Abstract
Background
Thrombotic thrombocytopenic purpura (TTP) is an uncommon haematological disease which can occur at any age and may present with COVID-19. This case describes a COVID-19 complication associated with a presentation resembling TTP.
Case description
A 51-year-old man who had received a kidney transplant and was on immunosuppressant medication, was admitted to a critical care unit with severe COVID-19 pneumonia/acute respiratory distress syndrome (ARDS) which required intubation, mechanical ventilation and inotropic support. The course was complicated by the classic pentad of thrombocytopenia, intravascular haemolysis, acute kidney injury, neurological symptoms and fever, which prompted the diagnosis of probable TTP. After five sessions of therapeutic plasma exchange, the patient’s general status improved, he was weaned off mechanical ventilation and his renal panel and haemolytic markers normalized.
Conclusion
TTP is a life-threatening condition which requires urgent management with therapeutic plasma exchange. This case highlights some possible complications of COVID-19 generally and in immunocompromised patients specifically. The potential role of plasma exchange in COVID-19 patients without a positive diagnosis of TTP (the so-called ‘TTP resembling presentation’) is an area of further research.
LEARNING POINTS
COVID-19 can manifest as a picture of thrombotic thrombocytopenic purpura (TTP) which requires therapeutic plasma exchange as in other cases of TTP.
Further research is required on the use of therapeutic plasma exchange in severe COVID-19 with cytokine storm.
Keywords: Thrombotic thrombocytopenic purpura, COVID-19, plasma exchange, cytokine storm
INTRODUCTION
Thrombotic thrombocytopenic purpura (TTP) is an invasive haematological condition characterized by the pentad of thrombocytopenia, microangiopathic haemolysis, fever, renal impairment and central nervous system involvement. It is the result of decreased activity of ADAMST13 which is responsible for the cleavage of ultra-large von Willebrand factor multimers [1]. On the other hand, COVID-19 is a new viral illness that may present with a picture of TTP or with actual TTP. Cytokine storm is a consequence of an impaired immune system and improper inflammatory response to COVID-19 and by itself can present to some extent as a TTP picture [2]. Irrespective of whether the TTP is real or a COVID-19 cytokine storm manifesting as TTP, plasma exchange is a very effective treatment. We report the case of an immunocompromised critically ill patient who had a severe COVID-19 infection complicated by haemolysis with a clinical picture of TTP which was treated with therapeutic plasma exchange after all other antiviral and immunosuppressive measures had failed.
CASE DESCRIPTION
The patient was a 51-year-old Saudi man who had received a kidney transplant in 2005 and who now had chronic graft dysfunction, dyslipidaemia, hyperuricaemia and hypertension. His medications included cyclosporine 50 mg orally twice daily, mycophenolate 1 g orally twice daily and prednisolone 5 mg orally once daily, allopurinol 100 mg once daily, amlodipine 5 mg once daily, bisoprolol 5 mg orally once daily and simvastatin 40 mg orally once daily. He presented to our emergency department with a 2-day history of subjective fever, sore throat, shortness of breath, nausea and general body aches. There was no vomiting, diarrhoea or cough. He had not been in contact with anyone who was ill or with any confirmed COVID-19 patients and had no history of recent travel. He was admitted to hospital. A nasopharyngeal swab for SARS nCoV-2 RNA was positive.
Physical Examination on Admission
The patient was conscious, alert and oriented to time, place and person, and was not in respiratory distress. His temperature was 38.6°C, pulse was 70 bpm, blood pressure was 112/65 mmHg, and respiratory rate was 15 bpm, Oxygen saturation was 97% on room air. Systemic examination was unremarkable apart from grade 1 lower limb oedema (bilateral pitting).
Laboratory and Radiological Investigations on Admission
A complete blood count showed haemoglobin of 13.1 g/dl, lymphocyte count 0.661×103/μl, white blood cell count 2.60×103/μl, platelet count 95×103/μl (baseline platelet 150–170×103/μl), neutrophil count 1.44×103/μl, high reticulocyte count and normal coagulation profile, procalcitonin 0.29 ng/ml, albumin 33.4 g/l, alkaline phosphatase 160.9 U/l, total bilirubin 19.1 μmol/l, conjugated bilirubin 14.36 μmol/l, BUN 14.6 mmol/l, creatinine 257 μmol/l (baseline creatinine 200), LDH 159 U/l, magnesium 0.52 mmol/l, sodium 127 mmol/l, D-dimer 0.35 mg/l and ferritin 661 ng/ml. Venous blood gas analysis showed metabolic acidosis: pH 7.29, pCO2 25.9, pO2 79.1 and HCO3– 15.1. A chest x-ray showed bilateral scattered airspace opacities especially in the left lower zone (Fig. 1).
Figure 1.
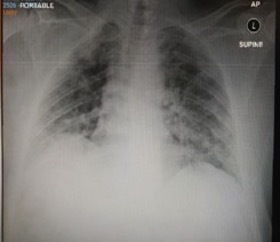
Chest x-ray on admission showing bilateral infiltration with left lower lobe consolidation
Hospital Course and Management
The patient was admitted to the COVID ward, and intravenous fluids were started along with an empiric intravenous antibiotic, hydroxychloroquine, NaHCO3, steroid and heparin. He was quite stable during his hospital stay until day 7 after admission when he became tachypnoeic and developed acute hypoxic respiratory failure. He was moved to the intensive care unit (ICU), intubated and put on high mechanical ventilation support because of severe acute respiratory distress syndrome (ARDS; initial P/F ratio 90). He was managed according to the ARDS Network protocol with a low tidal volume strategy, prone position and high PEEP of 14. Two doses of intravenous tocilizumab were given, immunosuppressive drugs were stopped and intravenous immunoglobulin 1 g/kg/day for 2 days (total of 2 g/kg) was administered. Antibiotics were escalated to meropenem and vancomycin as well acyclovir followed by ganciclovir which was added because of a high CMV titre. Continuous renal replacement therapy (CRRT) was initiated and continued for a few days because of a deterioration in acute kidney injury and refractory hyperkalaemia. A low Glasgow Coma Scale (GCS) score of 5–6 prompted CT of the brain which demonstrated left ill-defined occipital lobe hypodensity suggesting a new ischaemic stroke.
While in ICU, the patient’s haemoglobin level dropped from a baseline of 8–8.5 g/dl to 6 g/dl for which he required multiple blood transfusions, and heparin was held. Platelets continued to be around 45–50×103/ml (although they decreased to 20×103/ml at one point), the vitamin B12 level was 550 pg/ml, homocysteine was high at 20.6 μmol/l, and haptoglobin was <0.295 g/l. A peripheral blood smear showed anisocytosis, hypochromia, polychromasia, spherocytes, tear drop cells, occasional schistocytes, and normal neutrophil and lymphocyte morphology with reduced platelet distribution (Fig. 2). Haematology experts were consulted and suspicion of TTP was raised after exclusion of heparin-induced thrombocytopenia (HIT) and other possible causes of thrombocytopenia.
Figure 2.
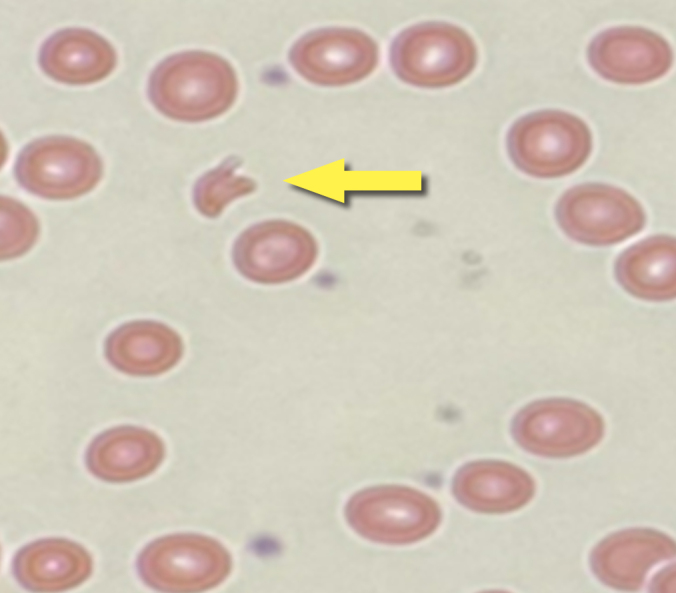
Peripheral blood smear with arrow indicating schistocytes
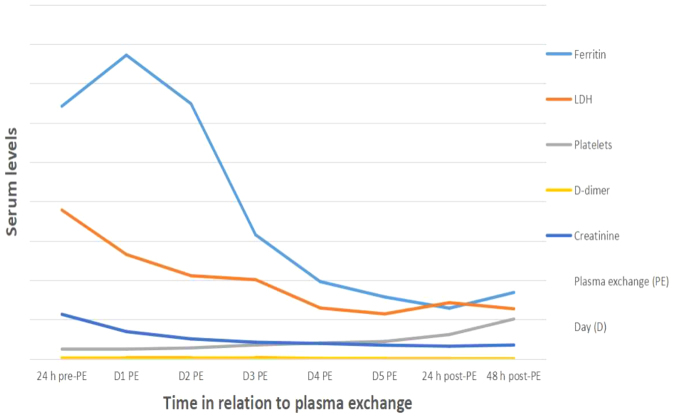
Plasma exchange was carried out in five sessions with fresh frozen plasma. Following the second session, the patient’s GCS score rose to 11 and the platelet count gradually improved over several days from 50×103/ml to 124×103/ml, stabilizing at 150×103/ml. After the last plasma exchange session, renal function returned to baseline and inflammatory markers normalized (Table 1 and Fig. 3). The patient was extubated, regained consciousness and was moved to a regular ward. Post-ICU rehabilitation support was provided for a few days before the patient was discharged home in a stable condition.
Table 1.
Haematological, renal and inflammatory markers before, during and after therapeutic plasma exchange
| Laboratory results | Reference range | 24 h pre-PE | D1 PE | D2 PE | D3 PE | D4 PE | D5 PE | 24 h post-PE | 48 h post-PE |
|---|---|---|---|---|---|---|---|---|---|
| WBC (×103μ/l) | 4–11 | 8.3 | 4.15 | 3.03 | 2.28 | 1.93 | 2.06 | 3.54 | 3.79 |
| RBC (×103μ/l) | 4.7–6.1 | 3.2 | 3.3 | 2.93 | 2.95 | 2.94 | 2.67 | 2.81 | 2.23 |
| Hemoglobin (g/dl) | 13–15 | 9.74 | 9.18 | 9.38 | 9.92 | 9.46 | 9.96 | 9.11 | 9.00 |
| Lymphocytes (×103/μl) | 1–5 | 0.76 | 0.58 | 0.28 | 0.60 | 0.52 | 0.57 | 0.61 | 0.76 |
| Neutrophils (×103/μl) | 2–7.5 | 7.16 | 3.44 | 2.61 | 2.61 | 1.17 | 1.19 | 1.62 | 2.45 |
| Platelets(×103/μl) | 140–450 | 50 | 50 | 56.5 | 71.8 | 81.3 | 89.7 | 124 | 203 |
| LDH (μ/l) | 85–227 | 757 | 531 | 423 | 403 | 259 | 229 | 286 | 255 |
| Ferritin (ng/ml) | 22–322 | 1284 | 1544 | 1297 | 631 | 393 | 315 | 258 | 338 |
| Procalcitonin (ng/ml) | <0.05 | 0.61 | 0.88 | 0.39 | 0.24 | 0.23 | 0.21 | 0.22 | 0.29 |
| CRP (mg/l) | 0–7 | 2.4 | 2.4 | 4.3 | 12.3 | 20 | 18 | 22.5 | 14 |
| D-dimer(mg/l) | 0–0.55 | 5.03 | 7.07 | 5.25 | 7.62 | 2.54 | 2.26 | 1.49 | 1.60 |
| Fibrinogen (g/l) | 2–4 | 1.61 | 2.28 | 1.86 | 2.66 | 2.62 | 2.70 | 2.85 | 3.00 |
| AST (μ/l) | 15–37 | 141 | 121 | 201 | 110 | 61 | 43 | 44 | 39 |
| BUN (mmol/l) | 2.5–6.4 | 39.5 | 31 | 23.8 | 16.8 | 13.5 | 11.6 | 11.1 | 10.9 |
| Creatinine (μmol/l) | 62–115 | 227 | 139 | 102 | 85 | 79 | 70 | 65 | 93 |
Abbreviation key;(h) hours, (D) Day, (WBC) White Blood Cell, (RBC) Red Blood Cell, (LDH) Lactic Acid Dehydrogenase, (CRP) C-Reactive Proteins, (AST) Aspartate Amino Transferase, (BUN) Blood Urea Nitrogen.
Figure 3.
Graph showing haematological, renal and inflammatory markers before, during and after therapeutic plasma exchange
DISCUSSION
The SARS-CoV-2 virus spread rapidly worldwide after December 2019 and WHO declared COVID-19 a pandemic in March 2020. Many studies and reports on the pathogenesis of this disease have since been published.
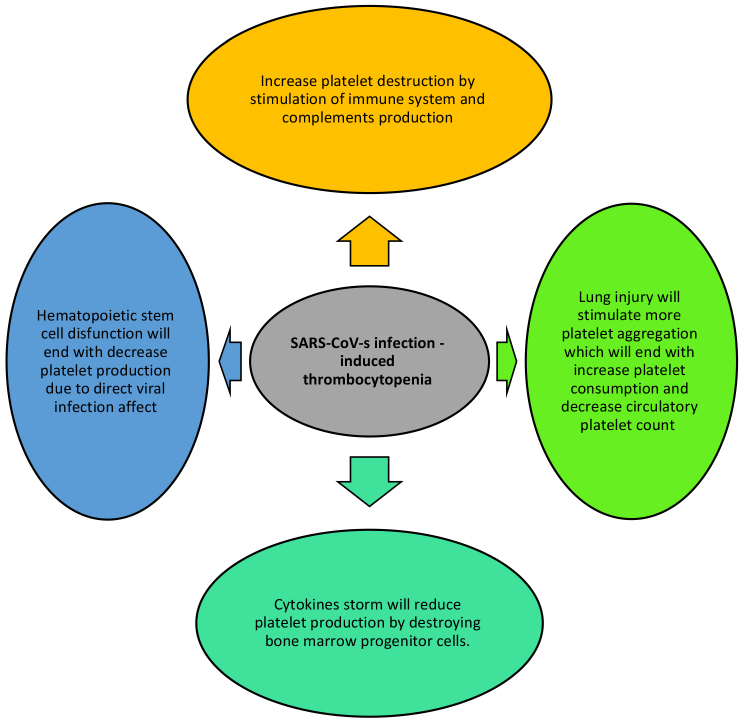
Reported haematological manifestations of COVID-19 include high D-dimer levels, lymphopenia, thrombocytopenia and, to a lesser extent, prolongation of activated partial thromboplastin time [3]. Some reports correlate the high mortality and morbidity rates seen in COVID-19 patients with the presence of lymphopenia and thrombocytopenia. Although thrombocytopenia in COVID-19 patients is very common, there are few reports on its pathogenesis. However, the main possible causes of thrombocytopenia in COVID-19 include sepsis, immune thrombocytopenia, disseminated intravascular coagulation (DIC) and drug-induced thrombocytopenia [4]. Cytokine storm can precipitate thrombocytopenia by destroying stem cells at the level of the bone marrow and decreasing platelet production. Other possible mechanisms of thrombocytopenia in COVID-19 are summarized in Fig. 4 [4].
Figure 4.
Possible mechanisms of thrombocytopenia in COVID-19 patients.
Adapted from Xu et al.[3}
TTP is an uncommon haematological disease that can be genetic or acquired. Acquired TTP can be drug-induced, occur after chemotherapy or after stem cell or solid organ transplantation, be secondary to autoimmune disease, or develop after viral illness. Although not yet documented, there is suspicion that COVID-19 may trigger the immune system and cause TTP. There are limited data on the co-occurrence of TTP and COVID-19. To our knowledge, there are a few reports of immune thrombocytopenic purpura (ITP) in association with COVID-19, but only one report of TTP in a COVID-19 patient [5].
The diagnosis of TTP is based on clinical and laboratory finding as the so-called TTP pentad is seen in less than 10% of cases. However, severe thrombocytopenia and microangiopathic haemolytic anaemia (MAHA) are the most constant signs [6]. In our case, TTP was suspected due to the presence of fever, severe thrombocytopenia, progressive renal impairment, ongoing haemolysis and new ischaemic stroke. HIT was excluded, PT and PTT were normal, and peripheral blood film was positive for some red blood cell fragmentation. Treatment for COVID-19 cytokine storm with antiviral medication, intravenous immunoglobulin, tocilizumab and corticosteroid was ineffective but the patient improved after five plasma exchange sessions.
The gold standard treatment for TTP is urgent therapeutic plasma exchange. This removes the immunological inhibitor and restores ADAMTS13 activity, thus improving survival rate and reducing mortality to 20% [7]. Other treatments include administration of corticosteroid, intravenous immunoglobulin, rituximab and the recently FDA-approved caplacizumab [8]. The role of plasma exchange to treat COVID-19 cytokine storm has not been widely studied. In their report, Khamis et al. conclude that the use of plasma exchange in critically ill COVID-19 patients improves outcome [9]. Zhang et al. presented a case series of three patients with severe COVID-19 and showed that therapeutic plasma exchange is a rapid and efficient method to overcome severe cytokine storm and respiratory distress syndrome [10].
In conclusion, while the diagnosis of TTP is based on both clinical and laboratory findings, it can be confirmed by measuring ADAMTS13 level and activity. However, we could not confirm the diagnosis in our patient using this method due to our inability to measure ADAMTS13. TTP is a life-threatening condition which requires urgent management with therapeutic plasma exchange.
This case highlights a possible complication of COVID-19, especially in immunocompromised patients. The potential role of plasma exchange in COVID-19 cases without a confirmed TTP diagnosis (the so-called ‘TTP resembling presentation’) is an area for further research.
Footnotes
Conflicts of Interests: The authors declare there are no competing interests.
REFERENCES
- 1.Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, et al. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339(22):1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 2.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2020 Jun 27; doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bomhof G, Mutsaers PGNJ, Leebeek FWG, Te Boekhorst PAW, Hofland J, Croles FN, et al. COVID-19-associated immune thrombocytopenia. Br J Haematol. 2020;190(2):e61–e64. doi: 10.1111/bjh.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hindilerden F, Yonal-Hindilerden I, Akar E, Kart-Yasar K. Covid-19 associated autoimmune thrombotic thrombocytopenic purpura: report of a case. Thromb Res. 2020;195:136–138. doi: 10.1016/j.thromres.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836–2846. doi: 10.1182/blood-2016-10-709857. [DOI] [PubMed] [Google Scholar]
- 7.Kempton CL, Antun AG. Thrombotic thrombocytopenic purpura. In: Shaz BH, Hillyer CD, Gil MR, editors. Transfusion medicine and hemostasis. 3rd ed. Elsevier; 2019. pp. 649–654. Available from https://www.sciencedirect.com/topics/nursing-and-health-professions/thrombotic-thrombocytopenic-purpura. [Google Scholar]
- 8.Hanlon A, Metjian A. Caplacizumab in adult patients with acquired thrombotic thrombocytopenic purpura. Ther Adv Hematol. 2020;11 doi: 10.1177/2040620720902904. 2040620720902904. Published online 2020 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khamis F, Al-Zakwani I, Al Hashmi S, Al Dowaiki S, Al Bahrani M, Pandak N, et al. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int J Infect Dis. 2020;99:214–218. doi: 10.1016/j.ijid.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Zhai H, Ma S, Chen J, Gao Y. Efficacy of therapeutic plasma exchange in severe COVID-19 patients. Br J Haematol. 2020 May 26; doi: 10.1111/bjh.16890. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]