 mL/min. The chromatographic separation was carried through an HP 5MS (30 m × 0.25 mm × 0.25 μm) column (Agilent). The conditions of the oven settings were as follows: 95 °C (1 min), 195 °C (15 °C/min), 206 °C (2 °C/min) with a hold until minute 13.2, then an increase to 320 °C (40 °C/min) and held to minute 24 with a total run time of 24 min. The tune parameters were emission, 35 μA, and energy, 69.9. SCAN mode (50–500 m/z) was employed to identify the compound, identification, and quantification ions were selected for SIM mode. The identified fragment ions were for 1-OH-NAP and 2-OH-NAP 201 and 216 m/z; for 2-OH-FLU and 9-OH-FLU 253 and 254 m/z; for 3-OH-FLU 253, 254 and 255 m/z; for 1-OH-PHE, 2-OH-PHE, 3-OH-PHE, and 4-OH-PHE 251 and 266 m/z; and for 1-OH-PYR 290 and 291 m/z and the internal standard 13C6 1-OH-PYR 281 and 296 m/z. Results were obtained and processed using Chemstation Software (Agilent®).
Analytical method validation
The method validation was performed according to the AOAC/FAO/IAEA/IUPAC Guidelines for the Validation of Analytical Methods for the Determination of Organic Compounds at Trace Levels (Alder et al. 2000) The parameters were linearity (correlation coefficient r2), sensitivity, the limit of detection and limit of quantification, precision measured by repeatability (n = 3), and reproducibility (n = 7) at low (10 ng/mL) and high (50 ng/mL) concentrations. The accuracy (percentage of recovery) of the analytical method was evaluated by the certified standard for 1-OH-PYR in urine CLINCHEK® Level I.
Statistical analysis
For the description of the quantitative variables, a test of normality of the data was performed using Shapiro-Wilk analysis. To establish the differences between the groups, Kruskal-Wallis’ tests with Dunn’s post hoc were applied.
To evaluate the OH-PAHs fingerprint of the study groups, principal component analysis (PCA) and canonical analysis of principal coordinates (CAP) were carried out. The PCA was obtained through the multivariate data cloud that was the best to discriminate between predefined groups (CAM, ESC, TER, and TOC). The CAP procedure included cross-validation to leave out a procedure to predict group belonging and thus obtain overall classification success rates. The analysis was performed using XLSTAT version 12.0 (StatSoft®, Tulsa, Oklahoma, USA) and the Primer 7+ Permanova add-on software package (v7.0.12 and v1.0.6; PRIMER-E Lt., Ivybridge, UK) multivariate analysis.
Results
General characteristics of the study population
One hundred and forty-nine precarious workers participated in the study, 31 miners from CAM, 36 stonemasons from ESC, 42 brickmakers from TER, and 40 indigenous workers from TOC, of whom 80% of the population were men and 20% women. The socioeconomic level of the participants is considered to be highly marginalized and the working conditions in all 4 scenarios of the study are considered precarious. The average age was 50.05 ± 13.83 years, the average body mass index was 27.18 ± 3.8 kg/m2 with 48.3% being overweight, 29.5% being obese, and 28.2% in normal weight (Table 1). With the information collected through the questionnaires, it is reported that 100% of the study population performs 3 or more risk activities at work associated with exposure to the evaluated environmental pollutants. As a result, 35.5, 77.7, 14.3, and 7.3% of CAM, ESC, TER, and TOC, respectively, were reported as active smokers, in parallel, an average of 9 h of work per day is reported.
Table 1.
Anthropometric characteristics and risk activities of study populations
| Parameter | Population | ||||
|---|---|---|---|---|---|
| Miners (CAM) | Stonemasons (ESC) | Brickmakers (TER) | Indigenous (TOC) | ||
| Number of subjects | n = 149 | 31 | 36 | 42 | 40 |
| Sex (%) | Male | 100 | 100 | 100 | 25 |
| Female | 0 | 0 | 0 | 75 | |
| Age (years) | 45.1 ± 14.7 | 43.8 ± 10.9 | 55.4 ± 15.8 | 53.8 ± 9.5 | |
| Height (m) | 1.65 ± 7.5 | 1.66 ± 5.8 | 1.64 ± 5.3 | 1.51 ± 6.4 | |
| Weight (Kg) | 72.5 ± 13.8 | 74.0 ± 8.9 | 78.7 ± 13.9 | 58.8 ± 6.1 | |
| BMI | 26.7 ± 4.3 | 26.9 ± 2.8 | 29.3 ± 4.5 | 25.5 ± 2.2 | |
| Obese (%) | 22.6 | 13.9 | 35.7 | 5 | |
| Normal (%) | 35.5 | 25 | 14.3 | 40 | |
| Overweight (%) | 41.9 | 61.1 | 50 | 55 | |
| Risk Activities (%) | Smoke exposure | 100 | 100 | 100 | 100 |
| Tobacco smoking | 35.5 | 77.7 | 14.3 | 7.3 | |
| Average smoke exposure hours (per day) | 6 | 8.1 | 13.1 | 8.6 | |
All data are presented as mean ± standard deviation and percentages
The analytical method for the determination of OH-PAHs in urine (GC-MS)
The following retention times (RT) were obtained for each of the compounds (RT ± 0.2 min), for 1-OH-NAP RT = 7.6 min, 2-OH-NAP RT = 7.9 min, 9-OH-FLU RT = 12.3 min, 3-OH-FLU RT = 12.5 min, 2-OH-FLU RT = 12.9 min, 4-OH-PHE RT = 14.8 min, 3-OH-PHE RT = 16.5, 1-OH-PHE RT = 16.7, 2-OH-PHE RT = 17.6 min, and 1-OH-PYR and 13C6 1-OH-PYR RT = 23.2 min.
The results of the validation of each compound are shown in Table 1 of the supplementary material. The analytical method achieved linearity of r2 = 0.99 for the 10 compounds for calibration curves of 0.25–100 μg/L. The limits of detection and quantification of the compounds on the range of 0.01–0.5 μg/L. The precision range of the method for the analytes measured as repeatability was obtained for low concentrations (10 μg/L) from 5.3 to 17.4% and high concentrations (50 μg/L) from 0.7 to 21.4% and as reproducibility from 4.2 to 23.6% and 5.2 to 21.4%, respectively. The recovery rate evaluated by the certified standard for 1-OH-PYR was 101.8%.
Assessment of OH-PAHs in urine
The results of exposure to OH-PAHs in urine are presented in Table 2; 100% of the study population presented urinary concentrations of at least nine of the assessed biomarkers. The biomarkers were shown in different concentrations and frequencies in the four-study populations, for CAM in the following order of frequency (most frequent to least frequent) 1-OH-PYR>2-OH-NAP>1-OH-NAP>2-OH-FLU>1-OH-PHE>3-OH-FLU>3-OH-PHE>4-OH-PHE>9-OH-FLU>2-OH-PHE; for ESC, 1-OH-PYR>2-OH-NAP>1-OH-NAP>4-OH-PHE>2-OH-PHE>3-OH-FLU>2-OH-FLU>9-OH-FLU>1-OH-PHE>3-OH-PHE; for TER, 1-OH-PYR>1-OH-NAP>2-OH-NAP>9-OH-FLU>2-OH-FLU>4-OH-PHE>1-OH-PHE>3-OH-FLU>3-OH-PHE>2-OH-PHE; and for TOC, 1-OH-PYR>4-OH-PHE>2-OH-NAP>1-OH-NAP>9-OH-FLU>3-OH-FLU>2-OH-FLU>3-OH-PHE>1-OH-PHE>2-OH-PHE. The populations presented a total sum of OH-PAHs concentrations of 9.20 (6.65–97.57), 14.8 (9.32–18.85), 15.7 (6.92–195.0), and 101.2 (8.02–134.4) μg/L for CAM, ESC, TER, and TOC, respectively (presented as median (IQR)). As can be seen, 1-OH-PYR was the most frequent biomarker in all four populations, and the indigenous population presented the higher concentrations of total OH-PAHs.
Table 2.
Concentrations of OH-PAHs in urine of study populations
| OH-PAHs | Population | |||||||
|---|---|---|---|---|---|---|---|---|
| Miners (n = 31) | > %LOD | Stonemasons (n = 36) | > %LOD | Brickmakers (n = 42) | > %LOD | Indigenous (n = 40) | > %LOD | |
| 1-OH-PYR | 1.80 (1.16–2.29)a | 96.7 | 6.54(3.90–8.70)a,b,c | 97.2 | 1.56 (1.20–2.97)b | 92.8 | 1.77 (1.17–2.65)c | 100 |
| 4-OH-PHE | 1.13 (0.86–1.41)a | 35.5 | 1.12 (0.96–1.54)b | 44.4 | 1.76 (1.36–2.07)c | 30.9 | 2.73 (2.51–2.88)a,b,c | 90 |
| 2-OH-NAP | 2.54 (1.98–3.48)a | 96.7 | 2.93 (2.40–3.60)b | 97.2 | 2.82 (2.26–3.5)b | 90.4 | 0.67 (0.61–1.06)a,b,c | 80 |
| 1-OH-NAP | 1.88 (1.47–2.45)a | 90.3 | 1.93 (1.45–2.47)b | 97.2 | 1.96 (1.24–3.56)c | 92.8 | 1.05 (0.85–1.58)a,b,c | 75 |
| 9-OH-FLU | 146.8 (87.46–342.3) | 29.0 | 171.2 (131.1–237.2) | 13.8 | 187.3 (109.1–220.0)a | 45.2 | 110.9 (93.07–136.3)a | 70 |
| 3-OH-FLU | 0.77 (0.36–1.3) | 45.2 | 1.82 (0.94–3.04)a | 41.6 | 1.74 (1.03–2.18)b | 28.5 | 0.23 (0.19–0.40)a,b | 32.5 |
| 2-OH-FLU | 0.80 (0.66–1.12) | 61.3 | 1.02 (0.91–1.72) | 25 | 0.75 (0.60–1.50) | 42.8 | 0.88 (0.67–1.49) | 15 |
| 3-OH-PHE | 0.86 (0.64–1.07) | 45.2 | 1.13 | 2.7 | 1.01 (0.66–1.46) | 23.8 | 0.96 (0.62–2.36) | 12.5 |
| 1-OH-PHE | 0.53 (0.45–0.75) | 54.8 | 0.75 (0.50–0.86) | 11.1 | 0.74 (0.44–0.99) | 30.9 | 0.756 | 2.5 |
| 2-OH-PHE | 0.82 (0.52–1.39) | 25.8 | 1.24 (1.06–1.54) | 44.4 | 0.95 (0.77–1.76) | 14.3 | < LOD | 0 |
| ∑-OH-PAHs | 9.20 (6.65–97.57) | 100 | 14.8 (9.32–18.85) | 100 | 15.7 (6.92–195.0) | 95.8 | 101.2 (8.02–134.4) | 100 |
All OH PAH concentrations are presented as median (IQR) in μg/L and frequencies in percentage (%). Miners (CAM), stonemasons (ESC), brickmakers (TER), and indigenous (TOC). The same letter indicates a statistically significant difference, Krsukall Wallis and pos hoc Dunn test. LOD, limit of detection, 1-OH-NAP 1-hydroxynaphtalene, 2-OH-NAP 2-hydroxynaphtalene, 2-OH-FLU, 3-OH-FLU, 9-OH-FLU 2-,3- and 9-hydroxyfluorene, 1-OH-PHE, 2-OH-PHE, 3-OH-PHE, 4-OH-PHE 1-,2-,3- and 4-hydroxyphenanthrene, and 1-OH-PYR 1-hydroxypyrene
Also, in Table 2 is shown the significant differences between each one of the OH-PAHs of the study populations. Significant differences were found between the concentrations of 1-OH-NAP, 2-OH-NAP, and 4-OH-PHE of the TOC community compared to the other populations in the study. Likewise, there were significant differences between the 1-OH-PYR concentrations of ESC and the concentrations presented by the other study populations. Another significant difference was presented between the 3-OH-FLU biomarker of the indigenous population, compared to ESC and TER, in the same way, 9-OH-FLU of TOC to TER. 2-OH-FLU, 3-OH-PHE, 1-OH-PHE, 2-OH-PHE, and ∑-OH-PAHs did not present significant differences in any of the populations of the study.
Multivariate analysis
Principal component analysis and canonical analysis of principal coordinates
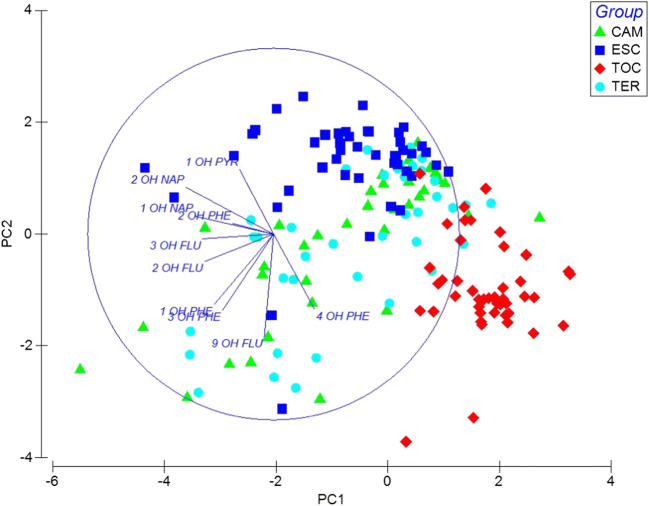
The PCA showed a separation between the concentrations of OH-PAHs in the ESC population compared to the TOC population, explaining 47.7% of the variability in two components. However, the CAM and TER populations presented similar concentrations of OH-PAHs and thus no separation was observed. (Fig. 1).
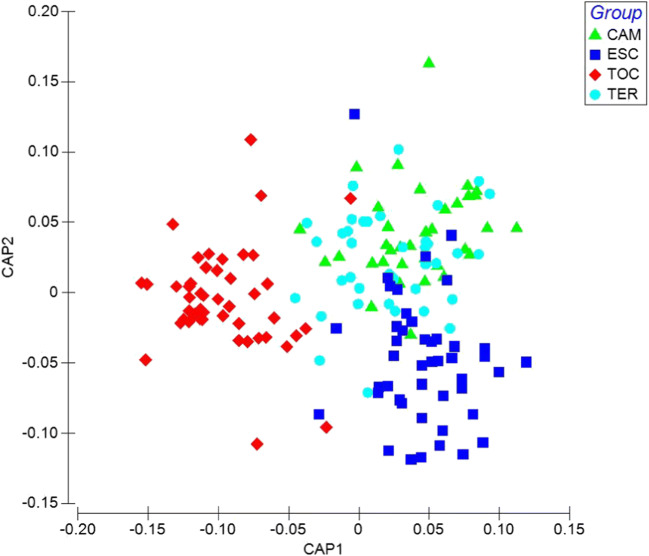
To evaluate the discrimination between study groups, a canonical principal coordinate analysis (PCA) was carried out, which achieved a separation of the populations due to exposure to different concentrations of PAHs through two PCA axes. The CAP 1 axis presented a correlation r2 = 0.88 with the OH-PAHs associated with the separation between TOC vs. ESC, TER, and CAM, while the CAP 2 axis presented a correlation r2 = 0.65 with the OH-PAHs associated with the separation between ESC vs. TOC, TER, and CAM with a correct classification 73.3% (p = 0.0001) (Fig. 2).
Fig. 2.
Principal component analysis (PCA) of study populations. Miners (CAM), brickmakers (TER), stonemasons (ESC), and indigenous people (TOC)
A Spearman correlation was conducted between the values of the CAP 1 and CAP 2 axis and the concentrations of the OH PAHs, and it was found that all the hydroxylated compounds correlate with CAP 1, indicating that the TOC population has significant differences in all the concentrations of the compounds compared to the other populations (Fig. 3). There is an increase in TOC of 2-OH-NAP, 1-OH-NAP, 2-OH-PHE, 1-OH-PYR, 1-OH-PHE, 2-OH-FLU, 3-OH-FLU, and 3-OH-PHE and a decrease of 4-OH-PHE and 9-OH-FLU. Moreover, seven OH-PAHs compounds correlate significantly with the CAP 2 axis associated with the separation between the ESC population and TOC, TER, and CAM. Results also show that 3-OH-PHE, 1-OH-PHE, 2-OH-FLU, and 9-OH-FLU increase in the ESC population and 1-OH-PHE, 2-OH-PHE and 4-OH-PHE decrease (Table 3).
Fig. 3.
Canonical analysis of principal coordinates (CAP) of PAHs exposure in different populations. Miners (CAM), brickmakers (TER), stonemasons (ESC), and indigenous people (TOC)
Table 3.
OH-PAHs that correlate with the axis CAP 1 and CAP 2
| OH-PAHs | CAP 1 | CAP 2 | ||
|---|---|---|---|---|
| Spearman r | p value | Spearman r | p value | |
| 1-OH-NAP | 0.5916 | 0.0000 | − 0.0600 | 0.4440 |
| 2-OH-NAP | 0.7325 | 0.0000 | − 0.1151 | 0.1409 |
| 9-OH-FLU | − 0.4487 | 0.0000 | 0.1637 | 0.0357 |
| 3-OH-FLU | 0.2669 | 0.0005 | 0.0321 | 0.6824 |
| 2-OH-FLU | 0.3249 | 0.0000 | 0.3715 | 0.0000 |
| 4-OH-PHE | − 0.7320 | 0.0000 | − 0.1990 | 0.0104 |
| 3-OH-PHE | 0.1919 | 0.0135 | 0.5133 | 0.0000 |
| 1-OH-PHE | 0.3153 | 0.0000 | 0.4849 | 0.0000 |
| 2-OH-PHE | 0.3857 | 0.0000 | − 0.1915 | 0.0138 |
| 1-OH-PYR | 0.3365 | 0.0000 | − 0.6959 | 0.0000 |
1-OH-NAP 1-hydroxynaphtalene, 2-OH-NAP 2-hydroxynaphtalene, 2-OH-FLU, 3-OH-FLU, 9-OH-FLU 2-,3- and 9-hydroxyfluorene, 1-OH-PHE, 2-OH-PHE, 3-OH-PHE, 4-OH-PHE 1-,2-,3- and 4-hydroxyphenanthrene, 1-OH-PYR 1-hydroxypyrene
Discussion
Precarious work has been increasingly recognized as prejudicial to health and well-being (Julia et al. 2017). According to data from the International Labor Organization, each year approximately 2.8 million workers around the world die due to unsafe or unhealthy working conditions (Hämäläinen et al. 2017). Despite clear human rights obligations to protect their health, workers around the world are in crisis, with an estimated one worker dying every 30 s from exposure to toxic chemicals, pesticides, radiation, and other hazardous substances (Hämäläinen et al. 2017, McKay et al. 2012). Nevertheless, since in some contexts and countries limited information is given on incidents resulting from such exposure, this number may be underestimated (Mendoza-González et al. 2020).
In a precarious work, the labor risks represent a major disadvantage due to not providing adequate training to workers on the various processes, this generates that the perception of risk by workers is zero and therefore do not follow specific protective measurements for the use of tools, control and exposure to substances (Schulte et al. 2020). Additionally, in these works, it is common the lack of provision of adequate protective personal equipment to workers, and in a specific manner to workers whose primary activities may generate exposure to substances that endanger their health (Pérez-Herrera et al. 2019, Quinlan et al. 2001).
Air quality in work environments is an occupational health and safety issue (Mandrioli et al. 2018), but it is further aggravated in works where in addition to high exposure to toxics, there is vulnerability due to high marginalization.
For over 30 years it has been shown that high exposure to PAHs is associated with several types of cancer (Boffetta et al. 1997), IARC has classified some occupational activities as carcinogenic, due to the association with exposure to carcinogenic compounds of which PAHs stand out. Regarding this, values allowed by international organizations such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency for emissions from different sources such as coke oven emissions and coal tar pitch volatiles (CTPVs) have been established, where they indicate concentrations in air of 0.15 mg/m3 and 0.1 mg/m3. Coke oven emissions are a mixture of coal tar, coal tar pitch, volatiles, creosote, polycyclic aromatic hydrocarbons (PAHs), and metals. Over 20 different PAHs are found in coke oven emissions, including benzo(a)pyrene, benzanthracene, chrysene, and phenanthrene. Approximately 80% of coal tar is unspecified carbon chains (C18-22); coal tar volatiles include benzene, toluene, and xylenes (EPA 1984).
The workers in our study are considered precarious workers. Our results indicate a high exposure to a mixture of PAHs (100% of the study population) due to the presence of the biomarkers of OH-PAHs in the urine. The presence of different OH-PAHs depends directly on the presence of the same PAHs in the smoke. In this regard, Table 1of supplementary material presents an overview of a different occupational population where the presence of OH-PAHs in urine has been documented.
Previous research by our working group has characterized exposure to PAHs through the biomarker of exposure 1-OH-PYR; studies in the indigenous population of the Huasteca Potosina (Rodriguez-Aguilar et al. 2019), exposed to wood smoke, have reported that 100% of the participants exceeded the reference values 0.24 μmol/mol creatinine for occupational populations (Jongeneelen 2001); brickmakers in the study area have reached higher concentrations 0.18 μg g−1 creatinine (Alegría-Torres et al. 2013). Pyrene is not carcinogenic, and its genotoxic effects are limited, but is present in most work environments where there is a potential release of PAHs. As a result, its metabolism product 1-OH-PYR, excreted in the urine, is considered in different scientific works as the best biological indicator of exposure to PAHs. However, because the composition of PAHs mixtures in different environments (e.g., wood smoke and biomass burning) is not always constant and is very complex, the risk may be over- or underestimated. In this context, we investigated several individual urinary biomarkers, which allows characterizing the exposure profile.
Concerning the 10 evaluated metabolites, we focused on the detection rate in those compounds higher than 60%. In mercury mining workers, the compounds with the highest detection in the population were 1-OH-NAP, 2-OH-NAP, 1-OH-PYR, and 2-OH FLU; this reflects the current exposure to PAHs in mining workers; previous occupational exposure studies indicate reports of 1-OH-NAP, 2-OH-NAP, and 2-OH FLU in steel smelter and galvanization in similar concentrations (Campo et al. 2016); however, the 1-OH-PYR in this scenario are up to 4 times less than the one obtained in our study. A possible explanation is the use of wood burning to extract the mercury; the ore is incinerated in clay ovens and workers are exposed for up to 8 h, where they are exposed to PAHs and elemental mercury (Camacho et al. 2016). Similar exposure to this PAHs fingerprint has been reported for workers exposed to wood burning (Bruschweiler et al. 2012). In this scenario, it is important to monitor the concentration of naphthalene, since this compound is classified as a possible carcinogen, and the reported concentrations are between 9 and 62 times for 1-OH-NAP and 2-OH-NAP, respectively, compared to the carcinogenic activity of coke oven workers (Du et al. 2020). Regarding the 1-OH-PYR in the mining population, it is found in 4 and 3.3 times higher concentrations than steel smelter and galvanization (Campo et al. 2016), respectively. and up to 26 times to asphalt workers (Xu et al. 2018).
In the stonemasons, the biomarkers that were present most often in the sampling were 1-OH-PYR, 1-OH-NAP, and 2-OH-NAP. This exposure indicates that the main pathway is inhalation; in this sense, naphthalene (two-ringed PAH) is a very volatile compound and most of its environmental levels enter the human body mainly in gaseous form, and therefore, the concentrations of 1-OH-NAP and 2-OH-NAP probably reflect the contribution from the air. Low molecular weight PAHs exist almost exclusively in the gas phase, while high molecular weight PAHs (5–6 rings, such as benzo[a]pyrene) are predominantly bound to particles; 4-ring PAHs (which include pyrene) are distributed between the gas and particle phases (Oliveira et al. 2016). Exposure to these PAHs are associated with gasoline combustion (Shao et al. 2019), what is suggested in this scenario, it is considered that the main source is the fuels used for their cutting equipment and also the vehicular traffic in the area. An important aspect is when comparing between the evaluated workers, in this area the highest concentrations of 1-OH-PYR were obtained; this result explained that most of the workers present tobacco smoking, 1-OH-PYR, a sensitive and specific biomarker has been proven to evaluate exposure to PAHs from the use of tobacco products (Wang et al. 2019).
The brickmakers showed similar concentrations and detection rates to the quarry population, except for 1-OH-PYR; the compounds 1-OH-PYR, 1-OH-NAP, and 2-OH-NAP were present in more than 90% of the population; it is important to note that as the mercury miners, detection rates for fluorene and phenanthrene metabolites are higher in these populations, considering that these compounds are associated with coke oven and diesel engines (in both areas diesel transport is used for material loading) (Khalili et al. 1995). This population reports similar concentrations to steel smelter, galvanization. and coke oven workers (Campo et al. 2016, Du et al. 2020).
As for the indigenous population, greater concentrations of 1-OH-PYR, 9-OH-FLU, 4-OH-PHE, 2-OH-NAP, and 1-OH-NAP were found. According to the results, naphthalene metabolites reached lower concentrations with significant differences compared to the other three occupations; the noteworthy value is 9-OH-FLU, which occurs in 70% of the population in average concentrations of 110.9 μg/L. Fluorene and its derivatives have been reported in air in gas phase and in particulate material; it has been demonstrated that under oxidizing conditions more toxic compounds are produced (e.g., 9-fluorenone) which can be oxidized to form dibenzo-p-dioxin drastically increasing the toxicity (Ding et al. 2019). Although biomonitoring data indicate a high exposure to Fluorene, 9-OH-FLU is one of the products of the biochemical reactions of 9-fluorenone (Kadlubar et al. 1992), which is important since it indicates that in the indigenous population, in addition to the PAHs, the exposure would also include the environmental degradation products caused by the combustion of the wood that are potentially more toxic than the precursors. On the other hand, in previous studies of our research group, we have reported that in these populations, plastics, or agricultural residues are generally used for the ignition initiation of wood (Estevez-Garcia et al. 2020, Flores-Ramirez et al. 2016), which could increase the toxicity of the formed chemical species; we consider that this aspect should be considered in further research.
Reference values for biomonitoring of PAHs in workers have been proposed by the American Conference of Governmental Industry Hygienists through the Biological Exposure Index and are limited to 1-OH-PYR, which should be 1 μg/L (ACGIH 2014). Based on this criterion, 100% of the workers in the 4 precarious activities were above the permitted level, with stonemasons being up to 6.5 times higher. However, given the toxicity of the mixture of compounds, the resulting chemical fingerprints must be evaluated.
The results of the multivariate analysis indicate differences between the several concentrations found in precarious workers; the indigenous population presents a different chemical fingerprint to the three scenarios, with 1-OH-NAP, 2-OH-NAP, 2-OH-PHE, 1-OH-PYR, 1-OH-PHE, 2-OH-FLU, 3-OH-FLU, and 3-OH-PHE increasing and 4-OH-PHE and 9-OH-FLU decreasing. Furthermore, the stonemason community presents different fingerprints, being that the 3-OH-PHE, 1-OH-PHE, 2-OH-FLU, and 9-OH-FLU increase in the population, and the 1-OH-PHE, 2-OH-PHE, and 4-OH-PHE decrease. The chemical fingerprints of PAHs exposure are similar among the brickmakers and mercury mining populations.
One important limitation of this study is that the environmental exposure of workers was not evaluated, so the different exposure profiles in this type of precarious work determined by biomonitoring cannot be confirmed independently. In addition, hydroxylated metabolites of heavy PAHs must be evaluated, which would provide more information on the exposure associated with the particulate material. Moreover, the punctual sample only indicates the exposure of one day; this added to the lack of data of environmental exposure prevents us from discussing in greater depth the chemical fingerprints of PAHs in these precarious work scenarios.
However, with our results, high exposure is demonstrated and the evidence on the evaluation of a wide panel of hydroxylated PAHs biomarkers is provided. Until the review of the current literature, this is one of the only investigations on occupational exposure to PAHs in precarious work in Mexico, it is important to highlight that, in our country, there are more than 30 million workers in precarious situations that include exposure to this kind of pollutants (INEGI 2020).
PAHs represent an important public health issue; assessing the risk of exposure of the working population is fundamental. The associated risks of PAHs mixtures from workplaces face the lack of knowledge of occupational exposure mixtures, the lack of accurate chemical characterization of occupational PAHs mixtures, and how to assess the carcinogenic and non-carcinogenic risk associated with mixtures. The identification of suitable biomarkers for the assessment of mixture toxicity is of great importance; nevertheless, due to the interactions, it is complicated to establish with certainty. Interesting approaches have been developed to assess the risk of diseases associated with pollutant exposure that includes an approach from the omic sciences; this could be applied in high exposure scenarios to establish the causal relationship (Martins et al. 2019, Rodriguez-Aguilar et al. 2020).
Despite the efforts and establishment of maximum permissible limits by international organizations to reduce exposure levels and thus the associated risks, PAHs continue to be a health threat to people who are exposed to these pollutants in work environments and even more so in precarious workers who do not have the minimum protective measurements.
Assessing the levels of hydroxylated PAHs and comparing them with workers in occupations recognized as carcinogenic (Table 2 supplementary material), we consider that these scenarios are very high-risk and should be addressed in an occupational health approach as proposed by the IARC; this would increase the importance and encourage surveillance systems by appropriate authorities.
Conclusion
In this study, high exposure to PAHs was found in people from four activities considered as precarious work. Of the 10 PAHs hydroxylated metabolites, 1-OH-PYR, 9-OH-FLU, 4-OH-PHE, 2-OH-NAP, and 1-OH-NAP were in higher concentrations, and differences between the mixtures reported in the indigenous population and stonemasons.
The exposure control in these kinds of work is of paramount importance due to the high exposure reported; surveillance through biological monitoring can be particularly useful, where in addition to 1-OH-PYR, other hydroxylated metabolites are included to better estimate the exposure in these working populations. On the other hand, the results of the concentrations in urine are similar and in some metabolites higher than workers in occupations classified as carcinogenic by the IARC. Our standpoint is that, in these populations, the precautionary principle should be applied, even considering the scientific uncertainties about the probability, causality, magnitude, and nature of the damage (Silva & Lizardi-Jiménez 2020). These occupations should be addressed as cancer-causing activities, which would imply the design of strategies to monitor and control these pollutants that our country currently lacks, particularly in precarious occupations.
The documented scenarios in this article are of a very high-risk to the health of precarious workers; in addition to the exposure to mixtures of PAHs, there are accumulated risks, for example, chemical risks where it is common to find exposure to other mixtures of pollutants such as metals, persistent organic compounds, and aflatoxins (Díaz de León-Martínez et al. 2019, Diaz de Leon-Martinez et al. 2020b, Flores-Ramirez et al. 2016); physical risks, for example, excessive material loads, mainly affecting the back and causing musculoskeletal disorders; biological risks, now with COVID-19 (Alahmad et al. 2020, Diaz de Leon-Martinez et al. 2020a), in addition to the high incidence of comorbidities such as diabetes, high blood pressure, chronic kidney disease, and psychosocial stress, among others (Cho 2020, Karasek et al. 2010, Pérez-Herrera et al. 2019), this in a context without social security and precarious socioeconomic conditions. It is imperative to develop strategies to protect the health of the precarious workers; these scenarios can no longer be ignored by labor and public health authorities.
Supplementary Information
(DOCX 33 kb)
Authors’ contributions
Lorena Díaz de León-Martínez: conceptualization, sampling, analytical methods, writing, and editing. Rogelio Flores-Ramírez: conceptualization, sampling, writing, editing, and funding. Maribel Rodríguez-Aguilar: analytical methods. Alejandra Berumen-Rodríguez: sampling and analytical methods. Francisco Javier Pérez-Vázquez: sampling and conceptualization. Fernando Díaz-Barriga: conceptualization, final editing, and funding
Funding
The authors acknowledge grants and fellowships from National Council on Science and Technology—Sectoral Research FOSEC SS/IMSS/ISSSTE # A3-S-38681.
Data availability
Not applicable
Compliance with ethical standards
Ethical approval
The protocol was approved by the Research Ethics Committee of the Faculty of Medicine of the Autonomous University of San Luis Potosi and the Bioethics Commission of the State of San Luis Potosi (CEI-2018-002).
Consent to participate
Not applicable
Consent to publish
Not applicable
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- ACGIH (2014): Threshold limit values and biological exposure indices for 2014. In: Hygienists ACoGI (Hrsg.), Cincinnati, Ohio, US
- Alahmad B, Kurdi H, Colonna K, Gasana J, Agnew J, Fox MA. COVID-19 stressors on migrant workers in Kuwait: cumulative risk considerations. BMJ Glob Health. 2020;5:e002995. doi: 10.1136/bmjgh-2020-002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder L, Hill A, Holland PT, Lantos J, Lee SM, MacNeil JD, O'Rangers J, Zoonen P, Ambrus A (2000) Guidelines for single-laboratory validation of analytical methods for trace-level concentrations of organic chemicals. Principles Pract Method Valid:179–252
- Alegría-Torres JA, Barretta F, Batres-Esquivel LE, Carrizales-Yáñez L, Pérez-Maldonado IN, Baccarelli A, Bertazzi PA. Epigenetic markers of exposure to polycyclic aromatic hydrocarbons in Mexican brickmakers: a pilot study. Chemosphere. 2013;91:475–480. doi: 10.1016/j.chemosphere.2012.11.077. [DOI] [PubMed] [Google Scholar]
- Alomirah H, Al-Zenki S, Al-Hooti S, Zaghloul S, Sawaya W, Ahmed N, Kannan K. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control. 2011;22:2028–2035. doi: 10.1016/j.foodcont.2011.05.024. [DOI] [Google Scholar]
- Armstrong B, Hutchinson E, Unwin J, Fletcher T. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: a review and meta-analysis. Environ Health Perspect. 2004;112:970–978. doi: 10.1289/ehp.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASTDR (1995): Toxicological profile for polycyclic aromatic hydrocarbons. In: Department of Health and Human Services PHS (Hrsg.), Atlanta, GA. US.
- Bhargava A, Khanna R, Bhargava S, Kumar S. Exposure risk to carcinogenic PAHs in indoor-air during biomass combustion whilst cooking in rural India. Atmos Environ. 2004;38:4761–4767. doi: 10.1016/j.atmosenv.2004.05.012. [DOI] [Google Scholar]
- Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997;8:444–472. doi: 10.1023/A:1018465507029. [DOI] [PubMed] [Google Scholar]
- Bruschweiler ED, Danuser B, Huynh CK, Wild P, Schupfer P, Vernez D, Boiteux P, Hopf NB. Generation of polycyclic aromatic hydrocarbons (PAHs) during woodworking operations. Front Oncol. 2012;2:148. doi: 10.3389/fonc.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho A, Van Brussel E, Carrizales L, Flores-Ramirez R, Verduzco B, Huerta SR, Leon M, Diaz-Barriga F. Mercury mining in Mexico: I. Community engagement to improve health outcomes from artisanal mining. Ann Glob Health. 2016;82:149–155. doi: 10.1016/j.aogh.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Campo L, Hanchi M, Olgiati L, Polledri E, Consonni D, Zrafi I, Saidane-Mosbahi D, Fustinoni S. Biological monitoring of occupational exposure to polycyclic aromatic hydrocarbons at an electric steel foundry in Tunisia. Ann Occup Hyg. 2016;60:700–716. doi: 10.1093/annhyg/mew024. [DOI] [PubMed] [Google Scholar]
- Campo L, Hanchi M, Sucato S, Consonni D, Polledri E, Olgiati L, Saidane-Mosbahi D, Fustinoni S. Biological monitoring of occupational exposure to metals in electric steel foundry workers and its contribution to 8-oxo-7, 8-dihydro-2′-deoxyguanosine levels. Int J Environ Res Public Health. 2020;17:1811. doi: 10.3390/ijerph17061811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2013): Monohydroxy-polycyclic aromatic hydrocarbons (OH-PAHs) in urine by isotope dilution gas chromatography/tandem mass spectrometry (GC-MS/MS). In: Sciences DoL (Hrsg.), pp. 1-38
- Cho Y (2020) The associations between patterns of precarious employment and workers’ health. Soc Sci J:1–14
- Dat N-D, Chang MB. Review on characteristics of PAHs in atmosphere, anthropogenic sources and control technologies. Sci Total Environ. 2017;609:682–693. doi: 10.1016/j.scitotenv.2017.07.204. [DOI] [PubMed] [Google Scholar]
- Diaz de Leon-Martinez L, de la Sierra-de la Vega L, Palacios-Ramirez A, Rodriguez-Aguilar M, Flores-Ramirez R. Critical review of social, environmental and health risk factors in the Mexican indigenous population and their capacity to respond to the COVID-19. Sci Total Environ. 2020;733:139357. doi: 10.1016/j.scitotenv.2020.139357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz de Leon-Martinez L, Ortega-Romero M, Grimaldo-Galeana JM, Barbier O, Vargas-Berrones K, Garcia-Arreola ME, Rodriguez-Aguilar M, Flores-Ramirez R. Assessment of kidney health and exposure to mixture pollutants in the Mexican indigenous population. Environ Sci Pollut Res Int. 2020;27:34557–34566. doi: 10.1007/s11356-020-09619-x. [DOI] [PubMed] [Google Scholar]
- Díaz de León-Martínez L, Díaz-Barriga F, Barbier O, Ortíz DLG, Ortega-Romero M, Pérez-Vázquez F, Flores-Ramírez R. Evaluation of emerging biomarkers of renal damage and exposure to aflatoxin-B1 in Mexican indigenous women: a pilot study. Environ Sci Pollut Res. 2019;26:12205–12216. doi: 10.1007/s11356-019-04634-z. [DOI] [PubMed] [Google Scholar]
- Ding Z, Yi Y, Zhang Q, Zhuang T. Theoretical investigation on atmospheric oxidation of fluorene initiated by OH radical. Sci Total Environ. 2019;669:920–929. doi: 10.1016/j.scitotenv.2019.02.400. [DOI] [PubMed] [Google Scholar]
- Du J, Pan B, Cao X, Li J, Yang J, Nie J. Urinary polycyclic aromatic hydrocarbon metabolites, peripheral blood mitochondrial DNA copy number, and neurobehavioral function in coke oven workers. Chemosphere. 2020;261:127628. doi: 10.1016/j.chemosphere.2020.127628. [DOI] [PubMed] [Google Scholar]
- EPA (1984) Carcinogen assessment of coke oven emissions. In: Agency UEP (Hrsg.) Final report no. EPA-600/6-82-003F. EPA, Washington, D.C.
- Estevez-Garcia JA, Schilmann A, Riojas-Rodriguez H, Berrueta V, Blanco S, Villasenor-Lozano CG, Flores-Ramirez R, Cortez-Lugo M, Perez-Padilla R. Women exposure to household air pollution after an improved cookstove program in rural San Luis Potosi, Mexico. Sci Total Environ. 2020;702:134456. doi: 10.1016/j.scitotenv.2019.134456. [DOI] [PubMed] [Google Scholar]
- Flores-Ramirez R, Perez-Vazquez FJ, Cilia-Lopez VG, Zuki-Orozco BA, Carrizales L, Batres-Esquivel LE, Palacios-Ramirez A, Diaz-Barriga F. Assessment of exposure to mixture pollutants in Mexican indigenous children. Environ Sci Pollut Res Int. 2016;23:8577–8588. doi: 10.1007/s11356-016-6101-y. [DOI] [PubMed] [Google Scholar]
- Flores-Ramirez R, Perez-Vazquez FJ, Medellin-Garibay SE, Aldrete AC, Vallejo-Perez MR, de Leon-Martinez LD, Yanez LC, Diaz-Barriga F. Exposure to mixtures of pollutants in Mexican children from marginalized urban areas. Ann Glob Health. 2018;84:250–256. doi: 10.29024/aogh.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grova N, Faÿs F, Hardy E, Appenzeller B. New insights into urine-based assessment of polycyclic aromatic hydrocarbon-exposure from a rat model: identification of relevant metabolites and influence of elimination kinetics. Environ Pollut. 2017;228:484–495. doi: 10.1016/j.envpol.2017.03.060. [DOI] [PubMed] [Google Scholar]
- Guo Y, Senthilkumar K, Alomirah H, Moon H-B, Minh TB, Mohd MA, Nakata H, Kannan K. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ Sci Technol. 2013;47:2932–2938. doi: 10.1021/es3052262. [DOI] [PubMed] [Google Scholar]
- Hämäläinen P, Takala J, Kiat TB. Global estimates of occupational accidents and work-related illnesses 2017. World. 2017;2017:3–4. [Google Scholar]
- Iamiceli AL, Abate V, Abballe A, Bena A, De Filippis SP, De Luca S, Fulgenzi AR, Iacovella N, Ingelido AM, Marra V. Biomonitoring of the adult population in the area of turin waste incinerator: baseline levels of polycyclic aromatic hydrocarbon metabolites. Environ Res. 2020;181:108903. doi: 10.1016/j.envres.2019.108903. [DOI] [PubMed] [Google Scholar]
- IARC (2010): IARC monographs on the evaluation of carcinogenic risks to humans. VOLUME 92 Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures In: IARC (Hrsg.). INTERNATIONAL AGENCY FOR RESEARCH ON CANCER, LYON, FRANCE [PMC free article] [PubMed]
- INEGI (2020): Estadísticas a propósito del día del trabajo datos nacionales. In: Geografía INEGI (Hrsg.), Ciudad de México, pp. 1-8
- Jongeneelen FJ. Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Ann Occup Hyg. 2001;45:3–13. doi: 10.1016/S0003-4878(00)00009-0. [DOI] [PubMed] [Google Scholar]
- Julia M, Vanroelen C, Bosmans K, Van Aerden K, Benach J. Precarious employment and quality of employment in relation to health and well-being in Europe. Int J Health Serv. 2017;47:389–409. doi: 10.1177/0020731417707491. [DOI] [PubMed] [Google Scholar]
- Kachi Y, Otsuka T, Kawada T (2014): Precarious employment and the risk of serious psychological distress: a population-based cohort study in Japan. Scandinavian journal of work, environment & health, 465-472 [DOI] [PubMed]
- Kadlubar FF, Butler MA, Kaderlik KR, Chou HC, Lang NP. Polymorphisms for aromatic amine metabolism in humans: relevance for human carcinogenesis. Environ Health Perspect. 1992;98:69–74. doi: 10.1289/ehp.929869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasek R, Collins S, Clays E, Bortkiewicz A, Ferrario M. Description of a large-scale study design to assess work-stress-disease associations for cardiovascular disease. Int J Occup Med Environ Health. 2010;23:293–312. doi: 10.2478/v10001-010-0035-2. [DOI] [PubMed] [Google Scholar]
- Khalili NR, Scheff PA, Holsen TM. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmos Environ. 1995;29:533–542. doi: 10.1016/1352-2310(94)00275-P. [DOI] [Google Scholar]
- Kim KH, Jahan SA, Kabir E, Brown RJC. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int. 2013;60:71–80. doi: 10.1016/j.envint.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Kogevinas M (2020): Bladder cancer, Occupational Cancers. Springer, pp 487-506
- Liu Y, Tao S, Yang Y, Dou H, Yang Y, Coveney RM. Inhalation exposure of traffic police officers to polycyclic aromatic hydrocarbons (PAHs) during the winter in Beijing, China. Sci Total Environ. 2007;383:98–105. doi: 10.1016/j.scitotenv.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Mandrioli D, Schlünssen V, Ádám B, Cohen RA, Colosio C, Chen W, Fischer A, Godderis L, Göen T, Ivanov ID, Leppink N, Mandic-Rajcevic S, Masci F, Nemery B, Pega F, Prüss-Üstün A, Sgargi D, Ujita Y, van der Mierden S, Zungu M, Scheepers PTJ. WHO/ILO work-related burden of disease and injury: protocol for systematic reviews of occupational exposure to dusts and/or fibres and of the effect of occupational exposure to dusts and/or fibres on pneumoconiosis. Environ Int. 2018;119:174–185. doi: 10.1016/j.envint.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Martins C, Dreij K, Costa PM. The state-of-the art of environmental toxicogenomics: challenges and perspectives of “omics” approaches directed to toxicant mixtures. Int J Environ Res Public Health. 2019;16:4718. doi: 10.3390/ijerph16234718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay S, Jefferys S, Paraksevopoulou A, Keles J. Study on precarious work and social rights. London: Working Lives Research Institute, London Metropolitan University; 2012. pp. 72–74. [Google Scholar]
- Medina-Gómez O, López-Arellano O. Informalidad laboral y derecho a la salud en México, un análisis crítico. Ciência & Saúde Coletiva. 2019;24:2583–2592. doi: 10.1590/1413-81232018247.14342017. [DOI] [PubMed] [Google Scholar]
- Mendoza-González MÁ, Cruz-Calderón SF, Valdivia-López M. Niveles y subniveles de precariedad extrema en México. Estudios Demográficos y Urbanos. 2020;35:405–448. doi: 10.24201/edu.v35i2.1784. [DOI] [Google Scholar]
- Oliveira M, Slezakova K, Delerue-Matos C, Pereira Mdo C, Morais S. Assessment of polycyclic aromatic hydrocarbons in indoor and outdoor air of preschool environments (3-5 years old children) Environ Pollut. 2016;208:382–394. doi: 10.1016/j.envpol.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Pérez-Herrera N, Díaz de León-Martínez L, Flores-Ramírez R, Barbier O, Ortega-Romero M, May-Euán F, Saldaña-Villanueva K, Perera-Rios J, Pérez-Vázquez FJ. Evaluation of benzene exposure and early biomarkers of kidney damage in children exposed to solvents due to precarious work in Ticul, Yucatán, México. Ann Glob Health. 2019;85:94. doi: 10.5334/aogh.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persoons R, Roseau L, Petit P, Hograindleur C, Montlevier S, Marques M, Ottoni G, Maitre A. Towards a recommended biomonitoring strategy for assessing the occupational exposure of roofers to PAHs. Toxicol Lett. 2020;324:54–64. doi: 10.1016/j.toxlet.2020.01.025. [DOI] [PubMed] [Google Scholar]
- Petit P, Maitre A, Persoons R, Bicout DJ. Lung cancer risk assessment for workers exposed to polycyclic aromatic hydrocarbons in various industries. Environ Int. 2019;124:109–120. doi: 10.1016/j.envint.2018.12.058. [DOI] [PubMed] [Google Scholar]
- Quinlan M, Mayhew C, Bohle P. The global expansion of precarious employment, work disorganization, and consequences for occupational health: a review of recent research. Int J Health Serv. 2001;31:335–414. doi: 10.2190/607H-TTV0-QCN6-YLT4. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Aguilar M, Diaz de Leon-Martinez L, Garcia-Luna S, Gomez-Gomez A, Gonzalez-Palomo AK, Perez-Vazquez FJ, Diaz-Barriga F, Trujillo J, Flores-Ramirez R. Respiratory health assessment and exposure to polycyclic aromatic hydrocarbons in Mexican indigenous population. Environ Sci Pollut Res Int. 2019;26:25825–25833. doi: 10.1007/s11356-019-05687-w. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Aguilar M, Diaz de Leon-Martinez L, Gorocica-Rosete P, Padilla RP, Thirion-Romero I, Ornelas-Rebolledo O, Flores-Ramirez R. Identification of breath-prints for the COPD detection associated with smoking and household air pollution by electronic nose. Respir Med. 2020;163:105901. doi: 10.1016/j.rmed.2020.105901. [DOI] [PubMed] [Google Scholar]
- Rota M, Bosetti C, Boccia S, Boffetta P, La Vecchia C. Occupational exposures to polycyclic aromatic hydrocarbons and respiratory and urinary tract cancers: an updated systematic review and a meta-analysis to 2014. Arch Toxicol. 2014;88:1479–1490. doi: 10.1007/s00204-014-1296-5. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Streit JM, Sheriff F, Delclos G, Felknor SA, Tamers SL, Fendinger S, Grosch J, Sala R. Potential scenarios and hazards in the work of the future: a systematic review of the peer-reviewed and gray literatures. Annals of Work Exposures and Health. 2020;64:786–816. doi: 10.1093/annweh/wxaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, Dubey A, Saini D, Singh M, Prasad CP, Roy S, Bharati SJ, Rinki M, Singh N, Seth T, Khanna M, Sethi N, Kumar S, Sirohi B, Mohan A, Guleria R, Rath GK. Environmental and occupational determinants of lung cancer. Transl Lung Cancer Res. 2019;8:S31–S49. doi: 10.21037/tlcr.2019.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C, Wang H, Atef N, Wang Z, Chen B, Almalki M, Zhang Y, Cao C, Yang J, Sarathy SM. Polycyclic aromatic hydrocarbons in pyrolysis of gasoline surrogates (n-heptane/iso-octane/toluene) Proc Combust Inst. 2019;37:993–1001. doi: 10.1016/j.proci.2018.06.087. [DOI] [Google Scholar]
- Silva VM, Lizardi-Jiménez MA (2020): Environmental problems and the state of compliance with the right to a healthy environment in a mining region of México. International Journal of Chemical Reactor Engineering 1
- Vimercati L, Bisceglia L, Cavone D, Caputi A, De Maria L, Delfino MC, Corrado V, Ferri GM (2020) Environmental monitoring of PAHs exposure, biomarkers and vital status in coke oven workers. Int J Environ Res Public Health 17 [DOI] [PMC free article] [PubMed]
- Wang Y, Wong LY, Meng L, Pittman EN, Trinidad DA, Hubbard KL, Etheredge A, del Valle-Pinero AY, Zamoiski R, van Bemmel DM, Borek N, Patel V, Kimmel HL, Conway KP, Lawrence C, Edwards KC, Hyland A, Goniewicz ML, Hatsukami D, Hecht SS, Calafat AM. Urinary concentrations of monohydroxylated polycyclic aromatic hydrocarbons in adults from the U.S. Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013-2014) Environ Int. 2019;123:201–208. doi: 10.1016/j.envint.2018.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudneh MB, Benskin JP, Grace R, Hamilton MC, Magee BH, Hoeger GC, Forsberg ND, Cosgrove JR. Quantitative determination of hydroxy polycylic aromatic hydrocarbons as a biomarker of exposure to carcinogenic polycyclic aromatic hydrocarbons. J Chromatogr A. 2016;1454:93–100. doi: 10.1016/j.chroma.2016.05.057. [DOI] [PubMed] [Google Scholar]
- Xu Y, Lindh CH, Jönsson BA, Broberg K, Albin M. Occupational exposure to asphalt mixture during road paving is related to increased mitochondria DNA copy number: a cross-sectional study. Environ Health. 2018;17:29. doi: 10.1186/s12940-018-0375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Pan B, Zhao X, Fu Y, Li X, Yang A, Li Q, Dong J, Nie J, Yang J. The interaction effects of smoking and polycyclic aromatic hydrocarbons exposure on the prevalence of metabolic syndrome in coke oven workers. Chemosphere. 2020;247:125880. doi: 10.1016/j.chemosphere.2020.125880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 33 kb)
Data Availability Statement
Not applicable