Abstract
Background:
Detection of germline RB1 mutations is critical for risk assessment of retinoblastoma (RB) patients. Assessment of somatic copy number alterations (SCNAs) is also critically important because of their prognostic significance. Herein we present a refined approach for the simultaneous identification of RB1 variants and SCNAs in the aqueous humor (AH) of RB eyes.
Materials and Methods:
Subjects included 7 eyes of 6 RB patients that underwent AH extraction, and 4 matched tumor samples. Cell-free DNA (cfDNA) was isolated and sequenced to assess genome-wide SCNAs. The same sequencing libraries then underwent targeted resequencing and mutation detection using a custom hybridization panel that targets RB1 and MYCN. Illumina paired-end 2×150bp sequencing was used to characterize single-nucleotide variants (SNVs) and loss of heterozygosity (LOH). Results were compared to peripheral blood RB1 testing. Tumor fraction (TFx) was calculated using ichorCNA.
Results:
Four of 7 AH samples contained clinically significant SCNAs. Of the 3 other samples, 1 showed focal MYCN amplification and 1 showed focal RB1 deletion. All 4 enucleated tumors contained SCNAs. Mutational analysis of tumor DNA identified all first hits (2 germline RB1 SNVs, 2 germline CNAs) and second hits (4 RB1 SNVs). RB1 variants in AH were concordant with those obtained from corresponding tumor tissue and blood. In AH samples without paired tumor, both RB1 hits were identified with high variant allele frequency, even in the absence of SCNAs.
Conclusions:
AH liquid biopsy is a minimally invasive, in vivo alternative to tissue analysis for the simultaneous identification of RB1 variants and SCNAs in RB eyes.
Keywords: Aqueous humor, mutational analysis, RB1 gene, Retinoblastoma, somatic copy number alterations
Introduction
Retinoblastoma (RB), a childhood intraocular cancer, is initiated by biallelic inactivation – usually from single-nucleotide variants (SNVs) or InDels – of the RB tumor suppressor gene (RB1) (1). RB1 was the first cloned tumor suppressor gene and this discovery has dramatically altered our understanding of cancer genetics in general and RB tumorigenesis particularly (2–4). In approximately 40% of RB patients, the initial RB1 inactivation event is due to a constitutional germline or mosaic mutation (i.e. hereditary RB) followed by a second somatic mutation which then leads to the tumor; in the other 60% of cases both RB1 mutations are somatic in nature. Children with hereditary RB are at increased risk for 1) developing other ocular and non-ocular tumors over time and for 2) transmission of the mutated gene to future offspring (5). In current clinical practice, testing of peripheral blood leukocytes for the presence of a constitutional pathogenic RB1 mutation is a crucial aspect of the management of children with RB and is used to determine surveillance strategies for RB patients and their families (6). While children with bilateral disease (e.g. retinoblastoma tumors in both eyes) can be presumed to have a constitutional mutation, upwards of 15% of patients with unilateral disease will also have a detectable mutation from peripheral blood which dramatically alters their care and prognosis (7). It is important to determine whether the detected mutation is a true germline mutation versus a mosaic mutation as the parents and siblings do not require screening in the setting of mosaicism (8); further, the percentage of mosaicism can also help to stratify the risk of second tumors to the child.
Somatic RB1 mutations are not routinely identified in RB although there is some evidence that particular mutations, notably deletions, acceptor splice sites and frameshift mutations, may present with more aggressive forms of the disease (9). Beyond biallelic RB1 inactivation, somatic copy number alterations (SCNAs) are thought to contribute to RB tumor growth and progression (10). Multiple studies have identified RB-related SCNAs from RB tumor tissue (10–13), and tumor-directed mutational analyses are also known to increase the accuracy of peripheral blood testing for RB1. However, access to this information is only available when RB tumor tissue is available from enucleated (surgically removed) eyes. This is because direct tissue biopsy can cause extraocular tumor spread and is therefore contraindicated in clinical practice (14–16). Only with our recent development of the AH liquid biopsy has in vivo detection of tumor derived cfDNA become a possibility for RB patients undergoing active, eye-salvaging therapy (17). In previous studies of the AH liquid biopsy, our group identified tumor-derived SCNAs with prognostic indications for therapeutic response and risk of enucleation (18). Independently, Gerrish et al. verified the presence of tumor derived cfDNA in the AH by identifying somatic RB1 mutations (19). Detection and identification of tumor-derived RB1 mutations in the AH has diagnostic significance, including the ability to more efficiently determine the presence of heritable germline RB1 mutations; additionally there is prognostic significance in whole-genomic evaluation of RB tumors for SCNAs. However, a streamlined process for the simultaneous identification of both RB1 mutations and largescale genomic alterations in an AH sample is yet to be described.
Herein, we demonstrate that the AH liquid biopsy is a minimally invasive, in vivo alternative to tissue analysis for the simultaneous identification of RB1 mutations and SCNAs for RB. Using a novel, optimized in-house pipeline, we demonstrate that both RB SCNAs and RB1 mutations can be interrogated from a single sample of <100ul of AH, and that the genetic and genomic alterations identified within AH match what is found by combined peripheral blood and tumor analyses with high accuracy.
Materials and methods
This research was conducted under Institutional Review Board approval and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the parents of all participants prior to inclusion in the study.
Patient and specimen characteristics
This study included 7 eyes from 6 patients diagnosed with retinoblastoma between December 2014 and July 2019 at the Children’s Hospital Los Angeles (CHLA) from whom written parental consent and AH sample(s) were obtained; genomic profiles from these cases have been previously published (18) and are presented here with additional mutation analyses.
Specimen collection and storage
A clear corneal paracentesis with a 32-gauge needle was performed to extract 0.1 mL of AH from retinoblastoma eyes (18). This procedure occurred either as part of the standard intravitreal melphalan (IVM) injection protocol (20), immediately following enucleation of the eye, or at diagnosis after diagnostic examination under anesthesia. During sampling, needles entered only the anterior chamber via the clear cornea and remained bevel up over the pharmacologically dilated iris; they did not make contact with the iris, lens, vitreous cavity or tumor. While the anterior chamber shallowed slightly it remained formed during paracentesis, and the needle site was examined after AH extraction for any leakage. No patient had a complication from the extraction.
Immediately following specimen extraction, AH samples were stored at −80°C. All samples underwent cfDNA isolation and sequencing within 1 month of extraction, as described previously (18,21). If an enucleation was required for clinical care of the patient, tumor tissue was subsequently obtained from formalin-fixed and paraffin embedded (FFPE) blocks for further genetic and genomic analyses.
Concentration of cfDNA
DNA concentration in the AH before isolation has been reported previously (17); to preserve the minimal volume of the AH sample, this was not repeated until after amplification using the Qubit Fluorometer system (Thermo Fisher).
Genomic analysis of AH samples
Analysis of the cfDNA from AH samples was previously outlined in depth and based on established SCNA analysis methods (18,21–23). Briefly, isolated cfDNA was constructed into a whole genome library followed by shallow sequencing at 0.3x for copy number profiling. As before, SCNAs were considered to be present at 20% deflection from a baseline human genome, consistent with previously established liquid biopsy analyses (18,22,23). Genomic analyses were also performed on matched tumor samples, when available, as described previously (18).
RB1 mutational analysis of AH samples
The same constructed whole genome libraries were further amplified to 500ng each for capture-based targeted next generation sequencing (NGS) for mutation detection. The Agilent SureSelect hybridization capture panel covers the full length including introns and exons of the RB1 gene, the MYCN gene and all exons of the BCOR and CREBBP genes (Agilent SureSelect Tier1). Illumina paired-end 2×150bp sequencing was carried out on the captured libraries to 100x.
Bioinformatics analysis
Bioinformatics analysis was performed to detect and to characterize single-nucleotide variant (SNV) using an in-house pipeline based on the bcbio pipeline at the CHLA Center for Personalized Medicine (24). The previously published pipeline was optimized for somatic mutational analyses. Briefly, the raw fastq data was trimmed off adaptors and low-quality bases with Atropos (25) and aligned to human GRCh37 reference with BWA-MEM and NovoAlign (v3) (26,27). Germline variants were called after marking duplicates with FreeBayes (28). The presence of somatic variants in the AH or tumor were called with VarDict without the paired normal blood sample (i.e. blinded to germline variant) (29). Variant annotation was conducted with Ensembl VEP (v96) (30). LOH was called if a region’s continuous variants had ≤ 3% alternative alleles.
Determination of cfDNA TFx
The fraction of tumor DNA (TFx) for each sequenced AH cfDNA sample was estimated using ichorCNA software (available at https://github.com/broadinstitute/ichorCNA), which is a standard tool for determining cfDNA TFx in blood-based liquid biopsies (31). The algorithm employed by ichorCNA to determine TFx of cfDNA in the serum has been described in detail (31,32). In short, ichorCNA utilizes a hidden Markov model to predict large-scale SCNAs within sequenced cfDNA. TFx estimations based on the presence of large-scale SCNAs are derived while accounting for differences in ploidy and subclonality at each locus, and an optimal TFx solution (as well as numerous alternative solutions) is provided by ichorCNA (31). Per ichorCNA recommendations, genomic profiles and corresponding TFx solutions were visually inspected to confirm the TFx estimate for each sample (https://github.com/broadinstitute/ichorCNA/wiki/Interpreting-ichorCNA-results). If the selected solution appeared suboptimal based on ichorCNA guidelines (i.e. a large proportion of SCNAs were being called subclonal, the majority of datapoints were falsely amplified, or two distinct copy number levels were being called neutral) (31), then a more accurate alternative solution was manually selected from the ichorCNA output (available upon request).
Clinical demographics
A data set with clinical characteristics of patients was compiled including germline RB1 status determined from peripheral blood cells as part of the routine clinical retinoblastoma work-up.
Results
AH samples were obtained from 7 eyes of 6 patients (labeled E1–7; Table 1). AH samples E1–4 were taken at the time of enucleation and matched tumor is available; 1 of the AH samples was taken during primary enucleation and 3 during secondary enucleation after failed attempts at eye salvaging therapy. AH samples E5, E6 and E7 were taken in vivo; E6 and E7 were from the right and left eyes of the same patient. There is no matched tumor available for samples E5, E6 or E7 as the eyes have not been enucleated. All patients underwent RB1 mutational analysis of the peripheral blood as part of routine clinical testing from the CHLA CLIA lab.
Table 1.
Mutational and somatic copy number alteration (SCNA) analyses for aqueous humor samples from 7 retinoblastoma eyes (E1–E7), including corresponding germline RB1 and tumor mutational analyses when available.
| Rb Eye | Timing of Sampling | Tumor_CNA (TFx) | AH_CNA (TFx) | gDNA RB1 mutation | RB1 mutation | tDNA |
AH_cfDNA |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| %VAF | (unaltered, altered) | 95% CI | %VAF | (unaltered, altered) | 95% CI | ||||||
| E1 | Secondary Enucleation | CNA+ (17.79%) | CNA+ (28.67%) | c.1758del, p.Glu587AsnfsTer24 | c.1758del, p.Glu587AsnfsTer24 c.689 C>G, p.Ser230* |
45.59% 29.10% |
543, 455 609, 250 |
(42.47% – 48.74%) (26.08% – 32.27%) |
65.71% 43.94% |
36, 69 37,29 |
(55.81% – 74.70%) (31.74% – 56.70%) |
| E2 | Primary Enucleation | CNA+ (83.03%) | CNA+ (45.25%) | 13qDel | 13qDel c.256 G > T, p.Gly86* |
13qDel/NA 97.19% |
… … 5, 173 |
… … (93.57% – 99.08%) |
13qDel/ NA 95.56% |
… … 2, 43 |
… … (84.85% – 99.46%) |
| E3 | Secondary Enucleation | CNA+ (91.42%) | CNA+ (45.01%) | c.958 C>T, p.Arg320* | c.958 C>T, p.Arg320* c.751 C>T, p.Arg251* |
30.74% 45.00% |
196, 87 374, 306 |
(25.42% – 36.48%) (41.22% – 48.83%) |
41.04% 55.56% |
102, 71 112, 140 |
(33.63% – 48.76%) (49.19% – 61.79%) |
| E4 | Secondary Enucleation | CNA+ (90.82%) | CNA+ (82.2%) | 13q deletion | 13q deletion c.1215 + 1 G>A, splice_donor_variant |
13qDel/NA 92.00% |
… … 4, 46 |
… … (80.77% – 97.78%) |
13qDel/ NA 92.45% |
… … 4, 49 |
… … (81.79% – 97.91%) |
| E5 | During Therapy | NA | CNA-with focal MYCNamp | c.2425del, p.Leu809* | c.2425del, p.Leu809* c.1024del, p.Thr342LeufsTer7 |
NA NA |
NA NA |
NA NA |
32.14% 15.79% |
19, 9 16, 3 |
(15.88% – 52.35%) (3.38% – 39.58%) |
| E6 | Diagnosis | NA | CNA- | c.1666 C>T, p.Arg455* | c.1666 C>T, p.Arg455* LOH |
NA NA |
NA NA |
NA NA |
66.67% LOH |
37,74 … … |
(57.09% – 75.33%) … … |
| E7 | Diagnosis | NA | CNA-with focal RB1 deletion | c.1666 C>T, p.Arg455* | c.1666 C>T, p.Arg455* Focal RB1 gene deletion |
NA NA |
NA NA |
NA NA |
100% Focal RB1 gene deletion |
0, 351 … … |
(98.95–100%)+ … … |
one-sided, 97.5% CI.
Abbreviations: AH, aqueous humor; amp, amplification; cfDNA, cell-free DNA; CNA, copy number alteration; gDNA, genomic DNA; NA, not available; Rb, retinoblastoma; tDNA, tumor DNA; TFx, tumor fraction; VAF, variant allele frequency.
Concentration of cfDNA in the AH
The DNA concentration in AH samples was reported previously (17); The DNA concentration before DNA isolation ranged from 0.084 to 56 ng/μL (median, 0.174 ng/μL). These results are within the range previously reported by Gerrish et al (19). To conserve the very limited AH sample volume, this was not repeated. After PCR amplification (14x) for whole genome library construction, the average final yield was 25.15 ng/ul (std dev ± 22.32). However, there is wide deviation with the highest yields in samples taken before therapy (diagnosis or primary enucleation) and the lowest yields from samples taken during active treatment due to a decrease in tumor-derived cfDNA in the AH as the intraocular tumor responds to treatment.
Whole genome SCNA analysis on tumor derived cfDNA from AH
Whole genome analyses were performed on all blood, AH samples, and matched tumor samples when available (Figure 1). Large-scale SCNAs were identified in AH samples E1–4. These alterations were concordant with the SCNAs in the tumor tissue as has been previously shown with other AH-tumor pairs (18). For E5 and E7, there were no large-scale SCNA detected, but focal MYCN amplification was identified in AH sample from E5 and focal RB1 gene loss was identified in AH from E7. These focal events were never detected in paired blood samples indicating they are somatic alterations.
Figure 1.
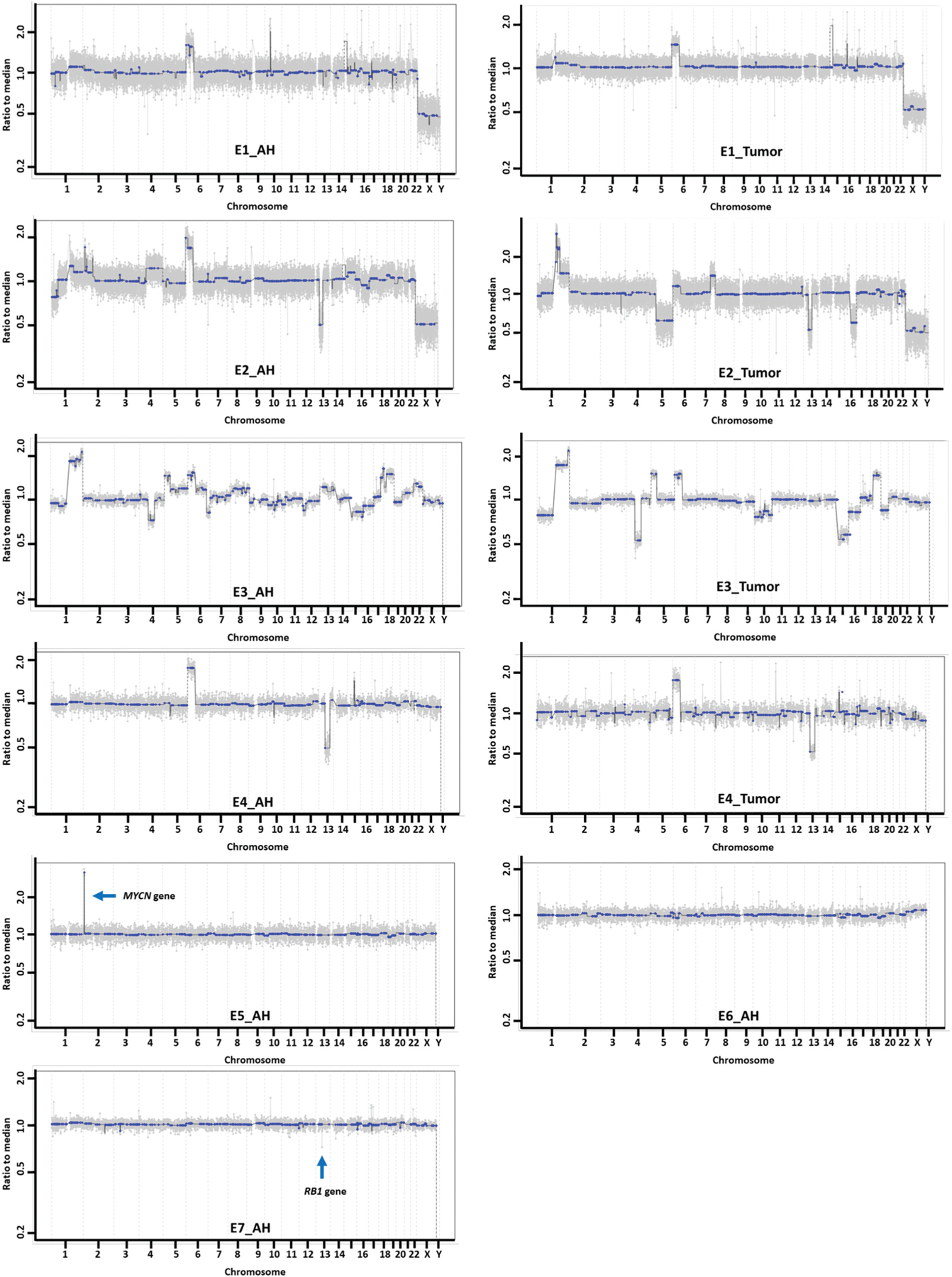
Whole genome copy number alteration profiling in AH cfDNA and tumor DNA.
RB1 variant detection in cfDNA from AH
From the same constructed whole genome libraries used in SCNA profiling, pathogenic mutations were identified in the AH cfDNA of all 7 eyes. These pathogenic mutations were identified in the AH in all 7 eyes by an customized bioinformatics pipeline at the CHLA Center for Personalized Medicine. The mutations were corroborated either by analyses of tumor tissue from enucleated eyes and/or from clinical testing of peripheral blood leukocytes. No pathogenic BCOR or CREBBP mutations were detected in these 7 eyes. RB1 variant allele fraction (%VAF) in both the tumor and AH are presented in Table 1, followed with number in parentheses of unaltered and altered reads on the specific variant, and corresponding 95% CI range, respectively.
Tumor fraction
The tumor fraction (TFx) or percentage of tumor-derived cfDNA in the AH was calculated with ichorCNA software (31) which optimizes the calculation of the fraction based on the presence of large-scale chromosomal alterations. Focal alterations like MYCN amplification in E5 and RB1 gene deletion in E7 have not been able to generate the TFx. The TFx ranged from 17.79–91.42% in tumor samples with +CNA for analysis (mean TFx 70.77%, Table 1).
In summary, our novel, optimized AH-based protocol demonstrates feasibility for the detection of both pathogenic RB1 mutations, genomic copy number alterations and tumor fraction in retinoblastoma from a single sample of AH. This has potential for numerous, clinically significant applications including diagnostic (RB1), prognostic (SCNAs) and therapeutic (TFx) implications. This streamlined approach facilitates in vivo detection of tumor-derived DNA, making it possible to identify underlying somatic RB1 mutations of RB – similar to Gerrish et al (19) – and SCNAs (18) from the same 100ul sample of AH. This can be done in the absence of a direct tumor biopsy or enucleation.
Discussion
Minimally invasive access to tumor-derived genetic and genomic information could be helpful not only for definitively diagnosing RB patients but also for prognostication – as identification of focal MYCN amplification (33) and gain of 6p (18) are poor prognostic indicators for overall globe salvage. We have demonstrated that our platform is sensitive for the detection of single gene alterations, such as MYCN amplification (E5) and focal RB1 loss (E7). Identification of somatic RB1 mutations also allows for improved specificity of peripheral blood testing for germline RB1 mutations, using tumor-directed mutational analyses. Such targeted genetic testing is especially important for identifying patients with low levels of germline RB1 mosaicism, as low-level mosaics may be missed by the routine methods of peripheral blood testing for RB (34). Additionally, targeted RB1 mutational analysis of the cerebrospinal fluid in patients with metastatic retinoblastoma has also shown promise in identifying minimal residual disease (35). Previously, identifications of these mutations would only be possible with tumor tissue in the setting of enucleation. Recent progress in the development of a liquid biopsy platform using the AH, however, has changed this paradigm (17–19). A larger prospective study of RB1 somatic and germline mutations within AH and peripheral blood would be useful for clarifying the diagnostic role of AH evaluation in the clinical setting and whether there is prognostic value in identifying the specific somatic RB1 mutations, or other pathogenic mutations such as BCOR or CREBBP (36), in vivo from every patient.
Another potential application of our approach is the determination of tumor fraction (TFx, i.e. the relative amount of tumor-derived DNA within a sample). Multiple studies of non-ocular cancers have shown a significant association between TFx and overall tumor burden, with increases in TFx reflecting progression of disease and vice versa (32,37). Although novel software, such as ichorCNA (31), allows for TFx estimation based on the detection of SCNAs within cfDNA, this approach is inherently limited for RB tumors that do not have any large-scale SCNAs, which account for nearly one-third of RB tumors, including samples E5, E6 and E7 here; in such tumors, accurate TFx estimation from current SCNA-based methods is not possible. However, with few exceptions (33), all RB tumors contain somatic mutations in RB1. Therefore, calculating TFx based on the presence of somatic RB1 variants with VAF may be a useful alternative approach for estimating TFx in the absence of SCNAs. As demonstrated in Table 1, there is a high VAF for the RB1 mutations identified in E6 and E7 which is on par with the tumor tissue, thus demonstrating the presence of tumor-derived DNA. Further investigation with implementing unique molecular identifiers (UMI) is necessary in order to develop a reliable RB1-based pipeline for TFx estimation in the setting of RB. The TFx can also be monitored overtime in the AH. Since TFx changes correlate with disease activity (32,37,38), this may be a novel way to monitor for minimal residual intraocular disease in retinoblastoma.
Given the potential prognostic significance of certain RB SCNAs, whole-genomic evaluation of RB tumors – in addition to RB1 testing – is becoming an increasingly important area of investigation for ocular oncologists and their pediatric patients. The refined approach presented herein allows for diagnostic and prognostic data to be generated in an optimized fashion from the same prepared cfDNA sequencing library. This maximizes the use of a very limited sample of AH, which – while less invasive than tumor biopsy – remains a more invasive sample to obtain than blood. Additionally, the maximum volume that can be taken from an eye in vivo is approximately 100ul and it cannot be directly repeated. Thus, future applications of this novel liquid biopsy platform must optimize their testing to obtain clinically relevant and impactful information from this otherwise small biospecimen.
Funding
This work was supported by the National Cancer Institute of the National Institute of Health Award under Grant [K08CA232344]; Hyundai Hope on Wheels under Grant [RGA012351]; Childhood Eye Cancer Trust; Knights Templar Eye Foundation; The American Cancer Society under Grant [#IRG-16-181-57]; Wright Foundation; the Larry and Celia Moh Foundation; the Institute for Families, Inc., Children’s Hospital Los Angeles; an unrestricted departmental grant from Research to Prevent Blindness; the National Eye Institute under Grant [P30EY029220]; the National Cancer Institute under Grant [P30CA014089]; the Vicky Joseph Research Fund; the Carol Vassiliadis Research Fund; and USC Dornsife College of Letters, Arts and Sciences.
Footnotes
Declaration of interest
Drs. Berry, Xu, and Hicks have filed a provisional patent application entitled, Aqueous Humor Cell Free DNA for Diagnostic and Prognostic Evaluation of Ophthalmic Disease. Otherwise the authors report no potential conflict of interest.
References
- 1.Tomar S, Sethi R, Sundar G, Quah TC, Quah BL, Lai PS. Mutation spectrum of RB1 mutations in retinoblastoma cases from Singapore with implications for genetic management and counselling. PLoS One. 2017;12(6):e0178776 10.1371/journal.pone.0178776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dryja TP, Rapaport JM, Joyce JM, Petersen RA. Molecular detection of deletions involving band q14 of chromosome 13 in retinoblastomas. Proc Natl Acad Sci U S A. 1986;83(19):7391–94. 10.1073/pnas.83.19.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323(6089):643–46. 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- 4.Berry JL, Polski A, Cavenee WK, Dryja TP, Murphree AL, Gallie BL. The RB1 story: characterization and cloning of the first tumor suppressor gene. Genes (Basel). 2019;10(11):879 10.3390/genes10110879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao R, Honavar SG. Retinoblastoma. Indian J Pediatr. 2017;84 (12):937–44. 10.1007/s12098-017-2395-0. [DOI] [PubMed] [Google Scholar]
- 6.Sippel KC, Fraioli RE, Smith GD, Schalkoff ME, Sutherland J, Gallie BL, Dryja TP. Frequency of somatic and germ-line mosaicism in retinoblastoma: implications for genetic counseling. Am J Hum Genet. 1998;62(3):610–19. 10.1086/301766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry JL, Lewis L, Zolfaghari E, Green S, Le BHA, Lee TC, Murphree AL, Kim JW, Jubran R. Lack of correlation between age at diagnosis and RB1 mutations for unilateral retinoblastoma: the importance of genetic testing. Ophthalmic Genet. 2018;39 (3):407–09. 10.1080/13816810.2017.1420807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Martin C, Robledo C, Gomez-Mariano G, Monzon S, Sastre A, Abelairas J, Sábado C, Martín-Begué N, Ferreres JC, Fernández-Teijeiro A, et al. Frequency of low-level and high-level mosaicism in sporadic retinoblastoma: genotype-phenotype relationships. J Hum Genet. 2020;65(2):165–74. 10.1038/s10038-019-0696-z. [DOI] [PubMed] [Google Scholar]
- 9.Mehyar M, Mosallam M, Tbakhi A, Saab A, Sultan I, Deebajah R, Jaradat I, AlJabari R, Mohammad M, AlNawaiseh I, et al. Impact of RB1 gene mutation type in retinoblastoma patients on clinical presentation and management outcome. Hematol Oncol Stem Cell Ther. 2020. 10.1016/j.hemonc.2020.02.006. [DOI] [PubMed]
- 10.Corson TW, Gallie BL. One hit, two hits, three hits, more? Genomic changes in the development of retinoblastoma. Genes Chromosomes Cancer. 2007;46(7):617–34. 10.1002/gcc.20457. [DOI] [PubMed] [Google Scholar]
- 11.Kooi IE, Mol BM, Massink MP, Ameziane N, Meijers-Heijboer H, Dommering CJ, van Mil SE, de Vries Y, van der Hout AH, Kaspers GJL, et al. Somatic genomic alterations in retinoblastoma beyond RB1 are rare and limited to copy number changes. Sci Rep. 2016;6:25264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kooi IE, Mol BM, Massink MP, de Jong MC, de Graaf P, van der Valk P, Meijers-Heijboer H, Kaspers GJL, Moll AC, Te Riele H, et al. A meta-analysis of retinoblastoma copy numbers refines the list of possible driver genes involved in tumor progression. PLoS One. 2016;11(4):e0153323 10.1371/journal.pone.0153323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowles E, Corson TW, Bayani J, Squire JA, Wong N, Lai PB, Gallie BL. Profiling genomic copy number changes in retinoblastoma beyond loss of RB1. Genes Chromosomes Cancer. 2007;46 (2):118–29. 10.1002/gcc.20383. [DOI] [PubMed] [Google Scholar]
- 14.Eide N, Walaas L. Fine-needle aspiration biopsy and other biopsies in suspected intraocular malignant disease: a review. Acta Ophthalmol. 2009;87(6):588–601. 10.1111/j.1755-3768.2009.01637.x. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson O, Hagmar B, Ryd W. Effects of fine-needle aspiration and other biopsy procedures on tumor dissemination in mice. Cancer. 1984;54(1):73–78. . [DOI] [PubMed] [Google Scholar]
- 16.Shields JA, Shields CL, Ehya H, Eagle RC Jr., De Potter P Fine-needle aspiration biopsy of suspected intraocular tumors. The 1992 Urwick Lecture. Ophthalmology. 1993;100(11):1677–84. 10.1016/S0161-6420(93)31418-1. [DOI] [PubMed] [Google Scholar]
- 17.Berry JL, Xu L, Murphree AL, Krishnan S, Stachelek K, Zolfaghari E, McGovern K, Lee TC, Carlsson A, Kuhn P, et al. Potential of aqueous humor as a surrogate tumor biopsy for retinoblastoma. JAMA Ophthalmol. 2017;135:1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry JL, Xu L, Kooi I, Murphree AL, Prabakar RK, Reid M, Stachelek K, Le BHA, Welter L, Reiser BJ, et al. Genomic cfDNA analysis of aqueous humor in retinoblastoma predicts eye salvage: the surrogate tumor biopsy for retinoblastoma. Mol Cancer Res. 2018;16(11):1701–12. 10.1158/1541-7786.MCR-18-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerrish A, Stone E, Clokie S, Ainsworth JR, Jenkinson H, McCalla M, Hitchcott C, Colmenero I, Allen S, Parulekar M, et al. Non-invasive diagnosis of retinoblastoma using cell-free DNA from aqueous humour. Br J Ophthalmol. 2019;103 (5):721–24. 10.1136/bjophthalmol-2018-313005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munier FL, Soliman S, Moulin AP, Gaillard MC, Balmer A, BeckPopovic M. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilisation of the needle track. Br J Ophthalmol. 2012;96(8):1084–87. 10.1136/bjophthalmol-2011-301016. [DOI] [PubMed] [Google Scholar]
- 21.Berry JL, Xu L, Murphree AL, Krishnan S, Stachelek K, Zolfaghari E, McGovern K, Lee TC, Carlsson A, Kuhn P, et al. Potential of aqueous humor as a surrogate tumor biopsy for retinoblastoma. JAMA Ophthalmol. 2017;135(11):1221–30. 10.1001/jamaophthalmol.2017.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baslan T, Kendall J, Rodgers L, Cox H, Riggs M, Stepansky A, Troge J, Ravi K, Esposito D, Lakshmi B, et al. Genome-wide copy number analysis of single cells. Nat Protoc. 2012;7(6):1024–41. 10.1038/nprot.2012.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baslan T, Kendall J, Rodgers L, Cox H, Riggs M, Stepansky A, Troge J, Ravi K, Esposito D, Lakshmi B, et al. Corrigendum: genome-wide copy number analysis of single cells. Nat Protoc. 2016;11(3):616 10.1038/nprot0316.616b. [DOI] [PubMed] [Google Scholar]
- 24.Chapman B, Kirchner R, Pantano L, De Smet M, Beltrame L, Khotiainsteva T, Naumenko S, Saveliev V, Guimera RV, Sytchey I, et al. bcbio-nextgen [Internet]. 2020. [accessed 2020 June 20]. http://bcbio-nextgen.readthedocs.io/en/latest/contents/citations.html.
- 25.Didion JP, Martin M, Collins FS. Atropos: specific, sensitive, and speedy trimming of sequencing reads. PeerJ. 2017;5:e3720 10.7717/peerj.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv Preprint. 2013;arXiv:1303.3997.
- 27.Novocraft Technologies. NovoAlign [Internet]. Selangor: Malaysia; 2014. [accessed 2020 June 20]. http://novocraft.com/products/novoalign/. [Google Scholar]
- 28.Garrison E, Marth G. Haplotype-based variant detection from short-read sequencing. arXiv Preprint. 2012;arXiv:1207.3907.
- 29.Lai Z, Markovets A, Ahdesmaki M, Chapman B, Hofmann O, McEwen R, Johnson J, Dougherty B, Barrett JC, Dry JR, et al. VarDict: a novel and versatile variant caller for next-generation sequencing in cancer research. Nucleic Acids Res. 2016;44(11): e108 10.1093/nar/gkw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, Flicek P, Cunningham F. The ensembl variant effect predictor. Genome Biol. 2016;17(1):122 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adalsteinsson VA, Ha G, Freeman SS, Choudhury AD, Stover DG, Parsons HA, Gydush G, Reed SC, Rotem D, Rhoades J, et al. Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat Commun. 2017;8 (1):1324 10.1038/s41467-017-00965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choudhury AD, Werner L, Francini E, Wei XX, Ha G, Freeman SS, Rhoades J, Reed SC, Gydush G, Rotem D, et al. Tumor fraction in cell-free DNA as a biomarker in prostate cancer. JCI Insight. 2018;3 (21). 10.1172/jci.insight.122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rushlow DE, Mol BM, Kennett JY, Yee S, Pajovic S, Theriault BL, Prigoda-Lee NL, Spencer C, Dimaras H, Corson TW, et al. Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies. Lancet Oncol. 2013;14 (4):327–34. 10.1016/S1470-2045(13)70045-7. [DOI] [PubMed] [Google Scholar]
- 34.Amitrano S, Marozza A, Somma S, Imperatore V, Hadjistilianou T, De Francesco S, Toti P, Galimberti D, Meloni I, Cetta F, et al. Next generation sequencing in sporadic retinoblastoma patients reveals somatic mosaicism. Eur J Hum Genet. 2015;23(11):1523–30. 10.1038/ejhg.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimaras H, Rushlow D, Halliday W, Doyle JJ, Babyn P, Abella EM, Williams J, Héon E, Gallie BL, Chan HSL, et al. Using RB1 mutations to assess minimal residual disease in metastatic retinoblastoma. Transl Res. 2010;156(2):91–97. 10.1016/j.trsl.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Afshar AR, Pekmezci M, Bloomer MM, Cadenas NJ, Stevers M, Banerjee A, et al. Next-generation sequencing of retinoblastoma identifies pathogenic alterations beyond rb1 inactivation that correlate with aggressive histopathologic features. Ophthalmology. 2019;127(6):804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stover DG, Parsons HA, Ha G, Freeman SS, Barry WT, Guo H, Choudhury AD, Gydush G, Reed SC, Rhoades J, et al. Association of cell-free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer. J Clin Oncol. 2018;36(6):543–53. 10.1200/JCO.2017.76.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Chang CW, Spoerke JM, Yoh KE, Kapoor V, Baudo C, Aimi J, Yu M, Liang-Chu MMY, Suttmann R, et al. Low-pass whole-genome sequencing of circulating cell-free DNA demonstrates dynamic changes in genomic copy number in a squamous lung cancer clinical cohort. Clin Cancer Res. 2019;25(7):2254–63. 10.1158/1078-0432.CCR-18-1593. [DOI] [PubMed] [Google Scholar]


