Bile from patients with chronic liver diseases contains antigens that activate natural killer T cells, an important subgroup of liver lymphocytes . Activation of this immunological pathway suggests that bile antigens may be important in inflammatory bile duct diseases.
Keywords: biliary microbiota, CD1d, cholangiocyte, PBC, PSC
Summary
Natural killer T (NKT) cells are an abundant subset of liver lymphocytes activated by lipid antigens presented on CD1d molecules that are expressed by cholangiocytes. We aimed to determine if bile from patients with chronic liver diseases contains antigenic lipids that can activate NKT cells. Using murine invariant (24.7, 24.8 and DN32.D3) and non‐invariant (14S.6, 14S.7 and 14S.10) NKT hybridomas we investigated the presence of lipid antigens in bile collected from the gallbladder of patients undergoing liver transplantation due to end‐stage liver disease. Biliary microbiota profiles were generated using 16S rRNA amplicon sequencing. We found that the patient bile samples contain antigens that activate both invariant and non‐invariant NKT hybridomas (24.7, 24.8, DN32.D3, 14S.6, 14S.7 and 14S.10), as demonstrated by activation of at least one hybridoma by eight of 10 bile samples. Activation at high dilutions suggests that some antigens are highly potent. We used the non‐invariant NKT hybridoma 14S.6 to screen 21 additional patient bile samples for NKT‐reactivity and demonstrated that 12 of 21 bile samples resulted in activation, three of which gave a strong activation. Four of 12 activating bile samples contained microbial DNA. Our results reveal an immunological pathway that could be of critical importance in biliary immunology.
Introduction
Inflammatory bile duct disorders such as primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) are major causes of liver‐related morbidity and mortality due to limited treatment options and a chronic and progressive disease course [1, 2]. The etiology and pathophysiology of these disorders are complex and believed to result from multiple environmental and genetic factors, but the exact pathophysiological processes remain unknown. Genome‐wide association studies of PSC and PBC have demonstrated strong associations to genetic variants within the human leukocyte antigen (HLA) region, and most uncovered risk loci are also associated with other immune‐mediated diseases [2, 3]. Environmental risk factors for PBC development include smoking, multiple urinary tract infections and hormone replacement therapy, whereas in PSC smoking and coffee consumption are probably disease‐protective [2, 4].
Natural killer T (NKT) cells are an abundant subpopulation of lymphocytes in human and murine livers that are activated by lipid antigens presented on CD1d, a non‐classical major histocompatibility complex (MHC) class I molecule [5]. NKT cells are commonly classified according to their T cell receptor (TCR) as invariant NKT (iNKT) cells, expressing a semi‐invariant TCR or non‐invariant NKT (niNKT) cells with a more diverse TCR repertoire [6]. NKT cells are important immunomodulators that bridge innate and adaptive immunity. They direct immune responses with recruitment and activation of other immune cells by rapid production of either pro‐ or anti‐inflammatory cytokines [7]. NKT cells may have protective or detrimental effects in response to microorganisms or in various liver diseases, as demonstrated in animal models of autoimmune hepatitis (AIH) with interleukin (IL)‐4‐mediated inflammation and anti‐inflammatory effects through production of IL‐17 [5, 8, 9, 10]. They are also found to promote disease in a PBC‐like disease model after activation by a microbial‐derived CD1d‐restricted lipid antigen in mice [11]. NKT cells are enriched in livers of patients with non‐alcoholic steatohepatitis‐related fibrosis and may promote steatosis and contribute to progression from steatohepatitis to hepatocellular carcinoma [12, 13]. NKT cells can also sense modified self‐lipids in hepatitis B virus (HBV)‐infected hepatocytes and reinforce the anti‐viral T and B cell immune response in HBV infection [14].
The main biological roles of cholangiocytes are protection against the toxic effects of bile and modification of its composition [15]. They also participate in liver injury, biliary inflammation, fibrogenesis, regeneration and repair through activation by endogenous or exogenous stimuli (e.g. microorganisms, drugs and xenobiotics) [16]. The expression level of CD1d on cholangiocytes correlates with liver disease severity, which suggests that NKT cells play a role in disease pathogenesis [17, 18]. We have previously shown that cholangiocytes function as antigen‐presenting cells (APCs) with activation of NKT cells in vitro [18]. Intrabiliary injection of the NKT cell‐activating agent oxazolone cause CD1d‐restricted inflammation in the bile ducts and suggest that cells in the bile duct function as APCs in vivo [19]. A potential role of NKT cells in biliary inflammation is further supported by the discovery of activated and more numerous hepatic NKT cells in a mouse model of spontaneous bile duct inflammation [20].
Bile is rich in lipids and contains several classes of lipids known to activate NKT cells, but to our knowledge a direct immunological role of such lipid antigens has not been demonstrated [21]. Herein, we report the probable presence of antigens in bile that are potentially of host and microbial origin which are capable of activating NKT cells implicating a potential role in biliary immunopathology.
Materials and methods
Patient bile samples
Bile was collected from the gallbladder of explanted livers directly after liver transplantation due to PSC (n = 15), PBC (n = 1), alcoholic cirrhosis (n = 3), acute intermittent porphyria (n = 1), hemochromatosis (n = 1), cryptogenic cirrhosis (n = 2), AIH (n = 5) or hepatocellular carcinoma (n = 3). A sterile scalpel was used to cut a small opening in the gallbladder and a minimum of 3 ml of bile was aspirated into sterile tubes with a sterile 20‐ml syringe, stored and aliquoted on ice until long‐term storage at −80°C. Written informed consent was obtained from all study participants. Ethical approval was obtained from the regional Committees for Medical and Health Research Ethics of South East Norway (reference no.2012‐286 and 2016‐1540), in accordance with the Declaration of Helsinki.
Cell lines
Murine iNKT (DN32.D3, 24.7 and 24.8) and niNKT (14S.6, 14S.7 and 14S.10) hybridomas were used to evaluate the presence of potential NKT cell‐activating antigens in bile [22, 23]. Stable CD1d‐transfected murine fibroblast L‐cells (L‐CD1d) were used as APCs in co‐culture assays [24]. All cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) high glucose supplemented with 10% fetal bovine serum and 1% gibco antibiotic–anti‐mycotic (10 000 units/ml penicillin, 10 000 μg/ml streptomycin and 25 μg/ml amphotericin B) (ThermoFisher Scientific, Waltham, MA, USA) and maintained in 37°C incubators with 5% CO2.
Plate‐based antigen presentation assay
A plate‐based assay was used to screen human bile samples for lipid antigens that may bind to CD1d and activate NKT hybridomas [25]. Cell culture plates were incubated overnight with 2·5 μg/ml murine CD1d monomers (National Institutes of Health Tetramer Core, Emory, GA, USA) in phosphate‐buffered saline (PBS) before unbound monomers were washed off. Bile samples in various dilutions were added to the coated cell culture plates followed by another overnight incubation. A synthetic analog (i.e. KRN 7000; Avanti Polar Lipids, Alabaster, AL, USA) of α‐galactosylceramide (α‐GalCer) was used as a positive control. Unbound lipids were washed off with PBS followed by addition of NKT hybridomas (105 cells per well). Supernatants were collected after 16 h incubation and IL‐2 was measured by enzyme‐linked immunosorbent assay (ELISA) (BD Biosciences, Franklin Lakes, NJ, USA).
Co‐culture assay
L‐CD1d cells were plated onto 96‐well flat‐bottomed plates (104 cells per well) and incubated with bile samples diluted in supplemented cell culture medium for 4 h before the plates were washed three times with DMEM. NKT hybridomas were then seeded onto the plates (105 cells per well) and incubated overnight, followed by centrifugation and collection of supernatant and measurement of IL‐2 by ELISA (BD Biosciences). In CD1d‐blocking experiments, 0·025 mg/ml of the monoclonal anti‐CD1d antibody clone 19G11 (BioXCell, West Lebanon, NH, USA) or an isotype control clone LTF‐2 (BioXCell) were used.
Bacterial DNA extraction
DNA from patient bile samples was extracted using QIAamp DNA mini kit (Qiagen, Hilden, Germany), as previously described [26]; 150 μl of each bile sample was resuspended in 1 ml InhibitEX buffer (Qiagen), vortexed and incubated at 95°C for 5 min followed by centrifugation at 21 500 g for 1 min to remove polymerase chain reaction (PCR) inhibitors. The pellets were then resuspended in 180 μl lysozyme solution [20 mg/ml lysozyme in 20 mM Tris HCl, pH 8.0; 2 mM ethylenediamine tetraacetic acid (EDTA); 1·2% Triton X‐100], 20 μl proteinase K and 200 μl of AL buffer (Qiagen). Homogenization was performed for 30 s in a bead beater (BioSpec Products, Bartlesville, OK, USA) with the addition of 0·5 g of 0·1‐mm Zirconia beads (BioSpec Products) and four 3‐mm Zirconia beads (PSP spin stool DNA kit; Stratec, Berlin, Germany). The samples were finally incubated at 70°C for 10 min before cleaning and elution of the extracted DNA using filter columns from the QIAamp DNA mini kit (Qiagen), according to the manufacturer’s protocol. DNA extracts were checked for the presence of high molecular DNA and purity using regular gel electrophoresis and Nanodrop (ThermoFisher Scientific).
Library preparations and sequencing
Libraries for sequencing were generated using barcoded primers in a dual‐indexing approach, as previously described [27]. The hypervariable regions V3 and V4 of the prokaryotic 16S rRNA gene were amplified using Phusion high‐fidelity (HF) PCR master mix with HF buffer (ThermoFisher Scientific). Cleaning and normalization of PCR products were performed using the SequalPrep Normalization Plate Kit (ThermoFisher Scientific). Quality control and quantification of pooled libraries were performed using Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and the Kapa Library Quantification Kit (Kapa Biosystems, London, UK). Sequencing was performed at the Norwegian Sequencing Centre (Oslo, Norway) applying the Illumina MiSeq platform and the version 3 kit from Illumina (Illumina, San Diego, CA, USA), which allows 300 base pairs paired‐end reads [28].
Bioinformatic processing
Remaining Illumina Universal Adapters and PhiX sequences added during sequencing were discarded using bbduk version 38.25 (https://sourceforge.net/projects/bbmap/) (parameters adaptor filter: k = 23 hdist = 1 tbo cf = TRUE ftm = 5; parameters phix filter: k = 31 hdist = 1), followed by sorting and separation of bacterial sequences according to sample IDs with je version 1.2 (parameters: MAX_MISMATCHES = 1 MIN_MISMATCH_DELTA = 2) [29]. Cutadapt version 1.18 [30] was subsequently used to trim indexes, heterogeneity spacers and primers added during PCR to be able to demultiplex reads (parameters: −overlap 20–discard‐untrimmed‐m 250‐j 0). The trimmed paired‐end reads were then quality trimmed and merged into single reads with bbmerge version 38.25 (parameters: qtrim = rl trimq = 15 maxlength = 440 mininsert = 390) [31]. Quality control, trimming to 400 base pairs and denoising of the merged reads were performed using the deblur plugin [32] in the Quantitative Insights Into Microbial Ecology (qiime)2 platform (version 2019.7) [33] to obtain amplicon sequence variants (ASVs). The ASVs were classified in qiime2 [34] with a naive Bayes classifier trained on the V3–V4 region of a preclustered version (99% sequence similarity) of the silva database version 132 [35]. In each bile sample, taxa with number of reads below 100 were discarded. There were no detectable levels of bacteria in the negative controls, and therefore no identified contaminants were removed from the data set before further analyses were performed.
Statistical analysis
All values are presented as mean ± standard error of the mean (s.e.m.) unless otherwise stated. Statistical significance was evaluated using Student’s t‐test for variables meeting the criteria of normal distribution. For experiments where multiple comparisons were included, one‐way analysis of variance (anova) was used followed by correction for multiple testing using Bonferroni’s method. P‐values below 0·05 were considered statistically significant. All statistical tests were performed using GraphPad Prism version 8.2 (GraphPad Software, La Jolla, CA, USA).
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Bile from patients with liver diseases contains antigens that activate NKT cells
To evaluate the presence of NKT cell‐activating antigens in human bile we used assays with plate‐bound CD1d [25] and screened bile samples from a panel of 10 patients with various liver diseases (labeled panel A; Table 1). Three iNKT and three niNKT hybridomas were used in these assays [22, 23] and eight of 10 bile samples activated at least one of the hybridomas, as demonstrated by increased levels of IL‐2 in the culture supernatant (Fig. 1). All the tested NKT hybridomas were activated by at least four bile samples, and the response varied markedly between the hybridomas for each bile sample. This diverse activation pattern suggests the presence of overlapping and diverging biliary antigens rather than a single antigen, such as a constitutively present physiological bile compound. This was further supported by the observation that two of the bile samples did not activate any of the NKT hybridomas, which rules out the ubiquitous presence of NKT cell‐activating antigens in bile.
Table 1.
Clinical characteristics of bile sample donors in panel A. Liver disease duration was defined as time from the first deviation of biochemical liver tests to the time of transplantation. Biochemistry values represent the values just prior to liver transplantation.
| Bile sample no. | Sex | Diagnosis | Liver cirrhosis | Disease duration (years) | Variables at time of transplantation | |||
|---|---|---|---|---|---|---|---|---|
| Bilirubin mg/dl | ALT U/l | ALP U/l | CRP mg/l | |||||
| A1 | M | PSC | No | 7·8 | 0·6 | 61 | 407 | 72 |
| A2 | M | Cryptogenic cirrhosis | Yes | 4·8 | 4·3 | 33 | 121 | 27 |
| A3 | M | Alcoholic cirrhosis, HCC | Yes | 0·8 | 0·8 | 28 | 92 | 1·1 |
| A4 | F | AIH | Yes | 15 | 0·6 | 266 | 152 | 0·77 |
| A5 | M | PSC, HCC | Yes | 31·8 | 0·9 | 44 | 142 | 2 |
| A6 | F | PSC | No | 2·6 | 0·4 | 31 | 69 | 1·1 |
| A7 | F | PSC | Yes | 11·6 | 19·1 | 214 | 663 | 16 |
| A8 | F | Cryptogenic cirrhosis, HCC | Yes | 5·8 | 2·4 | 38 | 184 | 1·6 |
| A9 | F | Cryptogenic cirrhosis | Yes | 7·8 | 6·3 | 41 | 140 | 1·4 |
| A10 | M | PSC | Yes | 5·7 | 1·4 | 104 | 238 | 15 |
PSC = primary sclerosing cholangitis; HCC = hepatocellular carcinoma; AIH = autoimmune hepatitis; ALT = alanine aminotransferase; ALP = alkaline phosphatase; INR = international normalized ratio; CRP = C‐reactive protein.
Fig. 1.
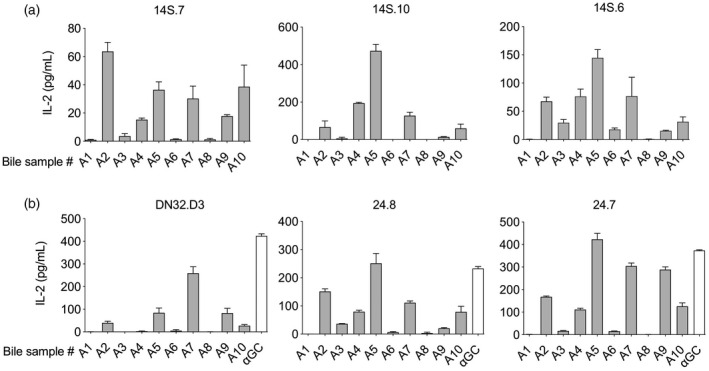
Human bile contains natural killer T (NKT) cell‐activating antigens. Bile samples from 10 patients with liver disease (A1–A10) were screened for NKT cell‐activating antigens in a CD1d‐coated plate assay with three invariant (DN32.D3, 24.8 and 24.7) and three non‐invariant (14S.7, 14S.10 and 14S.6) murine NKT cell hybridomas. All invariant (a) and non‐invariant (b) NKT hybridomas were activated by bile antigens and eight of 10 bile samples activated at least one of the hybridomas as indicated by an increased interleukin (IL)‐2‐production, with alpha‐galactosylceramide (αGC) as a positive control for the invariant NKT hybridomas. Representative results from two independent experiments are shown, presented as mean ± standard error of the mean (s.e.m.).
NKT‐activating antigens in bile are potent
To evaluate the potency of the bile antigens and to rule out non‐specific effects we performed serial dilution of bile samples to test for a dose–response relationship between NKT‐activation and bile antigens. We used the niNKT hybridomas 14S.10 and 14S.6 (Fig. 1) that have been previously reported to be activated by both self‐ and microbial‐derived lipids [23, 36]. We observed a clear dose–response relationship for the seven bile samples tested in dilutions from 1 : 100 to 1 : 1 000 000 (Fig. 2).
Fig. 2.
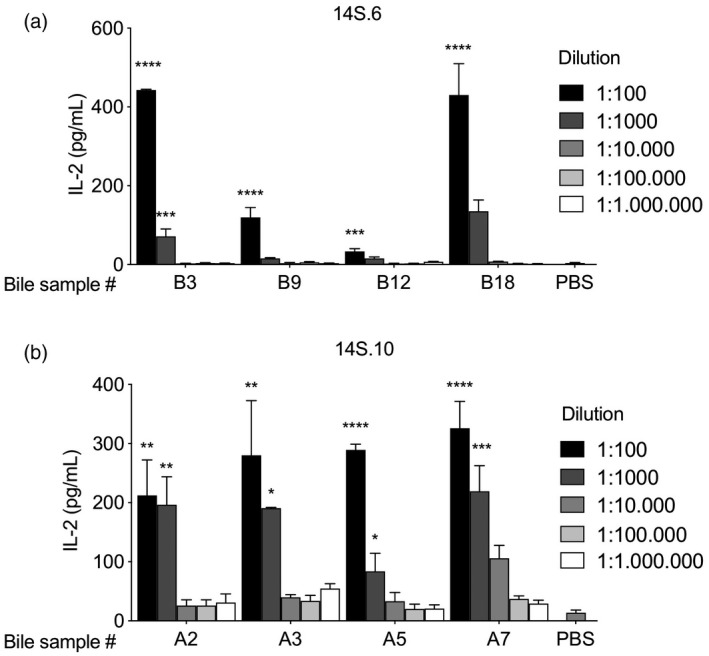
Bile antigens activate natural killer T (NKT) cells in a dose–response manner. Eight human bile samples from patients with liver disease were added in serial dilutions from 1 : 100 to 1 : 1.000 000 in a plate assay with murine CD1d monomers. Activation of the murine non‐invariant NKT hybridoma 14S.6 (a) and 14S.10 (b) are indicated by an increase of interleukin (IL)‐2 production. Representative results from two independent experiments are shown, presented as mean ± standard error of the mean (s.e.m.). Statistical significance was calculated by one‐way analysis of variance (anova) followed by correction for multiple testing using Bonferroni’s method within each bile sample in serial dilutions including the PBS control.Prepresents statistical difference between the individual bile samples compared to phosphate‐buffered saline (PBS).*P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001.
NKT cell antigens are frequently found in bile
To evaluate the frequency of NKT cell‐activating antigens in bile we tested bile samples from a second panel of 21 independent patients with various liver diseases (labeled panel B; Table 2) and used CD1d plate‐bound assays with NKT hybridomas, as previously described. Bile from 12 of 21 (57%) patients activated the niNKT hybridoma 14S.6, three of which (i.e. patients B3, B9 and B18) elicited a particularly high IL‐2‐response (Fig. 3). These bile samples originated from patients who were transplanted due to end‐stage PSC (i.e. bile samples B3 and B9) or AIH (i.e. bile sample B18) (Supporting information, Table S1).
Table 2.
Clinical characteristics of bile sample donors in panel B. Liver disease duration was defined as time from the first deviation of biochemical liver tests to the time of transplantation. Biochemistry values represent the values just prior to liver transplantation.
| Bile sample no. | Sex | Diagnosis | Liver cirrhosis | Disease duration (years) | Variables at time of transplantation | |||
|---|---|---|---|---|---|---|---|---|
| Bilirubin mg/dl | ALT U/l | ALP U/l | CRP mg/l | |||||
| B1 | M | PSC | No | 7·3 | 1·1 | 76 | 419 | 2·9 |
| B2 | M | PSC | No | 1·1 | 9·2 | 400 | 1520 | 4·2 |
| B3 | M | PSC | Yes | 18·1 | 2·4 | 171 | 473 | 11 |
| B4 | M | PSC | No | 7·7 | 3·2 | 358 | 635 | 11 |
| B5 | M | PSC | Yes | 8·2 | 0·6 | 229 | 337 | 3·5 |
| B6 | M | PSC | No | 8·1 | 1·5 | 71 | 249 | 6·1 |
| B7 | F | PSC | Yes | 17·4 | 17·6 | 61 | 307 | 174 |
| B8 | F | PSC | No | 4·2 | 1 | 64 | 193 | 5·7 |
| B9 | M | PSC | Yes | 11·1 | 1·2 | 128 | 344 | 3·1 |
| B10 | F | PSC | Yes | 14·3 | 3·2 | 82 | 325 | 1·7 |
| B11 | F | PSC | Yes | 25·8 | 0·3 | 38 | 107 | 4·9 |
| B12 | F | PBC | No | 1·8 | 1 | 89 | 413 | 2·7 |
| B13 | M | Hemochromatosis | Yes | 1·3 | 20·3 | 40 | 130 | 17 |
| B14 | F | AIH | Yes | 14·3 | 3·1 | 56 | 106 | 13 |
| B15 | F | AIH | Yes | 43·7 | 1 | 23 | 95 | 12 |
| B16 | M | Alcoholic cirrhosis | Yes | 2·2 | 1 | 25 | 205 | 37 |
| B17 | M | Alcoholic cirrhosis | Yes | 8 | 1·3 | 48 | 255 | 8·9 |
| B18 | F | AIH | Yes | 31·4 | 0·5 | 133 | 135 | 6·1 |
| B19 | F | AIP | No | 5 | 0·6 | 10 | 41 | 3·2 |
| B20 | M | Alcoholic cirrhosis | Yes | 2·5 | 1·8 | 22 | 111 | 36 |
| B21 | F | AIH | Yes | 50·8 | 39·7 | 78 | 52 | 41 |
PSC = primary sclerosing cholangitis; PBC = primary biliary cholangitis; AIP = acute intermittent porphyria; AIH = autoimmune hepatitis; ALT = alanine aminotransferase; ALP = alkaline phosphatase; INR = international normalized ratio; CRP = C‐reactive protein.
Fig. 3.
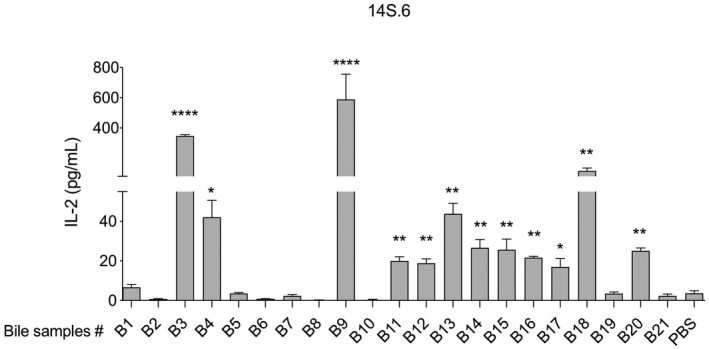
Natural killer T (NKT) cell‐activating antigens are commonly found in human bile. Twelve of twenty‐one bile samples collected from patients with liver disease activated the murine non‐invariantNKT hybridoma 14S.6, as demonstrated by an increased interleukin (IL)‐2‐production compared with phosphate‐buffered saline (PBS). Representative results from two independent experiments are shown, presented as mean ± standard error of the mean (s.e.m.). Statistical significance was calculated using the unpaired Student’st‐test.*P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001.
Uptake and CD1d‐presentation of bile antigens by antigen‐presenting cells
After having demonstrated the presence of NKT‐activating bile antigens, we aimed to explore whether these antigens could be taken up by APCs and presented on CD1d. The L‐CD1d cell was used as an APC in co‐culture assays with the niNKT hybridoma 14S.6 and incubated with the three most potent bile samples identified in previous experiments. All three bile samples activated the NKT hybridoma in a dose‐dependent manner, as demonstrated by an increased IL‐2‐response (Fig. 4a). Activation of the NKT hybridoma was entirely CD1d‐dependent for two of the three bile samples with abolishment of IL‐2 production upon blocking with a CD1d monoclonal antibody (19G11), compared to isotype control (Fig. 4b). For the third bile sample, CD1d‐blocking only partly reduced the IL‐2 production, which suggests that activation of the NKT hybridoma was not entirely CD1d‐dependent.
Fig. 4.
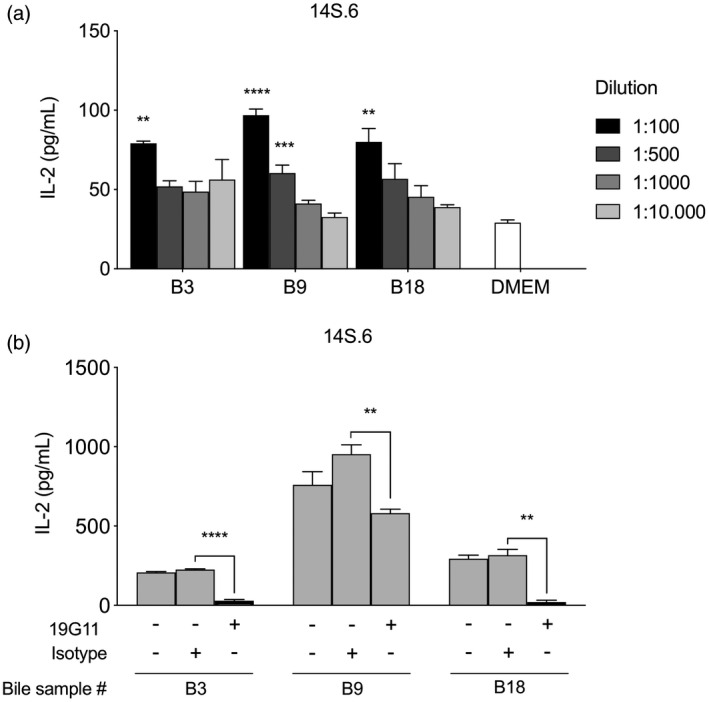
Natural killer T (NKT) cells are activated by biliary antigens presented by an antigen‐presenting cell. Bile samples from three patients with liver disease (B3, B9, B18) were co‐cultured in serial dilutions with a CD1d‐transfected antigen‐presenting cell (a). Activation of the non‐invariant NKT hybridoma 14S.6 is indicated by interleukin (IL)‐2‐production that follows a dose–response relationship, with phosphate‐buffered saline (PBS) as control. CD1d‐restriction was evaluated by addition of a monoclonal anti‐CD1d antibody (19G11) or an isotype control (b). Representative results from two independent experiments are shown, presented as mean ± standard error of the mean (s.e.m.). Statistical significance was calculated by one‐way analysis of variance (ANOVA) followed by correction for multiple testing using Bonferroni’s method within each bile sample in serial dilutions including the DMEM control (a) or using the unpaired Student’st‐test (b).*P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001.
The NKT cell‐activating bile antigens are potentially of both microbial and self‐derived origin
It has recently been demonstrated that bile is bacterially colonized in up to 50% of human bile samples, even in the setting of no clinically apparent infection [37]. To test whether the NKT cell‐activating bile antigens were of microbial origin we extracted microbial DNA and performed 16S rRNA sequencing of bile from the 21 patients shown in panel B; Table 2. Microbial DNA was found in eight of the 21 bile samples, four of which (bile sample B3, B4, B10 and B19) were among the 12 bile samples that activated the NKT hybridomas (Fig. 5). The sequenced microbial DNA included 14 genera from 14 families belonging to four phyla (Supporting information, Table S2). The most prominent phylum was Firmicutes. The bacteria Actinomyces, Bacteroides, Lactobacillus rhamnosus, Streptococcus sanguinis and Moryella (bile sample B3) and Enterobacteriaceae (bile sample B18) were not found in any of the non‐activating bile samples, which suggests that the NKT‐activating antigen in these bile samples could be of microbial origin. Interestingly, these bile samples (i.e. B3 and B18) also led to the strongest activation of the NKT hybridoma. Of the identified microbes, we found the genus Bacteroides (bile sample B3) and the species Streptococcus pneumoniae (bile sample B10) that have been previously shown to activate iNKT hybridomas (Fig. 5) [38, 39]. No other previously described bacteria associated with activation of NKT cells were found (Supporting information, Table S2). Taken together, these data suggest that some NKT cell‐activating bile antigens could be of microbial origin.
Fig. 5.
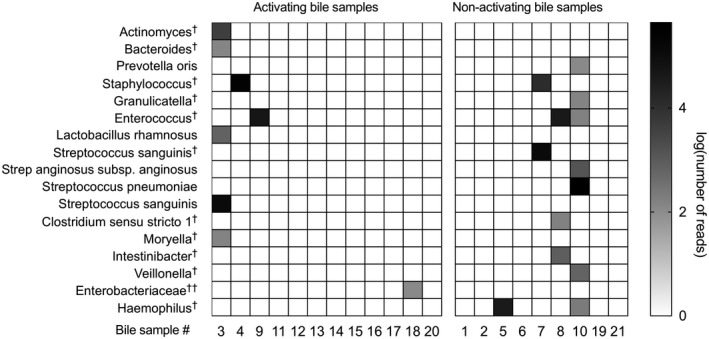
Bile antigens are of both endogenous and exogenous origin. Microbial DNA was extracted from bile of 21 patients with liver disease and sequenced by 16S rRNA. Four of 12 natural killer T (NKT) cell‐activating bile samples contained microbial DNA. Log‐transformed numbers of sequenced reads of microbiota at the most specified species level in bile samples by taxon‐based analysis arranged according to NKT cell‐activation are shown.†Family level;††genus level.
Discussion
In this study, we demonstrate the presence of NKT cell‐activating endogenous and exogenous antigens in human bile for the first time. The liver is constantly exposed to both harmless self and non‐self antigens through the portal vein and the bile ducts represent an extension of the gastrointestinal tract with exposure to the external environment. In this position the liver functions as an important immunological organ that needs to balance immune tolerance with targeted immune responses [40]. Compounds in bile can be of importance in liver immune responses, as cholangiocytes are constantly exposed to bile and represent the first line of defense in biliary immunity as well as a disease target in inflammatory bile duct diseases. Identification of novel bile antigens may provide clues to what is still unknown in the etiopathogenesis of bile duct disorders such as PBC and PSC.
Bile is secreted by hepatocytes with subsequent modification by cholangiocytes and contains lipids of both endogenous origin (i.e. phospholipids and cholesterol) as well as microbial and xenobiotic compounds [21, 41]. NKT cells recognize a broad spectrum of self and foreign antigens such as glycolipids, phospholipids, microbial antigens and even self‐peptide antigens [42]. Three iNKT and three niNKT hybridomas that express different TCRs, and thus cover a range of antigen‐specificities, were used in the experiments presented in this paper [22, 23]. All the investigated hybridomas were activated by biliary antigens and the activation patterns suggest that there is a diverse set of NKT cell‐activating antigens in human bile. Although the presence of biliary lipid antigens was detected using murine NKT hybridomas, human and murine NKT cells show a high level of conservation [7] and there is an overlap of activating lipids between human and murine NKT cells [43], and mechanisms for molecular recognition are conserved [44, 45]. Among the previously described classes of lipids in bile [21, 41], the iNKT hybridoma 24.8 has been found to be responsive to phosphatidylinositol (PI), phosphatidylglycerol (PG), phosphatidylcholine PC, phosphatidylethanolamine (PE), sphingomyelin (SM), the niNKT hybridoma 14S.6 has been found to respond to PI, PG, PE, lysophosphatidylcholine (LPC) and lysophosphatidylethanolamine (LPE) and the niNKT hybridoma 14S.7 has been found to respond to PI [14, 23, 46]. Thus, the activating lipids found in our experiments probably belong to one of these classes. We aimed to explore whether there are NKT cell‐activating antigens in bile, and did not study the presence of disease‐specific antigens. Although previous studies suggest no differences in phospholipids (i.e. phosphatidylcholine and lysophosphatidylcholine) in bile from PSC patients [47], future studies should aim to further characterize any potential antigenic differences in bile composition of patients with various liver and biliary diseases that may be important in the etiopathogenesis of these disorders.
Until recently bile was considered sterile, but increasing evidence demonstrates the presence of a bile microbiome [37, 48]. The findings in our study, with the presence of microbial DNA in eight of 21 bile samples, are in line with these observations. iNKT cells recognize microbial antigens from both pathogenic microbes (e.g. Borrelia burgdorferi, Sphingomonas and S. pneumonia) and commensal bacteria such as Bacteroides fragilis [49, 50, 51]. The presence of microbial DNA in bile correlated with a potent NKT hybridoma IL‐2 response. Most of the activating bile samples did not contain microbial DNA, which points to the presence of endogenous antigens as the source of the CD1d‐restricted NKT activation in these samples. One source of endogenous lipids may be products from damaged cholangiocytes due to cholestasis with consequent cell injury [52]. The existence of microbial‐derived antigens remains to be investigated further.
To avoid potential off‐target effects, we used a plate‐based assay where antigens bind to CD1d coated on a cell culture plate. This approach allows screening for bile antigens without interference by other bile compounds. The additional co‐culture assays with APCs that are dependent upon uptake of antigen and loading onto CD1d demonstrated similar results, which rules out assay‐based artifacts. The cell‐based assay using APCs also allowed evaluation of potential non‐CD1d‐dependent effects by using a monoclonal antibody that blocks CD1d‐presentation. Activation of NKT cells is complex, and unlike MHC‐restricted T cells they may be activated independently of TCR stimulation by exposure to microbial products or cytokines such as IL‐12 and IL‐18. Activation of the NKT hybridomas in vitro were not entirely blocked by the addition of a monoclonal antibody in all the experiments (Fig. 4b), which suggests additional pathways of activation, such as TLR stimulation, cytokine activation (i.e. IL‐12 and IL‐18) or a combination of these activation pathways [50, 53, 54].
The CD1d molecule is highly expressed in the liver, with expression on both circulating cells and multiple liver resident cells such as liver sinusoidal endothelial cells, Küpffer cells, dendritic cells, hepatocytes and cholangiocytes [17, 55, 56]. Most of these cells may be exposed to bile constituents in circumstances such as leakage of bile across the biliary epithelium in biliary diseases or during cholestasis [52]. The wide range of potential CD1d‐expressing APCs in close relationship with bile makes it interesting to study bile constituents in the context of biliary immunity. Herein, we demonstrate for the first time, to our knowledge, that bile contains immunoactive compounds that activate NKT cells in a CD1d‐dependent manner. Future studies should aim to further characterize the antigenic bile pool and investigate the importance of this immunological pathway in liver diseases that may introduce potentially novel therapeutic approaches.
Disclosures
The authors declare no relevant conflicts of interest.
Author contributions
L. V., N. L. B. and F. Z. performed the experiments. L. V., S. H. H. and N. L. B. performed data analysis. T. H. K. provided patient material. R. S. B. provided cell lines. E. M., J. R. H. and X. J. supervised different experiments and contributed with new ideas throughout the project. E. M., R. S. B., N. L. B., E. S and L. V. designed the experiments. L. V., N. L. B. and E. M. drafted the manuscript. All authors revised and approved of the final version of the manuscript.
Supporting information
Fig. S1. Princicpal coordinate plot (PCoA) showing the two primary axes PC1 and PC2, explaining 50.7% and 15.6% of the variance, respectively. The distances are calculated as Bray‐Curtis dissimilarities of enzyme commission features obtained with PICRUSt2. Only taxa with more than 100 reads are included in the plot.
Table S1. Clinical characteristics of bile sample donors B3, B9 and B18. The clinical data and biochemical values were acquired at the time of liver transplantation and any endoscopic retrograde cholangiography (ERC) procedures the year prior to liver transplantation were noted. MELD, Model for End‐stage Liver Disease; UC, Ulcerative colitis; UDCA, Ursodeoxycholic acid; ERC, endoscopic retrograde cholangiography; ALP, alkaline phosphatase; CRP, C‐reactive protein.
Table S2. Taxonomic overview of sequenced microbial DNA. The sequenced microbial DNA from the 21 bile samples in panel B included 14 genera from 14 families belonging to 4 phyla.
Acknowledgements
This study was supported by the South Eastern Norway Regional Health Authority (grant number 2015015 to E. M.), the Norwegian PSC Research Center and the National Institutes of Health (grant numbers: R01DK044319, R01DK053056, R01DK051362, R01DK088199 to R. S. B). The authors wish to thank the following members of the Norwegian PSC Research Center: Anne Pharo for technical assistance, Liv Wenche Thorbjørnsen for managing the biobank containing human tissues, Merete Tysdal for administrative support, Brian Chung for methodological discussion and Alexandra Goetz for technical assistance regarding microbiome analysis. Unloaded murine CD1d monomers were kindly provided by the NIH Tetramer Core, Emory, GA, USA.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet 2013; 382:1587–99. [DOI] [PubMed] [Google Scholar]
- 2. Hirschfield GM, Gershwin ME. The immunobiology and pathophysiology of primary biliary cirrhosis. Annu Rev Pathol Mech Dis 2013; 8:303–30. [DOI] [PubMed] [Google Scholar]
- 3. Jiang X, Karlsen TH. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol 2017; 14:279–95. [DOI] [PubMed] [Google Scholar]
- 4. Andersen IM, Tengesdal G, Lie BA, Boberg KM, Karlsen TH, Hov JR. Effects of coffee consumption, smoking, and hormones on risk for primary sclerosing cholangitis. Clin Gastroenterol Hepatol 2014; 12:1019–28. [DOI] [PubMed] [Google Scholar]
- 5. Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol 2011; 11:131–42. [DOI] [PubMed] [Google Scholar]
- 6. Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 2013; 13:101–17. [DOI] [PubMed] [Google Scholar]
- 7. Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1‐ and MR1‐restricted T cells. Annu Rev Immunol 2014; 32:323–66. [DOI] [PubMed] [Google Scholar]
- 8. Kaneko Y, Harada M, Kawano T et al Augmentation of Vα14 NKT cell‐mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin a‐induced hepatitis. J Exp Med 2000; 191:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wondimu Z, Santodomingo‐Garzon T, Le T, Swain MG. Protective role of interleukin‐17 in murine NKT cell‐driven acute experimental hepatitis. Am J Pathol 2010; 177:2334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Santodomingo‐Garzon T, Swain MG. Role of NKT cells in autoimmune liver disease. Autoimmun Rev 2011; 10:1–8. [DOI] [PubMed] [Google Scholar]
- 11. Mattner J, Savage PB, Leung P et al Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe 2008; 3:304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Syn W, Oo YH, Pereira TA et al Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology 2010; 51:1998–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolf MJ, Adili A, Piotrowitz K et al Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross‐talk with hepatocytes. Cancer Cell 2014; 26:549–64. [DOI] [PubMed] [Google Scholar]
- 14. Zeissig S, Murata K, Sweet L et al Hepatitis B virus‐induced lipid alterations contribute to natural killer T cell‐dependent protective immunity. Nat Med 2012; 18:1060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol 2019; 16:269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheung AC, Lorenzo Pisarello MJ, LaRusso NF. Pathobiology of biliary epithelia. Biochim Biophys Acta Mol Basis Dis 2018; 1864:1220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Canchis PW, Bhan AK, Landau SB, Yang L, Balk SP, Blumberg RS. Tissue distribution of the non‐polymorphic major histocompatibility complex class I‐like molecule, CD1d. Immunology 1993; 80:561–5. [PMC free article] [PubMed] [Google Scholar]
- 18. Schrumpf E, Tan C, Karlsen TH et al The biliary epithelium presents antigens to and activates natural killer T cells. Hepatology 2015; 62:1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berntsen NL, Fosby B, Tan C et al Natural killer T cells mediate inflammation in the bile ducts. Mucosal Immunol 2018; 11:1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schrumpf E, Jiang X, Zeissig S et al The role of natural killer T cells in a mouse model with spontaneous bile duct inflammation. Physiol Rep 2017; 5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tietz‐Bogert PS, Kim M, Cheung A et al Metabolomic profiling of portal blood and bile reveals metabolic signatures of primary sclerosing cholangitis. Int J Mol Sci 2018; 19:3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Behar SM, Podrebarac TA, Roy CJ, Wang CR, Brenner MB. Diverse TCRs recognize murine CD1. J Immunol 1999; 162:161–7. [PubMed] [Google Scholar]
- 23. Gumperz JE, Christopher R, Makowska A et al Murine CD1d‐restricted T cell recognition of cellular lipids. Immunity 2000; 12:211–21. [DOI] [PubMed] [Google Scholar]
- 24. Sugita M, Jackman RM, van Donselaar E et al Cytoplasmic tail‐dependent localization of CD1b antigen‐presenting molecules to MIICs. Science 1996; 273:349–352. [DOI] [PubMed] [Google Scholar]
- 25. Zeissig S, Olszak T, Melum E, Blumberg RS. Analyzing antigen recognition by natural killer T cells. Methods Mol Biol 2013; 960:557–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shimamura M, Yamamura M, Nabeshima T et al Simultaneous purification of DNA and RNA from microbiota in a single colonic mucosal biopsy. Microbiome 2016; 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fadrosh DW, Ma B, Gajer P et al An improved dual‐indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014; 2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kummen M, Holm K, Anmarkrud JA et al The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 2017; 66:611–619. [DOI] [PubMed] [Google Scholar]
- 29. Girardot C, Scholtalbers J, Sauer S, Su SY, Furlong EEM. Je, a versatile suite to handle multiplexed NGS libraries with unique molecular identifiers. BMC Bioinform 2016; 17:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin M. Cutadapt removes adapter sequences from high‐troughput sequencing reads. EMBnet.J 2011; 17:10–12. [Google Scholar]
- 31. Bushnell B, Rood J, Singer E. BBMerge – Accurate paired shotgun read merging via overlap. PLOS ONE 2017; 12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amir A, Daniel M, Navas‐Molina J et al Deblur rapidly resolves single‐nucleotide community sequence patterns. Am Soc Microbiol 2017; 2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bolyen E, Rideout JR, Dillon MR et al Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019; 37:852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bokulich NA, Kaehler BD, Rideout JR et al Optimizing taxonomic classification of marker‐gene amplicon sequences with QIIME 2’s q2‐feature‐classifier plugin. Microbiome 2018; 6:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quast C, Pruesse E, Yilmaz P et al The SILVA ribosomal RNA gene database project: improved data processing and web‐based tools. Nucleic Acids Res 2013; 41:590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tatituri RVV, Watts GFM, Bhowruth V et al Recognition of microbial and mammalian phospholipid antigens by NKT cells with diverse TCRs. Proc Natl Acad Sci USA 2013; 110:1827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liwinski T, Zenouzi R, John C et al Alterations of the bile microbiome in primary sclerosing cholangitis. Gut 2019;1–8. [DOI] [PubMed] [Google Scholar]
- 38. Wieland Brown LC, Penaranda C, Kashyap PC et al Production of α‐galactosylceramide by a prominent member of the human gut microbiota. PLOS Biol 2013; 11:e1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kinjo Y, Illarionov P, Vela JL et al Invariant natural killer T cells recognize glycolipids from pathogenic Gram‐positive bacteria. Nat Immunol 2011; 12:966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heymann F, Tacke F. Immunology in the liver – from homeostasis to disease. Nat Rev Gastroenterol Hepatol 2016; 13:88–110. [DOI] [PubMed] [Google Scholar]
- 41. Boyer JL. Bile formation and secretion. Compr Physiol 2013; 3:1035–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Godfrey DI, Uldrich AP, Mccluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol 2015; 16:1114–23. [DOI] [PubMed] [Google Scholar]
- 43. De Libero G. How T cells recognize lipids as antigens. Future Lipidol 2009; 4:189–203. [Google Scholar]
- 44. Borg NA, Wun KS, Kjer‐Nielsen L et al CD1d‐lipid‐antigen recognition by the semi‐invariant NKT T‐cell receptor. Nature 2007. Jul; 448:44–49. [DOI] [PubMed] [Google Scholar]
- 45. Kjer‐Nielsen L, Borg NA, Pellicci DG et al A structural basis for selection and cross‐species reactivity of the semi‐invariant NKT cell receptor in CD1d/glycolipid recognition. J Exp Med 2006; 203:661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rauch J, Gumperz J, Robinson C et al Structural features of the acyl chain determine self‐phospholipid antigen recognition by a CD1d‐restricted Invariant NKT (iNKT) cell. J Biol Chem 2003; 278:47508–47515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gauss A, Ehehalt R, Lehmann W‐D et al Biliary phosphatidylcholine and lysophosphatidylcholine profiles in sclerosing cholangitis. World J Gastroenterol 2013; 19:5454–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pereira P, Aho V, Arola J et al Bile microbiota in primary sclerosing cholangitis: Impact on disease progression and development of biliary dysplasia. PLOS ONE 2017; 12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kinjo Y, Tupin E, Wu D et al Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol 2006; 7:978–986. [DOI] [PubMed] [Google Scholar]
- 50. Mattner J, DeBord KL, Ismail N et al Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 2005; 434:525–529. [DOI] [PubMed] [Google Scholar]
- 51. An D, Oh SF, Olszak T et al Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 2014; 156:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jansen PLM, Ghallab A, Vartak N et al The ascending pathophysiology of cholestatic liver disease. Hepatology 2017; 65:722–738. [DOI] [PubMed] [Google Scholar]
- 53. Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d‐restricted natural killer T cell activation during microbial infection. Nat Immunol 2003; 4:1230–1237. [DOI] [PubMed] [Google Scholar]
- 54. Brigl M, Tatituri RVV, Watts GFM et al Innate and cytokine‐driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med 2011; 208:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Geissmann F, Cameron TO, Sidobre S et al Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLOS Biol 2005; 3:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Exley M, Garcia J, Wilson SB et al CD1d structure and regulation on human thymocytes, peripheral blood T cells. B cells and monocytes. Immunology 2000; 100:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Princicpal coordinate plot (PCoA) showing the two primary axes PC1 and PC2, explaining 50.7% and 15.6% of the variance, respectively. The distances are calculated as Bray‐Curtis dissimilarities of enzyme commission features obtained with PICRUSt2. Only taxa with more than 100 reads are included in the plot.
Table S1. Clinical characteristics of bile sample donors B3, B9 and B18. The clinical data and biochemical values were acquired at the time of liver transplantation and any endoscopic retrograde cholangiography (ERC) procedures the year prior to liver transplantation were noted. MELD, Model for End‐stage Liver Disease; UC, Ulcerative colitis; UDCA, Ursodeoxycholic acid; ERC, endoscopic retrograde cholangiography; ALP, alkaline phosphatase; CRP, C‐reactive protein.
Table S2. Taxonomic overview of sequenced microbial DNA. The sequenced microbial DNA from the 21 bile samples in panel B included 14 genera from 14 families belonging to 4 phyla.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


