Abstract
Mesenchymal stem or stromal cells (MSCs) are nonhematopoietic postnatal stem cells with self-renewal, multipotent differentiation, and potent immunomodulatory and anti-inflammatory capabilities, thus playing an important role in tissue repair and regeneration. Numerous clinical and preclinical studies have demonstrated the potential application of MSCs in the treatment of tissue inflammation and immune diseases, including inflammatory skin diseases. Therefore, understanding the biological and immunological characteristics of MSCs is important to standardize and optimize MSC-based regenerative therapy. In this review, we highlight the mechanisms underlying MSC-mediated immunomodulation and tissue repair/regeneration and present the latest development of MSC-based clinical trials on cutaneous diseases.
1. Introduction
Mesenchymal stem cells (MSCs) represent a unique population of postnatal multipotent stem cells existing in a variety of adult, perinatal, and fetal tissues [1]. Although the existence of MSCs was proposed by Cohnheim about 150 years ago, they were definitely identified in the bone marrow and designated as bone marrow stromal stem cells (BMSCs) by Friedenstein in the 1970s [2]. Subsequently, it has been further demonstrated that MSCs are immune privileged and possess potent self-renewal, multipotent differentiation, and immunomodulatory/anti-inflammatory capabilities [3]. Due to these unique properties of MSCs, they have been widely explored as a potential cell-based regenerative therapy for a large spectrum of diseases, such as myocardial infarction [4], inflammatory bowel disease [5], cancer [6], glaucoma [7], osteoarthritis [8], nervous system disorders [9], and oral and maxillofacial diseases [10]. Skin disorders caused by aging, various types of environmental and genetic factors, trauma, and systemic diseases, e.g., diabetes and graft versus host disease (GVHD), represent one of the major public health burdens worldwide that significantly affect the quality of life of patients [11]. Currently, there are limited treatment options for these dermatological disorders due to the complicated causes and our limited understanding of the mechanisms underlying their pathogenesis [11]. In the last decade, MSC-based therapy is emerging as a novel paradigm for the treatment of various skin disorders [12]. In this review, we will discuss our current understanding of the function and mechanism of actions as well as the potential application of MSCs in the treatment of cutaneous diseases.
2. Characterization of MSCs
MSCs can be easily isolated from human donors and rapidly expanded in vitro without loss of their main biological properties [13]. Up to date, MSCs have been isolated from various types of tissues, such as the bone marrow [14], adipose tissue [14], skeletal muscles [14], synovium [15], dental pulp [15], placenta [15], umbilical cord blood [15], umbilical cord [15], gingiva [16], amnion [17], umbilical cord Wharton's jelly [18], and skin [14, 15]. However, large variations exist in the quality and biological function of MSCs of different tissue origins due to the subtle differences in the isolation and ex vivo culture and expansion and the lack of consistent markers for MSC identification. To solve this issue, the Mesenchymal and Tissue Stem Cell Committee of the ISCT has proposed minimal criteria to define human MSCs: (a) plastic adherent; (b) the positive expression of CD105, CD73, and CD90 and the lack of expression of hematopoietic cell markers, e.g., CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA class II; and (c) the differentiation capability into osteoblasts, adipocytes, and chondroblasts in vitro [19, 20] (Figure 1(a)). In addition to these basic properties, MSCs possess potent self-renewal and immunomodulatory/anti-inflammatory functions but are immune privileged due to their expression of a high level of HLA class I molecule, the absent expression of HLA class II, and the T cell costimulatory molecules B7-1, B7-2, CD40, or CD40L [21], thus making MSCs tolerable to allogeneic T cells [22] and freshly isolated allogeneic natural killer (NK) cells [23] (Figure 1(b)). However, the expression of HLA class II molecule could be induced in undifferentiated MSC by treatment with IFN-γ or during MSC differentiation [21]. Therefore, both the autologous and allogeneic MSCs could be utilized for cell-based therapy in regenerative medicine.
Figure 1.
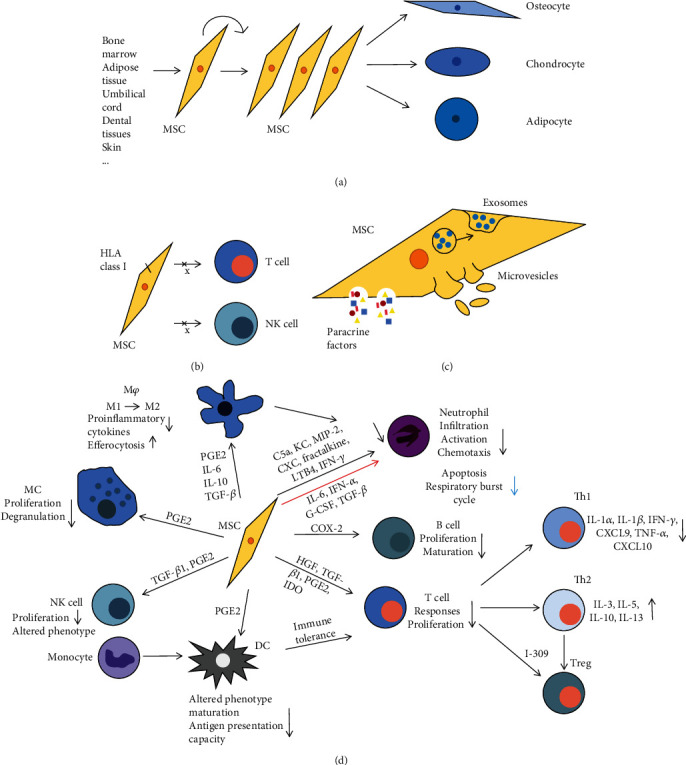
Schematic representation of the mechanisms of MSCs to promote tissue repair and immunosuppression. (a) Self-renewal and multipotent differentiation functions. (b) Low immunogenicity. (c) Transfer of biologically active substances by paracrine or EVs. (d) Immunomodulatory properties.
3. Immunomodulatory Properties of MSCs
MSCs have potent immunomodulatory effects on various types of innate and adaptive immune cells (Figure 1(d)). Among innate immune cells, MSCs of different tissue origins are capable of inhibiting the activation of proinflammatory M1 macrophages and promoting the polarization of macrophages toward anti-inflammatory M2 phenotypes as evidenced by decreased production of proinflammatory cytokines, increased secretion of anti-inflammatory mediators, and enhanced efferocytosis of apoptotic cells [24–27]. A recent study indicated that MSC-mediated reduction of CXC chemokines was achieved via enhancing the intracellular activation of p38 MAPK phosphorylation and inhibiting the NF-kappaB p65 phosphorylation in macrophages [28]. In terms of NK cells, MSCs alter their phenotypes and inhibit their proliferation and cytokine secretion either by cell-cell contact or through the mediation of soluble factors, including TGF-β1 and PGE2 [23]. In addition, MSCs regulate the functions of dendritic cells (DCs) through multiple modes of actions, such as direct cell-cell contact and paracrine secretion of various soluble factors, e.g., prostaglandin E2 (PGE2) [29, 30], leading to phenotype changes and inhibition of DC maturation and, consequently, reduced antigen presentation capacity [31–33] and increased T cell tolerance [34]. With regard to mast cells, several lines of evidence have shown that MSCs inhibit their proliferation and degranulation-mediated activation [35–37] through the secretion of PGE2 [30]. With regard to neutrophils, MSCs inhibited their apoptosis and respiratory burst cycle through the secretion of IL-6, IFN-α, G-CSF, and TGF-β [29, 38]. Meanwhile, MSCs have been shown to inhibit infiltration, activation, and chemotaxis of neutrophils as evidenced by reduced secretion of chemokines and proinflammatory cytokines, e.g., C5a (a keratinocyte chemoattractant), MIP-2, fractalkine, LTB4, IFN-γ, and CXC chemokines [39, 40].
Among adaptive immune cells, MSCs could inhibit immune responses and proliferation of T cells either through direct cell-cell contact or through secretion of a large panel of soluble factors, such as HGF, TGF-β1, PGE2, and IDO [23]. MSCs have potent inhibitory effects on activation of different subtypes of T helper cells, such as Th1 and Th17, as evidenced by reduced secretion of proinflammatory cytokines and chemokines, such as IL-1α, IL-1β, IFN-γ, CXCL9, TNF-α, CXCL10, and IL-17 [41, 42]. On the other hand, MSCs can promote the function of Th2 cells and regulatory T cells (Tregs) as evidenced by increased production of Th2 and anti-inflammatory cytokines, e.g., IL-3, IL-5, IL-10, IL-13, and the Th2 chemokine I-309 [41, 43–45]. Lastly, MSCs significantly suppressed the proliferation and maturation of B cells through the secretion of COX-2-dependent PGE2 [37].
4. Trophic Paracrine Effects of MSCs
Accumulating evidence supports the notion that MSCs exert their therapeutic effects under various pathological settings through their paracrine secretion of a panel of trophic factors with a wide range of biological functions (Figure 1(c)). A set of factors produced by MSCs, such as HGF, TGF-β, PGE2, IDO, IL-10, TSG-6, and human leukocyte antigen-G5, are involved in MSC-mediated immunosuppressive and anti-inflammatory functions [46]. Of note, certain proinflammatory cytokines produced by activated immune cells, e.g., IFN-γ and TNF-α, can stimulate the production of immunosuppressive mediators IDO and iNOS/NO in MSCs, which subsequently contribute to MSC-mediated immunosuppressive and anti-inflammatory functions [5, 47]. In addition, some of the soluble factors secreted by MSCs, such as MIP-1 and MCP-5, could promote the migration of fibroblasts [48], keratinocytes, endothelial cells, and macrophages [49], thus contributing to tissue repair and regeneration. On the other hand, some trophic factors secreted by MSCs, such as EGF, IGF, KGF, IGFBP-1, and VEGF, have proproliferative and antiapoptotic effects on various types of host tissue cells such as keratinocytes, hair follicle cells, and endothelial cells [49], while some other secreted factors by MSCs, such as MMPs, Ang-1, VEGF, serine proteases, and serine protease inhibitors, have proangiogenesis, antifibrosis, and antioxidant functions, all of which are beneficial to tissue regeneration [25, 50–54]. Taken together, MSCs can interact with various types of host cells through their paracrine secretion of a myriad of bioactive soluble factors, thus contributing to the establishment of a proregenerative microenvironment favorable to tissue regeneration and homeostasis.
5. MSC-Released Extracellular Vesicles
Cells release extracellular vesicles (EVs) as one of the intercellular communication platforms. EVs could be mostly classified based on their diameter, including apoptotic bodies (1-5 μm), microvesicles (MVs; 500-1000 nm), and exosomes (30-200 nm). EVs are widely distributed in body fluid and secreted in cell culture supernatants, which are rich in lipids, proteins, nucleic acids, and other components. Exosomes are released after fusion with the plasma membrane through forming multivesicular bodies, while MVs are released by directly shedding from the plasma membrane [5] (Figure 1(c)). In the following text, we collectively refer to exosomes and MVs as EVs.
In recent years, a growing body of evidence has shown that MSC-released EVs exhibit potent regulatory effects on various types of cells through transferring bioactive components to the recipient target cells. For example, MSC-EVs exert anti-inflammatory effects on inflammatory immune cells, such as M1 macrophages, DCs, and CD4+ Th1 and Th17 cells, through the intercellular transfer of immunoregulatory miRNAs and bioactive proteins, thus making them undergo phenotypic conversion into anti-inflammatory M2 macrophages, regulatory DCs, and Tregs. Additionally, MSC-EVs can promote tissue repair and regeneration through promoting the survival, proliferation, and regenerative potential of other types of resident cells. For instance, Wen et al. [55] showed that MSC-EVs inhibited hypoxia-induced apoptotic damages of cardiomyocytes by transferring miR-144 to target the PTEN/AKT pathway that plays an important role in protecting cardiomyocytes from ischemic/hypoxic damages. Moon et al. [56] found that MSC-EVs significantly mitigated stroke, possibly by promoting neurogenesis and angiogenesis by transfer of miR-184 and miR-210. Yan et al. [57] reported that MSC-EVs promoted the recovery of hepatocytes from oxidative stress-induced injuries through transferring GPX1 to suppress oxidative stress-induced apoptosis. Li et al. [58] investigated that MSC-EVs alleviated irradiation- (IR-) induced lung injury, possibly by inhibiting both the intrinsic and extrinsic apoptotic pathways in lung epithelial cells through the relay of miR-21-5p. Taken together, these findings suggest that MSC-released EVs exert therapeutic effects on various pathological conditions that are comparable to those conferred by MSC transplantation, thus serving as potential cell-free products for regenerative therapy of a variety of diseases.
6. Applications of MSCs in Cutaneous Diseases
Due to the potent regenerative potentials and trophic paracrine effects, MSCs and their derivative products are emerging as promising therapeutics for a spectrum of diseases, including inflammatory skin disorders [12]. Through searching the ClinicalTrials.gov database, 67 clinical trials on MSC-based therapies for cutaneous diseases were identified (Table 1), which were described in detail in the following sections.
Table 1.
MSC-based clinical trials for cutaneous diseases.
| ClinicalTrials.gov identifier | Phase/status/(start dates) | Conditions | Type of cells | Outcome | Country |
|---|---|---|---|---|---|
| NCT02824393 | Early phase 1 Completed (March 2017) |
Urticaria | Autologous MSCs | MSC therapy more effective than conventional treatment, resulted in longer recovery | Turkey |
| NCT01771679 | Phase 1 Phase 2 Suspended (July 2015) |
Photoaging | Allogeneic BMSCs | Ongoing | United States |
| NCT03765957 | Early phase 1 Not yet recruiting (February 2019) |
Psoriasis | MSCs | Ongoing | China |
| NCT02304562 | Phase 1 Unknown (January 2013) |
Sweat gland diseases | UCB-MSCs | Ongoing | China |
| NCT02491658 | Phase 1 Phase 2 Unknown (April 2015) |
Psoriasis vulgaris | UC-MSCs | Ongoing | China |
| NCT02213705 | Phase 1 Phase 2 Unknown (May 2014) |
Systemic scleroderma | Allogeneic MSCs | Ongoing | France |
| NCT03745417 | Phase 1 Phase 2 Recruiting (August 2018) |
Psoriasis | UC-MSCs | Ongoing | China |
| NCT03265613 | Phase 1 Phase 2 Active, not recruiting (September 2017) |
Psoriasis | ADSCs | Ongoing | China |
| NCT03564808 | Phase 2 Recruiting (June 2018) |
Skin pigmentation over contour deformities of the face, trauma, Romberg's disease | MSCs | Ongoing | Pakistan |
| NCT03392311 | Phase 1 Phase 2 Recruiting (August 2019) |
Psoriasis | ADSCs | Ongoing | China |
| NCT03424629 | Phase 1 Unknown (June 2018) |
Moderate and severe plaque psoriasis | UC-MSCs | Ongoing | China |
| NCT00962923 | Phase 1 Phase 2 Unknown (August 2009) |
Systemic sclerosis | Allogeneic MSCs | Ongoing | China |
| NCT04275024 | Not applicable Recruiting (April 2020) |
Psoriasis | ADSCs | Ongoing | China |
| NCT02034786 | Phase 1 Unknown (March 2015) |
Aesthetics procedure | Autologous MSCs | Ongoing | Brazil |
| NCT02685722 | Phase 1 Completed (January 2012) |
Skin ulcers | UC-MSCs | — | China |
| NCT04356287 | Phase 1 Phase 2 Not yet recruiting (October 2020) |
Systemic sclerosis | UC-MSCs | Ongoing | — |
| NCT04520022 | Phase 1 Phase 2 Completed (October 2016) |
Recessive dystrophic epidermolysis bullosa | UC-MSCs | — | Korea |
| NCT02975960 | Not applicable Completed (October 2016) |
Systemic sclerosis | Autologous ADSCs | — | Korea |
| NCT04153630 | Phase 1 Phase 2 Active, not recruiting (May 2018) |
Recessive dystrophic epidermolysis bullosa | BMSCs | Ongoing | Spain |
| NCT03529877 | Phase 1 Phase 2 Recruiting (January 2019) |
Recessive dystrophic epidermolysis bullosa | Allogeneic ABCB5-positive MSCs | Ongoing | United States, Austria, France, German, Italy, United Kingdom |
| NCT02888704 | Phase 1 Completed (July 2016) |
Atopic dermatitis | MSCs | — | Korea |
| NCT03887208 | Phase 1 Phase 2 Completed (January 2018) |
Skin, scar, cutis laxa, keloid, cicatrix | Autologous ADSCs | — | Poland |
| NCT02494752 | Not applicable Unknown (August 2015) |
Romberg's disease, craniofacial microsomia, lipodystrophy, mixed connective tissue disease | Fat graft enriched with MSCs | Ongoing | United Kingdom |
| NCT01033552 | Phase 2 Recruiting (January 2010) |
Epidermolysis bullosa | MSCs | Ongoing | United States |
| NCT02582775 | Phase 2 Recruiting (March 2016) |
Epidermolysis bullosa | Allogeneic MSCs | Ongoing | United States |
| NCT02834858 | Phase 1 Unknown (January 2016) |
Peripheral vascular disease, ischemia, diabetic foot | UC-MSCs | Ongoing | China |
| NCT01216865 | Phase 1 Phase 2 Unknown (January 2011) |
Diabetic foot, critical limb ischemia | UC-MSCs | Ongoing | China |
| NCT04464213 | Phase 1 Not yet recruiting (August 2020) |
Diabetic foot ulcer | pMSCs | Ongoing | — |
| NCT04432545 | — Available (-) |
Diffuse cutaneous systemic sclerosis with refractory pulmonary involvement | Allogeneic MSCs | Ongoing | China |
| NCT01932021 | Not applicable Unknown (April 2013) |
Skin ulcer | ADSCs | Ongoing | France |
| NCT02304588 | Phase 1 Unknown (January 2013) |
Diabetic foot, lower limb ischemia | MSCs | Ongoing | China |
| NCT03259217 | Phase 1 Unknown (October 2017) |
Diabetic foot ulcers | MSCs seeded in chitosan scaffold | Ongoing | Egypt |
| NCT04104451 | Phase 1 Recruiting (November 2019) |
Diabetic foot ulcer | CLMSCs | Ongoing | United States |
| NCT00955669 | Phase 1 Completed (August 2009) |
Diabetic critical limb ischemia and foot ulcer | Autologous BMSCs | Effective treatment and no recurrence in the next 10-year follow-up span | China |
| NCT04466007 | Phase 2 Not yet recruiting (October 2020) |
Diabetic foot with critical limb ischemia | Allogeneic ADSCs | Ongoing | — |
| NCT03676400 | Not applicable Completed (October 2018) |
Androgenic alopecia | UC-MSCs | — | Korea |
| NCT02672280 | Phase 1 Phase 2 Unknown (May 2016) |
Wounds, diabetic foot ulcers, burns | Medical collagen membrane with MSCs | Ongoing | China |
| NCT03865394 | Phase 1 Phase 2 Recruiting (September 2018) |
Diabetic foot ulcer | Autologous ADSCs | Ongoing | Poland |
| NCT04497805 | Phase 2 Not yet recruiting (August 2020) |
Diabetic foot ulcer | Allogeneic ADSCs | Ongoing | — |
| NCT03013049 | Not applicable Unknown (January 2016) |
Vitiligo | DMSCs | Ongoing | India |
| NCT02579369 | Phase 1 Phase 2 Unknown (October 2015) |
Dystrophic epidermolysis bullosa | Allogeneic MSCs | Ongoing | Korea |
| NCT02619877 | Phase 2 Completed (October 2015) |
Diabetic foot ulcer | Allogeneic ADSCs | Complete wound healing in the majority of patients | Korea |
| NCT02796079 | Phase 1 Unknown (January 2015) |
Peripheral vascular disease, ischemia, diabetic foot | MSCs | Ongoing | China |
| NCT03370874 | Phase 3 Unknown (July 2018) |
Diabetic foot ulcer | Allogeneic ADSCs | Ongoing | Korea |
| NCT03183726 | — Completed (January 2016) |
Diabetic foot ulcer | Allogeneic ADSCs | — | Korea |
| NCT03754465 | Phase 2 Recruiting (November 2018) |
Diabetic foot ulcer | Allogeneic ADSCs | Ongoing | United States |
| NCT03629002 | — Unknown (September 2018) |
Systemic scleroderma | MSCs | Ongoing | France |
| NCT03060551 | Early phase 1 Completed (July 2018) |
Systemic sclerosis | Autologous ADSCs | Improvement of skin fibrosis, hand edema, active ulcers, and quality of life | Korea |
| NCT03183804 | — Unknown (June 12, 2017) |
Diabetic foot ulcer | Allogeneic ADSCs | Ongoing | Korea |
| NCT03248466 | Early phase 1 Recruiting (August 2017) |
Diabetic foot ulcer | BMSCs | Ongoing | China |
| NCT03257098 | Phase 1 Phase 2 Recruiting (November 2017) |
Skin ulcer venous stasis chronic | Allogeneic ABCB5-positive MSCs | Ongoing | Germany |
| NCT03252340 | — Active, not recruiting (September 2017) |
Atopic dermatitis | MSCs | Ongoing | Korea |
| NCT02918123 | Phase 1 Recruiting (January 2018) |
Psoriasis | Allogeneic UCB-MSCs | Ongoing | Korea |
| NCT03183934 | — Unknown (July 2017) |
Dystrophic epidermolysis bullosa | Allogeneic ADSCs | Ongoing | Korea |
| NCT04173650 | Phase 1 Phase 2 Not yet recruiting (September 2020) |
Dystrophic epidermolysis bullosa | MSC-EVs | Ongoing | — |
| NCT03211793 | Phase 1 Phase 2 Unknown (November 2018) |
Systemic sclerosis Digital ulcer |
MSCs | Ongoing | Netherlands |
| NCT01065337 | Phase 2 Completed (August 2005) |
Diabetic foot | BMSCs | Improvement of microcirculation and complete wound healing in the majority of patients | Germany |
| NCT01686139 | Phase 1 Unknown (March 2016) |
Diabetic foot ulcers | MSCs | Ongoing | Israel |
| NCT04179760 | Phase 1 Phase 2 Recruiting (March 2020) |
Atopic dermatitis | Allogeneic BMSCs | Ongoing | Korea |
| NCT02394886 | Phase 1 Completed (November 2014) |
Diabetic foot ulcer | Allogeneic ADSCs | — | Korea |
| NCT028310752 | Phase 1 Unknown (January 2015) |
Diabetic foot | ADSCs | Ongoing | China |
| NCT04569409 | Phase 3 Recruiting (July 2020) |
Diabetic foot ulcer | Allogeneic ADSCs | Ongoing | Korea |
| NCT03276312 | Not applicable Completed (April 2015) |
Diabetic foot | Autologous ADSCs | Improvement of healing rate | Italy |
| NCT00815217 | Not applicable Unknown (February 2009) |
Diabetic wounds | Autologous ADSCs | Ongoing | United States |
| NCT04137562 | Phase 2 Recruiting (December 2019) |
Atopic dermatitis | ADSCs | Ongoing | Korea |
| NCT02742844 | Phase 1 Phase 2 Terminated (August 2016) |
Skin ulcer venous stasis chronic | ABCB5-positive MSCs | Ongoing | Germany |
| NCT02619734 | Phase 1 Unknown (August 2006) |
Chronic leg ulcer Sickle cell disease |
Autologous BMSCs | Ongoing | — |
6.1. Vitiligo
Vitiligo and alopecia areata (AA) are common chronic and recurrent autoimmune skin disorders characterized by white spots on the skin (vitiligo) and bald spots on the scalp (AA) because of selective destruction of melanocytes (MC) [2, 59, 60]. Most recently, Zhu et al. [60] found that vitiligo patients present with a high level of PTEN expression that may contribute to the impairment of melanocytes. In addition, it has been shown that MSCs could promote cell proliferation and suppress oxidative stress-induced apoptosis in human melanocytes by targeted inhibition of the PTEN/PI3K/AKT pathway, which may contribute to the therapeutic effects of MSCs on vitiligo. In a recent study, 23 vitiligo patients were treated by transplantation of autologous melanocytes, which showed that the transplantation efficiency of autologous melanocytes might be predicted by perilesional infiltration of CD8+ T cell activities [61]. Meanwhile, the results showed that dermal MSCs (DMSCs) could significantly inhibit the skin-homing activity of CD8+ T lymphocytes, suggesting that DMSCs might be concomitantly applied to improve the transplantation efficiency and therapeutic efficacy of autologous melanocytes in treating vitiligo [61].
6.2. Melanoma
Melanoma is the most malignant skin cancer, a process characterized by a linear transformation progression of normal melanocytes through various precursor lesions and ultimately to melanoma [62]. Several lines of evidence have shown that MSCs can be utilized as cellular vehicles to suppress the growth of melanoma through delivering IFN-β in the tumor microenvironment [63–65]. Wang et al. [66] reported that BMSCs transduced with pAd5-CMV-CYP2E1 recombinant adenovirus can act as an intermediate carrier to promote the killing effect of bystanders on melanoma cells in vitro and suppress the growth of cancer cells by activating 5-(3,3-dimethyl-1-triazeno)imidazole-4-carboxamide in an established mouse model of human melanoma. In addition, CM-FDMSC showed inhibitory effects on the tumorigenesis of A375 melanoma cells through promoting apoptosis, possibly by interfering with PI3K/AKT and mitogen-activated protein kinase (MAPK) signaling pathways, and a reduced BCL-2/BAX ratio [67]. Notwithstanding, some studies have shown that MSCs possess protective effects on melanoma cells. For instance, ADSCs could support proliferation and inhibit apoptosis and the response of melanoma cells to cytotoxic drugs in vitro. Meanwhile, ADSC-secreted soluble factors, e.g., VEGF, G-CSF, and SDF-1alpha/CXCR4, synergistically contribute to the formation of a proinflammatory tumor microenvironment, thus facilitating tumor growth [68]. Most recently, a study showed that genetically modified murine ADSCs producing IL-2 favored B16F10 melanoma cell proliferation in an immunocompetent mouse model of subcutaneous and lung metastatic melanoma [69].
6.3. Epidermolysis Bullosa
The successful use of hematopoietic cell transplantation (HCT) has previously been shown to treat pediatric patients with RDEB [70, 71], an intractable genetic blistering skin disease caused by mutations to the COL7A1 gene that deactivated the production of functional type VII collagen protein (C7) essential for skin integrity [72, 73]. Several lines of evidence have demonstrated that MSCs enhanced therapeutic effects of HCT graft on pediatric RDEB partly due to their intrinsic immunomodulatory, trophic properties and restorative effects on C7 production [71, 74–78]. Meanwhile, the tissue distress factor, high-mobility group box-1 (HMGB1) [79], Ccl27-Ccr10 chemotactic axis [80], and SDF-1α/CXCR4 signaling axis [72] have been shown to promote the recruitment of endogenous MSCs to skin lesions, thus attenuating the pathology of epidermolysis bullosa. Several clinical trials indicated that intradermal or intravenous administration of allogeneic MSCs showed therapeutic effects on recessive dystrophic epidermolysis bullosa [81, 82]. Currently, there are eight registered clinical trials on MSC-based therapy of epidermolysis bullosa as listed in the ClinicalTrials.gov (Table 1).
6.4. Photoaging
Photoaging refers to skin aging associated with ultraviolet radiation (UVR) exposure [83]. Jeong et al. [84–87] reported the successful use of MSCs and MSC-derived condition medium (CM) to improve wrinkling and reduce pigmentation on the photoaged skin in a mouse model through stimulating the expression of collagen and TGF-β, increasing dermal thickness, and decreasing MMP and IL-6 expression [48, 85, 87–89]. Additionally, MSCs and MSC-CM could decrease UVB-induced apoptotic cell death, upregulate antioxidant response element (ARE), increase SOD and GSH-Px activities, and attenuate the upregulation of malonaldehyde [85, 89, 90], all of which are beneficial to confining the photoaging process. Currently, there is one ongoing clinical trial on MSC-based therapy of photoaging (NCT01771679).
6.5. Psoriasis
Psoriasis is a T cell-mediated inflammatory autoimmune disease [91], characterized by an imbalance between the Th1/Th17 and Th2 cytokines [92]. Campanati et al. [93, 94] showed that MSCs isolated from psoriatic skin lesions exhibited compromised ability to inhibit T cell proliferation and activation, which might be attributed to their decreased capacity of secreting cytokines [94, 95]. Further studies indicated that the microenvironment in psoriasis could promote the expression of proinflammatory and angiogenetic factors by MSCs, thus contributing to the development of psoriatic skin lesions [96]. On the other hand, MSCs could mitigate psoriatic skin lesions by reducing the local levels of angiogenic and proinflammatory mediators [92, 97] and inhibiting the inflammatory responses of keratinocytes [98] and activation and differentiation of DC-mediated CD4+ T cells [99]. MSCs from skin lesions of psoriatic patients could promote proliferation and inhibit apoptosis of keratinocytes, which not only result in abnormal thickening of the epidermis [100] but also lead to dermal microvasculature formation/angiogenesis through increasing the expression of EDIL3, AMOT, and ECM [101]. Early clinical studies have demonstrated the safety and tolerance in psoriatic patients following transplantation of autologous MSCs [102]. In addition, Chen et al. [103] documented that the treatment of two cases of patients with psoriasis vulgaris with UCB-MSCs led to 4 or 5 years of remission. These findings suggest that replacing the abnormal resident MSCs in psoriatic skin lesions with autologous MSCs or allogeneic MSCs from healthy donors is worthy of further clinical studies on a large scale to develop safe and effective MSC-based therapy for psoriasis [104]. Currently, there are nine clinical trials on MSC-based therapy of psoriasis as listed in the ClinicalTrials.gov (Table 1).
6.6. Systemic Autoimmune Disease-Associated Skin Manifestations
Due to their potent immunosuppressive and anti-inflammatory properties, MSCs have been extensively explored as promising therapeutics for autoimmune skin diseases, such as dermatomyositis (DM) and alopecia areata (AA), and various systemic autoimmune/autoinflammatory diseases, such as systemic sclerosis (SSc), systemic lupus erythematosus (SLE), and graft versus host disease (GVHD) involved with severe skin manifestations (AA) [105–113]. Numerous clinical trials have been conducted to evaluate the therapeutic efficacy of MSCs in treating autoimmune disease-associated skin manifestations. For instance, Guiducci et al. [114, 115] reported that intravenous infusion of autologous MSCs improved vascularization, restored blood flow, and reduced skin necrosis in SSc patients. Liu et al. [116] reported that systemic infusion of MSCs significantly mitigated the severity of SLE as evidenced by attenuated proteinuria and hypocomplementemia. In addition, Sun et al. [117] reported that the disease activity index of SLE patients reduced by half within 6 months following systemic infusion of allogeneic MSCs from HLA-disparate family members. For GVHD patients, studies showed that HCT, together with the systemic infusion of MSCs after myeloablation, significantly reduced the incidence rate of acute and chronic GVHD at 6 months posttransplantation [118]. In addition, transplantation of allogeneic MSCs appears to be safe and effective in treating drug-resistant DM patients [113]. Regarding the treatment of AA, a few clinical trials showed that administration of ADSC-CM or ADSCs could increase hair density and thickness [119]. Currently, there are seven clinical trials on MSC-based therapy of systemic sclerosis as listed in the ClinicalTrials.gov (Table 1).
6.7. Cutaneous Ulcers
The therapeutic effects of MSCs on chronic cutaneous ulcers, such as pressure ulcers, radiation skin ulcers, diabetic skin ulcers, and leprosy skin ulcers, have been evaluated in both preclinical studies and clinical trials [120]. With regard to the treatment of pressure ulcers, MSCs promote ulcer healing in mice probably due to the reduced oxidative stress-mediated cellular apoptosis, vascular damages, and ER stress [121] and the induction of adipogenic differentiation and regeneration of the underlying architecture of the skin [122]. Of note, a recent study showed that transplantation of MSCs failed to promote pressure ulcer healing, possibly because of their transition retention and marginal differentiation capacity into tissue-specific cells [123]. With regard to diabetic skin ulcers, transplantation of allogeneic MSCs would improve the healing of diabetic skin ulcers by augmenting angiogenesis in a diabetic rabbit ear ulcer model [124]. Meanwhile, in a diabetic rat ulcer model, intramuscular transplantation of BMSCs showed increased survival ability and promoted the expression level of VEGF in the wound tissues at the later stage as compared to subcutaneously transplanted BMSCs [125]. In a radiation-induced skin ulcer model in rats, treatment with MSC-CM accelerated wound closure and healing, possibly by promoting angiogenesis and regeneration of sebaceous glands [126]. Furthermore, the therapeutic effects of MSCs on chronic skin ulcers have been reported in several clinical studies [120, 127]. For instance, in a study with 2 patients with radiation-induced skin ulcers, transplantation of autologous MSCs achieved complete epithelialization of the ulcer surface [128]. Mechanistically, MSCs promote the healing of radiation-induced skin ulcers through facilitating neovascularization and reepithelization due to the activation of the PI3K/AKT signaling pathway [129]. In another study with 22 patients with chronic plantar ulcers in leprosy, 21 patients showed improvement in ulcerous lesions following treatment with hAMMSC-CM [130]. Lastly, in one study with 53 patients with severe symptoms of Fontaine's II-IV diabetic foot ulcers together with varying degrees of lower extremity arterial abnormalities, transplantation of hUCB-MSCs after angioplasty increased neovascularization accompanied by complete or progressive healing of ulcers [131]. Currently, there are thirty-one registered clinical trials on MSC-based therapy of skin ulcers, among which twenty-six clinical trials on diabetic foot ulcers (Table 1).
6.8. Atopic Dermatitis
Atopic dermatitis (AD) is a typical abnormal T cell-mediated immune disorder characterized by a significant imbalance between Th2 and Th1/Th17, particularly in the early phase, whereas a mixed Th1/Th2 pattern appears in the chronic stage [132]. MSCs from the skin of AD patients showed an upregulation of a panel of Th1/Th17 cytokines, while Th2 cytokines were downregulated, suggesting that dysregulation of MSCs might also be involved in the pathogenesis of AD [132]. In a contact hypersensitivity (CHS) model in mice, MSCs in adipose tissues (ADSCs) may contribute to the self-limiting course of allergic contact dermatitis (ACD) by decreasing the expression of IFN-γ rather than increasing the expression of IL-10 [133]. On the other hand, treatment with exogenous ADSCs improved AD by decreasing the expression levels of cytokines and chemokines, such as IL-5, MIP-1ss, MIP-2, CCL5, and IL-17 [134]. Another study showed that allogeneic and syngeneic clonal BMSCs exhibited therapeutic effects on AD, possibly by suppressing T cell and B cell functions, decreasing the serum IgE level, and inhibiting cell infiltration in skin lesions and the expression of IL-4 in the lymph node and skin [135]. Most recently, it has been shown that MSC-derived EVs exerted potent therapeutic effects on AD in mice as evidenced by the improvement in pathological symptoms/clinical scores, decreased serum IgE level and number of eosinophils in the blood, and reduced infiltration of mast cells, CD86+ and CD206+ cells, and inflammatory cytokine levels in AD skin lesions [136, 137]. In a clinical trial, the safety and efficacy of hUCB-MSCs have been validated in the treatment of moderate-to-severe atopic dermatitis [138]. Currently, there are four registered clinical trials on MSC-based therapy of atopic dermatitis as listed in the ClinicalTrials.gov (Table 1).
6.9. Skin Wounds and Burns
Wound healing is a complex process involving multiple layers of integrated interactions among various cell types and bioactive molecules, whereas any aberrant change in this process can lead to compromised wound healing, such as abnormal scar formation [139]. In the last two decades, MSCs of diverse tissue origins have been extensively explored as a potential regenerative therapy to facilitate normal and abnormal skin wound healing through multiple modes of actions, such as promoting resolution of the inflammation, vascularization, migration, and proliferation of epithelial cells, matrix remodeling, and inhibition of apoptosis [139, 140]. For example, transplantation of autologous MSCs led to almost complete healing in the skin wound of pigs [141]. Zhang et al. showed that systemic administration of human gingiva-derived MSCs (GMSCs) facilitated full-thickness skin wound healing in mice through promoting reepithelialization, angiogenesis, and regenerative M2 macrophage polarization [27]. In the skin wound model of mice and rabbits, xenotransplantation of MSCs also promoted skin wound healing but did not cause any immunologic responses [142, 143]. In addition, a study showed that intradermal application of MSCs accelerated full-thickness skin wound healing in Albino rats [144]. In a rat diabetic skin wound model, transplanted MSCs could survive in the wounds and promote wound healing through angiogenesis [145]. In clinical studies, transplantation of MSCs could effectively promote the healing of skin burn wounds by regenerating functional sweat glands [146]. Clinical trials have also been conducted using autologous and allogeneic MSCs derived from adipose tissue and bone marrow to treat skin wounds and burns [147–149]. Most recently, the MSC secretome, including MSC-CM and MSC-derived EVs, has been shown to promote skin wound healing through multiple functions, e.g., regulating fibroblast functions/matrix remodeling and promoting reepithelialization, angiogenesis, resident cell proliferation, and polarization of regenerative M2 macrophages [48, 54, 150–154]. In addition, MSCs have been transplanted in combination with different types of biomaterials, such as the collagen-chitosan laser drilling acellular dermal matrix, microspheres, R120 nanofiber, graphene, silk fibroin, PVA, PLGA, and hydrogel, which improved the local retention and proliferation of transplanted MSCs and showed promising therapeutic effects on skin wound healing [155–163].
6.10. Keloids and Scars
Keloids are a skin disorder characterized by excessive collagen deposition into the extracellular matrix (ECM), while its pathogenesis remains largely unknown. Several lines of evidence have implied the potential role of MSCs with aberrant phenotypes and their special inflammatory niche in the keloid pathogenesis [164–166]. Even though there is still a lack of an animal model for human keloids, several in vitro studies have shown that human MSCs of different tissue origins, such as ADSCs, BMSCs, amnion-derived MSCs, fetal dermal MSCs, and human Wharton's jelly (umbilical cord) MSCs, had obvious paracrine inhibitory effects on the proliferation, profibrotic phenotype, the production of the extracellular matrix (ECM), and other bioactivities of fibroblasts derived from human keloids and hypertrophic scars [167–174]. In rabbit ear skin wound models, transplantation of autologous MSCs showed preventive effects on hypertrophic scar formation involving multiple potential mechanisms such as inhibition of the proliferation and transformation of fibroblasts into myofibroblasts; decreased expression of TGF-β1, type I and type III collagens, and inflammatory responses; and increased expression of decorin [175–177]. Recent studies indicated that the application of MSC-derived exosomes could also prevent scar formation through horizontal transfer of miRNAs to suppress differentiation of fibroblasts into myofibroblasts [178, 179]. Yates et al. [180] showed that cotransplantation of MSCs with fibroblasts could normalize matrix production, thus attenuating hypertrophic scarring. In addition, BMSCs genetically modified to overexpress TGF-β3 showed obvious effects to reduce the formation of scar tissue in a rabbit skin wound model [181]. Most recently, clinical trials have been performed to evaluate the antiscarring effects of MSCs on Cesarean section skin scars or acne scar formation [182, 183].
7. Challenges and Perspectives
To date, a growing body of preclinical and clinical studies has demonstrated the beneficial effect of MSC-based therapy for a wide spectrum of diseases, including various autoimmune and inflammatory skin disorders. However, there are several major challenges faced in the clinical translation of MSC-based regenerative therapies. One of the major challenges might be the large variations in the therapeutic efficacy of MSCs due to their heterogeneity caused by various intrinsic and extrinsic factors. Intrinsically, the different tissue origins, age, and health status (niche factors) can affect the property and function of MSCs. Extrinsically, the isolation, culture, and ex vivo expansion conditions can also affect the property and biological functions of MSCs [184]. Therefore, it would be critical to identify the appropriate donors and tissue sources of MSCs and optimize the isolation and ex vivo expansion conditions so as to obtain scalable MSC products with consistent quantity and quality. Recently, microfluidic single-cell characterization, an emerging technique, has offered particularly dramatic strategies for identifying and isolating the most effective cells for therapeutic use [185]. During the downstream application process, many factors such as the dosage, the route, the timing, and the frequency of cell delivery might significantly affect the therapeutic efficacy of MSC-based therapy [185]. Another concern is the safety of MSCs, particularly their tumorigenic potentials, following the long-term transplantation. To date, there is still no efficient way to follow up the fate and behavior MSCs following transplantation in vivo. Recently, a case study reported tumor formation at the site of spinal injury of a patient following local transplantation of adult olfactory mucosal cells [186]. Most recently, several studies have described the side effects of MSC therapy in different diseases, including graft versus host disease and cardiac, neurological, and orthopedic disorders [187].
Accumulating evidence has shown that MSC-derived EVs exhibited potent immunomodulatory/anti-inflammatory and pleiotropic effects as the parental MSCs did. More recently, some preclinical and early clinical studies have shown that MSC-EVs displayed therapeutic effects on several disease models, including cutaneous diseases [85, 119, 130, 136, 137]. Therefore, MSC-EVs hold great promises to be developed as potential cell-free therapeutic products that can avoid the major challenges faced in the use of MSCs [188].
8. Conclusions
In the last two decades, much progress has been made in delineating the molecular mechanisms of action of MSCs and their potential application in regenerative therapy for a wide spectrum of diseases, including various autoimmune and inflammatory skin disorders. To date, accumulating evidence supports the notion that MSCs exert their therapeutic effects under various pathological settings through multiple modes of functions mediated by their paracrine secretome containing a myriad of bioactive factors, including extracellular vesicles (EVs). However, much effort is still required to further investigate the specific cellular and molecular mechanisms by which MSCs of different tissue origins and their cell-free derivative products exert their unique therapeutic effects on certain cutaneous diseases.
Acknowledgments
This study was supported by grants from the Natural Science Foundation of Guangdong Province (2019A1515011713), “Group-type” Special Supporting Project for Educational Talents in Universities (4SG19221), Research Foundation of Guangdong Medical University for Ph.D. Staff (B2019038), and Key Cultivation Project of Guangdong Medical University (GDMUZ2019001).
Contributor Information
Qunzhou Zhang, Email: zqunzhou@upenn.edu.
Jincheng Zeng, Email: zengjc@gdmu.edu.cn.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Brown C., McKee C., Bakshi S., et al. Mesenchymal stem cells: cell therapy and regeneration potential. Journal of tissue engineering and regenerative medicine. 2019;13(9):1738–1755. doi: 10.1002/term.2914. [DOI] [PubMed] [Google Scholar]
- 2.Barbulescu C. C., Goldstein N. B., Roop D. R., Norris D. A., Birlea S. A. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. Journal of Investigative Dermatology. 2020;140(1):29–37. doi: 10.1016/j.jid.2019.03.1142. [DOI] [PubMed] [Google Scholar]
- 3.Liao L. M., Han Q., Zhao C. H. Application of mesenchymal stem cell in immunotherapy--review. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005;13(1):158–163. [PubMed] [Google Scholar]
- 4.Ko I. K., Kim B. S. Mesenchymal stem cells for treatment of myocardial infarction. International Journal of Stem Cells. 2008;1(1):49–54. doi: 10.15283/ijsc.2008.1.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao F., Tu Q., Wang L., et al. Mesenchymal stem cells and their therapeutic applications in inflammatory bowel disease. Oncotarget. 2017;8(23):38008–38021. doi: 10.18632/oncotarget.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javan M. R., Khosrojerdi A., Moazzeni S. M. New insights into implementation of mesenchymal stem cells in cancer therapy: prospects for anti-angiogenesis treatment. Frontiers in Oncology. 2019;9:p. 840. doi: 10.3389/fonc.2019.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrell C. R., Fellabaum C., Arsenijevic A., Markovic B. S., Djonov V., Volarevic V. Therapeutic potential of mesenchymal stem cells and their secretome in the treatment of glaucoma. Stem Cells International. 2019;2019:11. doi: 10.1155/2019/7869130.7869130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong L., Zheng L. Z., Qin L., Ho K. K. W. Role of mesenchymal stem cells in osteoarthritis treatment. Journal of Orthopaedic Translation. 2017;9:89–103. doi: 10.1016/j.jot.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez R., Jimenez-Luna C., Perales-Adan J., Perazzoli G., Melguizo C., Prados J. Differentiation of human mesenchymal stem cells towards neuronal lineage: clinical trials in nervous system disorders. Biomol Ther (Seoul) 2020;28:34–44. doi: 10.4062/biomolther.2019.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao X., Cao Z. Gingiva-derived mesenchymal stem cells and their potential applications in oral and maxillofacial diseases. Current Stem Cell Research & Therapy. 2019;15:43–53. doi: 10.2174/1574888X14666191107100311. [DOI] [PubMed] [Google Scholar]
- 11.Lim H. W., SAB C., Resneck J. S., Jr., et al. The burden of skin disease in the United States. Journal of the American Academy of Dermatology. 2017;76:958–972. doi: 10.1016/j.jaad.2016.12.043. e952. [DOI] [PubMed] [Google Scholar]
- 12.Shin T. H., Kim H. S., Choi S. W., Kang K. S. Mesenchymal stem cell therapy for inflammatory skin diseases: clinical potential and mode of action. International Journal of Molecular Sciences. 2017;18(2):p. 244. doi: 10.3390/ijms18020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K. H., Blasco-Morente G., Cuende N., Arias-Santiago S. Mesenchymal stromal cells: properties and role in management of cutaneous diseases. Journal of the European Academy of Dermatology and Venereology. 2017;31:414–423. doi: 10.1111/jdv.13934. [DOI] [PubMed] [Google Scholar]
- 14.Kozlowska U., Krawczenko A., Futoma K., et al. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World Journal of Stem Cells. 2019;11:347–374. doi: 10.4252/wjsc.v11.i6.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwatani S., Yoshida M., Yamana K., et al. Isolation and characterization of human umbilical cord-derived mesenchymal stem cells from preterm and term infants. Journal of Visualized Experiments. 2019;26(143) doi: 10.3791/58806. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q., Shi S., Liu Y., et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. Journal of Immunology. 2009;183(12):7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manochantr S., Tantrawatpan C., Kheolamai P., U Pratya Y., Supokawej A., Issaragrisil S. Isolation, characterization and neural differentiation potential of amnion derived mesenchymal stem cells. Journal of the Medical Association of Thailand. 2010;93(Supplement 7):S183–S191. [PubMed] [Google Scholar]
- 18.Peng J., Wang Y., Zhang L., et al. Human umbilical cord Wharton's jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Research Bulletin. 2011;84:235–243. doi: 10.1016/j.brainresbull.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Dominici M., Le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 20.Eleuteri S., Fierabracci A. Insights into the secretome of mesenchymal stem cells and its potential applications. International Journal of Molecular Sciences. 2019;20(18):p. 4597. doi: 10.3390/ijms20184597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Blanc K., Tammik C., Rosendahl K., Zetterberg E., Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Experimental Hematology. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 22.Tse W. T., Pendleton J. D., Beyer W. M., Egalka M. C., Guinan E. C. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 23.Sotiropoulou P. A., Perez S. A., Gritzapis A. D., Baxevanis C. N., Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 24.Cui Z., Feng Y., Li D., Li T., Gao P., Xu T. Activation of aryl hydrocarbon receptor (AhR) in mesenchymal stem cells modulates macrophage polarization in asthma. Journal of Immunotoxicology. 2020;17:21–30. doi: 10.1080/1547691X.2019.1706671. [DOI] [PubMed] [Google Scholar]
- 25.Ohnishi S., Sumiyoshi H., Kitamura S., Nagaya N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Letters. 2007;581:3961–3966. doi: 10.1016/j.febslet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Yokokawa K., Iwahara N., Hisahara S., et al. Transplantation of mesenchymal stem cells improves amyloid-beta pathology by modifying microglial function and suppressing oxidative stress. Journal of Alzheimer’s Disease. 2019;72:867–884. doi: 10.3233/JAD-190817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q. Z., Su W. R., Shi S. H., et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S., Zheng X., Li H., et al. Mesenchymal stem cells ameliorate hepatic ischemia/reperfusion injury via inhibition of neutrophil recruitment. Journal of Immunology Research. 2018;2018:10. doi: 10.1155/2018/7283703.7283703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L., Zhang W., Yue H., et al. Effects of human mesenchymal stem cells on the differentiation of dendritic cells from CD34+ cells. Stem Cells and Development. 2007;16:719–731. doi: 10.1089/scd.2007.0065. [DOI] [PubMed] [Google Scholar]
- 30.Su W. R., Zhang Q. Z., Shi S. H., Nguyen A. L., Le A. D. Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells. 2011;29:1849–1860. doi: 10.1002/stem.738. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X. X., Zhang Y., Liu B., et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 32.Nauta A. J., Kruisselbrink A. B., Lurvink E., Willemze R., Fibbe W. E. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. Journal of Immunology. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 33.Ramasamy R., Fazekasova H., Lam E. W., Soeiro I., Lombardi G., Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q., Sun B., Wang D., et al. Murine bone marrow mesenchymal stem cells cause mature dendritic cells to promote T-cell tolerance. Scandinavian Journal of Immunology. 2008;68:607–615. doi: 10.1111/j.1365-3083.2008.02180.x. [DOI] [PubMed] [Google Scholar]
- 35.Chehelcheraghi F., Abbaszadeh A., Tavafi M. Skin mast cell promotion in random skin flaps in rats using bone marrow mesenchymal stem cells and amniotic membrane. Iranian Biomedical Journal. 2018;22(5):322–330. doi: 10.29252/ibj.22.5.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chehelcheraghi F., Bayat M., Chien S. Effect of mesenchymal stem cells and chicken embryo extract on flap viability and mast cells in rat skin flaps. Journal of Investigative Surgery. 2020;33:123–133. doi: 10.1080/08941939.2018.1479006. [DOI] [PubMed] [Google Scholar]
- 37.Shin T.-H., Lee B.-C., Choi S. W., et al. Human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis via regulation of B lymphocyte maturation. Oncotarget. 2017;8:512–522. doi: 10.18632/oncotarget.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park Y. S., Lim G. W., Cho K. A., et al. Improved viability and activity of neutrophils differentiated from HL-60 cells by co-culture with adipose tissue-derived mesenchymal stem cells. Biochemical and Biophysical Research Communications. 2012;423:19–25. doi: 10.1016/j.bbrc.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 39.Jiang D., Muschhammer J., Qi Y., et al. Suppression of neutrophil-mediated tissue damage-a novel skill of mesenchymal stem cells. Stem Cells. 2016;34:2393–2406. doi: 10.1002/stem.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su W. H., Wang C. J., Fu H. C., et al. Human umbilical cord mesenchymal stem cells extricate bupivacaine-impaired skeletal muscle function via mitigating neutrophil-mediated acute inflammation and protecting against fibrosis. International Journal of Molecular Sciences. 2019;20(17):p. 4312. doi: 10.3390/ijms20174312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batten P., Sarathchandra P., Antoniw J. W., et al. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: relevance to tissue engineering human heart valves. Tissue Engineering. 2006;12:2263–2273. doi: 10.1089/ten.2006.12.2263. [DOI] [PubMed] [Google Scholar]
- 42.Byun J. W., Kim H. J., Na K., et al. Bone marrow-derived mesenchymal stem cells prevent alopecia areata development through the inhibition of NKG2D expression: a pilot study. Experimental Dermatology. 2017;26:532–535. doi: 10.1111/exd.13255. [DOI] [PubMed] [Google Scholar]
- 43.Boumaza I., Srinivasan S., Witt W. T., et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. Journal of Autoimmunity. 2009;32:33–42. doi: 10.1016/j.jaut.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Rey E., Gonzalez M. A., Varela N., et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2010;69:241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 45.Prevosto C., Zancolli M., Canevali P., Zocchi M. R., Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 46.Khubutiya M. S., Vagabov A. V., Temnov A. A., Sklifas A. N. Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models of acute organ injury. Cytotherapy. 2014;16:579–585. doi: 10.1016/j.jcyt.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Q. Z., Nguyen A. L., Yu W. H., Le A. D. Human oral mucosa and gingiva: a unique reservoir for mesenchymal stem cells. Journal of Dental Research. 2012;91:1011–1018. doi: 10.1177/0022034512461016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim W. S., Park B. S., Sung J. H., et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. Journal of Dermatological Science. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 49.Chen L., Tredget E. E., Wu P. Y., Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3(4, article e1886) doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbasi-Kangevari M., Ghamari S. H., Safaeinejad F., Bahrami S., Niknejad H. Potential therapeutic features of human amniotic mesenchymal stem cells in multiple sclerosis: immunomodulation, inflammation suppression, angiogenesis promotion, oxidative stress inhibition, neurogenesis induction, MMPs regulation, and remyelination stimulation. Frontiers in Immunology. 2019;10:p. 238. doi: 10.3389/fimmu.2019.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo S., Zhen Y., Wang A. Transplantation of bone mesenchymal stem cells promotes angiogenesis and improves neurological function after traumatic brain injury in mouse. Neuropsychiatric Disease and Treatment. 2017;13:2757–2765. doi: 10.2147/NDT.S141534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bak D. H., Choi M. J., Kim S. R., et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean Journal of Physiology & Pharmacology. 2018;22:555–566. doi: 10.4196/kjpp.2018.22.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maumus M., Pers Y. M., Ruiz M., Jorgensen C., Noel D. Mesenchymal stem cells and regenerative medicine: future perspectives in osteoarthritis. Medical Sciences (Paris) 2018;34:1092–1099. doi: 10.1051/medsci/2018294. [DOI] [PubMed] [Google Scholar]
- 54.Im G.-B., Kim Y. H., Kim Y.-J., et al. Enhancing the wound healing effect of conditioned medium collected from mesenchymal stem cells with high passage number using bioreducible nanoparticles. International Journal of Molecular Sciences. 2019;20 doi: 10.3390/ijms20194835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen Z., Mai Z., Zhu X., et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Research & Therapy. 2020;11:p. 36. doi: 10.1186/s13287-020-1563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moon G. J., Sung J. H., Kim D. H., et al. Application of mesenchymal stem cell-derived extracellular vesicles for stroke: biodistribution and microRNA study. Translational Stroke Research. 2019;10:509–521. doi: 10.1007/s12975-018-0668-1. [DOI] [PubMed] [Google Scholar]
- 57.Yan Y., Jiang W., Tan Y., et al. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Molecular Therapy. 2017;25:465–479. doi: 10.1016/j.ymthe.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J. W., Wei L., Han Z., Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. European Journal of Pharmacology. 2019;852:68–76. doi: 10.1016/j.ejphar.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 59.Frisoli M. L., Essien K., Harris J. E. Vitiligo: mechanisms of pathogenesis and treatment. Annual Review of Immunology. 2020;38:621–648. doi: 10.1146/annurev-immunol-100919-023531. [DOI] [PubMed] [Google Scholar]
- 60.Zhu L., Lin X., Zhi L., et al. Mesenchymal stem cells promote human melanocytes proliferation and resistance to apoptosis through PTEN pathway in vitiligo. Stem Cell Research & Therapy. 2020;11:p. 26. doi: 10.1186/s13287-019-1543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou M. N., Zhang Z. Q., Wu J. L., et al. Dermal mesenchymal stem cells (DMSCs) inhibit skin-homing CD8+ T cell activity, a determining factor of vitiligo patients' autologous melanocytes transplantation efficiency. PLoS One. 2013;8(4, article e60254) doi: 10.1371/journal.pone.0060254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varrone F., Caputo E. The miRNAs role in melanoma and in its resistance to therapy. International Journal of Molecular Sciences. 2020;21(3):p. 878. doi: 10.3390/ijms21030878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahn J., Lee H., Seo K., Kang S., Ra J., Youn H. Anti-tumor effect of adipose tissue derived-mesenchymal stem cells expressing interferon-beta and treatment with cisplatin in a xenograft mouse model for canine melanoma. PLoS One. 2013;8, article e74897 doi: 10.1371/journal.pone.0074897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Studeny M., Marini F. C., Champlin R. E., Zompetta C., Fidler I. J., Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Research. 2002;62(13):3603–3608. [PubMed] [Google Scholar]
- 65.Studeny M., Marini F. C., Dembinski J. L., et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. Journal of the National Cancer Institute. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 66.Wang J., Ma D., Li Y., et al. Targeted delivery of CYP2E1 recombinant adenovirus to malignant melanoma by bone marrow-derived mesenchymal stem cells as vehicles. Anticancer Drugs. 2014;25:303–314. doi: 10.1097/CAD.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 67.Sun B., Wang X., Pan Y., et al. Antitumor effects of conditioned media of human fetal dermal mesenchymal stem cells on melanoma cells. OncoTargets and Therapy. 2019;12:4033–4046. doi: 10.2147/OTT.S203910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kucerova L., Matuskova M., Hlubinova K., Altanerova V., Altaner C. Tumor cell behaviour modulation by mesenchymal stromal cells. Molecular Cancer. 2010;9:p. 129. doi: 10.1186/1476-4598-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bahrambeigi V., Ahmadi N., Salehi R., Javanmard S. H. Genetically modified murine adipose-derived mesenchymal stem cells producing interleukin-2 favor B16F10 melanoma cell proliferation. Immunological Investigations. 2015;44:216–236. doi: 10.3109/08820139.2014.988719. [DOI] [PubMed] [Google Scholar]
- 70.Boull C. L., Hylwa S. A., Sajic D., Wagner J. E., Tolar J., Hook K. P. Toxic epidermal necrolysis in recessive dystrophic epidermolysis bullosa following bone marrow transplantation. Journal of Pediatrics. 2016;173:242–244. doi: 10.1016/j.jpeds.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perdoni C., McGrath J. A., Tolar J. Preconditioning of mesenchymal stem cells for improved transplantation efficacy in recessive dystrophic epidermolysis bullosa. Stem Cell Research & Therapy. 2014;5(6):p. 121. doi: 10.1186/scrt511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iinuma S., Aikawa E., Tamai K., et al. Transplanted bone marrow-derived circulating PDGFRalpha+ cells restore type VII collagen in recessive dystrophic epidermolysis bullosa mouse skin graft. Journal of Immunology. 2015;194:1996–2003. doi: 10.4049/jimmunol.1400914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Webber B. R., Osborn M. J., AN M. E., et al. CRISPR/Cas9-based genetic correction for recessive dystrophic epidermolysis bullosa. NPJ Regenerative Medicine. 2016;1, article 16014 doi: 10.1038/npjregenmed.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alexeev V., Uitto J., Igoucheva O. Gene expression signatures of mouse bone marrow-derived mesenchymal stem cells in the cutaneous environment and therapeutic implications for blistering skin disorder. Cytotherapy. 2011;13:30–45. doi: 10.3109/14653249.2010.518609. [DOI] [PubMed] [Google Scholar]
- 75.Fujita Y., Abe R., Inokuma D., et al. Bone marrow transplantation restores epidermal basement membrane protein expression and rescues epidermolysis bullosa model mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14345–14350. doi: 10.1073/pnas.1000044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ganier C., Titeux M., Gaucher S., et al. Intradermal injection of bone marrow mesenchymal stromal cells corrects recessive dystrophic epidermolysis bullosa in a xenograft model. Journal of Investigative Dermatology. 2018;138:2483–2486. doi: 10.1016/j.jid.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 77.Kuhl T., Mezger M., Hausser I., Handgretinger R., Bruckner-Tuderman L., Nystrom A. High local concentrations of intradermal MSCs restore skin integrity and facilitate wound healing in dystrophic epidermolysis bullosa. Molecular Therapy. 2015;23:1368–1379. doi: 10.1038/mt.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Webber B. R., Kyle T O’Connor, McElmurry R. T., et al. Rapid generation of Col7a1(-/-) mouse model of recessive dystrophic epidermolysis bullosa and partial rescue via immunosuppressive dermal mesenchymal stem cells. Laboratory Investigation. 2017;97:1218–1224. doi: 10.1038/labinvest.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsu C. K., Wang S. P., Lee J. Y., McGrath J. A. Treatment of hereditary epidermolysis bullosa: updates and future prospects. American Journal of Clinical Dermatology. 2014;15:1–6. doi: 10.1007/s40257-013-0059-z. [DOI] [PubMed] [Google Scholar]
- 80.Alexeev V., Donahue A., Uitto J., Igoucheva O. Analysis of chemotactic molecules in bone marrow-derived mesenchymal stem cells and the skin: Ccl27-Ccr10 axis as a basis for targeting to cutaneous tissues. Cytotherapy. 2013;15:171–184. doi: 10.1016/j.jcyt.2012.11.006. e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Conget P., Rodriguez F., Kramer S., et al. Replenishment of type VII collagen and re-epithelialization of chronically ulcerated skin after intradermal administration of allogeneic mesenchymal stromal cells in two patients with recessive dystrophic epidermolysis bullosa. Cytotherapy. 2010;12:429–431. doi: 10.3109/14653241003587637. [DOI] [PubMed] [Google Scholar]
- 82.Rashidghamat E., Kadiyirire T., Ayis S., et al. Phase I/II open-label trial of intravenous allogeneic mesenchymal stromal cell therapy in adults with recessive dystrophic epidermolysis bullosa. Journal of the American Academy of Dermatology. 2019;83(2):447–454. doi: 10.1016/j.jaad.2019.11.038. [DOI] [PubMed] [Google Scholar]
- 83.Panich U., Sittithumcharee G., Rathviboon N., Jirawatnotai S. Ultraviolet radiation-induced skin aging: the role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells International. 2016;2016:14. doi: 10.1155/2016/7370642.7370642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jeong J. H., Fan Y., You G. Y., Choi T. H., Kim S. Improvement of photoaged skin wrinkles with cultured human fibroblasts and adipose-derived stem cells: a comparative study. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2015;68:372–381. doi: 10.1016/j.bjps.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 85.Kim W. S., Park B. S., Sung J. H. Protective role of adipose-derived stem cells and their soluble factors in photoaging. Archives of Dermatological Research. 2009;301:329–336. doi: 10.1007/s00403-009-0951-9. [DOI] [PubMed] [Google Scholar]
- 86.Kwon T. R., Oh C. T., Choi E. J., et al. Conditioned medium from human bone marrow-derived mesenchymal stem cells promotes skin moisturization and effacement of wrinkles in UVB-irradiated SKH-1 hairless mice. Photodermatol Photoimmunol Photomed. 2016;32:120–128. doi: 10.1111/phpp.12224. [DOI] [PubMed] [Google Scholar]
- 87.Ueda M. A novel approach for skin rejuvenation by regenerative medicine: delivery of stem cell-derived growth factors through an iontophoretic system. International Journal of Oral & Maxillofacial Implants. 2014;29:e59–e65. doi: 10.11607/jomi.te43. [DOI] [PubMed] [Google Scholar]
- 88.Son W. C., Yun J. W., Kim B. H. Adipose-derived mesenchymal stem cells reduce MMP-1 expression in UV-irradiated human dermal fibroblasts: therapeutic potential in skin wrinkling. Bioscience, Biotechnology, and Biochemistry. 2015;79:919–925. doi: 10.1080/09168451.2015.1008972. [DOI] [PubMed] [Google Scholar]
- 89.Li L., Ngo H. T. T., Hwang E., et al. Conditioned medium from human adipose-derived mesenchymal stem cell culture prevents UVB-induced skin aging in human keratinocytes and dermal fibroblasts. International Journal of Molecular Sciences. 2019;21 doi: 10.3390/ijms21010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Q., Luo Z., He S., et al. Conditioned serum-free medium from umbilical cord mesenchymal stem cells has anti-photoaging properties. Biotechnology Letters. 2013;35:1707–1714. doi: 10.1007/s10529-013-1242-2. [DOI] [PubMed] [Google Scholar]
- 91.Campanati A., Consales V., Orciani M., et al. Role of mesenchymal stem cells in the pathogenesis of psoriasis: current perspectives. Psoriasis (Auckl) 2017;7:73–85. doi: 10.2147/PTT.S108311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Campanati A., Orciani M., Sorgentoni G., et al. Indirect co-cultures of healthy mesenchymal stem cells restore the physiological phenotypical profile of psoriatic mesenchymal stem cells. Clinical and Experimental Immunology. 2018;193:234–240. doi: 10.1111/cei.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campanati A., Orciani M., Gorbi S., Regoli F., Di Primio R., Offidani A. Effect of biologic therapies targeting tumour necrosis factor-alpha on cutaneous mesenchymal stem cells in psoriasis. British Journal of Dermatology. 2012;167:68–76. doi: 10.1111/j.1365-2133.2012.10900.x. [DOI] [PubMed] [Google Scholar]
- 94.Liu R., Yang Y., Yan X., Zhang K. Abnormalities in cytokine secretion from mesenchymal stem cells in psoriatic skin lesions. European Journal of Dermatology. 2013;23:600–607. doi: 10.1684/ejd.2013.2149. [DOI] [PubMed] [Google Scholar]
- 95.Liu R., Wang Y., Zhao X., Yang Y., Zhang K. Lymphocyte inhibition is compromised in mesenchymal stem cells from psoriatic skin. European Journal of Dermatology. 2014;24:560–567. doi: 10.1684/ejd.2014.2394. [DOI] [PubMed] [Google Scholar]
- 96.Orciani M., Campanati A., Salvolini E., et al. The mesenchymal stem cell profile in psoriasis. British Journal of Dermatology. 2011;165:585–592. doi: 10.1111/j.1365-2133.2011.10438.x. [DOI] [PubMed] [Google Scholar]
- 97.Chang W. J., Niu X. P., Hou R. X., et al. LITAF, HHEX, and DUSP1 expression in mesenchymal stem cells from patients with psoriasis. Genetics and Molecular Research. 2015;14:15793–15801. doi: 10.4238/2015.December.1.31. [DOI] [PubMed] [Google Scholar]
- 98.Imai Y., Yamahara K., Hamada A., Fujimori Y., Yamanishi K. Human amnion-derived mesenchymal stem cells ameliorate imiquimod-induced psoriasiform dermatitis in mice. Journal of Dermatology. 2019;46:276–278. doi: 10.1111/1346-8138.14768. [DOI] [PubMed] [Google Scholar]
- 99.Lee Y. S., Sah S. K., Lee J. H., Seo K. W., Kang K. S., Kim T. Y. Human umbilical cord blood-derived mesenchymal stem cells ameliorate psoriasis-like skin inflammation in mice. Biochemistry and Biophysics Reports. 2017;9:281–288. doi: 10.1016/j.bbrep.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu R. F., Wang F., Wang Q., Zhao X. C., Zhang K. M. Research note mesenchymal stem cells from skin lesions of psoriasis patients promote proliferation and inhibit apoptosis of HaCaT cells. Genetics and Molecular Research. 2015;14:17758–17767. doi: 10.4238/2015.December.21.49. [DOI] [PubMed] [Google Scholar]
- 101.Niu X., Chang W., Liu R., et al. mRNA and protein expression of the angiogenesis-related genes EDIL3, AMOT and ECM1 in mesenchymal stem cells in psoriatic dermis. Clinical and Experimental Immunology. 2016;41:533–540. doi: 10.1111/ced.12783. [DOI] [PubMed] [Google Scholar]
- 102.De Jesus M. M., Santiago J. S., Trinidad C. V., et al. Autologous adipose-derived mesenchymal stromal cells for the treatment of psoriasis vulgaris and psoriatic arthritis: a case report. Cell Transplantation. 2016;25:2063–2069. doi: 10.3727/096368916X691998. [DOI] [PubMed] [Google Scholar]
- 103.Chen H., Niu J.-W., Ning H.-M., et al. Treatment of psoriasis with mesenchymal stem cells. American Journal of Medicine. 2016;129:e13–e14. doi: 10.1016/j.amjmed.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 104.Balato A., Caiazzo G. Mesenchymal stem cells participate to inflammatory skin process of atopic dermatitis. British Journal of Dermatology. 2017;176:1437–1438. doi: 10.1111/bjd.15412. [DOI] [PubMed] [Google Scholar]
- 105.Choi E. W., Shin I. S., Song J. W., et al. Transplantation of adipose tissue-derived mesenchymal stem cells prevents the development of lupus dermatitis. Stem Cells and Development. 2015;24:2041–2051. doi: 10.1089/scd.2015.0021. [DOI] [PubMed] [Google Scholar]
- 106.Guiducci S., Manetti M., Romano E., et al. Bone marrow-derived mesenchymal stem cells from early diffuse systemic sclerosis exhibit a paracrine machinery and stimulate angiogenesis in vitro. Annals of the Rheumatic Diseases. 2011;70:2011–2021. doi: 10.1136/ard.2011.150607. [DOI] [PubMed] [Google Scholar]
- 107.Lim J. Y., Ryu D. B., Lee S. E., Park G., Min C. K. Mesenchymal stem cells (MSCs) attenuate cutaneous sclerodermatous graft-versus-host disease (Scl-GVHD) through inhibition of immune cell infiltration in a mouse model. Journal of Investigative Dermatology. 2017;137:1895–1904. doi: 10.1016/j.jid.2017.02.986. [DOI] [PubMed] [Google Scholar]
- 108.Maria A. T. J., Toupet K., Bony C., et al. Antifibrotic, antioxidant, and immunomodulatory effects of mesenchymal stem cells in HOCl-induced systemic sclerosis. Arthritis & Rheumatology. 2016;68:1013–1025. doi: 10.1002/art.39477. [DOI] [PubMed] [Google Scholar]
- 109.Maria A. T., Toupet K., Maumus M., et al. Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. Journal of Autoimmunity. 2016;70:31–39. doi: 10.1016/j.jaut.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 110.Orciani M., Svegliati S., Gorbi S., et al. Alterations of ROS pathways in scleroderma begin at stem cell level. Journal of Biological Regulators and Homeostatic Agents. 2013;27:211–224. [PubMed] [Google Scholar]
- 111.Peltzer J., Aletti M., Frescaline N., Busson E., Lataillade J. J., Martinaud C. Mesenchymal stromal cells based therapy in systemic sclerosis: rational and challenges. Frontiers in Immunology. 2018;9:p. 2013. doi: 10.3389/fimmu.2018.02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tyndall A. Successes and failures of stem cell transplantation in autoimmune diseases. Hematology. 2011;2011(1):280–284. doi: 10.1182/asheducation-2011.1.280. [DOI] [PubMed] [Google Scholar]
- 113.Wang D., Zhang H., Cao M., et al. Efficacy of allogeneic mesenchymal stem cell transplantation in patients with drug-resistant polymyositis and dermatomyositis. Annals of the Rheumatic Diseases. 2011;70:1285–1288. doi: 10.1136/ard.2010.141804. [DOI] [PubMed] [Google Scholar]
- 114.Guiducci S., Porta F., Saccardi R., et al. Autologous mesenchymal stem cells foster revascularization of ischemic limbs in systemic sclerosis: a case report. Annals of Internal Medicine. 2010;153:650–654. doi: 10.7326/0003-4819-153-10-201011160-00007. [DOI] [PubMed] [Google Scholar]
- 115.Virzì F., Bianca P., Giammona A., et al. Combined platelet-rich plasma and lipofilling treatment provides great improvement in facial skin-induced lesion regeneration for scleroderma patients. Stem Cell Research & Therapy. 2017;8:p. 236. doi: 10.1186/s13287-017-0690-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu S., Guo Y. L., Yang J. Y., Wang W., Xu J. Efficacy of mesenchymal stem cells on systemic lupus erythematosus:a meta-analysis. Beijing Da Xue Xue Bao Yi Xue Ban. 2018;50(6):1014–1021. [PubMed] [Google Scholar]
- 117.Sun L., Akiyama K., Zhang H., et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27(6):1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reddy B. Y., Xu D. S., Hantash B. M. Mesenchymal stem cells as immunomodulator therapies for immune-mediated systemic dermatoses. Stem Cells and Development. 2012;21:352–362. doi: 10.1089/scd.2011.0404. [DOI] [PubMed] [Google Scholar]
- 119.Epstein G. K., Epstein J. S. Mesenchymal stem cells and stromal vascular fraction for hair loss: current status. Facial Plastic Surgery Clinics of North America. 2018;26:503–511. doi: 10.1016/j.fsc.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 120.Mejia-Barradas C. M., Cazares-Montanez J. E., Guerra-Marquez A., Hernandez-Chavez V. G., Caceres-Cortes J. R., Gutierrez-Iglesias G. Regenerative treatment with umbilical cord mesenchymal stem cells from Wharton's jelly in chronic ulcer caused by dermolipectomy. Cirugía y Cirujanos. 2019;87:8–16. doi: 10.24875/CIRU.18000515. [DOI] [PubMed] [Google Scholar]
- 121.Motegi S.-i., Sekiguchi A., Uchiyama A., et al. Protective effect of mesenchymal stem cells on the pressure ulcer formation by the regulation of oxidative and endoplasmic reticulum stress. Scientific Reports. 2017;7:p. 17186. doi: 10.1038/s41598-017-17630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Strong A. L., Bowles A. C., MacCrimmon C. P., et al. Adipose stromal cells repair pressure ulcers in both young and elderly mice: potential role of adipogenesis in skin repair. Stem Cells Translational Medicine. 2015;4:632–642. doi: 10.5966/sctm.2014-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de la Garza-Rodea A. S., Knaan-Shanzer S., van Bekkum D. W. Pressure ulcers: description of a new model and use of mesenchymal stem cells for repair. Dermatology. 2011;223(3):266–284. doi: 10.1159/000334628. [DOI] [PubMed] [Google Scholar]
- 124.O'Loughlin A., Kulkarni M., Creane M., et al. Topical administration of allogeneic mesenchymal stromal cells seeded in a collagen scaffold augments wound healing and increases angiogenesis in the diabetic rabbit ulcer. Diabetes. 2013;62:2588–2594. doi: 10.2337/db12-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wan J., Cai Q., Liu Y. Effect of different transplantations with bone-marrow derived mesenchymal stem cells on diabetic foot ulcers in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2013;38:347–355. doi: 10.3969/j.issn.1672-7347.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 126.Sun J., Zhang Y., Song X., Zhu J., Zhu Q. The healing effects of conditioned medium derived from mesenchymal stem cells on radiation-induced skin wounds in rats. Cell Transplantation. 2019;28:105–115. doi: 10.1177/0963689718807410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Benitez-Arvizu G., Palma-Lara I., Vazquez-Campos R., Sesma-Villalpando R. A., Parra-Barrera A., Gutierrez-Iglesias G. Autologous mesenchymal stem cells and cutaneus autograft as a treatment for chronic ulcer secondary to diabetes mellitus 2. Cirugía y Cirujanos. 2015;83:532–536. doi: 10.1016/j.circir.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 128.Kotenko K., Moroz B., Nadezhina N., et al. Successful treatment of localised radiation lesions in rats and humans by mesenchymal stem cell transplantation. Radiation Protection Dosimetry. 2012;151:661–665. doi: 10.1093/rpd/ncs177. [DOI] [PubMed] [Google Scholar]
- 129.Liu Z., Yu D., Xu J., et al. Human umbilical cord mesenchymal stem cells improve irradiation-induced skin ulcers healing of rat models. Biomedicine & Pharmacotherapy. 2018;101:729–736. doi: 10.1016/j.biopha.2018.02.093. [DOI] [PubMed] [Google Scholar]
- 130.Prakoeswa C. R. S., Natallya F. R., Harnindya D., et al. The efficacy of topical human amniotic membrane-mesenchymal stem cell-conditioned medium (hAMMSC-CM) and a mixture of topical hAMMSC-CM + vitamin C and hAMMSC-CM + vitamin E on chronic plantar ulcers in leprosy:a randomized control trial. Journal of Dermatological Treatment. 2018;29:835–840. doi: 10.1080/09546634.2018.1467541. [DOI] [PubMed] [Google Scholar]
- 131.Qin H. L., Zhu X. H., Zhang B., Zhou L., Wang W. Y. Clinical evaluation of human umbilical cord mesenchymal stem cell transplantation after angioplasty for diabetic foot. Experimental and Clinical Endocrinology & Diabetes. 2016;124:497–503. doi: 10.1055/s-0042-103684. [DOI] [PubMed] [Google Scholar]
- 132.Orciani M., Campanati A., Caffarini M., et al. T helper (Th)1, Th17 and Th2 imbalance in mesenchymal stem cells of adult patients with atopic dermatitis: at the origin of the problem. British Journal of Dermatology. 2017;176:1569–1576. doi: 10.1111/bjd.15078. [DOI] [PubMed] [Google Scholar]
- 133.Kikuchi S., Yanaba K., Nobeyama Y., et al. Suppressive effects of mesenchymal stem cells in adipose tissue on allergic contact dermatitis. Annals of Dermatology. 2017;29:391–399. doi: 10.5021/ad.2017.29.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim M., Lee S. H., Kim Y., et al. Human adipose tissue-derived mesenchymal stem cells attenuate atopic dermatitis by regulating the expression of MIP-2, miR-122a-SOCS1 axis, and Th1/Th2 responses. Frontiers in Pharmacology. 2018;9:p. 1175. doi: 10.3389/fphar.2018.01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Na K., Yoo H. S., Zhang Y. X., et al. Bone marrow-derived clonal mesenchymal stem cells inhibit ovalbumin-induced atopic dermatitis. Cell Death & Disease. 2014;5, article e1345 doi: 10.1038/cddis.2014.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cho B. S., Kim J. O., Ha D. H., Yi Y. W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Research & Therapy. 2018;9:p. 187. doi: 10.1186/s13287-018-0939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Song J. Y., Kang H. J., Ju H. M., et al. Umbilical cord-derived mesenchymal stem cell extracts ameliorate atopic dermatitis in mice by reducing the T cell responses. Scientific Reports. 2019;9:p. 6623. doi: 10.1038/s41598-019-42964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim H. S., Lee J. H., Roh K. H., Jun H. J., Kang K. S., Kim T. Y. Clinical trial of human umbilical cord blood-derived stem cells for the treatment of moderate-to-severe atopic dermatitis: phase I/IIa studies. Stem Cells. 2017;35:248–255. doi: 10.1002/stem.2401. [DOI] [PubMed] [Google Scholar]
- 139.Nourian Dehkordi A., Mirahmadi Babaheydari F., Chehelgerdi M., Raeisi Dehkordi S. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Research & Therapy. 2019;10:p. 111. doi: 10.1186/s13287-019-1212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jackson W. M., Nesti L. J., Tuan R. S. Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Translational Medicine. 2012;1:44–50. doi: 10.5966/sctm.2011-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ochiai H., Kishi K., Kubota Y., et al. Transplanted mesenchymal stem cells are effective for skin regeneration in acute cutaneous wounds of pigs. Regenerative Therapy. 2017;7:8–16. doi: 10.1016/j.reth.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee S. H., Lee J. H., Cho K. H. Effects of human adipose-derived stem cells on cutaneous wound healing in nude mice. Annals of Dermatology. 2011;23:150–155. doi: 10.5021/ad.2011.23.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stoff A., Rivera A. A., Banerjee N. S., et al. Promotion of incisional wound repair by human mesenchymal stem cell transplantation. Experimental Dermatology. 2009;18:362–369. doi: 10.1111/j.1600-0625.2008.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.El Sadik A. O., El Ghamrawy T. A., Abd El-Galil T. I. The effect of mesenchymal stem cells and chitosan gel on full thickness skin wound healing in albino rats: histological, immunohistochemical and fluorescent study. PLoS One. 2015;10, article e0137544 doi: 10.1371/journal.pone.0137544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Inoue H., Murakami T., Ajiki T., Hara M., Hoshino Y., Kobayashi E. Bioimaging assessment and effect of skin wound healing using bone-marrow-derived mesenchymal stromal cells with the artificial dermis in diabetic rats. Journal of Biomedical Optics. 2008;13, article 064036 doi: 10.1117/1.3042266. [DOI] [PubMed] [Google Scholar]
- 146.Sheng Z., Fu X., Cai S., et al. Regeneration of functional sweat gland-like structures by transplanted differentiated bone marrow mesenchymal stem cells. Wound Repair Regen. 2009;17:427–435. doi: 10.1111/j.1524-475X.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- 147.Caruana G., Bertozzi N., Boschi E., Pio Grieco M., Grignaffini E., Raposio E. Role of adipose-derived stem cells in chronic cutaneous wound healing. Annali Italiani di Chirurgia. 2015;86(1):1–4. [PubMed] [Google Scholar]
- 148.Maranda E. L., Rodriguez-Menocal L., Badiavas E. V. Role of mesenchymal stem cells in dermal repair in burns and diabetic wounds. Current Stem Cell Research & Therapy. 2017;12:61–70. doi: 10.2174/1574888x11666160714115926. [DOI] [PubMed] [Google Scholar]
- 149.Sung H. M., Suh I. S., Lee H. B., Tak K. S., Moon K. M., Jung M. S. Case reports of adipose-derived stem cell therapy for nasal skin necrosis after filler injection. Archives of Plastic Surgery. 2012;39:51–54. doi: 10.5999/aps.2012.39.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Joseph A., Baiju I., Bhat I. A., et al. Mesenchymal stem cell-conditioned media: a novel alternative of stem cell therapy for quality wound healing. Journal of Cellular Physiology. 2020;235:5555–5569. doi: 10.1002/jcp.29486. [DOI] [PubMed] [Google Scholar]
- 151.Kraskiewicz H., Paprocka M., Bielawska-Pohl A., et al. Can supernatant from immortalized adipose tissue MSC replace cell therapy? An in vitro study in chronic wounds model. Stem Cell Research & Therapy. 2020;11:p. 29. doi: 10.1186/s13287-020-1558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Saheli M., Bayat M., Ganji R., et al. Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Archives of Dermatological Research. 2019;312:325–336. doi: 10.1007/s00403-019-02016-6. [DOI] [PubMed] [Google Scholar]
- 153.Dalirfardouei R., Jamialahmadi K., Jafarian A. H., Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. Journal of Tissue Engineering and Regenerative Medicine. 2019;13:555–568. doi: 10.1002/term.2799. [DOI] [PubMed] [Google Scholar]
- 154.Wang X., Jiao Y., Pan Y., et al. Fetal dermal mesenchymal stem cell-derived exosomes accelerate cutaneous wound healing by activating Notch signaling. Stem Cells International. 2019;2019:11. doi: 10.1155/2019/2402916.2402916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Han Y., Sun T., Han Y., et al. Human umbilical cord mesenchymal stem cells implantation accelerates cutaneous wound healing in diabetic rats via the Wnt signaling pathway. European Journal of Medical Research. 2019;24:p. 10. doi: 10.1186/s40001-019-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huang S., Wu Y., Gao D., Fu X. Paracrine action of mesenchymal stromal cells delivered by microspheres contributes to cutaneous wound healing and prevents scar formation in mice. Cytotherapy. 2015;17:922–931. doi: 10.1016/j.jcyt.2015.03.690. [DOI] [PubMed] [Google Scholar]
- 157.Kalachaveedu M., Jenifer P., Pandian R., Arumugam G. Fabrication and characterization of herbal drug enriched guar galactomannan based nanofibrous mats seeded with GMSC's for wound healing applications. International Journal of Biological Macromolecules. 2020;148:737–749. doi: 10.1016/j.ijbiomac.2020.01.188. [DOI] [PubMed] [Google Scholar]
- 158.Lasocka I., Jastrzebska E., Szulc-Dabrowska L., et al. The effects of graphene and mesenchymal stem cells in cutaneous wound healing and their putative action mechanism. International Journal of Nanomedicine. 2019;14:2281–2299. doi: 10.2147/IJN.S190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li Z., Wang H., Yang B., Sun Y., Huo R. Three-dimensional graphene foams loaded with bone marrow derived mesenchymal stem cells promote skin wound healing with reduced scarring. Materials Science and Engineering C: Materials for Biological Applications. 2015;57:181–188. doi: 10.1016/j.msec.2015.07.062. [DOI] [PubMed] [Google Scholar]
- 160.Millan-Rivero J. E., Martinez C. M., Romecin P. A., et al. Silk fibroin scaffolds seeded with Wharton’s jelly mesenchymal stem cells enhance re-epithelialization and reduce formation of scar tissue after cutaneous wound healing. Stem Cell Research & Therapy. 2019;10:p. 126. doi: 10.1186/s13287-019-1229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ribeiro J., Pereira T., Amorim I., et al. Cell therapy with human MSCs isolated from the umbilical cord Wharton jelly associated to a PVA membrane in the treatment of chronic skin wounds. International Journal of Medical Sciences. 2014;11:979–987. doi: 10.7150/ijms.9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang G. X., Wang Y., Liu W. P., Zhang Z. H., Huang X. M., Xie A. Repair of skin damage with mesenchymal stem cells-poly (lactic-co-glycolic acid) scaffolds: experimental study with rabbits. Zhonghua Yi Xue Za Zhi. 2006;86:403–406. [PubMed] [Google Scholar]
- 163.Xu H., Huang S., Wang J., et al. Enhanced cutaneous wound healing by functional injectable thermo-sensitive chitosan-based hydrogel encapsulated human umbilical cord-mesenchymal stem cells. International Journal of Biological Macromolecules. 2019;137:433–441. doi: 10.1016/j.ijbiomac.2019.06.246. [DOI] [PubMed] [Google Scholar]
- 164.Lim K. H., Itinteang T., Davis P. F., Tan S. T. Stem cells in keloid lesions: a review. Plastic and Reconstructive Surgery – Global Open. 2019;7, article e2228 doi: 10.1097/GOX.0000000000002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Q., Yamaza T., Kelly A. P., et al. Tumor-like stem cells derived from human keloid are governed by the inflammatory niche driven by IL-17/IL-6 axis. PLoS One. 2009;4, article e7798 doi: 10.1371/journal.pone.0007798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Akino K., Akita S., Yakabe A., Mineda T., Hayashi T., Hirano A. Human mesenchymal stem cells may be involved in keloid pathogenesis. International Journal of Dermatology. 2008;47:1112–1117. doi: 10.1111/j.1365-4632.2008.03380.x. [DOI] [PubMed] [Google Scholar]
- 167.Fong C. Y., Biswas A., Subramanian A., Srinivasan A., Choolani M., Bongso A. Human keloid cell characterization and inhibition of growth with human Wharton's jelly stem cell extracts. Journal of Cellular Biochemistry. 2014;115:826–838. doi: 10.1002/jcb.24724. [DOI] [PubMed] [Google Scholar]
- 168.Arno A. I., Amini-Nik S., Blit P. H., et al. Effect of human Wharton's jelly mesenchymal stem cell paracrine signaling on keloid fibroblasts. Stem Cells Translational Medicine. 2014;3:299–307. doi: 10.5966/sctm.2013-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sato C., Yamamoto Y., Funayama E., et al. Conditioned medium obtained from amnion-derived mesenchymal stem cell culture prevents activation of keloid fibroblasts. Plastic and Reconstructive Surgery. 2018;141:390–398. doi: 10.1097/PRS.0000000000004068. [DOI] [PubMed] [Google Scholar]
- 170.Wang X., Ma Y., Gao Z., Yang J. Human adipose-derived stem cells inhibit bioactivity of keloid fibroblasts. Stem Cell Research & Therapy. 2018;9:p. 40. doi: 10.1186/s13287-018-0786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Arjunan S., Gan S. U., Choolani M., et al. Inhibition of growth of Asian keloid cells with human umbilical cord Wharton's jelly stem cell-conditioned medium. Stem Cell Research & Therapy. 2020;11:p. 78. doi: 10.1186/s13287-020-01609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fang F., Huang R. L., Zheng Y., Liu M., Huo R. Bone marrow derived mesenchymal stem cells inhibit the proliferative and profibrotic phenotype of hypertrophic scar fibroblasts and keloid fibroblasts through paracrine signaling. Journal of Dermatological Science. 2016;83:95–105. doi: 10.1016/j.jdermsci.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 173.Jiao Y., Wang X., Zhang J., Qi Y., Gong H., Jiang D. Inhibiting function of human fetal dermal mesenchymal stem cells on bioactivities of keloid fibroblasts. Stem Cell Research & Therapy. 2017;8:p. 170. doi: 10.1186/s13287-017-0624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu Y., Peng Y., Gao D., et al. Mesenchymal stem cells suppress fibroblast proliferation and reduce skin fibrosis through a TGF-beta3-dependent activation. International Journal of Lower Extremity Wounds. 2015;14:50–62. doi: 10.1177/1534734614568373. [DOI] [PubMed] [Google Scholar]
- 175.Liu S., Jiang L., Li H., et al. Mesenchymal stem cells prevent hypertrophic scar formation via inflammatory regulation when undergoing apoptosis. Journal of Investigative Dermatology. 2014;134:2648–2657. doi: 10.1038/jid.2014.169. [DOI] [PubMed] [Google Scholar]
- 176.Liu Y. L., Liu W. H., Sun J., et al. Mesenchymal stem cell-mediated suppression of hypertrophic scarring is p53 dependent in a rabbit ear model. Stem Cell Research & Therapy. 2014;5:p. 136. doi: 10.1186/scrt526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen L., Wang D. L., Wei Z. R., Wang B., Qi J. P., Sun G. F. Effects of local transplantation of autologous adipose-derived mesenchymal stem cells on the formation of hyperplastic scar on rabbit ears. Zhonghua Shao Shang Za Zhi. 2016;32:582–587. doi: 10.3760/cma.j.issn.1009-2587.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 178.Fang S., Xu C., Zhang Y., et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-beta/SMAD2 pathway during wound healing. Stem Cells Translational Medicine. 2016;5:1425–1439. doi: 10.5966/sctm.2015-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang L., Hu L., Zhou X., et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Scientific Reports. 2017;7, article 13321 doi: 10.1038/s41598-017-12919-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yates C. C., Rodrigues M., Nuschke A., et al. Multipotent stromal cells/mesenchymal stem cells and fibroblasts combine to minimize skin hypertrophic scarring. Stem Cell Research & Therapy. 2017;8:p. 193. doi: 10.1186/s13287-017-0644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li M., Qiu L., Hu W., et al. Genetically-modified bone mesenchymal stem cells with TGF-beta3 improve wound healing and reduce scar tissue formation in a rabbit model. Experimental Cell Research. 2018;367:24–29. doi: 10.1016/j.yexcr.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 182.Fan D., Xia Q., Wu S., et al. Mesenchymal stem cells in the treatment of Cesarean section skin scars: study protocol for a randomized, controlled trial. Trials. 2018;19:p. 155. doi: 10.1186/s13063-018-2478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.El-Domyati M., Moftah N. H., Nasif G. A., Ragaie M. H., Ibrahim M. R., Ameen S. W. Amniotic fluid-derived mesenchymal stem cell products combined with microneedling for acne scars: a split-face clinical, histological, and histometric study. Journal of Cosmetic Dermatology. 2019;18:1300–1306. doi: 10.1111/jocd.13039. [DOI] [PubMed] [Google Scholar]
- 184.Mushahary D., Spittler A., Kasper C., Weber V., Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93:19–31. doi: 10.1002/cyto.a.23242. [DOI] [PubMed] [Google Scholar]
- 185.Duscher D., Barrera J., Wong V. W., et al. Stem cells in wound healing: the future of regenerative medicine? A Mini-Review. Gerontology. 2016;62:216–225. doi: 10.1159/000381877. [DOI] [PubMed] [Google Scholar]
- 186.Dlouhy B. J., Awe O., Rao R. C., Kirby P. A., Hitchon P. W. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: case report. Journal of Neurosurgery: Spine. 2014;21:618–622. doi: 10.3171/2014.5.SPINE13992. [DOI] [PubMed] [Google Scholar]
- 187.Lukomska B., Stanaszek L., Zuba-Surma E., Legosz P., Sarzynska S., Drela K. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells International. 2019;2019:10. doi: 10.1155/2019/9628536.9628536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Golchin A., Farahany T. Z., Khojasteh A., Soleimanifar F., Ardeshirylajimi A. The clinical trials of mesenchymal stem cell therapy in skin diseases: an update and concise review. Current Stem Cell Research & Therapy. 2019;14:22–33. doi: 10.2174/1574888X13666180913123424. [DOI] [PubMed] [Google Scholar]


