Abstract
Interstitial cells of Cajal (ICC) generate pacemaker activity responsible for phasic contractions in colonic segmentation and peristalsis. ICC along the submucosal border (ICC-SM) contribute to mixing and more complex patterns of colonic motility. We show the complex patterns of Ca2+ signaling in ICC-SM and the relationship between ICC-SM Ca2+ transients and activation of smooth muscle cells (SMCs) using optogenetic tools. ICC-SM displayed rhythmic firing of Ca2+transients ~ 15 cpm and paced adjacent SMCs. The majority of spontaneous activity occurred in regular Ca2+ transients clusters (CTCs) that propagated through the network. CTCs were organized and dependent upon Ca2+ entry through voltage-dependent Ca2+ conductances, L- and T-type Ca2+ channels. Removal of Ca2+ from the external solution abolished CTCs. Ca2+ release mechanisms reduced the duration and amplitude of Ca2+ transients but did not block CTCs. These data reveal how colonic pacemaker ICC-SM exhibit complex Ca2+-firing patterns and drive smooth muscle activity and overall colonic contractions.
Research organism: Mouse
Introduction
Interstitial cells of Cajal (ICC) serve several important functions in the gastrointestinal tract, including generation of pacemaker activity (Langton et al., 1989; Ward et al., 1994; Huizinga et al., 1995), neurotransduction (Burns et al., 1996; Ward et al., 2000a; Sanders et al., 2010), and responses to stretch (Won et al., 2005). Electrical activity in ICC is transmitted to other cells in the tunica muscularis via gap junctions (Komuro, 2006). Smooth muscle cells (SMCs), ICC and another class of platelet-derived growth factor receptor alpha (PDGFRα)-positive interstitial cells are linked together in a coupled network known as the Smooth muscle cells, ICC and PDGFRα+ cells (SIP) syncytium (Sanders et al., 2012). The pacemaker function of ICC was deduced from morphological studies (Faussone Pellegrini et al., 1977; Thuneberg, 1982), dissection of pacemaker regions experiments (Smith et al., 1987a; Hara et al., 1986; Serio et al., 1991), studies on isolated ICC and SMCs (Langton et al., 1989; Sanders et al., 2014a; Zhu et al., 2009), studies of muscles from animals with loss-of-function mutations in c-Kit signaling (Ward et al., 1994; Ward et al., 1995; Huizinga et al., 1995) and simultaneous impalements of ICC and SMCs (Cousins et al., 2003). While these experiments were strongly indicative of the obligatory role of ICC as pacemakers in GI smooth muscles and essential role in normal electrical and contractile patterns, no experiments to date have measured pacemaker activity in ICC and responses of SMCs in terms of Ca2+ signaling and contraction simultaneously.
The anatomy and distribution of ICC varies from place to place throughout the GI tract. Some areas have only an intramuscular type of ICC (ICC-IM) that are closely aligned with and transduce inputs from excitatory and inhibitory enteric motor neurons (Burns et al., 1996; Beckett et al., 2004; Lies et al., 2014; Sanders et al., 2014b). Other regions contain ICC-IM and pacemaker types of ICC, that exist as a network in the myenteric plexus region of most areas of the gut (ICC-MY) (Rumessen and Thuneberg, 1982; Faussone-Pellegrini, 1992; Komuro et al., 1996; Burns et al., 1997; Komuro, 1999). The colon is more complex in that there are at least four types of ICC, distinguished by their anatomical locations and functions (Ishikawa and Komuro, 1996; Vanderwinden et al., 2000; Vanderwinden et al., 1996; Rumessen et al., 2013). One class of colonic ICC lies along the submucosal surface of the circular muscle (CM) layer (ICC-SM). These cells are known to provide pacemaker activity in colonic muscles, and their activity is integrated with a second frequency of pacemaker activity generated by ICC-MY (Smith et al., 1987a; Smith et al., 1987b; Durdle et al., 1983; Huizinga et al., 1988; Yoneda et al., 2002; Plujà et al., 2001). Pacemaker activities generated by ICC-SM and ICC-MY cause depolarization of SMCs, generation of Ca2+ action potentials, and excitation-contraction coupling (Yoneda et al., 2004).
ICC-SM generate slow waves in the canine colon (Smith et al., 1987a; Berezin et al., 1988). These are large amplitude and long-duration events that produce phasic contractions (Keef et al., 1992). The integrity of the ICC-SM network is required for regenerative propagation of slow waves, and disruption of the network causes passive decay of slow waves within a few millimeters (Sanders et al., 1990). Electrical coupling of ICC-SM in a network is an important feature allowing the pacemaker activity to coordinate the electrical activation of SMCs. ICC-SM in proximal colons of rodents also display pacemaker function; however, the frequency of the slow waves is higher (10–22 min−1, mean 14.8. min−1) (Yoneda et al., 2002). Slow waves, in this region of the GI tract, consist of a rapid upstroke phase, 148 mVs−1, that settles to a plateau phase lasting approximately 2 s. The slow waves are coupled to low-amplitude CM contractions (Yoneda et al., 2004). Colonic slow waves have been reported to depend upon both Ca2+ entry and intracellular Ca2+ release mechanisms; however, Ca2+ signaling in colonic pacemaker cells and the coupling of Ca2+ events to the electrical responses were not clarified.
Previous studies have shown that all classes of ICC in the GI tract express Ca2+-activated Cl- channels encoded by Ano1 (Gomez-Pinilla et al., 2009; Hwang et al., 2009). This conductance is required for slow wave activity (Hwang et al., 2009), and therefore Ca2+ dynamics in ICC are of fundamental importance in understanding pacemaker activity and electrical and mechanical rhythmicity in GI muscles. In the present study, we tested the hypothesis that Ca2+ transients in ICC-SM are linked to mechanical activation of the CM and that propagation of activity in ICC-SM is related to and controlled by Ca2+ entry via voltage-dependent Ca2+ conductances. Experiments were performed on tissues containing ICC-SM taken from mice with cell-specific expression of GCaMP6f in ICC, and changes in intracellular Ca2+ were monitored by confocal microscopy and digital video imaging.
Results
ICC-SM distribution within the submucosal plexus
We optimized a preparation in which the submucosal layer was separated from the tunica muscularis. We found that ICC-SM were adherent to the submucosal tissues, so this preparation allowed very clear high resolution of Ca2+ transients in ICC-SM in the complete absence of motion artifacts due to muscle contractions. We confirmed the presence and maintenance of ICC-SM networks, which occur in intact muscles, in these preparations.
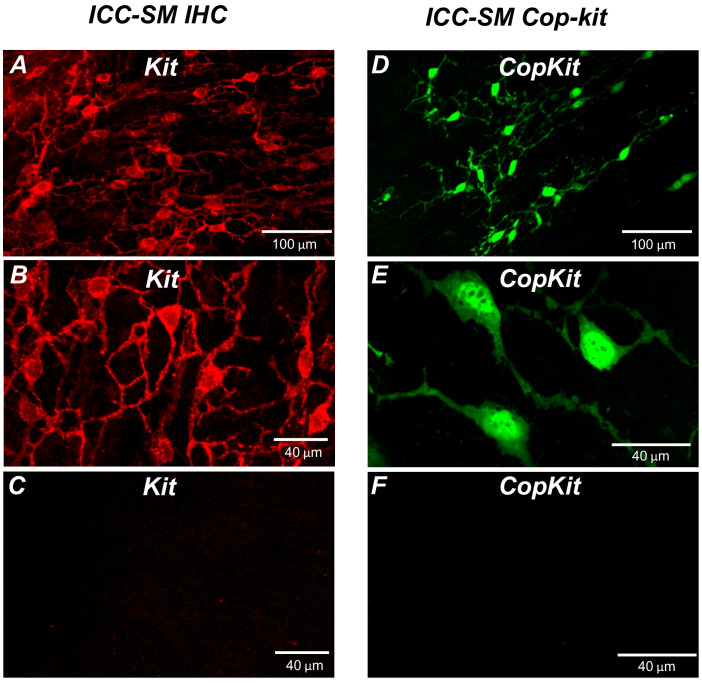
Kit immunoreactivity revealed a dense network of Kit-positive cells in the submucosal layer of the proximal colon (Figure 1A). The network consisted of ICC-SM interconnected with branching processes (Figure 1B). The average density of cell bodies was 312 ± 33 cells mm–2 (n = 6), and the average minimum separation between cell bodies was 49.3 ± 2.8 μm (Figure 1A&B; n = 6). Most of the ICC-SM network appeared to be adherent to the submucosal layer of the proximal colon. Isolation of the submucosa by sharp dissection showed that few ICC-SM (1.6 ± 2.1 cells mm–2; Figure 1C; n = 6) remained adherent to the muscularis in the uppermost region (1–2 μm) of the proximal colon.
Figure 1. Distribution of Kit+ submucosal interstitial cells of Cajal (ICC-SM) in the murine colon.
(A and B) Images of Kit+ (ICC-SM) in isolated submucosal layer of wild-type animals at ×20 and×40 magnifications (n = 6). Scale bars are 100 μm and 40 μm respectively. (C) Absence of ICC-SM on the submucosal surface of the tunica muscularis of the proximal colon after removing the submucosa. ICC-SM networks are intact in preparations of submucosal tissues removed from the muscle. (D and E) ICC-SM were present in Kit+/copGFP mice at ×20 and×60 magnifications (n = 6). Scale bars are 100 μm and 40 μm respectively. (F) Absence of Kit+ (ICC-SM) on the submucosal surface of the tunica muscularis after removing the submucosa from Kit+/copGFP proximal colon muscles (n = 6). Scale bars are 40 μm in both C and F. All image parameters were analyzed using Image J software.
Colonic muscles from Kit+/copGFP mice expressing the copGFP exclusively in ICC were also used to confirm the distribution of ICC-SM. copGFP-positive cells in the submucosal region were present at an average density of 284 ± 27 cells mm–2 and the average minimum separation between cell bodies was 52.7 ± 2.9 μm (Figure 1D and E; n = 6). ICC-SM were not resolved at the surface of the muscle layer after removing the submucosal layer (Figure 1F; n = 6). Colonic muscles expressing GCaMP6f exclusively in ICC were used to monitor Ca2+ signaling in ICC-SM. GCaMP6f-positive cells in the submucosal region were present at an average density of 291 ± 36 cells mm–2 (n = 10), and the average minimum separation between cell bodies was 50.6 ± 3.8 μm. Representative images of the GCaMP6f-expressing cells in ICC-SM are shown below in the Ca2+-imaging experiments (Figure 2A; Figure 3A and Figure 6A).
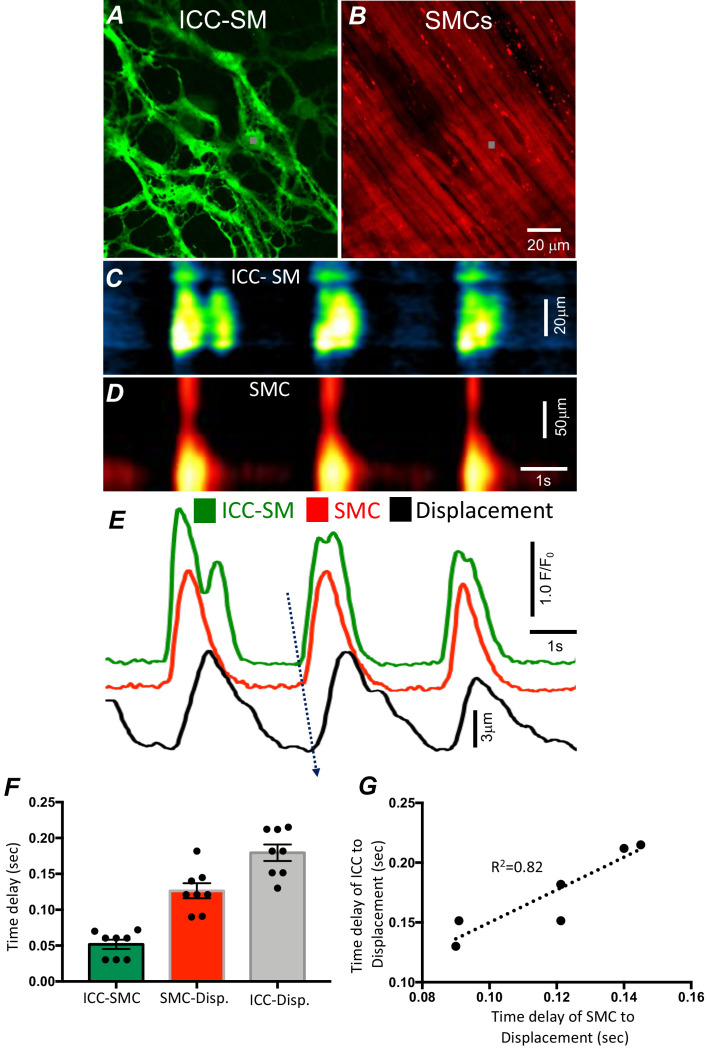
Figure 2. Temporal sequence of Ca2+ transients firing in submucosal interstitial cells of Cajal (ICC-SM) and smooth muscle cells (SMCs).
Representative dual-color imaging of ICC-SM (green; A) and SMCs (red; B) Ca2+ transients were recorded simultaneously from proximal colonic muscles of Kit-iCre-GCaMP6f/Acta2-RCaMP1.07 mice (see Materials and methods for details). C and D Spatiotemporal maps (STMaps) of Ca2+ signals in ICC-SM and SMCs during a recording period (gray boxes in A and B denote cell locations; see Video 1). The STMaps show coordinated firing of Ca2+ transients in both types of cells. Ca2+ transient traces are plotted in E (ICC-SM-green, SMCs-red). Tissue displacement was also monitored and plotted (black trace; E). The black dotted arrow depicts the sequence of activation firing of Ca2+ transients in ICC-SM, to activation of Ca2+ events in SMCs, and tissue displacement. (F) A comparison of latencies (ms) from the start of the initiation of Ca2+ transients in ICC-SM to SMC activation and tissue displacement (n = 8). (G) Correlation analysis of latencies between ICC-SM and SMC Ca2+ transients and tissue displacement (R2 = 0.82). All data graphed as mean ± SEM.
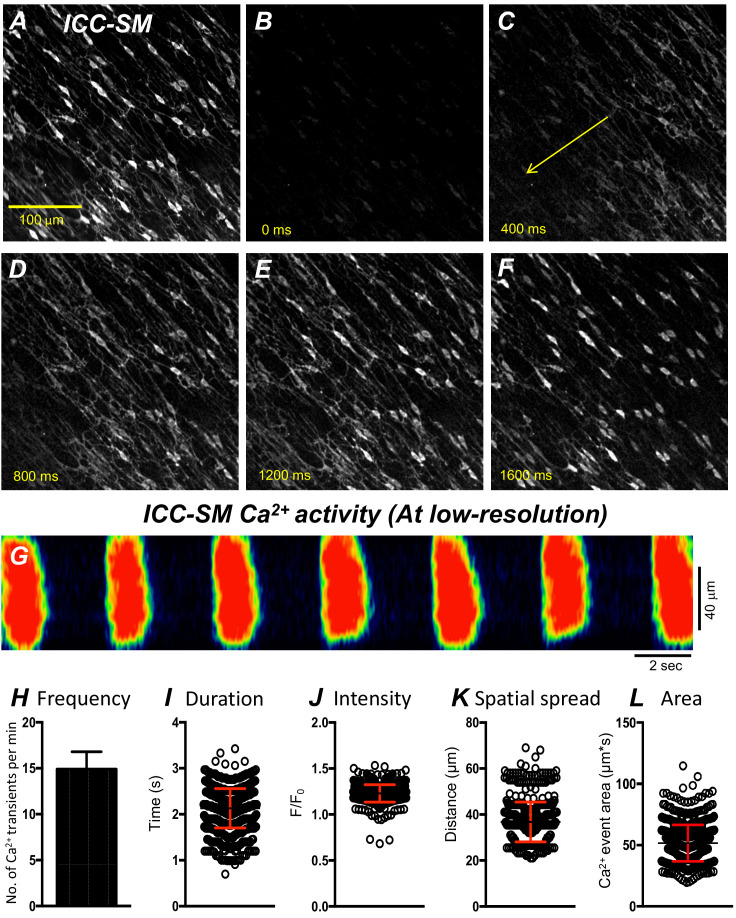
Figure 3. Propagating Ca2+ waves in submucosal interstitial cells of Cajal (ICC-SM) network.
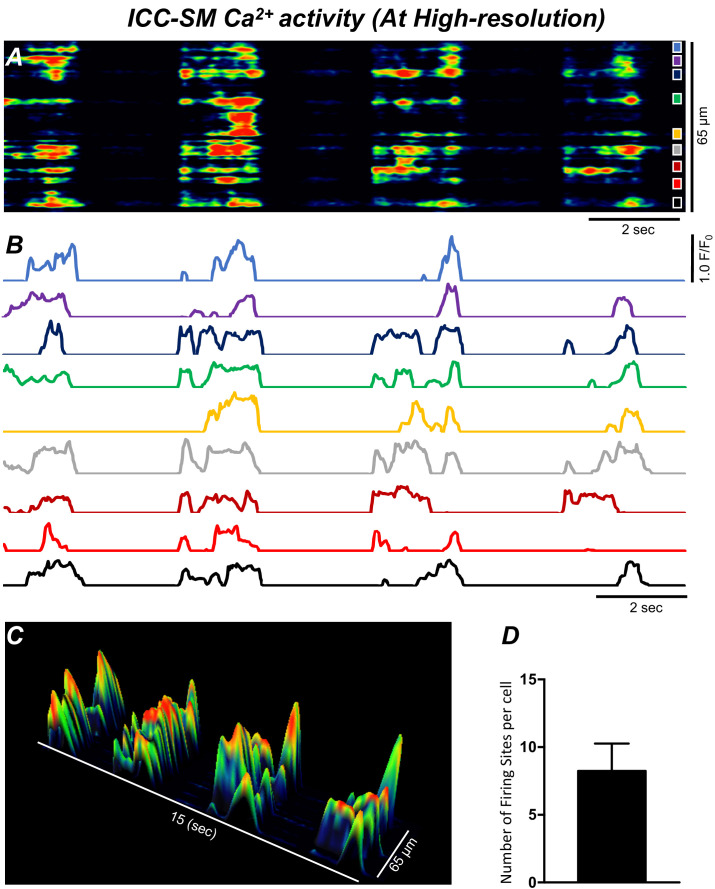
(A) An image of ICC-SM network from the proximal colon of a Kit-iCre-GCaMP6f mouse visualized at 10x (low resolution) magnification. Scale bar is 100 μm in A and pertains to all images in B-F. A-F Representative montage of the propagation of a Ca2+ wave throughout the ICC-SM network. The yellow arrow in panel C indicates the direction of Ca2+ transient propagation. (G) Spatiotemporal map (STMap) of Ca2+ signal in single ICC from the movie in panel A showing rhythmic firing of Ca2+ waves. Ca2+ activity is color coded with warm areas (red, orange) representing bright areas of Ca2+ fluorescence and cold colors (blue, black) representing dim areas of Ca2+ fluorescence. Summary data of multiple ICC-SM Ca2+-firing parameters (n = 15): frequency H, duration I, intensity J, spatial spread K and area of Ca2+ transients L. All data graphed as mean ± SEM.
Pacemaker activity of ICC-SM drives responses in SMCs
We developed a new mouse strain (Kit-iCre-GCaMP6f/Acta2-RCaMP1.07) that allowed simultaneous optical measurements of Ca2+ transients in ICC-SM and SMCs and tissue displacement (i.e. an optical means of monitoring muscle contractions). The dual-color mouse allowed simultaneous imaging of the two optogenetic Ca2+ sensors with different fluorescence characteristics (ensuring minimal spectral overlap). The sensors were expressed in a cell-specific manner in ICC and SMCs to characterize the coordination of signaling between ICC and SMCs (Figure 2 and Video 1). Ca2+ imaging of colonic muscles with attached submucosa showed a strong correlation between Ca2+ transients in the ICC-SM network and SMCs (Figure 2A–E; n = 8). Ca2+ transients recordings were obtained from ICC-SM and SMC at the exact coordinates in the pixel matched images (indicated by the gray box; Figure 2A&B) The sequence of activation began in ICC-SM and spread to SMCs with a latency of 56 ± 14 ms (Figure 2E&F; n = 8). Muscle displacement, an indicator of muscle contraction, was also measured and displayed latencies of 120 ± 17 ms between the rise of Ca2+ in SMCs and resolvable displacement (Figure 2E&F; n = 8) and 180 ± 15 ms between activation of Ca2+ transients in ICC-SM and contractile displacement (Figure 2E&F; n = 8). In a few instances, the time delay between ICC-SM and SMCs was not readily resolved. This could have resulted from a shift in the site of dominant pacemaker activity to a region outside the field of view (FOV). There was close correlation between the temporal latencies of ICC-SM and SMCs to tissue displacement (Figure 2G; R2 = 0.82), indicating the pacemaker nature of ICC-SM, the coupling between ICC-SM and SMCs and the management of Ca2+ signaling and contractions in SMCs by the pacemaker activity of ICC-SM.
Video 1. Simultaneous dual-color imaging of submucosal interstitial cells of Cajal (ICC-SM) and smooth muscle cells (SMCs) in the colon.
A video of propagating Ca2+ waves through an ICC-SM network in the proximal colon of Kit-iCre-GCaMP6f/Acta2-RCaMP1.07 strain imaged with a ×20 objective. simultaneous imaging of two optogenetic Ca2+ sensors: GCaMP6f in ICC-SM (left field of view [FOV]; green) and RCaMP1.07 in SMCs (right FOV; red) with different fluorescence characteristics (ensuring minimal spectral overlap). The signal coordination between ICC and SMCs showing the correlation between Ca2+ transients in the ICC-SM network and activation of SMCs adjacent to ICC-SM. ICC-SM transients (green trace) preceded Ca2+ signals in SMCs (red trace). The scale bar (yellow) is 25 µm.
Global Ca2+-firing patterns in ICC-SM
Movement generated by muscle contractions is always an issue when imaging cells in muscle preparations in situ. Therefore, we used preparations of ICC-SM adherent to submucosa that were separated from muscle strips in most experiments. At low-magnification (×10), rhythmic Ca2+ waves occurred and spread through ICC-SM networks in isolated submucosal preparations. The Ca2+ waves occurred at 8–22 cycle min−1 (Figure 3A–G) and averaged 14.9 ± 1.9 cycle min−1 (Figure 3H; n = 15; c = 120). Similar behavior occurred at similar frequencies (i.e. 16.2 ± 1.4 cycle min−1; n = 6) in ICC-SM attached to muscle tissues. Ca2+ waves propagated through the isolated ICC-SM networks with velocities of 219 ± 19 mm/s. Ca2+ transients imaged at ×10 appeared to be global and had average durations of 2.1 ± 0.4 s (Figure 3I; n = 15; c = 120) and amplitudes of 1.2 ± 0.3 ΔF/F0 (Figure 3J; n = 15; c = 120). The spatial spread of Ca2+ transients was 36.8 ± 0.4 µm (Figure 3K; n = 15; c = 120). The average area occupied by Ca2+ transients in the spatiotemporal maps was 51.6 ± 1.6 µm*s (Figure 3L; n = 15; c = 120).
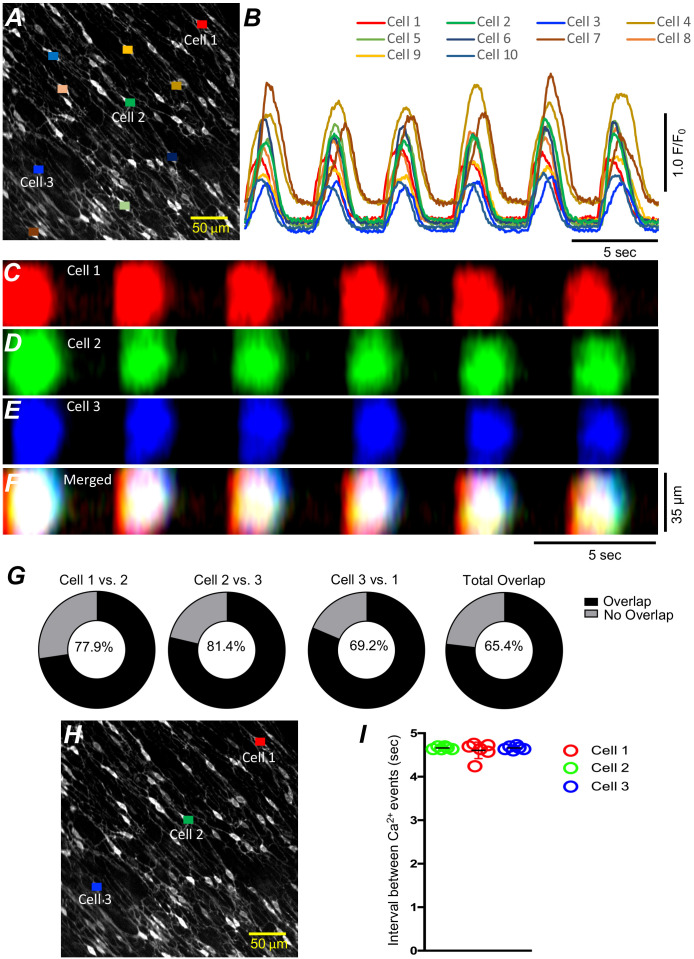
Cell-to-cell propagation of Ca2+ transients in ICC‐SM is shown in Figure 3 and Figure 4. Firing of global Ca2+ transients appeared to be sequential in nature (Figure 4A–G; n = 10; c = 60), as there was a strong correlation between the occurrence of global Ca2+ transients in multiple ICC-SM cells (Figure 4B; n = 10; c = 60). Overlays of STMaps of Ca2+ activity in adjacent cells running parallel to each other (Figure 4F&H) showed that the Ca2+ transients overlapped (65.4% of total Ca2+transients overlapped in the FOV during a 30-s recording period) (Figure 4G). Comparison between intervals of Ca2+ firing of multiple ICC-SM showed strong sequential firing as each cell demonstrated very close temporal firing intervals (Figure 4H&I; n = 6; c = 18). These results suggest that Ca2+ firing in ICC‐SM networks are entrained and propagate cell‐to‐cell.
Figure 4. Submucosal interstitial cells of Cajal (ICC-SM) Ca2+ signaling activity is entrained.
(A) Raw image of multiple colonic ICC-SM in a field of view (FOV). Ten cells were color coded, and their Ca2+ fluorescence activity traces were plotted in B. Three ICC-SM (coded as red, green and blue regions of interest; ROIs in A) were selected and STMaps of the average Ca2+ fluorescence intensity across the diameter of the cell during a 30-s recording were constructed C–E. STMaps from each cell were color coded to correspond to the red, green, and blue cells and merged into a summed STMap in F. Percentage of fluorescence area overlap of intracellular Ca2+ transients between ICC-SM cells is plotted in G and cell location is identified in H (n = 10). The durations of Ca2+ waves were such that there was a significant overlap of the Ca2+ events in individual cells across the FOVs at this magnification. Thus, each cell within the FOVs demonstrated similar temporal firing intervals (calculated as peak to peak intervals, n = 6) as shown in I.
ICC-SM Ca2+ signals composed of multiple Ca2+-firing sites
Low-power imaging suggested global changes in cell Ca2+, however, imaging of ICC-SM at higher power (×60–×100) allowed a more detailed visualization of the subcellular nature of the Ca2+ transients and revealed complex firing patterns. Imaging of ICC-SM networks with high spatial resolution showed that subcellular Ca2+ transients originated from distinct firing sites (Figure 5; n = 25). STMaps constructed from Ca2+ transients in single ICC-SM during 30 s of imaging (Figure 5A&B) identified the positions of all frequent firing sites within ICC-SM. Activity plots of individual firing sites showed they can fire once or multiple times during single Ca2+ waves (Figure 5B&C). The number of firing sites in a single ICC-SM ranged from 5 to 12 sites with an overall average of 8.2 ± 2 sites/cell (Figure 5D; n = 25).
Figure 5. Ca2+ transients in submucosal interstitial cells of Cajal (ICC-SM) arise from multiple firing sites.
(A) Spatiotemporal map (STMap) of Ca2+-firing sites from a single ICC-SM during four consecutive firing cycles. Ca2+ activity is color coded with warm areas (red, orange) representing bright areas of Ca2+ fluorescence and cold colors (blue, black) representing dim areas of Ca2+ fluorescence. Nine distinct firing sites were detected in this cell and are marked as color squares on the right of the STMap. (B) Traces of the Ca2+ transients at each of the nine Ca2+-firing sites shown on the STMap in panel A. (C) 3-D surface plots showing the Ca2+ activity at the Ca2+-firing sites shown on the STMap in panel A over four consecutive Ca2+-firing cycles. (D) Average number of the firing sites per cell (n = 25). Data graphed as mean ± SEM.
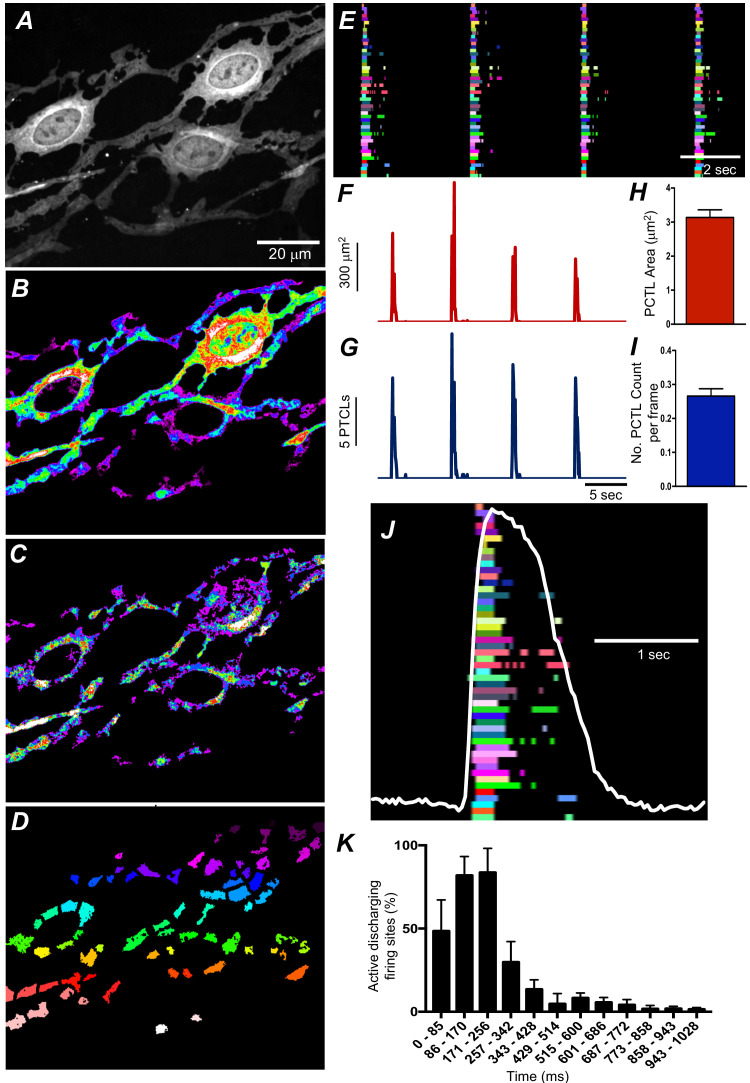
We employed Ca2+ particle analysis (Drumm et al., 2017) to identify and quantify Ca2+-firing sites in ICC-SM. Ca2+-firing sites were distinguished based on their spatial and temporal characteristics (Figure 6A–D and Video 2). The firing sites were color coded, as shown in the example in Figure 6D, to visualize and quantify all active sites firing Ca2+ transients. The activities of firing sites were plotted as occurrence maps (Figure 6E). Occurrence maps of all firing sites in a region of ICC-SM network demonstrated that the global Ca2+ waves resolved at low power resulted from summation of localized Ca2+ transients from a multitude of firing sites (Figure 6D–J). Ca2+ transients were clustered temporally as Ca2+ waves swept through ICC-SM networks (Figure 6E&J). Also apparent from the occurrence maps was that the firing sequence of sites changed from Ca2+ wave to Ca2+ wave. Not all firing sites discharged Ca2+ transients during each wave cycle, and some sites fired more than once (Figure 6E&J). From particle analysis the average particle area/frame of Ca2+ transients averaged 3.2 ± 0.4 µm2 (Figure 6H; n = 25) and particle count/frame averaged 0.27 ± 0.1 (Figure 6I; n = 25).
Figure 6. Submucosal interstitial cells of Cajal (ICC-SM) Ca2+ transient initiation sites.
(A) Representative image of an ICC-SM network from proximal colon of a Kit-iCre-GCaMP6f mouse at ×60 magnification. (B) Heat map of total Ca2+ PTCLs generated from the video shown in A (see Video 2). (C) Particles were thresholded temporally to generate a heat map indicating Ca2+-firing sites in the ICC-SM. (D) Image showing individually color-coded Ca2+-firing sites in the field of view (FOV) shown in C. (E) The temporal characteristics of each individual, color-coded firing site is displayed as an occurrence map, with each ‘lane’ representing the occurrence of firing PTCLs within each firing site. Activity traces for PTCLs for the duration of recording from the entire FOV are shown in F and G. Traces for PTCL area (F; red) and PTCL count (G; blue) are shown. H and I Summary graphs show average PTCL areas and counts for Ca2+-firing sites in ICC-SM (n = 25). (J) One Ca2+ wave in ICC-SM (white trace) is expanded and the numerous Ca2+ initiation sites that fired during this wave are superimposed. (K) Distribution plot showing average percentages of firing sites during a Ca2+ wave. Values are calculated for 1 s and plotted in 85 ms bins (n = 25). Data graphed as mean ± SEM.
Video 2. High spatial resolution ICC-SM Ca2+ signals composed of multiple Ca2+-firing sites.
A video showing subcellular Ca2+ transients in ICC-SM at high resolution imaged with a ×60 objective. Ca2+ signals were monitored using the genetically encoded Ca2+ indicator GCaMP6f. The left panel shows typical stellate-shaped ICC-SM with multiple interconnected processes. The scale bar (yellow) is 10 µm. The middle panel shows the Ca2+ particle (PTCL) activity, color coded in blue for raw PTCLs, and the centroids of particles are indicated in purple and green indicates Ca2+-firing sites. Note the multiple-site firing of Ca2+ transients in ICC-SM. The right panel shows initiation/firing sites accumulation map. The pattern of firing sites Ca2+ activity was temporally clustered as Ca2+ wave oscillations swept through ICC-SM networks.
Particle analysis also showed that Ca2+-firing sites were most active during the first ~256 ms of a Ca2+ wave, and activity decayed with time. This point was further illustrated by distribution plots showing the average percentage of firing sites discharging at various times during Ca2+ waves (Figure 6K; n = 25). Initially high firing and decay as a function of time suggest that Ca2+ entry mechanisms may be important for: (i) initiating Ca2+ transients and (ii) organizing the occurrence of Ca2+ transients into clusters. It is also possible that Ca2+ stores, loading during the diastolic period between Ca2+ waves, are more excitable at the onset of each Ca2+ wave.
Ca2+ influx mechanisms are required for initiation of clustered Ca2+ transients in ICC-SM
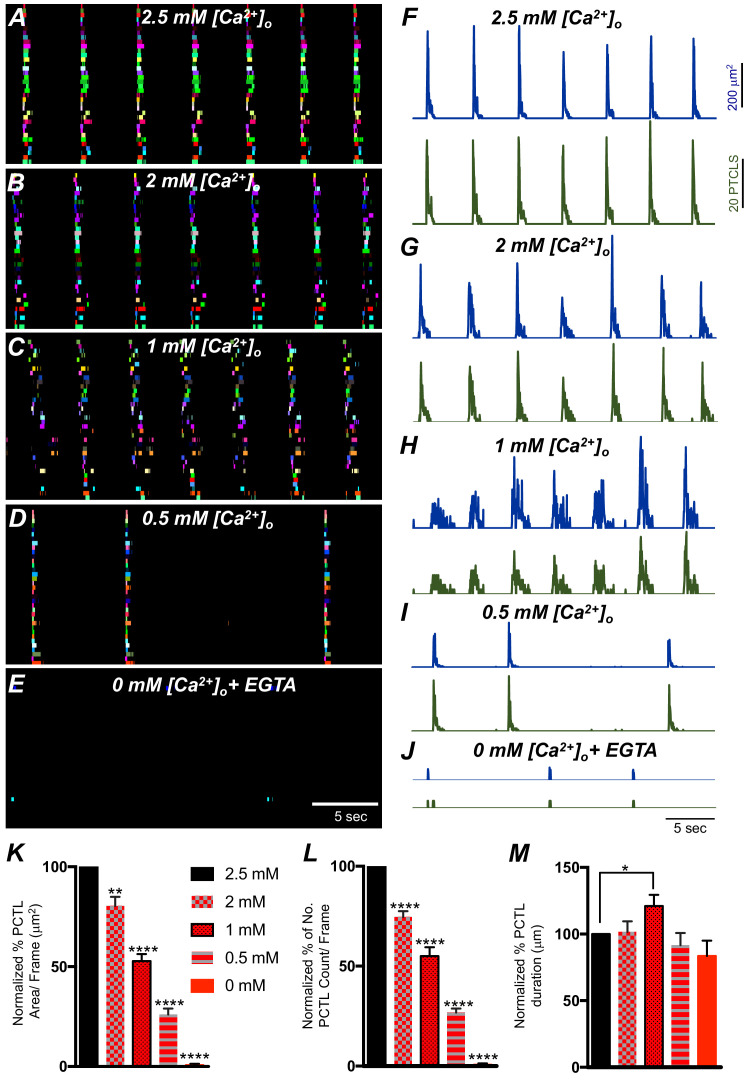
The effects of reducing extracellular Ca2+ ([Ca2+]o) on Ca2+ transients was investigated to evaluate the importance of Ca2+ influx for the patterning of Ca2+ signaling in ICC-SM. Removal of [Ca2+]o abolished Ca2+ transients in ICC-SM within 10 min (Figure 7E–L; n = 6). Stepwise reduction in [Ca2+]o (from 2.5 mM to 0 mM) showed that Ca2+ transients decreased in a concentration-dependent manner (Figure 7; n = 6). ICC-SM Ca2+ PTCL area and count of firing sites were reduced in response to lowering [Ca2+] o (Figure 7F–J; n = 6). Reducing [Ca2+]o from 2.5 mM (control conditions) to 2 mM caused a reduction in the average PTCL area by 19.6 ± 4.5% (Figure 7K; n = 6) and PTCL count by 25.4 ± 2.9% (Figure 7L; n = 6) with no significant change in the duration of PTCLs (Figure 7M; n = 7). Further reduction of [Ca2+] o to 1 mM reduced Ca2+ transient parameters by 47.2 ± 3.5% PTCL area (Figure 7K; n = 6) and 45.1 ± 4.4% PTCL count (Figure 7L; n = 6) and showed a significant change in the duration of PTCLs (Figure 7M; n = 7). Lowering [Ca2+] o to 0.5 mM also reduced Ca2+ PTCL area by 73.9 ± 3% (Figure 7K; n = 6) and PTCL count by 73.2 ± 2% (Figure 7L; n = 6) with no significant change in the duration of PTCLs (Figure 7M; n = 7). Removal of Ca2+ from the extracellular solution (Ca2+‐free KRB solution containing 0.5 mM EGTA) abolished Ca2+ signals 8–10 min after solution replacement. The Ca2+ transient PTCL area was reduced by 99.4 ± 0.6% (Figure 7K; n = 6) and PTCL count by 99.3 ± 0.7% (Figure 7L; n = 6) with no significant change in the duration of PTCLs (Figure 7M; n = 7). We also noted that the highly organized CTCs occurring in the presence of 2.5 mM [Ca2+] o became less organized as [Ca2+] o was reduced (e.g. compare the tight clusters in Figure 7A with the more diffuse clusters in Figure 7C). These experiments highlight the importance of Ca2+ entry mechanisms in Ca2+ signaling within ICC-SM.
Figure 7. The effect of lowering [Ca2+]o on Ca2+ transients in submucosal interstitial cells of Cajal (ICC-SM).
(A) ICC-SM Ca2+ transients and Ca2+-firing sites were color coded and plotted as an occurrence map under control conditions with [Ca2+]o = 2.5 mM. (B–E) showing the effects of reducing [Ca2+]o to 2 mM B; 1 mM C; 0.5 mM D and after Ca2+ removal of [Ca2+]o (0 mM added and final solution buffered with 0.5 mM EGTA) E. F-J Traces of Ca2+ PTCL activity in ICC-SM (PTCL area, blue and PTCL count, green) under control conditions F and after reducing [Ca2+]o to 2 mM G; 1 mM H; 0.5 mM I and after removal of [Ca2+]o as shown in J. Summary graphs of Ca2+ PTCLs in ICC-SM under control conditions and with reduced [Ca2+]o in K (PTCL area); L (PTCL count) and M (PTCL duration). Data were normalized to controls and expressed as percentages (%). Significance was determined using one-way ANOVA, * = p<0.1, ** = p<0.01, **** = p<0.0001, n = 6. All data graphed as mean ± SEM.
Molecular expression of Ca2+entry channels in ICC-SM
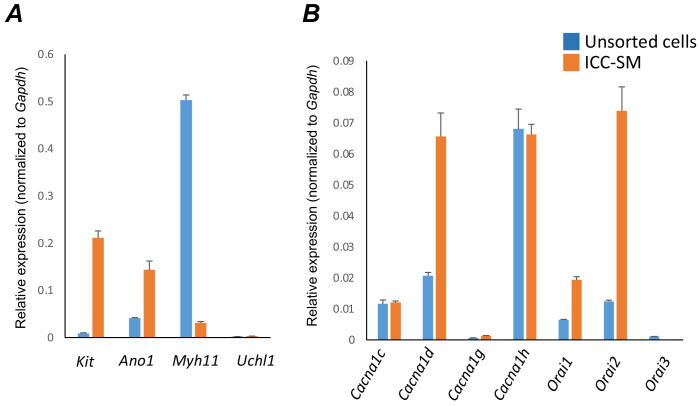
The apparent dependence on the Ca2+ gradient to maintain pacemaker function in ICC-SM suggests that Ca2+ entry mechanisms are critical for initiation and organization of CTCs. Therefore, we examined expression of several Ca2+ channels that might be responsible for Ca2+ entry in ICC-SM (Figure 8). After isolation of the submucosal layer from the proximal colon of Kit+/copGFP mice and subsequent cell dispersion, we sorted copGFP-positive ICC-SM with fluorescence activated cell-sorting (FACS) and evaluated the expression of voltage-dependent and voltage-independent Ca2+ channels by qPCR (Figure 8A&B). First, we confirmed the purity of sorted ICC-SM with cell-specific markers (Figure 8A). Kit receptors and ANO1 channels are signatures of ICC throughout the GI tract and enrichment of Kit and Ano1 expression was observed in sorted ICC-SM compared to unsorted cells (Kit expression was 0.21 ± 0.014; Ano1 expression was 0.14 ± 0.02 relative to Gapdh). The expression levels of Myh11 an SMC marker and Uch11 a pan neuronal marker encoding PGP9.5 were minimal (Myh11 expression was 0.03 ± 0.002; Uch11 expression was 0.002 ± 0.0001 relative to Gapdh), confirming the purity of ICC-SM sorted by FACS.
Figure 8. Molecular expression of genes related to Ca2+ entry channels.
(A) Relative expression of cellular-specific biomarker genes in submucosal interstitial cells of Cajal (ICC-SM; sorted to purity by FACS) and compared with unsorted cells dispersed from submucosal tissues obtained from Kit+/copGFP mice. Relative expression was determined by qPCR and normalized to Gapdh expression. Genes examined were Kit (tyrosine kinase receptor, found in ICC), Ano1 (Ca2+-activated Cl- channel), Uch11 (neural marker encoding PGP 9.5), Myh11 (smooth muscle myosin). (B) Relative expression of major Ca2+ entry channels considered most likely to be expressed in colonic ICC from RNAseq of total ICC in murine colon (Lee et al., 2017). L-Type Ca2+ channels (Cacna1c and Cacna1d) T-type Ca2+ channels (Cacna1g and Cacna1h) and Store‐operated Ca2+ entry (SOCE) channels (Orai1, Orai2 and Orai3) were evaluated. All data graphed as mean ± SEM (n = 4).
ICC-SM expressed L-type voltage-dependent Ca2+ channels encoded by Cacna1c and Cacna1d abundantly (CaV 1.2 and CaV 1.3 channels, respectively). Cacna1c showed a 0.012 ± 0.0005 and Cacna1d showed 0.066 ± 0.008 relative to Gapdh (Figure 8B; n = 4). ICC-SM also expressed Cacna1h (CaV 3.2) and to a lesser extent Cacna1g (CaV 3.1), both T-type voltage-dependent Ca2+ channels (Figure 8B; n = 4). Cacna1h expression was abundant in ICC-SM (0.07 ± 0.003 relative to Gapdh). Cacna1g expression was less than Cacna1h 0.0014 ± 0.0001 relative to Gapdh (Figure 8B; n = 4).
Maintenance and refilling of cellular Ca2+ stores may also be important for mediation and shaping of Ca2+ signals in ICC-SM. Store‐operated Ca2+ entry (SOCE) has been established as a mechanism for filling stores upon depletion (Putney, 2018; Gibson et al., 1998). Contributions from SOCE via STIM and Orai interactions are essential for maintenance of Ca2+ stores in other types of ICC (Zheng et al., 2018; Youm et al., 2019; Drumm et al., 2020a; Drumm et al., 2020b). Colonic ICC-SM showed enrichment in Orai1 and Orai2 but Orai3 was not resolved (Figure 8B; n = 4). Orai1 expression was 0.02 ± 0.001 relative to Gapdh and Orai2 was 0.074 ± 0.008 relative to Gapdh (Figure 8B; n = 4).
L-type Ca2+ channels are important for organization of Ca2+ transients in ICC-SM
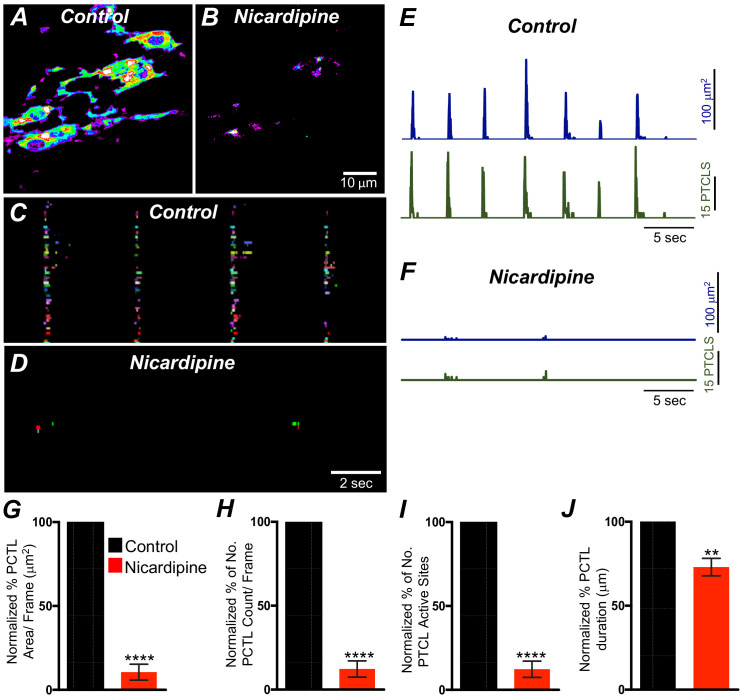
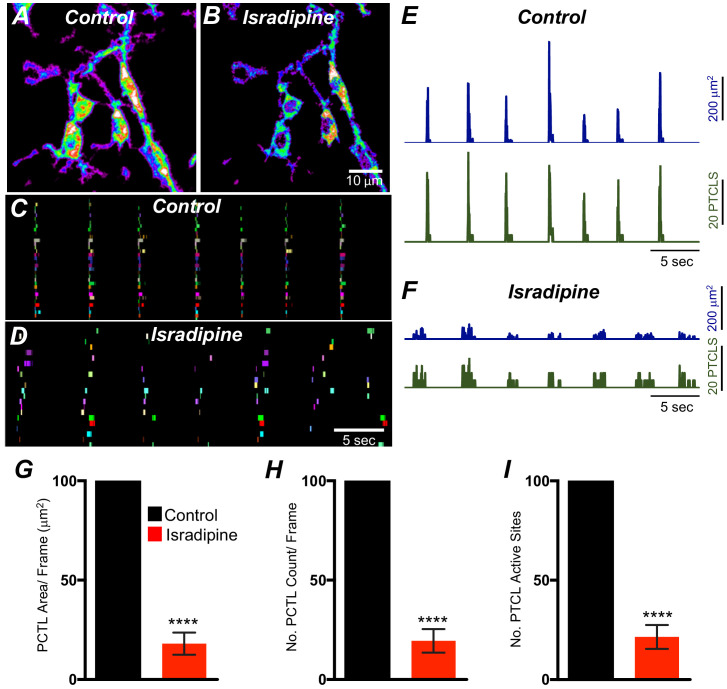
L-type Ca2+ channels (CaV 1.3, CaV 1.2) were expressed in ICC-SM, and Ca2+ transients showed dependence on the Ca2+ gradient. Previous studies have reported that L-type channel antagonists inhibit slow waves in the colon (Yoneda et al., 2002; Keef et al., 2002). Therefore, we evaluated the contributions of Ca2+ entry via L-type channels to Ca2+ transients in ICC-SM. Nicardipine (1 μM) abolished Ca2+ transients in ICC-SM (Figure 9A&B; n = 8). Firing site occurrence maps (Figure 9C&D; n = 8) describe the inhibitory effects of nicardipine on Ca2+ transients. Ca2+ PTCL area was reduced to 10.5 ± 4.7% (Figure 9E–G; n = 8) and PTCL count was reduced to 12.3 ± 4.8% (Figure 9H; n = 8). The number of firing sites also decreased to 8.4 ± 3% in the presence of nicardipine (Figure 9I; n = 8). Isradipine inhibits CaV 1.2 and CaV 1.3 equally (Rüegg and Hof, 1990; Koschak et al., 2001). Isradipine (1 μM) also reduced Ca2+ transients in ICC-SM (Figure 9—figure supplement 1A&B; n = 7). ICC-SM Ca2+-firing was reduced in the presence of isradipine as shown in the firing sites occurrence maps (Figure 9—figure supplement 1C&D). PTCL area was reduced to 18 ± 5% (Figure 9—figure supplement 1E–G; n = 7), and PTCL count was reduced to 19.5 ± 6% (Figure 9—figure supplement 1H; n = 7). The number of firing sites was inhibited by isradipine to 21.5 ± 6% (Figure 9—figure supplement 1I; n = 7). These data show that ICC-SM Ca2+ transients depend upon Ca2+ influx via L-type Ca2+ channels, and since isradipine had nearly the same or a lesser effect on Ca2+ transients than nicardipine, CaV 1.2 appear dominant as the Ca2+ entry mechanism.
Figure 9. L-type Ca2+ channel antagonist, nicardipine effects on submucosal interstitial cells of Cajal (ICC-SM) Ca2+ transients.
A and B Representative heat-map images of an ICC-SM network from the proximal colon of a Kit-iCre-GCaMP6f mouse showing active Ca2+ PTCLs under control conditions and in the presence of nicardipine (1 μM). Ca2+ activity is color coded with warm areas (white, red) representing bright areas of Ca2+ fluorescence and cold colors (purple, black) representing dim areas of Ca2+ fluorescence. Scale bar is 10 μm in both A and B. C and D Firing sites showing Ca2+ activity in ICC-SM. Firing sites were color coded and plotted as an occurrence map under control conditions and in the presence of nicardipine (1 μM). Traces of firing sites showing PTCL area (E; blue) and PTCL count (E; green) under control conditions and in the presence of nicardipine; PTCL area (F; blue) and PTCL count (F; green) show the inhibitory effects of nicardipine on Ca2+ transients in ICC-SM. Summary graphs of Ca2+ PTCL activity in ICC-SM before and in the presence of nicardipine are shown in G (PTCL area/frame), (H) (PTCL count/frame), (I) the number of PTCL active sites and J (PTCL duration). Data were normalized to controls and expressed as percentages (%). Significance determined using unpaired t-test, ** = p<0.01, **** = p<0.0001, n = 8. All data graphed as mean ± SEM.
Figure 9—figure supplement 1. Isradipine effects on Ca2+ transients in submucosal interstitial cells of Cajal (ICC-SM).
T-type Ca2+ channels contribute to Ca2+ transients in ICC-SM
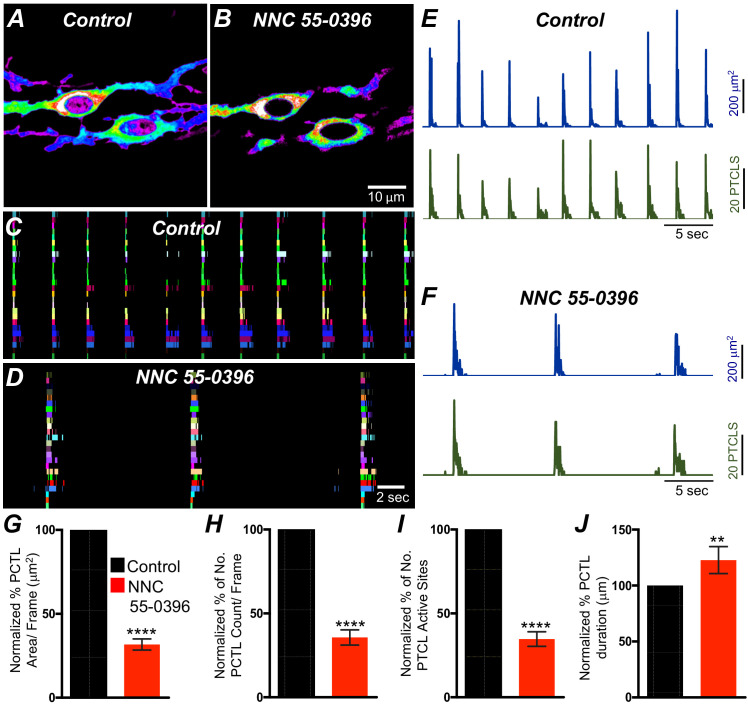
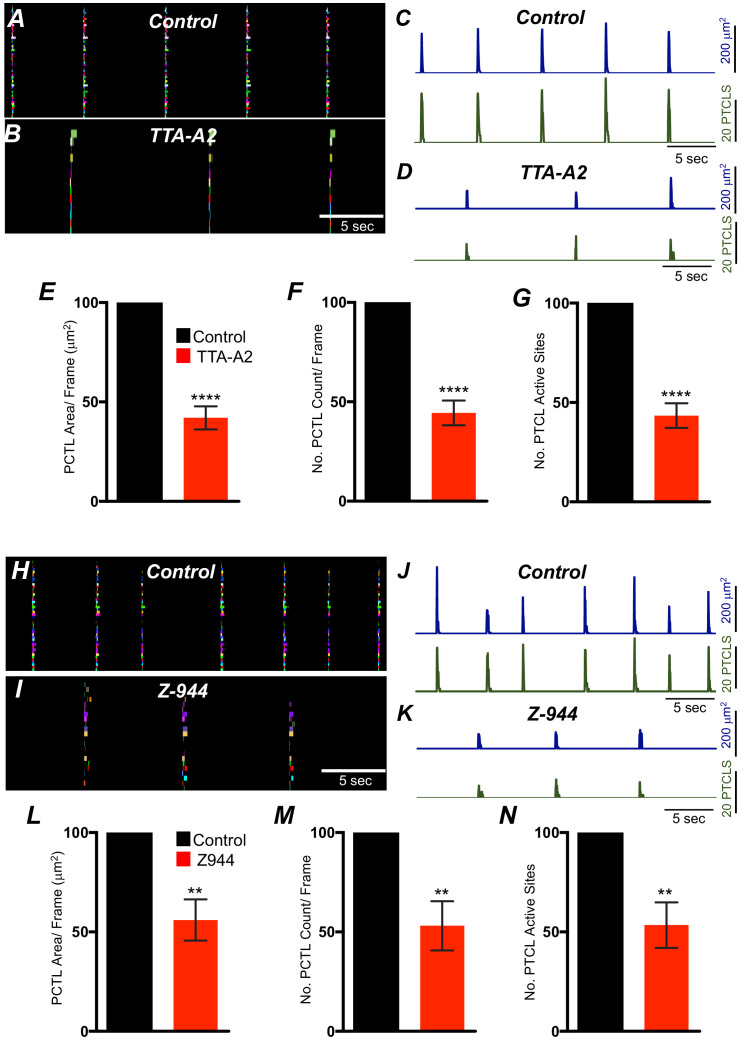
T-type Ca2+ channels have been identified as the dominant voltage-dependent Ca2+ conductance responsible for Ca2+ entry and propagation of slow waves in ICC-MY of the small intestine (Kito et al., 2005; Zheng et al., 2014; ). T-type channel antagonists (Ni2+ and mibefradil) reduced the rate-of-rise of the upstroke component of the slow waves and higher concentrations attenuated slow wave activity (Kito et al., 2005; Hotta et al., 2007; Yoneda et al., 2003). As reported above, ICC-SM express T-type channels Cacna1h and Cacna1g. Therefore, the role of T-type Ca2+ channels in modulating Ca2+signaling in ICC-SM was evaluated with specific T-type channel antagonists, NNC 55–0396 (10 μM), TTA-A2 (10 μM) and Z-944 (1 μM). NNC 55–0396 reduced Ca2+ transient firing (Figure 10A&B), Ca2+ transients firing sites occurrence maps (Figure 10C&D) and firing sites. PTCL area and PTCL count traces show a reduction in Ca2+ transient firing (Figure 10E&F). PTCL area was reduced to 31.7 ± 3.3% (Figure 10E–G; n = 9) and PTCL count was reduced to 35.6 ± 4.5% (Figure 10H; n = 9). The number of firing sites was reduced by NNC 55–0396 to 37.6 ± 4.3% (Figure 10I; n = 9). The duration of PTCLs was also reduced by NNC 55–0396 to 73 ± 5.2% (Figure 10J; n = 9). TTA-A2 showed similar inhibitory effects on ICC-SM Ca2+ transients (Figure 10—figure supplement 1). PTCL area was reduced to 42 ± 5.8% (Figure 10—figure supplement 1E; n = 7) and PTCL count was reduced to 44.4 ± 6.2% (Figure 10—figure supplement 1F; n = 7) The number of firing sites was also reduced by TTA-A2 to 43.4 ± 6% (Figure 10—figure supplement 1G; n = 7). Z-944 significantly reduced ICC-SM Ca2+ transients but was somewhat less effective than NNC 55–0396 or TTA-A2. PTCL area was reduced to 56 ± 10% (Figure 10—figure supplement 1E; n = 5), and PTCL count was reduced to 53 ± 12% (Figure 10—figure supplement 1F; n = 5) The number of firing sites was also reduced by Z-944 to 53.4 ± 11% (Figure 10—figure supplement 1G; n = 5). The data suggest that T-type Ca2+ channels also contribute to the initiation and organization of Ca2+ transients in ICC-SM.
Figure 10. T-type Ca2+ channel antagonist, NNC 55–0396 effects on ICC-SM Ca2+ transients.
A and B Representative heat-map images of Ca2+ transient particles in ICC-SM under control conditions A and in the presence of NNC 55–0396 (10 μM) (B). Active firing sites were color coded and plotted as an occurrence maps in the ICC-SM network under control C and NNC 55–0396 D conditions. Plots of Ca2+ transient particle activity of ICC-SM in control conditions and in the presence of NNC 55–0396 showing PTCL area (blue) and PTCL count (green) under control conditions E and in the presence of NNC 55–0396 F. Summary graphs of average percentage changes in PTCL area G, PTCL count H, the number of PTCL active sites I and PTCL duration J. Data were normalized to controls and expressed as percentages (%). Significance determined using unpaired t-test, **** = p<0.0001, n = 8. All data graphed as mean ± SEM.
Figure 10—figure supplement 1. Effects of T-type Ca2+ channel antagonists, TTA-A2 and Z-944 on submucosal interstitial cells of Cajal (ICC-SM) Ca2+ transients.
Effects of membrane hyperpolarization on Ca2+ transients in ICC-SM
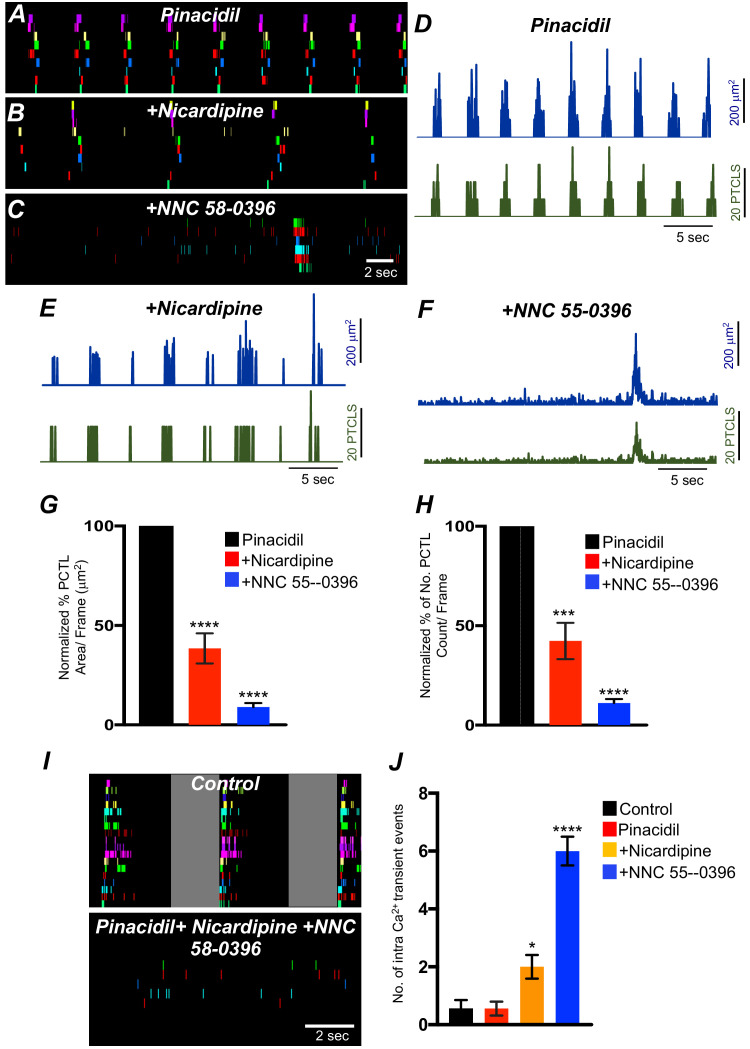
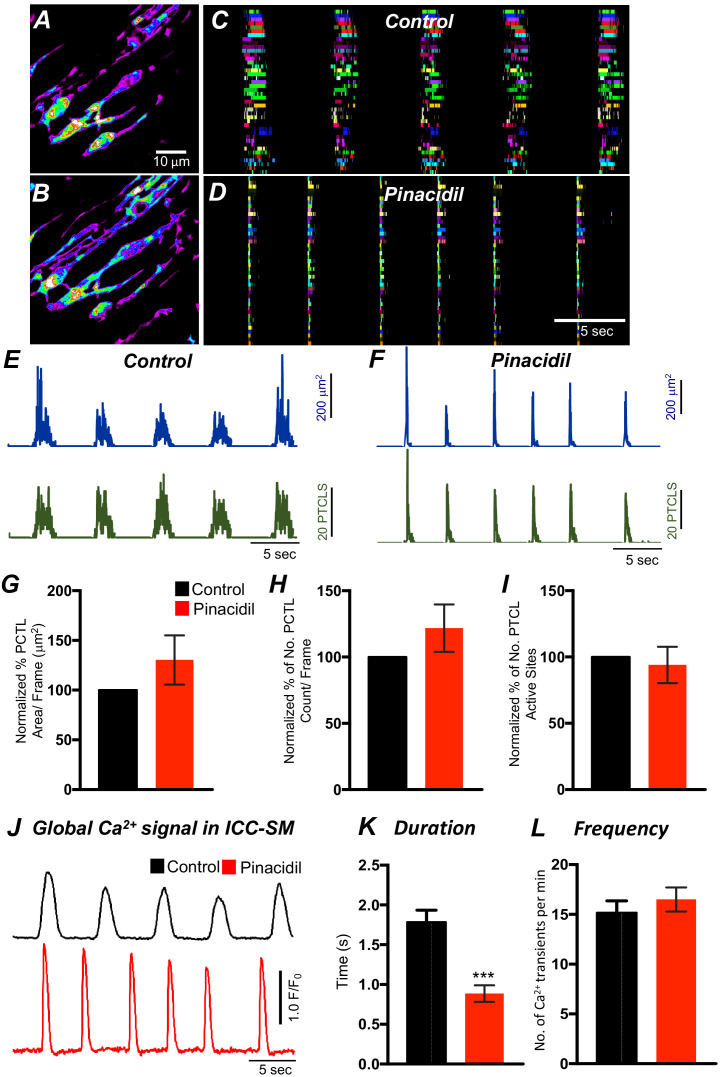
Experiments described above showed that Ca2+ transients in ICC-SM depend on voltage-dependent Ca2+ influx mechanisms (Figures 7–10). The role and contributions of the voltage-dependent channels expressed in ICC-SM (CaV 1.3 and CaV 1.2 and CaV 3.2) was further examined under conditions of membrane hyperpolarization induced by activation of KATP channels. KATP is functional in colonic SMCs but not in ICC (Huang et al., 2018). Therefore, these experiments were performed on preparations in which ICC-SM remained attached to the muscularis. Pinacidil produces hyperpolarization of SMCs via activation of KATP channels (Koh et al., 1998) and the hyperpolarization is expected to conduct to ICC, as they are electrically coupled. Pinacidil (10 μM), a selective KATP channel agonist, hyperpolarizes murine colonic muscles by ~10 mV (Kito et al., 2005; Koh et al., 1998; Kito and Suzuki, 2003). Pinacidil had no significant change to Ca2+ transient firing, except to reduce the duration of CTCs (Figure 11—figure supplement 1A–L). Ca2+ PTCL area was increased to 130.3 ± 24.3% (Figure 11—figure supplement 1G; p value = 0.24; n = 6) and PTCL count was increased to 121.8 ± 17.8% (Figure 11—figure supplement 1H; p value = 0.25; n = 6). The number of firing sites was not affected by pinacidil (Figure 11—figure supplement 1I; p value = 0.22; n = 6). The effects of pinacidil on global Ca2+ transients were evaluated. Pinacidil significantly reduced the duration of global Ca2+ transients from 1.78 ± 0.2 s to 0.89 ± 0.1 s (Figure 11—figure supplement 1J&K; n = 6). Reduction in the duration of Ca2+ transients was associated with a tendency for an increase in frequency, but this parameter did not reach significance. Ca2+ oscillation frequency under control conditions was 15.1 ± 1.1 cpm and in the presence of pinacidil was 16.5 ± 1.2 cpm (Figure 11—figure supplement 1J&L; p value = 0.45; n = 6).
The effects of nicardipine were tested in the presence of pinacidil. Under these conditions, nicardipine significantly reduced Ca2+ transients in ICC-SM (Figure 11G&H; n = 5). PTCL area was reduced to 38.5 ± 7.6% (Figure 11G; n = 5) and PTCL count was reduced to 42.3 ± 9.1% (Figure 11H; n = 5). In some regards, these results were surprising as membrane potential hyperpolarization might reduce contributions from L-type Ca2+ channels (CaV 1.2). One explanation is that CaV 1.3, which are abundant in ICC-SM and activate at more negative potentials than CaV 1.2 (Xu and Lipscombe, 2001) may contribute to Ca2+ entry at more hyperpolarized potentials. Further addition of NNC 55–0396 (10 μM) inhibited Ca2+ transients in ICC-SM to a greater extent. PTCL area was reduced to 9.0 ± 2% (Figure 11G; n = 5), and PTCL count was reduced to 11.2 ± 1.9% (Figure 11H; n = 5). The utilization of two Ca2+ conductances with different ranges of voltage-dependent activation for the initiation of CTCs provides a safety factor that insures persistence of pacemaker activity over a broad range of membrane potentials.
Figure 11. The effects of membrane hyperpolarization and voltage-dependent Ca2+ entry block on submucosal interstitial cells of Cajal (ICC-SM) Ca2+ transients.
(A) Ca2+-firing sites in ICC-SM are color coded and plotted in an occurrence map in the presence of pinacidil (10 μM). (B) Shows the effects of nicardipine (1 μM) in the continued presence of pinacidil. (C) shows effects of combining nicardipine and NNC 55–0396 (10 μM) in the continued presence of pinacidil. Traces of firing sites PTCL area (blue) and PTCL count (green) under each condition are shown in D pinacidil E pinacidil and nicardipine and F combination of pinacidil, nicardipine, and NNC 55–0396. Summary graphs of Ca2+ PTCL activity in ICC-SM in the presence of voltage-dependent Ca2+ channel antagonists (nicardipine and NNC 55–0396) are shown in G (PTCL area) and H (PTCL count). (I) The number of Ca2+-firing events were tabulated during 2 s intervals before the initiation of Ca2+ transient clusters in ICC-SM (period of tabulation indicated by the gray box) and summarized in J under control conditions, in pinacidil, in pinacidil and nicardipine and in a combination of pinacidil, nicardipine, and NNC 55–0396. Data were normalized to controls and expressed as percentages (%) in G and H. Significance determined using one-way-ANOVA, * = p<0.1, ** = p<0.01, *** = p<0.001 **** = p<0.0001, n = 5. All data graphed as mean ± SEM.
Figure 11—figure supplement 1. The effects of pinacidil of Ca2+ transients in submucosal interstitial cells of Cajal (ICC-SM).
Inhibiting voltage-dependent Ca2+ channels in the presence of pinacidil unmasked underlying Ca2+ transients that occurred more randomly than the clustered transients occurring normally (Figure 11F&I). We tabulated the number of Ca2+ events in the intervals between CTCs (calculated from a period of 2 s before the onset of a CTC). Underlying Ca2+ events were more frequent in the presence of pinacidil and nicardipine and increased again upon addition of NNC 55–0396 (Figure 11J; n = 5).
Contributions of intracellular Ca2+ stores and release channels in ICC-SM Ca2+ activity
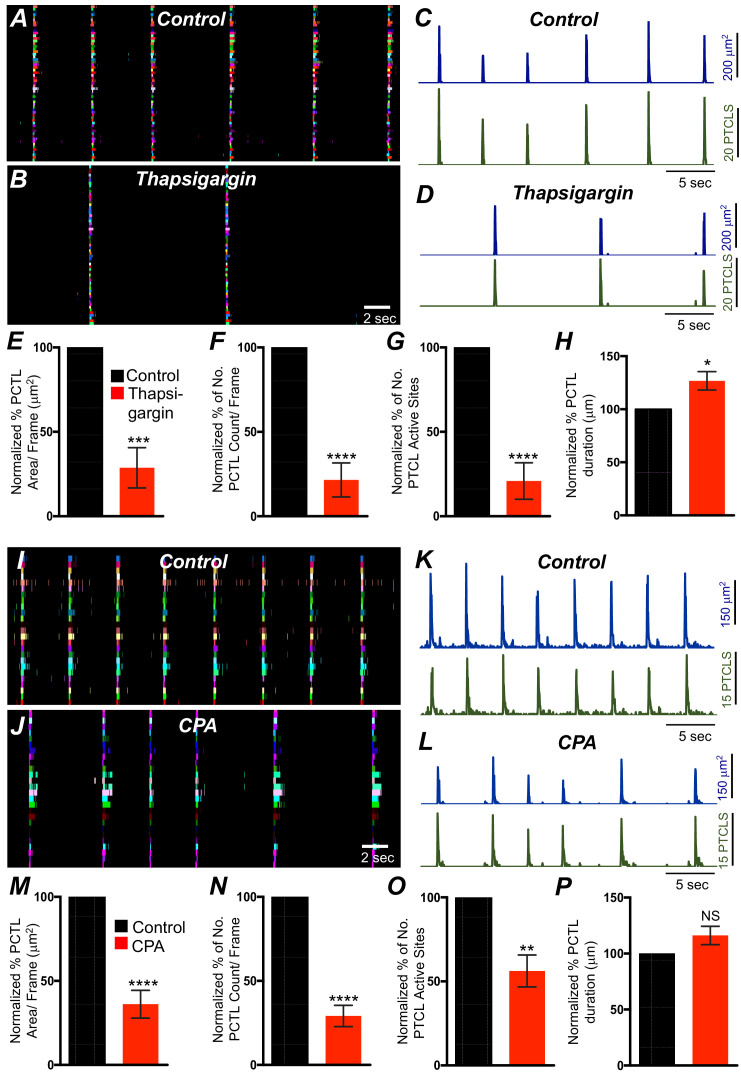
Previous studies have demonstrated that Ca2+ signaling in ICC-MY in the small intestine depends not only on Ca2+ influx but also on Ca2+ release from intracellular stores (Drumm et al., 2017). Ca2+ release from stores is also critical for generation of pacemaker currents and slow waves (Ward et al., 2000b; Zhu et al., 2015; Bayguinov et al., 2007). The role of Ca2+ release mechanisms in Ca2+ signaling in ICC-SM was also evaluated. Thapsigargin (1 μM; A SERCA pump antagonist) reduced, but did not block, Ca2+ transient firing in ICC-SM (Figure 12A–D). PTCL area was reduced to 29 ± 12% (Figure 12E; n = 6) and PTCL count was reduced to 22 ± 10% (Figure 12F; n = 6). The number of firing sites was reduced by thapsigargin to 21 ± 11% (Figure 12G; n = 6). The duration of PTCLs was increased by thapsigargin to 126.7 ± 9% (Figure 12H; n = 6). Cyclopiazonic acid (CPA, 10 μM), another SERCA antagonist, also reduced Ca2+ transient firing (Figure 12I–L). PTCL area was reduced to 36.1 ± 8.3% (Figure 12M; n = 5) and PTCL count was reduced to 29.1 ± 6.3% (Figure 12N; n = 5). The number of firing sites was reduced by CPA to 56 ± 9.5% (Figure 12O; n = 5). There was no significant change in the duration of PTCLs in the presence of CPA (Figure 12P; n = 5).
Figure 12. Intracellular Ca2+ stores contributions in submucosal interstitial cells of Cajal (ICC-SM) Ca2+ transients firing.
(A) Ca2+ activity of firing sites in ICC-SM are color coded and plotted in occurrence maps under control conditions and in the presence of thapsigargin (1 μM) (B). Traces of firing sites PTCL area (C; blue) and PTCL count (C; green) under control conditions and in the presence of thapsigargin PTCL area (D; blue) and PTCL count (D; green). Scale bars in C applies to traces in D. Summary graphs of Ca2+ PTCL activity in ICC-SM in the presence of thapsigargin are shown in E (PTCL area), (F) (PTCL count), (G) the number of PTCL active sites and H (PTCL duration; n = 6). CPA (SERCA pump inhibitor) reduced transients compared to control as shown in occurrence maps of firing sites I and J and Ca2+ activity traces K and L. Summary graphs of Ca2+ PTCL activity in ICC-SM in the presence of CPA are shown in M (PTCL area), (N) (PTCL count), (O) the number of PTCL active sites and P (PTCL duration; n = 5). Data were normalized to controls and expressed as percentages (%). Significance determined using unpaired t-test, * = p<0.1, ** = p<0.01, *** = p<0.001 **** = p<0.0001. All data graphed as mean ± SEM.
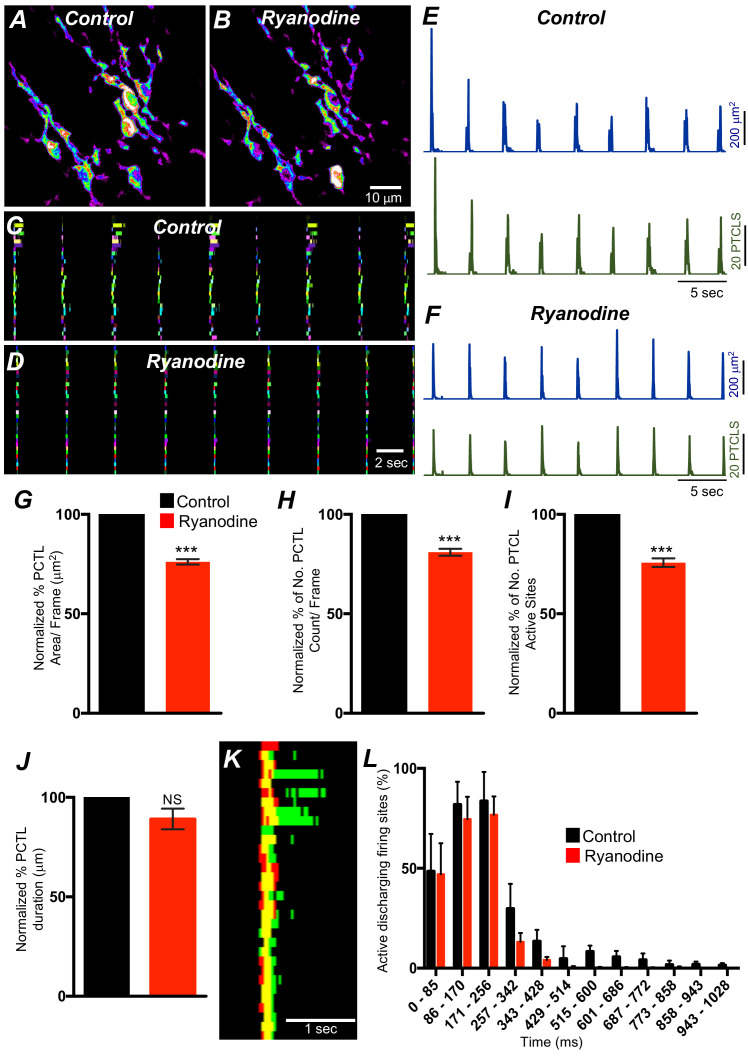
ER release channels RyRs and IP3Rs amplify and sustain Ca2+ signaling via direct localized release of Ca2+ or Ca2+-induced Ca2+ release (CICR) (Kaßmann et al., 2019; Vierra et al., 2019; Amberg et al., 2006; Takeda et al., 2011; Fill and Copello, 2002; Dupont and Goldbeter, 1994; Thomas et al., 1996; Berridge, 2009). Contributions from RyRs and IP3Rs to Ca2+ transients in ICC-SM were therefore investigated. Ryanodine (100 μM) significantly reduced Ca2+ event firing in ICC-SM (Figure 13A–F). PTCL area was reduced to 76.1 ± 1.6% (Figure 13G; n = 4) and PTCL count was reduced to 80.9 ± 2.7% (Figure 13H; n = 4). The number of firing sites was also reduced by ryanodine to 75.7 ± 2.1% (Figure 13I; n = 4). There was no significant change in the duration of PTCLs in the presence of ryanodine (Figure 13J; n = 5). We also noted that the greatest effects of ryanodine on Ca2+ transients occurred after the first ~300 ms of CTCs (Figure 13K&L; n = 4). Thus, ryanodine shortens the durations of the CTCs. Ca2+ release mechanisms via RyRs contribute to the overall patterns of Ca2+ waves in ICC-SM, as shown by the distribution plots of average percentages of firing sites during a Ca2+ wave (Figure 13L; n = 4).
Figure 13. Ryanodine receptors (RyRs) contributing to Ca2+ release in submucosal interstitial cells of Cajal (ICC-SM).
(A) Representative heat-map image of an ICC-SM network from proximal colon showing total active Ca2+ PTCLs under control conditions and in the presence of ryanodine (100 μM) (B). C and D Ca2+-firing sites are color coded and plotted in occurrence maps showing the effect of the ryanodine (100 μM), on Ca2+ transient clusters (CTCs) in ICC-SM. Traces of firing sites PTCL area (E; blue) and PTCL count (E; green) under control conditions and in the presence of ryanodine, PTCL area (F; blue) and PTCL count (F; green). Summary graphs of Ca2+ PTCL activity in ICC-SM in the presence of ryanodine are shown in G (PTCL area), (H) (PTCL count), (I) the number of PTCL active sites and J (PTCL duration; n = 4). (K) Overlaid occurrence maps showing Ca2+ firing during control conditions (all firing sites are in green) and in the presence of ryanodine (all firing sites are in red). Note how ryanodine shortened the duration of the total Ca2+ transient cluster (CTC). (L) Distribution plot of average percentages of firing sites during a Ca2+ wave, calculated for 1 s duration and plotted in 85 ms bins showing that ryanodine mainly blocked Ca2+ transients occurring after the first 257 ms intervals (n = 4). Data were normalized to controls and expressed as percentages (%). Significance determined using unpaired t-test, *** = p<0.001. All data graphed as mean ± SEM.
Figure 13—figure supplement 1. IP3 receptors (IP3Rs) contribution to Ca2+ transients in submucosal interstitial cells of Cajal (ICC-SM).
Figure 13—figure supplement 2. Effects of 2-APB and Tetracaine on submucosal interstitial cells of Cajal (ICC-SM) Ca2+ transients.
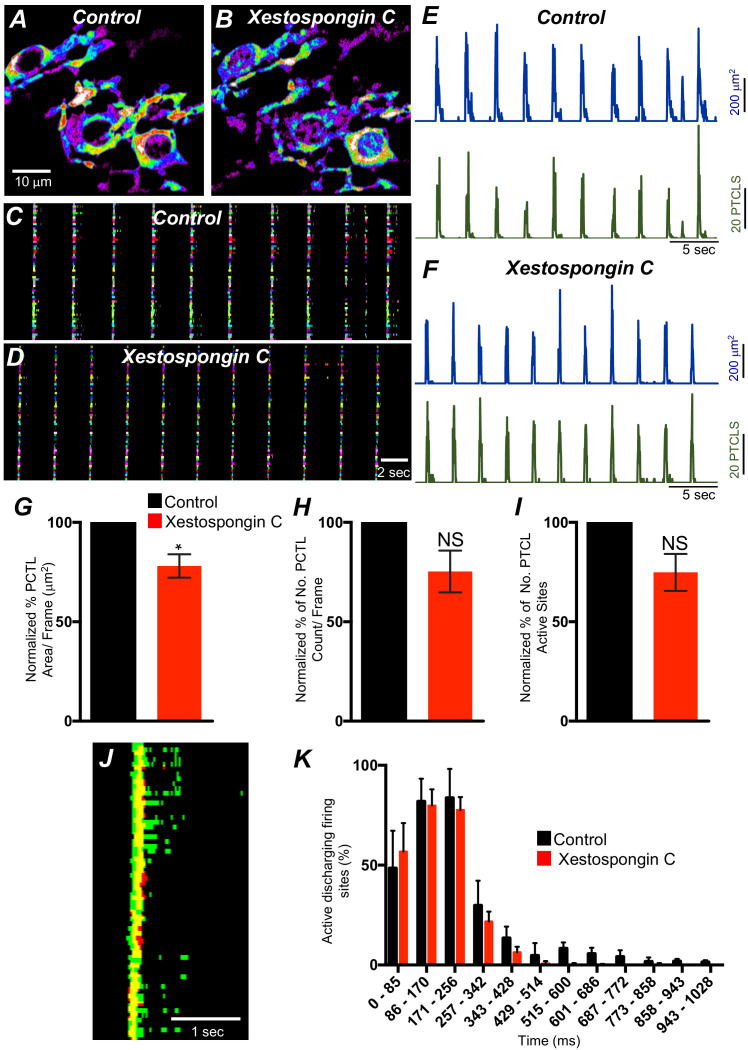
Xestospongin C (10 μM; An IP3R antagonist) also reduced Ca2+ events in ICC-SM (Figure 13—figure supplement 1A–I; n = 4). PTCL area was significantly reduced to 78 ± 5.8% (Figure 13—figure supplement 1G; n = 4) and although both PTCL count and number were reduced to 75.3 ± 10.5% and 74.8 ± 9.3%, respectively, these effects did not reach statistical significance (PTCL count: p value = 0.08 and PTCL number: p value = 0.06; Figure 13—figure supplement 1H&I; n = 4). Xestospongin C displayed inhibitory effects similar to ryanodine; most of the inhibition of Ca2+ events occurred after the first ~400 ms of CTCs (Figure 13—figure supplement 1J&K; n = 4). Thus, Ca2+ release via IP3Rs also contributes to the overall pattern of Ca2+ waves in ICC-SM, as shown by the distribution plots of average percentages of firing sites during a Ca2+ wave (Figure 13—figure supplement 1K; n = 4).
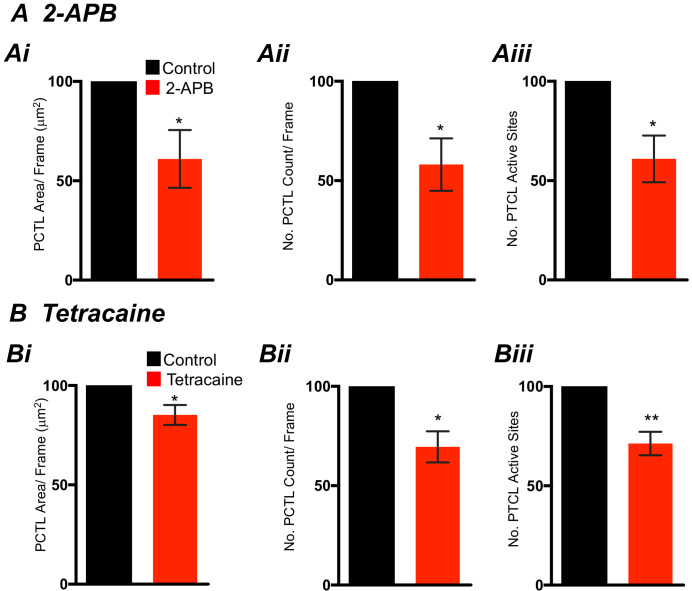
2-APB (100 μM) and tetracaine were also tested (100 μM; Figure 13—figure supplement 2A&B; n = 5) as secondary tests of the contributions of IP3Rs and RyRs in CTCs. 2-APB reduced Ca2+ PTCL area to 61.1 ± 14.5% (Figure 13—figure supplement 2 Ai; n = 5) and reduced PTCL count to 58 ± 13.2% (Figure 13—figure supplement 2 Aii; n = 5). The number of firing sites was also reduced by 2-APB to 63.7 ± 11.3% (Figure 13—figure supplement 2 Aiii; n = 5). Tetracaine reduced Ca2+ PTCL area to 85 ± 5.1% (Figure 13—figure supplement 2 Bi; n = 5) and reduced PTCL count to 69.5 ± 7.8% (Figure 13—figure supplement 2 Bii; n = 5). The number of firing sites was also reduced by tetracaine to 71.3 ± 5.9% (Figure 13—figure supplement 2 Biii; n = 5).
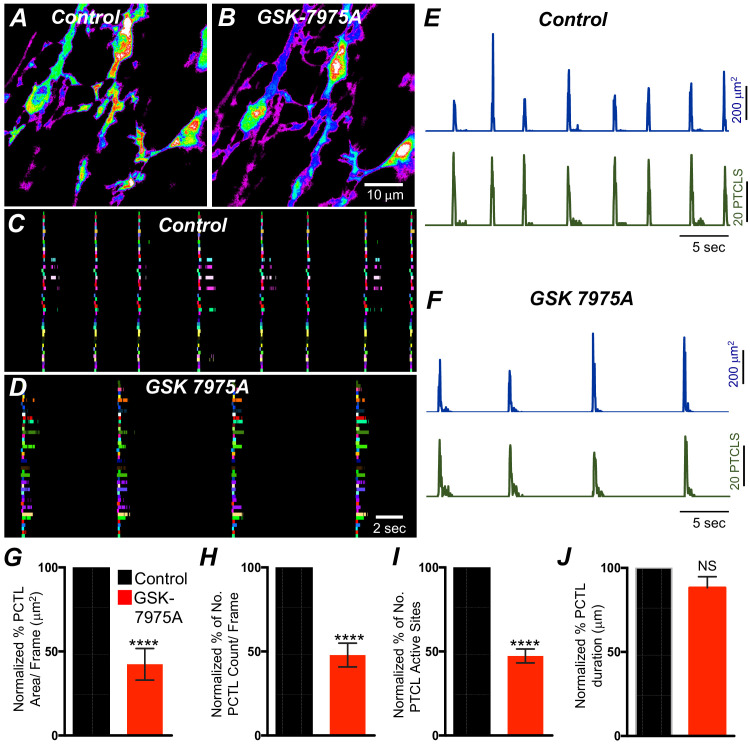
The effects of 2-APB could be non-specific and may include effects on store-operated Ca2+ entry channels (SOCE; e.g. by blocking Orai channels). Previous studies have shown SOCE to be important for maintenance of Ca2+ stores and sustaining Ca2+ release from the ER (Zheng et al., 2018; Lyfenko and Dirksen, 2008; Trebak et al., 2013; Chen and Sanderson, 2017; Putney, 2011; Prakriya and Lewis, 2015). ICC-SM express Orai channels (Orai1 and Orai2; Figure 8B), so the role of SOCE in maintenance of Ca2+ transients was examined using an Orai antagonist. GSK 7975A (10 μM; An Orai antagonist) reduced the firing frequency of CTCs (Figure 14A&B). Firing site occurrence (Figure 14C&D) and PTCL counts and areas were reduced (Figure 14E&F). Ca2+ PTCL area was reduced to 42.4 ± 9.4% (Figure 14E–G; n = 7), and PTCL count was reduced to 48 ± 7% (Figure 14H; n = 7). The number of firing sites was also inhibited by GSK 7975A to 47.5 ± 4.1% (Figure 14I; n = 7). There was no significant change in the duration of PTCLs in the presence of GSK 7975A (Figure 14J; n = 7).
Figure 14. SOCE role in maintaining submucosal interstitial cells of Cajal (ICC-SM) Ca2+ transients.
(A) Representative Heat-map image of an ICC-SM network showing total active Ca2+ PTCLs under control conditions and in the presence of GSK-7975A (10 μM, for 20 min) B. C and D Ca2+-firing sites are color coded and plotted in occurrence maps showing the effect of the SOCE channel antagonist, GSK-7975A (100 μM), on ICC-SM Ca2+ transients. Traces of PTCL area (E; blue) and PTCL count (E; green) under control conditions and in the presence of GSK-7975A, PTCL area (F; blue) and PTCL count (F; green). Summary graphs of Ca2+ PTCL activity in ICC-SM in the presence of GSK-7975A are shown in G (PTCL area), (H) (PTCL count), (I) the number of PTCL active sites, and J (PTCL duration; n = 7). Significance determined using unpaired t-test, **** = p<0.0001. All data graphed as mean ± SEM.
Discussion
This study characterized Ca2+ transients responsible for the pacemaker function of ICC-SM that contributes to contractile patterns in colonic motility. The sequence of activation from ICC-SM to colonic SMCs was quantified using two optogenetic sensors, expressed specifically in ICC or SMCs. Correlation analysis demonstrated a 1:1 relationship between CTCs in ICC-SM and Ca2+ signaling and contractile responses in SMCs. The CTCs in ICC-SM consisted of ~2 s bursts of activity from multiple sites within cells. Organization of the Ca2+ transients into clusters was due to voltage-dependent Ca2+ entry that appeared to be due to activation of both high-voltage activated Ca2+ channels (L-type encoded by Cacna1c and Cacna1d in ICC-SM) and low-voltage activated Ca2+ channels (T-type encoded by Cacna1h and possibly Cacna1g in ICC-SM). A portion of the Ca2+ transients making up CTCs were due to Ca2+ entry, as the earliest Ca2+ transients resolved in a cluster were not blocked by thapsigargin, CPA or antagonists of ryanodine and IP3 receptors. The earliest events were blocked by nicardipine, suggesting that L-type Ca2+ channels are the dominant Ca2+ entry pathway at basal resting potentials. Ca2+ transients in ICC-SM were not as sensitive to block by STIM and Orai, as in other ICC (Zheng et al., 2018; Drumm et al., 2019a), However, the Orai antagonist reduced the frequency of CTCs and may have blocked these events completely with longer treatment periods. Our data show that Ca2+ entry is fundamental in ICC-SM Ca2+ transients. In fact, localized Ca2+ influx via clusters of L-type Ca2+ channels may cause localized elevation in [Ca2+]i and activation of ANO1 channels directly. Localized Ca2+ entry events through L-type Ca2+ channels have been termed Ca2+sparklets, and these events could be involved in Ca2+ influx (Santana et al., 2008; Sonkusare et al., 2012; Navedo and Amberg, 2013a) and initiation of pacemaker activity in ICC-SM. However, the decrease in the frequency of CTCs by manipulations to reduce stored Ca2+ also suggest an important role for Ca2+ release in pacemaker activity, perhaps resulting from coupling between Ca2+ entry and CICR (Navedo and Santana, 2013b).
In this study, we developed a new preparation in which ICC-SM adherent to the submucosa was used to allow very high-resolution imaging without complications from muscular contractions. In situ preparations of this type in which the natural structure and connectivity between ICC are maintained may be valuable for future studies of cellular mechanisms responsible for pacemaker activity and factors that regulate or degrade pacemaker activity in pathophysiological conditions.
The pacemaker function of ICC-SM was demonstrated in a novel manner by simultaneous two-color optogenetic imaging with green (GCaMP6f) and red (RCaMP1.07) Ca2+ sensors expressed in ICC and SMCs, respectively. Imaging in this manner revealed the sequence of activation in ICC-SM and SMCs, showing clearly the frequency, onset and duration of Ca2+ transients in ICC-SM, the spatial spread of Ca2+ transients in ICC-SM networks, the development of Ca2+ transients in SMCs and tissue displacement (i.e. an optical indicator of muscle contraction). Correlation analysis demonstrated the coherence of these events. Ca2+ transients, lasting for about 2 s, propagated without decrement through networks of ICC-SM and preceded and likely initiated Ca2+ signaling and contractions in SMC, as was also suggested by intracellular microelectrode recordings from cells along the innermost surface of canine colonic muscles (Sanders et al., 1990). ICC-SM are electrically coupled to each other and the network of ICC-SM is coupled to SMCs via gap junctions, providing a means of electrical communication. Ca2+ transients initiate depolarization of ICC-SM via activation of ANO1 channels, depolarizing currents conduct to SMCs, and depolarization of SMCs activates Ca2+ entry, by increasing the open probability of L-type Ca2+ channels, and excitation-contraction coupling. Depolarizing signals from multiple ICC-SM influence the excitability of SMC, and the overall depolarization driving SMCs results from the summation of activity from the ICC-SM network.
Slow waves with characteristics similar to those found in the stomach and small intestine (i.e. relatively fast upstroke depolarization and a plateau phase) are generated along the submucosal surface of the CM layer in the colon (Smith et al., 1987a). It was discovered that peeling the submucosa from the innermost surface of CM blocked slow waves (Durdle et al., 1983). While the authors of that study thought this tissue was mostly connective tissue with possibly some adherent SMCs, it became clear that a population of pacemaker cells, ICC-SM, are present along the submucosal surface (Berezin et al., 1988; Ward et al., 1991). In the present study, we found that ICC-SM and the networks they form are preserved and remain functionally similar in isolated submucosal tissues to ICC-SM attached to the muscularis. It should be noted that ICC-SM were more adherent to CM in the distal colon, and it was more difficult to obtain ICC-SM/submucosal preparations from that region. Submucosal tissues with adherent ICC-SM were used in the current study to eliminate movement artifacts generated by muscle contractions that plague high-resolution Ca2+ imaging in most smooth muscle tissues.
While the frequency of pacemaker activity was relatively stable over time in a given preparation, the sequence of activation of individual ICC-SM within the network varied as a function of time, as previously observed in gastric (Hennig et al., 2004) and small intestinal (Park et al., 2006) ICC-MY networks. What appeared as global Ca2+ transients in low resolution imaging partitioned into clusters of localized Ca2+ transients when viewed with a ×60 objective at 30 frames/s or at higher acquisition speeds. Summation of the clustered events reproduced the frequency and duration of the Ca2+ waves observed at low resolution. Multiple firing sites, averaging ~8 per cell, were identified. This pattern of clustered Ca2+ transients (CTCs) was also observed in ICC-MY of the small intestine, the pacemaker cells in that region (Drumm et al., 2017). Organization of Ca2+ transients into clusters in ICC-SM was dependent upon voltage-dependent Ca2+ entry, and our data revealed that in contrast to ICC-MY of the small intestine, Ca2+ entry by both dihydropyridine-sensitive and insensitive mechanisms contributes to clustering and propagation of Ca2+ waves in intact networks. Nicardipine and isradipine reduced the occurrence of CTCs dramatically, and the T-channel antagonists, NNC 55–0396, TTA-A2, and Z-944 also reduced the occurrence and disordered the Ca2+ transients. Cacna1c, Cacna1d, and Cacna1h were expressed in purified ICC-SM, and the presence and function of these channels can explain the pharmacological observations. Channels resulting from Cacna1d (encoding CaVα1D) activate at relatively hyperpolarized membrane potentials and their currents are partially inhibited by dihydropyridines (~50–70% of current density block) in comparison to Cacna1c gene products (CaVα1C) (Xu and Lipscombe, 2001; Bell et al., 2001), but isradipine blocks CaVα1C and CaVα1D equally (Anekonda et al., 2011; Berjukow et al., 2000; Scholze et al., 2001). The fact that isradipine had no greater effect on the occurrence of clustered Ca2+ transients than nicardipine suggests that the L-type component of Ca2+ entry may be carried primarily by CaVα1C channels. We have observed relatively robust expression of Cacna1d in a variety of ICC in mice (Drumm et al., 2017; Lee et al., 2017); however, the function of these channels has not been identified specifically.
Having three independent voltage-dependent Ca2+ conductances with different voltage-dependent properties coordinate clustering of Ca2+ transients provides a safety factor for preservation of pacemaker activity over a broad range of membrane potentials. In spite of overarching changes in membrane potential that might influence the availability of ion channels with narrow voltage-ranges, the broader range of activation potentials offered by expression and function of both L-type and T-type Ca2+ channels might protect against voltage-dependent inhibition of pacemaker activity. L-type channels are activated at less polarized potentials than T-type channels (Nowycky et al., 1985). Thus, a factor producing tonic hyperpolarization of the SIP syncytium (e.g. purinergic inhibitory neurotransmission; Gallego et al., 2008; Hwang et al., 2012) may tend to switch the dominant voltage-dependent Ca2+ entry mechanism from L-type to T-type Ca2+ channels. This concept was demonstrated by the decreased inhibitory effects of nicardipine and increased effects of NNC 55–0396 on Ca2+ transients after exposure of tissues to pinacidil. The opposite might be true if the SIP syncytium experiences a depolarizing influence (e.g. neurogenic or humerogenic).
Pinacidil hyperpolarizes colonic muscles through activation of KATP channels in SMCs (Koh et al., 1998). This compound increased the frequency and decreased the duration of CTCs. These results are consistent with the effects of pinacidil on electrical pacemaker activity in the small intestine where it increases the dV/dtmax of the upstroke depolarization and decreases the duration of slow waves (Kito et al., 2005). The increase in frequency may have been due to reduced inactivation and increased availability of CaVα1D and T-type channels (CaVα1H) at more hyperpolarized potentials. The decrease in the duration of CTCs may be due to a relative shift in the importance of T-type vs. L-type Ca2+ channels with hyperpolarization. In the presence of pinacidil, NNC 55–0396 had increased antagonistic effects on CTCs. Ca2+ currents via T-type channels inactivate rapidly, whereas L-type channel inactivation is slower and incomplete (Hirano et al., 1989; Tseng and Boyden, 1989; Xu and Best, 1992). Thus, the Ca2+ entry period for T-type currents is likely to be more transient than with L-type currents. Channel density in proximity to Ca2+ release channels may also affect the degree of coupling between Ca2+ entry and CICR, but, as yet, little is known about the structure and functional components of microdomains in ICC.
The importance of Ca2+ entry as the primary means of activation and organization of pacemaker activity in ICC-SM was shown by the discoordination of Ca2+ transients when extracellular Ca2+ was decreased and the incomplete effects of thapsigargin and CPA on Ca2+ transients. We noted tight clustering of Ca2+ transients at 2.5 and 2.0 mM [Ca2+]o but the tightness of the CTCs disassociated when the driving force for Ca2+ entry (i.e. [Ca2+]o was reduced to 1 mM), and frequency of CTCs was greatly reduced at concentrations lower than 1 mM. Our concept is that Ca2+ entry couples to CICR in ICC. Reducing the driving force for Ca2+ entry would be expected to reduce the probability for effective coupling to CICR. CICR would be negligible when Ca2+ entry falls below threshold levels. Concentrations of thapsigargin and CPA that blocked Ca2+ transients quantitatively in other ICC (Drumm et al., 2020a; Drumm et al., 2019a; Drumm et al., 2017) caused a partial block of Ca2+ transients in ICC-SM. In fact, these drugs caused a marked narrowing of the duration of the CTCs, and this led us to analyze the temporal characteristics of Ca2+ transients within clusters. Ca2+ transients at the beginning of the CTCs were unaffected by ryanodine and xestospongin C, but transients toward the end of the clusters were blocked. These data suggest that the initial Ca2+ transients may result primarily from Ca2+ entry, and CTCs are sustained by Ca2+ release. This concept is also supported by previous studies showing inhibition of slow waves by blocking Ca2+ entry (L-type and T-type Ca2+ channels) (Drumm et al., 2017).
We have searched for a preparation of pacemaker ICC that would allow us to investigate the underlying pacemaker activity. We have speculated that stochastic Ca2+ release events, occurring in all ICC (Sanders et al., 2014a), are responsible for the spontaneous transient depolarizations (STDs) observed in patch clamp recordings from isolated ICC (Zhu et al., 2009; Zhu et al., 2011). No simultaneous recordings of Ca2+ transients and membrane currents or potentials changes have been achieved yet, and the expected link between Ca2+ transients and STDs is based on the fact that these events have common pharmacology and sensitivity to drugs that interfere with Ca2+ release (Zhu et al., 2015). Ca2+ transients and the spontaneous transient inward currents (STICs) due to activation of ANO1 channels result in spontaneous transient membrane depolarizations (STDs). Temporal summation of STDs is likely to be the generator potentials that activate T-type or L-type Ca2+ currents and initiate propagating slow wave events. In this concept it is logical to suggest that inhibition of Ca2+ release should reduce the duration of the CTCs, and inhibition of Ca2+ entry should inhibit the organizing influence of Ca2+ entry and block CTCs. When CTCs are blocked, stochastic Ca2+ transients may be unleashed, as occur in ICC-IM (Drumm et al., 2019a) and ICC-DMP (Baker et al., 2016) that lack expression of voltage-dependent Ca2+ entry mechanisms. Block of CTCs and unmasking of stochastic Ca2+ transients was accomplished by hyperpolarization with pinacidil and reduction in the availability of L-type and T-type Ca2+ channels with nicardipine and NNC 55–0396. ICC-SM, as imaged in the current experiments provide a potent model for investigating basic pacemaker mechanisms and what happens to these events in response to neurotransmission, hormonal and paracrine inputs and pathological or inflammatory conditions.
Previous studies have supported a role for store-operated Ca2+ entry (SOCE) in maintaining Ca2+ release events in ICC (Zheng et al., 2018; Drumm et al., 2019a). This is logical because Ca2+ release is extremely dynamic in ICC, and it is likely that Ca2+ stores would be depleted without an effective recovery mechanism. SOCE depends upon the expression of Orai channels and the ER delimited activator of Orai, STIM, that senses ER Ca2+ and binds to and activates Orai channels when Ca2+ depletion of the ER occurs (Butorac et al., 2020). STIM and Orai are expressed in colonic ICC (Lee et al., 2017). However, an antagonist of Orai, GSK7975A, reduced the frequency of CTCs, but failed to block these events at a concentration effective in blocking Ca2+ transients in small intestinal ICC-MY (Zheng et al., 2018). STIM/Orai interactions appear to have a role in Ca2+ store maintenance in ICC-SM, but our data suggest that Ca2+ entry via L-type and T-type Ca2+ channels also provide Ca2+ entry mechanisms that may contribute to store refilling. It could also be suggested that GSK7975A, a well-known antagonist for Orai1 (IC50 = 4 μM; Derler et al., 2013), is less effective on Orai2, the dominant isoform expressed in ICC-SM. However, studies on cortical neurons that express only Orai2 showed about 50% block of SOCE by GSK7975A (5 μM) (Chauvet et al., 2016).
In summary ICC-SM, as suggested from dissection and electrophysiological experiments (Smith et al., 1987a), are pacemaker cells distributed in an electrically coupled network along the submucosal surface of the CM layer. Our experiments demonstrate that Ca2+ transients in ICC-SM couple to activation of Ca2+ transients and contractions in neighboring SMCs. The contractile events, called ‘ripples’ by some authors in describing integrated colonic contractions (Corsetti et al., 2019; D'Antona et al., 2001), summate with the larger amplitude contractions emanating from the myenteric region of the tunica muscularis to produce mixing and propagated movements characteristic of colonic motility (Rosli et al., 2020). Data from this study suggest that voltage-dependent Ca2+ entry serves at least four important functions in the pacemaker activity of ICC-SM: (i) Propagation of activity within the ICC-SM network depends upon voltage-dependent Ca2+ entry, and the functions and voltage-dependent properties of three types of Ca2+ conductances appear to provide a safety factor that tends to preserve pacemaker activity over a broad range of membrane potentials (see Figure 15 and Figure 15—figure supplement 1). (ii) Ca2+ entry is the mechanism that organizes Ca2+ release events into CTCs. These events constitute the Ca2+ waves that propagate through ICC-SM networks, and cause slow wave depolarizations by activation of ANO1 channels. (iii) Ca2+ entry also appears to contribute to refilling of stores, as pacemaker activity was not as immediately dependent upon SOCE as in other ICC (Zheng et al., 2018; Drumm et al., 2019a). (iv) The observations that treatments expected to reduce Ca2+ release from stores and reduce coupling between Ca2+ entry and CICR reduced, but did not block CTCs, may indicate that transient Ca2+ entry (sparklets), possibly through activation of ANO1 channels and depolarization, may underlie the pacemaker functions of ICC-SM. Additional studies will be necessary to resolve these hypotheses in finer detail. The preparation of excised submucosal tissue with adherent ICC-SM removes movement artifacts from imaging and is likely to provide a powerful tool for improving resolution of pacemaker mechanisms and determining how regulatory and pathophysiological factors affect basic pacemaker mechanisms.
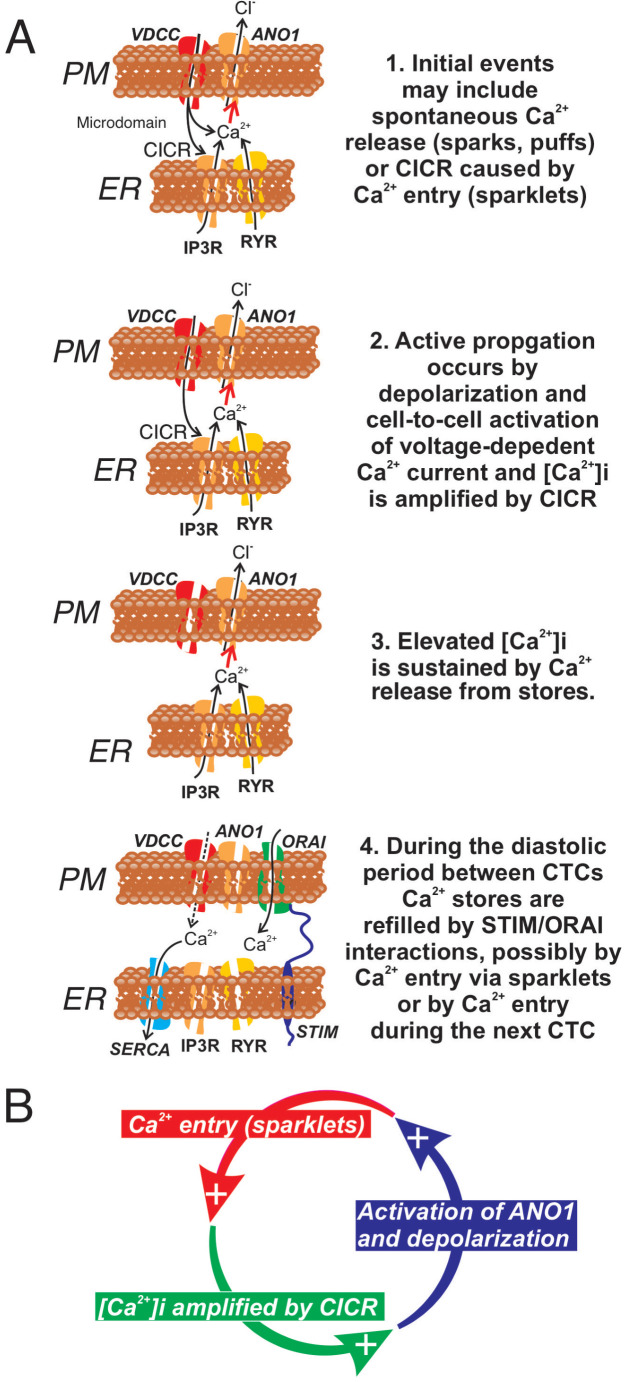
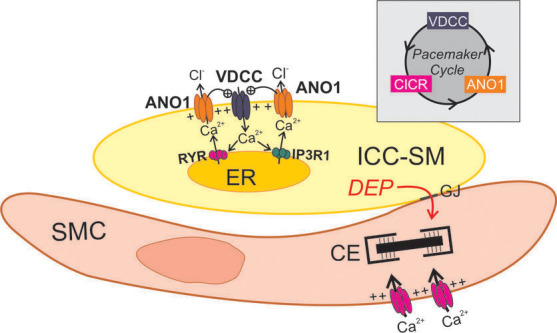
Figure 15. Role of voltage-dependent Ca2+ entry in the pacemaker function of submucosal interstitial cells of Cajal (ICC-SM).
(A) Shows segments of plasma membrane (PM) and endoplasmic reticulum membrane (ER) that form PM-ER junctions and microdomains. At least three types of voltage-dependent Ca2+ channels (VDCC) are expressed in ICC-SM, CaV1.2, CaV1.3, and CaV3.2. These conductances, with voltage-dependent activation and inactivation properties spanning a broad range of negative potentials, insure maintenance of pacemaker activity under conditions of hyperpolarization or depolarization in ICC-SM. Pacemaker activity (1. Initial events) in ICC-SM could be due to spontaneous release of Ca2+ from stores in the ER and utilize either IP3R or RYR receptors or both (Ca2+ sparks and puffs). However, our data cannot rule out the possibility that transient openings of voltage-dependent Ca2+ channels (sparklets) and amplification of Ca2+ in microdomains by CICR constitute the initial events of pacemaker activity. In this case, Ca2+ release from stores is not the primary pacemaker event but a secondary response to Ca2+ entry. Inhibition of Ca2+ release from stores would lead to reduced probability of CICR and decrease the frequency of CTCs. Our hypothesis is that Ca2+ entry and/or release from stores activates Ca2+-dependent Cl- current due to ANO1 channels in the plasma membrane. Active propagation between cells in interstitial cells of Cajal (ICC) networks (Phase 2) was inhibited by blocking voltage-dependent Ca2+ channels. Active propagation may also require or depend upon amplification of Ca2+ in microdomains by CICR. The duration of Ca2+ entry is likely to be brief due to voltage-dependent inactivation of L- and T-type Ca2+ channels. The duration of CTCs appears to be enhanced by CICR (Phase 3). Our data show that the duration of CTCs is reduced by several manipulations known to inhibit Ca2+ release from stores. In Phase four store reloading may occur by multiple mechanisms and may include: (i) transient Ca2+ entry via sparklets, (ii) activation of SOCE via STIM/ORAI interactions, and (iii) the increase in Ca2+ entry that occurs via depolarization and activation of Ca2+ entry at the onset of each CTC. (B) A novel hypothesis emerges from this study suggesting that the pacemaker mechanism in non-voltage-clamped cells includes a cyclical, positive-feedback phenomenon that may be responsible for initiation of CTCs and relies on: (i) Ca2+ entry through voltage-dependent Ca2+ channels. Openings of clusters of these channels would generate sparklets; (ii) Ca2+ entry initiates CICR which amplifies [Ca2+]i within microdomains; (iii) the rise in [Ca2+]i activates ANO1 channels in the PM causing depolarization; (iv) depolarization enhances the open probability of voltage-dependent Ca2+ channels, increasing Ca2+ entry. This cycle creates positive feedback for Ca2+ entry, clustering of localized Ca2+ transients due to Ca2+ entry during the first 350–450 ms of CTCs and development of slow wave depolarizations in ICC-SM.
Figure 15—figure supplement 1. Schematic showing how Ca2+ signaling in the pacemaker function of submucosal interstitial cells of Cajal (ICC) is coupled to smooth muscle cells (SMCs).
Materials and methods
Key resources table.
| Reagent type (species) or resource |
Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Anti- c-Kit (Goat polyclonal) | R and D Systems | Cat# AF1356, RRID:AB_354750 | IHC (1:500) |
| Antibody | Alexa-488 (donkey anti-goat IgG) | Invitrogen/ Thermo Fisher Scientific |
(Cat# A32814, RRID:AB_2762838) | IHC (1:1000) |
| Sequence-based reagent |
Kit, Ano1, Myh11,Uchl1, Cacna1c
Cacna1d, Cacna1g Cacna1h Orai1,Orai Orai3 |
This paper | PCR primers | Suppl. Table 1 |
| Chemical compound, drug | NNC 55–0396, TTA-A2 | Alomone Labs | Cat# N-206 Cat# T-140 |
|
| Chemical compound, drug | 2-APB, tetracaine, nicardipine, pinacidil, EGTA |
Millipore-Sigma | Cat# D9754; Cat# T7508; Cat# N7510; Cat# P154; Cat# E4378 | |
| Chemical compound, drug | Thapsigargin, isradipine, Z-944, CPA, ryanodine |
Tocris Bioscience | Cat# 1138/1; Cat# 2004/10; Cat# 6367/10; Cat# 1235/10; Cat# 1329/1 | |
| Chemical compound, drug | GSK 7975A | Aobious | Cat# AOB4124 | |
| Chemical compound, drug | Xestospongin C (XeC) | Cayman Chemical | Cat# 64950 | |
| Software, algorithm | STMapAuto, Ca2+ Analysis Software |
https://github.com/gdelvalle99/STMapAuto | https://doi.org/10.1016/j.ceca.2020.102260 |
Animals
Kit+/copGFP mice (B6.129S7-Kittm1Rosay/J; 5–8 wk old) were bred in house (Ro et al., 2010). GCaMP6f-floxed mice (Ai95 (RCL-GCaMP6f)-D) and C57BL/6 mice, their wild-type siblings, were purchased from Jackson Laboratories (Bar Harbor, MN, USA). Kit-iCre mice (c-Kit+/Cre-ERT2) were gifted from Dr. Dieter Saur (Technical University Munich, Munich, Germany).
Generation of Kit-iCre-GCaMP6f/Acta2-RCaMP1.07 mice
Acta2-RCaMP1.07 mice (tg(RP23-370F21-RCaMP1.07)B3-3Mik/J) express the fluorescent Ca2+ indicator RCaMP1.07 in SMCs under the control of the Acta2 locus promoter/enhancer regions were obtained from Jackson Laboratories (Bar Harbor, MN, USA). To generate cell-specific expression in two distinct cell types (ICC and SMCs) Acta2-RCaMP1.07 mice were bred with KitCre‐ERT2/GCaMP6ffl/fl mice. The offspring Kit-iCre-GCaMP6f/Acta2-RCaMP1.07 mice were identified by genotyping after receiving tamoxifen which served to delete the STOP cassette in the Cre-expressing cells; resulting in the expression of the fluorescent Ca2+ indicator protein, GCaMP6f. These mice allowed simultaneous, dual color imaging of ICC and SMCs. iCre mice were injected with tamoxifen (TAM; Intraperitoneal injection; IP) at 6–8 weeks of age (2 mg of TAM for three consecutive days), as described previously (Baker et al., 2016), to induce activation of the Cre recombinase and expression of optogenetic sensors. Mice were used for experiments 10–15 days after the tamoxifen injections. On days of experiments the mice were anaesthetized by inhalation of isoflurane (Baxter, Deerfield, IL, USA) and killed by cervical dislocation before excision of tissues.
The animals used, protocols performed and procedures in this study were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Use and Care Committee at the University of Nevada, Reno (IACUC; Protocol: 00053).
Tissue preparation
Colonic segments (2 cm in length, proximal region) were removed from mice after an abdominal incision and placed in Krebs-Ringer bicarbonate solution (KRB). The tissues were cut along the mesenteric border and intraluminal contents were washed away with KRB. Tissues were prepared by blunt dissection in two ways: (1) The submucosa layer was isolated after carefully removing the mucosal layer and the tunica muscularis. (2) The submucosa layer was left attached to the tunica muscularis after removal of the mucosa. The isolated submucosal layer preparation provided better imaging of ICC-SM by eliminating motion artifacts associated with muscle contractions. We used the isolated submucosal layer preparation in most cases in this study with the exception of two experiments where muscle attachments were necessary to test important questions (see Results).
Immunohistochemistry
Colonic tissues from wild-type mice were processed to assess distribution of c-Kit immunoreactivity. Whole mounts of submucosal layer after removing the mucosa and tunica muscularis were fixed in 4% paraformaldehyde and visualized as described previously (Sanders et al., 2014b). Briefly, after block with 1% bovine serum albumin, colonic tissues were incubated with a polyclonal antibody raised against c-Kit (mSCFR, R and D Systems, MN, USA; 1:500 dilution in 0.5% Triton-X working solution) for 48 hr. Immunoreactivity was detected using Alexa-488 labeled donkey anti-goat IgG (1:1000 in PBS; Invitrogen, NY, USA). Colonic tissues were visualized using a Zeiss LSM 510 confocal microscope and images were constructed using Image J software (National Institutes of Health, MD, USA, http://rsbweb.nih.gov/ij). ICC (copKit) images were visualized using a spinning-disk confocal system (CSU-W1; spinning disk, Yokogawa Electric, Tokyo, Japan).
Cell sorting and quantitative PCR
Kit+/copGFP mice (B6.129S7-Kittm1Rosay/J; 5–8 wks old) were used for evaluations of gene expression in ICC-SM. Cell-specific expression of the fluorescent reporter allows unequivocal identification of ICC (Ro et al., 2010). After cell dispersion, ICC-SM were sorted by fluorescence-activated cell sorting (FACS) and evaluated for purity as previously described (Baker et al., 2016). Total RNA was isolated using an Illustra RNAspin Mini RNA Isolation Kit (GE Healthcare). qScript cDNA SuperMix (Quanta Biosciences), used according to the manufacturer’s instructions, was used to synthesize first-strand cDNA. Quantitative PCR (qPCR) was performed using Fast Sybr Green chemistry on the 7900HT Fast Real-Time PCR System (Applied Biosystems) and gene-specific primers (Supplementary file 1). Regression analysis was performed to generate standard curves from the mean values of technical triplicate qPCRs of log10 diluted cDNA samples. Evaluation of gene expression in ICC-SM was compared with expression in the unsorted cells from the submucosal tissue of Kit+/copGFP mice.
Ca2+ imaging
The isolated/intact submucosal layers were pinned to Sylgard coated dish and perfused with KRB solution at 37°C for a 60-min equilibration period. Ca2+ imaging was performed using a spinning-disk confocal system (CSU-W1; spinning disk, Yokogawa Electric, Tokyo, Japan) mounted on an upright Nikon Eclipse FN1 microscope equipped with several water immersion Nikon CFI Fluor lenses (10 × 0.3 NA, 20 × 0.5 NA, 40 × 0.8 NA, 60 × 0.8 NA and 100 × 1.1 NA) (Nikon Instruments, New York, USA). The system is equipped with two solid-state laser lines of 488 nm and 561 nm. The laser lines are combined with a borealis system (ANDOR Technology, Belfast, UK) to increase laser intensity and uniformity throughout the imaging FOV. The system also has two high-speed electron multiplying charged coupled devices (EMCCD) cameras (Andor iXon-Ultra 897 EMCCD Cameras; ANDOR Technology, Belfast, UK) to allow dual-color imaging simultaneously and maintain sensitive and fast speed acquisition at full frame of 512 × 512 active pixels as previously described (Baker et al., 2015). Briefly, images were captured, and image sequences were collected at 33 to 50 fps using MetaMorph software (MetaMorph Inc, TN, USA). In experiments with pharmacological agents, a control activity period of (30 s) was recorded prior of drug application into the chamber for 15 min.
Ca2+ imaging analysis
Movies of Ca2+ transients in ICC-SM were imported, preprocessed and analyzed using a combination of three image analysis programs: (1) custom build software (Volumetry G8d, Dr. Grant Hennig); (2) Fiji/Image J (National Institutes of Health, MD, USA, http://rsbweb.nih.gov/ij); (3) Automated Spatio Temporal Map analysis plugin (STMapAuto), https://github.com/gdelvalle99/STMapAuto as described previously (Drumm et al., 2017; Drumm et al., 2019b; Leigh et al., 2020). Briefly, movies of Ca2+ transients (stacks of TIFF images) were imported into Volumetry G8d and motion stabilized, background subtracted and smoothed (Gaussian filter: 1.5 × 1.5 µm, StdDev 1.0). A particle analysis routine was employed using a flood-fill algorithm to enhance Ca2+ transient detection. particles (PTCLs) representing the areas of active Ca2+ signals in cells were saved as a coordinate-based particle movie and combined area and total number of PTCLs were calculated. To better isolate firing sites, only those particles that did not overlap with any particles in the previous frame but overlap with particles in the subsequent 70 ms were considered firing/initiation sites. To show the overall regions in cells where Ca2+ transients occurred, PTCLs were summed throughout the video to create an image of their occurrence. The spatial information in the Ca2+ occurrence maps data is an indication of each firing site that gives information on their temporal activation and provides no information on their spatial spread. This was implemented in our analysis to accommodate a large number of firing sites and effectively plot them in a 2D occurrence map.
Drugs and solutions
All tissues were perfused continuously with KRB solution containing (mmol/L): NaCl, 5.9; NaHCO3, 120.35; KCl, 1.2; MgCl2, 15.5; NaH2PO4,1.2; CaCl2, 2.5; and glucose, 11.5. The KRB solution was warmed to a physiological temperature of 37 ± 0.3°C and bubbled with a mixture of 97% O2 – 3% CO2. For experiments utilizing external solutions with 0 [Ca2+]o, CaCl2 was omitted and 0.5 mM ethylene glycol-bis (β-aminoethyl ether)-N, N, N’, N’–tetraacetic acid (EGTA) was added to the solution. NNC 55–0396 and TTA-A2 were purchased from Alomone Labs (Jerusalem, Israel). 2-aminoethyl-diphenylborinate (2-APB), tetracaine, nicardipine, pinacidil was purchased from Millipore-Sigma (St. Louis, Missouri, USA). Thapsigargin, isradipine, Z-944, CPA and ryanodine were purchased from Tocris Bioscience (Ellisville, Missouri, USA). GSK 7975A was purchased from Aobious (Aobious INC, MA, USA), and xestospongin C (XeC) was purchased from Cayman Chemical (Michigan, USA).
Statistical analysis
Data is presented as the mean ± standard error unless otherwise stated. Statistical analysis was performed using either a Students t-test or one-way ANOVA with a Tukey post hoc test where appropriate. In all tests, p<0.05 was considered significant. When describing data, n refers to the number of animals used in a dataset. Probabilities < 0.05 are represented by a single asterisk (*), probabilities < 0.01 are represented by two asterisks (**), probabilities < 0.001 are represented by three asterisks (***) and probabilities < 0.0001 are represented by four asterisks (****). All statistical tests were performed using GraphPad Prism 8.0.1 (San Diego, CA).
Acknowledgements
The authors extend their sincere appreciation to Yulia Bayguinov for assistance with immunohistochemical experiments, Lauren O’Kane for assistance with qPCR experiments and David White and Emily Fox for assistance with FACS.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Salah A Baker, Email: sabubaker@med.unr.edu.
Mark T Nelson, University of Vermont, United States.
Richard W Aldrich, The University of Texas at Austin, United States.
Funding Information
This paper was supported by the following grants:
National Institute of Diabetes and Digestive and Kidney Diseases R01 DK-120759 to Salah A Baker, Kenton M Sanders.
National Institute of Diabetes and Digestive and Kidney Diseases R01 DK-078736 to Caroline A Cobine.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Resources, Data curation, Software, Formal analysis, Supervision, Funding acquisition, Validation, Investigation, Visualization, Methodology, Writing - original draft, Project administration, Writing - review and editing.
Data curation, Formal analysis, Investigation, Methodology.
Formal analysis.
Data curation, Formal analysis, Investigation.
Validation, Writing - review and editing.
Data curation, Formal analysis, Methodology, Writing - review and editing.
Formal analysis, Investigation, Methodology, Writing - review and editing.
Conceptualization, Resources, Data curation, Supervision, Funding acquisition, Validation, Writing - original draft, Project administration, Writing - review and editing.
Ethics
Animal experimentation: The animals used, protocols performed and procedures in this study were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Use and Care Committee at the University of Nevada, Reno (IACUC; Protocol# 00053).
Additional files
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files.
References
- Amberg GC, Navedo MF, Santana LF. On the loose: uncaging Ca2+-induced Ca2+ release in smooth muscle. Journal of General Physiology. 2006;127:221–223. doi: 10.1085/jgp.200609510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anekonda TS, Quinn JF, Harris C, Frahler K, Wadsworth TL, Woltjer RL. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for alzheimer's disease. Neurobiology of Disease. 2011;41:62–70. doi: 10.1016/j.nbd.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Hennig GW, Ward SM, Sanders KM. Temporal sequence of activation of cells involved in Purinergic neurotransmission in the Colon. The Journal of Physiology. 2015;593:1945–1963. doi: 10.1113/jphysiol.2014.287599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Drumm BT, Saur D, Hennig GW, Ward SM, Sanders KM. Spontaneous ca(2+) transients in interstitial cells of cajal located within the deep muscular plexus of the murine small intestine. The Journal of Physiology. 2016;594:3317–3338. doi: 10.1113/JP271699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayguinov O, Ward SM, Kenyon JL, Sanders KM. Voltage-gated Ca2+ currents are necessary for slow-wave propagation in the canine gastric antrum. American Journal of Physiology. Cell Physiology. 2007;293:C1645–C1659. doi: 10.1152/ajpcell.00165.2007. [DOI] [PubMed] [Google Scholar]
- Beckett EA, Bayguinov YR, Sanders KM, Ward SM, Hirst GD. Properties of unitary potentials generated by intramuscular interstitial cells of cajal in the murine and guinea-pig gastric fundus. The Journal of Physiology. 2004;559:259–269. doi: 10.1113/jphysiol.2004.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DC, Butcher AJ, Berrow NS, Page KM, Brust PF, Nesterova A, Stauderman KA, Seabrook GR, Nürnberg B, Dolphin AC. Biophysical properties, pharmacology, and modulation of human, neuronal L-type (alpha(1D), Ca(V)1.3) voltage-dependent calcium currents. Journal of Neurophysiology. 2001;85:816–827. doi: 10.1152/jn.2001.85.2.816. [DOI] [PubMed] [Google Scholar]
- Berezin I, Huizinga JD, Daniel EE. Interstitial cells of cajal in the canine Colon: a special communication network at the inner border of the circular muscle. The Journal of Comparative Neurology. 1988;273:42–51. doi: 10.1002/cne.902730105. [DOI] [PubMed] [Google Scholar]
- Berjukow S, Marksteiner R, Gapp F, Sinnegger MJ, Hering S. Molecular mechanism of calcium channel block by isradipine role of a drug-induced inactivated channel conformation. The Journal of Biological Chemistry. 2000;275:22114–22120. doi: 10.1074/jbc.M908836199. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochimica Et Biophysica Acta (BBA) - Molecular Cell Research. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of cajal mediate inhibitory neurotransmission in the stomach. PNAS. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell and Tissue Research. 1997;290:11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- Butorac C, Krizova A, Derler I. Review: structure and activation mechanisms of CRAC channels. Advances in Experimental Medicine and Biology. 2020;1131:547–604. doi: 10.1007/978-3-030-12457-1_23. [DOI] [PubMed] [Google Scholar]
- Chauvet S, Jarvis L, Chevallet M, Shrestha N, Groschner K, Bouron A. Pharmacological characterization of the native Store-Operated calcium channels of cortical neurons from embryonic mouse brain. Frontiers in Pharmacology. 2016;7:486. doi: 10.3389/fphar.2016.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sanderson MJ. Store-operated calcium entry is required for sustained contraction and Ca2+ oscillations of airway smooth muscle. The Journal of Physiology. 2017;595:3203–3218. doi: 10.1113/JP272694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsetti M, Costa M, Bassotti G, Bharucha AE, Borrelli O, Dinning P, Di Lorenzo C, Huizinga JD, Jimenez M, Rao S, Spiller R, Spencer NJ, Lentle R, Pannemans J, Thys A, Benninga M, Tack J. First translational consensus on terminology and definitions of colonic motility in animals and humans studied by manometric and other techniques. Nature Reviews Gastroenterology & Hepatology. 2019;16:559–579. doi: 10.1038/s41575-019-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GD. Electrical coupling between the myenteric interstitial cells of cajal and adjacent muscle layers in the guinea-pig gastric antrum. The Journal of Physiology. 2003;550:829–844. doi: 10.1113/jphysiol.2003.042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antona G, Hennig GW, Costa M, Humphreys CM, Brookes SJ. Analysis of motor patterns in the isolated guinea-pig large intestine by spatio-temporal maps. Neurogastroenterology and Motility. 2001;13:483–492. doi: 10.1046/j.1365-2982.2001.00282.x. [DOI] [PubMed] [Google Scholar]
- Derler I, Schindl R, Fritsch R, Heftberger P, Riedl MC, Begg M, House D, Romanin C. The action of selective CRAC channel blockers is affected by the orai pore geometry. Cell Calcium. 2013;53:139–151. doi: 10.1016/j.ceca.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm BT, Hennig GW, Battersby MJ, Cunningham EK, Sung TS, Ward SM, Sanders KM, Baker SA. Clustering of Ca2+ transients in interstitial cells of cajal defines slow wave duration. Journal of General Physiology. 2017;149:703–725. doi: 10.1085/jgp.201711771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm BT, Hwang SJ, Baker SA, Ward SM, Sanders KM. Ca2+ signalling behaviours of intramuscular interstitial cells of cajal in the murine Colon. The Journal of Physiology. 2019a;597:3587–3617. doi: 10.1113/JP278036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm BT, Hennig GW, Baker SA, Sanders KM. Applications of Spatio-temporal mapping and particle analysis techniques to quantify intracellular Ca2+ signaling in situ. Journal of Visualized Experiments. 2019b;7:58989. doi: 10.3791/58989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm BT, Rembetski BE, Messersmith K, Manierka MS, Baker SA, Sanders KM. Pacemaker function and neural responsiveness of subserosal interstitial cells of cajal in the mouse Colon. The Journal of Physiology. 2020a;598:651–681. doi: 10.1113/JP279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm BT, Rembetski BE, Huynh K, Nizar A, Baker SA, Sanders KM. Excitatory cholinergic responses in mouse Colon intramuscular interstitial cells of cajal are due to enhanced ca. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2020b;34:10073–10095. doi: 10.1096/fj.202000672R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont G, Goldbeter A. Properties of intracellular Ca2+ waves generated by a model based on ca(2+)-induced Ca2+ release. Biophysical Journal. 1994;67:2191–2204. doi: 10.1016/S0006-3495(94)80705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durdle NG, Kingma YJ, Bowes KL, Chambers MM. Origin of slow waves in the canine Colon. Gastroenterology. 1983;84:375–382. doi: 10.1016/S0016-5085(83)80136-X. [DOI] [PubMed] [Google Scholar]
- Faussone Pellegrini MS, Cortesini C, Romagnoli P. Ultrastructure of the Tunica muscularis of the cardial portion of the human esophagus and stomach, with special reference to the so-called cajal's interstitial cells] Archivio Italiano Di Anatomia E Di Embriologia. Italian Journal of Anatomy and Embryology. 1977;82:157–177. [PubMed] [Google Scholar]
- Faussone-Pellegrini MS. Histogenesis, structure and relationships of interstitial cells of cajal (ICC): from morphology to functional interpretation. European Journal of Morphology. 1992;30:137–148. [PubMed] [Google Scholar]
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiological Reviews. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Gallego D, Gil V, Aleu J, Aulí M, Clavé P, Jiménez M. Purinergic and nitrergic junction potential in the human Colon. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008;295:G522–G533. doi: 10.1152/ajpgi.00510.2007. [DOI] [PubMed] [Google Scholar]
- Gibson A, McFadzean I, Wallace P, Wayman CP. Capacitative Ca2+ entry and the regulation of smooth muscle tone. Trends in Pharmacological Sciences. 1998;19:266–269. doi: 10.1016/s0165-6147(98)01222-x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of cajal in the human and mouse gastrointestinal tract. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;296:G1370–G1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Kubota M, Szurszewski JH. Electrophysiology of smooth muscle of the small intestine of some mammals. The Journal of Physiology. 1986;372:501–520. doi: 10.1113/jphysiol.1986.sp016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Hirst GD, Park KJ, Smith CB, Sanders KM, Ward SM, Smith TK. Propagation of pacemaker activity in the guinea-pig antrum. The Journal of Physiology. 2004;556:585–599. doi: 10.1113/jphysiol.2003.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Fozzard HA, January CT. Characteristics of L- and T-type Ca2+ currents in canine cardiac purkinje cells. American Journal of Physiology-Heart and Circulatory Physiology. 1989;256:H1478–H1492. doi: 10.1152/ajpheart.1989.256.5.H1478. [DOI] [PubMed] [Google Scholar]
- Hotta A, Okada N, Suzuki H. Mibefradil-sensitive component involved in the plateau potential in submucosal interstitial cells of the murine proximal Colon. Biochemical and Biophysical Research Communications. 2007;353:170–176. doi: 10.1016/j.bbrc.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Huang X, Lee SH, Lu H, Sanders KM, Koh SD. Molecular and functional characterization of inwardly rectifying K + currents in murine proximal Colon. The Journal of Physiology. 2018;596:379–391. doi: 10.1113/JP275234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Shin A, Chow E. Electrical coupling and pacemaker activity in colonic smooth muscle. American Journal of Physiology-Cell Physiology. 1988;255:C653–C660. doi: 10.1152/ajpcell.1988.255.5.C653. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of cajal is fundamental for slow wave activity in gastrointestinal muscles. The Journal of Physiology. 2009;587:4887–4904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. The Journal of Physiology. 2012;590:1957–1972. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Komuro T. Characterization of the interstitial cells associated with the submuscular plexus of the guinea-pig Colon. Anatomy and Embryology. 1996;194:49–55. doi: 10.1007/BF00196314. [DOI] [PubMed] [Google Scholar]
- Kaßmann M, Szijártó IA, García-Prieto CF, Fan G, Schleifenbaum J, Anistan YM, Tabeling C, Shi Y, le Noble F, Witzenrath M, Huang Y, Markó L, Nelson MT, Gollasch M. Role of ryanodine type 2 receptors in elementary ca. Journal of the American Heart Association. 2019;8:e010090. doi: 10.1161/JAHA.118.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keef KD, Ward SM, Stevens RJ, Frey BW, Sanders KM. Electrical and mechanical effects of acetylcholine and substance P in subregions of canine Colon. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1992;262:G298–G307. doi: 10.1152/ajpgi.1992.262.2.G298. [DOI] [PubMed] [Google Scholar]
- Keef KD, Anderson U, O'Driscoll K, Ward SM, Sanders KM. Electrical activity induced by nitric oxide in canine colonic circular muscle. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2002;282:G123–G129. doi: 10.1152/ajpgi.00217.2001. [DOI] [PubMed] [Google Scholar]
- Kito Y, Ward SM, Sanders KM. Pacemaker potentials generated by interstitial cells of cajal in the murine intestine. American Journal of Physiology-Cell Physiology. 2005;288:C710–C720. doi: 10.1152/ajpcell.00361.2004. [DOI] [PubMed] [Google Scholar]
- Kito Y, Suzuki H. Modulation of slow waves by hyperpolarization with potassium channel openers in antral smooth muscle of the guinea-pig stomach. The Journal of Physiology. 2003;548:175–189. doi: 10.1113/jphysiol.2002.035550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Bradley KK, Rae MG, Keef KD, Horowitz B, Sanders KM. Basal activation of ATP-sensitive potassium channels in murine colonic smooth muscle cell. Biophysical Journal. 1998;75:1793–1800. doi: 10.1016/S0006-3495(98)77621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro T, Tokui K, Zhou DS. Identification of the interstitial cells of cajal. Histology and Histopathology. 1996;11:769–786. [PubMed] [Google Scholar]
- Komuro T. Comparative morphology of interstitial cells of cajal: ultrastructural characterization. Microscopy Research and Technique. 1999;47:267–285. doi: 10.1002/(SICI)1097-0029(19991115)47:4<267::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Komuro T. Structure and organization of interstitial cells of cajal in the gastrointestinal tract. The Journal of Physiology. 2006;576:653–658. doi: 10.1113/jphysiol.2006.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. alpha 1d (Cav1.3) Subunits Can Form L-type Ca 2+Channels Activating at Negative Voltages. Journal of Biological Chemistry. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of cajal isolated from canine proximal Colon. PNAS. 1989;86:7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Ha SE, Park C, Park PJ, Fuchs R, Wei L, Jorgensen BG, Redelman D, Ward SM, Sanders KM, Ro S. Transcriptome of interstitial cells of cajal reveals unique and selective gene signatures. PLOS ONE. 2017;12:e0176031. doi: 10.1371/journal.pone.0176031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh WA, Del Valle G, Kamran SA, Drumm BT, Tavakkoli A, Sanders KM, Baker SA. A high throughput machine-learning driven analysis of ca. Cell Calcium. 2020;91:102260. doi: 10.1016/j.ceca.2020.102260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lies B, Gil V, Groneberg D, Seidler B, Saur D, Wischmeyer E, Jiménez M, Friebe A. Interstitial cells of cajal mediate nitrergic inhibitory neurotransmission in the murine gastrointestinal tract. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2014;307:G98–G106. doi: 10.1152/ajpgi.00082.2014. [DOI] [PubMed] [Google Scholar]
- Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. The Journal of Physiology. 2008;586:4815–4824. doi: 10.1113/jphysiol.2008.160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navedo MF, Amberg GC. Local regulation of L-type Ca²⁺ channel sparklets in arterial smooth muscle. Microcirculation. 2013a;20:290–298. doi: 10.1111/micc.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navedo MF, Santana LF. CaV1.2 sparklets in heart and vascular smooth muscle. Journal of Molecular and Cellular Cardiology. 2013b;58:67–76. doi: 10.1016/j.yjmcc.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, Sanders KM. Spatial and temporal mapping of pacemaker activity in interstitial cells of cajal in mouse ileum in situ. American Journal of Physiology-Cell Physiology. 2006;290:C1411–C1427. doi: 10.1152/ajpcell.00447.2005. [DOI] [PubMed] [Google Scholar]
- Plujà L, Albertí E, Fernández E, Mikkelsen HB, Thuneberg L, Jiménez M. Evidence supporting presence of two pacemakers in rat Colon. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2001;281:G255–G266. doi: 10.1152/ajpgi.2001.281.1.G255. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Store-Operated calcium channels. Physiological Reviews. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW. The physiological function of store-operated calcium entry. Neurochemical Research. 2011;36:1157–1165. doi: 10.1007/s11064-010-0383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW. Forms and functions of store-operated calcium entry mediators, STIM and orai. Advances in Biological Regulation. 2018;68:88–96. doi: 10.1016/j.jbior.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro S, Park C, Jin J, Zheng H, Blair PJ, Redelman D, Ward SM, Yan W, Sanders KM. A model to study the phenotypic changes of interstitial cells of cajal in gastrointestinal diseases. Gastroenterology. 2010;138:1068–1078. doi: 10.1053/j.gastro.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosli RM, Heitmann PT, Kumar R, Hibberd TJ, Costa M, Wiklendt L, Wattchow DA, Arkwright J, de Fontgalland D, Brookes SJH, Spencer NJ, Dinning PG. Distinct patterns of myogenic motor activity identified in isolated human distal Colon with high-resolution manometry. Neurogastroenterology & Motility. 2020;32:e13871. doi: 10.1111/nmo.13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg UT, Hof RP. Pharmacology of the calcium antagonist isradipine. Drugs. 1990;40:3–9. doi: 10.2165/00003495-199000402-00004. [DOI] [PubMed] [Google Scholar]
- Rumessen JJ, Vanderwinden JM, Hansen A, Horn T. Ultrastructure of interstitial cells in subserosa of human Colon. Cells Tissues Organs. 2013;197:322–332. doi: 10.1159/000346314. [DOI] [PubMed] [Google Scholar]
- Rumessen JJ, Thuneberg L. Plexus muscularis profundus and associated interstitial cells. I. light microscopical studies of mouse small intestine. The Anatomical Record. 1982;203:115–127. doi: 10.1002/ar.1092030111. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Stevens R, Burke E, Ward SW. Slow waves actively propagate at Submucosal surface of circular layer in canine Colon. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1990;259:G258–G263. doi: 10.1152/ajpgi.1990.259.2.G258. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. The Journal of Physiology. 2010;588:4621–4639. doi: 10.1113/jphysiol.2010.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility--insights from smooth muscle biology. Nature Reviews Gastroenterology & Hepatology. 2012;9:633–645. doi: 10.1038/nrgastro.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiological Reviews. 2014a;94:859–907. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Salter AK, Hennig GW, Koh SD, Perrino BA, Ward SM, Baker SA. Responses to enteric motor neurons in the gastric fundus of mice with reduced intramuscular interstitial cells of cajal. Journal of Neurogastroenterology and Motility. 2014b;20:171–184. doi: 10.5056/jnm.2014.20.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana LF, Navedo MF, Amberg GC, Nieves-Cintrón M, Votaw VS, Ufret-Vincenty CA. Calcium sparklets in arterial smooth muscle. Clinical and Experimental Pharmacology and Physiology. 2008;35:1121–1126. doi: 10.1111/j.1440-1681.2007.04867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholze A, Plant TD, Dolphin AC, Nürnberg B. Functional expression and characterization of a voltage-gated CaV1.3 (alpha1D) calcium channel subunit from an insulin-secreting cell line. Molecular Endocrinology. 2001;15:1211–1221. doi: 10.1210/mend.15.7.0666. [DOI] [PubMed] [Google Scholar]
- Serio R, Barajas-Lopez C, Daniel EE, Berezin I, Huizinga JD. Slow-wave activity in Colon: role of network of submucosal interstitial cells of cajal. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1991;260:G636–G645. doi: 10.1152/ajpgi.1991.260.4.G636. [DOI] [PubMed] [Google Scholar]
- Smith TK, Reed JB, Sanders KM. Origin and propagation of electrical slow waves in circular muscle of canine proximal Colon. American Journal of Physiology-Cell Physiology. 1987a;252:C215–C224. doi: 10.1152/ajpcell.1987.252.2.C215. [DOI] [PubMed] [Google Scholar]
- Smith TK, Reed JB, Sanders KM. Interaction of two electrical pacemakers in muscularis of canine proximal Colon. American Journal of Physiology-Cell Physiology. 1987b;252:C290–C299. doi: 10.1152/ajpcell.1987.252.3.C290. [DOI] [PubMed] [Google Scholar]
- Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Nystoriak MA, Nieves-Cintrón M, Santana LF, Navedo MF. Relationship between Ca2+ sparklets and sarcoplasmic reticulum Ca2+ load and release in rat cerebral arterial smooth muscle. American Journal of Physiology. Heart and Circulatory Physiology. 2011;301:H2285–H2294. doi: 10.1152/ajpheart.00488.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AP, Bird GS, Hajnóczky G, Robb-Gaspers LD, Putney JW. Spatial and temporal aspects of cellular calcium signaling. The FASEB Journal. 1996;10:1505–1517. doi: 10.1096/fasebj.10.13.8940296. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of cajal: intestinal pacemaker cells? Advances in Anatomy, Embryology, and Cell Biology. 1982;71:1–130. [PubMed] [Google Scholar]
- Trebak M, Zhang W, Ruhle B, Henkel MM, González-Cobos JC, Motiani RK, Stolwijk JA, Newton RL, Zhang X. What role for store-operated Ca²⁺ entry in muscle? Microcirculation. 2013;20:330–336. doi: 10.1111/micc.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng GN, Boyden PA. Multiple types of Ca2+ currents in single canine purkinje cells. Circulation Research. 1989;65:1735–1750. doi: 10.1161/01.RES.65.6.1735. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM, Rumessen JJ, Liu H, Descamps D, De Laet MH, Vanderhaeghen JJ. Interstitial cells of cajal in human Colon and in Hirschsprung's disease. Gastroenterology. 1996;111:901–910. doi: 10.1016/S0016-5085(96)70057-4. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM, Rumessen JJ, Bernex F, Schiffmann SN, Panthier JJ. Distribution and ultrastructure of interstitial cells of cajal in the mouse Colon, using antibodies to kit and kit(W-lacZ) mice. Cell and Tissue Research. 2000;302:155–170. doi: 10.1007/s004419900170. [DOI] [PubMed] [Google Scholar]
- Vierra NC, Kirmiz M, van der List D, Santana LF, Trimmer JS. Kv2.1 mediates spatial and functional coupling of L-type calcium channels and ryanodine receptors in mammalian neurons. eLife. 2019;8:e49953. doi: 10.7554/eLife.49953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Keller RG, Sanders KM. Structure and organization of electrical activity of canine distal Colon. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1991;260:G724–G735. doi: 10.1152/ajpgi.1991.260.5.G724. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. The Journal of Physiology. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. American Journal of Physiology-Cell Physiology. 1995;269:C1577–C1585. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of cajal mediate cholinergic neurotransmission from enteric motor neurons. The Journal of Neuroscience. 2000a;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. The Journal of Physiology. 2000b;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won KJ, Sanders KM, Ward SM. Interstitial cells of cajal mediate mechanosensitive responses in the stomach. PNAS. 2005;102:14913–14918. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Best PM. Postnatal changes in T-type calcium current density in rat atrial myocytes. The Journal of Physiology. 1992;454:657–672. doi: 10.1113/jphysiol.1992.sp019285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Lipscombe D. Neuronal ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. The Journal of Neuroscience. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda S, Takano H, Takaki M, Suzuki H. Properties of spontaneously active cells distributed in the submucosal layer of mouse proximal Colon. The Journal of Physiology. 2002;542:887–897. doi: 10.1113/jphysiol.2002.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda S, Takano H, Takaki M, Suzuki H. Effects of nifedipine and nickel on plateau potentials generated in submucosal interstitial cells distributed in the mouse proximal Colon. Journal of Smooth Muscle Research. 2003;39:55–65. doi: 10.1540/jsmr.39.55. [DOI] [PubMed] [Google Scholar]
- Yoneda S, Fukui H, Takaki M. Pacemaker activity from submucosal interstitial cells of cajal drives high-frequency and low-amplitude circular muscle contractions in the mouse proximal Colon. Neurogastroenterology and Motility. 2004;16:621–627. doi: 10.1111/j.1365-2982.2004.00546.x. [DOI] [PubMed] [Google Scholar]
- Youm JB, Zheng H, Koh SD, Sanders KM. Na-K-2Cl cotransporter and Store-Operated Ca2+ Entry in Pacemaking by Interstitial Cells of Cajal. Biophysical Journal. 2019;117:767–779. doi: 10.1016/j.bpj.2019.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Park KS, Koh SD, Sanders KM. Expression and function of a T-type Ca2+ conductance in interstitial cells of cajal of the murine small intestine. American Journal of Physiology-Cell Physiology. 2014;306:C705–C713. doi: 10.1152/ajpcell.00390.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Drumm BT, Earley S, Sung TS, Koh SD, Sanders KM. SOCE mediated by STIM and orai is essential for pacemaker activity in the interstitial cells of cajal in the gastrointestinal tract. Science Signaling. 2018;11:eaaq0918. doi: 10.1126/scisignal.aaq0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A ca(2+)-activated cl(-) conductance in interstitial cells of cajal linked to slow wave currents and pacemaker activity. The Journal of Physiology. 2009;587:4905–4918. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MH, Sung IK, Zheng H, Sung TS, Britton FC, O'Driscoll K, Koh SD, Sanders KM. Muscarinic activation of Ca2+-activated cl- current in interstitial cells of cajal. The Journal of Physiology. 2011;589:4565–4582. doi: 10.1113/jphysiol.2011.211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MH, Sung TS, O'Driscoll K, Koh SD, Sanders KM. Intracellular ca(2+) release from endoplasmic reticulum regulates slow wave currents and pacemaker activity of interstitial cells of cajal. American Journal of Physiology-Cell Physiology. 2015;308:C608–C620. doi: 10.1152/ajpcell.00360.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]