Mechanisms of acute cellular allograft rejection followed liver transplantation employs the activation, proliferation and differentiation of CD4+CD154+ and CD8+CD154+ T cells into effector cells triggered by direct allorecognition pathway, which is performed by both but is dominated by CD4+ T cells, and indirect allorecognition pathway virtually restricted to CD8+ T cells·
Keywords: acute cellular rejection, CD154+ T cells, cell‐mediated immunity (CMI), HLA, immunosuppression, liver transplantation
Summary
Decreasing graft rejection and increasing graft and patient survival are great challenges facing liver transplantation (LT). Different T cell subsets participate in the acute cellular rejection (ACR) of the allograft. Cell‐mediated immunity markers of the recipient could help to understand the mechanisms underlying acute rejection. This study aimed to analyse different surface antigens on T cells in a cohort of adult liver patients undergoing LT to determine the influence on ACR using multi‐parametric flow cytometry functional assay. Thirty patients were monitored at baseline and during 1 year post‐transplant. Two groups were established, with (ACR) and without (NACR) acute cellular rejection. Leukocyte, total lymphocyte, percentages of CD4+CD154+ and CD8+CD154+ T cells, human leukocyte antigen (HLA) mismatch between recipient–donor and their relation with ACR as well as the acute rejection frequencies were analysed. T cells were stimulated with concanavalin A (Con‐A) and surface antigens were analysed by fluorescence activated cell sorter (FACS) analysis. A high percentage of CD4+CD154+ T cells (P = 0·001) and a low percentage of CD8+CD154+ T cells (P = 0·002) at baseline were statistically significant in ACR. A receiver operating characteristic analysis determined the cut‐off values capable to stratify patients at high risk of ACR with high sensitivity and specificity for CD4+CD154+ (P = 0·001) and CD8+CD154+ T cells (P = 0·002). In logistic regression analysis, CD4+CD154+, CD8+CD154+ and HLA mismatch were confirmed as independent risk factors to ACR. Post‐transplant percentages of both T cell subsets were significantly higher in ACR, despite variations compared to pretransplant. These findings support the selection of candidates for LT based on the pretransplant percentages of CD4+CD154+ and CD8+CD154+ T cells in parallel with other transplant factors.
Introduction
Traditionally, the liver had been considered an organ with immunological privilege; however, it is still necessary to further decrease the frequency of rejection, improve graft and patient survival and modulate the immunosuppression (IS) [1, 2], all of which depend upon the patient’s sensitivity to the IS [3, 4].
High doses of IS are given to high immunological risk patients early post‐transplant to overcome the lack of pretransplant assessment for donor‐specific antibodies (DSA) and human leukocyte antigen (HLA) match [5]. Nevertheless, the safety and efficacy of the IS therapy remains a point of improvement in order to avoid immunosuppression‐associated side effects [6].
Extensive evidence indicates the participation of different T cell subsets during acute cellular rejection (ACR) of the allografts in kidney, heart, lung and liver transplantation [7, 8, 9]. Thus, the response after specific recognition of antigenic differences between the donor and the recipient and its relationship with IS has recently begun to be elucidated.
Cell‐mediated immunity (CMI) monitoring in the field of liver transplantation (LT) could provide new insights that may help to identify liver recipients at high immunological risk as well as to understand the pathophysiology underlying ACR [10, 11, 12].
The cellular adaptive immunity is a critical player of the alloresponse against the graft [13, 14] as a consequence of direct cell‐to‐cell contact between naive alloreactive T cells with antigen‐presenting cells (APCs). Subsequently, activated alloreactive T cells could be detected by the expression of different surface antigens with different functions (CD25, CD28, CD38, CD69, CD95 and CD154) [10, 15, 16]. The role of these surface antigens in alloreactive response is crucial to understand allograft injury, and therefore to validate them as predictive and prognosis biomarkers of ACR and post‐transplant outcome, respectively.
To date, numerous methodologies have been evaluated to measure the changes in the expression level of different immune antigens following the initiation of IS therapy to find biomarkers and establish reliable trials to monitor the individual response to IS therapy [17, 18, 19]. For instance, immunophenotyping of different T cell subsets, as well as the assessment of T cell activation status employing flow cytometry (FCM), is well accepted as current practice in transplantation medicine for routine CMI monitoring [20, 21].
Therefore, this study aimed to analyse the expression of CD25, CD38, CD69, CD95 and CD154 on CD4+ and CD8+ T cells in a cohort of patients undergoing LT to determine their influence on ACR using multi‐parametric FCM functional assay in an attempt to identify biomarkers of susceptibility to design new strategies to prevent, detect and reduce the ACR in LT.
Material and methods
Study design
A total of 30 consecutive liver transplant recipients (LTr) were recruited at the University Clinic Hospital Virgen de la Arrixaca (Spain). Socio‐demographic data (age, sex), main liver transplantation indications, post‐transplant complications (ACR) and immunological characteristics were studied (Table 1). All patients were immunologically monitored prior to transplantation when they were on the waiting‐list and followed up for 1 year after transplantation. The study design is represented in Fig. 1.
Table 1.
Socio‐demographic, clinical and immunological characteristics of patients undergoing liver transplantation and its relationship with acute cellular rejection
| Total LTr, N = 30 (%) | ACR n = 12 (%) | NACR n = 18 (%) | OR | 95% CI | P1 | P 2 | |
|---|---|---|---|---|---|---|---|
| Recipient gender, n (%) | |||||||
| Male | 23 (76·7) | 10 (83·3) | 13 (72·3) | 0·52 | 0·27–0·99 | 0·048 | 0·305 |
| Female | 7 (23·3) | 2 (16·7) | 5 (27·8) | ||||
| Age, mean ± s.e.m. (range)* | |||||||
| Recipient | 52·4 ± 1·9 (20–66) | 50·7 ± 3·6 (20–65) | 53·6 ± 2·0 (40–66) | 0·97 | 0·94–0·99 | 0·734 | 0·731 |
| Donor | 59·9 ± 2·8 (24–81) | 58·7 ± 4·8 (24–76) | 60·7 ± 3·4 (25–81) | 0·99 | 0·94–1·04 | 0·884 | 0·810 |
| Main indications for LTr, n (%) | |||||||
| AC | 18 (60) | 5 (41·6) | 13 (72·2) | ||||
| Viral AC** | 6 (20) | 1 (8·3) | 5 (27·8) | ||||
| Non‐viral AC | 12 (40) | 4 (33·3) | 8 (44·4) | 1·06 | 0·57–1–98 | 0·848 | 0·724 |
| HCV cirrhosis | 6 (20) | 4 (33·3) | 2 (11·1) | ||||
| Others*** | 6 (20) | 3 (25·1) | 3 (16·7) | ||||
| Immunosuppression therapy, n (%) | |||||||
| TRL | 17 (56·7) | 7 (58·3) | 10 (55·6) | 1·86 | 1·08–3·21 | 0·026 | 0·803 |
| TRL + MMF | 13 (43·3) | 5 (41·7) | 8 (44·4) | ||||
| HLA‐A mismatch, a n (%) | |||||||
| 0 MM | 0 (0) | 0 (0) | 0 (0) | ||||
| 1 MM | 13 (43·3) | 5 (41·7) | 8 (44·4) | 1·12 | 0·66–1·89 | 0·671 | 0·057 |
| 2 MM | 17 (56·7) | 7 (58·3) | 10 (55·6) | ||||
| HLA‐B mismatch, n (%) | |||||||
| 0 MM | 1 (3·3) | 1 (8·3) | 0 (0) | ||||
| 1 MM | 8 (26·7) | 1 (8·3) | 7 (38·9) | 1·66 | 0·99–2·78 | 0·05 | 0·083 |
| 2 MM | 21 (70) | 10 (83·3) | 11 (61·1) | ||||
| HLA‐DRB1 mismatch, n (%) | |||||||
| 0 MM | 0 (0) | 0 (0) | 0 (0) | ||||
| 1 MM | 11 (36·7) | 3 (25) | 8 (44·4) | 2·40 | 1·36–4·23 | 0·002 | < 0·001 |
| 2 MM | 19 (63·3) | 9 (75) | 10 (55·6) | ||||
| Pretransplant | |||||||
| cellular parameters | |||||||
| Total lymphocyte (%) | 12·9 ± 1·9 | 16·33 ± 2·68 | 7·97 ± 1·89 | 0·97 | 0·94–0·99 | 0·011 | 0·646 |
| Total lymphocyte (cells/mm3) | 1496·67 ± 110·91 | 1265 ± 122·63 | 1651·11 ± 158·04 | 0·99 | 0·99–1·00 | 0·633 | 0·073 |
| Total leukocyte (×109/l) | 6·77 ± 0·69 | 6·81 ± 0·77 | 6·75 ± 1·05 | 0·97 | 0·83–1·22 | 0·968 | 0·742 |
| CD4+CD154+ T cells (%) | 3·06 ± 0·28 | 4·28 ± 0·43 | 2·24 ± 0·21 | 2·58 | 1·31–5·09 | 0·006 | 0·004 |
| CD8+CD154+ T cells (%) | 0·85 ± 0·06 | 0·62 ± 0·08 | 0·99 ± 0·06 | 12·07 | 4·32–33·71 | < 0·001 | <0·001 |
N = total number of individuals; n = number of patients in each group· AC = alcoholic cirrhosis; ACR = acute cellular rejection; CI = confidence interval; HCV = hepatitis C viral infection; HLA = human leukocyte antigen; LTr = liver transplant recipients; MM = mismatch; MMF = mycophenolate mofetil; NACR = non–acute cellular rejection; OR = odds ratio; s.e.m. = standard error of the mean; TRL = tacrolimus. All comparisons were made between ACR and NACR groups.
Age is expressed in years;
HCV infection;
primary biliary cirrhosis, 1 (3·3%); Budd–Chiari syndrome, 1 (3·3%); primary sclerosing cholangitis, 2 (6·7%); cholangiocarcinoma, 2 (6·7%).
P 1 = P‐value obtained comparing total ACR patients versus NACR groups in univariate analysis. P 2 = P‐value obtained from logistic regression multivariable analysis for ACR. P‐values marked in bold type are statistically significant (P ≤ 0·05).
Match or mismatch of HLA alleles between liver recipient–donor pairs. Categorical variables were compared by the two‐sided Fisher’s exact test or two‐sided Pearson’s χ2 test. Continuous variables were compared by the two‐sided Mann–Whitney test and are expressed as mean ± s.e.m.
Fig. 1.
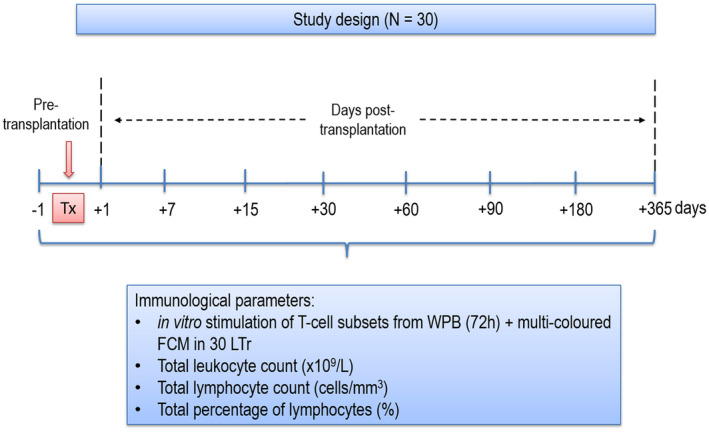
Illustrative chart for the sample collection and immunological determinations in this study. A total of 30 LTr were analysed in this prospective observational study for the expression of CD25, CD38, CD69, CD95 and CD154 antigens on CD4+ and CD8+ T cell at pretransplantation as well as at different time‐points during the first year post‐transplant. WPB samples were obtained by venepuncture in heparin tube for in‐vitro activation of T cells with Con‐A for 72 h. Following cell culture, patient samples were analysed in multi‐stain FCM to determine antigen expression on CD4+ and CD8+ T cells. Different immunological parameters were also obtained using routine Coulter analyser. Tx = transplantation; n = number of patients; WPB = whole peripheral blood; FCM = flow cytometry; LTr = liver transplant recipients; Con‐A = concanavalin A.
In our cohort, 76·7% (n = 23) of patients were male and 23·3% (n = 7) were female. The recipient mean age was 52·4 ± 1·9 (range = 20–66), while the donor mean age was 59·9 ± 2·8 (range = 24–81). All LT conducted in this study came from cadaveric donors.
Pediatric, retransplanted and combined transplant patients were excluded. The inclusion criteria were ABO compatibility, whole liver allograft, immunosuppressive therapy based on tacrolimus (TRL) with or without mycophenolate mofetil (MMF) as well as HIV‐negativity.
All patients gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of HUVA (PI19/01194).
Diagnostic criteria of liver transplant indications
Liver transplantation indications considered in this study were alcoholic cirrhosis (60%) with and without hepatitis infection (20 and 40%, respectively), viral cirrhosis due to hepatitis C infection (HCV) (20%) and other liver diseases, such as primary biliary cirrhosis (3·3%), Budd–Chiari syndrome (3·3%), primary sclerosing cholangitis (6·7%) and cholangiocarcinoma (6·7%) (Table 1). HCV and HBV pre‐infection diagnoses were determined according to Legaz et al., 2016 [2].
Acute cellular rejection diagnosis
The diagnosis of liver ACR was based on conventional clinical, biochemical and histological criteria. The Banff scheme was used for ACR diagnosis and for grading liver rejection [22, 23, 24].
For this study, two study groups were established: with acute cellular rejection (ACR; 40%, n = 12) and without acute cellular rejection (NACR; 60%, n = 18). Episodes of rejection were treated with high‐dose methylprednisolone (one bolus of 500 mg for 3 days).
Immunosuppression therapy
In this study, two groups of patients with different immunosuppressive therapies were analysed as follows: tacrolimus (TRL) in monotherapy (56·7%; n = 17) and TRL with mycophenolate mofetil (MMF) in double immunosuppression therapy (43·3%; n = 13).
Immunosuppression therapy was based in the administration of either TRL (Prograf®, Astellas Pharma, Addlestone, UK) with a target dose of 5 mg/day or MMF (CellCept®; Roche Pharma, Switzerland) with a target dose of 2000 mg/day. All patients in this study were under the same immunosuppressive conditions.
All patients also received methylprednisolone as the main corticosteroids‐based therapy (Dacortin® 20 mg/day) in patients with high immunological risk. Corticoids were gradually reduced to 5 mg/day until the third month post‐transplantation. Given the number of side effects with glucocorticoids, patients with stable graft function attempted to wean from steroids as early as possible.
Sample collection and DNA preparation
Samples were obtained from whole peripheral blood (WPB) or donor spleen. Genomic DNA was extracted using the Maxwell® 16 platform (Promega, Madison, WI, USA), according to the manufacturer’s instructions.
HLA tissue typing
Both patients and donors were Caucasian individuals genotyped for HLA. HLA‐A, ‐B and ‐DRB1 typing were analysed using a polymerase chain reaction sequence‐specific amplification (PCR–SSP) approach (Micro SSP™ Generic Trays; One Lambda, Thermo Fisher Scientific Inc., Waltham, MA, USA). Only HLA‐A, ‐B and ‐DRB1 antigens were studied, because of their higher expression level and high polymorphism. HLA mismatch (MM) between patient and donor was based at the allele level, as opposed to an HLA matching approach, as described previously [25, 26, 27, 28]. HLA mismatches between liver recipient–donor pairs in the rejection direction at any given loci were taken into account for the analysis. The mismatch was defined when an HLA allele carried by the donor was absent in the recipient (D+R−). For instance, a zero antigen mismatch was considered if a donor was homozygous at any given allele (i.e. HLA‐A2, A2) and the recipient was typed as HLA‐A2, A2 or HLA‐A2, A3. Conversely, if the donor was typed as HLA‐A2, A3 for a HLA‐A2, A23 or HLA‐A23, A66 recipients, a single or double mismatch at the HLA‐A locus was considered, grouping the recipients into 1 MM or 2 MM, respectively.
According to this analysis, three groups were established for HLA mismatch; 0 MM corresponded to a fully matched recipient–donor pair for HLA‐A, B and DRB1; 1 MM recipients with a single mismatch either at HLA‐A, ‐B or ‐DRB1 and 2 MM when recipient and donor had two allele mismatches at any given HLA loci (Table 1).
Immunological analysis
Total leukocyte count (×109/l), total lymphocyte count (cells/mm3), total percentage of lymphocytes (%) and CD25, CD38, CD69, CD95 and CD154 antigen expression levels (%) on CD4+ and CD8+ T cells were analysed in all patients in this study. For the analysis, a whole peripheral blood (WPB) sample was taken at pretransplant as well as at different time‐points post‐transplantation (7, 15, 30, 60, 90, 180 and 365 days), as shown in Fig. 1. Leukocyte count, total lymphocyte count and total lymphocyte percentage were always obtained prior to in‐vitro activation, whereas percentages of different T cell subsets were analysed upon stimulation in cell culture.
The WPB was diluted with RPMI‐1640 (1 : 10) (BioWhittaker, Lonza, Belgium) and added to flat‐bottomed 24‐well tissue culture microtitre plates. All WPB samples were polyclonal‐activated with lectin mitogenic concanavalin A (Con‐A), which is known for its ability to interact with certain components of the T cell receptor [29] (1 mg/ml; Sigma‐Aldrich, St Louis, MO, USA), reaching a final concentration of 15 µg/ml and a final volume of 2 ml per well (Fig. 2a).
Fig. 2.
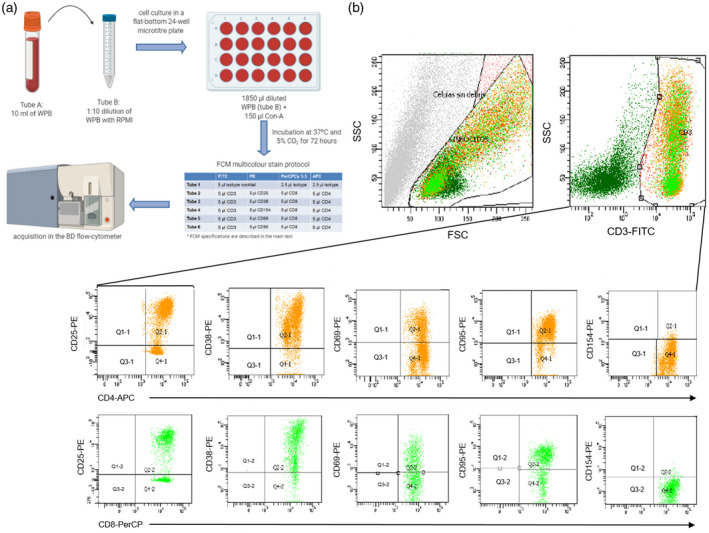
In‐vitro activation of WPB and posterior FACS analysis for the determination of activated CD4+CD154+ and CD8+CD154+ T cells. (a) Scheme of the procedure for the preparation of cells for in‐vitro activation with polyclonal mitogen (Con‐A) and the monoclonal antibody n(mAb) used in FCM assay. Cells were acquired in a BD FACSCanto™ II. (b) Gating strategy for the quantification of stimulated CD4+CD25+, CD4+CD38+, CD4+CD69+, CD4+CD95+, CD4+CD154+ and CD8+CD25+, CD8+CD38+, CD8+CD69+, CD8+CD95+, CD8+CD154+ T cells. Stimulated T cells show an augmented size along the x‐axis (FSC) and a more complex cytoplasm along the y‐axis (SSC) (top left plot). CD3+ activated T cells were delimited in SSC confronted with forward light 1‐FITC (FL1) plot (top right plot). T cell subsets were determined from the CD3+ T cell gate confronting FL2‐PE (anti‐CD25, anti‐CD38, anti‐CD–69, anti‐CD95 and anti‐CD154) against FL3‐PerCP (anti–CD8) and FL4‐APC (anti‐CD4) (bottom plots). From each patient sample at each time‐point, a minimum of 10 000 CD3+ T cells from tube 2 were acquired for the analysis. WPB = whole peripheral blood; RPMI = Roswell Park Memorial Institute (culture medium); Con‐A = concanavalin A; FITC = fluorescein isothiocyanate; PE = phycoerythrin; PerCP‐Cy5·5 = peridinin–chlorophyll–protein complex Cy5·5 conjugate; APC = allophycocyanin; SSC = side‐scatter; FSC = forward‐scatter.
Then, cell cultures were incubated for 72 h at 37°C in a humidified 5% CO2 incubator for in‐vitro cell stimulation (Fig. 2a), following the protocol described by Barten et al. [30]. Upon in‐vitro stimulation, activated WPB samples were analysed in a multi‐color FCM assay using monoclonal antibodies (mAb). Briefly, 600 µl of stimulated samples were centrifuged for 5 min at 390 g. After discarding the supernatant, the pellet was resuspended in 200 µl of phosphate‐buffered saline (PBS). Samples were then incubated with a combination of mAb, according to the manufacturer’s instructions, following erythrocyte lysis by adding 2 ml ×lysis buffer (BD FACS™ Lysing Solution). The panel of mAb included mouse IgG1K anti‐human CD3‐fluorescein isothiocyanate (FITC), CD4‐allophycocyanin (APC), CD8 peridinin–chlorophyll–protein complex Cy5.5 conjugate (PerCP‐Cy5·5), CD25‐phycoerythrin (PE), CD38‐PE, CD69‐PE and CD95‐PE (BD Biosciences BD, San Jose, CA, USA) and mouse IgG1K anti‐human CD154‐PE (Beckman Coulter, Marseille, France).
In all cases, isotype control antibodies were used in order to assess the positivity of each fluorochrome; IgG1‐FITC and IgG1‐PE (Beckman Coulter), mouse IgG1κ‐APC and mouse IgG1κ‐PerCP (BD Biosciences). Medium fluorescence intensity (MFI) was used as a relative measurement of molecule expression.
Finally, patient samples were analysed in a flow cytometer (FACScanto™ II; BD Biosciences). BD FACSDiva software version 6.1.3 was used to analyse the percentage of expression of CD25, CD38, CD69, CD95 and CD154 on CD4+ and CD8+ T cells, as shown in Fig. 2b.
Statistical analysis
Demographic, clinical and immunological data were collected in a database (Microsoft Access 11.0; Microsoft Corporation, Seattle, WA, USA), and statistical analysis was performed using spss version 20·0 software (SPSS Inc., Chicago, IL, USA). Quantitative data were reported as the mean ± standard error of the mean (s.e.m.), whereas qualitative data were reported as absolute and relative frequencies.
To detect differences, Pearson’s χ2 and two‐tailed Fisher’s exact tests were run to compare categorical variables between groups, and Wilcoxon’s and Mann–Whitney tests were used to compare paired and unpaired continues variables, respectively. Multivariable logistic regression analysis was applied to confirm positive associations [31]. A level of P ≤ 0·05 was accepted as statistically significant. Odds ratios (OR) and their 95% confidence intervals (CI) were calculated to estimate relative risk. The Kaplan–Meier method and log–rank test were used to compare between‐group differences [32].
Receiver operating characteristic (ROC) curves were used to identify the optimal cut‐off points for those surrogate biomarkers deemed significant (CD4+CD154+ and CD8+CD154+ T cells) to stratify patients at high risk of ACR. Cut‐off points were calculated based in the best Youden index (sensitivity + specificity – 1) [33]. The area under the ROC curve (AUC) were analysed: an area 0·7–0·8 was considered acceptable; an area of 0·8–0·9, excellent; an area > 0·9, outstanding [34].
Any demographic, clinical and immunological variable statistically significant at the univariate pretransplant cross‐sectional analysis as well as any known variable with clinical importance was finally assessed in a backward stepwise multivariate logistic regression.
Results
Patient enrollment and data acquisition
Donor and recipient socio‐demographic, clinical and immunological variables are given in Table 1, arranged by the presence or absence of ACR. Of the 30 patients, ACR occurred in 40% (n = 12), while the remaining 60% (n = 18) maintained a stable graft function throughout the whole study period. When comparing socio‐demographic characteristics, only recipient gender was statistically significant (P = 0·048) in univariate analysis. There was no further correlation regarding the pretransplant disease or recipient and donor age with ACR. All socio‐demographic did not reach statistical significance in multivariate logistic regression.
The immunosuppression therapy was statistically significant in univariate analysis (P = 0·026), but its effect was not shown to have any impact on the patient outcome when assessed with the multivariate analysis. Moreover, neither leukocyte count (×109/l) nor total count of lymphocytes (cells/mm3) were associated with rejection, whereas a lower percentage of total lymphocytes was shown to be associated with a protective effect against ACR development (16·33 ± 2·68 versus 7·97 ± 1·89; OR = 0·97, 95% CI = 0·94–0·99, P = 0·011).
Presence of HLA class I and class II mismatch between patient and donor increase the risk of acute cellular rejection
Univariate analysis of the effect of HLA‐A, ‐B and ‐DRB1 mismatch between patient and donor on the outcome of LT associated the presence of HLA‐B (OR = 1·66, 95% CI = 0·99–2·78, p = 0·05) and ‐DRB1 (OR = 2·40, 95% CI = 1·36–4·23, P = 0·002) mismatches with an increase in the frequency of ACR. HLA‐DRB1 withstood multivariate analysis as the only remaining statistically significant factor (Table 1; OR = 12·95, 95% CI = 3·18–52·65, P < 0·001). Interestingly, in multivariate analysis, HLA‐A and ‐B mismatch showed borderline significance with detrimental outcome.
Pretransplant distribution of the percentage of CD4+CD154+ and CD8+CD154+ T cells in patients awaiting LT
Prior to transplantation, stratification analysis of the association of activated CD3+ T cells with allograft ACR linked the proportion of CD4+CD154+ and CD8+CD154+ T cells with an increase in the frequency of ACR during the first year post‐transplant. None of the other surface antigens expressed on activated CD3+ T cells reached statistically significant differences between both study groups, ACR and NACR (data not shown). Patients who developed ACR had higher percentages of CD4+CD154+ T cells compared with patients from the NACR study group (Fig. 3a; 4·28 ± 0·43 versus 2·24 ± ·21; P = 0·001). Conversely, the percentage of CD8+CD154+ T cells was significantly lower in patients who developed ACR post‐transplantation (Fig. 3b; 0·62 ± 0·08 versus 0·99 ± ·06, P = 0·002).
Fig. 3.
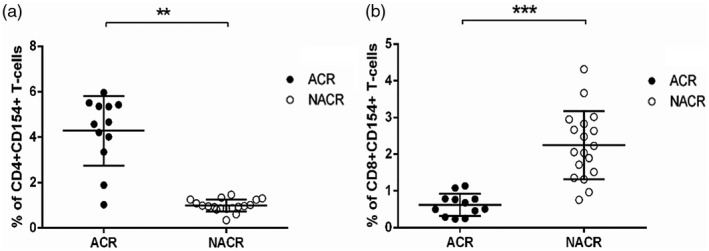
Pretransplant analysis for the expression of CD154 in activated CD4+ and CD8+ T cells in LT patients· (a) Pretransplant percentage of stimulated CD4+CD154+ T cells in ACR and NACR study groups·† (b) Pretransplant percentage of stimulated CD8+CD154+ T cells in ACR and NACR study groups.† ACR = acute cellular rejection; NACR = non‐acute cellular rejection; LT = liver transplant· **P ≤ 0·01· ***P ≤ 0·001· †The stratification analysis at pretransplantation was performed by the two‐sided Mann–Whitney test, where P < 0·05 was considered statistically significant.
Pretransplant percentages of CD4+CD154+ and CD8+CD154+ T cells can distinguish between patients with and without acute cellular rejection
Following stratification analysis, we aimed to study whether the percentages of the two T cell subsets were capable to stratify patients at high risk of ACR. For this purpose, ROC curve analysis was subsequently applied. A pretransplant percentage ≥ 3·18% of CD4+CD154+ T cells (Fig. 4a; AUC = 0·852, 95% CI = 0·682–1·017, P = 0·001) stratified liver patients at high risk of ACR. Similarly, patients with a percentage of CD8+CD154+ T cells ≤ 0·8% (Fig. 4b; AUC = 0·833, 95% CI = 0·671–0·996, P = 0·002) also were at high risk of developing allograft rejection.
Fig. 4.
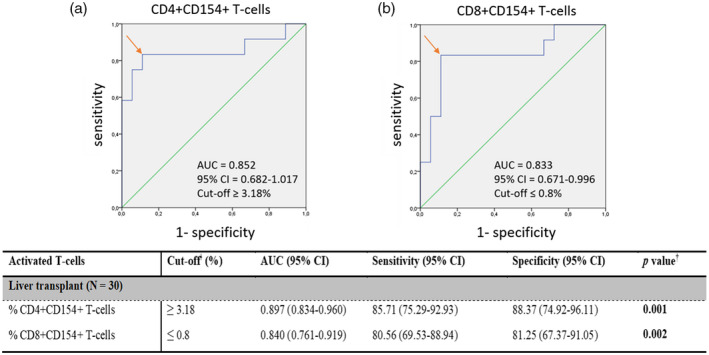
Pretransplant ROC curve analysis, cut‐off, AUC, sensitivity and specificity values for the percentage of activated CD4+CD154+ and CD8+CD154+ T cells in LTr. (a) The AUC˩ value determined a cut‐offǂ ≥ 3·18% of activated CD4+CD154+ T cells that accurately stratified LTr at high risk of ACR. (b) The AUC˩ value determined a cut‐offǂ < 0·8% of activated CD8+CD154+ T cells that accurately stratified LTr at high risk of ACR.‡ AUC = area under the curve; 95% CI = 95% confidence interval; ROC = receiver operating characteristic curve; LTr = liver transplant recipients; ACR = acute cellular rejection. ˩AUC = values considered in this study: 0·7–0·8 as acceptable; 0·8–0·9 as excellent and > 0·9 as outstanding. Both AUC values were considered excellent. ‡Youden index (sensitivity + specificity – 1) was carried out to obtained the most accurate cut‐off values, represented by the arrow, capable to stratify patients at high risk of ACR for the pretransplant percentages of activated CD4+CD154+ and CD8+CD154+ T cells. †Statistically significant P <0·05 is denoted in bold type.
Following observation that the pretransplant percentages of activated CD4+CD154+ and CD8+CD154+ T cells could stratify patients at high risk of ACR, we further analysed the power of these cut‐off values from our proposed predictive biomarkers to determine whether patients in our cohort who were above or below the cut‐off at baseline definitely developed ACR.
For the entire cohort, patients were classified at high risk of ACR either when the percentage of CD4+D154+ T cells was ≥ 3·18% or the percentage of CD8+CD154+ T cells was ≤ 0·8%. Based on these cut‐off values, 60% of LTr were at low risk of rejection, while the remaining 40% were classified as high risk of ACR.
Of the group of patients at low risk, 89% either with a percentage of CD4+CD154+ < 3·18% or CD8+CD154+ T cells > 0·8% did not reject their allograft; the remaining 11% developed ACR regardless of their biomarker values. Conversely, from the group of high‐risk patients, 83% with a biomarker percentage above or below cut‐off levels for CD4+CD154+ T cells for CD8+CD154+ T cells, respectively, developed ACR. The remaining 17% of patients showed stable graft function despite being included into the high‐risk group (sensitivity = 83·33%, 95% CI = 51·59–97·91; specificity = 88·89%, 95% CI = 65·29–98·62; P < 0·001).
HLA mismatched patients with a pretransplant percentage of activated CD4+CD154+ ≥ 3·18% and CD8+CD154+ T cells ≤ 0·8% had an increased risk of acute cellular rejection
Our pretransplant model of rejection was then assessed in logistic regression analysis in order to validate both the percentage of activated CD4+CD154+ and CD8+CD154+ T cells as pretransplant risk factors for ACR. Additionally, demographic, clinical and other immunological variables statistically significant in univariate analysis or those considered relevant were included in this analysis.
Patients who presented a percentage of activated CD4+CD154+ T cells ≥ 3·18% at pretransplant had a 2·58‐fold increase risk to develop ACR (P = 0·006). The impact on ACR based on the pretransplant percentage of CD4+CD154+ T cells remained statistically significant in multivariate analysis (OR = 8·10, 95% CI = 1·95–33·71, P = 0·004). Similarly, the pretransplant percentage of CD8+CD154+ T cells also was shown to be a significant risk factor to a worse post‐transplant outcome (P < 0·001). When the percentage of CD8+CD154+ T cells was analysed in multivariate logistic regression analysis, it was shown as the strongest pretransplant patient risk factor associated with ACR (OR = 22·43, 95% CI = 4·73–88·45, P < 0·001). These data are summarized in Table 1.
At pretransplant, HLA mismatch was shown to have a detrimental effect on the post‐transplant outcome. HLA–DRB1 mismatch between donor and recipient revealed a strong predictive effect towards a higher frequency of ACR (Table 1, OR = 12·95, 95% CI = 3·18–52·65, P < 0·001). HLA‐A and ‐B mismatch demonstrated borderline significance with ACR (P = 0·057 and 0·083, respectively).
The pretransplant percentage of activated CD4+CD154+ and CD8+CD154+ T cells, as well as HLA mismatch, were suggested as important predictive risk factors contributing to post‐transplant cellular rejection in patients awaiting LT.
The pretransplant percentage of CD4+CD154+ and CD8+CD154+ T cells was associated with a higher frequency of acute cellular rejection
The 1‐year probability of ACR for the whole cohort was 12%, with an accumulative probability of ACR‐free time at 12 months of 49%. The average estimated time of ACR was 5 months, ranging from 1·2 to 8·3.
Of the group of patients with ACR, those whose percentage of CD4+CD154+ T cells was ≥ 3·18% had a shorter 1‐year ACR‐free time compare to those with cut‐off values < 3·18% (Fig. 5a; 1‐year ACR‐free time: 85·7 versus 14·3%, P = 0·012). The average estimated time of ACR in this group of patients was 5·9 months, ranging from 3·5 to 8·4.
Fig. 5.
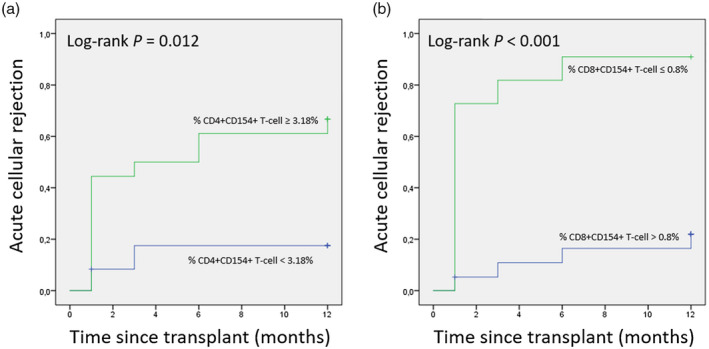
Probability curves of 1‐year ACR‐free time for LTr based on pretransplant percentages of (a) activated CD4+CD154+ T cells and (b) activated CD8+CD154+ T cells. This demonstrates that a significant increase in ACR is associated with liver patients having percentages of activated CD3+ T cell subsets out of range for the calculated cut‐off values by ROC analysis. Groups were compared for 12 months as end‐point. ACR = acute cellular rejection; LTr = liver transplant recipients.
Liver patients with a percentage of CD8+CD154+ T cells ≤ 0·8% also had a shorter 1‐year ACR‐free time compared to those whose cut‐off values were > 0·8% (Fig. 5b; 1‐year ACR‐free time: 71·4 versus 28·6%, P < 0·001). The average estimation time of ACR in this group was 2·6 months, ranging from 0·7 to 4·5.
Post‐transplant monitoring of CD4+CD154+ and CD8+CD154+ T cells in liver transplant recipients
Post‐transplant monitoring of LTr took place at several different time‐points for percentages and absolute numbers of total leukocytes and total lymphocytes as well as total percentages of both activated CD3+ T cells subsets. These data are summarized in Table 2.
Table 2.
Analysis of the percentage and absolute numbers of leukocyte and total lymphocytes as well as percentages of activated CD3+CD154+ T cell subsets during the first year post‐transplantation
| Follow‐up period | LTr (N = 30) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocyte (×109/l) | Total lymphocyte (cells/mm3) | Total lymphocyte (%) | CD4+CD154+ T cells (%) | CD8+CD154+ T cells (%) | |||||||||||
| ACR (n = 12) | NACR (n = 18) | P | ACR (n = 12) | NACR (n = 18) | P | ACR (n = 12) | NACR (n = 18) | P | ACR (n = 12) | NACR (n = 18) | P | ACR (n = 12) | NACR (n = 18) | P | |
| 7 days | 10·75 ± 1·02 | 13·17 ± 1·53 | 0·325 | 1066·38 ± 192·79 | 1766·67 ± 464·14 | 0·249 | 9·49 ± 1·22 | 12·42 ± 2·04 | 0·465 | 2·39 ± 0·56 | 0·87 ± 0·09 | <0·001 | 1·22 ± 0·07 | 0·67 ± 0·09 | < 0·001 |
| 15 days | 9·85 ± 0·97 | 8·94 ± 0·87 | 0·303 | 1125·00 ± 137·69 | 1644·39 ± 514·90 | 0·819 | 11·31 ± 0·72 | 13·77 ± 1·82 | 0·616 | 2·21 ± 0·43 | 0·99 ± 0·11 | 0·01 | 1·25 ± 0·12 | 0·76 ± 0·08 | 0·004 |
| 30 days | 7·31 ± 0·89 | 7·40 ± 0·74 | 0·647 | 1058·34 ± 156·41 | 1977·72 ± 526·29 | 0·346 | 15·83 ± 2·49 | 21·55 ± 2·57 | 0·195 | 1·51 ± 0·23 | 1·13 ± 0·28 | 0·08 | 1·04 ± 0·15 | 1·02 ± 0·10 | 0·819 |
| 60 days | 6·50 ± 0·60 | 6·59 ± 0·59 | 0·605 | 2798·17 ± 987·69 | 3141·78 ± 768·90 | 0·723 | 22·14 ± 4·00 | 27·84 ± 2·32 | 0·367 | 3·78 ± 1·92 | 6·48 ± 2·26 | 0·435 | 3·70 ± 1·12 | 4·60 ± 1·51 | 0·943 |
| 90 days | 5·83 ± 0·81 | 6·13 ± 0·89 | 0·976 | 2789·84 ± 987·72 | 4022·50 ± 912·87 | 0·172 | 24·87 ± 2·83 | 31·05 ± 2·53 | 0·101 | 6·73 ± 2·24 | 4·43 ± 1·79 | 0·222 | 5·37 ± 1·12 | 4·32 ± 1·23 | 0·435 |
| 180 days | 4·77 ± 0·64 | 6·26 ± 0·79 | 0·167 | 5166·25 ± 1237·54 | 4585·78 ± 943·01 | 0·755 | 33·14 ± 2·55 | 31·47 ± 2·89 | 0·592 | 5·43 ± 1·75 | 4·72 ± 1·20 | 0·943 | 6·93 ± 2·59 | 5·13 ± 1·03 | 0·833 |
| 365 days | 5·22 ± 0·66 | 6·96 ± 0·89 | 0·279 | 6574·42 ± 1224·11 | 4671·89 ± 924·95 | 0·439 | 34·40 ± 1·86 | 31·50 ± 3·69 | 0·574 | 8·21 ± 2·71 | 10·79 ± 3·73 | 0·921 | 8·38 ± 4·03 | 8·88 ± 2·29 | 0·921 |
N = total number of individuals; n = number of patients in each group; LTr = liver transplant recipients; ACR = acute cellular rejection; NACR = non‐acute cellular rejection; s.e.m. = standard error of the mean. All comparisons were made between ACR and NACR groups.
P = P‐values obtained by the univariate analysis between total ACR (n = 12) versus NACR (n = 18) liver patient groups. Data were compared by the two‐sided Mann–Whitney test and are expressed as mean ± s.e.m. Statistically significant P‐values are denoted in bold type.
Those LTr who rejected the allograft showed a statistically significant higher proportion of CD4+CD154+ T cells at 7 (2·39 ± 0·56 versus 0·87 ± 0·09, P < 0·001) and 15 (2·21 ± 0·43 versus 0·99 ± 0·11, P = 0·01) days post‐transplantation (Fig. 6a). The proportions of activated CD8+CD154+ T cells were statistically significantly higher in ACR at 7 (1·2 ± 0·07 versus 0·67 ± ·09, P < 0·001) and 15 (1·25 ± 0·12 versus 0·76 ± 0·08, P = 0·04) days post‐transplantation compared to NACR (Fig. 6b). Both T cell populations normalised their levels from day 15 onwards, and differences were not statistically significant by 1 month until the end of the follow‐up period of the study.
Fig. 6.
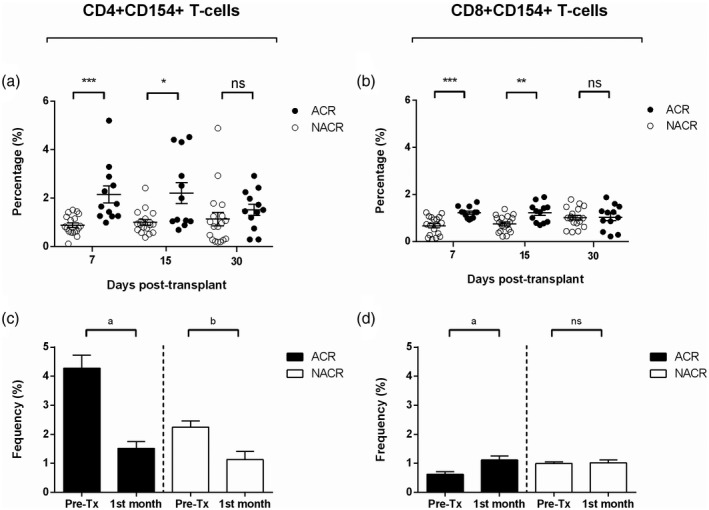
Post‐transplant monitoring of CD4+CD154+ and CD8+CD154+ T cells in LTr with and without ACR. (a) Percentages of CD4+CD154+ T cells between both study groups; black dots refer to ACR and white dots refer to NACR. (b) Percentages of CD8+CD154+ T cells between both study groups; black dots refer to ACR and white dots refer to NACR. (c) Differences in the percentage of CD4+CD154+ T cells between pretransplant and 1 month post‐transplantation; black bars refer to ACR and white bars refer to NACR. (d) Differences in the percentage of CD8+CD154+ T cells between pretransplant and 1 month post‐transplantation; black bars refer to ACR and white bars refer to NACR. ACR = acute cellular rejection; NACR = non‐acute cellular rejection; pre‐Tx = pretransplant· *P ≤ 0·05, **P ≤ 0·01, ***P ≤ 0·001. Differences in the percentage of both activated CD3+ T cell subsets at 7, 15 and 30 days post‐transplantation were analysed by two‐sided Mann–Whitney test, where P ≤ 0·05 was considered statistically significant. a P ≤ 0·05, b P ≤ 0·01. Differences in the percentage of both activated CD3+ T cell subsets between pr–transplant and 1 month post‐transplantation were analysed by two‐sided Wilcoxon’s test, where P ≤ 0·05 was considered statistically significant.
To further investigate the changes in the kinetics of both T cell populations described earlier, post‐transplantation in LTr, pretransplant proportions of CD4+CD154+ and CD8+CD154+ T cells were compared against its levels at 1 month.
Although LTr with ACR showed a higher percentage of CD4+CD154+ T cells at 7 and 15 days post‐transplantation compared to NACR, the proportion of this T cell subset was significantly lower at 1 month than its pretransplant levels in both study groups, ACR (Fig. 6c; 1·51 ± 0·23 versus 4·28 ± 0·44, P = 0·017) and NACR (Fig. 6c; 1·13 ± 0·27 versus 2·25 ± 0·22, P = 0·008).
A comparative analysis assessing the changes in the percentage of CD8+CD154+ T cells between 1 month and pretransplant was performed. Surprisingly, the analysis revealed that this activated CD3+ T cell subset experienced an expansion in ACR from day 1 post‐transplant throughout the following 1 month. The CD8+CD154+ T cells in recipients who rejected the graft had a statistically significant increased proportion when compared against baseline (Fig. 6d; 1·04 ± 0·15 versus 0·62 ± 0·08, P = 0·05). Interestingly, CD8+CD154+ T cells in NACR did not experience this proliferation, remaining similar throughout month 1 (Fig. 6d; 1·02 ± 0·09 versus 0·99 ± 0·06, P = 0·213).
Discussion
The relevance of the immunological characterization in the recipient and donor before LT has been well documented to date, and it might be a key determinant in LT success [35, 36]. However, in a day‐to‐day environment, this practice is not routinely considered in the LTr selection algorithm.
There have been substantial improvements in survival after LT, and IS therapy is largely responsible for this achievement. Nevertheless, LTr are exposed to lifelong IS, therefore substantially affecting patient wellbeing [37, 38]. Furthermore, ACR remains a considerable barrier to the long‐term post‐transplant outcome [14, 39, 40], where CD4 and CD8 T cells have been described as key players in the development of both acute and chronic cellular rejection [41].
In this study, in‐vitro activated CD3+ T cells were monitored for the expression of different surface antigens by means of multi‐parameter FCM assay in a cohort of LTr during 1 year post‐transplant identifying two different subsets significantly associated with ACR, CD4+CD154+ and CD8+CD154+ T cells.
Based on this premise, the main hypothesis of our study was that ACR is associated with the percentage of activated CD4+CD154+ and CD8+CD154+ T cells. Our findings confirmed that percentages of both CD3+ T cell populations correlate with post‐transplant outcome. Although there seem to be differences in the behavior between CD4+CD154+ and CD8+CD154+ T cells, it is clear that both subsets contribute to allograft rejection.
ACR is mainly carried out by allogeneic CD8+ cytotoxic T cells, which can directly recognize donor MHC class I molecules in the allograft inducing tissue injury [42, 43]. Indeed, the proportion of activated CD8+CD154+ T cells in ACR recipients from our cohort experienced a significant proliferation throughout 1 month post‐transplant compared to its levels at baseline. More importantly, this increment was not observed in NACR.
Conversely, activated CD4+ T cells seem to play a more relevant role in chronic cellular rejection through cytokine secretion, as well as inducing alloantibody production [44, 45, 46]. This type of response performs in a more delayed fashion, but equally affects LTr post‐transplant outcome. In our cohort, the pretransplant proportion of CD4+CD154+ T cells were significantly higher in ACR than NACR and this distribution withstood during 1 month, but as opposed to CD8+CD154+, CD4+CD154+ T cells did not experience any expansion.
In this study, the pretransplant percentages of activated CD4+CD154+ and CD8+CD154+ T cells were strongly associated with an increased probability of rejection. Recipients who rejected the graft had a higher percentage of CD4+CD154+ and a lower percentage of CD8+CD154+ T cells before transplantation. The strong association to ACR observed with pretransplant proportions of CD4+CD154+ and CD8+CD154+ T cells was used to build a model based on the most accurate cut‐off values capable to discriminate patients at high risk of rejection. This model showed high sensitivity and specificity, stratifying our patients at high risk of ACR. Patients with pretransplant percentages below the threshold for CD4+CD154+ and CD8+CD154+ T cells had a statistically significantly decreased 1‐year probability of ACR. Therefore, we propose both activated CD3+ T cells as surrogate pretransplant biomarkers.
The post‐transplant changes in the proportions of both activated CD3+ T cell subsets was also studied. The percentage of CD4+CD154+ T cells did not change substantially in rejectors, while CD8+CD154+ T cells experienced a significant proliferation compared to baseline, showing an important expansion along month 1, confirming the current hypothesis that brings forth activated CD8+CD154+ T cells as the first player of ACR [47, 48].
Our results confirm that other parameters also contribute to post‐transplant ACR. In fact, in this study one of the most important risk factors with a significant impact on clinical outcome was HLA matching between recipient and donor. Patients with HLA‐B and ‐DRB1 mismatches had an increased risk of ACR. HLA‐DRB1 mismatch carried the strongest risk, as opposed to HLA‐A and ‐B. Nevertheless, HLA‐A and ‐B mismatches were shown to trend towards increased risk in ACR. These results are in line with previous studies [27, 49, 50].
Recently, the role of both T cell subsets was studied as CMI biomarkers in an attempt to assess their usefulness in transplantation. The CD154+ T cytotoxic memory (TcM), as well as the CD4+CD154+ T helper memory (ThM) subsets, were studied in a cohort of pediatric recipients of small‐bowel transplantation [51] in pediatric recipients of liver allograft [52], as well as in kidney transplantation [53]. All these studies had the same conclusion regarding the role of TcM cells as a surrogate biomarker of ACR. Therefore, this effector memory CD8+CD154+ T cell subset was described as one of the extended driving participators in allograft rejection [54, 55], making it a perfect candidate for CMI monitoring.
The use of CMI has become a common approach in the search for the most appropriate pre‐ and post‐transplant biomarkers [21]. Our group has paid special attention to this strategy for more than a decade, not only to find predictive biomarkers but also to understand how the immune system behaves in heart, kidney and liver transplantation.
In our study, we used an adapted protocol [30] employing in‐vitro WPB stimulation with Con‐A followed by multi‐parameter FCM assay. This methodology, as well as similar approaches, have been applied in different single‐ and multi‐center studies to assess the in‐vitro expression of different T cell antigens and the response to the IS therapy, showing high sensitivity and specificity [10, 16, 56, 57]. These studies concluded that the evaluation of these antigens could be applied to assess the sensitivity of the patient to allograft rejection.
In this paper, in line with previous studies and the current dogma, we have described that the early steps of the ACR carried out by the donor‐allospecific effector‐memory T cell compartment can be detected even prior to transplantation. This T cell compartment is divided into CD4 and CD8 T cells, where each of them has a different role in orchestrating ACR. While CD8 T cells directly kill cells expressing donor HLA molecules on the graft, alloreactive CD4 T cells contribute to rejection by means of cytokine secretion as well as alloantibody production; a process where the CD154–CD40 cross‐over has a pivotal role [58, 59, 60].
Although the liver was traditionally considered an immunologically privileged organ [61], nowadays liver transplantation in sensitized patients would have a detrimental effect on both patient and graft survival [62].
Based on the results obtained from this study, we propose an algorithm that could be used to identify liver patients with high immunological risk awaiting LT. This algorithm will provide accurate information regarding the immune status of the patient before transplantation. With this information available, clinicians may highlight those patients at high immunological risk eligible to receive induction therapy [35, 63, 64, 65]. Importantly, these tests do not need to be performed immediately prior to transplantation, but in a screening fashion. In this regard, DSA screening plus CD4+CD154+ and CD8+CD154+ T cells should first be performed to assess the risk of rejection on a patient’s individual basis. Patient and donor HLA tissue typing also should be performed to identify the number of mismatches.
We believe that using the arsenal of tools currently available in all histocompatibility laboratories, such as HLA typing and DSA screening, together with the implementation of our screening assay for the CD4+CD154+ and CD8+CD154+ T cells in patient selection strategy, may benefit post‐transplant outcome.
For instance, if a candidate for a LT is assessed for ACR employing pretransplant percentages of CD4+CD154+ and CD8+CD154+ T cells, we would be able to stratify recipients either at high or low risk of rejection. This information could help clinicians to decide whether the patient should receive induction therapy as well as modulating the immediate post‐transplantation IS therapy accordingly. Also, the surveillance of both T cell subsets in post‐transplantation could provide valuable input regarding the prognosis of the transplant outcome. The information regarding the humoral alloresponse will also add a broader picture of the risk for a patient to develop an antibody‐mediated rejection.
We are aware of certain limitations derived from the design of our study. One of the major limiting factors is the reduced number of patients included in this study due to a relatively short follow‐up period, although this is a common caveat in unicenter studies in organ transplantation. Furthermore, this relatively small sample size hints at a small proportion of event incidence that may be underpowered to resolve the primary study outcome with clarity. Future, longer and well‐defined prospective studies may aid to clarify the roles of CD4+CD154+ and CD8+CD154+ T cells in ACR and ascertain their usefulness as biomarkers of CMI monitoring in LTr.
In summary, we have demonstrated that pretransplant recipient factors remain critical determinants of outcome in LT, despite the idea of immunological privilege. Specifically, a higher proportion of CD4+CD154+ and lower proportion of CD8+CD154+ T cells at baseline conveys a significant augmented probability of ACR.
We propose the current practice of the implementation of this non‐invasive assay that will accurately stratify patients at high risk of ACR. From our understanding, we highlight the importance of executing an extended algorithm in patient selection strategy for LT. Finally, our results suggest that these biomarkers could be used as an analytical tool to improve the prognosis of the post‐transplant outcome in adult liver recipients.
Disclosures
Consulting fees and/or non–financial support were reported from Diagnostica Longwood (F. B.), Astellas Pharma S.A. (F. B.) and Rafer (F. B.). The remaining authors certify that they have no competing interests in the subject matter or materials discussed in this manuscript.
Author contributions
Conceptualization: F. B. and M. M; methodology implementation: F. B. and R. A.; biomarkers determination and data acquisition: R. A., J. A. G. and H. M. B; data analysis: I. L. and V. J. C.; data validation: F. B., A. M. and M. R. M. Q.; statistical analysis: F. B., C. B., R. R.; clinical data investigation: J. A. C., A. M., P. R., A. M., J. A. P. and M. M.; resources, J. P. M; writing–preparation of original draft: F. B. and M. M.; writing–review and editing: A. M., I. L., F. B. and M. M.; visualization: F. B., A. M. and M. M; supervision: F. B. and M. M; project administration: M. M.; funding acquisition: M. M. All authors have read and agreed to the published version of the manuscript. The authors declare that research data are not shared.
Acknowledgements
All figures and the graphical abstract included in this manuscript were created with BioRender.com. Our work was possible thanks to support from Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Economy and Competitiveness. The authors who participated in this study report grants PI15/01370 and P19/01194 during the conduct of the study. This study was also co‐funded by the European Regional Development Fund (ERDF) with the principle of ‘A manner to build Europe’.
Data sharing statement
The authors declare that research data are not shared.
References
- 1. Legaz I, Bolarin JM, Campillo JA et al Pretransplant ascites or encephalopathy and their influence on survival and liver graft rejection in alcoholic cirrhosis disease. Arch Med Sci 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Legaz I, Navarro–Noguera E, Bolarín JM et al Epidemiology, evolution, and long‐term survival of alcoholic cirrhosis patients submitted to liver transplantation in Southeastern Spain. Alcohol Clin Exp Res 2016; 40:794–805. [DOI] [PubMed] [Google Scholar]
- 3. Pou L, Brunet M, Andres I, Rodamilans M, Lopez R, Corbella J. Influence of posttransplant time on dose and concentration of tacrolimus in liver transplant patients. Transpl Int [internet] 1998; 5:S270–S271. [DOI] [PubMed] [Google Scholar]
- 4. Millán O, Urtasun N, Brunet M. Biomarkers of the immunomodulatory effect of immunosuppressive drugs in transplant recipients. Transplant Rev 2009; 23:120–8. [DOI] [PubMed] [Google Scholar]
- 5. Choudhary NS, Saigal S, Bansal RK, Saraf N, Gautam D, Soin AS. Acute and chronic rejection after liver transplantation: what a clinician needs to know. J Clin Exp Hepatol 2017; 7:358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Durand F. How to improve long‐term outcome after liver transplantation? Liver Int 2018; 38:134–8. Available at: http://doi.wiley.com/10.1111/liv.13651 (accessed 3 May 2020). [DOI] [PubMed] [Google Scholar]
- 7. van de Berg PJEJ, Hoevenaars EC, La Yong S et al Circulating lymphocyte subsets in different clinical situations after renal transplantation. Immunology 2012; 136:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blanco‐García RM, López–Álvarez MR, Garrido IP et al CD28 and KIR2D receptors as sensors of the immune status in heart and liver transplantation. Hum Immunol 2011; 72:841–8. [DOI] [PubMed] [Google Scholar]
- 9. San Segundo D, Brunet M, Ballesteros MA et al Prospective study of biomarkers of immune response in lung transplant recipients. Transpl Proc 2012; 2666–8. [DOI] [PubMed] [Google Scholar]
- 10. Boix F, Millan O, Segundo DS et al High expression of CD38, CD69, CD95 and CD154 biomarkers in cultured peripheral T lymphocytes correlates with an increased risk of acute rejection in liver allograft recipients. Immunobiology 2016; 221:595–603. [DOI] [PubMed] [Google Scholar]
- 11. Boix‐Giner F, Millan O, Segundo DS et al High frequency of central memory regulatory T cells allows detection of liver recipients at risk of early acute rejection within the first month after transplantation. Int Immunol 2016; 28:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Millán O, Benitez C, Guillén D et al Biomarkers of immunoregulatory status in stable liver transplant recipients undergoing weaning of immunosuppressive therapy. Clin Immunol 2010; 137:337–46. [DOI] [PubMed] [Google Scholar]
- 13. Moreau A, Varey E, Anegon I, Cuturi MC. Effector mechanisms of rejection, vol. 3 Cold Spring Harbor Perspectives in Medicine. New York, NY: Cold Spring Harbor Laboratory Press, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wood KJ, Goto R. Mechanisms of rejection: current perspectives. Transplantation 2012; 93:1–10. [DOI] [PubMed] [Google Scholar]
- 15. Boix F, Bolarín JM, Mrowiec A et al CD28 biomarker quantification and expression level profiles in CD4+T–lymphocytes in solid organ transplantation. Transpl Immunol 2017; 42:9–17. [DOI] [PubMed] [Google Scholar]
- 16. Mancebo E, Castro MJ, Allende LM et al High proportion of CD95(+) and CD38(+) in cultured CD8(+) T cells predicts acute rejection and infection, respectively, in kidney recipients. Transpl Immunol 2016; 34:33–41. [DOI] [PubMed] [Google Scholar]
- 17. Oellerich M, Barten MJ, Armstrong VW. Biomarkers: the link between therapeutic drug monitoring and pharmacodynamics. Ther Drug Monit 2006; 28:35–8. [DOI] [PubMed] [Google Scholar]
- 18. Brunet M, Shipkova M, Van Gelder T et al Barcelona consensus on biomarker–based immunosuppressive drugs management in solid organ transplantation, vol. 38 Therapeutic Drug Monitoring. Philadelphia, PA: Lippincott Williams and Wilkins, 2016:S1–20. [DOI] [PubMed] [Google Scholar]
- 19. Barten MJ, Gummert JF. Biomarkers in transplantation medicine: prediction of pharmacodynamic drug effects. Transfus Med Hemother 2007; 34:182–7. [Google Scholar]
- 20. Nickel P, Bestard O, Volk H–D, Reinke P. Diagnostic value of T cell monitoring assays in kidney transplantation. Curr Opin Organ Transplant 2009; 14:426–31. [DOI] [PubMed] [Google Scholar]
- 21. Boix F, Trujillo C, Muro M. Cell‐mediated immunity (CMI) as the instrument to assess the response against the allograft: present and future. Curr Protein Pept Sci 2018; 19:1092–106. [DOI] [PubMed] [Google Scholar]
- 22. Legaz I, López–Álvarez MR, Campillo JA et al KIR gene mismatching and KIR/C ligands in liver transplantation: consequences for short‐term liver allograft injury. Transplantation 2013; 95:1037–44. [DOI] [PubMed] [Google Scholar]
- 23. Demetris AJ, Batts KP, Dhillon AP et al Banff schema for grading liver allograft rejection: an international consensus document. Hepatology 1997; 25:658–63. [DOI] [PubMed] [Google Scholar]
- 24. Mor E, Solomon H, Gibbs JF et al Acute cellular rejection following liver transplantation: clinical pathologic features and effect on outcome. Semin Liver Dis 1992; 12:28–40. [DOI] [PubMed] [Google Scholar]
- 25. Do Nguyen HT, Wong G, Chapman JR et al The association between broad antigen HLA mismatches, Eplet HLA mismatches and acute rejection after kidney transplantation. Transplant Direct 2016; 2:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petersdorf EW. In celebration of Ruggero Ceppellini: HLA in transplantation, vol. 89 HLA. Oxford, UK: Blackwell Publishing Ltd, 2017:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muro M, López‐Álvarez MR, Campillo JA et al Influence of human leukocyte antigen mismatching on rejection development and allograft survival in liver transplantation: is the relevance of HLA‐A locus matching being underestimated? Transpl Immunol 2012; 26:88–93. [DOI] [PubMed] [Google Scholar]
- 28. Ayala García MA, González Yebra B, López Flores AL, Guaní Guerra E. The major histocompatibility complex in transplantation. J Transplant 2012; 2012:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kanellopoulos JM, De Petris S, Leca G, Crumpton MJ. The mitogenic lectin from Phaseolus vulgaris does not recognize the T3 antigen of human T lymphocytes. Eur J Immunol 1985; 15:479–86. [DOI] [PubMed] [Google Scholar]
- 30. Barten MJ, Tarnok A, Garbade J et al Pharmacodynamics of T cell function for monitoring immunosuppression. Cell Prolif 2007; 40:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol 1989; 129:125–37. [DOI] [PubMed] [Google Scholar]
- 32. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–81. [Google Scholar]
- 33. Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3:32–5. [DOI] [PubMed] [Google Scholar]
- 34. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143:29–36. [DOI] [PubMed] [Google Scholar]
- 35. Cillo U, Bechstein WO, Berlakovich G et al Identifying risk profiles in liver transplant candidates and implications for induction immunosuppression. Transplant Rev 2018; 32:142–50. [DOI] [PubMed] [Google Scholar]
- 36. Kueht ML, Cotton RT, Galvan NTN, O’Mahony CA, Goss JA, Rana A. Profiling immunologic risk for acute rejection in liver transplantation: recipient age is an important risk factor. Transpl Immunol 2016; 38:44–9. [DOI] [PubMed] [Google Scholar]
- 37. Levitsky J, O’Leary JG, Asrani S et al Protecting the kidney in liver transplant recipients: practice‐based recommendations from the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant 2016; 16:2532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Watt KDS, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post‐liver transplant: results of the NIDDK long‐term follow‐up study. Am J Transplant 2010; 10:1420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heidt S, San Segundo D, Shankar S et al Peripheral blood sampling for the detection of allograft rejection: biomarker identification and validation. Transplantation 2011; 92:1–9. [DOI] [PubMed] [Google Scholar]
- 40. Germani G, Rodriguez‐Castro K, Russo FP et al Markers of acute rejection and graft acceptance in liver transplantation. World J Gastroenterol 2015; 21:1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gras S, Kjer‐Nielsen L, Chen Z, Rossjohn J, McCluskey J. The structural bases of direct T cell allorecognition: implications for T cell‐mediated transplant rejection. Immunol Cell Biol 2011; 89:388–95. [DOI] [PubMed] [Google Scholar]
- 42. DeWolf S, Sykes M. Alloimmune T cells in transplantation. J Clin Invest 2017; 127:2473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Millán O, Rafael‐Valdivia L, Torrademé E et al Intracellular IFN‐γ and IL‐2 expression monitoring as surrogate markers of the risk of acute rejection and personal drug response in de novo liver transplant recipients. Cytokine 2013; 61:556–64. [DOI] [PubMed] [Google Scholar]
- 44. Nagy ZA. Alloreactivity: an old puzzle revisited. Scand J Immunol 2012; 75:463–70. [DOI] [PubMed] [Google Scholar]
- 45. Le Moine A, Goldman M, Abramowicz D. Multiple pathways to allograft rejection. Transplantation 2002; 73:1373–81. [DOI] [PubMed] [Google Scholar]
- 46. Jucaud V. The immunogenicity of HLA class II mismatches: the predicted presentation of nonself allo‐HLA‐derived peptide by the HLA‐DR phenotype of the recipient is associated with the formation of DSA. J Immunol Res 2017; 2017:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Harper SJF, Ali JM, Wlodek E et al CD8 T cell recognition of acquired alloantigen promotes acute allograft rejection. Proc Natl Acad Sci USA 2015; 112:12788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Halamay KE, Kirkman RL, Sun L et al CD8 T cells are sufficient to mediate allorecognition and allograft rejection. Cell Immunol 2002; 216:6–14. [DOI] [PubMed] [Google Scholar]
- 49. Yagihashi A, Kobayashi M, Noguchi K et al HLA matching effect in liver transplantation. Transplant Proc 1992; 24:2432–3. [PMC free article] [PubMed] [Google Scholar]
- 50. Balan V, Ruppert K, Demetris AJ et al Long‐term outcome of human leukocyte antigen mismatching in liver transplantation: results of the National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation database. Hepatology 2008; 48:878–88. [DOI] [PubMed] [Google Scholar]
- 51. Ashokkumar C, Gupta A, Sun Q et al Allospecific CD154+ T cells identify rejection‐prone recipients after pediatric small‐bowel transplantation. Surgery 2009; 146:166–73. [DOI] [PubMed] [Google Scholar]
- 52. Ashokkumar C, Talukdar A, Sun Q et al Allospecific CD154+ T cells associate with rejection risk after pediatric liver transplantation. Am J Transplant 2009; 9:179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ashokkumar C, Shapiro R, Tan H et al Allospecific CD154+ T‐cytotoxic memory cells identify recipients experiencing acute cellular rejection after renal transplantation. Transplantation 2011; 92:433–8. [DOI] [PubMed] [Google Scholar]
- 54. Kirk AD, Blair PJ, Tadaki DK, Xu H, Harlan DM. The role of CD154 in organ transplant rejection and acceptance. Phil Trans R S B Biol Sci 2001; 356:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Benichou G, Gonzalez B, Marino J, Ayasoufi K, Valujskikh A. Role of memory T cells in allograft rejection and tolerance. Front Immunol 2017; 28:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Härtel C, Schumacher N, Fricke L, Ebel B, Kirchner H, Müller‐Steinhardt M. Sensitivity of whole‐blood T lymphocytes in individual patients to tacrolimus (FK 506): impact of interleukin‐2 mRNA expression as surrogate measure of immunosuppressive effect. Clin Chem 2004; 50:141–51. [DOI] [PubMed] [Google Scholar]
- 57. Boleslawski E, Benothman S, Grabar S et al CD25, CD28 and CD38 expression in peripheral blood lymphocytes as a tool to predict acute rejection after liver transplantation. Clin Transplant 2008; 22:494–501. [DOI] [PubMed] [Google Scholar]
- 58. Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T‐helper and a T‐killer cell. Nature 1998; 393:474–8. [DOI] [PubMed] [Google Scholar]
- 59. Aversa G, Punnonen J, Carballido JM, Cocks BG, de Vries JE. CD40 ligand–CD40 interaction in Ig isotype switching in mature and immature human B cells. Semin Immunol 1994; 6:295–301. [DOI] [PubMed] [Google Scholar]
- 60. Cerutti A, Zan H, Schaffer A et al CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+IgD+ B cell line. J Immunol 1998; 160:2145–57. [PMC free article] [PubMed] [Google Scholar]
- 61. Levitsky J. Does the liver provide immunosuppressive advantage? Clin Liver Dis 2019; 13:180–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Legaz I, Boix F, López M et al Influence of preformed antibodies in liver transplantation. J Clin Med 2020; 9:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kubal CA, Mangus RS, Saxena R et al Crossmatch‐positive liver transplantation in patients receiving thymoglobulin–rituximab induction. Transplantation 2014; 97:56–63. [DOI] [PubMed] [Google Scholar]
- 64. Pillai AA, Levitsky J. Overview of immunosuppression in liver transplantation. World J Gastroenterol 2009; 15:4225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bittermann T, Hubbard RA, Lewis JD, Goldberg DS. The use of induction therapy in liver transplantation is highly variable and is associated with posttransplant outcomes. Am J Transplant 2019; 19:3319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that research data are not shared.


